- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Synthesis, α-Amylase Inhibitory Activity and Molecular Docking Studies of 2,4-Thiazolidinedione Derivatives
Fawad Naeem1, Humaira Nadeem2, *, Aun Muhammad3, Muhammad Ammar Zahid1, Adil Saeed2
Abstract
Introduction:
2,4-Thiazolidinedione and its derivatives exhibit a variety of pharmacological activities including antidiabetic, antiviral, antifungal, anti-inflammatory, anti-cancer and aldose reductase inhibitory activities. Keeping in mind the pharmacological potential of 2,4-Thiazolidinedione derivatives as antidiabetic agents, seven arylidene derivatives of 2,4-thiazolidinedione 1(a-g) and four corresponding acetic acid derivatives 2(a-d) have been synthesized by a three-step procedure.
Methods:
All the synthesized compounds were characterized by elemental analysis, FTIR, 1HNMR, and 13CNMR and further screened for their α-amylase inhibitory potential.
Results:
All the compounds 1(a-g) and 2(a-d) showed varying degree of α-amylase inhibition, especially compound 1c (IC50 = 6.59μg/ml), 1d (IC50=2.03μg/ml) and 1g (IC50 = 3.14μg/ml) displayed significantly potent α-amylase inhibition as compared to the standard acarbose (IC50 = 8.26μg/ml). None of the acetic acid derivatives of 5-arylidene-2,4-thiazolidinedione showed prominent inhibitory activity. Docking results indicated that the best binding conformation was found inside the active site cleft of enzyme responsible for hydrolysis of carbohydrates.
Conclusion:
Therefore, it can be concluded that 2,4-thiazolidinedione derivatives can be used as effective lead molecules for the development of α-amylase inhibitors for the management of diabetes.
Article Information
Identifiers and Pagination:
Year: 2018Volume: 5
First Page: 134
Last Page: 144
Publisher Id: CHEM-5-134
DOI: 10.2174/1874842201805010134
Article History:
Received Date: 19/5/2018Revision Received Date: 11/10/2018
Acceptance Date: 21/10/2018
Electronic publication date: 30/11/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Riphah Institute of Pharmaceutical Sciences, G-7/4, Islamabad, Pakistan; Tel: 03235010850; E-mail: humaira.nadeem@riphah.edu.pk
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 19-5-2018 |
Original Manuscript | Synthesis, α-Amylase Inhibitory Activity and Molecular Docking Studies of 2,4-Thiazolidinedione Derivatives | |
1. INTRODUCTION
Alpha amylase is an important enzyme which is secreted primarily by the salivary glands and the pancreas contributing its fundamental role in the metabolism of starch and glycogen, which are commonly present in plants, microorganisms and also in higher organisms [1Kandra, L. α-Amylases of medical and industrial importance. J. Mol. Struct. THEOCHEM, 2003, 666, 487-498.
[http://dx.doi.org/10.1016/j.theochem.2003.08.073] , 2Whitcomb, D.C.; Lowe, M.E. Human pancreatic digestive enzymes. Dig. Dis. Sci., 2007, 52(1), 1-17.
[http://dx.doi.org/10.1007/s10620-006-9589-z] [PMID: 17205399] ].The enzyme α–Amylase belongs to family of endoamylases which are responsible for the initial hydrolysis of starch into shorter oligosaccharides by the breakage of α-D-(1, 4) glycosidic bonds [1Kandra, L. α-Amylases of medical and industrial importance. J. Mol. Struct. THEOCHEM, 2003, 666, 487-498.
[http://dx.doi.org/10.1016/j.theochem.2003.08.073] , 3Brayer, G.D.; Luo, Y.; Withers, S.G. The structure of human pancreatic alpha-amylase at 1.8 A resolution and comparisons with related enzymes. Protein Sci., 1995, 4(9), 1730-1742.
[http://dx.doi.org/10.1002/pro.5560040908] [PMID: 8528071] -5Tangphatsornruang, S.; Naconsie, M.; Thammarongtham, C.; Narangajavana, J. Isolation and characterization of an alpha-amylase gene in cassava (Manihot esculenta). Plant Physiol. Biochem., 2005, 43(9), 821-827.
[http://dx.doi.org/10.1016/j.plaphy.2005.07.014] [PMID: 16297635] ].Various amylolytic enzymes play their role in the breakdown of starch but activity of α-amylase is mandatory to start the process [5Tangphatsornruang, S.; Naconsie, M.; Thammarongtham, C.; Narangajavana, J. Isolation and characterization of an alpha-amylase gene in cassava (Manihot esculenta). Plant Physiol. Biochem., 2005, 43(9), 821-827.
[http://dx.doi.org/10.1016/j.plaphy.2005.07.014] [PMID: 16297635] ]. In order to formulate a successful treatment strategy in diabetes it is important to limit the postprandial hyperglycaemia which can be accomplished by the obstruction of carbohydrate hydrolysing enzymes. As a matter of fact, two enzymes that are involved in the metabolism of carbohydrates are alpha glucosidase and alpha amylase. Alpha glucosidase plays its part in the lysis of disaccharides and starch to produce glucose, whereas the alpha amylase causes the breakage of carbohydrates having long chains. These are considered as primary enzymes involved in the digestion process and they play their part in the absorption within the intestine. Inhibitors of this enzyme can slow down carbohydrate absorption prolonging total carbohydrate absorption time, decreasing the rate of glucose absorption and, subsequently, reducing the postprandial plasma glucose increase. Clinically used diabetes control inhibitors are acarbose and miglitol. By analyzing the role of these enzymes, it can be interpreted that the inhibition of these enzymes can be beneficial for the treatment of diabetes [6Subramanian, R.; Asmawi, M.Z.; Sadikun, A. In vitro alpha-glucosidase and alpha-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim. Pol., 2008, 55(2), 391-398.
