- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Efficient Removal of Methyl Orange from Wastewater by Polymeric Chitosan-iso-vanillin
Eman A. Alabbad1, *
Abstract
Introduction:
Water pollution is a serious issue in several countries. In addition, because of limited water resources, the recycling of wastewater is crucial. Consequently, new and effective sorbents are required to reduce the cost of wastewater treatment as well as to mitigate the health problems caused by water pollution.
Methods:
In this study, the removal of Methyl Orange (MO) dye from wastewater using a chitosan-iso-vanillin polymer was evaluated. The removal of MO from an aqueous solution was studied in a batch system, using the modified chitosan polymer.
Results:
The results indicate that the removal of MO by the modified chitosan was affected by the solution pH, sorbent dosage, initial MO concentration, contact time, and temperature. The experimental data were fitted to the Langmuir, Freundlich, and Temkin isotherms, and Freundlich isotherm showed the best fit. The kinetic data were fitted to the pseudo-first-order and pseudo-second-order rate equations. Thus, the removal of MO was controlled via chemisorption, and the removal rate was 97.9% after 3 h at an initial MO concentration of 100 ppm and a sorbent dose of 0.05 g. The adsorption behavior of the modified chitosan for the removal of MO was well-described using the pseudo-second-order kinetic model. Intraparticle diffusion analysis was also conducted, and the thermodynamic properties, including entropy (∆S), enthalpy (∆H), and free energy (∆G), were determined.
Conclusion:
The pH, initial MO concentration, sorbent dosage, adsorption temperature, and contact time had a significant effect on the adsorption of MO by chitosan-iso-vanillin.
Article Information
Identifiers and Pagination:
Year: 2020Volume: 7
First Page: 16
Last Page: 25
Publisher Id: CHEM-7-16
DOI: 10.2174/1874842202007010016
Article History:
Received Date: 31/12/2019Revision Received Date: 05/04/2020
Acceptance Date: 09/04/2020
Electronic publication date: 03/07/2020
Collection year: 2020
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Department of Chemistry, College of Science, Imam Abdulrahman Bin Faisal University, Dammam 31441, Saudi Arabia E-mail: ealabbad@iau.edu.sa
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 31-12-2019 |
Original Manuscript | Efficient Removal of Methyl Orange from Wastewater by Polymeric Chitosan-iso-vanillin | |
1. INTRODUCTION
In recent years, water pollution caused by organic pollutants has become a serious global environmental problem. Dyes are one of the most commonly used organic substances causing water pollution and harming human health when they leak into the aquatic environment [1Kang, D.; Yu, X.; Ge, M.; Xiao, F.; Xu, H. Novel Al-doped carbon nanotubes with adsorption and coagulation promotion for organic pollutant removal. J. Environ. Sci. (China), 2017, 54, 1-12.
[http://dx.doi.org/10.1016/j.jes.2016.04.022] [PMID: 28391917] ]. The removal of poisonous dyes from water bodies thus presents a major challenge. Several biological and physical processes have been used to eliminate dyes from wastewater. Importantly, these removal processes should be efficient, simple, and cost-effective [2Ramakrishna, K.R.; Viraraghavan, T. Dye removal using low cost adsorbents. Water Sci. Technol., 1997, 36, 189-196.
[http://dx.doi.org/10.2166/wst.1997.0516] ].
Sejie and Goncalves [3Fortunate, P.S.; Misael, S.N. Removal of Methyl Orange (MO) from Water by adsorption onto Modified Local Clay (Kaolinite). Physical Chemistry., 2016, 6(2), 39-48., 4Gonçalves, M.; Guerreiro, M.C.; Ramos, P.H.; de Oliveira, L.C.; Sapag, K. Activated carbon prepared from coffee pulp: potential adsorbent of organic contaminants in aqueous solution. Water Sci. Technol., 2013, 68(5), 1085-1090.
[http://dx.doi.org/10.2166/wst.2013.349] [PMID: 24037160] ] reported several methods to purify water, such as coagulation, ozonation, flocculation, adsorption, and reverse osmosis. The method of pollutant removal is determined according to the nature of the pollutants, which may originate from the pharmaceutical, printing, food, textile, and chemical industries [5Kaur, M.; Datta, M. Adsorption Characteristics of Acid Orange 10 from Aqueous Solutions onto Montmorillonite Clay. Adsorpt. Sci. Technol., 2011, 29(3), 301-318.
[http://dx.doi.org/10.1260/0263-6174.29.3.301] ].
Chitosan is a natural adsorbent for metal ions such as Ni, Pb, Zu, Cr, and Cu because it contains a large number of hydroxyl and amino groups. In addition, the flexible polymer structure increases the complexation of metal ions [6Wu, F.C.; Tseng, R.L.; Juang, R.S. A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J. Environ. Manage., 2010, 91(4), 798-806.
[http://dx.doi.org/10.1016/j.jenvman.2009.10.018] [PMID: 19917518] ]. Another cheap compound derived from biomass is vanillin. Vanillin derivatives have been used for the preparation of organometallic polymers by forming a Schiff base, followed by chelation with metal ions [7Kaya, I.; Bilici, A.; Gul, M. Schiff base substitute polyphenol and its metal complexes derived from o-vanillin with 2,3-diaminopyridine: synthesis, characterization, thermal, and conductivity properties. Polym. Adv. Technol., 2008, 19(4), 1154-1163.
[http://dx.doi.org/10.1002/pat.1073] ]. Recently, chitosan-ortho-vanillin polymers have been investigated for the removal of cadmium ions from aqueous solutions. It was found that the chemisorption of cadmium is the rate-limiting step, and the adsorption process is spontaneous, with a maximum removal capacity of 51.020 mg. g-1 [8Alabbad, E. Estimation the sorption capacity of chemically modified chitosan toward cadmium ion in wastewater effluents. Orient. J. Chem., 2019, 35(2), 757-765.
[http://dx.doi.org/10.13005/ojc/350236] ].
MO is a water-solution azo dye that is used in the textile industry, research laboratories, and the manufacturing of printing paper. It is also metabolized to aromatic amines by intestinal microorganisms [9Ma, J.; Yu, F.; Zhou, L.; Jin, L.; Yang, M.; Luan, J.; Tang, Y.; Fan, H.; Yuan, Z.; Chen, J. Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes. ACS Appl. Mater. Interfaces, 2012, 4(11), 5749-5760.
