- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Dinuclear Copper(II) 3,4,5-Tri-O-benzylgallate
Masahiro Mikuriya1, *, Chihiro Yamakawa1, Naoyuki Masuda1, Daisuke Yoshioka1, Sayuri Yamaguchi2, Hidetoshi Yamada2, Tsutomu Mizuta3, Naomi Kawata4, Hidekazu Tanaka5, Makoto Handa5
Abstract
Background:
Ellagitannins have attracted much attention because of the biological and pharmacological activities. In the total synthesis of ellagitannins, 3,4,5-tri-O-benzylgallic acid has been a key compound to introduce the protected galloyl group. From the perspective of coordination chemistry, 3,4,5-tri-O-benzylgallic acid is an interesting carboxylate ligand which might be capable of dinuclear carboxylate complex. Such a dinuclear carboxylate complex might be interesting as a new example of copper acetate type complexes.
Objectives:
The objective of the work is to synthesize a copper acetate type complex by using 3,4,5-tri-O-benzylgallic acid and to see a new feature of copper acetate compounds, by elucidating the crystal structure, magnetic property, and adsorption property for N2 gas.
Methods:
Copper(II) 3,4,5-tri-O-benzylgallate has been synthesized by a reaction of 3,4,5-tri-O-benzylgallic acid and copper(II) nitrate at pH=9 condition. The isolated complex was characterized using single-crystal X-ray structure analysis, XRD analysis, UV-visible spectroscopy, IR spectroscopy, and temperature dependence of magnetic susceptibility.
Results:
The crystal structure shows a crystallographically centrosymmetric dinuclear molecule with four carboxylato-bridges and axial dimethylformamide molecules and crystal dmf molecules [Cu···Cu 2.6345(12) Å]. In the crystal, 1D supramolecular assembly by π-π interaction between the benzyl aromatic rings of dinuclear molecules was observed. Temperature dependence of magnetic susceptibilities showed a considerable antiferromagnetic interaction between the two copper(II) ions (2J = –214 cm–1). Type-II gas-adsorption property was observed for N2.
Conclusion:
A key-compound for the total synthesis of ellagitannins, 3,4,5-tri-O-benzylgallic acid (Htbng), was shown to be a new ligand for the synthesis of dinuclear copper acetate analogue with a lantern-type core, extending the realm of copper acetate clusters.
Article Information
Identifiers and Pagination:
Year: 2019Volume: 6
Issue: Suppl-1, M3
First Page: 19
Last Page: 26
Publisher Id: CHEM-6-19
DOI: 10.2174/1874842201906010019
Article History:
Received Date: 15/01/2018Revision Received Date: 27/03/2018
Acceptance Date: 02/02/2019
Electronic publication date: 22/03/2019
Collection year: 2019
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Department of Applied Chemistry for Environment, School of Science and Technology, Kwansei Gakuin University, 2-1 Gakuen, Sanda, 669-1337, Japan; Tel: +81-79-565-8365; Fax: +81-79-565-9729; E-mail: junpei@kwansei.ac.jp
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 15-01-2018 |
Original Manuscript | Dinuclear Copper(II) 3,4,5-Tri-O-benzylgallate | |
1. INTRODUCTION
Ellagitannins are a class of tannins containing galloyl and hexahydroxydiphenoyl groups, in which more than 1000 analogues have been found in natural systems [1Yamada, H.; Hirokane, T.; Ikeuchi, K.; Wakamori, S. Fundamental methods in ellagitannin synthesis. Nat. Prod. Commun., 2017, 12, 1351-1358.]. These tannins have attracted much attention for a long period, because they have interesting biological and pharmacological activities. So far, total synthesis has been attempted for more than 20 ellagitannins. The benzyl group was often used as an effective protecting group of the phenol groups in the total syntheses [2Yamada, H.; Hirokane, T.; Asakura, N.; Kasai, Y.; Nagao, K. Strategies and methods for the total synthesis of ellagitannins. Curr. Org. Chem., 2012, 16, 578-604.
[http://dx.doi.org/10.2174/138527212799859426] , 3Yamaguchi, S.; Hirokane, T.; Yoshida, T.; Tanaka, T.; Hatano, T.; Ito, H.; Nonaka, G.; Yamada, H. Roxbin B is cuspinin: Structural revision and total synthesis. J. Org. Chem., 2013, 78(11), 5410-5417.
[http://dx.doi.org/10.1021/jo400562k] [PMID: 23656490] ], where 3,4,5-tri-O-benzylgallic acid (Fig. 1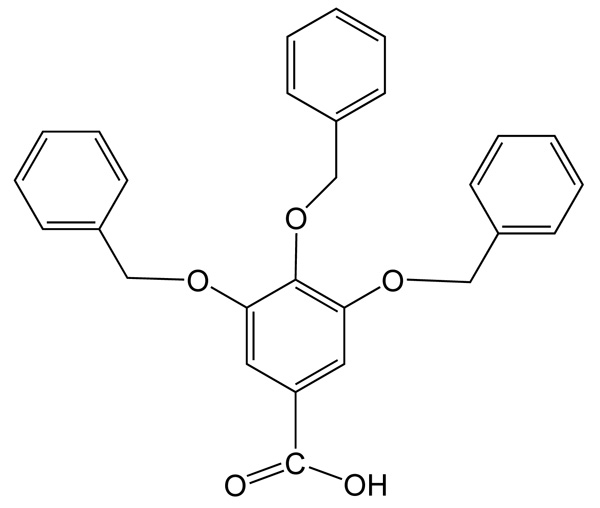 ) is a key compound to introduce the protected galloyl group. From the perspective of coordination chemistry, 3,4,5-tri-O-benzylgallic acid is an interesting carboxylate ligand which might be capable of dinuclear carboxylate complex. Such a dinuclear carboxylate complex might be interesting as a new example of copper acetate. Copper acetate is one of the oldest metal complexes with a unique Cu2 cluster and has attracted much attention since the discovery of the lantern-like acetato-bridged cluster and antiferromagnetic spin-coupling [4van Niekerk, J.N.; Schoening, F.R. A new type of copper complex as found in the crystal structure of cupric acetate, Cu2(CH3COO)4.2H2O. Acta Crystallogr., 1953, 6, 227-232.