[PMID: 18511986] ]. Despite all the development in the management of diabetes, currently the only agents which show their action in reducing the postprandial hyperglycaemia are the alpha-glucosidase inhibitors [7Mooradian, A.D.; Thurman, J.E. Drug therapy of postprandial hyperglycaemia. Drugs, 1999, 57(1), 19-29.
[http://dx.doi.org/10.2165/00003495-199957010-00003] [PMID: 9951949] ].
It has been documented that novel 2,4-thiazolidinedione derivatives (TZDs) have emerged as an important pharmacologically active class and have a special place in drug design and discovery [8Angajala, G.; Subashini, R. Diabetes Mellitus and Human Health Care., 2014, , 229-246.
[http://dx.doi.org/10.1201/b16415-6] ]. Compounds belonging to this class of heterocyclic compounds are being successfully used in various diabetic complexities and for the patients of Type-2 Diabetes [9Yoshioka, T.; Fujita, T.; Kanai, T.; Aizawa, Y.; Kurumada, T.; Hasegawa, K.; Horikoshi, H. Studies on hindered phenols and analogues. 1. Hypolipidemic and hypoglycemic agents with ability to inhibit lipid peroxidation. J. Med. Chem., 1989, 32(2), 421-428.
[http://dx.doi.org/10.1021/jm00122a022] [PMID: 2913302] ]. It has also been reported that TZDs also tend to show anti-bacterial [10Bozdağ-Dündar, O.; Verspohl, E.J.; Daş-Evcimen, N.; Kaup, R.M.; Bauer, K.; Sarikaya, M.; Evranos, B.; Ertan, R. Synthesis and biological activity of some new flavonyl-2,4-thiazolidinediones. Bioorg. Med. Chem., 2008, 16(14), 6747-6751.
[http://dx.doi.org/10.1016/j.bmc.2008.05.059] [PMID: 18565754] ], anti-fungal [11Mori, M.; Takagi, M.; Noritake, C.; Kagabu, S. 2,4-Dioxo-1,3-thiazolidine derivatives as a lead for new fungicides. J. Pestic. Sci., 2008, 33, 357-363.
[http://dx.doi.org/10.1584/jpestics.G08-15] ], anti-TB [12Visentini, P. Antituberculosis chemotherapeutic action of derivatives of 2-4-thiazolidinedione. I. Action in vitro of the 2-phenylhydrazone of 2,4-thiazolidinedione. Farmaco, Sci., 1954, 9(5), 274-277.
[PMID: 13191358] ], anti-convulsant [13Marshall, P.G.; Vallance, D.K. Anticonvulsant activity; derivatives of succinimide, glutarimide, thiazolidinedione and methanol, and some miscellaneous compounds. J. Pharm. Pharmacol., 1954, 6(10), 740-746.
[http://dx.doi.org/10.1111/j.2042-7158.1954.tb11011.x] [PMID: 13212661] ], anti-inflammatory [14Ceriello, A. Thiazolidinediones as anti-inflammatory and anti-atherogenic agents. Diabetes Metab. Res. Rev., 2008, 24(1), 14-26.
[http://dx.doi.org/10.1002/dmrr.790] [PMID: 17990280] ], pesticidal [15Eun, J.S.; Kim, K.S.; Kim, H.N.; Park, S.A.; Ma, T-Z.; Lee, K.A.; Kim, D.K.; Kim, H.K.; Kim, I.S.; Jung, Y.H.; Zee, O.P.; Yoo, D.J.; Kwak, Y.G. Synthesis of psoralen derivatives and their blocking effect of hKv1.5 channel. Arch. Pharm. Res., 2007, 30(2), 155-160.
[http://dx.doi.org/10.1007/BF02977688] [PMID: 17366735] ] and insecticidal activities [16Sahu, S.K.; Banerjee, M.; Mishra, S.K.; Mohanta, R.K.; Panda, P.K.; Misro, P.K. Synthesis, partition coefficients and antibacterial activity of 3′-phenyl (substituted)-6′-aryl-2′ (1H)-cis-3′,3‘a-dihydrospiro [3-H-indole-3,5’-pyrazolo (3′,4′-d)-thiazolo-2-(1H)-ones]. Acta Pol. Pharm., 2007, 64(2), 121-126. [3-H-indole-3,5’-pyrazolo (3’,4’-d)-thiazolo-2-(1H)-ones].
[PMID: 17665861] ]. Due to the potential of TZDs in the management of diabetes, a series of new thiazolidinedione derivatives have been synthesized in the current study and evaluated for their in vitro α-amylase inhibitory activity.
2. EXPERIMENTAL
2.1. General Chemistry
All the chemicals used in the synthesis were purchased from Sigma Aldrich and used without any further purification or distillation. Synthesized 5-substituted 2,4-Thiazolidinedione derivatives were purified by recrystallization in appropriate solvents and purity was checked by thin layer chromatography using silica gel 60 F254 Merck using ethyl acetate: petroleum ether (1:1) as solvent system. Melting points were determined in open glass capillaries by using Digital Gallenhamp (SANYO) model MPD.BM 3.5 apparatus and are uncorrected. Characterization of all the newly synthesized arylidene derivatives was done by using spectrophotometric analysis i.e. FTIR (Thermo-scientific NICOLET IS10 Spectrophotometer) and 1HNMR (Bruker AM-300 Spectrophotometer) using DMSO-d6 as solvent. Chemical shifts (δ) were indicated in parts per million downfield from tetramethylsilane, and the coupling constants (J) were recorded in Hertz. Molecular docking studies were carried out using AutoDock Vina software.
2.2. Synthesis of 2,4-Thiazolidinedione
2,4 Thiazolidinedione was synthesized following the standard procedure [17Nawale, S.L.; Dhake, A.S. Synthesis and evaluation of novel thiazolidinedione derivatives for antibacterial activity. Pharma Chem., 2012, 4, 2270-2277.].