[http://dx.doi.org/10.1021/am301053m] [PMID: 23062571] ]. Because MO is soluble in water, and is resistant to degradation [10Suciu, N.A.; Ferrari, T.; Ferrari, F.; Trevisan, M.; Capri, E. Pesticide removal from waste spray-tank water by organoclay adsorption after field application: An approach for a formulation of cyprodinil containing antifoaming/defoaming agents. Environ. Sci. Pollut. Res. Int., 2012, 19(4), 1229-1236.
[http://dx.doi.org/10.1007/s11356-011-0643-9] [PMID: 22057850] ], its removal from aqueous solutions by existing water treatment methods is difficult. Thus, MO persists in the environment if not treated correctly and presents a hazard to living organisms [11Bhatnagar, A.; Jain, A.K. A comparative adsorption study with different industrial wastes as adsorbents for the removal of cationic dyes from water. J. Colloid Interface Sci., 2005, 281(1), 49-55.
[http://dx.doi.org/10.1016/j.jcis.2004.08.076] [PMID: 15567379] ]. The leakage of dyes into wastewater has been identified as a factor affecting human health because of the carcinogenic and mutagenic effects of dyes [12Wang, S.; Boyjoo, Y.; Choueib, A.; Zhu, Z.H. Removal of dyes from aqueous solution using fly ash and red mud. Water Res., 2005, 39(1), 129-138.
[http://dx.doi.org/10.1016/j.watres.2004.09.011] [PMID: 15607172] ].
In this study, we investigated the use of a low-cost chitosan-iso-vanillin biosorbent material for the removal of MO from wastewater and conducted batch equilibrium studies to determine the thermodynamic and kinetic parameters. The main objective of this study was to evaluate the removal of MO dye from wastewater using chitosan-iso-vanillin. Thus, we estimated the adsorption capacity and mechanism of MO sorption on chitosan-iso-vanillin and studied its removal efficiency with respect to aqueous MO.
2. MATERIALS AND METHODS
Chemicals were obtained from commercial sources and used as received. Chitosan (not less than 85% glucosamine), iso-vanillin (99%), MO (Merck), acetone (99%), ethanol (99%), glacial acetic acid (99%), and methanol (99%) were used. An orbital shaker (Steadyshake, 757), UV-visible spectrometer (UVD-2950, Labomed, Inc.), and pH meter (Metrohm, 525A) were used for our analyses.
2.1. Synthesis of Chitosan-iso-vanillin Biosorbent
Chitosan-iso-vanillin was prepared using a previously reported method [13Alakhras, F.; Al-Shahrani, H.; Al-Abbad, E.; Al-Rimawi, F.; Ouerfelli, N. Removal of Pb(II) metal ions from aqueous solutions using chitosan–vanillin derivatives chelating polymers. Pol. J. Environ. Stud., 2019, 28(3), 1523-1534.
[http://dx.doi.org/10.15244/pjoes/89545] ]. Briefly, 5.70 g of chitosan was refluxed with 9 mL of glacial acetic acid, 90 mL of methanol, and 13.69 g of iso-vanillin monomer. The resulting material was filtered and washed with ethanol and acetone. Subsequently, purification was carried out by Soxhlet extraction. Finally, the sorbent was dried overnight at 70°C.
2.2. Preparing the MO Dye Solution
MO powder was used without any additional purification (water-soluble anionic azo dye). The chemical formula of MO is C14H14N3NaO3S, and its structural formula is shown in Fig. (1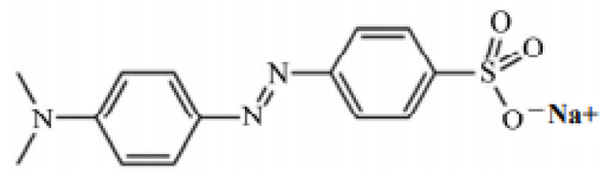 ). The stock dye solution was prepared by dissolving the appropriate mass of MO in purified water. Serial dilution was used to prepare dye concentrations of 5, 10, 15, 20, 30, 40, and 50 ppm. The purpose of these different concentrations was to obtain a calibration absorption curve versus concentration at the predetermined wavelength of 472 nm. The calibration curve was used to determine the exact concentrations of the unknown dye solutions.
). The stock dye solution was prepared by dissolving the appropriate mass of MO in purified water. Serial dilution was used to prepare dye concentrations of 5, 10, 15, 20, 30, 40, and 50 ppm. The purpose of these different concentrations was to obtain a calibration absorption curve versus concentration at the predetermined wavelength of 472 nm. The calibration curve was used to determine the exact concentrations of the unknown dye solutions.
 |
Fig. (1) Structure of MO. |
2.3. Sorption of MO onto the Polymer
Batch equilibrium adsorption experiments were used to investigate dye removal. In these experiments, 25 mL of MO solution was placed in a 100 mL flask containing 0.05 g of the suspended sorbent at 30°C for 3 h. The pH range (3.0-10.0) was adjusted using 1 M HCl or 1 M NaOH. Sorption experiments were carried out for 5, 10, 20, 30, 60, 120, 240, 480, and 1440 min to evaluate the effect of time on dye removal. In addition, these tests were performed at different MO dye concentrations (50, 75, 100, 125, 150, 200, and 300 mg. L-1) and at various temperatures (30, 50, and 70°C). The effect of the amount of chitosan-iso-vanillin polymer was also studied with respect to 0.01, 0.05, 0.1, 0.2, and 1 g of the dry adsorbent. After the experiments, the samples were filtered, and the residual MO concentration in solution was determined by UV-Vis spectrophotometry. At equilibrium, the concentration of the adsorbed MO ions, expressed by the equilibrium adsorption capacity (Qe, mg. g-1) was evaluated using Eq. (1),
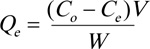 |
(1) |
where Qe has units of milligrams of MO per gram of sorbent, Co and Ce represent the initial and equilibrium MO concentration (mg.L-1), V is the volume of MO solution (L), and W is the amount of chitosan-iso-vanillin (g).
From the adsorption experiments, the amount of MO removed over time (Qt, mg. g-1) was evaluated using Eq. (2),
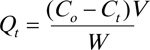 |
(2) |
Ct is the concentration of MO in the liquid (mg.L-1) at time t.
3. RESULTS AND DISCUSSION
3.1. Synthesis and Characterization of the Biosorbent
After cellulose, chitosan is one of the most abundant biopolymers [14Ravi Kumar, M.N.V. A review of chitin and chitosan application. React. Funct. Polym., 2000, 46(1), 1-27.