) is a key compound to introduce the protected galloyl group. From the perspective of coordination chemistry, 3,4,5-tri-O-benzylgallic acid is an interesting carboxylate ligand which might be capable of dinuclear carboxylate complex. Such a dinuclear carboxylate complex might be interesting as a new example of copper acetate. Copper acetate is one of the oldest metal complexes with a unique Cu2 cluster and has attracted much attention since the discovery of the lantern-like acetato-bridged cluster and antiferromagnetic spin-coupling [4van Niekerk, J.N.; Schoening, F.R. A new type of copper complex as found in the crystal structure of cupric acetate, Cu2(CH3COO)4.2H2O. Acta Crystallogr., 1953, 6, 227-232.
[http://dx.doi.org/10.1107/S0365110X53000715] -13Mikuriya, M. Copper(II) acetate as a motif of metal-assembled complexes. Bull. Jpn. Soc. Coord. Chem., 2008, 52, 17-28. [In Japanese].
[http://dx.doi.org/10.4019/bjscc.52.17] ]. Recently, a unique di-µ-acetato-bridged dinuclear cluster, [Cu2(CH3CO2)4(EtimH)2] (EtimH = 2-ethylimidazole), was reported by Hernandez et al. [14Hernandez, J.; Avila, M.; Jimenez-Vazquez, H.A.; Duque, J.; Reguera, E. Copper dimer with acetate-2-ethylimidazole as ligands. Synth. React. Inorg. Met.-Org. Nano-Met. Chem., 2015, 45, 342-350.
[http://dx.doi.org/10.1080/15533174.2013.832322] ]. They aimed to attempt to form porous copper acetate-2-ethylimdazole. We have been engaged in the synthesis of copper acetate analogues and their metal-assembled complexes [13Mikuriya, M. Copper(II) acetate as a motif of metal-assembled complexes. Bull. Jpn. Soc. Coord. Chem., 2008, 52, 17-28. [In Japanese].
[http://dx.doi.org/10.4019/bjscc.52.17] , 15Mikuriya, M.; Kida, S.; Ueda, I.; Tokii, T.; Muto, Y. Crystal structure and magnetic property of Di-µ-propionato-O,O’-bis[N-p-tolylsalicylideneaminatocopper(II)]. Bull. Chem. Soc. Jpn., 1977, 50, 2464-2470.
[http://dx.doi.org/10.1246/bcsj.50.2464] -31Mikuriya, M.; Yamakawa, C.; Tanabe, K.; Yoshioka, D.; Mitsuhashi, R.; Tanaka, H.; Handa, M. Synthesis, crystal structure, magnetic property, and N2-gas-adsorption property of dinuclear copper(II) 3,4,5-trimethoxybenzoate. J. Turkish Chem. Soc., Sect. A 5(sp.is.1), 2018, 103-110.
[http://dx.doi.org/10.18596/jctcsa.370793] ]. During the course of our studies, we found that copper(II) benzoate forms a chain compound with pyrazine and the assembled chain complex has a porous structure and an adsorption property for N2, where the aromatic benzoate groups form a hydrophobic micropore [19Nukada, R.; Mori, W.; Takamizawa, S.; Mikuriya, M.; Handa, M.; Naono, H. Microporous structure of a chain compound of copper(II) benzoate bridged by pyrazine. Chem. Lett., 1999, 28, 367-368.
[http://dx.doi.org/10.1246/cl.1999.367] , 30Nukada, R.; Mikuriya, M.; Handa, M.; Naono, H. Hydrophobic micropore in a chain compound of dinuclear copper(II) benzoate with pyrazine—Adsorption properties for N2. CCl4, H2O, CO2, and CH3CN. 2015. The Proceedings of the 2nd International Porous and Powder Materials Symposium and Exhibition PPM.Izmir, Turkey, September 15-18, 2015 The Organizing Committee of The International Porous and Power Materials Symposium and Exhibition: Izmir, Turkey, 2015, pp. 77-81.]. This is another interesting feature of copper acetate analogues. In this study, we synthesized a new copper acetate analogue by the reaction of 3,4,5-tri-O-benzylgallic acid (abbreviated as Htbng) with a copper(II) salt. The bulky aromatic groups of tbng– ligand may be expected to form enough space for gas adsorption property in the copper carboxylate.
2. MATERIALS AND METHODS
All the chemicals except for the Htbng ligand were commercial products and were used as supplied. Elemental analyses for C, H, and N were performed using a Thermo-Finnigan FLASH EA1112 series CHNO-S analyzer. Infrared spectra were measured with a JASCO MFT-2000 FT-IR Spectrophotometer in the 4000-600 cm–1 region. Diffused reflectance spectrum was measured with a Shimadzu UV-vis-NIR Recording Spectrophotometer Model UV-3100 in the 200-1500 nm region. Magnetic susceptibilities were measured with a Quantum Design MPMS-XL7 SQUID susceptometer over a temperature range of 4.5-300 K. Powder X-ray Diffraction (PXRD) data were collected in the θ range of 5-40°on a RIGAKU RINT2000/PC diffractometer with Cu Kα radiation (λ = 1.5418 Å). Adsorption measurement for N2 was performed by a MicrotracBEL BELSORP-mini II. Prior to the adsorption, the sample was evacuated at 298 K for 2h.