Yield 62%, m.p (obs) 123-125oC, m.p (lit) 123-125oC, R f = 0.34 (ethyl acetate: pet. ether 1:1), IR (cm-1): 3217 (N-H), 1680 (C=O), 1648 (C=O), 1472 (CH2).
2.3. General Procedure for the Synthesis of 5-Arylidene-2,4-Thiazolidinediones 1(a-g)
A mixture of 2,4-Thiazolidinedione (3.0 mmol), diethyl amine (3.0 mmol) and respective aldehydes (3.0 mmol) in 20-25 ml of distilled water was stirred at room temperature for 3-5 hours. The progress of the reaction was monitored by thin layer chromatography (ethyl acetate: pet. ether 1:1). After reaction completion as indicated by TLC, the crude product separated was filtered and recrystallized from ethanol [18Barakat, A.; Al-Majid, A.M.; AL-Najjar, H.J.; Mabkhot, Y.N.; Ghabbour, H.A.; Fun, H-K. An efficient and green procedure for synthesis of rhodanine derivatives by aldol-thia-Michael protocol using aqueous diethylamine medium. RSC Adv., 2014, 4, 4909-4916.
[http://dx.doi.org/10.1039/c3ra46551a] ] (Table 1).
2.3.1. 5-(3-Phenylallylidene) Thiazolidine-2,4-Dione (1a)
Yield 67%, m.p. 215-217°C, Rf = 0.25 (ethyl acetate: pet. Ether 1:1), IR (cm-1): 3217 (N-H), 1656 (C=O), 1648 (C=O), 1511 (C=C), 1HNMR (300 MHz, DMSO): 12.37 (s, 1H, NH), 7.26-7.42 (m, 5H, Aryl-H), 7.99 (d, 1H, J = 14.5 Hz, HC=CH), 7.02 (d, 1H, J = 13.0 Hz, C=CH), 6.71 (d, 1H, J = 14.5 Hz, HC=CH). 13CNMR (100 MHz, CCl4): 169.3 (C=O), 167.1 (C=O), 140.2 (CH), 136.6, 131.2, 131.9, 127.4 (Aryl-C), 128.7 (C). Elemental Anal. Calc.: C = 62.26%, N = 6.05%, H = 3.92%, Found: C = 62.32%, N = 6.13%, H = 4.03%.
2.3.2. 5-(2-Hydroxybenzylidene) Thiazolidine-2,4-Dione (1b)
Yield 69%, m.p. 195-197°C, Rf = 0.36 (ethyl acetate: pet. Ether 1:1), IR (cm-1): 3121 (N-H), 1671 (C=O), 1656 (C=O), 1591 (C=C), 1HNMR (300 MHz, DMSO): 12.31 (s, 1H, NH), 10.27 (s, 1H, OH), 8.22 (s, 1H, C=CH), 6.72-7.49 (m, 4H, Aryl-H). 13CNMR (100 MHz, CCl4): 168.7 (C=O), 166.5 (C=O), 129.0 (C), 127.2 (CH),156.5-117.1 (Aryl-C). Elemental Anal. Calc.: C = 54.24%, N = 6.32%, H = 3.19% Found: C = 53.98%, N = 6.12%, H = 3.23%.
2.3.3. 5-(3-Hydroxybenzylidene) Thiazolidine-2,4-Dione (1c)
Yield 70%, m.p. 240-242°C, Rf = 0.38 (ethyl acetate: pet. Ether 1:1), IR (cm-1): 3293 (N-H), 1675 (C=O), 1664 (C=O), 1587 (C=C), 1HNMR (300 MHz, DMSO): 12.58 (s, 1H, NH), 9.85 (s, 1H, OH), 7.68 (s, 1H, C=CH), 6.70-7.31 (m, 4H, Aryl-H). 13CNMR (100 MHz, CCl4): 168.3 (C=O), 165.9 (C=O), 133.1 (CH), 128.4 (C), 158.4-107.4 (Aryl-C). Elemental Anal. Calc.: C = 54.24%, N = 6.32%, H = 3.19%, Found: C = 54.11%, N = 6.22%, H = 3.26%.
2.3.4. 5-Benzylidenethiazolidine-2,4-Dione (1d)
Yield 72%, m.p. 230-232°C, Rf = 0.26 (ethyl acetate: pet. Ether 1:1), IR (cm-1): 3127 (N-H), 1664 (C=O), 1645 (C=O), 1524 (C=C), 1HNMR (300 MHz, DMSO): 12.31 (s, 1H, NH), 7.97 (s, 1H, C=CH), 7.33-7.65 (m, 5H, Aryl-H). 13CNMR (100 MHz, CCl4): 169.1 (C=O), 166.9 (C=O), 131.7 (CH), 129.8 (C), 133.1-130.0 (Aryl-C). Elemental Anal. Calc.: C = 58.47%, N = 6.82%, H = 3.43%, Found: C = 58.52%, N = 6.78%, H = 3.38%.
2.3.5. 5-(4-Chlorobenzylidene) Thiazolidine-2,4-Dione (1e)
Yield 81%, m.p. 210-212°C, Rf = 0.19 (ethyl acetate: pet. Ether 1:1), IR (cm-1): 3250 (N-H), 1678 (C=O), 1660 (C=O), 1544 (C=C), 1HNMR (300 MHz, DMSO): 12.34 (s, 1H, NH), 7.91 (s, 1H, C=CH), 7.61-7.64 (m, 5H, Aryl-H). 13CNMR (100 MHz, CCl4): 169.5 (C=O), 167.0 (C=O), 133.6 (CH), 127.8 (C), 136.1-129.3 (Aryl-C). Elemental Anal. Calc.: C = 50.06%, N = 5.84%, H = 2.52% Found: C = 50.21%, N = 5.98%, H = 2.59%.