[http://dx.doi.org/10.1016/S1381-5148(00)00038-9] ]. Because of its properties such as good biocompatibility, non-toxicity, ability to form a mechanical membrane, and antimicrobial activity, chitosan has been widely used in the food industry and for metal chelation from wastewater, water purification, fruit juice clarification and solid removal, edible film formation, and preservation of foods against microbial degradation [15Shahidi, F.; Kamil, J.; Jeon, Y.; Kim, S. Antioxidant role of chitosan in a cooked cod (GADUS MORHUA) model system. J. Food Lipids, 2002, 9(1), 57-64.
[http://dx.doi.org/10.1111/j.1745-4522.2002.tb00208.x] -18Shahidi, F.; Synowiecki, J. Isolation and characterization of nutrients and value-added products from snow crab (Chionoecetes opilio) and shrimp (Pandalus borealis) processing discards. J. Agric. Food Chem., 1991, 39(8), 1527-1532.
[http://dx.doi.org/10.1021/jf00008a032] ]. In addition, chitosan in the form of nanospheres or microspheres can also be used as a controlled delivery system for active agents. To delay the controlled release of the active agents, chitosan microcrystals usually require cross-linking, for which glyoxal, formaldehyde, glutaraldehyde, and other reactive binding agents can be used [19Ajit, P.R.; Namdev, B.S.; Sangamesh, A.P.; Tejraj, M.A. Novel interpenetrating polymer network microspheres of chitosan and methylcellulose for controlled release of theophylline. Carbohydr. Polym., 2007, 69(4), 678-687.
[http://dx.doi.org/10.1016/j.carbpol.2007.02.008] ].
Vanillin, obtained from the pods or bean of the tropical vanilla orchid, is often used as a flavoring and purifying agent for nutrients and is Generally Recognized as a Safe (GRAS) substance [20Karathanos, V.T.; Mourtzinos, I.; Yannakopoulou, K.; Andrikopoulos, N.K. Study of the solubility, antioxidant activity and structure of inclusion complex of vanillin with b-cyclodextrin. Food Chem., 2007, 101(2), 652-658.
[http://dx.doi.org/10.1016/j.foodchem.2006.01.053] ]. Apart from its flavor, vanillin also exhibits bioactivity [21Sinha, A.K.; Sharma, U.K.; Sharma, N. A comprehensive review on vanilla flavor: extraction, isolation and quantification of vanillin and others constituents. Int. J. Food Sci. Nutr., 2008, 59(4), 299-326.
[http://dx.doi.org/10.1080/09687630701539350] [PMID: 17886091] ]. Moreover, vanillin is also used for other purposes, such as in cosmetics and medicines. The aldehyde groups of vanillin and the amino groups of chitosan can undergo a base-catalyzed nucleophilic substitution reaction, forming imines, and continued polymerization results in a network structure that is useful for the controlled release applications [22Mourtzinos, I.; Konteles, S.; Kalogeropoulos, N.; Karathanos, V.T. Thermal oxidation of vanillin affects its antioxidant and antimicrobial properties. Food Chem., 2009, 114(3), 791-797.
[http://dx.doi.org/10.1016/j.foodchem.2008.10.014] ].
The condensation reaction between the carbonyl group of vanillin and primary amine of chitosan results in the formation of a Schiff base, i.e., an imine. In our study, the reaction of iso-vanillin with chitosan yielded a polymeric absorbent material, as shown in Fig. (2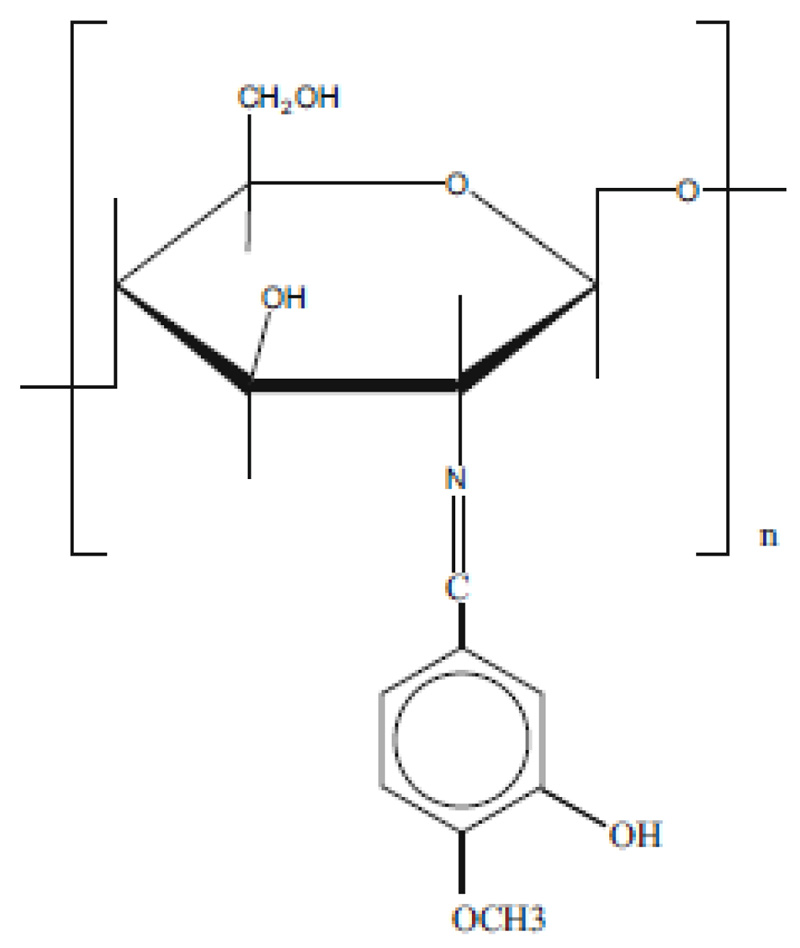 ). The obtained biosorbent has been characterized previously [13Alakhras, F.; Al-Shahrani, H.; Al-Abbad, E.; Al-Rimawi, F.; Ouerfelli, N. Removal of Pb(II) metal ions from aqueous solutions using chitosan–vanillin derivatives chelating polymers. Pol. J. Environ. Stud., 2019, 28(3), 1523-1534.
). The obtained biosorbent has been characterized previously [13Alakhras, F.; Al-Shahrani, H.; Al-Abbad, E.; Al-Rimawi, F.; Ouerfelli, N. Removal of Pb(II) metal ions from aqueous solutions using chitosan–vanillin derivatives chelating polymers. Pol. J. Environ. Stud., 2019, 28(3), 1523-1534.
[http://dx.doi.org/10.15244/pjoes/89545] ].
