- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
C-C Forming Reactions with Palladium and Platinum: Lessons Learned in Our Group
Areej Hashim1, Abdullah Al-Hemyari1, Muna Bufaroosha1, Masataka Watanabe2, Krishna G. Dongol2, Thies Thiemann1, *
Abstract
This short paper details our experiences with Pd- and Pt-catalyzed cross-coupling and cyclization reactions in investigations covering the years from 1997 until now. Pd-catalysed Suzuki-Miyaura-, Heck- and Sonogashira-type cross coupling reactions in conjunction with other transformations in one pot, especially with the Wittig reaction, are highlighted. This is followed by a description of a combination of Heck- and cyclization reactions. Finally, Pt-catalyzed diene-yne cyclization reactions are discussed.
Article Information
Identifiers and Pagination:
Year: 2018Volume: 5
First Page: 76
Last Page: 88
Publisher Id: CHEM-5-76
DOI: 10.2174/1874842201805010076
Article History:
Received Date: 26/4/2018Revision Received Date: 29/7/2018
Acceptance Date: 6/8/2018
Electronic publication date: 28/09/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Department of Chemistry, College of Science, United Arab Emirates University, PO Box 15551, Al Ain, United Arab Emirates; Tel: +971-(0)56-692-5943; E-mail: thies@uaeu.ac.ae
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 26-4-2018 |
Original Manuscript | C-C Forming Reactions with Palladium and Platinum: Lessons Learned in Our Group | |
1. INTRODUCTION
Our first contact with Pd-catalyzed cross-coupling reactions came when attempting to construct functionalized estradiol derivatives as potential diagnostic agents for estrogen receptor-positive breast cancer [1Oliveira, M.C.; Neto, C.; Gano, L.; Marques, F.; Santos, I.; Thiemann, T.; Santos, A.C.; Botelho, F.; Oliveira, C.F. Estrogen receptor ligands for targeting breast tumours: A brief outlook on radioiodination strategies. Curr. Radiopharm., 2012, 5(2), 124-141.
[http://dx.doi.org/10.2174/1874471011205020124] [PMID: 22280114] -3Neto, C.; Oliveira, M.C.; Gano, L.; Marques, F.; Yasuda, T.; Thiemann, T.; Kniess, T.; Santos, I. Novel 7α-alkoxy-17α-(4′-halophenylethynyl)estradiols as potential SPECT/PET imaging agents for estrogen receptor expressing tumours: Synthesis and binding affinity evaluation. Steroids, 2012, 77(11), 1123-1132.
[http://dx.doi.org/10.1016/j.steroids.2012.05.004] [PMID: 22633985] ]. Initially, this involved Wittig olefination reactions of formylestrane derivatives with stabilized phosphoranes [4Thiemann, T.; Thiemann, C.; Sasaki, S.; Vill, V.; Mataka, S.; Tashiro, M. Synthesis and photochemical behavior of C-16 substituted steroids. J. Chem. Res., 1997, 21 (S), 248-249; 21 (M), 1736-1750.]. In order to have a platform to diversify the molecules that could be prepared, a synthetic sequence was developed by constructing stabilized phosphoranes with a terminal haloaryl unit on a polymer backbone stemming from triphenylphosphine-polystyrene, derivatising the phosphoranes by C-C cross-coupling reaction and finally reacting the phosphoranes thus prepared with a steroidal carbaldehyde [5Thiemann, T.; Umeno, K.; Wang, J.; Arima, K.; Watanabe, M.; Tabuchi, Y.; Tanaka, Y.; Gorohmaru, H.; Mataka, S. Elongated phosphoranes by C-C coupling of haloaroylmethylidenephosphoranes – synthesis and applications. J. Chem. Soc., Perkin Trans. 1, 2002, 2090-2110.
[http://dx.doi.org/10.1039/B202745N] ]. This clearly showed that stabilized phosphoranes are compatible with reaction conditions needed to carry out many of the C-C cross-coupling reactions and would lead to the development of one-pot reactions involving Pd-catalyzed cross-coupling reactions and Wittig olefinations [6Thiemann, T. Wittig- and Horner-Wadsworth-Emmons Olefination in Aqueous Media with and without Phase Transfer Catalysis. Mini Rev. Org. Chem., 2018, 15, 412-432.
[http://dx.doi.org/10.2174/1570193X15666180221152118] -8Al Jasem, Y.; El-Esawi, R.; Thiemann, T. Wittig- and Horner-Wadsworth-Emmons (HWE) olefination reactions with stabilized and semi-stabilized phosphoranes and phosphonates under non-classical conditions. J. Chem. Res., 2014, 38, 453-463.
[http://dx.doi.org/10.3184/174751914X14044684705710] ].
One of our Heck-reaction cyclization sequences also came from the desire to functionalize estrane derivatives, this time at C7, by submitting a η6-estra-1,3,5(10),6-trien-17-one 17,17-acetal tricarbonylchromium(0) [9Gopal Dongol, K.; Melo e Silva, M.C.; Matsubara, K.; Matsumoto, T.; Mataka, S.; Thiemann, T. π-Facial Selectivity in the Formation of η6-Tricarbonylchromium(0) Complexes of Estra-1,3,5(10),6-tetraenes. Z. Anorg. Allg. Chem., 2003, 629, 945-947.
[http://dx.doi.org/10.1002/zaac.200300017] ] to a Heck reaction, which led to a surprising triarylation of the compound with concurrent cyclization. This was found to be a general reaction of η6-dihydronaphthalene tricarbonylchromium (0) complexes [10Gopal Dongol, K.; Matsubara, K.; Mataka, S.; Thiemann, T. Trialkylation of η6-Dihydronaphthalene-Cr(CO)3 complexes. J. Chem. Soc. Chem. Commun., 2002, (24), 3060-3061.
[http://dx.doi.org/10.1039/B209494K] ]. In contrast, a further Heck reaction–cyclization combination, a Heck-triene-cyclization-aromatisation sequence, was a desired transformation [11Thiemann, T.; Watanabe, M.; Mataka, S. Areno-annelated Estra-1,3,5(10),6,8,11,14,16-octaenes. New J. Chem., 2001, 25, 1104-1107.
[http://dx.doi.org/10.1039/b104648a] ]. Finally, a Pd-catalyzed arene-ene-yne cyclization was investigated [12Watanabe, M.; Mataka, S.; Thiemann, T. Benzothieno and benzofurano annelated estranes. Steroids, 2005, 70(13), 856-866.
[http://dx.doi.org/10.1016/j.steroids.2005.06.001] [PMID: 16045951] ]. Both of the latter reactions led to novel areno-annelations, and both were used to synthesize areno-annelated steroids.
Initially, Pd(PPh3)4 in a biphasic medium of aq. Na2CO3 and 1,2-dimethoxyethane (DME) was found to be the catalyst of choice for many of the reactions detailed here. Later on, the slightly cheaper Pd(PPh3)2Cl2 was used as a pre-catalyst, in the presence of PPh3 (3 equiv.). Interestingly, these were sufficiently active to catalyze Suzuki-Miyaura reactions with chloroarenes, where the chloro group was activated by a nitro substituent or as part of a quinoid system as in chloroanthraquinones [13Thiemann, T.; Tanaka, Y.; Iniesta, J.; Varghese, H.T.; Panicker, C.Y. Arylation of Chloroanthraquinones by surprisingly facile Suzuki-Miyaura cross-coupling reactions. J. Chem. Res., 2009, 33, 732-736.
[http://dx.doi.org/10.3184/030823409X12586584303880] ] and 1-chloro-2,4-dinitrobenzene [14Thiemann, T.; Tanaka, Y.; Hisaindee, S.; Kaabi, M. A study of the Suzuki-Miyaura reaction of chloroarenes using Pd(PPh3)4 as catalyst. J. Chem. Res., 2010, 34, 34-38.
[http://dx.doi.org/10.3184/030823410X12624523028293] ]. Nevertheless, at a later time, other reaction systems were used. Reactions were run under Jeffery conditions [15Jeffery, T. Highly Stereospecific palladium-catalysed vinylation of vinylic halides under solid-liquid phase transfer conditions. Tetrahedron Lett., 1985, 26, 2667-2668.
[http://dx.doi.org/10.1016/S0040-4039(00)98131-0] -17Jeffery, T. Palladium-catalyzed vinylation of acetylenic iodides under solid-liquid phase-transfer conditions. Synthesis, 1987, (01), 70-71.
[http://dx.doi.org/10.1055/s-1987-27851] ]. Also, Pd freshly deposited on carbon nanofibers was used as catalyst [18Yamamoto, K.; Thiemann, T. Suzuki-Miyaura cross-coupling and Heck-Mizoroki reactions catalysed by Pd on carbon nanofibers. J. Chem. Res., 2011, 35, 246-250.
[http://dx.doi.org/10.3184/174751911X13025306846745] ] in Suzuki and Heck reactions.
The following gives a short overview of above reactions. At the end, a number of experimental procedures can be found as representative examples.
2. RESULTS AND DISCUSSION
2.1. One-Pot Cross-Coupling Reaction: Wittig Olefination Procedures
We realized that haloaroylmethylidenetriphenylphosphoranes such as 1 and 8 would be stable under conditions necessary for Suzuki- [5Thiemann, T.; Umeno, K.; Wang, J.; Arima, K.; Watanabe, M.; Tabuchi, Y.; Tanaka, Y.; Gorohmaru, H.; Mataka, S. Elongated phosphoranes by C-C coupling of haloaroylmethylidenephosphoranes – synthesis and applications. J. Chem. Soc., Perkin Trans. 1, 2002, 2090-2110.
[http://dx.doi.org/10.1039/B202745N] , 19Thiemann, T.; Umeno, K.; Ohira, D.; Inohae, E.; Sawada, T.; Mataka, S. Suzuki-Kumada coupling with bromoaroylmethylidenephosphoranes. New J. Chem., 1999, 23, 1067-1070.
[http://dx.doi.org/10.1039/a907470h] ], Sonogashira [5Thiemann, T.; Umeno, K.; Wang, J.; Arima, K.; Watanabe, M.; Tabuchi, Y.; Tanaka, Y.; Gorohmaru, H.; Mataka, S. Elongated phosphoranes by C-C coupling of haloaroylmethylidenephosphoranes – synthesis and applications. J. Chem. Soc., Perkin Trans. 1, 2002, 2090-2110.
[http://dx.doi.org/10.1039/B202745N] , 19Thiemann, T.; Umeno, K.; Ohira, D.; Inohae, E.; Sawada, T.; Mataka, S. Suzuki-Kumada coupling with bromoaroylmethylidenephosphoranes. New J. Chem., 1999, 23, 1067-1070.
[http://dx.doi.org/10.1039/a907470h] , 20Thiemann, T.; Umeno, K.; Inohae, E.; Mataka, S. Novel Elongated Phosphoranes by Heck-reaction and Pd(0)-Catalysed Alkynylation and their Use in C-7 Group Functionalisation in Estrones. Rep. Inst. Adv. Mat. Kyushu Univ., 2000, 14(1) Chem. Abstr.,2000, 133, 335-380.] and Heck [5Thiemann, T.; Umeno, K.; Wang, J.; Arima, K.; Watanabe, M.; Tabuchi, Y.; Tanaka, Y.; Gorohmaru, H.; Mataka, S. Elongated phosphoranes by C-C coupling of haloaroylmethylidenephosphoranes – synthesis and applications. J. Chem. Soc., Perkin Trans. 1, 2002, 2090-2110.