2.1. Synthetic Procedures
The Htbng ligand was synthesized according to the previously reported method [32Halkes, S.B.A.; Vrasidas, I.; Rooijer, G.R.; van den Berg, A.J.J.; Liskamp, R.M.J.; Pieters, R.J. Synthesis and biological activity of polygalloyl-dendrimers as stable tannic acid mimics. Bioorg. Med. Chem. Lett., 2002, 12(12), 1567-1570.
[http://dx.doi.org/10.1016/S0960-894X(02)00245-7] [PMID: 12039 563] ]. Copper(II) carboxylate was synthesized by the following method. [Cu2(tbng)4(dmf)2]·H2O: A 0.198 g (0.45 mmol) portion of Htbng and 10 cm3 of methanol were added to a 6 cm3 of 0.10 M sodium hydroxide solution. The mixed solution was adjusted to pH = 9 by adding a small amount of nitric acid. To this solution, a solution of copper(II) nitrate trihydrate (0.0618 g, 0.25 mmol) in water (3 cm3) was added with stirring to give a yellowish-green precipitate. The precipitate was collected and dried under vacuum. Yield, 0.204 g (85% based on the metal salt). Anal. Found: C, 68.93; H, 5.42%. Calcd for C112H100Cu2O24 (Cu2(tbng)4·4H2O): C, 68.74; H, 5.15%. The precipitate was recrystallized from dmf-methanol to give green crystals. Anal. Found: C, 69.03; H, 4.96; N, 1.55%. Calcd for C118H108Cu2N2O23 ([Cu2(tbng)4(dmf)2]·H2O): C, 69.16; H, 5.31; N, 1.37%. IR (KBr, cm–1): 3083, 3061, 3030 (νC(aromatic)H, 2924 (νasCH2), 2860 (νsCH2), 1577 (νasCOO), 1407 (νsCOO). Diffuse reflectance spectra: λmax 272, 293, 298, 375sh, 716 nm.
 |
Fig. (1) 3,4,5-Tri-O-benzylgallic acid, Htbng. |
2.2. Crystallography
Single-crystals suitable for X-ray analysis were obtained due to very few amount of small crystals by slow diffusion of a dmf solution of [Cu2(tbng)4(dmf)2]·H2O. Single-crystal diffraction data were measured on a Bruker Smart APEX-II ULTRA diffractometer equipped with a multilayered confocal mirror monochrometer and a Mo-Kα radiation source (λ = 0.71073 Å). Crystal data and details concerning data collection are given in Table 1. The structure was solved by direct methods, and refined by full-matrix least-squares methods. The hydrogen atoms were inserted at their calculated positions and fixed there. All of the calculations were carried out utilizing the SHELXTL software package [33Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A, 2008, 64(Pt 1), 112-122.
[http://dx.doi.org/10.1107/S0108767307043930] [PMID: 18156677] ]. Crystallographic data have been deposited with Cambridge Crystallographic Data Centre: Deposit number CCDC-1815964. Copies of the data can be obtained free of charge viahttp://www.ccdc.cam.ac.uk/conts/ retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge, CB2 1EZ, UK; Fax: +44 1223 336033; e-mail: deposit@ccdc.cam.ac.uk).
3. RESULTS AND DISCUSSIONS
Elemental analysis data of the isolated compound are in accordance with the formulation [Cu2(tbng)4(dmf)2]·H2O. IR data showed two COO stretching bands at 1577 and 1407 cm–1 with the difference in energy characteristic of syn-syn-bridging carboxylate [34Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B, 2009, ]. As shown in Fig. (2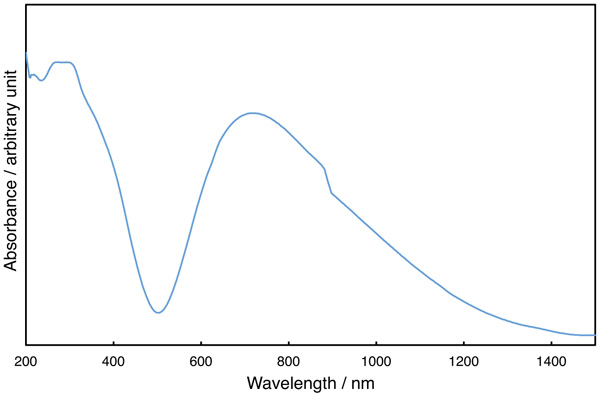 ), the diffuse reflectance spectrum of [Cu2(tbng)4(dmf)2]·H2O shows strong bands at 272, 293, and 298 nm, and a shoulder at 370 nm in the UV region, and a broad band at 716 nm with a shoulder at lower energy side in the vis-NIR region. The former four bands can be assigned to LMCT bands from the carboxylato-oxygen to the CuII d orbital. The vis-NIR region band can be associated with d-d transitions, confirming a square-pyramidal coordination environment of the copper(II) atoms [35Murakami, Y.; Sakata, K. Kireto Kagaku., 1976, Vol. 1, 91-396. (in Japanese)].
), the diffuse reflectance spectrum of [Cu2(tbng)4(dmf)2]·H2O shows strong bands at 272, 293, and 298 nm, and a shoulder at 370 nm in the UV region, and a broad band at 716 nm with a shoulder at lower energy side in the vis-NIR region. The former four bands can be assigned to LMCT bands from the carboxylato-oxygen to the CuII d orbital. The vis-NIR region band can be associated with d-d transitions, confirming a square-pyramidal coordination environment of the copper(II) atoms [35Murakami, Y.; Sakata, K. Kireto Kagaku., 1976, Vol. 1, 91-396. (in Japanese)].