2.3.6. 5-(4-Hydroxybenzylidene) Thiazolidine-2,4-Dione (1f)
Yield 62%, m.p. 276-278°C, Rf = 0.40 (ethyl acetate: pet. Ether 1:1), IR (cm-1): 3144 (N-H), 1677 (C=O), 1663 (C=O), 1573 (C=C), 1HNMR (300 MHz, DMSO): 12.44 (s, 1H, NH), 9.68 (s, 1H, OH), 7.94 (s, 1H, C=CH), 6.59-7.45 (m, 4H, Aryl-H). 13CNMR (100 MHz, CCl4): 168.9 (C=O), 166.5 (C=O), 130.9 (CH), 129.1 (C), 157.9-116.3 (Aryl-C). Elemental Anal. Calc.: C = 54.24%, N = 6.32%, H = 3.19%, Found: C = 54.16%, N = 6.25%, H = 3.17%.
2.3.7. 5-(4-Hydroxy-3-Methoxybenzylidene) Thiazolidine-2,4-Dione (1g)
Yield 65%, m.p. 320-322°C, Rf = 0.37 (ethyl acetate: pet. Ether 1:1), IR (cm-1): 3266 (N-H), 1656 (C=O),1667 (C=O), 1527 (C=C), 1HNMR (300 MHz, DMSO): 12.39 (s, 1H, NH), 9.55 (s, 1H, OH), 7.93 (s, 1H, C=CH), 7.01-7.29 (m, 3H, Aryl-H), 3.83 (s, 3H, OCH3). 13CNMR (100 MHz, CCl4): 169.4 (C=O), 167.5 (C=O), 132.4 (CH), 128.7 (C), 149.0-108.2 (Aryl-C). Elemental Anal. Calc.: C = 52.53%, N = 5.57%, H = 3.61%, Found: C = 52.48%, N = 5.63%, H = 3.70%.
2.4. General Procedure for the Synthesis of 5-Arylidene 2,4 Thiazolidinediones-2-Acetic Acid Derivatives 2(a-e)
To a suspension of 6.0 mmoles of the respective arylidene derivative in ethanol, 7.0 mmol of potassium hydroxide was added and the mixture was stirred for two hours at room temperature. The precipitates of corresponding potassium salt were separated, filtered and washed with ethanol. In the next step, a mixture of 0.02 mole of respective salt and 0.03 mole of sodium chloroacetate in ethanol was refluxed for 5-6 hours. The reaction mixture was then diluted with 40 ml of water and acidified with dilute hydrochloric acid to pH 3. The precipitates formed were separated by filtration and washed with ether. The crude product was recrystallized from DMF-ethanol [19Zimenkovskii, B.S.; Kutsyk, R.V.; Lesyk, R.B.; Matyichuk, V.S.; Obushak, N.D.; Klyufinska, T.I. Synthesis and antimicrobial activity of 2,4-dioxothiazolidine-5-acetic acid amides. Pharm. Chem. J., 2006, 40, 303-306.
[http://dx.doi.org/10.1007/s11094-006-0115-6] ] (Table 2).
2.4.1. 2-(2,4-dioxo-5-(3-phenylallylidene) thiazolidin-3-yl) acetic acid (2a)
Yield 42%, m.p. 218-220°C, Rf = 0.34 (ethyl acetate: pet. Ether 1:1), IR (cm-1): 3560 (O-H), 1710 (C=O), 1676 (C=O), 1640 (C=O), 1500(C=C), 1HNMR (300 MHz, DMSO): 12.52 (s, 1H, COOH), 7.99 (d, 1H, J = 12.4 Hz, C=CH), 7.33-7.46 (m, 5H, Aryl-H), 7.02 (d, 1H, J = 13.5 Hz HC=CH), 6.71 (d, 1H, J = 13.4 Hz, HC=CH), 4.65 (s, 2H, CH2-CO). 13CNMR (100 MHz, CCl4): 173.5 (COOH), 170.0 (C=O), 165.7 (C=O), 138.9, 133.8, 132.7 (CH), 136.5-127.1 (Aryl C). 52.6 (CH2). Elemental Anal. Calc.: C = 58.06%, N = 4.83%, H = 3.83%, Found: C = 58.12%, N = 4.77%, H = 3.80%.
2.4.2. 2-(5-(2-Hydroxybenzylidene)-2,4-Dioxothiazolidin-3-yl) Acetic acid (2b)
Yield 40%, m.p. 232-234°C, Rf = 0.39 (ethyl acetate: pet. Ether 1:1), IR (cm-1): 3418 (O-H), 1715 (C=O), 1680 (C=O), 1664 (C=O), 1594(C=C), 1HNMR (300 MHz, DMSO): 12.53(s, 1H, COOH), 10.25 (s, 1H, OH), 8.02 (s, 1H, C=CH), 6.91-7.30 (m, 4H, Aryl-H), 3.39 (s, 2H, CH2). 13CNMR (100 MHz, CCl4): 171.4 (COOH), 169.0 (C=O), 164.6 (C=O), 127.8 (CH), 157.1-117.1 (Aryl-C), 50.7 (CH2). Elemental Anal. Calc.: C = 51.56%, N = 5.01%, H = 3.24%, Found: C = 51.64%, N = 5.07%, H = 3.31%.
2.4.3. 2-(5-(3-Hydroxybenzylidene)-2,4-Dioxothiazolidin-3-yl) Acetic Acid (2c)
Yield 54%, m.p. 246-248°C, Rf = 0.64 (ethyl acetate: pet. Ether 1:1), IR (cm-1): 3289 (O-H), 1705 (C=O), 1648 (C=O), 1675 (C=O), 1583(C=C), 1HNMR (300MHz, DMSO): 12.6 (s, 1H, COOH), 9.85 (s, 1H, OH), 7.69 (s, 1H, C=CH), 6.87-7.34 (m, 4H, Aryl-H), 3.38 (s, 2H, CH2). 13CNMR (300MHz, DMSO): 173.3 (COOH), 169.6 (C=O), 164.4 (C=O), 132.9 (CH), 158.4-107.3 (Aryl-C), 52.3 (CH2). Elemental Anal. Calc.: C = 51.56%, N = 5.01%, H = 3.24%, Found: C = 51.60%, N = 5.11%, H = 3.27%.