[http://dx.doi.org/10.1039/B202745N] , 21Watanabe, M.; Mataka, S.; Thiemann, T. One Pot sonogashira-coupling / wittig olefination procedures. J. Chem. Res., 2005, 29, 636-639.
[http://dx.doi.org/10.3184/030823405774663057] ] reactions and hence it was possible to derivatize 1 and 8 to a plethora of phosphoranes, such as 3, 5, 7, and 10 (Scheme 1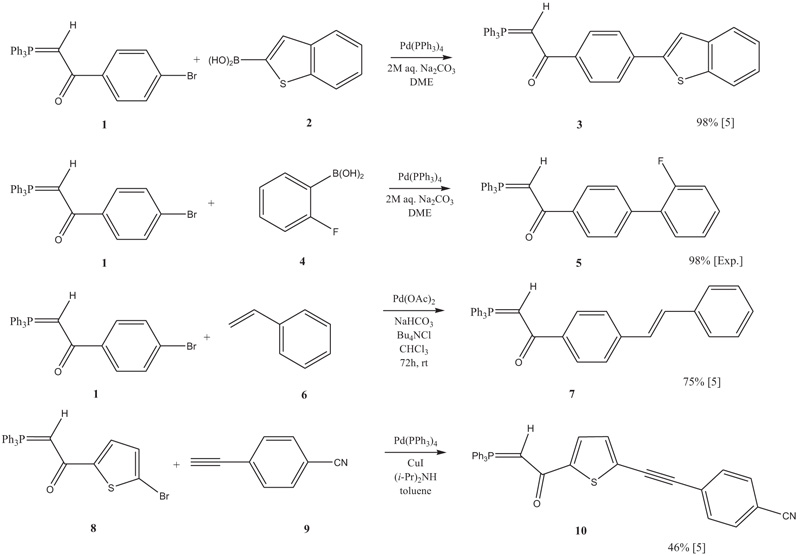 ). These could be reacted subsequently with carbaldehydes in a Wittig olefination [5Thiemann, T.; Umeno, K.; Wang, J.; Arima, K.; Watanabe, M.; Tabuchi, Y.; Tanaka, Y.; Gorohmaru, H.; Mataka, S. Elongated phosphoranes by C-C coupling of haloaroylmethylidenephosphoranes – synthesis and applications. J. Chem. Soc., Perkin Trans. 1, 2002, 2090-2110.
). These could be reacted subsequently with carbaldehydes in a Wittig olefination [5Thiemann, T.; Umeno, K.; Wang, J.; Arima, K.; Watanabe, M.; Tabuchi, Y.; Tanaka, Y.; Gorohmaru, H.; Mataka, S. Elongated phosphoranes by C-C coupling of haloaroylmethylidenephosphoranes – synthesis and applications. J. Chem. Soc., Perkin Trans. 1, 2002, 2090-2110.
[http://dx.doi.org/10.1039/B202745N] , 19Thiemann, T.; Umeno, K.; Ohira, D.; Inohae, E.; Sawada, T.; Mataka, S. Suzuki-Kumada coupling with bromoaroylmethylidenephosphoranes. New J. Chem., 1999, 23, 1067-1070.
[http://dx.doi.org/10.1039/a907470h] , 21Watanabe, M.; Mataka, S.; Thiemann, T. One Pot sonogashira-coupling / wittig olefination procedures. J. Chem. Res., 2005, 29, 636-639.
[http://dx.doi.org/10.3184/030823405774663057] ]. The reactions proceed with the corresponding phosphonium salts also [5Thiemann, T.; Umeno, K.; Wang, J.; Arima, K.; Watanabe, M.; Tabuchi, Y.; Tanaka, Y.; Gorohmaru, H.; Mataka, S. Elongated phosphoranes by C-C coupling of haloaroylmethylidenephosphoranes – synthesis and applications. J. Chem. Soc., Perkin Trans. 1, 2002, 2090-2110.
[http://dx.doi.org/10.1039/B202745N] ]. Here, even Na2CO3 has been found to be a base strong enough to convert the phosphonium salts into the respective phosphoranes. For the most part, Suzuki-Wittig reactions were run with Pd(PPh3)4 as the catalyst in a solvent mixture of aq. Na2CO3 and DME [22Thiemann, T.; Watanabe, M.; Tanaka, Y.; Mataka, S. One-Pot wittig-olefination / suzuki-reaction - the Compatibility of conjugated phosphoranes in Pd(0) catalysed C-C-bond forming reactions. New J. Chem., 2006, 30, 359-369.
[http://dx.doi.org/10.1039/b516724h] ], and Sonogashira coupling-Wittig reactions were carried out with Pd(PPh3)4/CuI as catalysts and diisopropylamine (DIPA) as base in toluene [5Thiemann, T.; Umeno, K.; Wang, J.; Arima, K.; Watanabe, M.; Tabuchi, Y.; Tanaka, Y.; Gorohmaru, H.; Mataka, S. Elongated phosphoranes by C-C coupling of haloaroylmethylidenephosphoranes – synthesis and applications. J. Chem. Soc., Perkin Trans. 1, 2002, 2090-2110.
[http://dx.doi.org/10.1039/B202745N] ]. For Heck-Wittig reactions various conditions were used with Pd(OAc)2 or Pd(Cl2)dppe [23Burmester, C.; Mataka, S.; Thiemann, T. Synthesis of non-symmetric Divinylarenes by a Heck/Wittig reaction combination. Synth. Commun., 2010, 40, 3196-3208.
[http://dx.doi.org/10.1080/00397910903395235] ] as the catalyst and with Et3N or K2CO3 as base in DMF at 100 °C. However, here Jeffery-type PTC conditions in aq. Na2CO3/CHCl3 and either Bu4NCl or BnMe3NCl as PTC catalyst were found to be favorable, where the reactions were run at room temperature (rt). Haloaroylmethylidenetriphenylphosphoranes such as 1 could also be reacted with a boronic acid, and a carbaldehyde in a one-pot Suzuki-Wittig reaction (eg. to 13 Scheme 2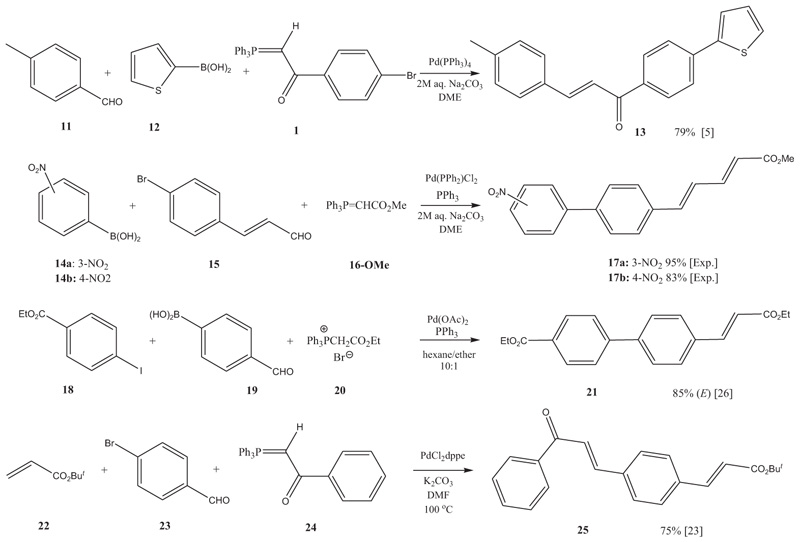 ) [5Thiemann, T.; Umeno, K.; Wang, J.; Arima, K.; Watanabe, M.; Tabuchi, Y.; Tanaka, Y.; Gorohmaru, H.; Mataka, S. Elongated phosphoranes by C-C coupling of haloaroylmethylidenephosphoranes – synthesis and applications. J. Chem. Soc., Perkin Trans. 1, 2002, 2090-2110.
) [5Thiemann, T.; Umeno, K.; Wang, J.; Arima, K.; Watanabe, M.; Tabuchi, Y.; Tanaka, Y.; Gorohmaru, H.; Mataka, S. Elongated phosphoranes by C-C coupling of haloaroylmethylidenephosphoranes – synthesis and applications. J. Chem. Soc., Perkin Trans. 1, 2002, 2090-2110.
[http://dx.doi.org/10.1039/B202745N] ].
 |
Scheme 1 Derivatisation of phosphoranes with different Pd-catalysed C-C cross coupling reactions. |
 |
Scheme 2 Wittig olefinations with C-C cross coupling reactions in one pot, using a.) a phosphorane (1); b.) a haloarylaldehyde (15); or c.) a formylarylboronic acid as the central building block. |
Alternatively, Wittig and C-C coupling reactions can be carried out with a haloaryl/hetarylcarbaldehyde (eg., with 15 and 23) as the central building block. Recently, for instance, one pot Suzuki cross coupling–Wittig olefination procedures have been carried out with 4-bromocinnamaldehyde (15) and alkoxycarbonylmethylidenetriphenylphosphorane like 16-OMe giving biphenylpentadienoates in acceptable yields (see Experimental part and Scheme 2 ). In this type of reaction, there is a choice of phosphoranes, both stabilized and semi-stabilized, that can be used. In contrast to above transformations, where the Wittig olefination with the rather sparingly reactive phosphoranes 1, 8 and 24 is more sluggish than the concomitant Suzuki reaction, reactions with semi-stabilized phosphoranes and with the stabilized alkoxycarbonylmethylideenetriphenylphosphoranes 16 are rapid [24Thiemann, T. Solventless wittig reactions with fluorinated benzaldehydes. J. Chem. Res., 2007, 31, 336-341.
). In this type of reaction, there is a choice of phosphoranes, both stabilized and semi-stabilized, that can be used. In contrast to above transformations, where the Wittig olefination with the rather sparingly reactive phosphoranes 1, 8 and 24 is more sluggish than the concomitant Suzuki reaction, reactions with semi-stabilized phosphoranes and with the stabilized alkoxycarbonylmethylideenetriphenylphosphoranes 16 are rapid [24Thiemann, T. Solventless wittig reactions with fluorinated benzaldehydes. J. Chem. Res., 2007, 31, 336-341.
[http://dx.doi.org/10.3184/030823407X225464] ] and the Suzuki transformation determines the completion of the process. Lastly, in the case of Suzuki-Wittig reactions in one pot, the boronic acids can act as the central building block, that is when formylarylboronic acids (e.g.19) are employed [25Thiemann, T.; Watanabe, M.; Tanaka, Y.; Mataka, S. One pot Suzuki coupling - Wittig olefination reactions. J. Chem. Res., 2004, 28, 723-727.
[http://dx.doi.org/10.3184/0308234043431609] , 26Yamamoto, K.; Watanabe, M.; Ideta, K.; Mataka, S.; Thiemann, T. Combined Suzuki coupling – Wittig olefination reaction in aqueous medium. Z. Naturforsch. B, 2005, 60, 1299-1307.
