- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Chemical Constituents and Uses of Calotropis Procera and Calotropis Gigantea – A Review (Part I – The Plants as Material and Energy Resources)
Mazen A. M. Al Sulaibi1, 2, Carolin Thiemann1, Thies Thiemann1, *
Abstract
The traditional and current use of Calotropis procera and C. gigantea, two soft-wooded, xerophytic shrubs of the family Apocynaceae, are reviewed against the background of the plants' chemical constituents and their biological properties. The focus is on the usage of the plants for building materials, natural pesticides, animal feed and bioremediative purposes.
Article Information
Identifiers and Pagination:
Year: 2020Volume: 7
First Page: 1
Last Page: 15
Publisher Id: CHEM-7-1
DOI: 10.2174/1874842202007010001
Article History:
Received Date: 13/01/2020Revision Received Date: 27/02/2020
Acceptance Date: 01/03/2020
Electronic publication date: 17/04/2020
Collection year: 2020
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Department of Chemistry, Faculty of Science, United Arab Emirates University, Al Ain, Abu Dhabi, UAE;
Tel: 0502213686; E-mail: thies@uaeu.ac.ae
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 13-01-2020 |
Original Manuscript | Chemical Constituents and Uses of Calotropis Procera and Calotropis Gigantea – A Review (Part I – The Plants as Material and Energy Resources) | |
1. INTRODUCTION
Plants growing in arid regions have elicited increased attention, because the hostile environment, in which these plants survive, forces them to develop chemical protective systems rarely found in vegetation of other ecosystems. Furthermore, many of the plants grow in areas, where the dependence on traditional, plant-based medicines over industrially produced pharmaceuticals persists to this day. The two plants, Calotopris procera (giant milkweed, also named C. persica) and Calotropis gigantea (crown flower), have been used widely in traditional medicine in North Africa, the Middle East, and South and South-East Asia. This has led to extensive research on the chemical constituents of the plants. Both plants are known to be sources of cardenolides, and newer research has yielded a number of interesting cancer-active constituents. In addition, extracts of both plants have remarkable nematocidal, molluscidal and insecticidal activities. In many regions, the wood of Calotropis plants has been used as a building material and as a source of fuel. In addition, certain parts of the plants have been used as feed for livestock. In other regions, Calotropis plants are seen as invasive species that threaten local plant life and that due to their toxicity also pose a threat to grazing field animals. The complexity of the plants’ properties and chemical constituents combined with the wide geographic distribution and regional use of C. procera and C. gigantea has led to a fast-growing body of research on the two plants. For C. gigantea alone, approximately 120 research ent-
ries have appeared in 2006 (Australian New Crop Website),
while in 2018, the database Web of Knowledge listed 55 research papers for C. procera and 30 research papers for C. gigantea. Short reviews on the two plants have appeared previously [1Mueen Ahmed, K.K.; Rana, A.C.; Dixit, V.K. Calotropis Species (Ascelpediaceace) - A Comprehensive Review. Pharmacogn. Mag., 2005, 1, 48-52.-9Yogi, B.; Gupta, S.K.; Mishra, A. Calotropis procera (Madar): a medicinal plant of various therapeutic uses – a review. Bull. Env. Pharmacol. Life Sci., 2016, 5, 74-81.]. Two more comprehensive reviews have been given on ethnopharmaceutical aspects of C. gigantea [10Kumar, S.P.; Suresh, E.; Kalavathy, S. Review on a potential herb Calotropis gigantea (L.) R. Br. Sch. Acad. J. Pharm., 2013, 2, 135-143., 11Kadiyala, M.; Ponnusankar, S.; Elango, K. Calotropis gigantiea (L.) R. Br (Apocynaceae): a phytochemical and pharmacological review. J. Ethnopharmacol., 2013, 150(1), 32-50.
[http://dx.doi.org/10.1016/j.jep.2013.08.045] [PMID: 24012528] ]. The current review provides a comprehensive, up-to-date picture of the uses and chemical constituents of both plants. The review comes in two parts, where the current part focuses on the usage of the plants for building materials, natural pesticides, animal feed and bioremediative purposes.
1.1. Geographic Distribution and Habitat
Calotropis procera (Ait.) R. Br. and Calotropis gigantea R. Br. are two species of soft-wooded, evergreen, perennial shrubs of the family Apocynaceae, and subfamily Asclepiadoideae (milkweeds). Traditionally, the two plants have developed in two separate regions of the world, one, C. procera, predominately in Africa and in the Middle East, the other, C. gigantea, in Asia. Due to rapid expansion, often with human help, today they share some of the same habitats in the same regions.
C. gigantea is native to much of South Asia, such as to Iran, Pakistan, Nepal, Sri Lanka and India, and much of South East Asia such as to Malaysia, Myanmar, the Philippines, Indonesia, China, Laos, Thailand, and Vietnam. C. gigantea has been introduced to many of the Pacific Islands, including the Hawaiian Islands and New Guinea, whereas in some areas, such as in Palau [12Space, J.C.; Lorence, D.H.; LaRosa, A.M. Report to the Republic of Palau: 2008 update on Invasive Plant Species., 2009, , 18.] and on the Solomon Islands [13Hancock, I.R.; Henderson, C.P. Flora of the Solomon Islands.Research Bulletin No. 7, Ministry of Agriculture and Lands, Honiara, 1988, 54.], it has been brought under cultivation. Also, in northern Australia, it has been introduced and cultivated. In the Americas, it has also been brought under cultivation in Barbados [14Howard, R.A. Flora of the Lesser Antilles, Leeward and Windward Islands, 1989, 6] and has established itself in Cuba [15Krings, A.; Areces Berazain, F.; Lazcano Lara, J.C. New and re-discovered milkweeds from Cuba: Calotropis gigantea and Gonolobus stephanotrichus. Willdenowia, 2005, 35, 315-318.
[http://dx.doi.org/10.3372/wi.35.35213] ] as well as in Brazil, where it is said to be an aggressive invader of the Caatinga ecoregion [16Cavalcante, A.; Major, I. Invasion of alien plants in the Caatinga biome. Ambio, 2006, 35(3), 141-143.
[http://dx.doi.org/10.1579/0044-7447(2006)35[141:IOAPIT]2.0.CO;2] [PMID: 16846205] ]. With Indian immigrants, C. gigantea has also been introduced to Africa, including the Seychelles and Mauritius.
As a native plant, C. procera is found in many parts of tropical Africa, including Madagascar and in far south as Angola, in arid zones of Northern Africa and the Middle East. It has spread to South Asia and South-Eastern Asia. It has been introduced into parts of South America [6Silva, M.C.C.; da Silva, A.B.; Texeira, F.M.; de Sousa, P.C.P.; Rondon, R.M.M.; Honorio, J.E.R., Junior; Sampaio, L.R.L.; Oliveira, S.L.; Holonda, A.N.M.; de Vasconcelos, S.M.M. Therapeutic and biological activities of Calotropis procera (Ait.) R. Br. Asian Pac. J. Trop. Med., 2010, 332-336.
[http://dx.doi.org/10.1016/S1995-7645(10)60081-8] , 17Gracia C, A.; Rangel-Buitrago, N.; Castro-Barros, J-D. Non-native plant species in the Atlantico Department Coastal Dune Systems, Caribbean of Colombia: A new management challenge. Mar. Pollut. Bull., 2019, 141, 603-610.
[http://dx.doi.org/10.1016/j.marpolbul.2019.03.009] [PMID: 30955773] ] and Australia, where it is considered to be an undesirable weed. Also, it now occurs on the Caribbean and Hawaiian Islands, in Central America, South Africa as well as on the Spanish Canary Islands. In the Philippines, it has been reported as a relative new-comer. C. procera is relatively salt-tolerant, although the reduced dry mass of the plant is associated with the salinity of the soil [18Ibrahim, A.H. Tolerance and avoidance responses to salinity and water stresses in Calotropis procera and Suaeda aegyptiaca. Turk. J. Agric. For., 2013, 37, 352-360., 19Al-Zahrani, H.S. Effects on salinity stress on growth of Calotropis procera seedlings. Bull. Pure Appl. Sci., 2002, 21, 109-122.], and grows well on degraded lands. The rootstock has been reported to be resistant against fires [20Karschon, R. Contributions to the arboreal flora of Israel: Calotropis procera (Willd.) R.Br La-Yaaron, 1970, 20, 1-6,41-48. (Hebrew and English).] and temporary flooding. Both plants thrive in an arid environment [21Boutraa, T. Effects of water stress on growth, water use efficiency, leaf area and chlorophyll content in the desert shrub Calotropis procera. J. Int. Environ. Appl. Sci., 2010, 5, 124-132.], and C. procera can be seen at the edge of the desert in the United Arab Emirates (UAE) (see Fig. 1 ). In the UAE, it has been estimated that on desert land dominated by C. procera, about 0.66 tons of soil organic carbon (SOC) per hectar is sequestered annually [22Ksiksi, T.S. Acacia tortilis and Calotropis procera: do they substantially promote soil carbon sequestration? Open J. Soil Sci., 2012, 2, 116-122.
). In the UAE, it has been estimated that on desert land dominated by C. procera, about 0.66 tons of soil organic carbon (SOC) per hectar is sequestered annually [22Ksiksi, T.S. Acacia tortilis and Calotropis procera: do they substantially promote soil carbon sequestration? Open J. Soil Sci., 2012, 2, 116-122.
[http://dx.doi.org/10.4236/ojss.2012.22017] ]. In Mauretania, C. procera has been used in sand encroachment management, as it was found to be the first species to colonize sand dunes [23Migongo-Bake, E.; Elskamp, F.; Mwangi, A.; Cherogony, L. Success stories in the struggle against desertification – a holistic and integrated approach to environmental conservation and sustainable livelihoods., 2002, ]. In the Indian subcontinent, C. procera has been seen at up to 1000 m elevation [24Parrotta, J.A. Healing plants of Peninsular India., 2001,
[http://dx.doi.org/10.1079/9780851995014.0000] ]. As the propagation of Calotropis plants is aided by human activity [25Sharma, G.P.; Kumar, M.; Raghubanshi, A.S. Urbanization and road-use determines Calotropis procera distribution in the eastern Indo-Gangetic plain, India. Ambio, 2010, 39(2), 194-197.
[http://dx.doi.org/10.1007/s13280-010-0026-3] [PMID: 20653282] ], it has been used as an indicator of man-induced historical perturbation of the environment [26Neumann, K. Zur Vegetationsgeschichte der Ostsahara im Holozaen; Holzkohlen aus praehistorischen Fundstellen.Forschungen zur Umweltgeschichte der Ostsahara., 1989, , 13-182.]. It is also an indication of overgrazing in many areas.
 |
Fig. (1) Photo of a Calotropis procera bush in Al Ain, UAE, showing the size to which C. procera can grow. |
1.2. General Description
C. gigantea can grow to 4 m high, while C. procera is usually smaller, although in the Al Ain area in the United Arab Emirates, the plants can also grow up to 4.5 m in height (Fig. 1). The stem can be up to 25 cm in diameter [27Little, E.L., Jr; Woodbury, R.O.; Wadsworth, F.H. Trees of Puerto Rico and the Virgin Islands, 1974, 2, 1024.]. The opposite leaves of C. procera are large, up to 18 cm and up to 13 cm broad. Leaves of C. gigantea are up to 10 cm long and 8 cm wide [28Forster, P.I.; Little, D.J.; Nicholas, A. Asclepiadaceae, Flora of Australia., 1996, Vol. 8, 214.]. C. procera has a profusion of five-petaled, sweet-smelling white flowers, 3.8 to 5.1 cm in size, with a marked purple tip. The equally five-petaled flowers of C. gigantea are white to lavender in color, and in contrast to those of C. procera, without fragrance. The grey-green fruits of C. procera are 8 – 12 cm long, containing 350-500 seeds with tufts of white, silky hair. Similarly, C. gigantea exhibits single or paired fruits, 7-10 cm long, with a profusion of white tufted, brown seeds, 2.5 – 3.5 cm in length [29Jagtap, V.A.; Usman, M.R.M.; Salunkhe, P.S.; Gagrani, M.B. Anti-inflammatory activity of Calotropis gigantea Linn. leaves extract on in-vitro models Int. J. Curr. Pharm. Rev. Res, 2010, 1-5.]. The seeds of both plants are distributed by wind and water, but also by birds when the ripe pods burst. Flowering takes place all through the year. The plants propagate through suckering as well. Thus, crowns and roots form suckers, where also broken stems can take root and regenerate. Pollination happens through insects, mostly through bees or butterflies.
1.3. Historical Use of the Calotropis
The name “Calotropis” stems from Greek with the meaning of “beautiful boat keel”. Historically, Calotropis has been of economic interest as many parts of the plant are usable. It is a sacred plant to many Hindi, associated with the observances of the maruts (winds), demigods of the rigvedic god Rudra. In the Arabian world, Calotropis was associated with sun-worship in ancient times, and also in Vedic times, the leaves of Calotropis, in form of arkapattra (sun-leaf) or arkaparna (lightning leaf), have been associated with sun-worship.
The Roman Jewish historian Titus Flavius Josephus (37-c.100) mentioned C. procera as the apple of Sodom. As such it is listed in the Mishnah and the Talmud. Abu Hanifa Dinawari (815-896) included Calotropis in his Book of Plants [30Dymock, W. A history of the principal drugs of vegetable originPharmacographia Indica, 1972, , 15.]. Also, Ibn Sina (980-1037) has made note of the plant. In Renaissance Europe, the plant has been described by Prosper Alpinus (1553 – 1617) (De Plant. Aegpyti, Venet, 1592, Ch. XXV). Prosper Alpinus had studied the plant in Egypt (1580-1584) [31Bertreau, A.; Calotropis, Les. Bibliothèque d’Agriculture coloniale, 1913, , 1-87.-33Neuwinger, H.D. Afrikanische Arzneipflanzen und Jagdgifte, Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart 1994, 224.].
However, human interaction with the plant dates back to much earlier times. Thus, charcoal remains of C. procera have been found at archaeological sites associated with pre-dynastic settlements in the Nile Valley of Upper Egypt [34Newton, C.; Midant-Reynes, B. Environmental change and settlement shifts in Upper Egypt during the Predynastic: charcoal analysis at Adaima. Holocene, 2007, 17, 1109-1118.
[http://dx.doi.org/10.1177/0959683607082551] ], although C. procera was not a predominant source of firewood in early Egypt [35Bard, K.A.; Fattovich, R.; Borojevic, K.; Berna, F.; Zazzaro, C. Harbor of Pharaohs to the Land of Punt (Marsa/Wadi Gawasis Report)., 2008, , 22-38.]. Nevertheless, an increase in the abundance of C. procera with an increase of human agricultural activity has been noted [34Newton, C.; Midant-Reynes, B. Environmental change and settlement shifts in Upper Egypt during the Predynastic: charcoal analysis at Adaima. Holocene, 2007, 17, 1109-1118.
[http://dx.doi.org/10.1177/0959683607082551] ]. Interestingly, in much later times, charcoal derived from Calotropis was utilized in gun powder, especially in Indo-China [36Maroyi, A. In Medicinal Plants 2Plant Resources of Tropical Africa, 2013, 11, 31.].
In some early civilizations, C. procera fibers served in textile making, such as in Cyprus in 2000 BC [37Belgiorno, M.R.; Lentini, A.; Scala, G. The textile industry (2000 BC) of Pyrgos-Mavroraki (Cyprus). Archaelogical Textiles Newsletter, 2006, 25, 30.]. According to Kramer, the fibers from the inner bark were used in the manufacture of cloth for princes and nobles. Later, Calotropis was used to make string and cord in Oman [38Potts, D.T.; Reade, W.J. New evidence for late third millennium linen from Tell Abraq, Umm-Al-Qaiwain, UAE. Paéorient, 1993, 19, 96-106.], and in Borneo, seed hairs of the plant were made into threads. In India, the fibers have been called the bow-strings of India and have been used for rug and net-making as well as for sewing threads.
Always, the flowers of the plant have served decorative purposes. This continues to this day, even in countries where Calotropis has been introduced only recently, such as in Hawaii, where the flowers of C. gigantea are included in garlands (leis), and in the Philippines, where the flowers are used to decorate rosaries. In India, the maruts (storm deities) have been greeted with a garland of flowers of Calotropis on Saturdays for centuries. Nowadays, C. gigantea is sold as a plant for home and garden, specifically as a butterfly attractor in a number of countries [39Mikula, R. Butterfly plants for your garden, www.butterflybreeders.com/pages/bflygdning/butterflyplants.html2001.]. The ornamental potential of C. procera has been highlighted in a recent article [40Petrie, J.M.M. Arabian Desert Primer: Ornamental potential of hyperarid adapted plants from Saudi Arabia. Desert Plants, 2007, 23, 19-32.].
In general, the asclepias (milkweeds), which apart from the Calotropis species, include more than 140 species of plants, have long been included in traditional medicine and have been named after the Greek god of healing. The Greek-Arabian traditional medicine knows the Calotropis plant under the name of Madar and Ushar, where from ancient times, extracts and powders of different parts of Calotropis have been used. Both C. procera [under the name of Raktha Arka] and C. gigantea [under the name of Arka kalpna and Sweta Arka] have been described in the Aryuvedic classics [41Arya, S.K.; Agarwal, V.D. Antiquity of arka kalpna in Ayurvedic classics. Sacitra Ayurveda, 1985, 38, 477-480.]. C. procera was used medically by the ancient Egyptians [42Ebbell, B. A contribution to the earliest history of leprosy. Int. J. Lepr., 1935, 3, 257-263.], seemingly going back as far as the Neolithic period [43Greiss, A.M.E. Anatomical identification of plant remains and other materials from (1) El-Omari excavation at Helwan from the first dynasty. Bull. Inst. Egypte, 1955, 36, 227-235.], and the plant can also be found in the Sudanese traditional medicine [44Dieye, A.M.; Tidjani, M.A.; Diouf, A.; Bassene, E.; Faye, B. Senegalese pharmacopeia: study of acute toxicity and antitussive activity of C. procera (Ait.). Dakar Méd., 1993, 38, 69-72.
[PMID: 7882852] ] as well as in the traditional medicine of North and Central Africa and the Middle East in general and of Central Asia.
Lastly, in Africa, arrow poisons have been derived from the plants. It was this fact that led to the first modern, extensive investigations (Hesse) on the chemical constituents of C. procera, carried out in order to identify the chemical structures of the poisonous components, specifically the cardiac glucoside components.
2. CURRENT NON-MEDICINAL USE OF THE PLANT
2.1. Use as a Building Material
The Calotropis plant has been used for materials, where, when cultivated at 1 to 1.5 m spacing as done in certain regions in South America and the Caribbean, annual yields of up to 500 kg fibre production per ha can be expected [45www.worldagroforestry.org/treedb2/AFTPDFS/Calotropis_procera.pdf]. At 525 trees per ha, Nasser et al. have calculated an annual branch yield of even up to 5 tons per ha [46Nasser, R.A.; Al-Mefarrej, H.A.; Khan, P.R.; Alhafta, K.H. Technological Properties of Calotropis procera (Ait.) wood and its relation to utilization. Am.-Eurasian J. Agric. Environ. Sci., 2012, 12, 5-16.]. The wood is light-weight with a typical air-dried wood density of 0.39 g/cm3 [46Nasser, R.A.; Al-Mefarrej, H.A.; Khan, P.R.; Alhafta, K.H. Technological Properties of Calotropis procera (Ait.) wood and its relation to utilization. Am.-Eurasian J. Agric. Environ. Sci., 2012, 12, 5-16.]. The stem of the plant is used for roof-making. The use of the stem fibre for paper, nags and nets has also been reported [47Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Anthony, S. Agroforestree Database: a tree reference and selection guide version 4.0., 2009, www.worldagroforestry.org/resources/databases/agroforestree]. It has been found that chemomechanical pulping of C. procera leads to a high yield of pulp, suitable for paperboard [48Khristova, P.; Tissot, M. Soda-anthraquinone pulping of Hibiscus sabdariffa (Karkadeh) and Calotropis procera from Sudan. Bioresour. Technol., 1995, 53, 67-72.
[http://dx.doi.org/10.1016/0960-8524(95)00067-O] ]. Currently, the fibers are still used for rope making [49Oun, A.A.; Rhim, J.W. Characterization of nanocelluloses isolated from Ushar (Calotropis procera) seed fiber: effect of isolation method. Mater. Lett., 2016, 168, 146-150.
[http://dx.doi.org/10.1016/j.matlet.2016.01.052] ] in both Africa and South America. Floss (silky hair, akund floss, 2-3 cm long, 12-42 microns wide) from seed stands have been used as stuffing for pillows and mattresses. This use was known in the Acient Egypt and by 1910, Calotropis procera was cultivated in Djibouti, producing about 1200 kg seed hair per hectare [31Bertreau, A.; Calotropis, Les. Bibliothèque d’Agriculture coloniale, 1913, , 1-87., 33Neuwinger, H.D. Afrikanische Arzneipflanzen und Jagdgifte, Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart 1994, 224.]. Even recently, seed hair from Calotropis has been presented as a potential silk replacer [50Batello, C.; Marzot, M.; Toure, A.H. The future is an ancient lake: traditional knowledge, biodiversity and genetic resources for food and agriculture in Lake Chad Basin ecosystems. FAO Interdepartmental Working Group on biological diversity for food and agriculture, Rome, 2004.www.fao.org/docrep/010/y5118e/y5118e00.htm]. Seed hair has been tested as a thermal insulating material and has been found comparable to Rockwool mineral fibers in its insulating properties. Tests have also been performed with insulating boards made from seed hair, mixed with either phenol-formaldehyde resin or a cornstarch based binder [51Ali, M.E-S.; Zeitoun, O.M. Discovering and manufacturing a new natural insulating material extracted from a plant growing up in Saudi Arabia. J. Eng. Fibers Fabrics, 2012, 7, 88-94.
[http://dx.doi.org/10.1177/155892501200700405] ]. Insulating material from a composite of C. procera fibres and phenol-formaldehyde resins has been shown to have high water repellency [52Ali, M.E-S. US Pat. 20130193365 (Aug. 1, 2013), Chem. Abstr, 2013, 159, 294814.. Insulation material based on natural fibers from flowering plant seeds in a phenol-formaldehyde resin or cornstarch binder]. C. procera flax has been forwarded as a binding material for the improvement of acoustic plaster [53Bamberger, J.J. GB398547 (March 18, 1933) Improvements in or relating to acoustic plaster, ]. Lately, the use of fibers from C. procera as a reinforcement material for thermoplastic composites was suggested, with polypropylene (PP) as matrix polymer and maleated polypropylene Epolene G-3003 as coupling agent [54Nourbakhsh, A.; Ashori, A.; Kouhpayehzadeh, M. Giant Milkweed (Calotropis persica) Fibers — A Potential Reinforcement Agent for Thermoplastics Composites. J. Reinf. Plast. Compos., 2009, 28, 2143-2149.