3.2. Effect of pH on MO Sorption
The effects of the solution pH on MO dye sorption from pH 3.0 to 10.0 are plotted in Fig. (3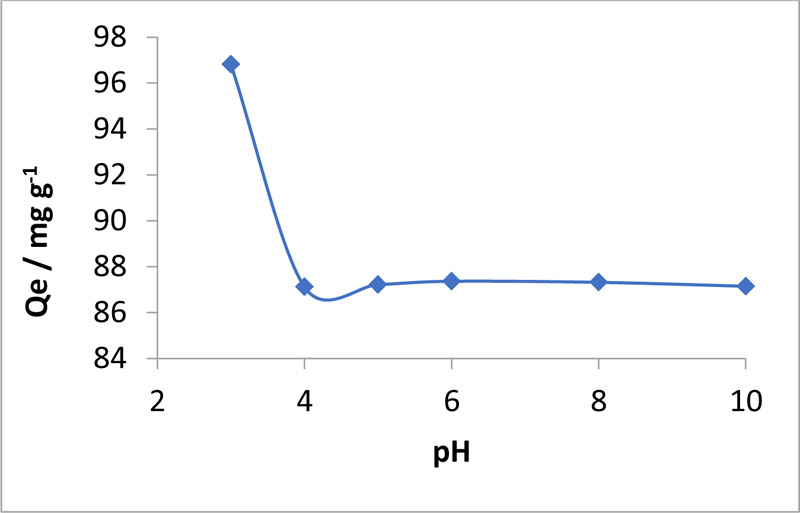 ). The pH of the MO solution plays an important role in the adsorption process because it affects the dissociation of the functional groups on the sorbent active sites and consequently, the surface charge of the adsorbent. In addition, the pH affects the chemistry of the MO solution [23Huang, R.; Liu, Q.; Huo, J.; Yang, B. Adsorption of methyl orange onto protonated cross-linked chitosan. Arab. J. Chem., 2017, 10(1), 24-32.
). The pH of the MO solution plays an important role in the adsorption process because it affects the dissociation of the functional groups on the sorbent active sites and consequently, the surface charge of the adsorbent. In addition, the pH affects the chemistry of the MO solution [23Huang, R.; Liu, Q.; Huo, J.; Yang, B. Adsorption of methyl orange onto protonated cross-linked chitosan. Arab. J. Chem., 2017, 10(1), 24-32.
[http://dx.doi.org/10.1016/j.arabjc.2013.05.017] ]. The adsorption of MO by chitosan-iso-vanillin decreased as the solution pH increased. The highest MO removal (96.8%) was recorded at pH 3 when the sorbent dose was 0.05 g. According to previous studies [24Chiou, M.S.; Li, H.Y. Equilibrium and kinetic modeling of adsorption of reactive dye on cross-linked chitosan beads. J. Hazard. Mater., 2002, 93(2), 233-248.
[http://dx.doi.org/10.1016/S0304-3894(02)00030-4] [PMID: 12117469] -26Iqbal, J.; Wattoo, F.H.; Wattoo, M.H.S.; Malik, R.; Tirmizi, S.A.; Imran, R.; Ghangro, A.B. Adsorption of acid yellow dye on flakes of chitosan prepared from fishery wastes. Arab. J. Chem., 2011, 4, 389-395.
[http://dx.doi.org/10.1016/j.arabjc.2010.07.007] ], the optimum pH range for sorption by chitosan-iso-vanillin is 3 - 6. As shown in Fig. (3 ), the sorbent showed a relatively stable adsorption capacity for MO over a wide range of pH levels, but, this process was the most effective at pH 3 - 6. These results suggest that in an aqueous solution, the MO dye dissociates, yielding negatively charged sulfonate groups and positively charged sodium ions, i.e., negatively charged MO ions. Second, the electrostatic attraction between the MO anion and sorbent surface results in adsorption. Notably, when the pH of the MO solution was increased, the adsorption decreased [27Deepika, R.; Venkateshprabhu, M.; Pandimdevi, M. Studies on the behaviour of reactive dyes onto the cross-linked chitosan using adsorption isotherms. Int. J. Environ. Sci., 2013, 4(3), 323-351.] because of the competition for adsorption sites between the OH- ions in the basic solution and MO anions.
), the sorbent showed a relatively stable adsorption capacity for MO over a wide range of pH levels, but, this process was the most effective at pH 3 - 6. These results suggest that in an aqueous solution, the MO dye dissociates, yielding negatively charged sulfonate groups and positively charged sodium ions, i.e., negatively charged MO ions. Second, the electrostatic attraction between the MO anion and sorbent surface results in adsorption. Notably, when the pH of the MO solution was increased, the adsorption decreased [27Deepika, R.; Venkateshprabhu, M.; Pandimdevi, M. Studies on the behaviour of reactive dyes onto the cross-linked chitosan using adsorption isotherms. Int. J. Environ. Sci., 2013, 4(3), 323-351.] because of the competition for adsorption sites between the OH- ions in the basic solution and MO anions.
 |
Fig. (2) Structure of the chitosan-iso-vanillin biosorbent. |
 |
Fig. (3) Effect of pH on MO removal by chitosan-iso-vanillin (MO = 100 ppm, T = 30°C, W = 0.05 g, and t = 3 h). |
3.3. Influence of Initial MO Concentration and Isothermal Study
The influence of the initial MO concentration at pH 3 and at 30°C on the adsorption process is shown in Fig. (4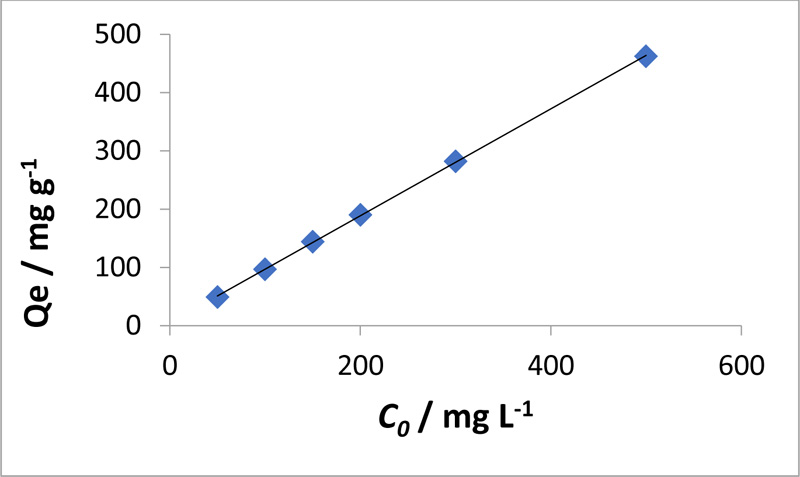 ). Increasing initial MO concentration led to an increase in the equilibrium adsorption capacity of chitosan-iso-vanillin for MO. The ratio of dye molecules to adsorption sites is low at low initial dye concentrations and, as a result, there are more sites for the adsorption of the dye [28Kyaw, T.T.; Wint, D.S.; Naing, K.M. Studies on the sorption behavior of dyes on cross-linked chitosan beads in acid medium. International Conference on Biomedical Engineering and Technology IPCBEE, 2011, 174-178.].