[http://dx.doi.org/10.1515/znb-2005-1215] ]. When dihaloarenes (e.g.26) are utilized in these reactions, p-terphenyl derivatives 28 can be prepared easily (Scheme 3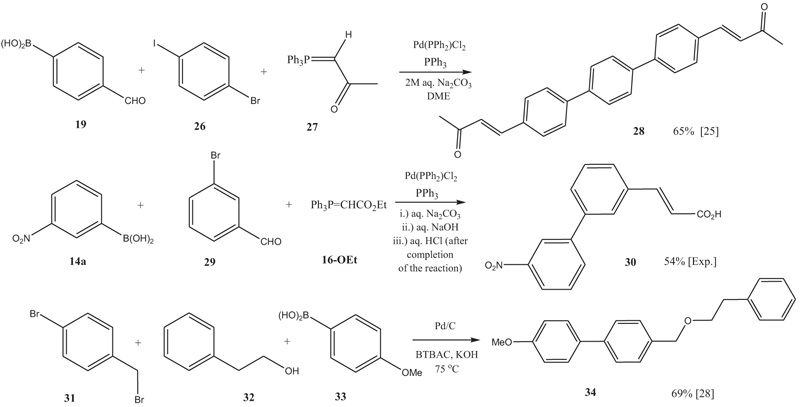 ) [25Thiemann, T.; Watanabe, M.; Tanaka, Y.; Mataka, S. One pot Suzuki coupling - Wittig olefination reactions. J. Chem. Res., 2004, 28, 723-727.
) [25Thiemann, T.; Watanabe, M.; Tanaka, Y.; Mataka, S. One pot Suzuki coupling - Wittig olefination reactions. J. Chem. Res., 2004, 28, 723-727.
[http://dx.doi.org/10.3184/0308234043431609] ]. Instead of the phosphoranes, phosphonium salts 20 also can be used as the starting material (Scheme 2 ). An easy method of separation was found for those reactions in which the Wittig-Suzuki coupling was run in a mixture of hexane/ether (10:1) aq. 2M Na2CO3 in the presence of either Pd(OAc)2-PPh3 (eg., Scheme 2
). An easy method of separation was found for those reactions in which the Wittig-Suzuki coupling was run in a mixture of hexane/ether (10:1) aq. 2M Na2CO3 in the presence of either Pd(OAc)2-PPh3 (eg., Scheme 2 , to 21) or Pd2(dba)3 as catalyst, under ultrasonication. After completion of the reaction the products are found in the organic phase, which is separated from the aq. phase and concentrated in vacuo to give the products in sufficient purity to be used in further transformations [26Yamamoto, K.; Watanabe, M.; Ideta, K.; Mataka, S.; Thiemann, T. Combined Suzuki coupling – Wittig olefination reaction in aqueous medium. Z. Naturforsch. B, 2005, 60, 1299-1307.
, to 21) or Pd2(dba)3 as catalyst, under ultrasonication. After completion of the reaction the products are found in the organic phase, which is separated from the aq. phase and concentrated in vacuo to give the products in sufficient purity to be used in further transformations [26Yamamoto, K.; Watanabe, M.; Ideta, K.; Mataka, S.; Thiemann, T. Combined Suzuki coupling – Wittig olefination reaction in aqueous medium. Z. Naturforsch. B, 2005, 60, 1299-1307.
[http://dx.doi.org/10.1515/znb-2005-1215] ].
The one-pot Suzuki-Wittig procedure with 16 can be run with a concomitant hydrolysis step (see Experimental Part). Previously, it has been noted that Wittig reaction of aldehydes with 16 can be performed in 10w% aq. NaOH, which leads to the hydrolysis of the acrylates formed and makes a simple work-up possible. In the case of solid products, simple filtration of triphenylphosphine oxide and acidification followed by a second filtration are enough to obtain the acrylic acids [27Thiemann, T.; Elshorbagy, M.; Ahmadani, S.; Al Jasem, Y.; Al Azani, M.; al-Sulaibi, M.; al Hindawi, B. Facile, direct reaction of benzaldehydes to 3-phenyl-prop-2-enoic acids and 3-arylprop-2-ynoic acids in aqueous medium. IJOC, 2016, 6, 126-141.
[http://dx.doi.org/10.4236/ijoc.2016.62014] ]. In the case of combining the hydrolysis step with the Suzuki-Wittig procedure, the presence of unreacted boronic acid and the metal catalyst makes a chromatographic separation of the product 30 necessary (Scheme 3 ).
).
A further reaction that the authors have carried out with the Suzuki cross-coupling reaction in one pot is a Williamson-type ether synthesis, eg., to 34 (Scheme 3 ). This procedure was run as a PTC reaction with solid KOH and benzyltributylammonium chloride (BTABC) as the PT catalyst and with Pd/C as the coupling catalyst (Scheme 3
). This procedure was run as a PTC reaction with solid KOH and benzyltributylammonium chloride (BTABC) as the PT catalyst and with Pd/C as the coupling catalyst (Scheme 3 ) [28Thiemann, T.; Al Jasem, Y.; Butt, H.; al Hindawi, B.; Barkhad, M.; al Khazali, M.; al Azani, M. New heat transfer fluids (HTFs) for solar thermal applications, 3rd World Sustainability Forum Sciforum electronic conference Series, 2013, 3, d-002.
) [28Thiemann, T.; Al Jasem, Y.; Butt, H.; al Hindawi, B.; Barkhad, M.; al Khazali, M.; al Azani, M. New heat transfer fluids (HTFs) for solar thermal applications, 3rd World Sustainability Forum Sciforum electronic conference Series, 2013, 3, d-002.
[http://dx.doi.org/10.3390/wsf3-d002] ].
 |
Scheme 3 Double Wittig–Suzuki reaction leading to terphenyl 28; Wittig-Suzuki-hydrolysis reaction leading to phenylcinnamic acid 30 and Suzuki-coupling-etherfication leading to benzyl ether 34. |
2.2. Pt- and Pd-Catalyzed Cyclization Reactions
Foremost Heck-, but also Suzuki-type C-C cross-coupling reactions lead to systems that when heated undergo cyclization reactions [29Watanabe, M.; Matsumoto, T.; Mataka, S.; Thiemann, T. Facile access to condensed arenothiophenes – preparation of dihydrophenanthrothiophenes and dihydrophenanthrobenzo[b]thiophenes. J. Chem. Res., 2005, 29, 564-571.
[http://dx.doi.org/10.3184/030823405774308925] , 30Watanabe, M.; Matsumoto, T.; Mataka, S.; Thiemann, T. Heteroareno-annelated estranes by triene cyclization. CEJC, 2006, 4, 375-402.]. In some cases, the catalyst, such as “Pd” is essential for the reaction to proceed as in the reaction of 17-bromo-3-methoxy-16-E-(β-nitroethenyl)estra-1,3,5(10),16-tetraene with butyl acrylate (26), where the primary Heck product 37 undergoes a triene cyclization, and the cyclization product 38 subsequently aromatizes under extrusion of the nitro group (Scheme 4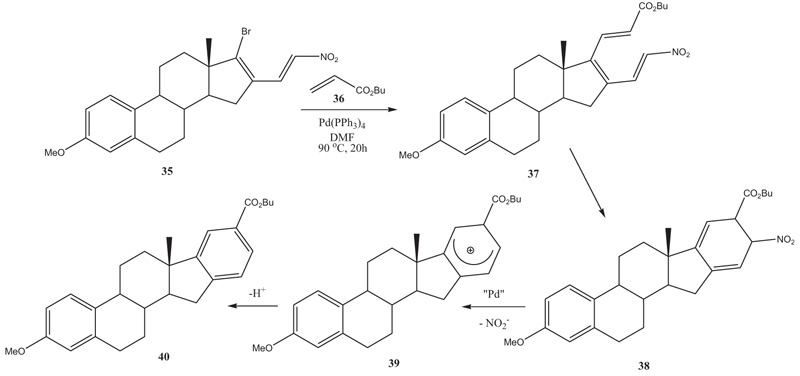 ) [11Thiemann, T.; Watanabe, M.; Mataka, S. Areno-annelated Estra-1,3,5(10),6,8,11,14,16-octaenes. New J. Chem., 2001, 25, 1104-1107.
) [11Thiemann, T.; Watanabe, M.; Mataka, S. Areno-annelated Estra-1,3,5(10),6,8,11,14,16-octaenes. New J. Chem., 2001, 25, 1104-1107.
[http://dx.doi.org/10.1039/b104648a] ], giving benzoannelated estrane 40, albeit in low yield (26-39%). This reaction has also been carried with styrene and acrylonitrile as olefin component [11Thiemann, T.; Watanabe, M.; Mataka, S. Areno-annelated Estra-1,3,5(10),6,8,11,14,16-octaenes. New J. Chem., 2001, 25, 1104-1107.
[http://dx.doi.org/10.1039/b104648a] ]. Subsequently the benzoannelated estranes such as 40 can be dehydrogenated easily with DDQ to give benzoannelated estra-1,3,5(10),6,8,11,14,16-octatetraenes. These have one sp3-hybridized carbon left in their framework, which at the same time remains a controlled stereocenter.
 |
Scheme 4 Heck-reaction-triene-cyclization-aromatisation to benzo-annelated estranes. |
A Pt-mediated process is the cyclization of 16-ethynyl-17-hetarylestra-l,3,5(10),16-tetraen-3-ols to hetarenoestranes. The 16-ethynyl-17-hetarylestra-l,3,5(10),16-tetraen-3-ols can easily be prepared from the 17-ketosteroids by consecutively performed Vilsmeier-Arnold formylation, Suzuki-cross coupling, and Corey-Fuchs reaction. Initially, the 16-ethynyl-17-hetarylestra-l,3,5(10),16-tetraen-3-ols were cyclized to heteroestranes with Ru(p-cymene)Cl2PPh3 as the catalyst (Scheme 5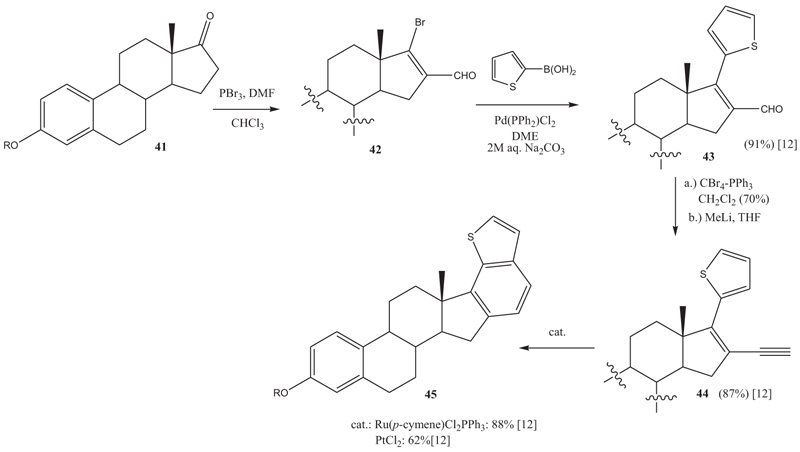 ). In slightly lower yields, the cyclization proceeds with PtCl2 as well [12Watanabe, M.; Mataka, S.; Thiemann, T. Benzothieno and benzofurano annelated estranes. Steroids, 2005, 70(13), 856-866.
). In slightly lower yields, the cyclization proceeds with PtCl2 as well [12Watanabe, M.; Mataka, S.; Thiemann, T. Benzothieno and benzofurano annelated estranes. Steroids, 2005, 70(13), 856-866.
[http://dx.doi.org/10.1016/j.steroids.2005.06.001] [PMID: 16045951] ]. Pt-, Pt-Ru nanoparticles, and PtCl2 immobilized on carbon black gave the cyclization products in much lower yield under otherwise identical reaction conditions [12Watanabe, M.; Mataka, S.; Thiemann, T. Benzothieno and benzofurano annelated estranes. Steroids, 2005, 70(13), 856-866.