[http://dx.doi.org/10.1177/0731684408091902] ]. C. procera fibers were tested as reinforcement material in an epoxy matrix, too [55Yoganandam, K.; Ganeshan, P. NagarajaGanesh, B.; Raja, K. Characterization studies on Calotropis procera fibers and their performance as reinforcements in epoxy matrix. J. Nat. Fibers, 2019.
[http://dx.doi.org/10.1080/15440478.2019.1588831] ]. Fibers of C. gigantea have gained attention as well, with studies on purifying the fibers for mass production [56Liu, D. (Hunan Yunjin Group Co. Ltd.), CN 103031625 (Jan. 24, 2013) Apparatus for removing seeds from Calotropis gigantea fibre, ], and on new bleaching [57Zhao, T.; Wang, M.; Zhou, J. CN 103397508A (July 30, 2013), Low-carbon low damage bleaching method of Calotropis gigantea fiber fabric ] and dyeing techniques [58Chen, Q.; Zhao, T.; Wang, M.; Wang, J. Studies of the fibre structure and dyeing properties of Calotropis gigantea, kapok, and cotton fibres., 2013, , 448-453., 59Gao, J.; Zhao, T. Dyeing behavior of Calotropis gigantea fibre with direct dyes., 2012, , 33-35.] to make them commercial products, although dye uptake and dye fixation on the fibres remains a challenge [58Chen, Q.; Zhao, T.; Wang, M.; Wang, J. Studies of the fibre structure and dyeing properties of Calotropis gigantea, kapok, and cotton fibres., 2013, , 448-453.]. C. gigantea fibers from bark and seeds have been promoted as promising raw materials for fiber-reinforced composites [60Ashori, A.; Bahreini, Z. Evaluation of C. gigantea as a promising raw material for fiber-reinforced composite. J. Compos. Mater., 2009, 43, 1297-1304.
[http://dx.doi.org/10.1177/0021998308104526] -63Ganeshan, P. NagarajaGanesh, B.; Ramshankar, P.; Raja, K. Calotropis gigantea fibers: A potential reinforcement for polymer matrices. Int. J. Polym. Anal. Charact., 2018, 23, 271-277.
[http://dx.doi.org/10.1080/1023666X.2018.1439560] ]. Polylactic acid (PLA) and polyester have been used as matrix polymers in combination with C. gigantea fibres as reinforcement material [64Karthik, T.; Ganesan, P. Light-weight bio-composites from Calotropis gigantea stem fiber. Melliand International, 2012, 18, 214-215., 65Babu, D.G.; Babu, S.K.; Kishore, N.P. Tensile and wear behavior of Calotropis gigantea fruit fiber reinforced polyester composites. Proc. Eng., 2014, 97, 531-535.
[http://dx.doi.org/10.1016/j.proeng.2014.12.279] ]. C. gigantea fibres exhibit a high degree of tubular hollowness (80–90%). Along the fiber, no natural twist exists. In one study, the crystallinity of C. gigantea fibres was found to be 42.5% with a crystallinity orientation index of 85.4% [58Chen, Q.; Zhao, T.; Wang, M.; Wang, J. Studies of the fibre structure and dyeing properties of Calotropis gigantea, kapok, and cotton fibres., 2013, , 448-453.]. Bast fibres of C. gigantea have been noted to have increased crystallinity upon treatment with 5w% alkali solution, leading to higher tensile strength of the material [66Ramasamy, R.; Reddy, K.O.; Rajulu, A.V. Extraction and characterization of Calotropis gigantea bast fibers as novel reinforcement for composites materials. J. Nat. Fibers, 2018, 15, 527-538.
[http://dx.doi.org/10.1080/15440478.2017.1349019] ].
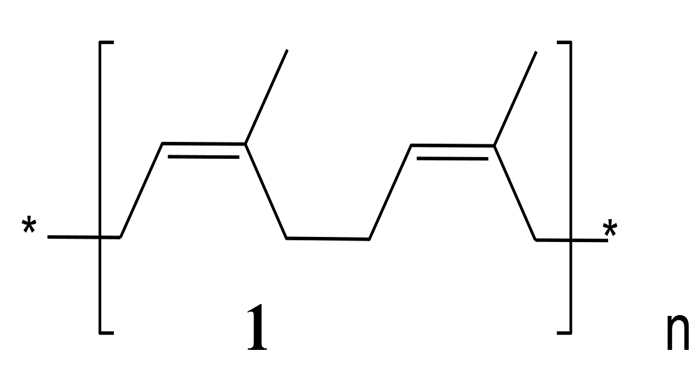 |
Fig. (2) Polyisoprene (1). |
During World War 1, rubber for tyres was produced from the C. procera’s latex [33Neuwinger, H.D. Afrikanische Arzneipflanzen und Jagdgifte, Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart 1994, 224.]. Although, the composition of the latex of the plant is very complex, it can consist of up to 25-35% of natural rubber [poly(cis-1,4-isoprene, 1), Fig. 2] [67Mohamed, N.H.; Ismail, M.A.; Abdel-Mageed, W.M.; Shoreit, A.A.M. Biodegradation of natural rubber latex of Calotropis procera by two endophytic fungal species. J. Bioremediat. Biodegrad., 2017, 8, 1-5.
[http://dx.doi.org/10.4172/2155-6199.1000380] ]. This polyisoprene has coagulative-like properties that lead to an increased adhesiveness of the latex, e.g., when secreted as a response to herbivory [68Ramos, M.V.; Demarco, D.; da Costa Souza, I.C.; de Freitas, C.D.T. Lactifiers, latex, and their role in plant defense. Trends Plant Sci., 2019, 24(6), 553-567.
[http://dx.doi.org/10.1016/j.tplants.2019.03.006] [PMID: 30979674] ].
2.2. Use as Adsorbent
The water-resistance and hydrophobicity of Calotropis fibers, treated and untreated, lend them the potential to be used as adsorbents for hydrocarbons, such as for oil [69Zheng, Y.; Zhu, Y.; Wang, A.; Hu, H. Potential of Calotropis gigantea fiber as an absorbent for removal of oil from water. Ind. Crops Prod., 2016, 83, 387-390.
[http://dx.doi.org/10.1016/j.indcrop.2016.01.009] , 70Zheng, Y.; Cao, E.; Zhu, Y.; Wang, A.; Hu, H. Perfluorosilane treated Calotropis giganteafiber: Instant hydrophobic–oleophilic surface with efficient oil-absorbing performance. Chem. Eng. J., 2016, 295, 477-483.
[http://dx.doi.org/10.1016/j.cej.2016.03.074] ]. Typically, the derivatization of the fibers with hydrophobic alkylsilyl and perfluoroalkylsilyl moieties such as with octadecyltrichlorosilane [71Zheng, Y.; Cao, E.J.; Tu, L.X.; Wang, A.Q.; Hu, H.M. A comparative study for oil-absorbing performance of octadecyltrichlorosilane treated Calotropis gigantea fiber and kapok fiber. Cellulose, 2017, 24, 989-1000.
[http://dx.doi.org/10.1007/s10570-016-1155-z] ] or with 1H,1H,2H,2H-perfluorooctyltriethoxysilane [70Zheng, Y.; Cao, E.; Zhu, Y.; Wang, A.; Hu, H. Perfluorosilane treated Calotropis giganteafiber: Instant hydrophobic–oleophilic surface with efficient oil-absorbing performance. Chem. Eng. J., 2016, 295, 477-483.
[http://dx.doi.org/10.1016/j.cej.2016.03.074] ] enhances their oil adsorbent properties. Cao et al. fabricated a roughened Calotropis gigantea fiber by immobilizing copper and nickel nanoparticles on the original fiber by the impregnation method, using NaBH4 as reductant. The treated fibers were noted to readily adsorb oils from model oil–water mixtures [72Cao, E.; Xiao, W.; Duan, W.; Wang, N.; Wang, A.; Zheng, Y. Metallic nanoparticles roughened Calotropis gigantea fiber enables efficient absorption of oils and organic solvents. Ind. Crops Prod., 2018, 115, 275-279.
[http://dx.doi.org/10.1016/j.indcrop.2018.02.052] ]. Excellent and selective oil sorption behavior properties of C. procera and C. gigantea blended with cotton and polypropylene fibers were observed by Thilagavathi et al. [73Thilagavathi, G.; Praba Karan, C.; Das, D. Oil sorption and retention capacities of thermally-bonded hybrid nonwovens prepared from cotton, kapok, milkweed and polypropylene fibers. J. Environ. Manage., 2018, 219, 340-349.
[http://dx.doi.org/10.1016/j.jenvman.2018.04.107] [PMID: 29753978] ]. The maximum oil sorption capacity of the developed thermally-bonded nonwovens was 40.2 g/g for high density (HD) oil and 23.0 g/g for diesel oil. Pyrolysis of C. gigantea fibers leads to carbon fibers that have a take-up capacity of 130 g/g for model oils in water-oil mixtures [74Tu, L.X.; Duan, W.Z.; Xiao, W.L.; Fu, C.X.; Wang, A.Q.; Zheng, Y. Calotropis gigantea fiber derived carbon fiber enable fast and efficient absorption of oils and organic solvents. Separ. Purif. Tech., 2018, 192, 30-35.
[http://dx.doi.org/10.1016/j.seppur.2017.10.005] ]. Sheets of C. gigantea fibers are also sandwiched between thin polyether sulfone layers leading to a shapable construct which exhibits an oil sorption capacity of 4.3 – 9.3 g oil/ g sorbent [75Xiao, W.; Wang, N.; Niu, B.; Fu, C.; Zhou, L.; Zheng, Y. Polyethylene sulfone assisted shape construction of Calotropis gigantea fiber for preparing a sustainable and reusable oil sorbent. Cellulose, 2019, 26, 3923-3933.
[http://dx.doi.org/10.1007/s10570-019-02356-6] ].
The surface of C. gigantea fibers can be functionalized such as with poly(m-phenylenediamine) (2) to be used as selective adsorbents. In the case of poly(m-phenylenediamine) functionalized C. gigantea fibers, these can be prepared easily by oxidative polymerization of the diamine on the surface of the fibers by immersing the fibers into an aqueous solution of m-phenylenediamine in the presence of ammonium persulfate. The functionalized fibers are excellent reductive adsorbents of Cr(VI) [76Cao, E.; Duan, W.; Yi, L.; Wang, A.; Zheng, Y. Poly(m-phenylenediamine) functionalized Calotropis gigantean fiber for coupled adsorption reduction for Cr(VI). J. Mol. Liq., 2017, 240, 225-232.
[http://dx.doi.org/10.1016/j.molliq.2017.05.087] ]. Poly(m-phenylenediamine) 2 functionalized C. gigantea fibers have also been used for the adsorption of ciprofloxacin (4), a fluoroquinolone antibiotic, which is a major pharmaceutical effluent, from wastewater [77Cao, E.; Duan, W.; Wang, A.; Zheng, Y. Oriented growth of poly(m-phenylenediamine) on Calotropis gigantea fiber for rapid adsorption of ciprofloxacin. Chemosphere, 2017, 171, 223-230.
[http://dx.doi.org/10.1016/j.chemosphere.2016.12.087] [PMID: 28024207] ]. Treating C. gigantea fibers with aq. NaClO2 and doping them subsequently with polydopamine 3 allows for the adsorption of ciprofloxacin (4) and norfloxacin (5), a further fluoroquinolone antibiotic [78Yi, L.S.; Liang, G.W.; Xiao, W.L.; Duan, W.Z.; Wang, A.Q.; Zheng, Y. Rapid nitrogen-rich modification of Calotropis gigantea fiber for highly efficient removal fluoroquinolone antibiotics. J. Mol. Liq., 2018, 256, 408-415.
[http://dx.doi.org/10.1016/j.molliq.2018.02.060] ] (Fig. 2). Dried leaf powder of C. procera has been used successfully as adsorbent for the dyes malachite green (6) [79Kaur, R.; Kaur, H. Calotropis procera as effective adsorbent for removal of malachite green dye: a comprehensive study. Desalination Water Treat., 2017, 78, 252-262.] and Congo red (7) [80Kaur, R.; Kaur, H. Calotropis procera an effective adsorbent for removal of Congo red dye: isotherm and kinetc modelling. Model. Earth Syst. Environ., 2017, 3
[http://dx.doi.org/10.1007/s40808-017-0274-3] ] from aqueous solutions (Fig. 3).
2.3. Usage as Fuel
Within limits, the wood of C. procera is utilized as cooking fuel in some areas [81Varshney, A.C.; Bhoi, K.L. Cloth from bast fiber of the Calotropis procera (Aak) plant. Biol. Wastes, 1988, 26, 229-232.
[http://dx.doi.org/10.1016/0269-7483(88)90168-1] , 82Ganaba, S.; Ouadba, J.M.; Bognounou, O. Fuelwood in the Sahelian region of Burkina Faso: Ethnic preferences. Sécheresse, 1998, 9, 261-268.]. Also, the plant is being studied extensively as a source of biofuel [83Padmaja, K.V.; Atheya, N.; Bhatnagar, A.K.; Singh, K.K. Conversion of Calotropis procera biocrude to liquid fuels using thermal and catalytic cracking. Fuel, 2009, 88, 780-785.
[http://dx.doi.org/10.1016/j.fuel.2008.11.020] ] with the gross heat content of the plant estimated at 6.1 kcal/g [84Kalita, D.; Saikia, C.N. Chemical constituents and energy content of some latex bearing plants. Bioresour. Technol., 2004, 92(3), 219-227.
[http://dx.doi.org/10.1016/j.biortech.2003.10.004] [PMID: 14766154] ]. Erdman et al. give a heat value of the whole plant of 4.2 kcal/g [85Erdman, M.D.; Erdman, B.A. Calotropis procera as an alternative source of plant hydrocarbons. Econ. Bot., 1981, 35, 467-472.
[http://dx.doi.org/10.1007/BF02858597] ], Radhaboy et al. put it at 5.2 kcal/g [86Radhaboy, G.; Pugazhvadivu, M.; Ganeshan, P.; Ramshankar, P. Analysis of Thermochemical behaviour of Calotropis procera parts for their potentiality International Journal of Ambient Energy, 2019.
[http://dx.doi.org/10.1080/01430750.2019.1630309.] ]. The heat values of whole plant fractions extracted with benzene, with petroleum ether and with ethyl acetate have been given as 9.6 kcal/g, 13.7 kcal/g, and 7.4 kcal/g, respectively [87De, S.; Bag, A.; Mukherji, S. Potential use of Pedilanthus tithymaloides Poit. as a renewable resource of plant hydrocarbons. Bot. Bull. Acad. Sin., 1997, 38, 105-108.]. Both the seeds of C. procera and C. gigantea are judged as having the potential of providing biodiesel conforming to European and ASTM standards [88Razon, L.F. Philippine plant oils as feedstock for Biodiesel. Philipp. Agric. Sci., 2008, 91, 278-286.] and have a relatively high oil-content (C. gigantea: 31%; C. procera: 26%) [89Sundar Rao, K.; Pantulu, A.J.; Lakshminarayana, G. Analysis of C. gigantea, Acia caesia, and Abelmoschus ficulneus seeds. J. Am. Oil Chem. Soc., 1983, 60, 1259-1261.
[http://dx.doi.org/10.1007/BF02702095] ]. In all published seed oil analyses [89Sundar Rao, K.; Pantulu, A.J.; Lakshminarayana, G. Analysis of C. gigantea, Acia caesia, and Abelmoschus ficulneus seeds. J. Am. Oil Chem. Soc., 1983, 60, 1259-1261.
[http://dx.doi.org/10.1007/BF02702095] -91Phoo, Z.W.M.M.; Razon, L.F.; Knothe, G.; Ilham, Z.; Goembira, F.; Madrazo, C.F.; Roces, S.A.; Saka, S. Evaluation of Indian milkweed (Calotropis gigantea) seed oil as alternative feedstock for biodiesel. Ind. Crops Prod., 2014, 54, 226-232.
[http://dx.doi.org/10.1016/j.indcrop.2014.01.029] ] of both C. procera and C. gigantea, oleic acid (10), palmitic acid (12), linoleic acid (9) and stearic acid (13) were the main constituents. Seed oil from C. procera of North Eastern Brazil has been shown to have an oil content of 21%, with a range between 19.7% and 24.0% [90Barbosa, M.O.; de Almeida-Cortez, J.S.; da Silva, S.I.; de Oliveira, A.F.M. Seed oil content and fatty acid composition from different populations of Calotropis procera (Aiton) W. T. Aiton (Apocynaceae). J. Am. Oil Chem. Soc., 2014, 91, 1433-1441.
[http://dx.doi.org/10.1007/s11746-014-2475-5] ], with constituent unsaturated palmitoleic (1.7%, 8), linoleic (35.3%, 9), oleic (33.3%, 10) and elaidic (4.2%, 11) acids as well as saturated palmitic (15.8%, 12) and stearic (9.5%, 13) acids [92de Sousa, L.V.; Santos, A.P.B.; di Souza, L.; Dias Santos, A.G.; Beatriz, A. Evaluation of the properties of Calotropis procera oil aiming the production of Biodiesel, Orbital: The Electronic. J. Chem., 2018, 10, 147-152., 93Traore, A.S. Biogas production from C. procera: a latex plant found in West Africa. Bioresour. Technol., 1992, 41, 105-109.
[http://dx.doi.org/10.1016/0960-8524(92)90178-Z] ], some bound in triglycerides [92de Sousa, L.V.; Santos, A.P.B.; di Souza, L.; Dias Santos, A.G.; Beatriz, A. Evaluation of the properties of Calotropis procera oil aiming the production of Biodiesel, Orbital: The Electronic. J. Chem., 2018, 10, 147-152.]. Seeds of C. gigantea, collected in Shwebo District, Myanmar, were equally analyzed to have 33.3 w% oil content with palmitic (15.5%, 12), linoleic (36.3%, 9), oleic (30.3%, 10) and stearic (10.5%, 13) acids along with palmitoleic (0.3%, 8), asclepic (0.8%, 14), linolenic (0.8%, 15), arachidic (0.6%, 16), behenic (0.1%, 17), and lignoceric (0.4%, 18) acids as minor constituents [91Phoo, Z.W.M.M.; Razon, L.F.; Knothe, G.; Ilham, Z.; Goembira, F.; Madrazo, C.F.; Roces, S.A.; Saka, S. Evaluation of Indian milkweed (Calotropis gigantea) seed oil as alternative feedstock for biodiesel. Ind. Crops Prod., 2014, 54, 226-232.
[http://dx.doi.org/10.1016/j.indcrop.2014.01.029] ] (Fig. 4).
The use of dry biomass of C. procera and C. gigantea to produce biogas has been studied closely since 1992. The fermentation of a suspension of 4% (w/v) of dried leaves of C. procera in water at an initial pH of 7.5 has been found to give 2·9 to 3·6 litres of biogas day−1 litre−1. The fermentation was found to be fast with 66% of dry material loaded being degraded during the first 2 days of incubation at 30°C with the resulting biogas containing 56–59% (v/v) methane [93Traore, A.S. Biogas production from C. procera: a latex plant found in West Africa. Bioresour. Technol., 1992, 41, 105-109.
[http://dx.doi.org/10.1016/0960-8524(92)90178-Z] ]. P. Gourdon et al. record 280 mL volatiles /g solid of C. procera leaves [94Gourdon, R.; Leger, P.; Vermande, P. Methane recovery by anaerobic digestion of cellulosic material available in Sahel. Biol. Wastes, 1989, 30, 181-187.
[http://dx.doi.org/10.1016/0269-7483(89)90120-1] ]. Biogas production with a mixture of fresh, chopped leafy biomass of both C. procera and C. gigantea with cow and buffalo dung was tried, also [95Manikandan, M.; Arumugam, R. Potentiality of Calotropis procera on the yield of biocrudes and biogas production. J. Phytol., 2010, 2, 33-40., 96Shilpkar, P.; Shah, M.; Chaudhry, D.R. An alternate use of Calotropis gigantea: biomethanation. Curr. Sci. India, 2007, 92, 435-437.]. This co-digestion led to an increased volume of biogas produced, albeit with a lower methane and higher carbon dioxide content [96Shilpkar, P.; Shah, M.; Chaudhry, D.R. An alternate use of Calotropis gigantea: biomethanation. Curr. Sci. India, 2007, 92, 435-437.].
Also, C. procera and C. gigantea have been studied as potential petrocrops. Extraction of the plant itself, especially with hexane and heptane, has led to high-yielding hydrocarbon fractions, which have been forwarded as potentially useful chemical feedstock [83Padmaja, K.V.; Atheya, N.; Bhatnagar, A.K.; Singh, K.K. Conversion of Calotropis procera biocrude to liquid fuels using thermal and catalytic cracking. Fuel, 2009, 88, 780-785.
[http://dx.doi.org/10.1016/j.fuel.2008.11.020] , 85Erdman, M.D.; Erdman, B.A. Calotropis procera as an alternative source of plant hydrocarbons. Econ. Bot., 1981, 35, 467-472.
[http://dx.doi.org/10.1007/BF02858597] , 97Erdman, M.D. Nutrient and cardenolide composition of unextracted and solvent-extracted Calotropis procera. J. Agric. Food Chem., 1983, 31(3), 509-513.
[http://dx.doi.org/10.1021/jf00117a012] [PMID: 6886206] -99Kalita, D. Hydrocarbon plant – new source of energy for the future. Renew. Sustain. Energy Rev., 2008, 12, 455-471.
[http://dx.doi.org/10.1016/j.rser.2006.07.008] ]. Within this context, transgenic C. gigantea plants have been grown with alkane contents that were up to 30% higher than in the wild-form of C. gigantea [100Zhao, B.; Sun, J.; Wang, X. (Institute of Process Engineering, Chinese Academy of Sciences) CN 202586271 (Jan 11, 2012) Method of generating high-alkane content in Calotropis gigantea through genetic transformation ]. The biocrude thus obtained was subjected to thermal and catalytic cracking to deliver gas and liquid fuels containing mono- and diaromatics, olefins and saturated alkanes [83Padmaja, K.V.; Atheya, N.; Bhatnagar, A.K.; Singh, K.K. Conversion of Calotropis procera biocrude to liquid fuels using thermal and catalytic cracking. Fuel, 2009, 88, 780-785.
[http://dx.doi.org/10.1016/j.fuel.2008.11.020] ]. The gas products from the catalytic cracking process were seen to be propene (22.0% – 26.7%), isobutene (8.1w% - 15.0w%) and pentanes/pentenes (22.7w% - 28.9w%) at temperatures between 460 °C and 520 °C [83Padmaja, K.V.; Atheya, N.; Bhatnagar, A.K.; Singh, K.K. Conversion of Calotropis procera biocrude to liquid fuels using thermal and catalytic cracking. Fuel, 2009, 88, 780-785.
[http://dx.doi.org/10.1016/j.fuel.2008.11.020] ].