). Increasing initial MO concentration led to an increase in the equilibrium adsorption capacity of chitosan-iso-vanillin for MO. The ratio of dye molecules to adsorption sites is low at low initial dye concentrations and, as a result, there are more sites for the adsorption of the dye [28Kyaw, T.T.; Wint, D.S.; Naing, K.M. Studies on the sorption behavior of dyes on cross-linked chitosan beads in acid medium. International Conference on Biomedical Engineering and Technology IPCBEE, 2011, 174-178.].
In addition, the initial dye concentration affects the rate of adsorption, which also affects the adsorption capacity of the sorbent [24Chiou, M.S.; Li, H.Y. Equilibrium and kinetic modeling of adsorption of reactive dye on cross-linked chitosan beads. J. Hazard. Mater., 2002, 93(2), 233-248.
[http://dx.doi.org/10.1016/S0304-3894(02)00030-4] [PMID: 12117469] ]. This is because of the increased concentration gradient with the increase in the initial dye concentration, which acts as the driving force for adsorption. In contrast, at very high initial dye concentrations, the number of available adsorption sites decreases with an increase in the initial concentrations of the dye molecules. As a result, the number of available sites where adsorption occurs is rapidly reduced, resulting in a corresponding reduction in dye removal [29El-Sayed, E.M.; Tamer, T.M.; Omer, A.M.; Mohy Eldin, M.S. Development of novel chitosan schiff base derivatives for cationic dye removal: methyl orange model. Desalination Water Treat., 2016, 57(47), 22632-22645.
[http://dx.doi.org/10.1080/19443994.2015.1136694] ]. In other words, in the case of low concentrations, the ratio of the initial number of dye molecules to the available adsorption sites is low, and more adsorption sites are available for the dye molecules, which increase the removal rate. However, at higher concentrations, the ratio of the initial number of available adsorption sites to the dye molecules is low; thus, the number of available adsorption sites is reduced, resulting in decreased dye removal. Protonation improved the adsorption of the anionic dye onto chitosan.
 |
Fig. (4) Effect of initial MO concentration on the equilibrium adsorption capacity of chitosan-iso-vanillin (W= 0.05 g, pH = 3, T = 30°C, t = 3 h). |
The linear plots of MO adsorption by chitosan-iso-vanillin were fitted to the Langmuir, Freundlich, and Temkin isotherms. These models relate the distribution of adsorbed molecules between solid and liquid phases at equilibrium. The Langmuir isotherm model is expressed in Eq. (3):
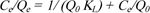 |
(3) |
Here, Q0 is the adsorption capacity (mg. g-1), and KL is the Langmuir constant (L. mg-1), which is related to the energy of sorption. In the Langmuir model, an additional parameter, the dimensionless separation factor RL given in Eq. (4), can be used to understand adsorption. If RL=1, the adsorption relationship is linear; if RL>1, adsorption is unfavorable; if 0 <RL <1, adsorption is favorable; and if RL = 0, adsorption is irreversible. The obtained value of RL is listed in Table 1.
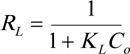 |
(4) |
The data were also fitted to the Freundlich adsorption isotherm, which is suitable for modelling low concentrations and for heterogeneous surfaces; the Freundlich isotherm is given by Eq. (5):
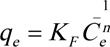 |
(5) |
The Freundlich constants KF and n are related to the degree of adsorption and adsorption capacity, respectively, and can be calculated using the slope and intercept of the linear plot of log qe vs. log Ce.
The Temkin model (Eq. (6)) assumes that the heat of adsorption decreases linearly with increasing coverage of the adsorbent surface because of the adsorbate-adsorbate interactions.
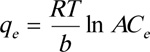 |
(6) |
Here, the constant B = RT/b, where b is the Temkin constant related to the heat of sorption, T is the temperature (K), R is the gas constant (8.314 J/mol K), A is the Temkin equilibrium binding constant (L. mg-1) which is related to the maximum binding energy (∆E = -∆H, i.e., the change in adsorption energy (J. mol-1), and Q0 is the adsorption (maximum) capacity [30Kundu, S.; Gupta, A.K. Arsenic adsorption onto iron oxide-coated cement (IOCC): regression analysis of equilibrium data with several isotherm models and their optimization. Chem. Eng., 2006, 122(1-2), 93-106.
[http://dx.doi.org/10.1016/j.cej.2006.06.002] , 31Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon. Part I. Two-parameter models and equations allowing determination of thermodynamic parameters. J. Hazard. Mater., 2007, 147(1-2), 381-394.
[http://dx.doi.org/10.1016/j.jhazmat.2007.01.021] [PMID: 17276594] ]. A and B can be obtained from the intercept and the slope of the plot qe against ln Ce, and these values are listed in Table 1. In this study, the fitting of the data to the Langmuir, Freundlich, and Temkin isotherms yielded a coefficient of variation values (R2 = 0.9151, 0.9999, and 0.8886, respectively). The Langmuir coefficient (KL) was found to be 0.049 and Q0 to be 666.6 mg.g-1. The Freundlich isotherm KF, which reflects the adsorption capacity was 45.3 mg.g-1. Further, the Freundlich isotherm yielded a better fit to the experimental data than the Langmuir or Temkin isotherms [32Saha, T.K.; Bhounil, N.C.; Karmaker, S.; Ahmed, M.G.; Ichikawa, H.; Fukumori, Y. Adsorption of methyl orange noto chitosan from aqueous solution. J. Water Resource Prot., 2010, 2, 898-906.
[http://dx.doi.org/10.4236/jwarp.2010.210107] ]. Table 2 lists Q0 for the removal of MO from aqueous solution using several different polymeric sorbents at 25°C. In a previous study, self-assembled gels were prepared from Exfoliated Montmorillonite and Chitosan (EMCG) and used to remove MO in the presence of Methylene Blue (MB), where the adsorption capacity was 545 mg. g-1 [33Kang, S.; Qin, L.; Zhao, Y.; Wang, W.; Zhang, T.; Yang, L.; Rao, F.; Song, S. Enhanced removal of methyl orange on exfoliated montmorillonite/chitosan gel in presence of methylene blue. Chemosphere, 2020, 238,124693.