[http://dx.doi.org/10.1016/j.steroids.2005.06.001] [PMID: 16045951] ]. For both the platinum and the ruthenium-mediated reactions a metal carbine was postulated as the intermediate in the cyclization.
 |
Scheme 5 Thienyl-ene-yne cyclization to benzothieno[b]estrane derivative 45. |
In the case where the terminal alkene moiety is substituted with an electron withdrawing substituent such as an ester group instead of being part of a heterocyclic system, the cyclization takes a different pathway leading to fulvenes. The reactions are run with Pt(PPh3)4 as catalyst in mesitylene at elevated temperatures [31Watanabe, M.; Shiine, K.; Ideta, K.; Matsumoto, T.; Thiemann, T. Diene-yne cyclization reactions of 1-ethynyl-2-vinyl-3,4-dihydronaphthalenes and 1-ethynyl-2-vinylnaphthalenes. J. Chem. Res., 2008, 32, 669-678.
[http://dx.doi.org/10.3184/030823408X375214] ]. Here it is irrelevant whether the central alkene moiety is part of an aromatic system as in 49, or not, as in 47. The reactions produce two well-separable, relatively polar, reddish products, E/Z-48 and E/Z-50, respectively (Scheme 6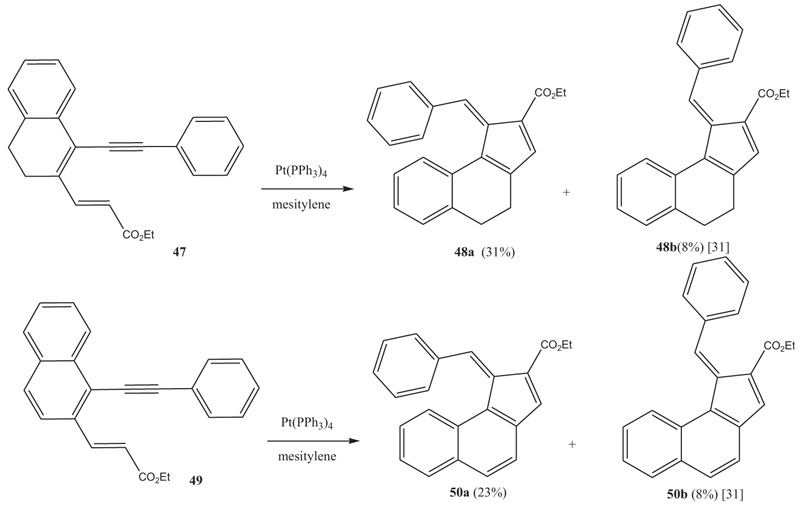 ). The phenyl group of the phenylethynyl can carry substituents such as a cyano or methyl group.
). The phenyl group of the phenylethynyl can carry substituents such as a cyano or methyl group.
 |
Scheme 6 Diene-yne cyclization with Pt(PPh3)4 as catalyst leading to fulvenes 48 and 50. |
In our final mediated Pd-catalyzed reaction, η6-dihydronaphthalene tricarbonylchromium(0) complexes were reacted with haloarenes. As mentioned above, the idea was to introduce an aryl substituent selectively at C7 of an estra-1,3,5(10),6-tetraene derivative by means of a Pd-catalyzed Heck reaction. The reaction with the non-complexed estra-1,3,5(10),6-tetraen-3-ol-17-one led to a mixture of C6- and C-7 arylated estra-1,3,5(10),6-tetraenes in poor yield. 3-O-Methylestra-1,3,5(10),16-tetraen-17-one (51) could be converted to the respective chromiumtricarbonyl complex 52 easily by reaction with chromium hexacarbonyl [Cr(CO)6] in a mixture of dibutyl ether–THF. The complexation proceeds stereoselectively to give the β-facial complex 52 exclusively. The stereochemistry was established by single crystal X-ray crystallography of the complex [9Gopal Dongol, K.; Melo e Silva, M.C.; Matsubara, K.; Matsumoto, T.; Mataka, S.; Thiemann, T. π-Facial Selectivity in the Formation of η6-Tricarbonylchromium(0) Complexes of Estra-1,3,5(10),6-tetraenes. Z. Anorg. Allg. Chem., 2003, 629, 945-947.
[http://dx.doi.org/10.1002/zaac.200300017] ]. The stereochemistry can be explained by the directing effect of the alkene-moiety at C6/C7 [9Gopal Dongol, K.; Melo e Silva, M.C.; Matsubara, K.; Matsumoto, T.; Mataka, S.; Thiemann, T. π-Facial Selectivity in the Formation of η6-Tricarbonylchromium(0) Complexes of Estra-1,3,5(10),6-tetraenes. Z. Anorg. Allg. Chem., 2003, 629, 945-947.
[http://dx.doi.org/10.1002/zaac.200300017] ]. When the steroidal chromium complex 52 is reacted with iodobenzene under Pd(OAc)2 catalysis under PTC conditions, a triarylation happens with a concomitant ring-closure reaction and 53 is obtained after decomplexation (Scheme 7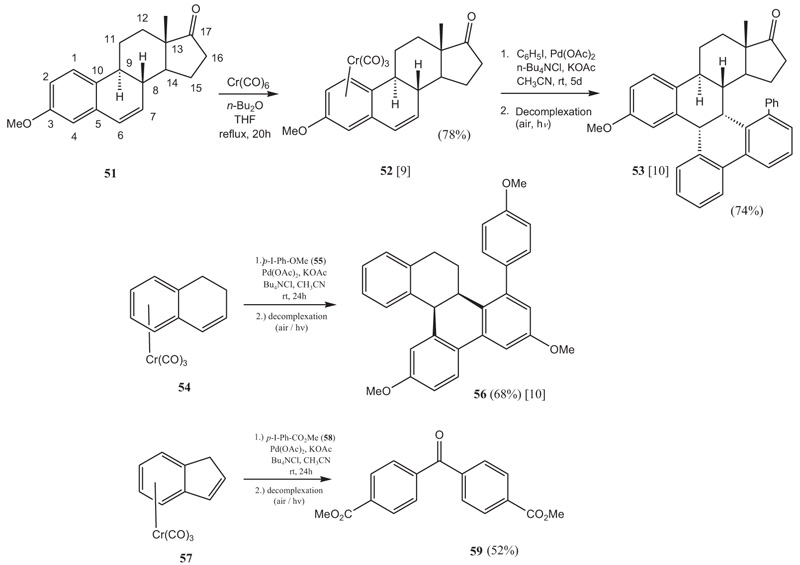 ) [10Gopal Dongol, K.; Matsubara, K.; Mataka, S.; Thiemann, T. Trialkylation of η6-Dihydronaphthalene-Cr(CO)3 complexes. J. Chem. Soc. Chem. Commun., 2002, (24), 3060-3061.
) [10Gopal Dongol, K.; Matsubara, K.; Mataka, S.; Thiemann, T. Trialkylation of η6-Dihydronaphthalene-Cr(CO)3 complexes. J. Chem. Soc. Chem. Commun., 2002, (24), 3060-3061.
[http://dx.doi.org/10.1039/B209494K] ]. The transformation also works with other η6-dihydronaphthalene tricarbonyl- chromium(0) complexes, e.g. with 54 (Scheme 7 ). The reason for this reaction pathway over the common Heck-type arylation of the alkene moiety can be envisaged to be the difficulty in cyclic alkenes for a single bond rotation to occur after the syn-addition of the arylpalladium halide species to the double bond.This rotation is needed in most cases for a subsequent syn-hydridopalladium elimination to occur, a pathway that can easily be followed by non-cyclic alkenes. Interestingly, this has been found to lead to cascade reactions also in selected other, non-complexed cyclic alkenes, as was shown by de Meijere [32de Meijere, A.; Bräse, S. Palladium in action: Domino coupling and allylic substitution reactions for the efficient construction of complex organic molecules. J. Organomet. Chem., 1999, 576, 88-110.
). The reason for this reaction pathway over the common Heck-type arylation of the alkene moiety can be envisaged to be the difficulty in cyclic alkenes for a single bond rotation to occur after the syn-addition of the arylpalladium halide species to the double bond.This rotation is needed in most cases for a subsequent syn-hydridopalladium elimination to occur, a pathway that can easily be followed by non-cyclic alkenes. Interestingly, this has been found to lead to cascade reactions also in selected other, non-complexed cyclic alkenes, as was shown by de Meijere [32de Meijere, A.; Bräse, S. Palladium in action: Domino coupling and allylic substitution reactions for the efficient construction of complex organic molecules. J. Organomet. Chem., 1999, 576, 88-110.
[http://dx.doi.org/10.1016/S0022-328X(98)01087-0] , 33Reiser, O.; Weber, M.; de Meijere, A. Selective 1:3 coupling of norbornene and iodobenzene: A facile synthesis of benzo[e]pyrenes with annelated cycloaliphatic moieties. Angew. Chem. Int. Ed. Engl., 1989, 28, 1037-1038.
[http://dx.doi.org/10.1002/anie.198910371] ], Catellani [34Catellani, M. Selective organometallic syntheses from molecular pools. Pure Appl. Chem., 2002, 74, 63-68.
[http://dx.doi.org/10.1351/pac200274010063] -36Catellani, M. Catalytic Multistep Reactions via Palladacycles. Synlett, 2003, (03), 298-312.
[http://dx.doi.org/10.1055/s-2003-37102] ] and Carretero [37Mauleón, P.; Alonso, I.; Carretero, J.C. Unusual palladium-catalyzed cascade arylation of α,β-unsaturated phenyl sulfones under Heck reaction conditions. Angew. Chem. Int. Ed. Engl., 2001, 40(7), 1291-1293.
[http://dx.doi.org/10.1002/1521-3773(20010401)40:7<1291::AID-ANIE1291>3.0.CO;2-9] [PMID: 11301455] ].
 |
Scheme 7 Reaction cascades with η6-dihydronaphthalene tricarbonylchromium(0) complexes. |
We believe that a possible mechanism in our case starts with a standard syn-addition of the arylpalladium iodide to the double bond of the η6-dihydronaphthalene tricarbonylchromium(0). As there is no possibility for a syn-hydridopalladium elimination, the Pd inserts into the neighboring aryl C-H bond leading to a palladacycle, e.g. to 61 (Scheme 8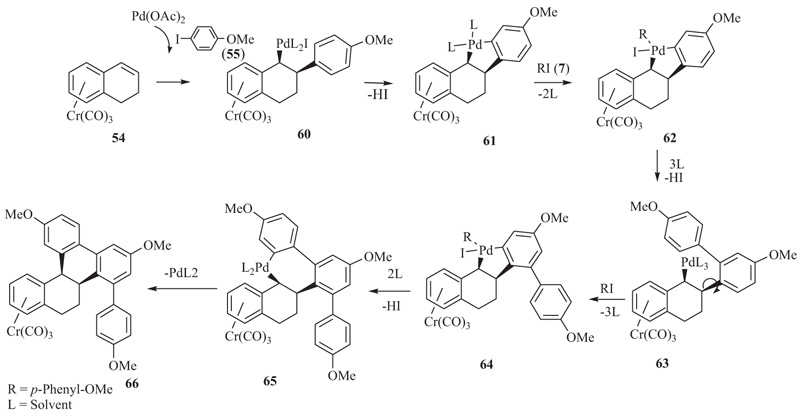 ). Then, follows an oxidative addition to the Pd of the palladacyle, followed by the first aryl-aryl coupling. This step is repeated after a C-C single bond rotation linking the aryl unit to the tetrahydronaphthalene system. A reductive elimination on the Pd leads to the final ring closure. The exact intermediates and the oxidation states of Pd in these intermediates still need to be ascertained. The chromium complexes, e.g.66, can be purified by column chromatography under inert atmosphere. In the solid state, they are fairly stable over a short period of time. They can be decomplexed by exposing the substances in solution to air and light, eg., to UV irradiation. The regiochemistry of the arylations was determined by single crystal X-ray crystallography of the chromium complexes of type 66 [10Gopal Dongol, K.; Matsubara, K.; Mataka, S.; Thiemann, T. Trialkylation of η6-Dihydronaphthalene-Cr(CO)3 complexes. J. Chem. Soc. Chem. Commun., 2002, (24), 3060-3061.