The molecular structure for [Cu2(tbng)4(dmf)2]·4dmf was drawn as an ORTEP diagram in Fig. (3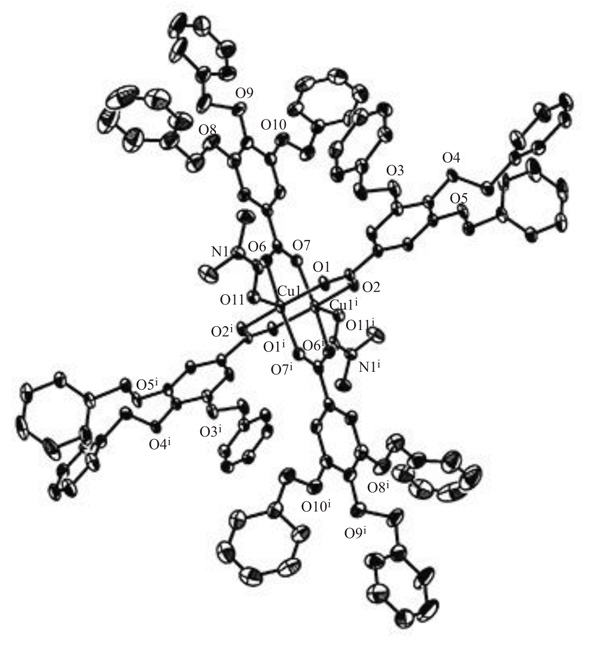 ). The asymmetric unit contains two crystal dmf molecules and one-half of dinuclear [Cu2(tbng)4(dmf)2] unit with a crystallographic inversion center at the midpoint of the Cu1 and Cu1i atoms. The dinuclear unit has a lantern-like dinuclear core bridged by four tbng– ligands in a syn-syn fashion [13Mikuriya, M. Copper(II) acetate as a motif of metal-assembled complexes. Bull. Jpn. Soc. Coord. Chem., 2008, 52, 17-28. [In Japanese].
). The asymmetric unit contains two crystal dmf molecules and one-half of dinuclear [Cu2(tbng)4(dmf)2] unit with a crystallographic inversion center at the midpoint of the Cu1 and Cu1i atoms. The dinuclear unit has a lantern-like dinuclear core bridged by four tbng– ligands in a syn-syn fashion [13Mikuriya, M. Copper(II) acetate as a motif of metal-assembled complexes. Bull. Jpn. Soc. Coord. Chem., 2008, 52, 17-28. [In Japanese].
[http://dx.doi.org/10.4019/bjscc.52.17] ]. The Cu1···Cu1i distance is 2.6345(12) Å, which is in the range found in dinuclear copper(II) carboxylates [6Doedens, R.J. Structure and metal-metal interactions in copper(II) carboxylate complexes. Prog. Inorg. Chem., 1976, 21, 209-231.-13Mikuriya, M. Copper(II) acetate as a motif of metal-assembled complexes. Bull. Jpn. Soc. Coord. Chem., 2008, 52, 17-28. [In Japanese].
[http://dx.doi.org/10.4019/bjscc.52.17] ]. The coordination geometry around each copper atom is an elongated square-pyramid. The bond distances of the Cu1 and basal O atoms are 1.957(3)-1.977(3) Å, which are within the normal range found in copper(II) carboxylates [6Doedens, R.J. Structure and metal-metal interactions in copper(II) carboxylate complexes. Prog. Inorg. Chem., 1976, 21, 209-231.-13Mikuriya, M. Copper(II) acetate as a motif of metal-assembled complexes. Bull. Jpn. Soc. Coord. Chem., 2008, 52, 17-28. [In Japanese].
[http://dx.doi.org/10.4019/bjscc.52.17] ]. The fifth position of the Cu1 atom is occupied by a DMF molecule with the Cu1-O11 distance of 2.166(3) Å, which is also in the normal range as axial bonding for the copper(II) carboxylates [6Doedens, R.J. Structure and metal-metal interactions in copper(II) carboxylate complexes. Prog. Inorg. Chem., 1976, 21, 209-231.-13Mikuriya, M. Copper(II) acetate as a motif of metal-assembled complexes. Bull. Jpn. Soc. Coord. Chem., 2008, 52, 17-28. [In Japanese].
[http://dx.doi.org/10.4019/bjscc.52.17] ]. In the crystal, DMF molecules are trapped into the small space between the dinuclear units (Fig. 4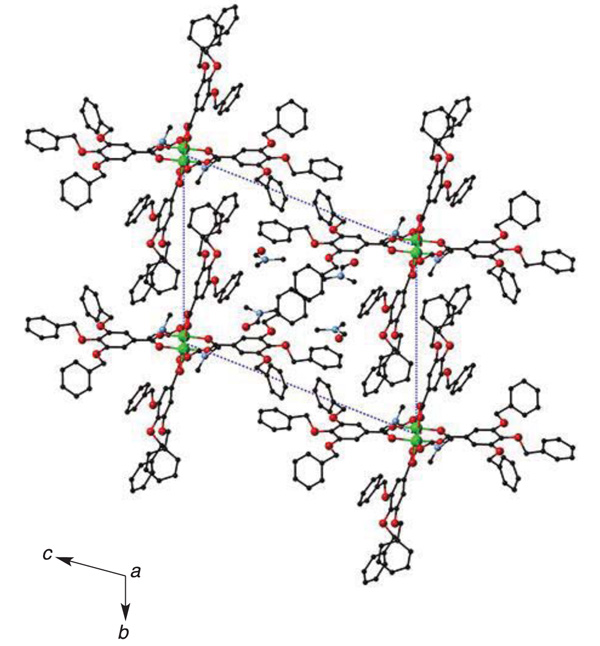 ). Each dinuclear molecule is associated with π···π interaction of one of the three benzyl aromatic rings to form one-dimensional chain supramolecular network as shown in Fig. (5
). Each dinuclear molecule is associated with π···π interaction of one of the three benzyl aromatic rings to form one-dimensional chain supramolecular network as shown in Fig. (5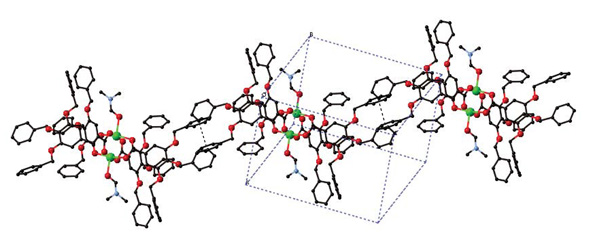 ).