Pyrolysis of C. procera derived biomass has been attempted, also. Thus, co-cracking of petroleum vacuum residue with polypropylene and C. procera derived biomass has been found to lead to a lowering of the activation energy as compared to cracking petroleum vacuum residue alone [101Ahmaruzzaman, M.; Sharma, D.K. Coprocessing of petroleum vacuum residue with plastics, coal, and biomass and its synergistic effects. Energy Fuels, 2007, 21, 891-897.
[http://dx.doi.org/10.1021/ef060102w] , 102Ahmaruzzaman, M.; Sharma, D.K. Characterization of liquid products from the co-cracking of ternary and quaternary mixture of petroleum vacuum residue, polypropylene, Samla coal and Calotropis procera. Fuel, 2008, 87, 1967-1973.
[http://dx.doi.org/10.1016/j.fuel.2008.01.007] ].
2.4. Animal Feed
In many regions, livestock feeding on Calotropis plants is limited, and toxicity associated with animal feeding on the plants is a constant problem [103Mahmoud, O.M.; Adam, S.E.I.; Tartour, G. The effects of Calotropis procera on small ruminants. I. Effects of feeding sheep with the plant. J. Comp. Pathol., 1979, 89(2), 241-250.
[http://dx.doi.org/10.1016/0021-9975(79)90063-X] [PMID: 457943] -105el Badwi, ; Samia, M.A.; Adam, S.E.; Shigidi, M.T.; Hapke, H.J. Studies on laticiferous plants: toxic effects in goats of Calotropis procera latex given by different routes of administration. Dtsch. Tierarztl. Wochenschr., 1998, 105(11), 425-427.
[PMID: 9857566] ], with sheep fatalities having been reported at 5-10 g of latex feed per kg of bodyweight. Wildlife grazing of the plant is scant [106Sharma, G.K. Calotropis procera and Calotropis gigantea. Indian J. Veterinary Sci., 1934, 4, 63-74.], but browsing of leaves and flowers by gazelles has been seen, especially in times of drought [107Heuzé, V.; Tran, G.; Baumont, R.; Bastianelli, D. Calotropis (Calotropis procera) Feedipedia, 2016.http://www.feedipedia.org/node/588]. The grazing of leaf and flower was observed to some extent also with goats, sheep and cattle in the arid region of Cholistan rangelands in Pakistan [108Abdullah, M.; Rafay, M.; Hussain, T.; Ahmad, H.; Tahir, U.; Rasheed, F.; Ruby, T.; Khalil, S. Nutritive potential and palatability preference of browse foliage by livestock in arid rangelands of Cholistan desert (Pakistan). J. Anim. Plant Sci., 2017, 27, 1656-1664.]. It has been suggested that the toxicity of the plants for animals may depend on biotypes and on the environmental conditions during the plants' growth [109Radunz, B.L.; Wilson, G.; Beere, G. Feeding rubberbush (Calotropis procera) to cattle and sheep. Aust. Vet. J., 1984, 61(7), 243-244.
[http://dx.doi.org/10.1111/j.1751-0813.1984.tb06006.x] [PMID: 6497815] , 110Lottermoser, B.G. Colonisation of the rehabilitated Mary Kathleen uranium mine site (Australia) by Calotropis procera: toxicity risk to grazing animals. J. Geochem. Explor., 2011, 111, 39-46.
[http://dx.doi.org/10.1016/j.gexplo.2011.07.005] ]. It has also been noted that drying may result in the loss of some of the more toxic components such as flavonoid and cardiotonic glycosides of the plant material [111dos Santos Belém, C.; de Souza, A.M.; de Lima, P.R.; de Carvalho, F.A.L.; Queiroz, M.A.A.; da Costa, M.M. Digestibility, fermentation and microbiological characteristics of Calotropis procera silage with different quantities of grape pomace. Cienc. Agrotec., 2016, 40, 698-705.
[http://dx.doi.org/10.1590/1413-70542016406020916] , 112Mello, M.M.; Vaz, F.A.; Gonҫalves, L.C.; Saturnino, H.M. Estudo fitoquímíco da Calotropis procera Ait., sua utilizaҫão na alimentaҫão de caprinos: Efitos clínicos e bioquímicos séericoos. Belo Horizonte, MG. Rev. Bras. Saúda Prod. An, 2001, 2, 15-20.]. Additionally, there have been recent efforts to find safe limits to include the plants in hay and silage as an alternative feed in arid and semi-arid regions [111dos Santos Belém, C.; de Souza, A.M.; de Lima, P.R.; de Carvalho, F.A.L.; Queiroz, M.A.A.; da Costa, M.M. Digestibility, fermentation and microbiological characteristics of Calotropis procera silage with different quantities of grape pomace. Cienc. Agrotec., 2016, 40, 698-705.
[http://dx.doi.org/10.1590/1413-70542016406020916] , 113Costa, R.G.; de Medeiros, A.N.; Alves, A.R.; de Medeiros, G.R. Prospects for use of rooster tree (Calotropis procera) in animal production. Rev. Caatinga, 2009, 22, 1-9.]. In NW Brazil [114Madruga, M.S.; Arruda, S.G.B.; Narain, N.; Souza, J.G. Castration and slaughter age effects on panel assessment and aroma compounds of the “mestiço” goat meat. Meat Sci., 2000, 56(2), 117-125.
[http://dx.doi.org/10.1016/S0309-1740(00)00025-5] [PMID: 22061898] ], and to a smaller extent in parts of India [115Nehra, O.P.; Oswal, M.C.; Faroda, A.S. Management of fodder tree in Haryana. Indian Farming, 1987, 37, 31-33.], silk flower hay (SHF, C. procera SW.) has been reported to be suitable for feeding goats, sheep, and camels. In sheep and goat farming, it was noted that substitution of corn and soybean by silk flower hay of up to 30% did not decrease the nutrient uptake by Morada Nova lambs [116Torres, J.F.; Braga, A.P.; Lima, G.F.C.; Rangel, A.H.; de Lima Júnior, D.M.; do Vale Maciel, M.; Oliveira, S.E.O. Utilizaҫão do feno de flor-de-seda (Calotropis procera ait. r. br.) na alimentaҫão de ovinos. Acta Vet. Bras., 2010, 4, 42-50.] and did not decrease the sensory attributes of their meat [117Costa, R.G.; da Silva, N.V.; de Azevedo, P.S.; de Medeiros, A.N.; de Carvalho, F.F.R.; Queiroga, R.C.R.E.; de Medeiros, G.R. Meat quality of lambs fed silk flower hay (Calotropis procera SW.) in their diet. Rev. Bras. Zootec., 2011, 40, 1266-1271.
[http://dx.doi.org/10.1590/S1516-35982011000600015] ] or important attributes of the milk of the goats [118Pereira, G.F.; Gracindo, A.P.A.C.; Tinoco, A.F.D.; de Oliveria, P.H.M.; Rangel, A.H.D. Fatty acid profile of milk of goats fed with growing levels of flor-de-seda hay. Rev. Caatinga, 2009, 22, 206-210.].
There has been a study carried out with C. procera grown in the southern coastal region of Puerto Rico, combining the use of C. procera as a biofuel and the use of the processed residues as animal feed [97Erdman, M.D. Nutrient and cardenolide composition of unextracted and solvent-extracted Calotropis procera. J. Agric. Food Chem., 1983, 31(3), 509-513.
[http://dx.doi.org/10.1021/jf00117a012] [PMID: 6886206] ]. It was found that the crude protein content of unextracted and extracted C. procera was comparable to that of Euphorbia, and was found to be of sufficient level for most goat, sheep, and cattle maintenance.
For human consumption, it has been reported that nectar crystals from dried flowers of C. procera have been used as a sugar substitute. Thus, in Java, the central part of the flower has been processed into a sweetmeat named Chinese candy [119Uphof, J.C.Th. Dictionary of Economic Plants., 1959, ]. On the other hand, it must be recognized that the nectar can contain poisonous components as well. Cases of fatal poisonings of humans with both C. procera and C. gigantea [120Bengal: Report of the Chemical Examiner for 1940 The British Pharmaceutical Codex, 1934, .] are known. In general, the cardenolides found in C. procera are toxic to vertebrates.
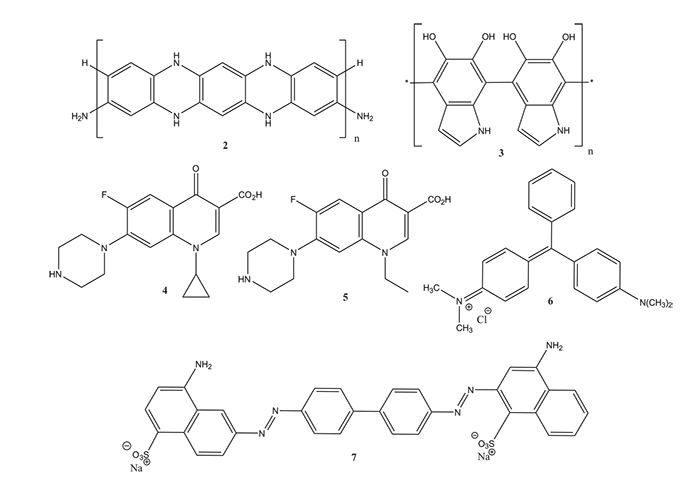 |
Fig. (3) Dried leaf powder of C. procera has been used successfully as adsorbents for the dyes malachite green (6) and Congo red (7) from aqueous solutions. |
 |
Fig. (4) Carboxylic acid content of C. procera and C. gigantea seed oils. |
2.5. Pesticidal Use of the Plant
The reports on the bioactivity of extracts from both species are numerous, where the extracts have been utilized as herbicide [121Al-Zahrani, H.S.; Al-Robai, S.A. Allelopathic effect of Calotropis procera leaves extract on seed germination of some plants. JKAU: Sci., 2007, 19, 115-126.
[http://dx.doi.org/10.4197/Sci.19-1.9] ], fungicide [122Larhsini, M.; Lazrek, H.B.; Bousaid, M.; Jana, M.; Amarouch, H. Evaluation of antifungal and molluscicidal properties of extracts of Calotropis procera. Fitoterapia, 1997, 68, 371-373.], insecticide [123Ahmed, U.A.M.; Shi, Z.; Bashier, N.H.H.; Muafi, K.; Hao, Z.; Guo, Y. Evaluation of insecticidal potentials of aqueous extracts of C. procera Ait. against Henosepilachna elaterii Rossi. J. Appl. Sci. (Faisalabad), 2006, 6, 2466-2470.
[http://dx.doi.org/10.3923/jas.2006.2466.2470] -128Barati, R.; Golmohammadi, G.; Ghajarie, H.; Zarabi, M.; Mansouri, R. Efficiency of some herbal pesticides on reproductive parameters of silverleaf whitefly, Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Arch. Phytopathol. Pflanzenschutz, 2014, 47, 212-221.
[http://dx.doi.org/10.1080/03235408.2013.807035] ], nematicide [129Rakesh, P.; Alok, K.; Neetu, K. Nematicidal activity in flowers of some medicinal and aromatic plants. Indian J. Entomol., 2001, 31, 96-98.-133Verma, B.S.; Verma, K.K. Toxicity of some indigenous plant extracts to root-knot, seed gall and citrus nematodes. Pesticides, 1989, 23, 25-27.], acaricide [134Chungsamarnyart, N.; Ratanakreetakul, C.; Jansawan, W. Acaricidal activity of the combination of plant crude extracts to cattle ticks. Kasetsart J. Natl. Sci., 1994, 28, 649-660.], and as molluscicide [122Larhsini, M.; Lazrek, H.B.; Bousaid, M.; Jana, M.; Amarouch, H. Evaluation of antifungal and molluscicidal properties of extracts of Calotropis procera. Fitoterapia, 1997, 68, 371-373., 135Hussein, H.I.; Kamel, A.; Abou-Zeid, M.; Abdel-Khalek, ; El-Sebae, H.; Saleh, M.A. Uscharin, the most potent molluscicidal compound tested against land snails. J. Chem. Ecol., 1994, 20(1), 135-140.
[http://dx.doi.org/10.1007/BF02065996] [PMID: 24241704] , 136Al-Sarar, A.; Hussein, H.; Abobakr, Y.; Bayoumi, A. Molluscicidal activity of methomyl and cardenolide extracts from Calotropis procera and Adenium arabicum against the land snail Monacha cantiana. Molecules, 2012, 17(5), 5310-5318.
[http://dx.doi.org/10.3390/molecules17055310] [PMID: 22565481] ]. Traditionally, extracts from C. procera have been used alone or in combination with that of other plants, such as in a combination of the extract of C. procera flowers, Azadirachta indica and Nicotiana tabacum leaves, and Trachyspermum ammi seeds used against the common cattle tick Rhipicephalus microplus (Boophilus) [137Zaman, M.A.; Iqbal, Z.; Abbas, R.Z.; Khan, M.N.; Muhammad, G.; Younus, M.; Ahmed, S. In vitro and in vivo acaricidal activity of a herbal extract. Vet. Parasitol., 2012, 186(3-4), 431-436.
[http://dx.doi.org/10.1016/j.vetpar.2011.11.018] [PMID: 22305296] ].
2.6. Insecticidal Use – Insects Associated with Calotropis plants
The larvicidal role of C. procera in mosquito control was reported already more than 25 years ago [127Girdhar, G.; Deval, K.; Mittal, P.K.; Vasudevan, P. Mosquito control by Calotropis latex. Pesticides, 1984, 18, 26-29.]. The latex from the green parts of the plant, which in effect represents a system of defense of the plant itself against insects [68Ramos, M.V.; Demarco, D.; da Costa Souza, I.C.; de Freitas, C.D.T. Lactifiers, latex, and their role in plant defense. Trends Plant Sci., 2019, 24(6), 553-567.
[http://dx.doi.org/10.1016/j.tplants.2019.03.006] [PMID: 30979674] , 122Larhsini, M.; Lazrek, H.B.; Bousaid, M.; Jana, M.; Amarouch, H. Evaluation of antifungal and molluscicidal properties of extracts of Calotropis procera. Fitoterapia, 1997, 68, 371-373., 138Konno, K. Plant latex and other exudates as plant defense systems: roles of various defense chemicals and proteins contained therein. Phytochemistry, 2011, 72(13), 1510-1530.
[http://dx.doi.org/10.1016/j.phytochem.2011.02.016] [PMID: 21450319] ], severely affects larvae development and mortality and suppresses egg hatching [139Ramos, M.V.; Bandeira, Gde.P.; de Freitas, C.D.; Nogueira, N.A.P.; Alencar, N.M.N.; de Sousa, P.A.; Carvalho, A.F.U. Latex constituents from Calotropis procera (R. Br.) display toxicity upon egg hatching and larvae of Aedes aegypti (Linn.). Mem. Inst. Oswaldo Cruz, 2006, 101(5), 503-510.
[http://dx.doi.org/10.1590/S0074-02762006000500004] [PMID: 17072453] ] in the mosquito Aedes aegypti, which is a vector of the dengue virus. At the same time, mixing water with aqueous latex extract leads to reduced ovipositioning of gravid A. aegypti females [140Singhi, M.; Joshi, V.; Sharma, R.C.; Sharma, K. Ovipositioning behavior of Aedes aegypti in different concentrations of latex of Calotropis procera: studies on refractory behavior and its sustenance across genotrophic cycles. Dengue Bull., 2004, 28, 184-188.]. Laboratory experiments using water without and with various concentrations of larvicidal latex extract seem to indicate that the ovipositioning female can distinguish among the extract concentrations to lay its eggs in the medium with the least larvicidal concentration [140Singhi, M.; Joshi, V.; Sharma, R.C.; Sharma, K. Ovipositioning behavior of Aedes aegypti in different concentrations of latex of Calotropis procera: studies on refractory behavior and its sustenance across genotrophic cycles. Dengue Bull., 2004, 28, 184-188.]. Aqueous latex extracts, but not those of the flowers, were shown to also have a larvacidal activity in the mosquito Anopheles labranchiae [141Markouk, M.; Bekkouche, K.; Larhsini, M.; Bousaid, M.; Lazrek, H.B.; Jana, M. Evaluation of some Moroccan medicinal plant extracts for larvicidal activity. J. Ethnopharmacol., 2000, 73(1-2), 293-297.
[http://dx.doi.org/10.1016/S0378-8741(00)00257-9] [PMID: 11025168] ] and Culex qinquefasciatus [142Shahi, M.; Hanafi-Bojd, A.A.; Iranshahi, M.; Vatandoost, H.; Hanafi-Bojd, M.Y. Larvicidal efficacy of latex and extract of Calotropis procera (Gentianales: Asclepiadaceae) against Culex quinquefasciatus and Anopheles stephensi (Diptera: Culicidae). J. Vector Borne Dis., 2010, 47(3), 185-188.
[PMID: 20834091] , 143Tahir, H.M.; Ishaq, T.; Mukhtar, M.K.; Khan, S.Y.; Ahmed, L. Potential use of Calotropis procera (milk weed) to control Culex quinquefasciatus (Diptera: culicidae). Pak. J. Zool., 2013, 45, 615-621.]. Aqueous leaf extracts were found to have larvacidal activity against Anopheles arabiensis [144Elimam, A.M.; Elmalik, K.H.; Ali, F.S. Efficacy of leaves extract of Calotropis procera Ait. (Asclepiadaceae) in controlling Anopheles arabiensis and Culex quinquefasciatus mosquitoes. Saudi J. Biol. Sci., 2009, 16(2), 95-100.
[http://dx.doi.org/10.1016/j.sjbs.2009.10.007] [PMID: 23961048] ]. Also, the extracts exhibited oviposition deterrent activity in the mosquitoes C. qinquefasciatus and A. arabiensis [144Elimam, A.M.; Elmalik, K.H.; Ali, F.S. Efficacy of leaves extract of Calotropis procera Ait. (Asclepiadaceae) in controlling Anopheles arabiensis and Culex quinquefasciatus mosquitoes. Saudi J. Biol. Sci., 2009, 16(2), 95-100.
[http://dx.doi.org/10.1016/j.sjbs.2009.10.007] [PMID: 23961048] ]. Moderate larvacidal activity of aq. extracts of C. procera was found against C. quinquefasciatus Say [142Shahi, M.; Hanafi-Bojd, A.A.; Iranshahi, M.; Vatandoost, H.; Hanafi-Bojd, M.Y. Larvicidal efficacy of latex and extract of Calotropis procera (Gentianales: Asclepiadaceae) against Culex quinquefasciatus and Anopheles stephensi (Diptera: Culicidae). J. Vector Borne Dis., 2010, 47(3), 185-188.
[PMID: 20834091] , 144Elimam, A.M.; Elmalik, K.H.; Ali, F.S. Efficacy of leaves extract of Calotropis procera Ait. (Asclepiadaceae) in controlling Anopheles arabiensis and Culex quinquefasciatus mosquitoes. Saudi J. Biol. Sci., 2009, 16(2), 95-100.
[http://dx.doi.org/10.1016/j.sjbs.2009.10.007] [PMID: 23961048] , 145Rahuman, A.A.; Bagavan, A.; Kamaraj, C.; Saravanan, E.; Zahir, A.A.; Elango, G. Efficacy of larvicidal botanical extracts against Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol. Res., 2009, 104(6), 1365-1372.
[http://dx.doi.org/10.1007/s00436-009-1337-9] [PMID: 19198882] ] and Anopholes stephensi [142Shahi, M.; Hanafi-Bojd, A.A.; Iranshahi, M.; Vatandoost, H.; Hanafi-Bojd, M.Y. Larvicidal efficacy of latex and extract of Calotropis procera (Gentianales: Asclepiadaceae) against Culex quinquefasciatus and Anopheles stephensi (Diptera: Culicidae). J. Vector Borne Dis., 2010, 47(3), 185-188.
[PMID: 20834091] ]. Methanolic latex extracts of C. procera have been proven to be the most effective, however, as larvacide against such dengue vectors as A. aegypti. Thus, Singhi et al. have carried out a field study in selected areas of Jodphur City, India, dispersing an aq. solution of a dried methanolic extract of C. procera latex in water tanks and containers that normally function as breeding areas of different mosquito species such as C. quinquefasciatus, A. stephensi, and A. aegypti. A 100% larval mortality was found throughout when applying the solution at a concentration of 100 ppm [146Singhi, M.; Purohit, A.; Chattopadhyay, S. Effectiveness and feasibility of methanol extracted latex of Calotropis procera as larvicide against dengue vectors of western Rajasthan, India. J. Vector Borne Dis., 2015, 52(2), 142-146.
[PMID: 26119546] ]. Also, methanolic extracts of C. gigantea leaves have larvicidal (1st-4th instar tested) and pupicidal effect on C. quinquefasciatus, A. stephensi, and A. aegypti [147Kovendan, K.; Murugan, K.; Prasanna Kumar, K.; Panneerselvam, C.; Mahesh Kumar, P.; Amerasan, D.; Subramaniam, J.; Vincent, S. Mosquitocidal properties of Calotropis gigantea (Family: Asclepiadaceae) leaf extract and bacterial insecticide, Bacillus thuringiensis, against the mosquito vectors. Parasitol. Res., 2012, 111(2), 531-544.
[http://dx.doi.org/10.1007/s00436-012-2865-2] [PMID: 22382205] ].
The extracts of C. procera leaves have been shown to have a profound effect on a diminished survival of the fifth instar larvae of the desert locust Schistocerca gregaria. Additionally, the arrest of ovarian growth and the absence of sexual maturity in adult Schistocerca gregaria were noted [148Abbassi, K.; Kadiri, Z.A.; Ghaout, S. Biological activity of Calotropis procera (Ait. R. Br) leaves on the desert locust (Schistocerca gregaria, Forsk. 1775) Zoologica baetica, 2004, 15, 153–166.]. Also, latex extracts of C. procera are effective against S. gregaria [149Al Robai, A.A. Toxicological studies on the latex of the uscher plant C. procera (Ait) in Saudi Arabia. IV. Effects of partly purified uscher latex and of the poison gland secretion of the uscherhopper; Poekilocerus bufonius Klug on the desert locust, Schistocerca gregaria Forskal. (Orthoptera: Acrididae). Arab Gulf J. Sci. Res., 1997, 15, 709-716.]. Interestingly, the extracts of C. gigantea have been found to be active against S. gregaria as well, with an isolated plant component, a non-protein amino acid, forwarded as an antifeedant [150Pari, K.; Rao, P.J.; Devakumar, C.; Rastogi, J.N. A novel insect antifeedant nonprotein amino acid from Calotropis gigantea. J. Nat. Prod., 1998, 61(1), 102-104.
[http://dx.doi.org/10.1021/np970255z] [PMID: 9548837] ], thought to be the active principle. Later, the structure of this amino acid could not be confirmed [151Suparpprom, C.; Vilaivan, T. Synthesis of 2-[4′-(ethylcarbamoyl)phenyl]-N-acetylglycine, the proposed structure for giganticine. J. Nat. Prod., 2001, 64(8), 1114-1116.