). Then, follows an oxidative addition to the Pd of the palladacyle, followed by the first aryl-aryl coupling. This step is repeated after a C-C single bond rotation linking the aryl unit to the tetrahydronaphthalene system. A reductive elimination on the Pd leads to the final ring closure. The exact intermediates and the oxidation states of Pd in these intermediates still need to be ascertained. The chromium complexes, e.g.66, can be purified by column chromatography under inert atmosphere. In the solid state, they are fairly stable over a short period of time. They can be decomplexed by exposing the substances in solution to air and light, eg., to UV irradiation. The regiochemistry of the arylations was determined by single crystal X-ray crystallography of the chromium complexes of type 66 [10Gopal Dongol, K.; Matsubara, K.; Mataka, S.; Thiemann, T. Trialkylation of η6-Dihydronaphthalene-Cr(CO)3 complexes. J. Chem. Soc. Chem. Commun., 2002, (24), 3060-3061.
[http://dx.doi.org/10.1039/B209494K] ].
 |
Scheme 8 Tentative mechanism of the triarylation of η6-dihydronaphthalene tricarbonylchromium(0). |
η6-Indene tricarbonylchromium(0) (57) behaves differently under the reaction conditions, where the tricarbonyl chromium moiety offers a carbon monoxide ligand for a carbonylation of the haloarene, leading to benzophenones such as 59 after complete decomplexation, exposing the reaction mixtures in solution to air and light after completion of the carbonylation. Enquist et al. had noted a direct carbonylation of aryl iodides to benzophenones when they were reacted with dicobalt octacarbonyl [Co2(CO)8] under microwave irradiation [38Enquist, P.A.; Nilsson, P.; Larhed, M. Ultrafast chemistry: Cobalt carbonyl-mediated synthesis of diaryl ketones under microwave irradiation. Org. Lett., 2003, 5(25), 4875-4878.
[http://dx.doi.org/10.1021/ol036091x] [PMID: 14653696] ].
2.3. A short Word on Pd-Catalysts
Many Pd- and Pt-catalysts have been developed [39de Meijere, A.; Diedrich, F., Eds.; Metal-catalyzed cross-coupling reactions., 2004,
[http://dx.doi.org/10.1002/9783527619535] -41Molnár, Á., Ed.; Palladium-catalyzed coupling reactions., 2013,
[http://dx.doi.org/10.1002/9783527648283] ] for C-C cross-coupling reactions. Recyclability of the catalyst, low catalyst loading and high turnover frequencies are some important characteristics of the catalyst. Equally important, however, are versatility, cost, and ease of preparation. Many of the reactions above were run with commercially available Pd(PPh3)4, Pd(PPh3)2Cl2 and Pt(PPh3)4 or with Pd(OAc)2 under Jeffery [15Jeffery, T. Highly Stereospecific palladium-catalysed vinylation of vinylic halides under solid-liquid phase transfer conditions. Tetrahedron Lett., 1985, 26, 2667-2668.
[http://dx.doi.org/10.1016/S0040-4039(00)98131-0] -17Jeffery, T. Palladium-catalyzed vinylation of acetylenic iodides under solid-liquid phase-transfer conditions. Synthesis, 1987, (01), 70-71.
[http://dx.doi.org/10.1055/s-1987-27851] ] conditions. It must be noted that the commonly used reaction system with the commercially available Pd(PPh3)4 in DME/aq. Na2CO3 satisfactorily facilitates the Suzuki cross-coupling of chloroarenes with differently substituted arylboronic acids, when the chloro-substituent is activated as in 1-chloro-2,4-dinitrobenzene (70b) [14Thiemann, T.; Tanaka, Y.; Hisaindee, S.; Kaabi, M. A study of the Suzuki-Miyaura reaction of chloroarenes using Pd(PPh3)4 as catalyst. J. Chem. Res., 2010, 34, 34-38.
[http://dx.doi.org/10.3184/030823410X12624523028293] ] and chloroanthraquinones [13Thiemann, T.; Tanaka, Y.; Iniesta, J.; Varghese, H.T.; Panicker, C.Y. Arylation of Chloroanthraquinones by surprisingly facile Suzuki-Miyaura cross-coupling reactions. J. Chem. Res., 2009, 33, 732-736.
[http://dx.doi.org/10.3184/030823409X12586584303880] ] such as in 67 (Scheme 9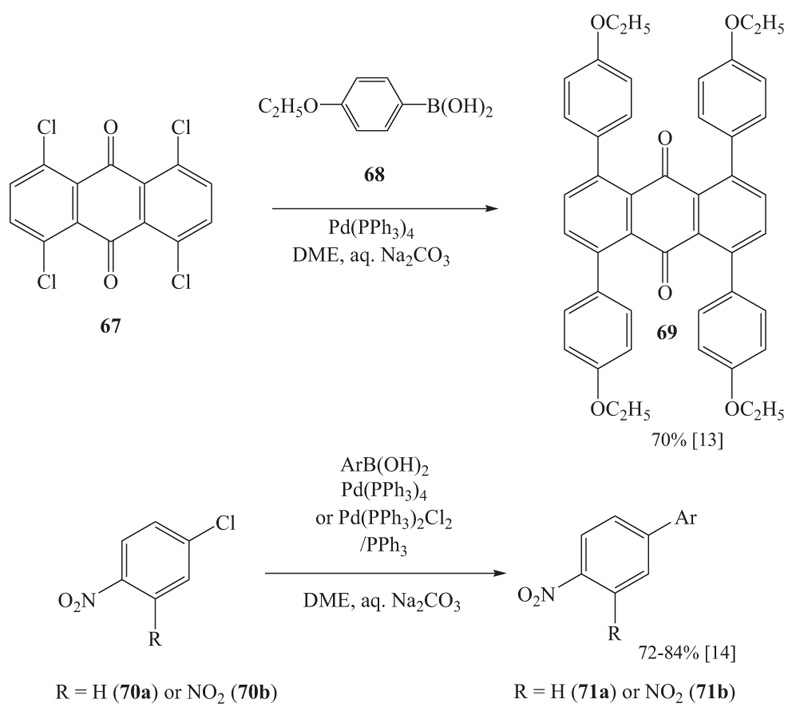 ). It must be noted that 1-chloro-2,4-dinitrobenzene (70b) is often used as a substrate to test the activity of new Pd-catalysts in Suzuki reactions and thus it must be realized that 70b undergoes cross-coupling reactions with ease and other substrates would put the new catalyst to a more rigorous test.
). It must be noted that 1-chloro-2,4-dinitrobenzene (70b) is often used as a substrate to test the activity of new Pd-catalysts in Suzuki reactions and thus it must be realized that 70b undergoes cross-coupling reactions with ease and other substrates would put the new catalyst to a more rigorous test.
 |
Scheme 9 Facile arylations of tetrachloroanthraquinone (67) and chloronitrobenzenes (70). |
Lastly, we studied the catalytic activity of Pd immobilized as nanoparticles on carbon nanofibers (CNFs) [42Lim, S.; Shimizu, A.; Yoon, S-H.; Korai, Y.; Mochida, I. High Yield Preparation of Tubular Carbon Nanofibers over Supported Co-Mo Catalysts. Carbon, 2004, 42, 1279-1283.
[http://dx.doi.org/10.1016/j.carbon.2004.01.027] ]. These vapor-grown carbons over different catalysts [eg., with reactant gases CO/H2 (4:1) or ethylene/H2 (4:1) over [reduced] Co-Mo (9:1) cat., prepared from (NH4)6Mo7O24 and Co(NO3)2.6H2O, carbon nanofibers grown in a flow reactor at 480–600 °C are made of graphene layers, formed into stacked cones, cups or plates. The positioning of the stacks in relation to each other then leads to platelet-type and herring (fish-bone)-type CNFs. In addition, there are the tubular-type CNFs (Fig. 1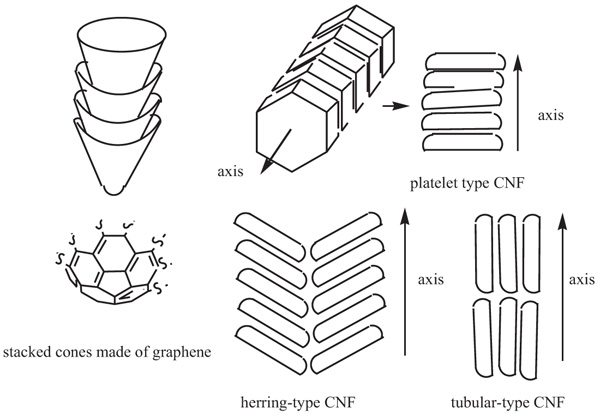 ).
).
 |
Fig. (1) Schematic representation of different types of carbon nanofibers (CNFs). |
With these CNFs in hand, Pd was immobilized on them by reduction, starting out from either PdCl2 [microwave irradiation, 650 W, 2 min. (free-running temperature), CNF, in ethylene glycol with or without the surfactant polyvinylpyrrolidone K-30 (PVP)], from H2PdCl4 prepared from PdCl2 and 1.5 N aq. HCl solution [immobilization of Pd on CNF using sodium formate, hydrazine or NaBH4 as reductant in H2O with and without the surfactant PVP] or from Pd2(dba)3.CHCl3 [reduction: CNF, H2 [1 atm], toluene]. Good results in the Heck reaction of 4-bromobenzaldehyde and tert-butyl acrylate were obtained with Pd on tubular CNF, which had been prepared by the permeation technique described above, starting out from PdCl2 in aq. HCl, reducing the Pd(II) with hydrazine in H2O in the presence of surfactant PVP. This Pd-doped tubular CNF was found to carry 4.6-5.6wt% Pd with a Pd-particle size of 10-15 nm. For the Heck reaction, this catalyst gave a turnover frequency of 44.6 h-1 in DMF at 100 °C, where solid K2CO3 was used as the base. This compared well with pre-catalysts PdCl2 (TOF 1.9 h-1), Pd(OAc)2 (TOF >10.8 h-1), and Pd(PPh3)2Cl2 (TOF 29.0 h-1) [18Yamamoto, K.; Thiemann, T. Suzuki-Miyaura cross-coupling and Heck-Mizoroki reactions catalysed by Pd on carbon nanofibers. J. Chem. Res., 2011, 35, 246-250.