2.4.4. 2-(5-benzylidene-2,4-Dioxothiazolidin-3-yl) Acetic Acid (2d)
Yield 62%, m.p. 270-272°C, Rf = 0.14 (ethyl acetate: pet. Ether 1:1), IR (cm-1): 3470 (O-H), 1717 (C=O), 1674 (C=O),1665 (C=O), 1561 (C=C),1HNMR (300 MHz, DMSO): 12.54 (s, 1H, COOH), 7.85 (s, 1H, C=CH), 7.33-7.65 (m, 4H, Aryl-H), 4.75 (s, 2H, CH2). 13CNMR (300MHz, DMSO): 171.3 (COOH), 170.2 (C=O), 164.6 (C=O), 133.6 (CH), 133.0-128.5 (Aryl-C), 52.8 (CH2). Elemental Anal. Calc.: C = 54.69%, N = 5.31%, H = 3.44%, Found: C = 54.73%, N = 5.39%, H = 3.40%.
2.5. In-vitro α-Amylase Inhibitory Activity
In-vitro α-amylase inhibitory activity was performed by following reported procedure. 100 µL reaction mixture was prepared using 70 µL of 50 mM phosphate buffer pH 6.8, 50 µL of test compound followed by the addition of 10 µL (0.057 units) enzyme solution in the buffer. The contents were mixed, pre-incubated for 10 minutes at 25°C and absorbance measured at 400nm. The 10 µL of 0.5 mM p-nitrophenyl glucopyranoside and incubated at 25°C, the absorbance of p-nitrophenol was measured at 400nm using the 96-well plate reader. Acarbose was used as standard. All the experiments were carried out in triplicates. The percent inhibition was calculated by the given formula:
 |
Active compounds were suitably diluted and their inhibition studies were determined. Results were obtained as %inhibition. The IC50 values of samples were calculated by linear regression analysis.
2.6. Molecular Docking Studies
Exploration of mechanism of action and molecular interaction of synthesized derivatives with α-amylase was performed by molecular docking studies using AutoDock Vina software. To study the molecular interaction of compounds, crystallographic data of human pancreatic α-amylase (PDB:1HNY) was retrieved from Protein Data Bank. Retrieved crystal structure of α-amylase was cleaned and hydrogen atoms were added. All the heteroatoms were removed before docking study. The samples used in the molecular docking studies were compounds 1(a-g) and 2(a-d) (Fig. 2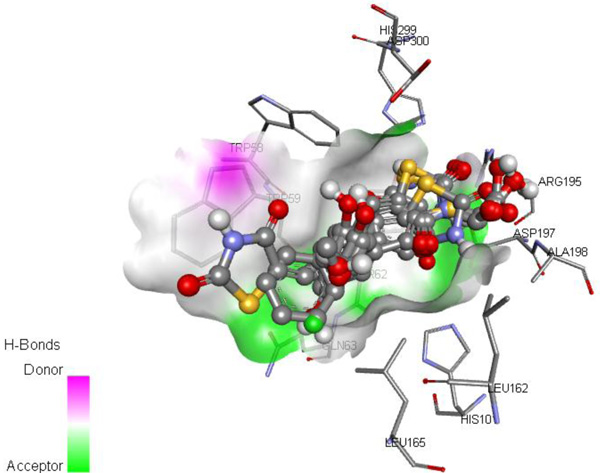 ). The 2D structures were sketched using ChemDraw-ultrav14.0 followed by 3D structure conversion and energy minimization by ChemBioOffice modelling software (Cambridgesoft, UK). The 2D and 3D interactions were visualized using discovery studio 4.0.
). The 2D structures were sketched using ChemDraw-ultrav14.0 followed by 3D structure conversion and energy minimization by ChemBioOffice modelling software (Cambridgesoft, UK). The 2D and 3D interactions were visualized using discovery studio 4.0.
3. RESULTS AND DISCUSSION
3.1. Chemistry
In our study, synthesis of 2,4-thiazolidinedione was carried out by reacting chloroacetic acid and thiourea in the presence of concentrated HCl (Scheme 1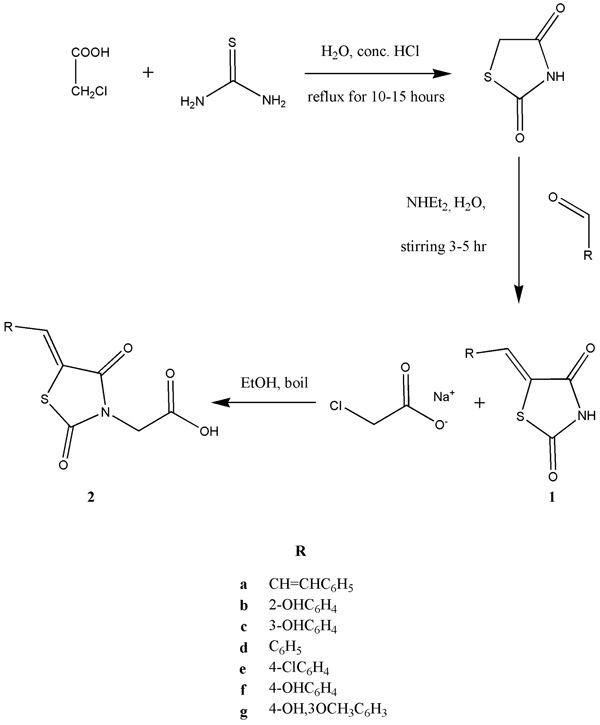 ). Formation of thiazolidinedione nucleus was confirmed by comparing its melting point with the reported literature [13Marshall, P.G.; Vallance, D.K. Anticonvulsant activity; derivatives of succinimide, glutarimide, thiazolidinedione and methanol, and some miscellaneous compounds. J. Pharm. Pharmacol., 1954, 6(10), 740-746.