[http://dx.doi.org/10.1021/np010046l] [PMID: 11520243] ]. Leaf extracts of C. procera were found to be effective against the flesh fly, Sarcophaga haemorrhoidales fallen [126Moursy, L.E. Insecticidal activity of Calotropis procera extracts of the flesh fly, Sarcophaga haemorrhoidalis fallen. J. Egypt. Soc. Parasitol., 1997, 27(2), 505-514.
[PMID: 9257990] ] and have been advocated as an insect control against the two moths Clostera cupreata (Noctuidae) and Plecoptera reflexa (Noctuidae), where the 3rd instar larvae were targeted [152Rashmi, S. K.P.; Arya, S. Phytochemical profile and evaluation of insecticidal efficacy of Calotropis procera against defoliators. J. Med. Plants Res., 2011, 5, 6738-6743.
[http://dx.doi.org/10.5897/JMPR11.774] ]. Calotropis procera has been used for the control of the ladybird beetle Henosepilachna elaterii [123Ahmed, U.A.M.; Shi, Z.; Bashier, N.H.H.; Muafi, K.; Hao, Z.; Guo, Y. Evaluation of insecticidal potentials of aqueous extracts of C. procera Ait. against Henosepilachna elaterii Rossi. J. Appl. Sci. (Faisalabad), 2006, 6, 2466-2470.
[http://dx.doi.org/10.3923/jas.2006.2466.2470] ], the painted grasshopper Poecilocerus pictus Fab [153Chandra, H.; Lal, P. Food preference studies of “AK” grasshopper Poekilocerus pictus Fab. Plant Protect. Bull. Faridabad, 1993, 45, 3-10.], the lesser grain borer Rhizoperta dominica [124Jacob, S.; Sheila, M.K. A note on the protection of stored rice from the lesser grain borer Rhizoperta dominica Fabr. By indigenous plant products. Indian J. Entomol., 1993, 55, 337-339., 154Amin, M.R.; Shahjahan, M.; El-Taj, H.F.; Iqbal, T.M.T.; Hussain, M.A. Use of akanda, biskatali, and neem leaves as botanical insecticides against lesser grain borer. Bangladesh J. Entomol., 2000, 10, 1-13., 155Neenah, G. Potential of using flavonoids, latex and extracts from Calotropis procera (Ait.) as grain protectants against two coleopteran pests of stored rice. Ind. Crops Prod., 2013, 45, 327-334.
[http://dx.doi.org/10.1016/j.indcrop.2012.12.043] ], the rice weevil Sitophilus oryzae and the silver leaf whitefly Bemisia tabaci Gennadius [156Reihaneh, B.; Golamrezah, G.; Hamid, G.; Mehdi, Z.; Raziyeh, M. The effects of some botanical insecticides and pymetrozine on life table parameters of silver leaf whitefly Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae). Pestic. Phytomed., 2013, 28, 47-55.
[http://dx.doi.org/10.2298/PIF1301047B] ]. The root bark of C. gigantea [157Alam, P.; Ali, M. Phytochemical investigation of C. procera Ait. roots. Indian J. Chem., 2009, 48B, 443-446.] and aq. leaf extracts of C. procera [158Abbasi, A.B.; Bibi, R.; Khan, A.A.; Iqbal, M.S.; Sherani, J.; Khan, A.M. Assessment of Calotropis procera Aiton and Datura alba Nees leaves extracts as bio-insecticides against Tribolium castaneum Herbst in stored wheat Triticium aetivum L., J. Biofertil. Biopest., 2012, 3, 126-129.] show insecticidal activity against the red flour beetle Triboleum castaneum, while the root bark of C. procera is active against the confused flour beetle Triboleum confusum [159Jahan, S.; Maman, A.; Khan, A.R. Insecticidal effect of akanda (Calotropis procera) on Tribolium confusum Duval (Coleoptera: Tenebrionidae). Bangladesh J. Zool., 1991, 19, 261-268.]. While mixed extacts from C. procera and other plants have been found most effective against the common cattle tick Rhipicephalus microplus (Boophilus) [137Zaman, M.A.; Iqbal, Z.; Abbas, R.Z.; Khan, M.N.; Muhammad, G.; Younus, M.; Ahmed, S. In vitro and in vivo acaricidal activity of a herbal extract. Vet. Parasitol., 2012, 186(3-4), 431-436.
[http://dx.doi.org/10.1016/j.vetpar.2011.11.018] [PMID: 22305296] , 160Nithya, V.; Kamalam, M.; Umakanthan, T. Screening of indigenous medicinal plants for their acaricidal activity against catlle ticks under in vivo condition. Int. J. Pharm. Sci. Res., 2015, 6, 3049-3052.] (see above), leaf extracts and whole plant extracts of C. procera alone are successful against the common cattle tick [160Nithya, V.; Kamalam, M.; Umakanthan, T. Screening of indigenous medicinal plants for their acaricidal activity against catlle ticks under in vivo condition. Int. J. Pharm. Sci. Res., 2015, 6, 3049-3052., 161Shyma, K.P.; Gupta, J.P.; Ghosh, S.; Patel, K.K.; Singh, V. Acaricidal effect of herbal extracts against cattle tick Rhipicephalus (Boophilus) microplus using in vitro studies. Parasitol. Res., 2014, 113(5), 1919-1926.
[http://dx.doi.org/10.1007/s00436-014-3839-3] [PMID: 24633906] ] and the Asian blue tick (Rhipcephalus microplus), affecting both the oviposition of female ticks and the larval mortality rate [161Shyma, K.P.; Gupta, J.P.; Ghosh, S.; Patel, K.K.; Singh, V. Acaricidal effect of herbal extracts against cattle tick Rhipicephalus (Boophilus) microplus using in vitro studies. Parasitol. Res., 2014, 113(5), 1919-1926.
[http://dx.doi.org/10.1007/s00436-014-3839-3] [PMID: 24633906] , 162Khan, A.; Nasreen, N.; Niaz, S.; Ayaz, S.; Naeem, H.; Muhammad, I.; Said, F.; Mitchell, R.D., III; de León, A.A.P.; Gupta, S.; Kumar, S. Acaricidal efficacy of Calotropis procera (Asclepiadaceae) and Taraxacum officinale (Asteraceae) against Rhipicephalus microplus from Mardan, Pakistan. Exp. Appl. Acarol., 2019, 78(4), 595-608.
[http://dx.doi.org/10.1007/s10493-019-00406-z] [PMID: 31367977] ]. Cardiac glycosides in the extracts of C. procera can be seen as one of the active principles according to the work by D. H. Al-Rajhy et al. on the efficacy of the extracts on the camel tick Hyalomma dromedarii (Acari: Ixodidae) [163Al-Rajhy, D.H.; Alahmed, A.M.; Hussein, H.I.; Kheir, S.M. Acaricidal effects of cardiac glycosides, azadirachtin and neem oil against the camel tick, Hyalomma dromedarii (Acari: Ixodidae). Pest Manag. Sci., 2003, 59(11), 1250-1254.
[http://dx.doi.org/10.1002/ps.748] [PMID: 14620053] ].
Nevertheless, by far, not all insects are affected negatively by the plant. The African monarch, Danaus chrysippus, a butterfly common to many areas in Africa and Asia, thrives on the plant and its caterpillars (1st – 5th instar) utilize some of the latex proteins in their diet. It is suggested that the caterpillars’ proteolytic digestive system destroys the toxic proteins of the Calotropis latex, including the peptidases, and thus makes them immune to the toxic principles of the plant [68Ramos, M.V.; Demarco, D.; da Costa Souza, I.C.; de Freitas, C.D.T. Lactifiers, latex, and their role in plant defense. Trends Plant Sci., 2019, 24(6), 553-567.
[http://dx.doi.org/10.1016/j.tplants.2019.03.006] [PMID: 30979674] , 164Pereira, D.A.; Ramos, M.V.; Souza, D.P.; Portela, T.C.L.; Guimarȁes, J.A.; Madeira, S.V.F.; Texeira de Freitas, C.D. Digestibility of defense proteins in latex of milkweeds by digestive proteases of Monarch butterflies, Danaus plexippus L.: a potential determinant of plant-herbivore interactions. Plant Sci., 2010, 179, 348-355.
[http://dx.doi.org/10.1016/j.plantsci.2010.06.009] ]. There also seems to be a fine balance in the feeding behavior of the Danaus chrysippus caterpillars, notably of the younger instars, where the need for nourishment is off-set by the exposure to de facto poisonous cardenolides in the latex, where caterpillars cut the leaves, wait for the exuded latex to dry, and progress on feeding off the leaves while avoiding the latex for the most part [68Ramos, M.V.; Demarco, D.; da Costa Souza, I.C.; de Freitas, C.D.T. Lactifiers, latex, and their role in plant defense. Trends Plant Sci., 2019, 24(6), 553-567.
[http://dx.doi.org/10.1016/j.tplants.2019.03.006] [PMID: 30979674] , 164Pereira, D.A.; Ramos, M.V.; Souza, D.P.; Portela, T.C.L.; Guimarȁes, J.A.; Madeira, S.V.F.; Texeira de Freitas, C.D. Digestibility of defense proteins in latex of milkweeds by digestive proteases of Monarch butterflies, Danaus plexippus L.: a potential determinant of plant-herbivore interactions. Plant Sci., 2010, 179, 348-355.
[http://dx.doi.org/10.1016/j.plantsci.2010.06.009] ].
C. procera and C. gigantea are host to a larger number of other insects such as the cossid moth, Semitocossus johannes (Staudinger) [165Al Dhafer, H.M.; Aldryhim, Y.N.; Elgharbawy, A.A. Aspects of the life-history of Semitococcus johannes (Staudinger) (Lepidoptera: Cossidae) feeding on the milkweed Calotropis procera (Aiton) W.T., Aiton in the kingdom of Saudi Arabia. J. Kans. Entomol. Soc., 2013, 86, 133-144.
[http://dx.doi.org/10.2317/JKES120824.1] ], the larvae of which can cause severe damage to the plant. Especially, the nectar of the flowers attracts insects, including ants. The large Arabian carpenter bee, Xylocopa sulcatipes Maa. is noted to build its nests in dry stems of C. procera [166Hannan, M.A.; Alqarni, A.S.; Owayss, A.A.; Engel, M.S. The large carpenter bees of central Saudi Arabia, with notes on the biology of Xylocopa sulcatipes Maa (Hymenoptera, Apidae, Xylocopinae). ZooKeys, 2012, 201(201), 1-14.
[PMID: 22768000] ]. Here, even a X. sulcatipes – C. procera coevolutionary pattern has been suggested with X. sulcatipes as the natural co-adapted pollinator [167Willmer, P.G. The role of insect water balance in pollination ecology: Xylocopa and Calotropis. Oecologia, 1988, 76(3), 430-438.
[http://dx.doi.org/10.1007/BF00377039] [PMID: 28312024] ]. It is known that many species of Asclepias plants, in general, attract insects such as butterflies [168Brower, L.P. Ecological chemistry. Sci. Am., 1969, 220(2), 22-29.
[http://dx.doi.org/10.1038/scientificamerican0269-22] [PMID: 5767170] , 169Parsons, J.A. A digitalis-like toxin in the monarch butterfly, Danaus plexippus L. J. Physiol., 1965, 178, 290-304.
[http://dx.doi.org/10.1113/jphysiol.1965.sp007628] [PMID: 14298120] ] and milkweed bugs [170Duffey, S.S.; Scudder, G.E.E. Cardiac glycosides in North American Asclepiadaceae, a basis of unpalatability in brightly colored Hemiptera and Coleoptera. J. Insect Physiol., 1972, 18, 63-78.
[http://dx.doi.org/10.1016/0022-1910(72)90065-0] ]. Ten insect species were recorded in India alone to feed on C. gigantea [171Pugalenthi, P.; Livingstone, D. Susceptibility-linked population dynamics of insects associated with Calotropis gigantea (L) R. Br. (Asclepiadaceae) of the Maruthamalai scrub jungle, India. Ann. Entomol., 1993, 11, 39-42., 172Saikia, H.C.; Das, B.K.; Kalita, J. Studies on the Insects associated with calotropis gigantea in Guwahati city of Assam, India. J. Zool. Stud., 2015, 2, 6-13.]. Altogether, 65 species of insects and 5 species of mites have been documented on C. procera and C. gigantea [173Dhileepan, K. Prospects for the classical biological control of Calotropis procera (Apocynaceae) using coevolved insects. Biocontrol Sci. Technol., 2014, 24, 977-998.
[http://dx.doi.org/10.1080/09583157.2014.912611] ]. In the United Arab Emirates, the black carpenter bee (Xylocopa ctenoxylocopa fenetrata), canary carpenter bee (Xylocopa koptortosoma aestuans), oriental wasp (Vespa orientalis), and scoliid wasp (Vobalayca flavifrons) are typical insects associated with C. procera. Butterflies will lay eggs on milkweeds as will moths [165Al Dhafer, H.M.; Aldryhim, Y.N.; Elgharbawy, A.A. Aspects of the life-history of Semitococcus johannes (Staudinger) (Lepidoptera: Cossidae) feeding on the milkweed Calotropis procera (Aiton) W.T., Aiton in the kingdom of Saudi Arabia. J. Kans. Entomol. Soc., 2013, 86, 133-144.
[http://dx.doi.org/10.2317/JKES120824.1] ], and it is the caterpillars which incorporate the sequestered cardenolides from the plants in their defense systems. Within this context, the cardenolide fingerprint of monarch butterflies, Danaus plexippus L., reared on different milkweeds has been studied [174Malcolm, S.B.; Cockrell, B.J.; Brower, L.P. Cardenolide fingerprint of monarch butterflies reared on common milkweed,Asclepias syriaca L. J. Chem. Ecol., 1989, 15(3), 819-853.
[http://dx.doi.org/10.1007/BF01015180] [PMID: 24271887] -176Martin, R.A.; Lynch, S.P. Cardenolide content and thin-layer chromatography profiles of monarch butterflies,Danaus plexippus L., and their larval host-plant milkweed,Asclepias asperula subsp.Capricornu (woods.) woods., in north central Texas. J. Chem. Ecol., 1988, 14(1), 295-318.
[http://dx.doi.org/10.1007/BF01022548] [PMID: 24277011] ]. The transfer of cardiac toxins from the caterpillar feeding on C. procera to the tissue of the adult butterfly has also been noted in the common tiger butterfly, Danaus chrysippus. The grasshopper Poecilocerus pictus feeds on C. gigantea. Again, it was found that cardiac glycosides are taken up by the insect [177Livingstone, D.; Pugalenthi, P. Biology of Poecilocerus pictus (Orthoptera: Pyrgomorphidae) on the basis of its nutritional ecology. J. Entomol. Res., 1992, 16, 267-272. [New Dehli]., 178Pugalenthi, P.; Livingstone, D. Cardenolides (heart poisons) in the painted grasshopper Poecilocerus pictus (Orthoptera: Pyrgomorphidae) feeding on the milkweed Calotropis gigantea L. (Asclepiadaceae). J. N.Y. Entomol. Soc., 1995, 103, 191-196.]. Here, however, a comparative characterization of the cardiac glycosides of C. gigantea and the feeding P. pictus revealed partial metabolisation of some of the substances in the insect extract. It was found that the insect extract reduces the viability of A549 (carcinomic human a lveolar basal epithelial) and COLO 205 (human caucasian colon adenocarcinoma) cells, inducing apoptosis in COLO 205, where the extract distinguishes between normal human cells and cancer cells [179Mathen, C.; Hardikar, B. Cytotoxic compounds from Poecilocerus pictus feeding on Calotropis gigantea. J. Exp. Ther. Oncol., 2010, 8(3), 177-185.
[PMID: 20734917] , 180Mathen, C.; Peter, S.M.; Hardikar, B.P. Comparative evaluation of the cytotoxic and apoptotic potential of Poecilocerus pictus and Calotropis gigantea. J. Environ. Pathol. Toxicol. Oncol., 2011, 30(1), 83-92.
[http://dx.doi.org/10.1615/JEnvironPatholToxicolOncol.v30.i1.80] [PMID: 21609318] ]. The unpalatability of insects reared on cardiac glycoside containing milkweeds was demonstrated in feeding experiments of Asclepias currasavica L. reared monarchs to the American blue jay Cyanocitta cristata bromia [181Brower, L.P.; van Brower, J.; Corvino, J.M. Plant poisons in a terrestrial food chain. Proc. Natl. Acad. Sci. USA, 1967, 57(4), 893-898.
[http://dx.doi.org/10.1073/pnas.57.4.893] [PMID: 5231352] ]. The aphid Aphis nerii is another insect that thrives on C. gigantea. Aphids feeding on C. gigantea leaves were found to have longer life-spans, with higher fecundity of the females, while the lady-beetle Menochilus sexmaculatus, the main predator of Aphis nerii, exhibited a shorter life-span when on a diet of C. gigantea fed aphids [182Murugan, K.; Jeyabalan, D.; Kumar, N.S.; Nathan, S.S.; Sivaramakrishnan, S. Influence of host plant on growth and reproduction of Aphis nerii and feeding and prey utilization of its predator Menochilus sexmaculatus. Indian J. Exp. Biol., 2000, 38(6), 598-603.
[PMID: 11116532] ]. The Calotropis plants are not only hosts to insects, but to birds such as sunbirds as well.
On the other hand, insects lead also to possible ways for a classical biological control of Calotropis. It has been noted that three pre-dispersal seed predators, the Aak weevil Paramecops farinosus, the Aak fruit fly Dacus persicus in the Indian subcontinent and the Sodom apple fruit fly Dacus longistylus in the Middle East can be seen as prospective biological control agents of the plant [173Dhileepan, K. Prospects for the classical biological control of Calotropis procera (Apocynaceae) using coevolved insects. Biocontrol Sci. Technol., 2014, 24, 977-998.
[http://dx.doi.org/10.1080/09583157.2014.912611] ].
2.7. Nematicidal/Schistosomicidal/Antihelminthic Activity
Calotropis procera has been used for the control of plant-pathogenic nematodes Meloidogyne javanica [183Ahmad, R.; Shahab, M.Z.; Inam-ul-Haq, M.; Javed, N.; Dogar, M.A.; Khan, M.Y. Effect of soil amendment with Calotropis procera for the control of Meloidogyne javanica infection on egg plant. Pak. J. Nematol., 1996, 14, 55-59., 184Khurma, U.R.; Chaudhary, P. Comparative effects of extracts of different parts of Calotropis procera, Cassia fistula, Ricinus communis, and Sesbania sesban on Meloidogyne javanica juveniles. J. Environ. Biol., 1999, 20, 287-288.] and Meloidogyne incognita [132Maqbool, M.A.; Hashmi, S.; Ghaffar, A. Effect of latex extracts from Euphorbia caducifolia and Calotropis procera on root-knot nematode Meloidogyne incognita infesting tomato and egg plant. Pak. J. Nematol., 1987, 43-47., 185Parvatha, R.P.; Rao, M.S. Eco-friendly management of Meloidogyne incognita on tomato by integration of Verticillium chlamydosporium with neem and Calotropis leaves, Zeitschr. f. Pflanzenkrankheiten u. Pflanzenschutz, 1999, 106, 530-533., 186Rehman, B.; Ahmad, F.; Babalola, O.O.; Ganai, M.A.; Parihar, K.; Siddiqui, M.A. Usages of botanical extracts for the management of the root-knot nematode, Meloidogyne incognita in chickpea. J. Pure Appl. Microbiol., 2013, 7, 2385-2388.]. It must be remarked that the mortality rates of Meloidogyne incognita juveniles induced by the leaf extracts of C. procera are not as high as that induced by the extracts of some other plants such as Acacia nilotica, Aristilochia bracteolate or Chenopodium album [187Elbadri, G.A.; Lee, D.W.; Park, J.C.; Yu, H.B.; Choo, H.Y. Evaluation of various plant extracts for their nematicidal efficacies against juveniles of Meloidogyne incognita. J. Asia Pac. Entomol., 2008, 11, 99-102.
[http://dx.doi.org/10.1016/j.aspen.2008.04.004] ]. Latex extracts of C. gigantea have also been used against Meloidogyne incognita as well as against the cowpea cyst nematode Heterodera cajani [188Devi, L.S.; Gupta, P. Evaluation of some plant lattices against Heterodera cajani in cowpea (Vigna sinensis). Natl. Acad. Sci. Lett., 2000, 23, 65-67.]. Interestingly, the methanolic extracts of Calotopis procera flowers also exhibit schistosomicidal activity [189Yousif, F.; Hifnawy, M.S.; Soliman, G.; Boulos, L.; Labib, T.; Mahmoud, S.; Ramzy, F.; Yousif, M.; Hassan, I.; Mahmoud, K.; El-Hallouty, S.M.; El-Gendy, M.; Gohar, L.; El-Manawaty, M.; Fayyad, W.; El-Menshawi, B.S. Large scale in vitro screening of Egyptian native and cultivated plants for schistosomicidal activity. Pharm. Biol., 2007, 45, 501-510.
[http://dx.doi.org/10.1080/13880200701389425] ]. Aqueous and methanolic extracts of dried flowers of C. procera have antihelmintic effect as studied on live Haemonchus contortas [190Iqbal, Z.; Lateef, M.; Jabbar, A.; Muhammad, G.; Khan, M.N. Anthelmintic activity of Calotropis procera (Ait.) Ait. F. flowers in sheep. J. Ethnopharmacol., 2005, 102(2), 256-261.
[http://dx.doi.org/10.1016/j.jep.2005.06.022] [PMID: 16085379] ]. Sheep infected with gastrointestinal nematodes were treated with the extracts and the egg counts in treated sheep decreased significantly [190Iqbal, Z.; Lateef, M.; Jabbar, A.; Muhammad, G.; Khan, M.N. Anthelmintic activity of Calotropis procera (Ait.) Ait. F. flowers in sheep. J. Ethnopharmacol., 2005, 102(2), 256-261.
[http://dx.doi.org/10.1016/j.jep.2005.06.022] [PMID: 16085379] ] although the activity was found to be lower than that of levamisole. Also, the latex of C. procera has been shown to have antihelmintic effect in sheep [191Al-Qarawi, A.A.; Mahmoud, O.M.; Sobaih, M.A.; Haroun, E.M.; Adam, S.E.I. A preliminary study on the anthelmintic activity of Calotropis procera latex against Haemonchus contortus infection in Najdi sheep. Vet. Res. Commun., 2001, 25(1), 61-70.
[http://dx.doi.org/10.1023/A:1026762002947] [PMID: 11214673] , 192Shivkar, Y.M.; Kumar, V.L. Antihelmintic effect of latex of Calotropis procera. Pharm. Biol., 2003, 41, 263-265.
[http://dx.doi.org/10.1076/phbi.41.4.263.15666] ]. Ethyl acetate solutions of lyophilized C. procera latex inhibited egg hatching of H. contortas, showed a pronounced larvicidal effect (EC50 = 0.22 mg/mL) and inhibited the motility of the parasites by over 80% after 6h contact time [193Cavalcante, G.S.; de Morais, S.M.; Andre, W.P.P.; Ribeiro, W.L.C.; Rodrigues, A.L.M.; De Lira, F.C.; Viana, J.M.; Bevilaqua, C.M.L. Chemical composition and in vitro activity of Calotropis procera (Ait.) latex on Haemonchus contortus. Vet. Parasitol., 2016, 226, 22-25.