[http://dx.doi.org/10.3184/174751911X13025306846745] , 43Yamamoto, N.; Kondo, S.; Chikaoka, T.; Nakashima , H.; Masui, H. Effects of magnetic field configuration on thrust performance in a miniature microwave discharge ion thruster. J App Phys, 2007, 102(12), 123304..]. Also, Pd immobilized on tubular CNF served as a satisfactory catalyst for the Suzuki reaction of 4-methoxyphenylboronic acid and 4-bromobenzaldehyde (Scheme 10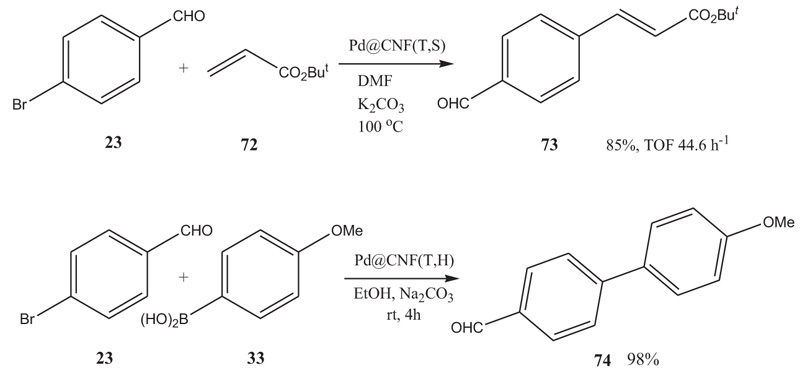 ), providing 4-methoxy-4’-formylbiphenyl in more than 98% yield, when the reaction was run with a catalyst loading of 0.02 mol% in Pd at rt for 4h, utilizing ethanol as solvent and Na2CO3 as base. The Pd@CNF catalysts could be recycled.
), providing 4-methoxy-4’-formylbiphenyl in more than 98% yield, when the reaction was run with a catalyst loading of 0.02 mol% in Pd at rt for 4h, utilizing ethanol as solvent and Na2CO3 as base. The Pd@CNF catalysts could be recycled.
 |
Scheme 10 Heck and Suzuki reactions with Pd-doped carbon nanofibers. |
3. EXPERIMENTAL
3.1. General
Melting points were measured on a Stuart SMP 10 melting point apparatus or on a Yanaco microscopic hotstage and are uncorrected. Infrared spectra were measured with a Thermo/Nicolet Nexus 470 FT-IR ESP or a JASCO IR-700 spectrometer. 1H and 13C NMR spectra were recorded with a Varian 400 NMR spectrometer (1H at 395.7 MHz, 13C at 100.5 MHz) or a JEOL EX-270 spectrometer (1H at 270 MHz, 13C at 67.8 MHz). The assignments of the carbon signals were aided by DEPT 90 and DEPT 135 experiments (DEPT = Distortionless Enhancement by Polarisation Transfer). The chemical shifts are relative to tetramethylsilane (TMS) (solvent CDCl3, unless otherwise noted). Mass spectra were measured with a JMS-01-SG-2 or with an Agilent QTOF 6540 UHD. Column chromatography, when necessary, was performed on silica gel (S, 0.063 mm - 0.1 mm, Riedel de Haen and Merck grade 9385 or on Wakogel 300).
3.2. Representative Procedures
3.2.1. 4-(2’-Fluorophenyl)benzoylmethylidenetriphenylphosphorane (5):
A mixture of 1 (153 mg, 0.33 mmol), 2-fluorophenylboronic acid (4, 100 mg, 0.70 mmol) and Pd(PPh3)4 (20 mg, 1.7 X 10-2 mmol) in aq. Na2CO3 (2M, 2.3 mL) and DME (4.5 mL) was heated at 70 ºC for 14 h. Then, water (15 mL) was added to the cooled solution, and the mixture was extracted with chloroform (3 X 10 mL). The organic phase was dried over anhydrous MgSO4 and concentrated in vacuo. The residue was subjected to column chromatography on silica gel (ether) to give 5 (155 mg, 98%) as colorless needles; mp. 244–245 ºC (ether); IR (KBr/cm-1) ν = 3048, 1581, 1518, 1481, 1436, 1408, 1385, 1103, 881, 756, 747, 692; 1H NMR (270 MHz, CDCl3) 4.48 (1H, d, 2JP-H 23.7 Hz), 7.10 – 7.77 (21H, m), 8.04 (2H, d, 3J 8.2 Hz); 13C NMR (67.8 MHz, CDCl3, DEPT 90, DEPT 135) 51.08 (+, CH, 1JC=P 110.8 Hz), 116.03 (+, CH, 3JC-F 23.1 Hz), 124.25 (+, CH, J 3.6 Hz), 126.78 (Cquat, 1JC-P 97.7 Hz), 127.03 (+, CH), 128.44 (+, CH, J 2.5 Hz), 128.87 (+, CH, JC-P 12.2 Hz), 130.74 (+, CH, J 3.6 Hz), 132.07 (+, CH, J 2.4 Hz), 133.15 (+, CH, JC-P 9.8 Hz), 133.44 (Cquat), 136.69 (Cquat), 140.41 (Cquat, J 14.7 Hz), 159.79 (Cquat, 1JC-F 248 Hz), 184.32 (Cquat, J 3.7 Hz, C=O). MS (70 eV) m/z (%) 474 (M+, 40), 445 (11), 303 (65), 183 (100). HRMS Found: 474.1550. Calcd. for C32H24OFP: 474.1549. Found: C, 81.13; H, 5.18%. Calcd. for C32H24OFP: C, 81.00; H, 5.10%.
3.2.2. Methyl (E,E)-5-(3’-nitrobiphenyl)penta-2,4-dienoate (17a):
A reaction mixture of 3-nitrophenylboronic acid (14a, 270 mg, 1.62 mmol), 4-bromocinnamaldehyde (15, 260 mg, 1.23 mmol), methoxycarbonylmethylidenetriphenylphosphorane (16-OMe, 600 mg, 1.80 mmol), Pd(PPh3)2Cl2 (25 mg, 3.6 X 10-5 mol), and triphenylphosphine (PPh3, 25 mg, 9.5 X 10-5 mol) in the biphasic solvent system aq. Na2CO3 (7 mL, 2.32 g Na2CO3 in 10 mL H2O) and 1,2-dimethoxyethane (DME, 10 mL) was stirred at 70 ºC for 24 h. Thereafter, the cooled mixture was extracted with chloroform (3 X 20 mL) and water (50 mL). The organic phase was dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The residue was subjected to column chromatography on silica gel to give 17a (362 mg, 95%) as a bright-yellow solid; IR (KBr/cm-1) ν = 1685, 1618, 1529, 1354, 1242, 1144, 997; 1H NMR (400 MHz, CDCl3) 3.78 (3H, s, OCH3), 6.08 (1H, d, 3J = 15.6 Hz), 6.93 – 6.95 (2H, m), 7.45 – 7.59 (1H, m), 7.58 (2H, d, 3J = 8.4 Hz), 7.62 (dd, 3J = 8.0 Hz, 3J = 8.0 Hz), 7.63 (2H, d, 3J = 8.4 Hz), 7.92 (1H, d, 3J = 8.0 Hz), 8.20 (1H, d, 3J = 8.0 Hz), 8.45 (1H, s); 13C NMR (68.7 MHz, CDCl3) 51.7 (OCH3), 121.4, 121.7, 122.3, 127.0, 127.5 (2C), 127.9 (2C), 129.9, 132.8, 136.3, 138.9, 139.4, 141.9, 144.5, 148.7, 167.4 (Cquat, CO); UV (CH2Cl2) λmax = 330 nm (ε = 36690 M-1cm-1).
3.2.3. Methyl (E,E)-5-(4’-nitrobiphenyl)penta-2,4-dienoate (17b):
A reaction mixture of 4-nitrophenylboronic acid (14b, 375 mg, 2.25 mmol), 4-bromocinnamaldehyde (15, 360 mg, 1.70 mmol), methoxycarbonylmethylidenetriphenylphosphorane (16-OMe, 830 mg, 2.49 mmol), Pd(PPh3)2Cl2 (25 mg, 3.6 X 10-5 mol), and triphenylphosphine (PPh3, 25 mg, 9.5 X 10-5 mol) in the biphasic solvent system aq. Na2CO3 (2.32 g Na2CO3 in 10 mL H2O) and 1,2-dimethoxyethane (DME, 10 mL) was stirred at 70 ºC for 24 h. Thereafter, the cooled mixture was extracted with chloroform (3 X 20 mL) and water (50 mL). The organic phase was dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The residue was subjected to column chromatography on silica gel to give 17b (431 mg, 83%) as a bright-yellow solid; IR (KBr/cm-1) ν = 1707, 1618, 1591, 1514, 1341, 1239, 1134, 1010, 844, 757, 731; 1H NMR (400 MHz, CDCl3) 3.79 (3H, s, OCH3), 6.05 (1H, d, 3J = 15.2 Hz), 6.95 (2H, m), 7.44 – 7.51 (1H, m), 7.61 (2H, d, 3J = 8.4 Hz), 7.63 (2H, d, 3J = 8.4 Hz), 7.74 – 7.77 (2H, m), 8.29 – 8.32 (2H, m); 13C NMR (67.8 MHz, CDCl3) 51.7 (OCH3), 121.6, 124.2 (2C), 127.2, 127.2(5), 127.6 (2C), 127.8, 136.6, 139.0, 139.3, 144.4, 146.7, 147.2, 167.4 (Cquat, CO); UV (CH2Cl2) λmax = 345 nm (ε = 45980 M-1cm-1).
3.2.4. 3-(3”-Nitrobiphen-3’-yl)acrylic acid (21):
To a mixture of 3-bromobenzaldehyde (29, 858 mg, 4.64 mmol), ethoxycarbonylmethylidenetriphenylphosphorane (16-OEt, 2.0 g, 5.74 mmol), 3-nitrophenylboronic acid (771 mg, 4.64 mmol), bistriphenylphosphinopalladium dichloride (38 mg, 0.05 mmol) and triphenylphosphine (35 mg, 0.13 mmol) aq. Na2CO3 (2.52 g Na2CO3 on 10 mL H2O) was added, and the resulting mixture was stirred for 24 h at 75 ºC. Thereafter, aq. NaOH (2.05 g NaOH on 10 mL H2O) was added and the mixture was stirred a further 24 h at 95ºC. The mixture was cooled, and water was added (20 ml). Thereafter, the ensuing precipitate was filtered off. The aqueous filtrate was acidified with half conc. HCl, and the aqueous layer was extracted with EtOAc (2 X 75 mL). The combined organic extracts were dried over anhydrous MgSO4, evaporated in vacuo and the residue was subjected to column chromatography on silica gel (EtOAc/hexane: 1/3) to give 21 (670 mg, 54%) as a colorless solid (mp. > 230 °C); IR (KBr/cm-1) ν = 3500 – 2550 (bs, OH), 3019, 1690 (s, C=O), 1631, 1532, 1221, 790, 682; 1H-NMR (400 MHz, DMSO-d6) 6.70 (1H, d, 3J = 16.0 Hz), 7.54 – 7.84 (7H, m), 8.12 (1H, m), 8.21 (1H, m); 13 C-NMR (100.5 MHz, CDCl3): 120.7, 121.8, 122.9, 127.3, 128.7, 129.1, 130.2, 130.9, 133.9, 135.7, 138.9, 141.5, 143.9, 148.9, 168.1.
3.2.5. 3-Methoxy-2’-butoxycarbonyl- [[17Jeffery, T. Palladium-catalyzed vinylation of acetylenic iodides under solid-liquid phase-transfer conditions. Synthesis, 1987, (01), 70-71.
[http://dx.doi.org/10.1055/s-1987-27851] , 16Jeffery, T. Palladium-catalysed vinylation of organic halides under solid liquid phase transfer conditions. J. Chem. Soc. Chem. Commun., 1984, (19), 1287-1289.