). Formation of thiazolidinedione nucleus was confirmed by comparing its melting point with the reported literature [13Marshall, P.G.; Vallance, D.K. Anticonvulsant activity; derivatives of succinimide, glutarimide, thiazolidinedione and methanol, and some miscellaneous compounds. J. Pharm. Pharmacol., 1954, 6(10), 740-746.
[http://dx.doi.org/10.1111/j.2042-7158.1954.tb11011.x] [PMID: 13212661] ]. In the next step, 2,4-thiazolidinedione was reacted with various aldehydes in the presence of diethyl amine and distilled water [14Ceriello, A. Thiazolidinediones as anti-inflammatory and anti-atherogenic agents. Diabetes Metab. Res. Rev., 2008, 24(1), 14-26.
[http://dx.doi.org/10.1002/dmrr.790] [PMID: 17990280] ] to get 5-arylidene derivatives of 2,4-thiazolidinediones 1 (a-g). The synthesized compounds were characterized by FTIR and NMR spectroscopic data. The yields of compounds were obtained in the range of 40-70%. Purity in each case was established by thin layer chromatography. In the IR spectra characteristic peaks of amide carbonyl of thiazolidinedione moiety were observed in the range 1648-1686 cm-1. NH stretchings were present at 3121-3266 cm-1. Aromatic C=C stretchings were obsereved at 1500-1594 cm-1 which indicated the condensation of aromatic aldehydes with thiazolidinedione nucleus. In the 1HNMR spectra of 1 (a-g) characteristic singlet peaks of methine protons were observed downfield in the range 7.02-8.22 ppm confirming the formation of arylidene derivatives. In case of 1a having cinnamoyl moiety two doublets of methine protons appeared at 6.71 and 7.99 ppm with trans coupling constant of 14.0 Hz. NH singlet peak was observed in all derivatives at 12.31-12.58 ppm. The 13CNMR data showed two peaks for carbonyl carbons of thiazolidinedione nucleus. Methine carbon of arylidene moiety appeared at 143.3 ppm as expected.
In the last step compounds 2(a-d) were synthesized by the reaction of respective arylidene derivative of thiazolidinedione 1(a-d) with sodium chloroacetate [19Zimenkovskii, B.S.; Kutsyk, R.V.; Lesyk, R.B.; Matyichuk, V.S.; Obushak, N.D.; Klyufinska, T.I. Synthesis and antimicrobial activity of 2,4-dioxothiazolidine-5-acetic acid amides. Pharm. Chem. J., 2006, 40, 303-306.
[http://dx.doi.org/10.1007/s11094-006-0115-6] ]. In case of other three arylidene derivatives 1(e-g) the reaction was not successful due to very low solubility of these compounds. The yields of isolated acetic acid derivatives were also low. The target compounds 2(a-d) were characterized by FTIR and NMR spectroscopic data. The IR data exhibited characteristic carbonyl stretching of carboxylic acid moiety in the range 1732-1750 cm-1, while amide cabonyl stretchings were observed at lower frequency. The 1HNMR data of these derivatives exhibited characteristic singlet peak for methylene protons of acetic acid moiety in the range 3.38-4.75 ppm. Also, NH singlets were absent in all the spectra. Singlets of carboxylic OH were observed downfield at 12.52-12.60 ppm. In the 13CNMR spectra carboxylic carbon resonated at 169.0 ppm while carbonyl groups of thiazolidinedione moiety appeared at 164.4 and 173.4 ppm. Methylene carbon appeared upfield at 47.9 ppm. All the other carbons resonated in the expected region.
3.2. In-Vitro α-Amylase Inhibitory Activity
The synthesized compounds were tested for their inhibitory potential against α-amylase enzyme. Different concentrations of the test compounds i.e. 20 µg/mL, 30 µg/mL, 40 µg/mL and 50 µg/mL were used for the assay. The concentrations were plotted against % inhibition and IC50 of each compound was calculated by non-linear regression method by using Graphpad Prism V6.0. All tested compounds showed good to excellent inhibitory potential with IC50 values ranging from 2.03 µg/mL to 32.16 µg/mL (Fig. 1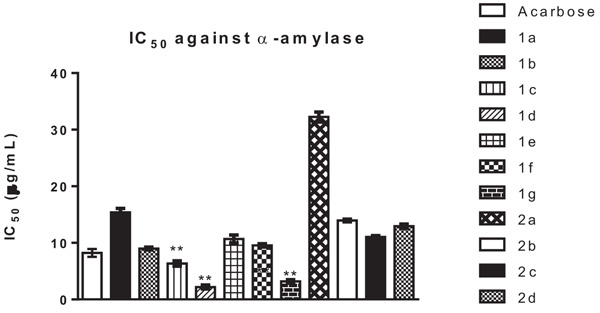 ). Compounds 1c, 1d and 1g were found to be most potent inhibitors among all the tested compounds with IC50 value of 6.59 µg/mL, 2.03 µg/mL and 3.14 µg/mL respectively. The inhibition was significant (P < 0.01) when compared to positive control. It can be concluded from the results that unsubstituted benzene derivative exhibits the lowest IC50 value, whereas 3-hydroxy and 3-methoxy derivatives were also more active as compared to the standard Acarbose. This may be due to hydrogen bond formation capacity of these derivatives in the active site of the enzyme. In case of 2-hydroxy and 4-hydroxy arylidene derivatives the inhibitory potential was slightly lower as compared to the standard. All of the 5-arylidene of 2,4-thiazolidinediones showed good inhibition as compared to acetic acid derivatives of 5-arylidene 2,4 thiazolidinediones. The inhibitory potential of all the acetic acid derivatives of 2,4-thiazolidinediones was low. This can be attributed to the polar nature of these derivatives due to the carboxylic group present in 2(a-d) which might result in less permeability across cell membranes.