[http://dx.doi.org/10.1016/j.vetpar.2016.06.012] [PMID: 27514877] ].
2.8. Molluscicidal Activity
Much of the here-mentioned activity can be attributed to the cardenolide content of the extracts as could also be shown by the molluscicidal activity of cardenolide extracts from C. procera against the land snail Monacha cantania [136Al-Sarar, A.; Hussein, H.; Abobakr, Y.; Bayoumi, A. Molluscicidal activity of methomyl and cardenolide extracts from Calotropis procera and Adenium arabicum against the land snail Monacha cantiana. Molecules, 2012, 17(5), 5310-5318.
[http://dx.doi.org/10.3390/molecules17055310] [PMID: 22565481] ] and single-compound toxicity studies of uscharin in the snail Thepa pisana [135Hussein, H.I.; Kamel, A.; Abou-Zeid, M.; Abdel-Khalek, ; El-Sebae, H.; Saleh, M.A. Uscharin, the most potent molluscicidal compound tested against land snails. J. Chem. Ecol., 1994, 20(1), 135-140.
[http://dx.doi.org/10.1007/BF02065996] [PMID: 24241704] ]. Even fresh leaves of C. gigantea at 200 g/ha have been used to control the golden apple snail, Pomacea canaliculata, in rice [194Lobo, P.P.G.; Llagas, F.D. Evaluation of starflower (Calotropis gigantea) against golden apple snail (Pomaceae canaliculata) in lowland transplanted rice. Philipp. J. Crop Sci., 1991, 16, 103-107.]. Alcoholic extracts of fruits, leaves, stems, and roots of C. procera were found to show molluscicidal activity against Biomphalaria arabica [195Abo Zaid, K.H.; El-Wakil, H.; El-Hussein, A.; Jomaa, S.; Shohayeb, M. Evaluation of the molluscicidal activity of Punica granatum, Calotropis procera, Solanum incantum, and Citrullus colocynthis against Biomphalaria arabica. World Appl. Sci. J., 2013, 26, 873-879.], the intermediate host of the trematode Schistsoma mansoni in Saudi Arabia.
2.9. Plant Fungicidal Use
Different aerial parts of C. gigantea, such as the leaves, have been used as anti-fungicide, especially against Phyllactinia corylea (powdery mildew), Peridiopsora mori (brown rust), Pseudocercospora mori (black leaf spot), Myrothecium roridum (brown leaf spot), Colletotrichum graminicola, Drechslera sorokiniana (Drechslera leaf blight), Fusarium solani, Macrophomina phaseolina, and Phomopsis sojae (soybean stem blight). Mixtures of extracts of C. gigantea, Azadirachta indica (neem), Datura stramonium and cow manure have been seen to be effective against Fusarim mangiferae, a fungus leading to floral malformation in mango [196Usha, K.; Singh, B.; Praseetha, P.; Deepa, N.; Agarwal, D.K.; Agarwal, R.; Nagaraja, A. Antifungal activity of Datura stramonium, Calotropis gigantea, and Azadirachta indica against Fusarium mangiferae and floral malformation in mango. Eur. J. Plant Pathol., 2009, 124, 637-657.
[http://dx.doi.org/10.1007/s10658-009-9450-2] ]. Extracts of C. procera were found to inhibit growth, sporulation, and the conidial germination of Drechslera biseptata and Fusarium solani [197Abu-Taleb, A.M.; El-Deeb, K.; Al-Otibi, F.O. Bioactivity of some plant extracts against Drechslera biseptata and Fusarium solani. J. Food Agric. Environ., 2011, 9, 769-774.]. Others have reported the fungi toxic properties of extracts of the plant [198Shivpuri, A.; Sharma, O.P.; Jhamaria, S.L. Fungitoxic properties of plant extracts against pathogenic fungi. J. Mycol. Plant Pathol., 1997, 27, 29-31.], especially also against seed-borne mycoflora of wheat, which includes Altemaria alternata, A. clamydophor, Aspergillus niger, A. flavus, Rhizopus oryzae, Mucor spp., Fusarium spp., Drechslera australiensis, Penicillium spp., Curvularia lunata and Cladosporium [199Pathak, N.; Zaidi, R.K. Comparative study of seed dressing fungicides and Calotropis procera latex for the control of seed-borne mycoflora of wheat. Ann. Biol. Res., 2013, 4, 1-6.]. Recently, it has been found that methanolic C. procera leaf extracts are active against Macrophomina phaseolina, a fungus prevalent in arid tropical or semitropical regions causing stalk and fruit rot in many crop plants [200Jabeen, K.; Waheed, N.; Iqbal, S. Antifungal potential of Calotropis procera against Macrophomina phaseolina. Life Sci. J., 2013, 10, 572-576.]. Extracts of Calotropis procera were found to be active against the fungal pathogen Alternaria solani, which causes early blight disease in tomatoes [201Sadana, D.; Didwania, N. Integrated disease management of bull eye’s pathogen infecting Lycopersicon esculentum (tomato). J. Microbiol. Biotechnol. Food Sci., 2019, 9, 53-57.
[http://dx.doi.org/10.15414/jmbfs.2019.9.1.53-57] ].
2.10. Allelopathic Activity
Aqueous extracts of dried leaves have a strong adverse effect on the germination of wheat (Triticum aestivum, Graminaea) [121Al-Zahrani, H.S.; Al-Robai, S.A. Allelopathic effect of Calotropis procera leaves extract on seed germination of some plants. JKAU: Sci., 2007, 19, 115-126.
[http://dx.doi.org/10.4197/Sci.19-1.9] , 202Abdel-Farid, I.; El-Sayed, M.; Mohamed, E. Allelopathic potential of Calotropis procera and Morettia philaeana. Int. J. Agric. Biol., 2013, 15, 130-134., 203Qasem, J.R. A survey on the phytotoxicity of common weeds, wild grown species and medicinal plants on wheat. Allelopathy J., 2017, 42, 179-193.
[http://dx.doi.org/10.26651/allelo.j./2017-42-2-1115] ] and influence the germination of barley (Hordeum vulgare, Graminaea), cucumber (Cucumis satires, Curcurbitaceae), radish (Raphanus sativus) [202Abdel-Farid, I.; El-Sayed, M.; Mohamed, E. Allelopathic potential of Calotropis procera and Morettia philaeana. Int. J. Agric. Biol., 2013, 15, 130-134.], and Fenugreek (Trigonella foenumgraecum, Leguminosae) to a lesser degree [121Al-Zahrani, H.S.; Al-Robai, S.A. Allelopathic effect of Calotropis procera leaves extract on seed germination of some plants. JKAU: Sci., 2007, 19, 115-126.
[http://dx.doi.org/10.4197/Sci.19-1.9] ]. Also, aqueous leaf extracts were reported to exhibit an allelopathic effect on the germination and seedling growth of maize, where inhibition of radicle and plumule growth of four maize cultivars were noted [204Kayode, J. Allelopathic effect of aqueous extracts of Calotropis procera on germination and seedling growth of maize. Pak. J. Sci. Ind. Res., 2004, 47, 69-72.]. Flower extracts of C. procera were found to have an effect on the germination of wheat and canola (Brassica napus), not only delaying the germination but also leading to a reduction of root and shoot lengths of the plants. Also, the germination, seedling growth and biomass yield of acacia species such as of Acacia tortilis (Forssk.) are detrimentally influenced by aq. C. procera leaf extract [205Alshahrani, N.D.S.T.S.; Aref, I.M.; Nasser, R.A. Allelopathic potential of Calotropis procera and Eucalyptus species on germination and growth of some timber trees. Allelopathy J., 2017, 40, 81-94.
[http://dx.doi.org/10.26651/2017-40-1-1068] ]. Aq. whole plant extract mixtures of Datura stramonium and C. procera have been advocated for the management of the invasive parthenium weed Parthenium hysterophorus L [206Raza, A.; Kamran, M.; Safdar, M.E.; Ali, H.H.; Abbas, T.; Asif, M.; Ali, L.; Rehman, A. Management tactics for the handling of Parthenium hysterophorus L. in non-native environment through phytotoxic compounds of local species. Int. J. Agric. Biol., 2019, 21, 215-222.]. In this case, the allelopathic properties of the extracts were attributed to quercetin (19), p-coumaric acid (20), gallic acid (21), sinapinic acid (22), and chlorogenic acid (23) (Fig. 5). In another study, caffeic acid (24), gentisic acid (25), catechol (26), syringic acid (27), ellagic acid (28), resorcinol (29), and p-hydroxybenzoic acid (30) in addition to gallic and p-coumaric acid, 20 and 21, were the isolated compounds made responsible for the allelopathic behavior of aq. leaf extracts of C. procera, this time on Cassia sophera and Allium cepa L. (onion) (Fig. 6) [207Gulzar, A.; Siddiqui, M.B.; Bi, S. Phenolic acid allelochemicals induced morphological, ultrastructural, and cytological modification on Cassia sophera L. and Allium cepa L. Protoplasma, 2016, 253(5), 1211-1221.
[http://dx.doi.org/10.1007/s00709-015-0862-x] [PMID: 26387115] ]. Nevertheless, even in the arid wasteland, other plants can be seen growing in close proximity to C. procera. Reversely, it has been noted that buffel grass (Cenchrus ciliaris) has allelopathic toxicity towards the roots of Calotropis [208Parsons, W.T.; Cuthbertson, E.G. Noxious weeds of Australia., 2001, ].
2.11. Calotropis in Environmental Monitoring and Bioremediation
Calotropis plants can grow under adverse conditions and have been found to be tolerant of pollution. This suggests that the species can be grown in the neighborhood of polluted sites as a remedial measure [209Achakzai, K.; Khalid, S.; Adrees, M.; Bibi, A.; Ali, S.; Nawaz, R.; Rizwan, M. Air pollution tolerance index of plants around brick kilns in Rawalpindi, Pakistan. J. Environ. Manage., 2017, 190, 252-258.
[http://dx.doi.org/10.1016/j.jenvman.2016.12.072] [PMID: 28061409] ]. Thus, the uptake of metals by C. procera, both from the soil via the roots and from the air via the leaves [210Gajbhiye, T.; Pandey, S.K.; Kim, K.H. Factors controlling the deposition of airborne metals on plant leaves in a subtropical industrial environment. Asian J. Atmos. Environ., 2016, 10, 162-167.
[http://dx.doi.org/10.5572/ajae.2016.10.3.162] ], has received some interest. The results have been found to vary, where both the conditions on site and the matrix incorporating the heavy metals may well play a role. C. procera has been used for the monitoring of Pb in automobile-
 |
Fig. (5) Allelopathic compounds of aq. whole plant extracts of C. procera [206Raza, A.; Kamran, M.; Safdar, M.E.; Ali, H.H.; Abbas, T.; Asif, M.; Ali, L.; Rehman, A. Management tactics for the handling of Parthenium hysterophorus L. in non-native environment through phytotoxic compounds of local species. Int. J. Agric. Biol., 2019, 21, 215-222.]. |
 |
Fig. (6) Allelopathic compounds of aq. leaf extracts of C. procera [207Gulzar, A.; Siddiqui, M.B.; Bi, S. Phenolic acid allelochemicals induced morphological, ultrastructural, and cytological modification on Cassia sophera L. and Allium cepa L. Protoplasma, 2016, 253(5), 1211-1221. [http://dx.doi.org/10.1007/s00709-015-0862-x] [PMID: 26387115] ]. |
exhaust pollution [211Singh, N.; Yunus, M.; Srivastava, K.; Singh, S.N.; Pandey, V.; Misra, J.; Ahmad, K.J. Monitoring of auto exhaust pollution by roadside plants. Environ. Monit. Assess., 1995, 34(1), 13-25.
[http://dx.doi.org/10.1007/BF00546243] [PMID: 24201905] , 212Khalid, N.; Hussain, M.; Young, H.S.; Ashraf, M.; Hameed, M.; Ahmad, R. Lead concentrations in soils and some wild plant species along two busy roads in Pakistan. Bull. Environ. Contam. Toxicol., 2018, 100(2), 250-258.
[http://dx.doi.org/10.1007/s00128-017-2247-7] [PMID: 29248955] ]. C. procera has been under discussion as a phytoremediator of soils polluted with metal wastes [213d’Souza, R.; Varun, M.; Pratas, J.; Paul, M.S. Spatial distribution of heavy metals in soil and flora associated with the glass industry in North Central India: implications for phytoremediation. Soil Sediment Contam., 2013, 22, 1-20.
[http://dx.doi.org/10.1080/15320383.2012.697936] -215Nawab, J.; Khan, S.; Shah, M.T.; Gul, N.; Ali, A.; Khan, K.; Huang, Q. Heavy metal bioaccumulation in native plants in chromite impacted sites: a search for effective remediating plant species, Clean – Soil, Air. Water, 2016, 44, 37-46.]. In some cases, it could be shown that C. procera shows a high transfer factor for Cd, Ni and Pb from the soil to the plant, with the highest concentrations of Pb and Cd found in the fruits, and of Ni in the stem of the plant [216Gupta, A.K.; Sinha, S. Phytoextraction capacity of the plants growing on tannery sludge dumping sites. Bioresour. Technol., 2007, 98(9), 1788-1794.
[http://dx.doi.org/10.1016/j.biortech.2006.06.028] [PMID: 16973356] ]. The accumulation of Cd in the leaves has also been demonstrated with plants growing on contaminated soil near Riyadh, Saudi Arabia [217Al-Qahtani, K.M. Assessment of heavy metals accumulation in native plant species from soils contaminated in Riyadh City, Saudi Arabia. Life Sci. J., 2012, 2, 384-392.] and on contaminated soil near Agra in the urban North Central area of India [218D’Souza, R.J.; Varun, M.; Masih, J.; Paul, M.S. Identification of Calotropis procera L. as a potential phytoaccumulator of heavy metals from contaminated soils in Urban North Central India. J. Hazard. Mater., 2010, 184(1-3), 457-464.
[http://dx.doi.org/10.1016/j.jhazmat.2010.08.056] [PMID: 20843602] ]. In the same general region, C. procera has been found to have a good bioconcentration factor for Zn, Mn, Cd and Cu, but a low translocation factor, where concentrations of these heavy metals were higher in the roots than in the leaves of the plant [219Varun, M.; D’Souza, R.; Pratas, J.; Paul, M.S. Metal contamination of soils and plants associated with the glass industry in North Central India: prospects of phytoremediation. Environ. Sci. Pollut. Res. Int., 2012, 19(1), 269-281.
[http://dx.doi.org/10.1007/s11356-011-0530-4] [PMID: 21735162] ]. This study is juxtaposed by the findings of M. Singh et al., who showed that C. procera has a moderate bioaccumulation capacity for Co, Cr, Cu, Pb, and Zn with a high translocation capacity of these metals to the shoots of the plant [220Singh, M.; Verma, M.; Kumar, R.N. Effects of open dumping of MSW on metal contamination of soil, plants, and earthworms in Ranchi, Jharkhand, India. Environ. Monit. Assess., 2018, 190(3), 139.
[http://dx.doi.org/10.1007/s10661-018-6492-y] [PMID: 29442190] ]. On the other hand, a third study has found no accumulation at all of Zn and Ni in leaves, shoots or roots from contaminated soils in the Unnao, Uttar Pradeh area [221Chandra, R.; Kumar, V.; Tripathi, S.; Sharma, P. Heavy metal phytoextraction potential of native weeds and greases from endocrine-disrupting chemicals rich complex distillery sludge and their histological observations during in-situ phytoremediation. Ecol. Eng., 2018, 111, 143-156.
[http://dx.doi.org/10.1016/j.ecoleng.2017.12.007] ]. The results delineating the Cd-uptake of the plant are also not clear-cut. Investigations have shown that Cd(II) salts are readily adsorbed by C. procera at both pH 5.0 and pH 8.0, with a maximum biosorptive capacity of 40 – 50 mg Cd(II) / g of plant material [222Pandey, P.K.; Verma, Y.; Choubey, S.; Pandey, M.; Chandrasekhar, K. Biosorptive removal of cadmium from contaminated groundwater and industrial effluents. Bioresour. Technol., 2008, 99(10), 4420-4427.
[http://dx.doi.org/10.1016/j.biortech.2007.08.026] [PMID: 17892931] ]. Interestingly, though, a study of the metal accumulation in C. procera in polluted areas within Lucknow, India, showed little uptake of Cd [223Barthwal, J.; Nair, S.; Kakkar, P. Heavy metal accumulation in medicinal plants collected from environmentally different sites. Biomed. Environ. Sci., 2008, 21(4), 319-324.
[http://dx.doi.org/10.1016/S0895-3988(08)60049-5] [PMID: 18837296] ] and the use of C. procera as indicator plants in heavily contaminated soils surrounding the Mahad AD'Dahab mine, Saudi Arabia, led to no accumulation of Cd in the plants at all, though high concentrations of Cd could be observed in neighboring Pergularia tomentosa and Salsola sp. plants. In this case, however, C. procera could be used as an indicator of As, Pb, Zn (also ref. 224Tripathi, A.; Misra, D.R. Bioaccumalation of Pb, Ni and Zn in some plant species growing in and around a municipal waste dumpsite at Allahabad, India. J. Solid Waste Technol. Manag., 2013, 39, 1-12.
[http://dx.doi.org/10.5276/JSWTM.2013.1] ), and Cu [225Al-Farraj, A.S.; Al-Wabel, M.I. Heavy metals accumulation of some plant species grown on mining area at Mahad AD’Dahab, Saudi Arabia. J. Appl. Sci. (Faisalabad), 2007, 7, 1170-1175.
[http://dx.doi.org/10.3923/jas.2007.1170.1175] ]. The use of C. procera for the phytostabilisation of Cu and Zn was also suggested by Al-Qahtani [217Al-Qahtani, K.M. Assessment of heavy metals accumulation in native plant species from soils contaminated in Riyadh City, Saudi Arabia. Life Sci. J., 2012, 2, 384-392.] and Dan-Badjo et al. have remarked on the significant uptake by C. procera of both As and Zn from the soil of a gold mining area in Niger [226Dan-Badjo, A.T.; Ibrahim, O.Z.; Guéro, Y.; Morel, L.; Feidt, C.; Echevarria, G. Impacts of artisanal gold mining on soil, water and plant contamination by trace elements at Komabangou, Western Niger J. Geochem. Explor, 2019, 205 ID 106328]. From a location in Punjab, a significant correlation was reported between the Cr and Pb content in the soil and the content of these metals in C. gigantea plants growing in the area [227Khan, Z.I.; Ahmad, K.; Rasheed, M.J.Z.; Nawaz, R.; Ayub, M.; Zahoor, A.F.; Anjum, A.; Yousaf, M.; Dogar, Z.U.H.; Ur Rahman, K.; Rauf, A.; Mukhtar, M.K.; Naqvi, S.H.; Shaheen, M.; Fardous, A.; Gondal, S.; Naheed, S.; Ahmad, S.; Hussain, G.; Sher, M.; Arshad, F.; Ali, K.G.; Parveen, B. Toxic and some essential metals in medicinal plants used in herbal medicines” a case study in Pakistan. Afr. J. Pharm. Pharmacol., 2013, 7, 1389-1395.
[http://dx.doi.org/10.5897/AJPP2012.1894] ]. Pb and Cd at levels of 32 to 80 mg/kg (Pb) and of 2.8 to 3.6 mg/kg (Cd) in the leaves of C. procera were found to exhibit toxicity to the plant as evidenced by lower chlorophyll contents in the leaves [218D’Souza, R.J.; Varun, M.; Masih, J.; Paul, M.S. Identification of Calotropis procera L. as a potential phytoaccumulator of heavy metals from contaminated soils in Urban North Central India. J. Hazard. Mater., 2010, 184(1-3), 457-464.
[http://dx.doi.org/10.1016/j.jhazmat.2010.08.056] [PMID: 20843602] ], leading to reduced photosynthesis of the plant. It must be noted that some additional stress factors may have been contributing, such as the proximity of the plants to heavy road traffic resulting in exposure to various other pollutants. There is some concern regarding the good metal-extraction capability of the plant that often also serves medicinal purposes as the effect of the metal concentration in the plant on the plant's medicinal extracts is not known. Also, the use of Calotropis as fodder in more highly contaminated areas should be avoided [225Al-Farraj, A.S.; Al-Wabel, M.I. Heavy metals accumulation of some plant species grown on mining area at Mahad AD’Dahab, Saudi Arabia. J. Appl. Sci. (Faisalabad), 2007, 7, 1170-1175.
[http://dx.doi.org/10.3923/jas.2007.1170.1175] ].
Processed parts of C. procera have also been used in environmental remediation. Thus, C. procera roots have been utilized in the removal of Cr(III), As, and Cu from water, incl. waste-water [228Taskeen, A.; Naeem, I.; Mubeen, H.; Moeen, T. Comparison of Biomasses of Different Plants for Phytoremediation of Arsenic. Asian J. Chem., 2009, 21, 2857-2860.-231Mubeen, H.; Naeem, I.; Taskeen, A. Phytoremediation of Cu(II) by Calotropis procera roots. New York Sci. J., 2010, 3, 1-5.]. Oven dried, ground and sieved C. procera roots of 100 mesh size in fixed-bed columns have been used to remove Pb(II) from water [231Mubeen, H.; Naeem, I.; Taskeen, A. Phytoremediation of Cu(II) by Calotropis procera roots. New York Sci. J., 2010, 3, 1-5.]. The results obtained when using C. procera latex in the purification of domestic and industrial wastewater in Nigeria, on the reduction of turbidity, color, odor, microbial load and total coliforms were comparable to those using already proved coagulants such as Moringa oleifera, Jatropha curcas, FeCl3, and aluminum sulfate (alum) [232Okonko, I.O.; Shittu, O.B. Bioremediation and municipal water treatment using latex exudate from Calotropis procera (Sodom apple). Electronic J. Env. Agr. Food Chem., 2007, 6, 1890-1904.]. Simple carbonization of C. procera leaves in the presence of sulfuric acid at 150oC left charcoal of 40-60 mesh, which was used to remove Zn(II) from water [233Vaishnav, V.; Chandra, S.; Daga, K. Adsorption studies of Zn(II) ions from wastewater using Calotropis procera as an adsorbent. Int. J. Sci. Eng. Res., 2011, 2, 1-6.]. Also, polyvinyl-coated activated carbon prepared from C. procera leaves was used for the removal of Zn(II) from synthetic wastewater [233Vaishnav, V.; Chandra, S.; Daga, K. Adsorption studies of Zn(II) ions from wastewater using Calotropis procera as an adsorbent. Int. J. Sci. Eng. Res., 2011, 2, 1-6.]. Cut C. procera leaves have been studied in water purification in Nigeria, where it was found that treatment significantly reduced the water's turbidity and coliform count [234Shittu, B.O.; Popoola, T.O.S.; Taiwo, O. Potentials of Calotropis procera leaves for wastewater treatment Proc. Int. Conf. Sci. Nat. Develop., 2004, 97-101.]. Biosorption studies of Cd(II) on leaf biomass have also been carried out [235Babalola, J.O.; Overah, L.C.; Babarinde, A.; Oninla, V.O.; Olatunde, A. Kinetic, equilibrium and thermodynamic studies on the biosorption of Cd (II) from aqueous solutions by the leaf biomass of Calotropis procera - ‘Sodom apple’. J. Appl. Sci. Environ. Manag., 2011, 15, 607-615.]. Polyvinyl coated activated carbon prepared from C. procera leaves was used for the removal of Cd(II) from synthetic wastewater [236Vaishnav, V.; Dega, K.; Lal, M. Adsorption of metal (Cd) from synthetic wastewater by plant material. Int. J. Res. Chem. Environ., 2013, 3, 148-154.].