[http://dx.doi.org/10.1016/j.chemosphere.2019.124693] [PMID: 31524627] ]. In another study, an environment-friendly sorbent was used as a cheap method to remove textile dyes, especially MO from wastewater, yielding a Q0 of 44.8 mg. g-1 [34Wong, V.L.; Tay, S.Y.; Lim, S.S. Enhanced removal of methyl orange from aqueous solution by chitosan-CaCl2 beads. Mater. Sci. Eng. J., 2020, 736, 1-14.
[http://dx.doi.org/10.1088/1757-899X/736/2/022049] ]. For a system of protonated cross-linked chitosan in a batch system, a Q0 of 89.29 mg. g-1 was obtained [35Huang, R.; Liu, Q.; Huo, J.; Yang, B. Adsorption of methyl orange onto protonated cross- linked chitosan. Arab. J. Chem., 2017, 10, 24-32.
[http://dx.doi.org/10.1016/j.arabjc.2013.05.017] ], and the use of chitosan biomass yielded a Q0 of 29 mg. g-1 [36Allouche, F.; Yassaa, N.; Lonuici, H. Sorption of methyl orange from aqueous solution on chitosan biomass. Procedia Earth Planet. Sci., 2015, 15, 596-601.
[http://dx.doi.org/10.1016/j.proeps.2015.08.109] ]. Thus, the chitosan-iso-vanillin polymer sorbent developed in this study exhibits the highest Q0 amongst all previously reported sorbents for MO at 25°C.
3.4. Effect of Adsorbent Dosage
The effect of the adsorbent dosage on the adsorption of MO was investigated to determine the minimum sorbent dosage that enabled the maximum dye adsorption. The dose of the sorbent was increased from 0.01 to 0.2 g, and the effect on the equilibrium adsorption capacity for MO was determined, as listed in Table 3. At a sorbent dosage of 0.01 g, 93.64% of the MO was removed, which is the lowest value of the dosage tested. In contrast, 97.91% removal was obtained with sorbent dosages of 0.05 and 0.1 g. Thus, as shown by the data in Table 3 and Fig. (5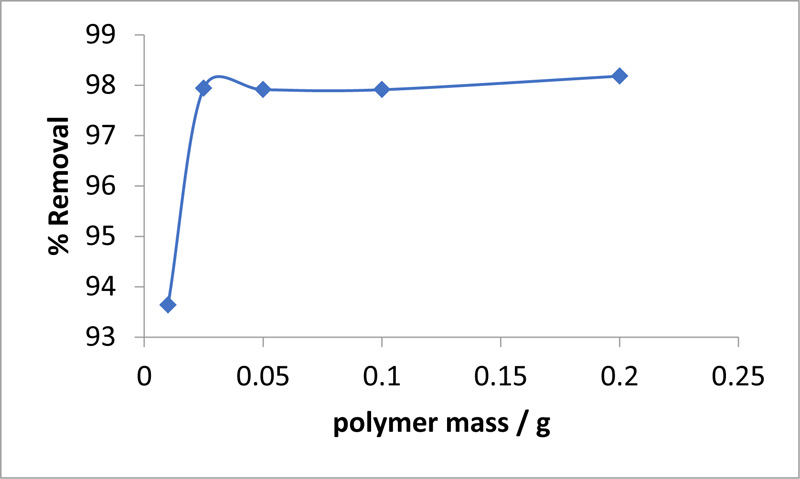 ), the MO removal rate increased with increasing the biosorbent’s dose until it reached a plateau at 0.2 g, where the removal rate was 98.18%. Despite the marked improvement in the rate of removal of the MO with increasing adsorbent dosage, the effect was not proportional, even though the number of sites available for adsorption increased. This can be attributed to the possible overlapping or aggregation of adsorption sites resulting in an increase in the diffusion path length.
), the MO removal rate increased with increasing the biosorbent’s dose until it reached a plateau at 0.2 g, where the removal rate was 98.18%. Despite the marked improvement in the rate of removal of the MO with increasing adsorbent dosage, the effect was not proportional, even though the number of sites available for adsorption increased. This can be attributed to the possible overlapping or aggregation of adsorption sites resulting in an increase in the diffusion path length.
 |
Fig. (5) Effect of biosorbent dose on MO removal (MO = 100 ppm, pH = 3, T = 30 °C, t = 3 h). |
3.5. Influence of Sorption Period and Kinetic Analysis
Another factor affecting the adsorption processes is the contact time between the sorbent and dye. The result of the kinetic analysis is shown in Fig. (6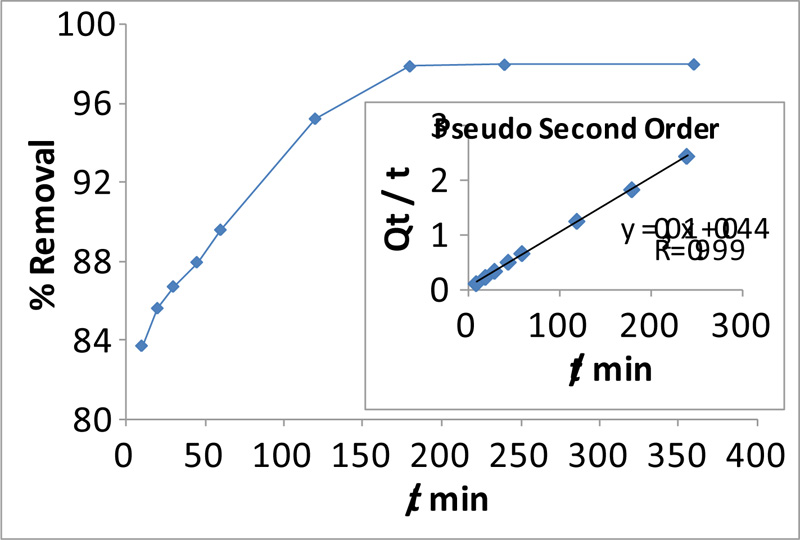 ). Initially, the uptake of MO by chitosan-iso-vanillin was rapid because of the electrostatic attraction between the surface of the sorbent and the dye. Notably, with an increase in the initial MO concentration, the time required to reach equilibrium increased; hence, contact time is an important variable in adsorption processes [37Maleki, A.; Pajootan, E.; Hayati, B. Ethyl acrylate grafted chitosan for heavy metal removal from wastewater: Equilibrium, kinetic and thermodynamic studies. J. Taiwan Inst. Chem. Eng., 2015, 51, 127-134.