[http://dx.doi.org/10.1039/C39840001287] ]benzoestra-1,3,5(10),16-tetraene (40):
A deaerated solution of 35 (126 mg, 0.3 mmol), Pd(PPh3)4 (35 mg, 0.03 mmol), butyl acrylate (615 mg, 4.8 mmol) and Et3N (215 mg, 2.1 mmol) in DMF (0.7 mL) was heated at 90 °C for 20 h. Thereafter, the cooled solution was poured into water (60 mL) and extracted with ether (3 X 80 mL). The organic phase was dried over anhydrous MgSO4 and concentrated in vacuo. The residue was subjected to column chromatography on silica gel (hexane/ether 5:1) to give 40 as a yellowish solid (33 mg, 26%), mp. 195–197 °C; 1H-NMR (270 MHz, CDCl3) 0.96 (3H, s, CH3), 0.93 – 3.02 (20H, m), 3.79 (3H, s, OCH3), 4.32 (2H, t, OCH2,3J = 6.5 Hz), 6.66 (1H, d, 4J = 2.6 Hz), 6.74 (1H, d, 3J = 8.4 Hz, 4J = 2.6 Hz), 7.21 (1H, d, 3J = 8.4 Hz), 7.30 (1H, d, 3J = 7.6 Hz), 7.79 (1H, s), 7.85 (1H, d, 3J = 7.6 Hz); 13 C-NMR (67.8 MHz, CDCl3, DEPT 90, DEPT 135): 13.8 (+, CH3), 19.3 (+, CH3), 26.4 (-), 27.8m (-), 29.7 (-), 30.8 (-), 32.2 (-), 35.0 (-), 37.7 (+, CH), 44.3 (+, CH), 45.5 (Cquat), 55.2 (+, CH), 56.5 (+, OCH3), 64.7 (-, OCH2), 111.5 (+, CH), 113.9 +, CH), 121.9 (+, CH), 124.9 (+, CH), 126.1 (+, CH), 127.8 (+, CH), 128.7 (Cquat), 132.6 (Cquat), 137.8 (Cquat), 148.4 (Cquat), 154.8 (Cquat), 157.5 (Cquat), 167.2 (Cquat); MS (70 eV) m/z (%) 418 (M+, 100). HRMS Found: 418.2510. Calcd. for C28H34O3: 418.2508.
3.2.6. 2,12-Dimethoxy-8-(4’-methoxyphenyl)-8b,9,10,14b-tetrahydrobenzo[b]chrysene (56):
A mixture of η6-1,2-dihydronaphthalene chromiumtricarbonyl complex (54, 70 mg, 0.26 mmol), palladium acetate (Pd(OAc)2, 4.5 mg, 0.02 mmol), p-iodo-anisole (55, 190 mg, 0.80 mmol), n-tetra-butyl ammonium chloride (195 mg, 0.70 mmol), and potassium acetate (50 mg, 0.50 mmol) in acetonitrile (1 mL) was stirred for 24 h at rt. After evaporation of the solvent in vacuo, a yellow solid was obtained. The solid was decomplexed by exposing it to air and light. The decomplexed mixture was purified by column chromatography (SiO2, hexane/Et2O) and compound 56 (80 mg, 0.18 mmol, 68%) was obtained as a colorless solid, mp. 167 °C; IR (KBr/cm-1) ν = 3437, 2953, 2925, 2855, 2359, 1727, 1599, 1511, 1462, 1428, 1340, 1289, 1247, 1203, 1173, 1100, 1049, 835; 1H-NMR (270 MHz, CDCl3) 1.67 (2H, m), 2.64 (2H, m), 3.12 (1H, m), 3.68 (3H, s, OCH3), 3.83 (3H, s, OCH3), 3.88 (3H, s OCH3), 4.11 (1H, d, 3J = 4.3 Hz), 6.34 (1H, d, J = 2.6 Hz), 6.72 (1H, d, J = 2.6 Hz), 6.78 (2H, dd, 3J = 5.9 Hz, J = 2.6 Hz), 6.93 (d, 1H, 3J = 8.57 Hz), 7.10 (1H, s), 7.14 (2H, d, 3J = 6.9 Hz), 7.19 (1H, d, J = 4.0 Hz), 7.29 (2H, d, 3J = 3.6 Hz), 7.70 (2H, dd, 3J = 6.2 Hz, J = 3.6 Hz); 13 C-NMR (67.8 MHz, CDCl3): 23.7, 28.5, 35.1, 43.2, 55.0, 55.3, 55.4, 108.6, 110.9, 113.5, 114.3, 115.0, 125.5, 125.1, 125.5, 126.7, 128.8, 129.7, 130.0, 130.7, 130.9, 131.4, 132.4, 133.8, 134.8, 136.4, 141.1, 157.9, 159.5. EI-MS: m/z (%) = 448 [M +1](100), 405, (M+-CH3CO, 4), 358 (M+-CH3CO,C2H6O +1, 53), 342 (23), 327 (5), 295 (5), 230 (15), 215 (6), 189 (9), 149 (44), 141 (20), 111 (15), 83 (26), 71 (22). HRMS Found: 448.2037. Calcd. for C31H28O3: 448.2034. Found: C, 65.55; H, 3.48%. Calcd. For C31H28O3: C, 66.14; H, 3.48%.
3.2.7. Dimethyl benzophenone-4,4’-dicarboxylate (59):
A deaerated mixture of η6- indenetricarbonylchromium(0) complex (57, 100 mg, 0.40 mmol), palladium acetate (Pd(OAc)2, 4.5 mg, 0.02 mmol), methyl 4-iodobenzoate (58, 205 mg, 0.8 mmol), n-tetra-butyl ammonium chloride (195 mg, 0.70 mmol), and potassium acetate (50 mg, 0.50 mol) in acetonitrile (1 mL) was stirred at rt for 24 h. After evaporation of the solvent in vacuo, a yellow solid was obtained, which was decomplexed by exposing it to light and air. Column chromatography on silica gel (hexane/ether) gave 59 (60 mg, 0.2 mmol, 52%) as a colorless solid (mp. 221 °C [Lit. 228 °C [44Ulbrich, H.; Luxenburger, A.; Prech, P.; Eriksson, E.E.; Soehnlein, O.; Rotzius, P.; Lindbom, L.; Dannhardt, G. A novel class of potent nonglycosidic and nonpeptidic pan-selectin inhibitors. J. Med. Chem., 2006, 49(20), 5988-5999.
[http://dx.doi.org/10.1021/jm060468y] [PMID: 17004713] ]]); IR (neat): νmax = 2958 (w), 1720 (s), 1610 (w), 1559 (w), 1433 (m), 1397 (w), 1285 (s), 1180 (m), 1112 (s), 1017 (w), 954 (m), 849 (m), 757 (m); 1H-NMR (CDCl3, 270 MHz) δ = 3.97 (6H, s, CO2CH3), 7.85 (dd, 4H, 3J = 6.6 Hz, 4J = 2.0 Hz), 8.15 (dd, 4H, 3J = 6.6 Hz, J = 2.0 Hz). 13 C-NMR (CDCl3, 67.8 MHz) δ = 52.2, 127.3, 129.8, 130.2, 144.4, 166.8, 196.0; MS (EI, 70 eV) m/z (%) = 298 [M+] (22), 267 (M+- OCH3, 15), 239 (M +- OCH3, -CO, 10), 208 (M +- OCH3, -CO, -OCH3, 3), 180 (M +- OCH3,-CO, -OCH3, -CO,11), 163 (C6H4-CO2CH3, 100), 152 (15), 135 (23), 120 (17), 104 (46), 76 (69), 59 (5). HRMS Found: 298.0846. Calcd. for C17H14O5: 298.0841.
CONCLUSION
This was a short tour-de-force of some of the work on Pd- and Pt-catalysis that we have carried out over the years. The focus has been to develop strategies of combining various reactions with Pd- or Pt-catalyzed C-C cross coupling reactions in one pot. Furthermore, diene-yne cyclizations were studied under Pt-catalysis that led either to arenes or fulvenes. A triarylation of η6-dihydronaphthalene tricarbonylchromium complexes with concomitant ring closure was more of a serendipitous discovery.
The authors believe that studies of metal-catalyzed directed cascade reactions will continue to open up new synthetic strategies to complex molecules of value. The recycling and reuse of Pd- and Pt-catalysts will remain to be of great importance for their use in industrial processes. Equally important may be continued investigations on the mechanisms of cross-coupling reactions with heterogeneous Pt- and Pd-catalysts as well as a better understanding of leaching phenomena of these catalysts and their mitigation. This should lead to more facile work-up procedures and to even lower levels of metal contamination of the products.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
I am indebted to those who contributed to this chemistry as collaborators in addition to the co-authors of this communication: Dr. Christian Burmester, Ms. Kyoko Yamamoto, Mr. Kodai Shiine, Mr. Taisuke Matsumoto (X-ray crystallography), Prof. Dr. Shuntaro Mataka, Prof. Dr. Matsubara (X-ray crystallography), Prof. Dr. Isao Mochida, Prof. Dr. Yukihiro Motoyama, and Prof. Dr. Seong Ho Yoon. Especially, we thank Prof. I. Mochida and S.-H. Yoon for the CNF material. The investigations of using Pd and Pt-doped CNFs as catalysts were part of a JST-CREST project on carbon nanofibres (2005 - 2007).