CONCLUSION
Calotropis procera and Calotropis gigantea are undemanding plants that, while being undesirable weeds in some regions, have economic potential as a source of fuel and chemical feedstock. The review, which is seen as part 1 of a two-part series, showed their use in providing construction materials and, in limitations, their utilization as animal feed. Extracts of C. procera and C. gigantea are employed widely as natural pesticides. The application of the two plants in bioremediation efforts, including in the monitoring of environmental pollutants in the soil was discussed, also.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors thank the UAEU e-Research Support & Consultations specialist Mr. Ahmed Taha.
REFERENCES
| [1] | Mueen Ahmed, K.K.; Rana, A.C.; Dixit, V.K. Calotropis Species (Ascelpediaceace) - A Comprehensive Review. Pharmacogn. Mag., 2005, 1, 48-52. |
| [2] | Meena, A.K.; Yadav, A.K.; Nirjanan, U.S.; Singh, B.; Nagariya, A.K.; Sharma, K.; Gaurav, A.; Sharma, S.; Rao, M.M. A review on Calotropis procera Linn. and its ethnobotany, phytochemical, pharmacological profile. Drug Invention Today, 2010, 2, 185-190. |
| [3] | Kumar, G.; Karthik, L.; Rao, K.V.B. A review on the pharmacological and phytochemical profile of Calotropis gigantea Linn. Pharmacologyonline, 2011, 1, 1-8. |
| [4] | Qureshi, A.A.; Shaista, O.; Sanghai, D.B.; Setty, S.R.; Bhajipale, N.S. Phytochemical constituents and pharmacological activities of Calotropis procera. Plant Arch., 2008, 8, 23-27. |
| [5] | Juncker, T.; Schumacher, M.; Dicato, M.; Diederich, M. UNBS1450 from Calotropis procera as a regulator of signaling pathways involved in proliferation and cell death. Biochem. Pharmacol., 2009, 78(1), 1-10. [http://dx.doi.org/10.1016/j.bcp.2009.01.018] [PMID: 19447218] |
| [6] | Silva, M.C.C.; da Silva, A.B.; Texeira, F.M.; de Sousa, P.C.P.; Rondon, R.M.M.; Honorio, J.E.R., Junior; Sampaio, L.R.L.; Oliveira, S.L.; Holonda, A.N.M.; de Vasconcelos, S.M.M. Therapeutic and biological activities of Calotropis procera (Ait.) R. Br. Asian Pac. J. Trop. Med., 2010, 332-336. [http://dx.doi.org/10.1016/S1995-7645(10)60081-8] |
| [7] | Himanshu, J.; Gururaja, M.P.; Divya, S. Calotropis gigantea R. Br. (Asclepiadaceae). Int. J. Pharm. Res., 2011, 3, 10-14. |
| [8] | Quazi, S.; Mathur, K.; Arora, S. Calotropis procera: an overview of its phytochemistry and pharmacology. Indian J. Drugs, 2013, 1, 63-69. [IJOD]. |
| [9] | Yogi, B.; Gupta, S.K.; Mishra, A. Calotropis procera (Madar): a medicinal plant of various therapeutic uses – a review. Bull. Env. Pharmacol. Life Sci., 2016, 5, 74-81. |
| [10] | Kumar, S.P.; Suresh, E.; Kalavathy, S. Review on a potential herb Calotropis gigantea (L.) R. Br. Sch. Acad. J. Pharm., 2013, 2, 135-143. |
| [11] | Kadiyala, M.; Ponnusankar, S.; Elango, K. Calotropis gigantiea (L.) R. Br (Apocynaceae): a phytochemical and pharmacological review. J. Ethnopharmacol., 2013, 150(1), 32-50. [http://dx.doi.org/10.1016/j.jep.2013.08.045] [PMID: 24012528] |
| [12] | Space, J.C.; Lorence, D.H.; LaRosa, A.M. Report to the Republic of Palau: 2008 update on Invasive Plant Species., 2009, , 18. |
| [13] | Hancock, I.R.; Henderson, C.P. Flora of the Solomon Islands.Research Bulletin No. 7, Ministry of Agriculture and Lands, Honiara, 1988, 54. |
| [14] | Howard, R.A. Flora of the Lesser Antilles, Leeward and Windward Islands, 1989, 6 |
| [15] | Krings, A.; Areces Berazain, F.; Lazcano Lara, J.C. New and re-discovered milkweeds from Cuba: Calotropis gigantea and Gonolobus stephanotrichus. Willdenowia, 2005, 35, 315-318. [http://dx.doi.org/10.3372/wi.35.35213] |
| [16] | Cavalcante, A.; Major, I. Invasion of alien plants in the Caatinga biome. Ambio, 2006, 35(3), 141-143. [http://dx.doi.org/10.1579/0044-7447(2006)35[141:IOAPIT]2.0.CO;2] [PMID: 16846205] |
| [17] | Gracia C, A.; Rangel-Buitrago, N.; Castro-Barros, J-D. Non-native plant species in the Atlantico Department Coastal Dune Systems, Caribbean of Colombia: A new management challenge. Mar. Pollut. Bull., 2019, 141, 603-610. [http://dx.doi.org/10.1016/j.marpolbul.2019.03.009] [PMID: 30955773] |
| [18] | Ibrahim, A.H. Tolerance and avoidance responses to salinity and water stresses in Calotropis procera and Suaeda aegyptiaca. Turk. J. Agric. For., 2013, 37, 352-360. |
| [19] | Al-Zahrani, H.S. Effects on salinity stress on growth of Calotropis procera seedlings. Bull. Pure Appl. Sci., 2002, 21, 109-122. |
| [20] | Karschon, R. Contributions to the arboreal flora of Israel: Calotropis procera (Willd.) R.Br La-Yaaron, 1970, 20, 1-6,41-48. (Hebrew and English). |
| [21] | Boutraa, T. Effects of water stress on growth, water use efficiency, leaf area and chlorophyll content in the desert shrub Calotropis procera. J. Int. Environ. Appl. Sci., 2010, 5, 124-132. |
| [22] | Ksiksi, T.S. Acacia tortilis and Calotropis procera: do they substantially promote soil carbon sequestration? Open J. Soil Sci., 2012, 2, 116-122. [http://dx.doi.org/10.4236/ojss.2012.22017] |
| [23] | Migongo-Bake, E.; Elskamp, F.; Mwangi, A.; Cherogony, L. Success stories in the struggle against desertification – a holistic and integrated approach to environmental conservation and sustainable livelihoods., 2002, |
| [24] | Parrotta, J.A. Healing plants of Peninsular India., 2001, [http://dx.doi.org/10.1079/9780851995014.0000] |
| [25] | Sharma, G.P.; Kumar, M.; Raghubanshi, A.S. Urbanization and road-use determines Calotropis procera distribution in the eastern Indo-Gangetic plain, India. Ambio, 2010, 39(2), 194-197. [http://dx.doi.org/10.1007/s13280-010-0026-3] [PMID: 20653282] |
| [26] | Neumann, K. Zur Vegetationsgeschichte der Ostsahara im Holozaen; Holzkohlen aus praehistorischen Fundstellen.Forschungen zur Umweltgeschichte der Ostsahara., 1989, , 13-182. |
| [27] | Little, E.L., Jr; Woodbury, R.O.; Wadsworth, F.H. Trees of Puerto Rico and the Virgin Islands, 1974, 2, 1024. |
| [28] | Forster, P.I.; Little, D.J.; Nicholas, A. Asclepiadaceae, Flora of Australia., 1996, Vol. 8, 214. |
| [29] | Jagtap, V.A.; Usman, M.R.M.; Salunkhe, P.S.; Gagrani, M.B. Anti-inflammatory activity of Calotropis gigantea Linn. leaves extract on in-vitro models Int. J. Curr. Pharm. Rev. Res, 2010, 1-5. |
| [30] | Dymock, W. A history of the principal drugs of vegetable originPharmacographia Indica, 1972, , 15. |
| [31] | Bertreau, A.; Calotropis, Les. Bibliothèque d’Agriculture coloniale, 1913, , 1-87. |
| [32] | Lewin, L. Calotropis procera, ein neues digitalisartig wirkendes Herzmittel Arch. Exp. Path. Pharmakol., 1913, 71, 142-156. [http://dx.doi.org/10.1007/BF01865395] |
| [33] | Neuwinger, H.D. Afrikanische Arzneipflanzen und Jagdgifte, Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart 1994, 224. |
| [34] | Newton, C.; Midant-Reynes, B. Environmental change and settlement shifts in Upper Egypt during the Predynastic: charcoal analysis at Adaima. Holocene, 2007, 17, 1109-1118. [http://dx.doi.org/10.1177/0959683607082551] |
| [35] | Bard, K.A.; Fattovich, R.; Borojevic, K.; Berna, F.; Zazzaro, C. Harbor of Pharaohs to the Land of Punt (Marsa/Wadi Gawasis Report)., 2008, , 22-38. |
| [36] | Maroyi, A. In Medicinal Plants 2Plant Resources of Tropical Africa, 2013, 11, 31. |
| [37] | Belgiorno, M.R.; Lentini, A.; Scala, G. The textile industry (2000 BC) of Pyrgos-Mavroraki (Cyprus). Archaelogical Textiles Newsletter, 2006, 25, 30. |
| [38] | Potts, D.T.; Reade, W.J. New evidence for late third millennium linen from Tell Abraq, Umm-Al-Qaiwain, UAE. Paéorient, 1993, 19, 96-106. |
| [39] | Mikula, R. Butterfly plants for your garden, www.butterflybreeders.com/pages/bflygdning/butterflyplants.html2001. |
| [40] | Petrie, J.M.M. Arabian Desert Primer: Ornamental potential of hyperarid adapted plants from Saudi Arabia. Desert Plants, 2007, 23, 19-32. |
| [41] | Arya, S.K.; Agarwal, V.D. Antiquity of arka kalpna in Ayurvedic classics. Sacitra Ayurveda, 1985, 38, 477-480. |
| [42] | Ebbell, B. A contribution to the earliest history of leprosy. Int. J. Lepr., 1935, 3, 257-263. |
| [43] | Greiss, A.M.E. Anatomical identification of plant remains and other materials from (1) El-Omari excavation at Helwan from the first dynasty. Bull. Inst. Egypte, 1955, 36, 227-235. |
| [44] | Dieye, A.M.; Tidjani, M.A.; Diouf, A.; Bassene, E.; Faye, B. Senegalese pharmacopeia: study of acute toxicity and antitussive activity of C. procera (Ait.). Dakar Méd., 1993, 38, 69-72. [PMID: 7882852] |
| [45] | www.worldagroforestry.org/treedb2/AFTPDFS/Calotropis_procera.pdf |
| [46] | Nasser, R.A.; Al-Mefarrej, H.A.; Khan, P.R.; Alhafta, K.H. Technological Properties of Calotropis procera (Ait.) wood and its relation to utilization. Am.-Eurasian J. Agric. Environ. Sci., 2012, 12, 5-16. |
| [47] | Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Anthony, S. Agroforestree Database: a tree reference and selection guide version 4.0., 2009, www.worldagroforestry.org/resources/databases/agroforestree |
| [48] | Khristova, P.; Tissot, M. Soda-anthraquinone pulping of Hibiscus sabdariffa (Karkadeh) and Calotropis procera from Sudan. Bioresour. Technol., 1995, 53, 67-72. [http://dx.doi.org/10.1016/0960-8524(95)00067-O] |
| [49] | Oun, A.A.; Rhim, J.W. Characterization of nanocelluloses isolated from Ushar (Calotropis procera) seed fiber: effect of isolation method. Mater. Lett., 2016, 168, 146-150. [http://dx.doi.org/10.1016/j.matlet.2016.01.052] |
| [50] | Batello, C.; Marzot, M.; Toure, A.H. The future is an ancient lake: traditional knowledge, biodiversity and genetic resources for food and agriculture in Lake Chad Basin ecosystems. FAO Interdepartmental Working Group on biological diversity for food and agriculture, Rome, 2004.www.fao.org/docrep/010/y5118e/y5118e00.htm |
| [51] | Ali, M.E-S.; Zeitoun, O.M. Discovering and manufacturing a new natural insulating material extracted from a plant growing up in Saudi Arabia. J. Eng. Fibers Fabrics, 2012, 7, 88-94. [http://dx.doi.org/10.1177/155892501200700405] |
| [52] | Ali, M.E-S. US Pat. 20130193365 (Aug. 1, 2013), Chem. Abstr, 2013, 159, 294814.. Insulation material based on natural fibers from flowering plant seeds in a phenol-formaldehyde resin or cornstarch binder |
| [53] | Bamberger, J.J. GB398547 (March 18, 1933) Improvements in or relating to acoustic plaster, |
| [54] | Nourbakhsh, A.; Ashori, A.; Kouhpayehzadeh, M. Giant Milkweed (Calotropis persica) Fibers — A Potential Reinforcement Agent for Thermoplastics Composites. J. Reinf. Plast. Compos., 2009, 28, 2143-2149. [http://dx.doi.org/10.1177/0731684408091902] |
| [55] | Yoganandam, K.; Ganeshan, P. NagarajaGanesh, B.; Raja, K. Characterization studies on Calotropis procera fibers and their performance as reinforcements in epoxy matrix. J. Nat. Fibers, 2019. [http://dx.doi.org/10.1080/15440478.2019.1588831] |
| [56] | Liu, D. (Hunan Yunjin Group Co. Ltd.), CN 103031625 (Jan. 24, 2013) Apparatus for removing seeds from Calotropis gigantea fibre, |
| [57] | Zhao, T.; Wang, M.; Zhou, J. CN 103397508A (July 30, 2013), Low-carbon low damage bleaching method of Calotropis gigantea fiber fabric |
| [58] | Chen, Q.; Zhao, T.; Wang, M.; Wang, J. Studies of the fibre structure and dyeing properties of Calotropis gigantea, kapok, and cotton fibres., 2013, , 448-453. |
| [59] | Gao, J.; Zhao, T. Dyeing behavior of Calotropis gigantea fibre with direct dyes., 2012, , 33-35. |
| [60] | Ashori, A.; Bahreini, Z. Evaluation of C. gigantea as a promising raw material for fiber-reinforced composite. J. Compos. Mater., 2009, 43, 1297-1304. [http://dx.doi.org/10.1177/0021998308104526] |
| [61] | Velusamy, K.; Navaneethakrishnan, P.; Rajeshkumar, G.; Sathishkumar, T.P. The influenxe of fiber content and length on mechanical and water absorption properties of Calotropis gigantea fiber reinforced epoxy composites. J. Ind. Text., 2019, 48, 1274-1290. [http://dx.doi.org/10.1177/1528083718763778] |
| [62] | Vinod, A.; Vijay, R.; Singaravelu, D.; Lenin, D. Thermomechanical characterization of Calotropis gigantea stem powder-filled jute fiber-reinforced epoxy composites. J. Nat. Fibers, 2018, 15, 648-657. [http://dx.doi.org/10.1080/15440478.2017.1354740] |
| [63] | Ganeshan, P. NagarajaGanesh, B.; Ramshankar, P.; Raja, K. Calotropis gigantea fibers: A potential reinforcement for polymer matrices. Int. J. Polym. Anal. Charact., 2018, 23, 271-277. [http://dx.doi.org/10.1080/1023666X.2018.1439560] |
| [64] | Karthik, T.; Ganesan, P. Light-weight bio-composites from Calotropis gigantea stem fiber. Melliand International, 2012, 18, 214-215. |
| [65] | Babu, D.G.; Babu, S.K.; Kishore, N.P. Tensile and wear behavior of Calotropis gigantea fruit fiber reinforced polyester composites. Proc. Eng., 2014, 97, 531-535. [http://dx.doi.org/10.1016/j.proeng.2014.12.279] |
| [66] | Ramasamy, R.; Reddy, K.O.; Rajulu, A.V. Extraction and characterization of Calotropis gigantea bast fibers as novel reinforcement for composites materials. J. Nat. Fibers, 2018, 15, 527-538. [http://dx.doi.org/10.1080/15440478.2017.1349019] |
| [67] | Mohamed, N.H.; Ismail, M.A.; Abdel-Mageed, W.M.; Shoreit, A.A.M. Biodegradation of natural rubber latex of Calotropis procera by two endophytic fungal species. J. Bioremediat. Biodegrad., 2017, 8, 1-5. [http://dx.doi.org/10.4172/2155-6199.1000380] |
| [68] | Ramos, M.V.; Demarco, D.; da Costa Souza, I.C.; de Freitas, C.D.T. Lactifiers, latex, and their role in plant defense. Trends Plant Sci., 2019, 24(6), 553-567. [http://dx.doi.org/10.1016/j.tplants.2019.03.006] [PMID: 30979674] |
| [69] | Zheng, Y.; Zhu, Y.; Wang, A.; Hu, H. Potential of Calotropis gigantea fiber as an absorbent for removal of oil from water. Ind. Crops Prod., 2016, 83, 387-390. [http://dx.doi.org/10.1016/j.indcrop.2016.01.009] |
| [70] | Zheng, Y.; Cao, E.; Zhu, Y.; Wang, A.; Hu, H. Perfluorosilane treated Calotropis giganteafiber: Instant hydrophobic–oleophilic surface with efficient oil-absorbing performance. Chem. Eng. J., 2016, 295, 477-483. [http://dx.doi.org/10.1016/j.cej.2016.03.074] |
| [71] | Zheng, Y.; Cao, E.J.; Tu, L.X.; Wang, A.Q.; Hu, H.M. A comparative study for oil-absorbing performance of octadecyltrichlorosilane treated Calotropis gigantea fiber and kapok fiber. Cellulose, 2017, 24, 989-1000. [http://dx.doi.org/10.1007/s10570-016-1155-z] |
| [72] | Cao, E.; Xiao, W.; Duan, W.; Wang, N.; Wang, A.; Zheng, Y. Metallic nanoparticles roughened Calotropis gigantea fiber enables efficient absorption of oils and organic solvents. Ind. Crops Prod., 2018, 115, 275-279. [http://dx.doi.org/10.1016/j.indcrop.2018.02.052] |
| [73] | Thilagavathi, G.; Praba Karan, C.; Das, D. Oil sorption and retention capacities of thermally-bonded hybrid nonwovens prepared from cotton, kapok, milkweed and polypropylene fibers. J. Environ. Manage., 2018, 219, 340-349. [http://dx.doi.org/10.1016/j.jenvman.2018.04.107] [PMID: 29753978] |
| [74] | Tu, L.X.; Duan, W.Z.; Xiao, W.L.; Fu, C.X.; Wang, A.Q.; Zheng, Y. Calotropis gigantea fiber derived carbon fiber enable fast and efficient absorption of oils and organic solvents. Separ. Purif. Tech., 2018, 192, 30-35. [http://dx.doi.org/10.1016/j.seppur.2017.10.005] |
| [75] | Xiao, W.; Wang, N.; Niu, B.; Fu, C.; Zhou, L.; Zheng, Y. Polyethylene sulfone assisted shape construction of Calotropis gigantea fiber for preparing a sustainable and reusable oil sorbent. Cellulose, 2019, 26, 3923-3933. [http://dx.doi.org/10.1007/s10570-019-02356-6] |
| [76] | Cao, E.; Duan, W.; Yi, L.; Wang, A.; Zheng, Y. Poly(m-phenylenediamine) functionalized Calotropis gigantean fiber for coupled adsorption reduction for Cr(VI). J. Mol. Liq., 2017, 240, 225-232. [http://dx.doi.org/10.1016/j.molliq.2017.05.087] |
| [77] | Cao, E.; Duan, W.; Wang, A.; Zheng, Y. Oriented growth of poly(m-phenylenediamine) on Calotropis gigantea fiber for rapid adsorption of ciprofloxacin. Chemosphere, 2017, 171, 223-230. [http://dx.doi.org/10.1016/j.chemosphere.2016.12.087] [PMID: 28024207] |
| [78] | Yi, L.S.; Liang, G.W.; Xiao, W.L.; Duan, W.Z.; Wang, A.Q.; Zheng, Y. Rapid nitrogen-rich modification of Calotropis gigantea fiber for highly efficient removal fluoroquinolone antibiotics. J. Mol. Liq., 2018, 256, 408-415. [http://dx.doi.org/10.1016/j.molliq.2018.02.060] |
| [79] | Kaur, R.; Kaur, H. Calotropis procera as effective adsorbent for removal of malachite green dye: a comprehensive study. Desalination Water Treat., 2017, 78, 252-262. |
| [80] | Kaur, R.; Kaur, H. Calotropis procera an effective adsorbent for removal of Congo red dye: isotherm and kinetc modelling. Model. Earth Syst. Environ., 2017, 3 [http://dx.doi.org/10.1007/s40808-017-0274-3] |
| [81] | Varshney, A.C.; Bhoi, K.L. Cloth from bast fiber of the Calotropis procera (Aak) plant. Biol. Wastes, 1988, 26, 229-232. [http://dx.doi.org/10.1016/0269-7483(88)90168-1] |
| [82] | Ganaba, S.; Ouadba, J.M.; Bognounou, O. Fuelwood in the Sahelian region of Burkina Faso: Ethnic preferences. Sécheresse, 1998, 9, 261-268. |
| [83] | Padmaja, K.V.; Atheya, N.; Bhatnagar, A.K.; Singh, K.K. Conversion of Calotropis procera biocrude to liquid fuels using thermal and catalytic cracking. Fuel, 2009, 88, 780-785. [http://dx.doi.org/10.1016/j.fuel.2008.11.020] |
| [84] | Kalita, D.; Saikia, C.N. Chemical constituents and energy content of some latex bearing plants. Bioresour. Technol., 2004, 92(3), 219-227. [http://dx.doi.org/10.1016/j.biortech.2003.10.004] [PMID: 14766154] |
| [85] | Erdman, M.D.; Erdman, B.A. Calotropis procera as an alternative source of plant hydrocarbons. Econ. Bot., 1981, 35, 467-472. [http://dx.doi.org/10.1007/BF02858597] |
| [86] | Radhaboy, G.; Pugazhvadivu, M.; Ganeshan, P.; Ramshankar, P. Analysis of Thermochemical behaviour of Calotropis procera parts for their potentiality International Journal of Ambient Energy, 2019. [http://dx.doi.org/10.1080/01430750.2019.1630309.] |
| [87] | De, S.; Bag, A.; Mukherji, S. Potential use of Pedilanthus tithymaloides Poit. as a renewable resource of plant hydrocarbons. Bot. Bull. Acad. Sin., 1997, 38, 105-108. |