). Initially, the uptake of MO by chitosan-iso-vanillin was rapid because of the electrostatic attraction between the surface of the sorbent and the dye. Notably, with an increase in the initial MO concentration, the time required to reach equilibrium increased; hence, contact time is an important variable in adsorption processes [37Maleki, A.; Pajootan, E.; Hayati, B. Ethyl acrylate grafted chitosan for heavy metal removal from wastewater: Equilibrium, kinetic and thermodynamic studies. J. Taiwan Inst. Chem. Eng., 2015, 51, 127-134.
[http://dx.doi.org/10.1016/j.jtice.2015.01.004] ]. The kinetic data were fitted to the pseudo-first-order, as shown in Eqs. (7) and (8), respectively. These models have been shown to stimulate the dye removal by different sorbents well [8Alabbad, E. Estimation the sorption capacity of chemically modified chitosan toward cadmium ion in wastewater effluents. Orient. J. Chem., 2019, 35(2), 757-765.
[http://dx.doi.org/10.13005/ojc/350236] ].
 |
Fig. (6) Effect of contact time on MO removal by chitosan-iso-vanillin (MO =100 ppm, pH = 3, T = 30 °C,W = 0.05 g). |
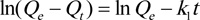 |
(7) |
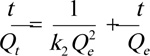 |
(8) |
The experimentally observed Qe of 98.00 mg. L-1, was reached in 180 min, reflecting the occupation of the remaining active sites on the adsorbent surface. As shown in Table 4, the coefficient of variation for fitting to the pseudo-second-order model (
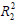 = 0.9990) was greater than that for fitting to the pseudo-first-order (
= 0.9990) was greater than that for fitting to the pseudo-first-order (
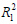 = 0.9524). As shown in Fig. (6
= 0.9524). As shown in Fig. (6 ), the MO chitosan-iso-vanillin system rapidly reached equilibrium. Thus, the surface was rapidly covered with dye molecules, which blocked further adsorption of the dye molecules that remained in the solution. As a result, adsorption slowed down, resulting in the plateau at equilibrium adsorption. These results are consistent with those reported in Ref [38Gibbs, G.; Tobin, J.M.; Guibal, E. Sorption of acid green 25 on chitosan: influence of experimental parameters on uptake kinetics and sorption isotherms. J. Appl. Polym. Sci., 2003, 90(4), 1073-1080.
), the MO chitosan-iso-vanillin system rapidly reached equilibrium. Thus, the surface was rapidly covered with dye molecules, which blocked further adsorption of the dye molecules that remained in the solution. As a result, adsorption slowed down, resulting in the plateau at equilibrium adsorption. These results are consistent with those reported in Ref [38Gibbs, G.; Tobin, J.M.; Guibal, E. Sorption of acid green 25 on chitosan: influence of experimental parameters on uptake kinetics and sorption isotherms. J. Appl. Polym. Sci., 2003, 90(4), 1073-1080.
[http://dx.doi.org/10.1002/app.12761] ], in which a greater concentration required a longer time to reach equilibrium, implying that there is a direct relationship between these factors.
Pseudo-first-order and pseudo-second-order kinetic parameters. To examine the adsorption mechanism further, the Weber-Morris intraparticle diffusion rate constant was calculated using Eq (9):
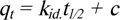 |
(9) |
Here qt is the mass of adsorbed MO at contact time t (min), kid (mg. g-1 min-½) is the Weber-Morris intraparticle diffusion rate constant, and c is the boundary layer. Fig. (7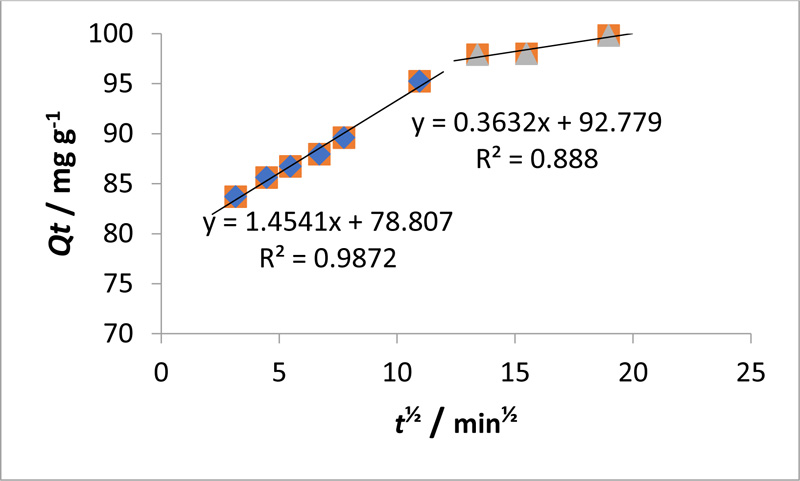 ). shows the linear plot of qt versus t1/2 for MO based on the intraparticle diffusion mechanism, which does not determine the rate-determining step, as shown by the correlation coefficient (R2) of 0.9870. In addition, adsorption occurs by both surface and intraparticle diffusion. In this study, the pseudo-second-order model was the most suitable kinetic model for the data. Therefore, direct chemisorption dominated the sorption kinetics, which is consistent with the findings of a study [39Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical models of sorption kinetics including a surface reaction mechanism: A review. Adv. Colloid Interface Sci., 2009, 152(1-2), 2-13.
). shows the linear plot of qt versus t1/2 for MO based on the intraparticle diffusion mechanism, which does not determine the rate-determining step, as shown by the correlation coefficient (R2) of 0.9870. In addition, adsorption occurs by both surface and intraparticle diffusion. In this study, the pseudo-second-order model was the most suitable kinetic model for the data. Therefore, direct chemisorption dominated the sorption kinetics, which is consistent with the findings of a study [39Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical models of sorption kinetics including a surface reaction mechanism: A review. Adv. Colloid Interface Sci., 2009, 152(1-2), 2-13.
[http://dx.doi.org/10.1016/j.cis.2009.07.009] [PMID: 19735907] ]. This finding indicates that electron sharing between the adsorbates and adsorbent was the key factor driving adsorption [40Habiba, U.; Siddique, T.A.; Joo, T.C.; Salleh, A.; Ang, B.C.; Afifi, A.M. Synthesis of chitosan/polyvinyl alcohol/zeolite composite for removal of methyl orange, Congo red and chromium(VI) by flocculation/adsorption. Carbohydr. Polym., 2017, 157, 1568-1576.