REFERENCES
| [1] | Oliveira, M.C.; Neto, C.; Gano, L.; Marques, F.; Santos, I.; Thiemann, T.; Santos, A.C.; Botelho, F.; Oliveira, C.F. Estrogen receptor ligands for targeting breast tumours: A brief outlook on radioiodination strategies. Curr. Radiopharm., 2012, 5(2), 124-141. [http://dx.doi.org/10.2174/1874471011205020124] [PMID: 22280114] |
| [2] | Oliveira, M.C.; Neto, C.; Ribeiro Morais, G.; Thiemann, T. Steroid receptor ligands for breast cancer targeting: An insight into their potential role as PET imaging agents. Curr. Med. Chem., 2013, 20(2), 222-245. [http://dx.doi.org/10.2174/092986713804806658] [PMID: 23231090] |
| [3] | Neto, C.; Oliveira, M.C.; Gano, L.; Marques, F.; Yasuda, T.; Thiemann, T.; Kniess, T.; Santos, I. Novel 7α-alkoxy-17α-(4′-halophenylethynyl)estradiols as potential SPECT/PET imaging agents for estrogen receptor expressing tumours: Synthesis and binding affinity evaluation. Steroids, 2012, 77(11), 1123-1132. [http://dx.doi.org/10.1016/j.steroids.2012.05.004] [PMID: 22633985] |
| [4] | Thiemann, T.; Thiemann, C.; Sasaki, S.; Vill, V.; Mataka, S.; Tashiro, M. Synthesis and photochemical behavior of C-16 substituted steroids. J. Chem. Res., 1997, 21 (S), 248-249; 21 (M), 1736-1750. |
| [5] | Thiemann, T.; Umeno, K.; Wang, J.; Arima, K.; Watanabe, M.; Tabuchi, Y.; Tanaka, Y.; Gorohmaru, H.; Mataka, S. Elongated phosphoranes by C-C coupling of haloaroylmethylidenephosphoranes – synthesis and applications. J. Chem. Soc., Perkin Trans. 1, 2002, 2090-2110. [http://dx.doi.org/10.1039/B202745N] |
| [6] | Thiemann, T. Wittig- and Horner-Wadsworth-Emmons Olefination in Aqueous Media with and without Phase Transfer Catalysis. Mini Rev. Org. Chem., 2018, 15, 412-432. [http://dx.doi.org/10.2174/1570193X15666180221152118] |
| [7] | Merza, F.; Taha, A.; Thiemann, T. Tandem-, Domino- and one-pot reactions involving Wittig- and Horner-Wadsworth-Emmons olefination. INTECH OPEN., 2018, [http://dx.doi.org/10.5772/intechopen.70364] |
| [8] | Al Jasem, Y.; El-Esawi, R.; Thiemann, T. Wittig- and Horner-Wadsworth-Emmons (HWE) olefination reactions with stabilized and semi-stabilized phosphoranes and phosphonates under non-classical conditions. J. Chem. Res., 2014, 38, 453-463. [http://dx.doi.org/10.3184/174751914X14044684705710] |
| [9] | Gopal Dongol, K.; Melo e Silva, M.C.; Matsubara, K.; Matsumoto, T.; Mataka, S.; Thiemann, T. π-Facial Selectivity in the Formation of η6-Tricarbonylchromium(0) Complexes of Estra-1,3,5(10),6-tetraenes. Z. Anorg. Allg. Chem., 2003, 629, 945-947. [http://dx.doi.org/10.1002/zaac.200300017] |
| [10] | Gopal Dongol, K.; Matsubara, K.; Mataka, S.; Thiemann, T. Trialkylation of η6-Dihydronaphthalene-Cr(CO)3 complexes. J. Chem. Soc. Chem. Commun., 2002, (24), 3060-3061. [http://dx.doi.org/10.1039/B209494K] |
| [11] | Thiemann, T.; Watanabe, M.; Mataka, S. Areno-annelated Estra-1,3,5(10),6,8,11,14,16-octaenes. New J. Chem., 2001, 25, 1104-1107. [http://dx.doi.org/10.1039/b104648a] |
| [12] | Watanabe, M.; Mataka, S.; Thiemann, T. Benzothieno and benzofurano annelated estranes. Steroids, 2005, 70(13), 856-866. [http://dx.doi.org/10.1016/j.steroids.2005.06.001] [PMID: 16045951] |
| [13] | Thiemann, T.; Tanaka, Y.; Iniesta, J.; Varghese, H.T.; Panicker, C.Y. Arylation of Chloroanthraquinones by surprisingly facile Suzuki-Miyaura cross-coupling reactions. J. Chem. Res., 2009, 33, 732-736. [http://dx.doi.org/10.3184/030823409X12586584303880] |
| [14] | Thiemann, T.; Tanaka, Y.; Hisaindee, S.; Kaabi, M. A study of the Suzuki-Miyaura reaction of chloroarenes using Pd(PPh3)4 as catalyst. J. Chem. Res., 2010, 34, 34-38. [http://dx.doi.org/10.3184/030823410X12624523028293] |
| [15] | Jeffery, T. Highly Stereospecific palladium-catalysed vinylation of vinylic halides under solid-liquid phase transfer conditions. Tetrahedron Lett., 1985, 26, 2667-2668. [http://dx.doi.org/10.1016/S0040-4039(00)98131-0] |
| [16] | Jeffery, T. Palladium-catalysed vinylation of organic halides under solid liquid phase transfer conditions. J. Chem. Soc. Chem. Commun., 1984, (19), 1287-1289. [http://dx.doi.org/10.1039/C39840001287] |
| [17] | Jeffery, T. Palladium-catalyzed vinylation of acetylenic iodides under solid-liquid phase-transfer conditions. Synthesis, 1987, (01), 70-71. [http://dx.doi.org/10.1055/s-1987-27851] |
| [18] | Yamamoto, K.; Thiemann, T. Suzuki-Miyaura cross-coupling and Heck-Mizoroki reactions catalysed by Pd on carbon nanofibers. J. Chem. Res., 2011, 35, 246-250. [http://dx.doi.org/10.3184/174751911X13025306846745] |
| [19] | Thiemann, T.; Umeno, K.; Ohira, D.; Inohae, E.; Sawada, T.; Mataka, S. Suzuki-Kumada coupling with bromoaroylmethylidenephosphoranes. New J. Chem., 1999, 23, 1067-1070. [http://dx.doi.org/10.1039/a907470h] |
| [20] | Thiemann, T.; Umeno, K.; Inohae, E.; Mataka, S. Novel Elongated Phosphoranes by Heck-reaction and Pd(0)-Catalysed Alkynylation and their Use in C-7 Group Functionalisation in Estrones. Rep. Inst. Adv. Mat. Kyushu Univ., 2000, 14(1) Chem. Abstr.,2000, 133, 335-380. |
| [21] | Watanabe, M.; Mataka, S.; Thiemann, T. One Pot sonogashira-coupling / wittig olefination procedures. J. Chem. Res., 2005, 29, 636-639. [http://dx.doi.org/10.3184/030823405774663057] |
| [22] | Thiemann, T.; Watanabe, M.; Tanaka, Y.; Mataka, S. One-Pot wittig-olefination / suzuki-reaction - the Compatibility of conjugated phosphoranes in Pd(0) catalysed C-C-bond forming reactions. New J. Chem., 2006, 30, 359-369. [http://dx.doi.org/10.1039/b516724h] |
| [23] | Burmester, C.; Mataka, S.; Thiemann, T. Synthesis of non-symmetric Divinylarenes by a Heck/Wittig reaction combination. Synth. Commun., 2010, 40, 3196-3208. [http://dx.doi.org/10.1080/00397910903395235] |
| [24] | Thiemann, T. Solventless wittig reactions with fluorinated benzaldehydes. J. Chem. Res., 2007, 31, 336-341. [http://dx.doi.org/10.3184/030823407X225464] |
| [25] | Thiemann, T.; Watanabe, M.; Tanaka, Y.; Mataka, S. One pot Suzuki coupling - Wittig olefination reactions. J. Chem. Res., 2004, 28, 723-727. [http://dx.doi.org/10.3184/0308234043431609] |
| [26] | Yamamoto, K.; Watanabe, M.; Ideta, K.; Mataka, S.; Thiemann, T. Combined Suzuki coupling – Wittig olefination reaction in aqueous medium. Z. Naturforsch. B, 2005, 60, 1299-1307. [http://dx.doi.org/10.1515/znb-2005-1215] |
| [27] | Thiemann, T.; Elshorbagy, M.; Ahmadani, S.; Al Jasem, Y.; Al Azani, M.; al-Sulaibi, M.; al Hindawi, B. Facile, direct reaction of benzaldehydes to 3-phenyl-prop-2-enoic acids and 3-arylprop-2-ynoic acids in aqueous medium. IJOC, 2016, 6, 126-141. [http://dx.doi.org/10.4236/ijoc.2016.62014] |
| [28] | Thiemann, T.; Al Jasem, Y.; Butt, H.; al Hindawi, B.; Barkhad, M.; al Khazali, M.; al Azani, M. New heat transfer fluids (HTFs) for solar thermal applications, 3rd World Sustainability Forum Sciforum electronic conference Series, 2013, 3, d-002. [http://dx.doi.org/10.3390/wsf3-d002] |
| [29] | Watanabe, M.; Matsumoto, T.; Mataka, S.; Thiemann, T. Facile access to condensed arenothiophenes – preparation of dihydrophenanthrothiophenes and dihydrophenanthrobenzo[b]thiophenes. J. Chem. Res., 2005, 29, 564-571. [http://dx.doi.org/10.3184/030823405774308925] |
| [30] | Watanabe, M.; Matsumoto, T.; Mataka, S.; Thiemann, T. Heteroareno-annelated estranes by triene cyclization. CEJC, 2006, 4, 375-402. |
| [31] | Watanabe, M.; Shiine, K.; Ideta, K.; Matsumoto, T.; Thiemann, T. Diene-yne cyclization reactions of 1-ethynyl-2-vinyl-3,4-dihydronaphthalenes and 1-ethynyl-2-vinylnaphthalenes. J. Chem. Res., 2008, 32, 669-678. [http://dx.doi.org/10.3184/030823408X375214] |
| [32] | de Meijere, A.; Bräse, S. Palladium in action: Domino coupling and allylic substitution reactions for the efficient construction of complex organic molecules. J. Organomet. Chem., 1999, 576, 88-110. [http://dx.doi.org/10.1016/S0022-328X(98)01087-0] |
| [33] | Reiser, O.; Weber, M.; de Meijere, A. Selective 1:3 coupling of norbornene and iodobenzene: A facile synthesis of benzo[e]pyrenes with annelated cycloaliphatic moieties. Angew. Chem. Int. Ed. Engl., 1989, 28, 1037-1038. [http://dx.doi.org/10.1002/anie.198910371] |
| [34] | Catellani, M. Selective organometallic syntheses from molecular pools. Pure Appl. Chem., 2002, 74, 63-68. [http://dx.doi.org/10.1351/pac200274010063] |
| [35] | Catellani, M.; Motti, E.; Faccini, F.; Ferraccioli, R. New catalytic methods for the synthesis of selectively substituted aromatics through palladacycles. Pure Appl. Chem., 2005, 77, 1243-1248. [http://dx.doi.org/10.1351/pac200577071243] |
| [36] | Catellani, M. Catalytic Multistep Reactions via Palladacycles. Synlett, 2003, (03), 298-312. [http://dx.doi.org/10.1055/s-2003-37102] |
| [37] | Mauleón, P.; Alonso, I.; Carretero, J.C. Unusual palladium-catalyzed cascade arylation of α,β-unsaturated phenyl sulfones under Heck reaction conditions. Angew. Chem. Int. Ed. Engl., 2001, 40(7), 1291-1293. [http://dx.doi.org/10.1002/1521-3773(20010401)40:7<1291::AID-ANIE1291>3.0.CO;2-9] [PMID: 11301455] |
| [38] | Enquist, P.A.; Nilsson, P.; Larhed, M. Ultrafast chemistry: Cobalt carbonyl-mediated synthesis of diaryl ketones under microwave irradiation. Org. Lett., 2003, 5(25), 4875-4878. [http://dx.doi.org/10.1021/ol036091x] [PMID: 14653696] |
| [39] | de Meijere, A.; Diedrich, F., Eds.; Metal-catalyzed cross-coupling reactions., 2004, [http://dx.doi.org/10.1002/9783527619535] |
| [40] | Tsuji, J. Palladium reagents and catalysts: new perspective for the 21st century., 2002, |
| [41] | Molnár, Á., Ed.; Palladium-catalyzed coupling reactions., 2013, [http://dx.doi.org/10.1002/9783527648283] |
| [42] | Lim, S.; Shimizu, A.; Yoon, S-H.; Korai, Y.; Mochida, I. High Yield Preparation of Tubular Carbon Nanofibers over Supported Co-Mo Catalysts. Carbon, 2004, 42, 1279-1283. [http://dx.doi.org/10.1016/j.carbon.2004.01.027] |
| [43] | Yamamoto, N.; Kondo, S.; Chikaoka, T.; Nakashima , H.; Masui, H. Effects of magnetic field configuration on thrust performance in a miniature microwave discharge ion thruster. J App Phys, 2007, 102(12), 123304.. |
| [44] | Ulbrich, H.; Luxenburger, A.; Prech, P.; Eriksson, E.E.; Soehnlein, O.; Rotzius, P.; Lindbom, L.; Dannhardt, G. A novel class of potent nonglycosidic and nonpeptidic pan-selectin inhibitors. J. Med. Chem., 2006, 49(20), 5988-5999. [http://dx.doi.org/10.1021/jm060468y] [PMID: 17004713] |