| [88] | Razon, L.F. Philippine plant oils as feedstock for Biodiesel. Philipp. Agric. Sci., 2008, 91, 278-286. |
| [89] | Sundar Rao, K.; Pantulu, A.J.; Lakshminarayana, G. Analysis of C. gigantea, Acia caesia, and Abelmoschus ficulneus seeds. J. Am. Oil Chem. Soc., 1983, 60, 1259-1261. [http://dx.doi.org/10.1007/BF02702095] |
| [90] | Barbosa, M.O.; de Almeida-Cortez, J.S.; da Silva, S.I.; de Oliveira, A.F.M. Seed oil content and fatty acid composition from different populations of Calotropis procera (Aiton) W. T. Aiton (Apocynaceae). J. Am. Oil Chem. Soc., 2014, 91, 1433-1441. [http://dx.doi.org/10.1007/s11746-014-2475-5] |
| [91] | Phoo, Z.W.M.M.; Razon, L.F.; Knothe, G.; Ilham, Z.; Goembira, F.; Madrazo, C.F.; Roces, S.A.; Saka, S. Evaluation of Indian milkweed (Calotropis gigantea) seed oil as alternative feedstock for biodiesel. Ind. Crops Prod., 2014, 54, 226-232. [http://dx.doi.org/10.1016/j.indcrop.2014.01.029] |
| [92] | de Sousa, L.V.; Santos, A.P.B.; di Souza, L.; Dias Santos, A.G.; Beatriz, A. Evaluation of the properties of Calotropis procera oil aiming the production of Biodiesel, Orbital: The Electronic. J. Chem., 2018, 10, 147-152. |
| [93] | Traore, A.S. Biogas production from C. procera: a latex plant found in West Africa. Bioresour. Technol., 1992, 41, 105-109. [http://dx.doi.org/10.1016/0960-8524(92)90178-Z] |
| [94] | Gourdon, R.; Leger, P.; Vermande, P. Methane recovery by anaerobic digestion of cellulosic material available in Sahel. Biol. Wastes, 1989, 30, 181-187. [http://dx.doi.org/10.1016/0269-7483(89)90120-1] |
| [95] | Manikandan, M.; Arumugam, R. Potentiality of Calotropis procera on the yield of biocrudes and biogas production. J. Phytol., 2010, 2, 33-40. |
| [96] | Shilpkar, P.; Shah, M.; Chaudhry, D.R. An alternate use of Calotropis gigantea: biomethanation. Curr. Sci. India, 2007, 92, 435-437. |
| [97] | Erdman, M.D. Nutrient and cardenolide composition of unextracted and solvent-extracted Calotropis procera. J. Agric. Food Chem., 1983, 31(3), 509-513. [http://dx.doi.org/10.1021/jf00117a012] [PMID: 6886206] |
| [98] | Carruthers, I.B.; Griffiths, D.J.; Home, V.; Williams, I.R. Hydrocarbons from Calotropis procera in Northern Australia. Biomass, 1984, 4, 275-282. [http://dx.doi.org/10.1016/0144-4565(84)90040-4] |
| [99] | Kalita, D. Hydrocarbon plant – new source of energy for the future. Renew. Sustain. Energy Rev., 2008, 12, 455-471. [http://dx.doi.org/10.1016/j.rser.2006.07.008] |
| [100] | Zhao, B.; Sun, J.; Wang, X. (Institute of Process Engineering, Chinese Academy of Sciences) CN 202586271 (Jan 11, 2012) Method of generating high-alkane content in Calotropis gigantea through genetic transformation |
| [101] | Ahmaruzzaman, M.; Sharma, D.K. Coprocessing of petroleum vacuum residue with plastics, coal, and biomass and its synergistic effects. Energy Fuels, 2007, 21, 891-897. [http://dx.doi.org/10.1021/ef060102w] |
| [102] | Ahmaruzzaman, M.; Sharma, D.K. Characterization of liquid products from the co-cracking of ternary and quaternary mixture of petroleum vacuum residue, polypropylene, Samla coal and Calotropis procera. Fuel, 2008, 87, 1967-1973. [http://dx.doi.org/10.1016/j.fuel.2008.01.007] |
| [103] | Mahmoud, O.M.; Adam, S.E.I.; Tartour, G. The effects of Calotropis procera on small ruminants. I. Effects of feeding sheep with the plant. J. Comp. Pathol., 1979, 89(2), 241-250. [http://dx.doi.org/10.1016/0021-9975(79)90063-X] [PMID: 457943] |
| [104] | Mahmoud, O.M.; Adam, S.E.I.; Tartour, G. The effects of Calotropis procera on small ruminants. II. Effects of administration of the latex to sheep and goats. J. Comp. Pathol., 1979, 89(2), 251-263. [http://dx.doi.org/10.1016/0021-9975(79)90064-1] [PMID: 457944] |
| [105] | el Badwi, ; Samia, M.A.; Adam, S.E.; Shigidi, M.T.; Hapke, H.J. Studies on laticiferous plants: toxic effects in goats of Calotropis procera latex given by different routes of administration. Dtsch. Tierarztl. Wochenschr., 1998, 105(11), 425-427. [PMID: 9857566] |
| [106] | Sharma, G.K. Calotropis procera and Calotropis gigantea. Indian J. Veterinary Sci., 1934, 4, 63-74. |
| [107] | Heuzé, V.; Tran, G.; Baumont, R.; Bastianelli, D. Calotropis (Calotropis procera) Feedipedia, 2016.http://www.feedipedia.org/node/588 |
| [108] | Abdullah, M.; Rafay, M.; Hussain, T.; Ahmad, H.; Tahir, U.; Rasheed, F.; Ruby, T.; Khalil, S. Nutritive potential and palatability preference of browse foliage by livestock in arid rangelands of Cholistan desert (Pakistan). J. Anim. Plant Sci., 2017, 27, 1656-1664. |
| [109] | Radunz, B.L.; Wilson, G.; Beere, G. Feeding rubberbush (Calotropis procera) to cattle and sheep. Aust. Vet. J., 1984, 61(7), 243-244. [http://dx.doi.org/10.1111/j.1751-0813.1984.tb06006.x] [PMID: 6497815] |
| [110] | Lottermoser, B.G. Colonisation of the rehabilitated Mary Kathleen uranium mine site (Australia) by Calotropis procera: toxicity risk to grazing animals. J. Geochem. Explor., 2011, 111, 39-46. [http://dx.doi.org/10.1016/j.gexplo.2011.07.005] |
| [111] | dos Santos Belém, C.; de Souza, A.M.; de Lima, P.R.; de Carvalho, F.A.L.; Queiroz, M.A.A.; da Costa, M.M. Digestibility, fermentation and microbiological characteristics of Calotropis procera silage with different quantities of grape pomace. Cienc. Agrotec., 2016, 40, 698-705. [http://dx.doi.org/10.1590/1413-70542016406020916] |
| [112] | Mello, M.M.; Vaz, F.A.; Gonҫalves, L.C.; Saturnino, H.M. Estudo fitoquímíco da Calotropis procera Ait., sua utilizaҫão na alimentaҫão de caprinos: Efitos clínicos e bioquímicos séericoos. Belo Horizonte, MG. Rev. Bras. Saúda Prod. An, 2001, 2, 15-20. |
| [113] | Costa, R.G.; de Medeiros, A.N.; Alves, A.R.; de Medeiros, G.R. Prospects for use of rooster tree (Calotropis procera) in animal production. Rev. Caatinga, 2009, 22, 1-9. |
| [114] | Madruga, M.S.; Arruda, S.G.B.; Narain, N.; Souza, J.G. Castration and slaughter age effects on panel assessment and aroma compounds of the “mestiço” goat meat. Meat Sci., 2000, 56(2), 117-125. [http://dx.doi.org/10.1016/S0309-1740(00)00025-5] [PMID: 22061898] |
| [115] | Nehra, O.P.; Oswal, M.C.; Faroda, A.S. Management of fodder tree in Haryana. Indian Farming, 1987, 37, 31-33. |
| [116] | Torres, J.F.; Braga, A.P.; Lima, G.F.C.; Rangel, A.H.; de Lima Júnior, D.M.; do Vale Maciel, M.; Oliveira, S.E.O. Utilizaҫão do feno de flor-de-seda (Calotropis procera ait. r. br.) na alimentaҫão de ovinos. Acta Vet. Bras., 2010, 4, 42-50. |
| [117] | Costa, R.G.; da Silva, N.V.; de Azevedo, P.S.; de Medeiros, A.N.; de Carvalho, F.F.R.; Queiroga, R.C.R.E.; de Medeiros, G.R. Meat quality of lambs fed silk flower hay (Calotropis procera SW.) in their diet. Rev. Bras. Zootec., 2011, 40, 1266-1271. [http://dx.doi.org/10.1590/S1516-35982011000600015] |
| [118] | Pereira, G.F.; Gracindo, A.P.A.C.; Tinoco, A.F.D.; de Oliveria, P.H.M.; Rangel, A.H.D. Fatty acid profile of milk of goats fed with growing levels of flor-de-seda hay. Rev. Caatinga, 2009, 22, 206-210. |
| [119] | Uphof, J.C.Th. Dictionary of Economic Plants., 1959, |
| [120] | Bengal: Report of the Chemical Examiner for 1940 The British Pharmaceutical Codex, 1934, . |
| [121] | Al-Zahrani, H.S.; Al-Robai, S.A. Allelopathic effect of Calotropis procera leaves extract on seed germination of some plants. JKAU: Sci., 2007, 19, 115-126. [http://dx.doi.org/10.4197/Sci.19-1.9] |
| [122] | Larhsini, M.; Lazrek, H.B.; Bousaid, M.; Jana, M.; Amarouch, H. Evaluation of antifungal and molluscicidal properties of extracts of Calotropis procera. Fitoterapia, 1997, 68, 371-373. |
| [123] | Ahmed, U.A.M.; Shi, Z.; Bashier, N.H.H.; Muafi, K.; Hao, Z.; Guo, Y. Evaluation of insecticidal potentials of aqueous extracts of C. procera Ait. against Henosepilachna elaterii Rossi. J. Appl. Sci. (Faisalabad), 2006, 6, 2466-2470. [http://dx.doi.org/10.3923/jas.2006.2466.2470] |
| [124] | Jacob, S.; Sheila, M.K. A note on the protection of stored rice from the lesser grain borer Rhizoperta dominica Fabr. By indigenous plant products. Indian J. Entomol., 1993, 55, 337-339. |
| [125] | Khan, S.M.; Siddiqui, M.N. Potential of some indigenous plants as pesticides against larvae of cabbage butterfly Pieris brassicae L. Sarhad J. Agric., 1994, 10, 291-301. |
| [126] | Moursy, L.E. Insecticidal activity of Calotropis procera extracts of the flesh fly, Sarcophaga haemorrhoidalis fallen. J. Egypt. Soc. Parasitol., 1997, 27(2), 505-514. [PMID: 9257990] |
| [127] | Girdhar, G.; Deval, K.; Mittal, P.K.; Vasudevan, P. Mosquito control by Calotropis latex. Pesticides, 1984, 18, 26-29. |
| [128] | Barati, R.; Golmohammadi, G.; Ghajarie, H.; Zarabi, M.; Mansouri, R. Efficiency of some herbal pesticides on reproductive parameters of silverleaf whitefly, Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Arch. Phytopathol. Pflanzenschutz, 2014, 47, 212-221. [http://dx.doi.org/10.1080/03235408.2013.807035] |
| [129] | Rakesh, P.; Alok, K.; Neetu, K. Nematicidal activity in flowers of some medicinal and aromatic plants. Indian J. Entomol., 2001, 31, 96-98. |
| [130] | Anver, S.; Alam, M.M. Effect of latex seed dressing on interacting root-knot and reniform nematodes. Afro-Asian J. Nematol., 1992, 2, 17-20. |
| [131] | Charu, J.; Trivedi, P.C. Nematicidal activity of certain plants against root-knot nematode, Meloidogyne incognita, infecting chickpea, Cicer arietinum. Ann. Plant Prot. Sci., 1997, 5, 171-174. |
| [132] | Maqbool, M.A.; Hashmi, S.; Ghaffar, A. Effect of latex extracts from Euphorbia caducifolia and Calotropis procera on root-knot nematode Meloidogyne incognita infesting tomato and egg plant. Pak. J. Nematol., 1987, 43-47. |
| [133] | Verma, B.S.; Verma, K.K. Toxicity of some indigenous plant extracts to root-knot, seed gall and citrus nematodes. Pesticides, 1989, 23, 25-27. |
| [134] | Chungsamarnyart, N.; Ratanakreetakul, C.; Jansawan, W. Acaricidal activity of the combination of plant crude extracts to cattle ticks. Kasetsart J. Natl. Sci., 1994, 28, 649-660. |
| [135] | Hussein, H.I.; Kamel, A.; Abou-Zeid, M.; Abdel-Khalek, ; El-Sebae, H.; Saleh, M.A. Uscharin, the most potent molluscicidal compound tested against land snails. J. Chem. Ecol., 1994, 20(1), 135-140. [http://dx.doi.org/10.1007/BF02065996] [PMID: 24241704] |
| [136] | Al-Sarar, A.; Hussein, H.; Abobakr, Y.; Bayoumi, A. Molluscicidal activity of methomyl and cardenolide extracts from Calotropis procera and Adenium arabicum against the land snail Monacha cantiana. Molecules, 2012, 17(5), 5310-5318. [http://dx.doi.org/10.3390/molecules17055310] [PMID: 22565481] |
| [137] | Zaman, M.A.; Iqbal, Z.; Abbas, R.Z.; Khan, M.N.; Muhammad, G.; Younus, M.; Ahmed, S. In vitro and in vivo acaricidal activity of a herbal extract. Vet. Parasitol., 2012, 186(3-4), 431-436. [http://dx.doi.org/10.1016/j.vetpar.2011.11.018] [PMID: 22305296] |
| [138] | Konno, K. Plant latex and other exudates as plant defense systems: roles of various defense chemicals and proteins contained therein. Phytochemistry, 2011, 72(13), 1510-1530. [http://dx.doi.org/10.1016/j.phytochem.2011.02.016] [PMID: 21450319] |
| [139] | Ramos, M.V.; Bandeira, Gde.P.; de Freitas, C.D.; Nogueira, N.A.P.; Alencar, N.M.N.; de Sousa, P.A.; Carvalho, A.F.U. Latex constituents from Calotropis procera (R. Br.) display toxicity upon egg hatching and larvae of Aedes aegypti (Linn.). Mem. Inst. Oswaldo Cruz, 2006, 101(5), 503-510. [http://dx.doi.org/10.1590/S0074-02762006000500004] [PMID: 17072453] |
| [140] | Singhi, M.; Joshi, V.; Sharma, R.C.; Sharma, K. Ovipositioning behavior of Aedes aegypti in different concentrations of latex of Calotropis procera: studies on refractory behavior and its sustenance across genotrophic cycles. Dengue Bull., 2004, 28, 184-188. |
| [141] | Markouk, M.; Bekkouche, K.; Larhsini, M.; Bousaid, M.; Lazrek, H.B.; Jana, M. Evaluation of some Moroccan medicinal plant extracts for larvicidal activity. J. Ethnopharmacol., 2000, 73(1-2), 293-297. [http://dx.doi.org/10.1016/S0378-8741(00)00257-9] [PMID: 11025168] |
| [142] | Shahi, M.; Hanafi-Bojd, A.A.; Iranshahi, M.; Vatandoost, H.; Hanafi-Bojd, M.Y. Larvicidal efficacy of latex and extract of Calotropis procera (Gentianales: Asclepiadaceae) against Culex quinquefasciatus and Anopheles stephensi (Diptera: Culicidae). J. Vector Borne Dis., 2010, 47(3), 185-188. [PMID: 20834091] |
| [143] | Tahir, H.M.; Ishaq, T.; Mukhtar, M.K.; Khan, S.Y.; Ahmed, L. Potential use of Calotropis procera (milk weed) to control Culex quinquefasciatus (Diptera: culicidae). Pak. J. Zool., 2013, 45, 615-621. |
| [144] | Elimam, A.M.; Elmalik, K.H.; Ali, F.S. Efficacy of leaves extract of Calotropis procera Ait. (Asclepiadaceae) in controlling Anopheles arabiensis and Culex quinquefasciatus mosquitoes. Saudi J. Biol. Sci., 2009, 16(2), 95-100. [http://dx.doi.org/10.1016/j.sjbs.2009.10.007] [PMID: 23961048] |
| [145] | Rahuman, A.A.; Bagavan, A.; Kamaraj, C.; Saravanan, E.; Zahir, A.A.; Elango, G. Efficacy of larvicidal botanical extracts against Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol. Res., 2009, 104(6), 1365-1372. [http://dx.doi.org/10.1007/s00436-009-1337-9] [PMID: 19198882] |
| [146] | Singhi, M.; Purohit, A.; Chattopadhyay, S. Effectiveness and feasibility of methanol extracted latex of Calotropis procera as larvicide against dengue vectors of western Rajasthan, India. J. Vector Borne Dis., 2015, 52(2), 142-146. [PMID: 26119546] |
| [147] | Kovendan, K.; Murugan, K.; Prasanna Kumar, K.; Panneerselvam, C.; Mahesh Kumar, P.; Amerasan, D.; Subramaniam, J.; Vincent, S. Mosquitocidal properties of Calotropis gigantea (Family: Asclepiadaceae) leaf extract and bacterial insecticide, Bacillus thuringiensis, against the mosquito vectors. Parasitol. Res., 2012, 111(2), 531-544. [http://dx.doi.org/10.1007/s00436-012-2865-2] [PMID: 22382205] |
| [148] | Abbassi, K.; Kadiri, Z.A.; Ghaout, S. Biological activity of Calotropis procera (Ait. R. Br) leaves on the desert locust (Schistocerca gregaria, Forsk. 1775) Zoologica baetica, 2004, 15, 153–166. |
| [149] | Al Robai, A.A. Toxicological studies on the latex of the uscher plant C. procera (Ait) in Saudi Arabia. IV. Effects of partly purified uscher latex and of the poison gland secretion of the uscherhopper; Poekilocerus bufonius Klug on the desert locust, Schistocerca gregaria Forskal. (Orthoptera: Acrididae). Arab Gulf J. Sci. Res., 1997, 15, 709-716. |
| [150] | Pari, K.; Rao, P.J.; Devakumar, C.; Rastogi, J.N. A novel insect antifeedant nonprotein amino acid from Calotropis gigantea. J. Nat. Prod., 1998, 61(1), 102-104. [http://dx.doi.org/10.1021/np970255z] [PMID: 9548837] |
| [151] | Suparpprom, C.; Vilaivan, T. Synthesis of 2-[4′-(ethylcarbamoyl)phenyl]-N-acetylglycine, the proposed structure for giganticine. J. Nat. Prod., 2001, 64(8), 1114-1116. [http://dx.doi.org/10.1021/np010046l] [PMID: 11520243] |
| [152] | Rashmi, S. K.P.; Arya, S. Phytochemical profile and evaluation of insecticidal efficacy of Calotropis procera against defoliators. J. Med. Plants Res., 2011, 5, 6738-6743. [http://dx.doi.org/10.5897/JMPR11.774] |
| [153] | Chandra, H.; Lal, P. Food preference studies of “AK” grasshopper Poekilocerus pictus Fab. Plant Protect. Bull. Faridabad, 1993, 45, 3-10. |
| [154] | Amin, M.R.; Shahjahan, M.; El-Taj, H.F.; Iqbal, T.M.T.; Hussain, M.A. Use of akanda, biskatali, and neem leaves as botanical insecticides against lesser grain borer. Bangladesh J. Entomol., 2000, 10, 1-13. |
| [155] | Neenah, G. Potential of using flavonoids, latex and extracts from Calotropis procera (Ait.) as grain protectants against two coleopteran pests of stored rice. Ind. Crops Prod., 2013, 45, 327-334. [http://dx.doi.org/10.1016/j.indcrop.2012.12.043] |
| [156] | Reihaneh, B.; Golamrezah, G.; Hamid, G.; Mehdi, Z.; Raziyeh, M. The effects of some botanical insecticides and pymetrozine on life table parameters of silver leaf whitefly Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae). Pestic. Phytomed., 2013, 28, 47-55. [http://dx.doi.org/10.2298/PIF1301047B] |
| [157] | Alam, P.; Ali, M. Phytochemical investigation of C. procera Ait. roots. Indian J. Chem., 2009, 48B, 443-446. |
| [158] | Abbasi, A.B.; Bibi, R.; Khan, A.A.; Iqbal, M.S.; Sherani, J.; Khan, A.M. Assessment of Calotropis procera Aiton and Datura alba Nees leaves extracts as bio-insecticides against Tribolium castaneum Herbst in stored wheat Triticium aetivum L., J. Biofertil. Biopest., 2012, 3, 126-129. |
| [159] | Jahan, S.; Maman, A.; Khan, A.R. Insecticidal effect of akanda (Calotropis procera) on Tribolium confusum Duval (Coleoptera: Tenebrionidae). Bangladesh J. Zool., 1991, 19, 261-268. |
| [160] | Nithya, V.; Kamalam, M.; Umakanthan, T. Screening of indigenous medicinal plants for their acaricidal activity against catlle ticks under in vivo condition. Int. J. Pharm. Sci. Res., 2015, 6, 3049-3052. |
| [161] | Shyma, K.P.; Gupta, J.P.; Ghosh, S.; Patel, K.K.; Singh, V. Acaricidal effect of herbal extracts against cattle tick Rhipicephalus (Boophilus) microplus using in vitro studies. Parasitol. Res., 2014, 113(5), 1919-1926. [http://dx.doi.org/10.1007/s00436-014-3839-3] [PMID: 24633906] |
| [162] | Khan, A.; Nasreen, N.; Niaz, S.; Ayaz, S.; Naeem, H.; Muhammad, I.; Said, F.; Mitchell, R.D., III; de León, A.A.P.; Gupta, S.; Kumar, S. Acaricidal efficacy of Calotropis procera (Asclepiadaceae) and Taraxacum officinale (Asteraceae) against Rhipicephalus microplus from Mardan, Pakistan. Exp. Appl. Acarol., 2019, 78(4), 595-608. [http://dx.doi.org/10.1007/s10493-019-00406-z] [PMID: 31367977] |
| [163] | Al-Rajhy, D.H.; Alahmed, A.M.; Hussein, H.I.; Kheir, S.M. Acaricidal effects of cardiac glycosides, azadirachtin and neem oil against the camel tick, Hyalomma dromedarii (Acari: Ixodidae). Pest Manag. Sci., 2003, 59(11), 1250-1254. [http://dx.doi.org/10.1002/ps.748] [PMID: 14620053] |