).
The magnetic data of [Cu2(tbng)4(dmf)2]·H2O are shown in the form of χAT vs. T plot in Fig. (6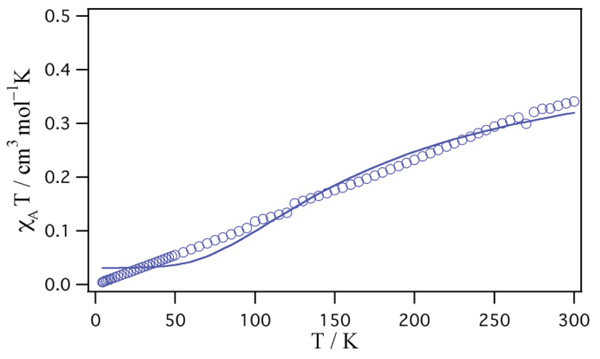 ). The χAT value is 0.341 cm3 mol–1 K (per CuII unit) at 300 K. This corresponds to the magnetic moment of 1.65 µB, which is lower than the spin-only value (1.73 µB) as CuII (d9, S = 1/2) ion. The χAT decreases with lowering of the temperature and reaches value of 0.0046 cm3 mol–1 K at 4.5 K, showing an antiferromagnetic interaction between the two copper(II) ions. The magnetic data were analyzed by the Bleaney-Bowers equation based on the Heisenberg model (H = –2JS1·S2 (S1 = S2 = 1/2)) [5Bleaney, B.; Bowers, K.D. Anomalous paramagnetism of copper acetate. Proc. R. Soc. Lond., 1952, A214, 451-465.]:
). The χAT value is 0.341 cm3 mol–1 K (per CuII unit) at 300 K. This corresponds to the magnetic moment of 1.65 µB, which is lower than the spin-only value (1.73 µB) as CuII (d9, S = 1/2) ion. The χAT decreases with lowering of the temperature and reaches value of 0.0046 cm3 mol–1 K at 4.5 K, showing an antiferromagnetic interaction between the two copper(II) ions. The magnetic data were analyzed by the Bleaney-Bowers equation based on the Heisenberg model (H = –2JS1·S2 (S1 = S2 = 1/2)) [5Bleaney, B.; Bowers, K.D. Anomalous paramagnetism of copper acetate. Proc. R. Soc. Lond., 1952, A214, 451-465.]:
χA = (1–p)[Ng2µB2/kT][3+exp(–2J/kT)]–1 + pNg2µB2/4kT + Nα,
 |
Fig. (2) Diffused reflectance spectrum of [Cu2(tbng)4(dmf)2]·H2O. |
Where J is an exchange coupling constant for the two copper(II) ions, p is the fraction of mononuclear impurity, and Nα is the temperature-independent paramagnetism, which was set to 60 x 10–6 cm3 mol–1 for each copper(II) ion [8Kato, M.; Muto, Y. Factors affecting the magnetic properties of dimeric copper(II) complexes. Coord. Chem. Rev., 1988, 92, 45-83.
[http://dx.doi.org/10.1016/0010-8545(88)85005-7] , 25Mikuriya, M.; Azuma, H.; Sun, J.; Yoshioka, D.; Handa, M. Dinuclear copper(II) complexes of free radical carboxylic acids. Chem. Lett., 2002, 31, 608-609.
[http://dx.doi.org/10.1246/cl.2002.608] , 30Nukada, R.; Mikuriya, M.; Handa, M.; Naono, H. Hydrophobic micropore in a chain compound of dinuclear copper(II) benzoate with pyrazine—Adsorption properties for N2. CCl4, H2O, CO2, and CH3CN. 2015. The Proceedings of the 2nd International Porous and Powder Materials Symposium and Exhibition PPM.Izmir, Turkey, September 15-18, 2015 The Organizing Committee of The International Porous and Power Materials Symposium and Exhibition: Izmir, Turkey, 2015, pp. 77-81.]. The best-fitting parameters were 2J = –214 cm–1, g = 2.13, p = 0.07), showing an antiferromagnetic coupling between the two copper(II) ions. The –2J value is a little smaller than those of copper(II) acetate (~ 284 cm–1) [6Doedens, R.J. Structure and metal-metal interactions in copper(II) carboxylate complexes. Prog. Inorg. Chem., 1976, 21, 209-231.] and most of copper(II) benzoates (–2J = 316-350 cm–1) [10Kawata, T.; Uekusa, H.; Ohba, S.; Furukawa, T.; Tokii, T.; Muto, Y. Magneto-structural correlation in dimeric copper(II) benzoates. Acta Crystallogr. B, 1992, 48, 253-261.
[http://dx.doi.org/10.1107/S0108768191012697] ], showing a relatively weak antiferromagnetic coupling. It is known that the larger bending angle of the OCO moiety of the benzoate group relative to the Cu-O···O-Cu plane of the carboxylate-bridge, ϕbend, plays an important role to weaken the antiferromagnetic coupling via the benzoate bridges [10Kawata, T.; Uekusa, H.; Ohba, S.; Furukawa, T.; Tokii, T.; Muto, Y. Magneto-structural correlation in dimeric copper(II) benzoates. Acta Crystallogr. B, 1992, 48, 253-261.