). Compounds 1c, 1d and 1g were found to be most potent inhibitors among all the tested compounds with IC50 value of 6.59 µg/mL, 2.03 µg/mL and 3.14 µg/mL respectively. The inhibition was significant (P < 0.01) when compared to positive control. It can be concluded from the results that unsubstituted benzene derivative exhibits the lowest IC50 value, whereas 3-hydroxy and 3-methoxy derivatives were also more active as compared to the standard Acarbose. This may be due to hydrogen bond formation capacity of these derivatives in the active site of the enzyme. In case of 2-hydroxy and 4-hydroxy arylidene derivatives the inhibitory potential was slightly lower as compared to the standard. All of the 5-arylidene of 2,4-thiazolidinediones showed good inhibition as compared to acetic acid derivatives of 5-arylidene 2,4 thiazolidinediones. The inhibitory potential of all the acetic acid derivatives of 2,4-thiazolidinediones was low. This can be attributed to the polar nature of these derivatives due to the carboxylic group present in 2(a-d) which might result in less permeability across cell membranes.
 |
Scheme 1 |
 |
Fig. (1) Comparison of IC50 of the test compounds. Significance vs. Positive control group: **P < 0.01. |
3.3. Molecular Docking Analysis
Molecular docking study was carried out to determine the potential interactions and binding affinity between the ligand and the target enzyme, α-amylase. All the ligands tend to bind in the same binding site of α-amylase within an RMSD of 1.5Å when compared with acarbose. Three significant residues i.e., Asp300, Glu233 and Asp197 are present at the active site cleft which are mainly involved in the hydrolysis of glycosidic bonds present in carbohydrates. The most acceptable docked poses of ligands with the best binding affinity for human pancreatic α-amylase at the active site were selected and are shown in Fig. (2 ).
).
 |
Fig. (2) Binding cavity of α-amylase with docked ligands and the interacting residues around. |
The binding affinities of the synthesized compounds and acarbose against α-amylase enzyme were determined using Autodock vina. All the compounds gave good binding affinities ranging from -7.2 to -6.3 KJ/mol due to hydrogen bond formation capacity at multiple sites especially the carbonyl groups present in the ring are involved in hydrogen bonding in the active site. Acetic acid derivatives have good binding affinity due to extra pi-anion interactions with the aspartic acid residues present in the enzyme’s active site. IC50 value of 1d is lowest whereas it showed lower binding affinity due to absence of any hydrogen bond formation group on benzene ring. Acarbose showed binding affinity of -7.5 KJ/mol. The binding affinities of the compounds and the interactions with the protein are given in Table 3.
The compound 2a showed the lowest binding affinity of -7.2 KJ/mol (Fig. 3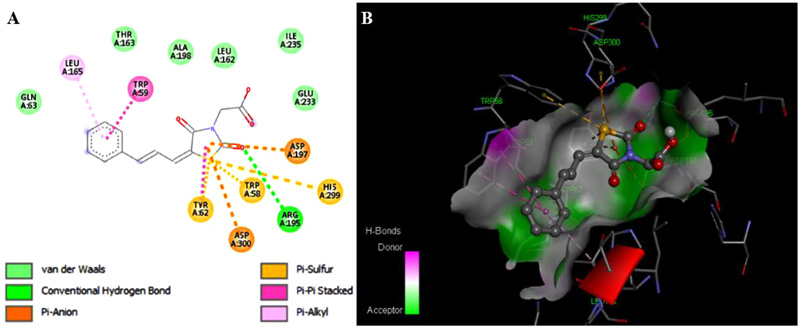 ). The binding affinities of acetic acid derivatives were found to be better as compared to 1(a-g) but inhibitory potential was low which can be attributed to the polar carboxylic group present in 2(a-d) making them less permeable across cell membranes.
). The binding affinities of acetic acid derivatives were found to be better as compared to 1(a-g) but inhibitory potential was low which can be attributed to the polar carboxylic group present in 2(a-d) making them less permeable across cell membranes.
CONCLUSION
In this study eleven new 2,4-thiazolidinedione derivatives have been synthesized and screened for their α-amylase inhibitory activity. Non-linear regression analysis was used to statistically compute the IC50 values. Compounds 1c, 1d and 1g were found to be most potent inhibitors among all the tested compounds with IC50 value of 6.59 µg/mL, 2.03 µg/mL and 3.14 µg/mL respectively. Docking results indicated that the best binding conformation was found inside the active site cleft of HPA responsible for hydrolysis of carbohydrates. Therefore, it can be concluded that 2,4-thiazolidinedione derivatives can be used as effective lead molecules for the development of α-amylase inhibitors for the management of diabetes.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Not applicable.