| [164] | Pereira, D.A.; Ramos, M.V.; Souza, D.P.; Portela, T.C.L.; Guimarȁes, J.A.; Madeira, S.V.F.; Texeira de Freitas, C.D. Digestibility of defense proteins in latex of milkweeds by digestive proteases of Monarch butterflies, Danaus plexippus L.: a potential determinant of plant-herbivore interactions. Plant Sci., 2010, 179, 348-355. [http://dx.doi.org/10.1016/j.plantsci.2010.06.009] |
| [165] | Al Dhafer, H.M.; Aldryhim, Y.N.; Elgharbawy, A.A. Aspects of the life-history of Semitococcus johannes (Staudinger) (Lepidoptera: Cossidae) feeding on the milkweed Calotropis procera (Aiton) W.T., Aiton in the kingdom of Saudi Arabia. J. Kans. Entomol. Soc., 2013, 86, 133-144. [http://dx.doi.org/10.2317/JKES120824.1] |
| [166] | Hannan, M.A.; Alqarni, A.S.; Owayss, A.A.; Engel, M.S. The large carpenter bees of central Saudi Arabia, with notes on the biology of Xylocopa sulcatipes Maa (Hymenoptera, Apidae, Xylocopinae). ZooKeys, 2012, 201(201), 1-14. [PMID: 22768000] |
| [167] | Willmer, P.G. The role of insect water balance in pollination ecology: Xylocopa and Calotropis. Oecologia, 1988, 76(3), 430-438. [http://dx.doi.org/10.1007/BF00377039] [PMID: 28312024] |
| [168] | Brower, L.P. Ecological chemistry. Sci. Am., 1969, 220(2), 22-29. [http://dx.doi.org/10.1038/scientificamerican0269-22] [PMID: 5767170] |
| [169] | Parsons, J.A. A digitalis-like toxin in the monarch butterfly, Danaus plexippus L. J. Physiol., 1965, 178, 290-304. [http://dx.doi.org/10.1113/jphysiol.1965.sp007628] [PMID: 14298120] |
| [170] | Duffey, S.S.; Scudder, G.E.E. Cardiac glycosides in North American Asclepiadaceae, a basis of unpalatability in brightly colored Hemiptera and Coleoptera. J. Insect Physiol., 1972, 18, 63-78. [http://dx.doi.org/10.1016/0022-1910(72)90065-0] |
| [171] | Pugalenthi, P.; Livingstone, D. Susceptibility-linked population dynamics of insects associated with Calotropis gigantea (L) R. Br. (Asclepiadaceae) of the Maruthamalai scrub jungle, India. Ann. Entomol., 1993, 11, 39-42. |
| [172] | Saikia, H.C.; Das, B.K.; Kalita, J. Studies on the Insects associated with calotropis gigantea in Guwahati city of Assam, India. J. Zool. Stud., 2015, 2, 6-13. |
| [173] | Dhileepan, K. Prospects for the classical biological control of Calotropis procera (Apocynaceae) using coevolved insects. Biocontrol Sci. Technol., 2014, 24, 977-998. [http://dx.doi.org/10.1080/09583157.2014.912611] |
| [174] | Malcolm, S.B.; Cockrell, B.J.; Brower, L.P. Cardenolide fingerprint of monarch butterflies reared on common milkweed,Asclepias syriaca L. J. Chem. Ecol., 1989, 15(3), 819-853. [http://dx.doi.org/10.1007/BF01015180] [PMID: 24271887] |
| [175] | Martin, R.A.; Lynch, S.P.; Brower, L.P.; Malcolm, S.B.; Van Hook, T. Cardenolide content, emetic potency, and thin-layer chromatography profiles of monarch butterflies, Danaus plexippus, and their larval host-plant milkweed, Asclepias humistrata, in Florida. Chemoecol., 1992, 3, 1-13. [http://dx.doi.org/10.1007/BF01261450] |
| [176] | Martin, R.A.; Lynch, S.P. Cardenolide content and thin-layer chromatography profiles of monarch butterflies,Danaus plexippus L., and their larval host-plant milkweed,Asclepias asperula subsp.Capricornu (woods.) woods., in north central Texas. J. Chem. Ecol., 1988, 14(1), 295-318. [http://dx.doi.org/10.1007/BF01022548] [PMID: 24277011] |
| [177] | Livingstone, D.; Pugalenthi, P. Biology of Poecilocerus pictus (Orthoptera: Pyrgomorphidae) on the basis of its nutritional ecology. J. Entomol. Res., 1992, 16, 267-272. [New Dehli]. |
| [178] | Pugalenthi, P.; Livingstone, D. Cardenolides (heart poisons) in the painted grasshopper Poecilocerus pictus (Orthoptera: Pyrgomorphidae) feeding on the milkweed Calotropis gigantea L. (Asclepiadaceae). J. N.Y. Entomol. Soc., 1995, 103, 191-196. |
| [179] | Mathen, C.; Hardikar, B. Cytotoxic compounds from Poecilocerus pictus feeding on Calotropis gigantea. J. Exp. Ther. Oncol., 2010, 8(3), 177-185. [PMID: 20734917] |
| [180] | Mathen, C.; Peter, S.M.; Hardikar, B.P. Comparative evaluation of the cytotoxic and apoptotic potential of Poecilocerus pictus and Calotropis gigantea. J. Environ. Pathol. Toxicol. Oncol., 2011, 30(1), 83-92. [http://dx.doi.org/10.1615/JEnvironPatholToxicolOncol.v30.i1.80] [PMID: 21609318] |
| [181] | Brower, L.P.; van Brower, J.; Corvino, J.M. Plant poisons in a terrestrial food chain. Proc. Natl. Acad. Sci. USA, 1967, 57(4), 893-898. [http://dx.doi.org/10.1073/pnas.57.4.893] [PMID: 5231352] |
| [182] | Murugan, K.; Jeyabalan, D.; Kumar, N.S.; Nathan, S.S.; Sivaramakrishnan, S. Influence of host plant on growth and reproduction of Aphis nerii and feeding and prey utilization of its predator Menochilus sexmaculatus. Indian J. Exp. Biol., 2000, 38(6), 598-603. [PMID: 11116532] |
| [183] | Ahmad, R.; Shahab, M.Z.; Inam-ul-Haq, M.; Javed, N.; Dogar, M.A.; Khan, M.Y. Effect of soil amendment with Calotropis procera for the control of Meloidogyne javanica infection on egg plant. Pak. J. Nematol., 1996, 14, 55-59. |
| [184] | Khurma, U.R.; Chaudhary, P. Comparative effects of extracts of different parts of Calotropis procera, Cassia fistula, Ricinus communis, and Sesbania sesban on Meloidogyne javanica juveniles. J. Environ. Biol., 1999, 20, 287-288. |
| [185] | Parvatha, R.P.; Rao, M.S. Eco-friendly management of Meloidogyne incognita on tomato by integration of Verticillium chlamydosporium with neem and Calotropis leaves, Zeitschr. f. Pflanzenkrankheiten u. Pflanzenschutz, 1999, 106, 530-533. |
| [186] | Rehman, B.; Ahmad, F.; Babalola, O.O.; Ganai, M.A.; Parihar, K.; Siddiqui, M.A. Usages of botanical extracts for the management of the root-knot nematode, Meloidogyne incognita in chickpea. J. Pure Appl. Microbiol., 2013, 7, 2385-2388. |
| [187] | Elbadri, G.A.; Lee, D.W.; Park, J.C.; Yu, H.B.; Choo, H.Y. Evaluation of various plant extracts for their nematicidal efficacies against juveniles of Meloidogyne incognita. J. Asia Pac. Entomol., 2008, 11, 99-102. [http://dx.doi.org/10.1016/j.aspen.2008.04.004] |
| [188] | Devi, L.S.; Gupta, P. Evaluation of some plant lattices against Heterodera cajani in cowpea (Vigna sinensis). Natl. Acad. Sci. Lett., 2000, 23, 65-67. |
| [189] | Yousif, F.; Hifnawy, M.S.; Soliman, G.; Boulos, L.; Labib, T.; Mahmoud, S.; Ramzy, F.; Yousif, M.; Hassan, I.; Mahmoud, K.; El-Hallouty, S.M.; El-Gendy, M.; Gohar, L.; El-Manawaty, M.; Fayyad, W.; El-Menshawi, B.S. Large scale in vitro screening of Egyptian native and cultivated plants for schistosomicidal activity. Pharm. Biol., 2007, 45, 501-510. [http://dx.doi.org/10.1080/13880200701389425] |
| [190] | Iqbal, Z.; Lateef, M.; Jabbar, A.; Muhammad, G.; Khan, M.N. Anthelmintic activity of Calotropis procera (Ait.) Ait. F. flowers in sheep. J. Ethnopharmacol., 2005, 102(2), 256-261. [http://dx.doi.org/10.1016/j.jep.2005.06.022] [PMID: 16085379] |
| [191] | Al-Qarawi, A.A.; Mahmoud, O.M.; Sobaih, M.A.; Haroun, E.M.; Adam, S.E.I. A preliminary study on the anthelmintic activity of Calotropis procera latex against Haemonchus contortus infection in Najdi sheep. Vet. Res. Commun., 2001, 25(1), 61-70. [http://dx.doi.org/10.1023/A:1026762002947] [PMID: 11214673] |
| [192] | Shivkar, Y.M.; Kumar, V.L. Antihelmintic effect of latex of Calotropis procera. Pharm. Biol., 2003, 41, 263-265. [http://dx.doi.org/10.1076/phbi.41.4.263.15666] |
| [193] | Cavalcante, G.S.; de Morais, S.M.; Andre, W.P.P.; Ribeiro, W.L.C.; Rodrigues, A.L.M.; De Lira, F.C.; Viana, J.M.; Bevilaqua, C.M.L. Chemical composition and in vitro activity of Calotropis procera (Ait.) latex on Haemonchus contortus. Vet. Parasitol., 2016, 226, 22-25. [http://dx.doi.org/10.1016/j.vetpar.2016.06.012] [PMID: 27514877] |
| [194] | Lobo, P.P.G.; Llagas, F.D. Evaluation of starflower (Calotropis gigantea) against golden apple snail (Pomaceae canaliculata) in lowland transplanted rice. Philipp. J. Crop Sci., 1991, 16, 103-107. |
| [195] | Abo Zaid, K.H.; El-Wakil, H.; El-Hussein, A.; Jomaa, S.; Shohayeb, M. Evaluation of the molluscicidal activity of Punica granatum, Calotropis procera, Solanum incantum, and Citrullus colocynthis against Biomphalaria arabica. World Appl. Sci. J., 2013, 26, 873-879. |
| [196] | Usha, K.; Singh, B.; Praseetha, P.; Deepa, N.; Agarwal, D.K.; Agarwal, R.; Nagaraja, A. Antifungal activity of Datura stramonium, Calotropis gigantea, and Azadirachta indica against Fusarium mangiferae and floral malformation in mango. Eur. J. Plant Pathol., 2009, 124, 637-657. [http://dx.doi.org/10.1007/s10658-009-9450-2] |
| [197] | Abu-Taleb, A.M.; El-Deeb, K.; Al-Otibi, F.O. Bioactivity of some plant extracts against Drechslera biseptata and Fusarium solani. J. Food Agric. Environ., 2011, 9, 769-774. |
| [198] | Shivpuri, A.; Sharma, O.P.; Jhamaria, S.L. Fungitoxic properties of plant extracts against pathogenic fungi. J. Mycol. Plant Pathol., 1997, 27, 29-31. |
| [199] | Pathak, N.; Zaidi, R.K. Comparative study of seed dressing fungicides and Calotropis procera latex for the control of seed-borne mycoflora of wheat. Ann. Biol. Res., 2013, 4, 1-6. |
| [200] | Jabeen, K.; Waheed, N.; Iqbal, S. Antifungal potential of Calotropis procera against Macrophomina phaseolina. Life Sci. J., 2013, 10, 572-576. |
| [201] | Sadana, D.; Didwania, N. Integrated disease management of bull eye’s pathogen infecting Lycopersicon esculentum (tomato). J. Microbiol. Biotechnol. Food Sci., 2019, 9, 53-57. [http://dx.doi.org/10.15414/jmbfs.2019.9.1.53-57] |
| [202] | Abdel-Farid, I.; El-Sayed, M.; Mohamed, E. Allelopathic potential of Calotropis procera and Morettia philaeana. Int. J. Agric. Biol., 2013, 15, 130-134. |
| [203] | Qasem, J.R. A survey on the phytotoxicity of common weeds, wild grown species and medicinal plants on wheat. Allelopathy J., 2017, 42, 179-193. [http://dx.doi.org/10.26651/allelo.j./2017-42-2-1115] |
| [204] | Kayode, J. Allelopathic effect of aqueous extracts of Calotropis procera on germination and seedling growth of maize. Pak. J. Sci. Ind. Res., 2004, 47, 69-72. |
| [205] | Alshahrani, N.D.S.T.S.; Aref, I.M.; Nasser, R.A. Allelopathic potential of Calotropis procera and Eucalyptus species on germination and growth of some timber trees. Allelopathy J., 2017, 40, 81-94. [http://dx.doi.org/10.26651/2017-40-1-1068] |
| [206] | Raza, A.; Kamran, M.; Safdar, M.E.; Ali, H.H.; Abbas, T.; Asif, M.; Ali, L.; Rehman, A. Management tactics for the handling of Parthenium hysterophorus L. in non-native environment through phytotoxic compounds of local species. Int. J. Agric. Biol., 2019, 21, 215-222. |
| [207] | Gulzar, A.; Siddiqui, M.B.; Bi, S. Phenolic acid allelochemicals induced morphological, ultrastructural, and cytological modification on Cassia sophera L. and Allium cepa L. Protoplasma, 2016, 253(5), 1211-1221. [http://dx.doi.org/10.1007/s00709-015-0862-x] [PMID: 26387115] |
| [208] | Parsons, W.T.; Cuthbertson, E.G. Noxious weeds of Australia., 2001, |
| [209] | Achakzai, K.; Khalid, S.; Adrees, M.; Bibi, A.; Ali, S.; Nawaz, R.; Rizwan, M. Air pollution tolerance index of plants around brick kilns in Rawalpindi, Pakistan. J. Environ. Manage., 2017, 190, 252-258. [http://dx.doi.org/10.1016/j.jenvman.2016.12.072] [PMID: 28061409] |
| [210] | Gajbhiye, T.; Pandey, S.K.; Kim, K.H. Factors controlling the deposition of airborne metals on plant leaves in a subtropical industrial environment. Asian J. Atmos. Environ., 2016, 10, 162-167. [http://dx.doi.org/10.5572/ajae.2016.10.3.162] |
| [211] | Singh, N.; Yunus, M.; Srivastava, K.; Singh, S.N.; Pandey, V.; Misra, J.; Ahmad, K.J. Monitoring of auto exhaust pollution by roadside plants. Environ. Monit. Assess., 1995, 34(1), 13-25. [http://dx.doi.org/10.1007/BF00546243] [PMID: 24201905] |
| [212] | Khalid, N.; Hussain, M.; Young, H.S.; Ashraf, M.; Hameed, M.; Ahmad, R. Lead concentrations in soils and some wild plant species along two busy roads in Pakistan. Bull. Environ. Contam. Toxicol., 2018, 100(2), 250-258. [http://dx.doi.org/10.1007/s00128-017-2247-7] [PMID: 29248955] |
| [213] | d’Souza, R.; Varun, M.; Pratas, J.; Paul, M.S. Spatial distribution of heavy metals in soil and flora associated with the glass industry in North Central India: implications for phytoremediation. Soil Sediment Contam., 2013, 22, 1-20. [http://dx.doi.org/10.1080/15320383.2012.697936] |
| [214] | Kumar, N.; Bauddh, K.; Kumar, S.; Dwivedi, N.; Singh, D.P.; Barman, S.C. Accumulation of metals in weed species grown on the soil contaminated with industrial wasteand their phytoremediation potential. Ecol. Eng., 2013, 61, 491-495. [http://dx.doi.org/10.1016/j.ecoleng.2013.10.004] |
| [215] | Nawab, J.; Khan, S.; Shah, M.T.; Gul, N.; Ali, A.; Khan, K.; Huang, Q. Heavy metal bioaccumulation in native plants in chromite impacted sites: a search for effective remediating plant species, Clean – Soil, Air. Water, 2016, 44, 37-46. |
| [216] | Gupta, A.K.; Sinha, S. Phytoextraction capacity of the plants growing on tannery sludge dumping sites. Bioresour. Technol., 2007, 98(9), 1788-1794. [http://dx.doi.org/10.1016/j.biortech.2006.06.028] [PMID: 16973356] |
| [217] | Al-Qahtani, K.M. Assessment of heavy metals accumulation in native plant species from soils contaminated in Riyadh City, Saudi Arabia. Life Sci. J., 2012, 2, 384-392. |
| [218] | D’Souza, R.J.; Varun, M.; Masih, J.; Paul, M.S. Identification of Calotropis procera L. as a potential phytoaccumulator of heavy metals from contaminated soils in Urban North Central India. J. Hazard. Mater., 2010, 184(1-3), 457-464. [http://dx.doi.org/10.1016/j.jhazmat.2010.08.056] [PMID: 20843602] |
| [219] | Varun, M.; D’Souza, R.; Pratas, J.; Paul, M.S. Metal contamination of soils and plants associated with the glass industry in North Central India: prospects of phytoremediation. Environ. Sci. Pollut. Res. Int., 2012, 19(1), 269-281. [http://dx.doi.org/10.1007/s11356-011-0530-4] [PMID: 21735162] |
| [220] | Singh, M.; Verma, M.; Kumar, R.N. Effects of open dumping of MSW on metal contamination of soil, plants, and earthworms in Ranchi, Jharkhand, India. Environ. Monit. Assess., 2018, 190(3), 139. [http://dx.doi.org/10.1007/s10661-018-6492-y] [PMID: 29442190] |
| [221] | Chandra, R.; Kumar, V.; Tripathi, S.; Sharma, P. Heavy metal phytoextraction potential of native weeds and greases from endocrine-disrupting chemicals rich complex distillery sludge and their histological observations during in-situ phytoremediation. Ecol. Eng., 2018, 111, 143-156. [http://dx.doi.org/10.1016/j.ecoleng.2017.12.007] |
| [222] | Pandey, P.K.; Verma, Y.; Choubey, S.; Pandey, M.; Chandrasekhar, K. Biosorptive removal of cadmium from contaminated groundwater and industrial effluents. Bioresour. Technol., 2008, 99(10), 4420-4427. [http://dx.doi.org/10.1016/j.biortech.2007.08.026] [PMID: 17892931] |
| [223] | Barthwal, J.; Nair, S.; Kakkar, P. Heavy metal accumulation in medicinal plants collected from environmentally different sites. Biomed. Environ. Sci., 2008, 21(4), 319-324. [http://dx.doi.org/10.1016/S0895-3988(08)60049-5] [PMID: 18837296] |
| [224] | Tripathi, A.; Misra, D.R. Bioaccumalation of Pb, Ni and Zn in some plant species growing in and around a municipal waste dumpsite at Allahabad, India. J. Solid Waste Technol. Manag., 2013, 39, 1-12. [http://dx.doi.org/10.5276/JSWTM.2013.1] |
| [225] | Al-Farraj, A.S.; Al-Wabel, M.I. Heavy metals accumulation of some plant species grown on mining area at Mahad AD’Dahab, Saudi Arabia. J. Appl. Sci. (Faisalabad), 2007, 7, 1170-1175. [http://dx.doi.org/10.3923/jas.2007.1170.1175] |
| [226] | Dan-Badjo, A.T.; Ibrahim, O.Z.; Guéro, Y.; Morel, L.; Feidt, C.; Echevarria, G. Impacts of artisanal gold mining on soil, water and plant contamination by trace elements at Komabangou, Western Niger J. Geochem. Explor, 2019, 205 ID 106328 |
| [227] | Khan, Z.I.; Ahmad, K.; Rasheed, M.J.Z.; Nawaz, R.; Ayub, M.; Zahoor, A.F.; Anjum, A.; Yousaf, M.; Dogar, Z.U.H.; Ur Rahman, K.; Rauf, A.; Mukhtar, M.K.; Naqvi, S.H.; Shaheen, M.; Fardous, A.; Gondal, S.; Naheed, S.; Ahmad, S.; Hussain, G.; Sher, M.; Arshad, F.; Ali, K.G.; Parveen, B. Toxic and some essential metals in medicinal plants used in herbal medicines” a case study in Pakistan. Afr. J. Pharm. Pharmacol., 2013, 7, 1389-1395. [http://dx.doi.org/10.5897/AJPP2012.1894] |
| [228] | Taskeen, A.; Naeem, I.; Mubeen, H.; Moeen, T. Comparison of Biomasses of Different Plants for Phytoremediation of Arsenic. Asian J. Chem., 2009, 21, 2857-2860. |
| [229] | Taskeen, A.; Naeem, I. A comparative Study of Removal of Arsenic by Calotropis procera, Eichohornia crassipus and Pteris vitata Proceedings of International Seminar on Medicinal Plants, Lahore College for Women University, 2008, 256-257. |
| [230] | Mubeen, H.; Naeem, I. Removal of Cu from Water and Waste Water using Calotropis procera roots, Proceedings of International Seminar on Medicinal Plants, Lahore College for Women University 2008, 245-250. |
| [231] | Mubeen, H.; Naeem, I.; Taskeen, A. Phytoremediation of Cu(II) by Calotropis procera roots. New York Sci. J., 2010, 3, 1-5. |
| [232] | Okonko, I.O.; Shittu, O.B. Bioremediation and municipal water treatment using latex exudate from Calotropis procera (Sodom apple). Electronic J. Env. Agr. Food Chem., 2007, 6, 1890-1904. |
| [233] | Vaishnav, V.; Chandra, S.; Daga, K. Adsorption studies of Zn(II) ions from wastewater using Calotropis procera as an adsorbent. Int. J. Sci. Eng. Res., 2011, 2, 1-6. |
| [234] | Shittu, B.O.; Popoola, T.O.S.; Taiwo, O. Potentials of Calotropis procera leaves for wastewater treatment Proc. Int. Conf. Sci. Nat. Develop., 2004, 97-101. |
| [235] | Babalola, J.O.; Overah, L.C.; Babarinde, A.; Oninla, V.O.; Olatunde, A. Kinetic, equilibrium and thermodynamic studies on the biosorption of Cd (II) from aqueous solutions by the leaf biomass of Calotropis procera - ‘Sodom apple’. J. Appl. Sci. Environ. Manag., 2011, 15, 607-615. |
| [236] | Vaishnav, V.; Dega, K.; Lal, M. Adsorption of metal (Cd) from synthetic wastewater by plant material. Int. J. Res. Chem. Environ., 2013, 3, 148-154. |