[http://dx.doi.org/10.1107/S0108768191012697] ]. In the case of the most related copper(II) benzoate with 3,4,5-trimethoxybenzote, [Cu23,4,5- [Cu2{(CH3O)3C6H2CO2}4(CH3OH)2]·2dmf, the ϕbend values are 2.8 and 2.9°, and shows a stronger antiferromagnetic interaction between the two copper(II) ions (2J = –292 cm–1) [31Mikuriya, M.; Yamakawa, C.; Tanabe, K.; Yoshioka, D.; Mitsuhashi, R.; Tanaka, H.; Handa, M. Synthesis, crystal structure, magnetic property, and N2-gas-adsorption property of dinuclear copper(II) 3,4,5-trimethoxybenzoate. J. Turkish Chem. Soc., Sect. A 5(sp.is.1), 2018, 103-110.
[http://dx.doi.org/10.18596/jctcsa.370793] ]. The crystal structure of the present complex revealed that the benzoate group has a considerable bending with ϕbend = 9.4 and 5.9° possibly because of the packing effect of the dinuclear clusters with bulky galloyl groups, resulting in a weaker antiferromagnetic coupling between the two copper(II) ions.
 |
Fig. (4) Packing diagram of [Cu2(tbng)4(dmf)2]·4dmf. |
 |
Fig. (5) View of the 1D supramolecular assembly of [Cu2(tbng)4(dmf)2]·4dmf, showing π-π interaction between the benzyl aromatic rings of dinuclear Cu2(tbng)4(dmf)2 molecules by dashed lines. |
 |
Fig. (6) Temperature dependence of χAT of [Cu2(tbng)4(dmf)2]·H2O. The solid line represents the best fit of the data. |
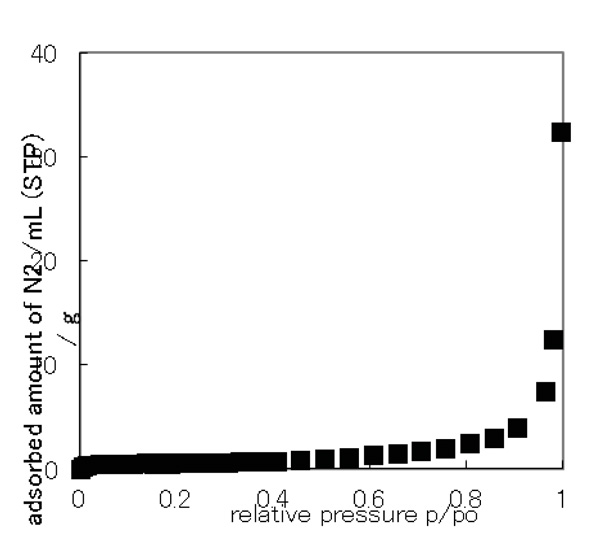 |
Fig. (7) N2 adsorption isotherm of [Cu2(tbng)4(dmf)2]·H2O. |
The crystal structure of [Cu2(tbng)4(dmf)2]·4dmf did not show large voids, although it has many small cavities. The PXRD data of [Cu2(tbng)4(dmf)2]·H2O did not coincide with the simulated data from the crystal structure of [Cu2(tbng)4 (dmf)2]·4dmf, suggesting that the packing of the dinuclear molecules was influenced by the incorporation of the solvent. Therefore, we expected an adsorbing property for the present complex, if the bulky galloyl groups work well for porous structure. However, the adsorption isotherm on [Cu2(tbng)4 (dmf)2]·H2O showed that the isotherm belongs to Type II in the IUPAC classification (SBET = 2.0 m2g–1) as shown in Fig. (7 ), suggesting the crystals to be non-porous. This is similar to the case for [Cu2{3,4,5-(CH3O)3C6H2CO2}4(CH3OH)2]·2dmf [31Mikuriya, M.; Yamakawa, C.; Tanabe, K.; Yoshioka, D.; Mitsuhashi, R.; Tanaka, H.; Handa, M. Synthesis, crystal structure, magnetic property, and N2-gas-adsorption property of dinuclear copper(II) 3,4,5-trimethoxybenzoate. J. Turkish Chem. Soc., Sect. A 5(sp.is.1), 2018, 103-110.
), suggesting the crystals to be non-porous. This is similar to the case for [Cu2{3,4,5-(CH3O)3C6H2CO2}4(CH3OH)2]·2dmf [31Mikuriya, M.; Yamakawa, C.; Tanabe, K.; Yoshioka, D.; Mitsuhashi, R.; Tanaka, H.; Handa, M. Synthesis, crystal structure, magnetic property, and N2-gas-adsorption property of dinuclear copper(II) 3,4,5-trimethoxybenzoate. J. Turkish Chem. Soc., Sect. A 5(sp.is.1), 2018, 103-110.
[http://dx.doi.org/10.18596/jctcsa.370793] ].
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The measurement of X-ray diffraction was made using Bruker SMART APEX-II ULTRA at the Natural Science Center for Basic Research and Development (N-BARD), Hiroshima University. The present work was partially supported by Grant-in-Aid for Scientific Research No. 17K05820 from the Ministry of Education, Culture, Sports, Science and Technology (MEXT, Japan) and the MEXT Supported Program for the Strategic Research Foundation at Private Universities, 2010-2014.