REFERENCES
| [1] | Kandra, L. α-Amylases of medical and industrial importance. J. Mol. Struct. THEOCHEM, 2003, 666, 487-498. [http://dx.doi.org/10.1016/j.theochem.2003.08.073] |
| [2] | Whitcomb, D.C.; Lowe, M.E. Human pancreatic digestive enzymes. Dig. Dis. Sci., 2007, 52(1), 1-17. [http://dx.doi.org/10.1007/s10620-006-9589-z] [PMID: 17205399] |
| [3] | Brayer, G.D.; Luo, Y.; Withers, S.G. The structure of human pancreatic alpha-amylase at 1.8 A resolution and comparisons with related enzymes. Protein Sci., 1995, 4(9), 1730-1742. [http://dx.doi.org/10.1002/pro.5560040908] [PMID: 8528071] |
| [4] | Iulek, J.; Franco, O.L.; Silva, M.; Slivinski, C.T.; Bloch, C., Jr; Rigden, D.J.; Grossi de Sá, M.F. Purification, biochemical characterisation and partial primary structure of a new α-amylase inhibitor from Secale cereale (rye). Int. J. Biochem. Cell Biol., 2000, 32(11-12), 1195-1204. [http://dx.doi.org/10.1016/S1357-2725(00)00053-4] [PMID: 11137459] |
| [5] | Tangphatsornruang, S.; Naconsie, M.; Thammarongtham, C.; Narangajavana, J. Isolation and characterization of an alpha-amylase gene in cassava (Manihot esculenta). Plant Physiol. Biochem., 2005, 43(9), 821-827. [http://dx.doi.org/10.1016/j.plaphy.2005.07.014] [PMID: 16297635] |
| [6] | Subramanian, R.; Asmawi, M.Z.; Sadikun, A. In vitro alpha-glucosidase and alpha-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim. Pol., 2008, 55(2), 391-398. [PMID: 18511986] |
| [7] | Mooradian, A.D.; Thurman, J.E. Drug therapy of postprandial hyperglycaemia. Drugs, 1999, 57(1), 19-29. [http://dx.doi.org/10.2165/00003495-199957010-00003] [PMID: 9951949] |
| [8] | Angajala, G.; Subashini, R. Diabetes Mellitus and Human Health Care., 2014, , 229-246. [http://dx.doi.org/10.1201/b16415-6] |
| [9] | Yoshioka, T.; Fujita, T.; Kanai, T.; Aizawa, Y.; Kurumada, T.; Hasegawa, K.; Horikoshi, H. Studies on hindered phenols and analogues. 1. Hypolipidemic and hypoglycemic agents with ability to inhibit lipid peroxidation. J. Med. Chem., 1989, 32(2), 421-428. [http://dx.doi.org/10.1021/jm00122a022] [PMID: 2913302] |
| [10] | Bozdağ-Dündar, O.; Verspohl, E.J.; Daş-Evcimen, N.; Kaup, R.M.; Bauer, K.; Sarikaya, M.; Evranos, B.; Ertan, R. Synthesis and biological activity of some new flavonyl-2,4-thiazolidinediones. Bioorg. Med. Chem., 2008, 16(14), 6747-6751. [http://dx.doi.org/10.1016/j.bmc.2008.05.059] [PMID: 18565754] |
| [11] | Mori, M.; Takagi, M.; Noritake, C.; Kagabu, S. 2,4-Dioxo-1,3-thiazolidine derivatives as a lead for new fungicides. J. Pestic. Sci., 2008, 33, 357-363. [http://dx.doi.org/10.1584/jpestics.G08-15] |
| [12] | Visentini, P. Antituberculosis chemotherapeutic action of derivatives of 2-4-thiazolidinedione. I. Action in vitro of the 2-phenylhydrazone of 2,4-thiazolidinedione. Farmaco, Sci., 1954, 9(5), 274-277. [PMID: 13191358] |
| [13] | Marshall, P.G.; Vallance, D.K. Anticonvulsant activity; derivatives of succinimide, glutarimide, thiazolidinedione and methanol, and some miscellaneous compounds. J. Pharm. Pharmacol., 1954, 6(10), 740-746. [http://dx.doi.org/10.1111/j.2042-7158.1954.tb11011.x] [PMID: 13212661] |
| [14] | Ceriello, A. Thiazolidinediones as anti-inflammatory and anti-atherogenic agents. Diabetes Metab. Res. Rev., 2008, 24(1), 14-26. [http://dx.doi.org/10.1002/dmrr.790] [PMID: 17990280] |
| [15] | Eun, J.S.; Kim, K.S.; Kim, H.N.; Park, S.A.; Ma, T-Z.; Lee, K.A.; Kim, D.K.; Kim, H.K.; Kim, I.S.; Jung, Y.H.; Zee, O.P.; Yoo, D.J.; Kwak, Y.G. Synthesis of psoralen derivatives and their blocking effect of hKv1.5 channel. Arch. Pharm. Res., 2007, 30(2), 155-160. [http://dx.doi.org/10.1007/BF02977688] [PMID: 17366735] |
| [16] | Sahu, S.K.; Banerjee, M.; Mishra, S.K.; Mohanta, R.K.; Panda, P.K.; Misro, P.K. Synthesis, partition coefficients and antibacterial activity of 3′-phenyl (substituted)-6′-aryl-2′ (1H)-cis-3′,3‘a-dihydrospiro [3-H-indole-3,5’-pyrazolo (3′,4′-d)-thiazolo-2-(1H)-ones]. Acta Pol. Pharm., 2007, 64(2), 121-126. [3-H-indole-3,5’-pyrazolo (3’,4’-d)-thiazolo-2-(1H)-ones]. [PMID: 17665861] |
| [17] | Nawale, S.L.; Dhake, A.S. Synthesis and evaluation of novel thiazolidinedione derivatives for antibacterial activity. Pharma Chem., 2012, 4, 2270-2277. |
| [18] | Barakat, A.; Al-Majid, A.M.; AL-Najjar, H.J.; Mabkhot, Y.N.; Ghabbour, H.A.; Fun, H-K. An efficient and green procedure for synthesis of rhodanine derivatives by aldol-thia-Michael protocol using aqueous diethylamine medium. RSC Adv., 2014, 4, 4909-4916. [http://dx.doi.org/10.1039/c3ra46551a] |
| [19] | Zimenkovskii, B.S.; Kutsyk, R.V.; Lesyk, R.B.; Matyichuk, V.S.; Obushak, N.D.; Klyufinska, T.I. Synthesis and antimicrobial activity of 2,4-dioxothiazolidine-5-acetic acid amides. Pharm. Chem. J., 2006, 40, 303-306. [http://dx.doi.org/10.1007/s11094-006-0115-6] |