REFERENCES
| [1] | Yamada, H.; Hirokane, T.; Ikeuchi, K.; Wakamori, S. Fundamental methods in ellagitannin synthesis. Nat. Prod. Commun., 2017, 12, 1351-1358. |
| [2] | Yamada, H.; Hirokane, T.; Asakura, N.; Kasai, Y.; Nagao, K. Strategies and methods for the total synthesis of ellagitannins. Curr. Org. Chem., 2012, 16, 578-604. [http://dx.doi.org/10.2174/138527212799859426] |
| [3] | Yamaguchi, S.; Hirokane, T.; Yoshida, T.; Tanaka, T.; Hatano, T.; Ito, H.; Nonaka, G.; Yamada, H. Roxbin B is cuspinin: Structural revision and total synthesis. J. Org. Chem., 2013, 78(11), 5410-5417. [http://dx.doi.org/10.1021/jo400562k] [PMID: 23656490] |
| [4] | van Niekerk, J.N.; Schoening, F.R. A new type of copper complex as found in the crystal structure of cupric acetate, Cu2(CH3COO)4.2H2O. Acta Crystallogr., 1953, 6, 227-232. [http://dx.doi.org/10.1107/S0365110X53000715] |
| [5] | Bleaney, B.; Bowers, K.D. Anomalous paramagnetism of copper acetate. Proc. R. Soc. Lond., 1952, A214, 451-465. |
| [6] | Doedens, R.J. Structure and metal-metal interactions in copper(II) carboxylate complexes. Prog. Inorg. Chem., 1976, 21, 209-231. |
| [7] | Melnik, M. Study of the relation between the structural data and magnetic interaction in oxo-bridged binuclear copper(II) compounds. Coord. Chem. Rev., 1982, 42, 259-293. [http://dx.doi.org/10.1016/S0010-8545(00)80537-8] |
| [8] | Kato, M.; Muto, Y. Factors affecting the magnetic properties of dimeric copper(II) complexes. Coord. Chem. Rev., 1988, 92, 45-83. [http://dx.doi.org/10.1016/0010-8545(88)85005-7] |
| [9] | Yamanaka, M.; Uekusa, H.; Ohba, S.; Saito, Y.; Iwata, S.; Kato, M.; Tokii, T.; Muto, Y.; Steward, O.W. Correlation of electron density and spin-exchange interaction in dimeric copper(II) formates, acetates and silanecarboxylates. Acta Crystallogr. B, 1991, 47, 344-355. [http://dx.doi.org/10.1107/S0108768190012459] |
| [10] | Kawata, T.; Uekusa, H.; Ohba, S.; Furukawa, T.; Tokii, T.; Muto, Y. Magneto-structural correlation in dimeric copper(II) benzoates. Acta Crystallogr. B, 1992, 48, 253-261. [http://dx.doi.org/10.1107/S0108768191012697] |
| [11] | Uekusa, H.; Ohba, S.; Tokii, T.; Muto, Y.; Kato, M.; Husebye, S.; Steward, O.W.; Chang, S-C.; Rose, J.P.; Pletcher, J.F.; Suzuki, I. Magneto-structural correlations of dimeric copper(II) trichloroacetates. Acta Crystallogr. B, 1992, 48, 650-667. [http://dx.doi.org/10.1107/S0108768192002908] |
| [12] | Sundberg, M.R.; Uggla, R.; Melnik, M. Comparison of the structural parameters in copper(II) acetate-type dimers containing distorted square pyramidal Cu4O and CuO4N chromophores. Polyhedron, 1996, 15, 1157-1163. [http://dx.doi.org/10.1016/0277-5387(95)00352-5] |
| [13] | Mikuriya, M. Copper(II) acetate as a motif of metal-assembled complexes. Bull. Jpn. Soc. Coord. Chem., 2008, 52, 17-28. [In Japanese]. [http://dx.doi.org/10.4019/bjscc.52.17] |
| [14] | Hernandez, J.; Avila, M.; Jimenez-Vazquez, H.A.; Duque, J.; Reguera, E. Copper dimer with acetate-2-ethylimidazole as ligands. Synth. React. Inorg. Met.-Org. Nano-Met. Chem., 2015, 45, 342-350. [http://dx.doi.org/10.1080/15533174.2013.832322] |
| [15] | Mikuriya, M.; Kida, S.; Ueda, I.; Tokii, T.; Muto, Y. Crystal structure and magnetic property of Di-µ-propionato-O,O’-bis[N-p-tolylsalicylideneaminatocopper(II)]. Bull. Chem. Soc. Jpn., 1977, 50, 2464-2470. [http://dx.doi.org/10.1246/bcsj.50.2464] |
| [16] | Nakashima, M.; Mikuriya, M.; Muto, Y. Structural, magnetic and IR spectroscopic characterization of dinuclear copper(II) trichloroacetate adduct with benzonitrile. Nature of the copper(II)- benzonitrile bond. Bull. Chem. Soc. Jpn., 1985, 58, 968-973. [http://dx.doi.org/10.1246/bcsj.58.968] |
| [17] | Mikuriya, M.; Nukada, R.; Morishita, H.; Handa, M. Chain compounds formed by the reaction of copper(II) carboxylate [Cu2(O2CR)4] (R = C(CH3)3, CCl3) and bridging ligand L (L = Pyrazine, 4,4′-Bipyridine, and 1,4-Diazabicyclo[2.2.2]octane). Chem. Lett., 1995, 24, 617-618. [http://dx.doi.org/10.1246/cl.1995.617] |
| [18] | Mikuriya, M.; Azuma, H.; Nukada, R.; Handa, M. Synthesis, x-ray structures, and magnetic properties of [Cu2(piv)4(Et3N)2] and [Cu6(piv)6(EtO)6] (Hpiv = Pivalic Acid): Role of base for dinuclear adduct and oligonuclear formation. Chem. Lett., 1999, 28, 57-58. [http://dx.doi.org/10.1246/cl.1999.57] |
| [19] | Nukada, R.; Mori, W.; Takamizawa, S.; Mikuriya, M.; Handa, M.; Naono, H. Microporous structure of a chain compound of copper(II) benzoate bridged by pyrazine. Chem. Lett., 1999, 28, 367-368. [http://dx.doi.org/10.1246/cl.1999.367] |
| [20] | Matsushima, H.; Koikawa, M.; Nukada, R.; Mikuriya, M.; Tokii, T. Structural characterization and magnetic properties of triply carboxylato-bridged dinuclear copper(II) complexes. Bull. Chem. Soc. Jpn., 1999, 72, 1025-1035. [http://dx.doi.org/10.1246/bcsj.72.1025] |
| [21] | Mikuriya, M.; Azuma, H.; Nukada, R.; Sayama, Y.; Tanaka, K.; Lim, J-W.; Handa, M. Antiferromagnetic adducts of copper(II) propionate with pyridyl nitronyl nitroxides. Bull. Chem. Soc. Jpn., 2000, 73, 2493-2498. [http://dx.doi.org/10.1246/bcsj.73.2493] |
| [22] | Mikuriya, M.; Azuma, H.; Handa, M. Coordination polymers of copper(II) and copper(I) trifluoroacetates with pyrazine. Mol. Cryst. Liq. Cryst. (Phila. Pa.), 2000, 342, 205-210. [http://dx.doi.org/10.1080/10587250008038266] |
| [23] | Nukada, R.; Mikuriya, M.; Yamashita, A.; Handa, M. Mononuclear and polynuclear chain compounds of copper(II) pivalate with N,N′-Didentate ligands.Chalenges for Coordination Chemistry in the New Century., 2001, , 83-88. |
| [24] | Mikuriya, M.; Azuma, H.; Handa, M. Coordination polymers of copper(II) propionate with linking ligands. Mol. Cryst. Liq. Cryst. (Phila. Pa.), 2002, 379, 205-210. [http://dx.doi.org/10.1080/713738673] |
| [25] | Mikuriya, M.; Azuma, H.; Sun, J.; Yoshioka, D.; Handa, M. Dinuclear copper(II) complexes of free radical carboxylic acids. Chem. Lett., 2002, 31, 608-609. [http://dx.doi.org/10.1246/cl.2002.608] |
| [26] | Horikoshi, R.; Mikuriya, M. One-Dimensional coordination polymers from the self-assembly of copper(II) carboxylates and 4,4′- Dithiobis(pyridine). Bull. Chem. Soc. Jpn., 2005, 78, 827-834. [http://dx.doi.org/10.1246/bcsj.78.827] |
| [27] | Lorinc, S.; Koman, M.; Melnik, M.; Mikuriya, M. Mono-, di- and polymeric copper(II) complexes with diclofenic acid (nsaid drug), structures, spectral and magnetic properties. Advances in Coordination, Bioinorganic and Inorganic Chemistry, 2005, , 176-186. |
| [28] | Mikuriya, M.; Yano, M.; Takahashi, N.; Yoshioka, D.; Tanaka, H.; Handa, M. Synthesis and crystal structure of a chain complex of copper(II) pivalate and 1,2-Bis(4-pyridyl)ethane in relation to adsorption property for N2, X-ray struct. Anal. Online, 2015, 31, 47-48. |
| [29] | Mikuriya, M.; Yano, M.; Takahashi, N.; Yoshioka, D.; Tanaka, H.; Handa, M. Synthesis, crystal structure, magnetic property, and N2-gas-adsorption property of chain componds of dinuclear copper(II) pivalate with N,N’-bidentate lgands. 2015. The Proceedings of the 2nd International Porous and Powder Materials Symposium and Exhibition PPM.Izmir, Turkey, September 15-18, 2015 The Organizing Committee of The International Porous and Power Materials Symposium and Exhibition: Izmir, Turkey, 2015, pp. 72-76. |
| [30] | Nukada, R.; Mikuriya, M.; Handa, M.; Naono, H. Hydrophobic micropore in a chain compound of dinuclear copper(II) benzoate with pyrazine—Adsorption properties for N2. CCl4, H2O, CO2, and CH3CN. 2015. The Proceedings of the 2nd International Porous and Powder Materials Symposium and Exhibition PPM.Izmir, Turkey, September 15-18, 2015 The Organizing Committee of The International Porous and Power Materials Symposium and Exhibition: Izmir, Turkey, 2015, pp. 77-81. |
| [31] | Mikuriya, M.; Yamakawa, C.; Tanabe, K.; Yoshioka, D.; Mitsuhashi, R.; Tanaka, H.; Handa, M. Synthesis, crystal structure, magnetic property, and N2-gas-adsorption property of dinuclear copper(II) 3,4,5-trimethoxybenzoate. J. Turkish Chem. Soc., Sect. A 5(sp.is.1), 2018, 103-110. [http://dx.doi.org/10.18596/jctcsa.370793] |
| [32] | Halkes, S.B.A.; Vrasidas, I.; Rooijer, G.R.; van den Berg, A.J.J.; Liskamp, R.M.J.; Pieters, R.J. Synthesis and biological activity of polygalloyl-dendrimers as stable tannic acid mimics. Bioorg. Med. Chem. Lett., 2002, 12(12), 1567-1570. [http://dx.doi.org/10.1016/S0960-894X(02)00245-7] [PMID: 12039 563] |
| [33] | Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A, 2008, 64(Pt 1), 112-122. [http://dx.doi.org/10.1107/S0108767307043930] [PMID: 18156677] |
| [34] | Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B, 2009, |
| [35] | Murakami, Y.; Sakata, K. Kireto Kagaku., 1976, Vol. 1, 91-396. (in Japanese) |