[http://dx.doi.org/10.1016/j.carbpol.2016.11.037] [PMID: 27987870] ].
 |
Fig. (7) Fitting of MO adsorption data to the Weber-Morris intraparticle diffusion model. |
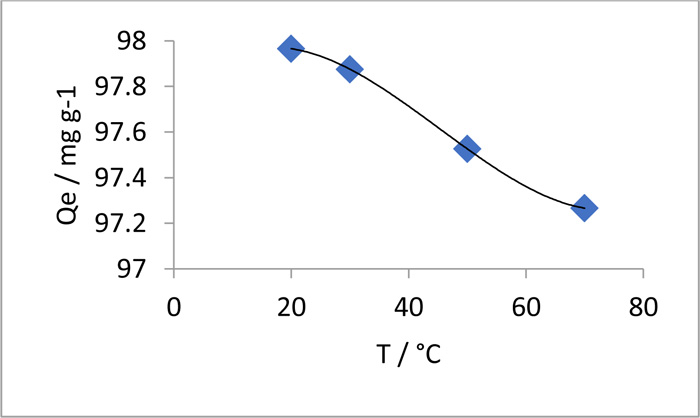 |
Fig. (8) Effect of temperature on MO removal by chitosan-iso-vanillin. (MO = 100 ppm, pH = 3, W = 0.05 g, t = 3 h). |
3.6. Influence of Temperature and Thermodynamic Investigation
The effect of temperature on MO adsorption by chitosan-iso-vanillin at pH 3 is shown in Fig. (8 ). The removal of MO was studied at 20, 30, 50, and 70°C. The adsorption process is affected by temperature, which affects the equilibrium capacity of the adsorbent for MO. With increasing temperature, MO adsorption decreased, indicating an exothermic adsorption reaction. Thus, the interaction between dye molecules and adsorption sites weakened with increasing temperature. In addition, a lower temperature led to a reduction in the number of available active sites for adsorption [41Theydan, S.K. Effect of Process Variables, Adsorption kinetics and equilibrium studies of hexavalent chromium removal from aqueous solution by date seeds and its activated carbon by ZnCl2. Iraqi Academic Sci. J., 2018, 19(1), 1-12., 42Alqaragully, M.B. Removal of textile dyes (maxilon blue, and methyl orange) by date stones activated carbon. Int. J. Adv. Res. Chem. Sci., 2014, 1(1), 48-59.]. To determine if the adsorption process was endothermic or exothermic, the thermodynamic parameters were determined for the dye adsorbent systems, i.e., enthalpy (∆H), entropy (∆S), and free energy (∆G) changes, and the results are listed in Table 5. The increase in the absolute value of ΔG with increasing temperature indicates that elevated temperatures facilitated adsorption. The negative value of ΔH indicates that the adsorption process is exothermic. In other studies, these parameters have been used to identify the underlying adsorption mechanism [43Li, Q.; Yue, Q.Y.; Su, Y.; Gao, B.Y.; Sun, H.J. Equilibrium, thermodynamics and process design to minimize adsorbent amount for the adsorption of acid dyes onto cationic polymer-loaded bentonite. Chem. Eng., 2010, 158(3), 489-497.
). The removal of MO was studied at 20, 30, 50, and 70°C. The adsorption process is affected by temperature, which affects the equilibrium capacity of the adsorbent for MO. With increasing temperature, MO adsorption decreased, indicating an exothermic adsorption reaction. Thus, the interaction between dye molecules and adsorption sites weakened with increasing temperature. In addition, a lower temperature led to a reduction in the number of available active sites for adsorption [41Theydan, S.K. Effect of Process Variables, Adsorption kinetics and equilibrium studies of hexavalent chromium removal from aqueous solution by date seeds and its activated carbon by ZnCl2. Iraqi Academic Sci. J., 2018, 19(1), 1-12., 42Alqaragully, M.B. Removal of textile dyes (maxilon blue, and methyl orange) by date stones activated carbon. Int. J. Adv. Res. Chem. Sci., 2014, 1(1), 48-59.]. To determine if the adsorption process was endothermic or exothermic, the thermodynamic parameters were determined for the dye adsorbent systems, i.e., enthalpy (∆H), entropy (∆S), and free energy (∆G) changes, and the results are listed in Table 5. The increase in the absolute value of ΔG with increasing temperature indicates that elevated temperatures facilitated adsorption. The negative value of ΔH indicates that the adsorption process is exothermic. In other studies, these parameters have been used to identify the underlying adsorption mechanism [43Li, Q.; Yue, Q.Y.; Su, Y.; Gao, B.Y.; Sun, H.J. Equilibrium, thermodynamics and process design to minimize adsorbent amount for the adsorption of acid dyes onto cationic polymer-loaded bentonite. Chem. Eng., 2010, 158(3), 489-497.
[http://dx.doi.org/10.1016/j.cej.2010.01.033] ]. As shown in Table 5, at all temperatures, ΔG is negative, indicating that the adsorption process is spontaneous. In addition, an increase in the temperature increased the absolute value of ΔG and may facilitate the adsorption process. The positive ΔS values suggest increased disorder at the liquid-solid interface. Our findings are in agreement with earlier studies [44Kora, A.J.; Rastogi, L. Catalytic degradation of anthropogenic dye pollutants using palladium nanoparticles synthesized by gum olibanum, a glucuronoarabinogalactan biopolymer. Ind. Crops Prod., 2016, 81, 1-10.
[http://dx.doi.org/10.1016/j.indcrop.2015.11.055] ].
CONCLUSION
In this study, the adsorption of MO dye by chitosan-iso-vanillin from water was evaluated. The results showed that the pH, initial MO concentration, mass of sorbent, adsorption temperature, and contact time had a significant effect on the adsorption of MO by chitosan-iso-vanillin. Fitting of the data to three isothermal models confirmed that the adsorption of MO by chitosan-iso-vanillin was best fitted by the Freundlich isotherm. Compared with other sorbents reported in previous literature, chitosan-iso-vanillin achieved a higher adsorption capacity for MO and the pseudo-second-order model. In addition, the adsorption process was exothermic and spontaneous.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICTS OF INTEREST
The author declares that there are no conflicts of interest regarding the publication of this paper.
ACKNOWLEDGEMENTS
I thank my colleagues at Imam Abdulrahman Bin Faisal University for their patience, constant encouragement, and assistance during this work.
REFERENCES
| [1] | Kang, D.; Yu, X.; Ge, M.; Xiao, F.; Xu, H. Novel Al-doped carbon nanotubes with adsorption and coagulation promotion for organic pollutant removal. J. Environ. Sci. (China), 2017, 54, 1-12. [http://dx.doi.org/10.1016/j.jes.2016.04.022] [PMID: 28391917] |
| [2] | Ramakrishna, K.R.; Viraraghavan, T. Dye removal using low cost adsorbents. Water Sci. Technol., 1997, 36, 189-196. [http://dx.doi.org/10.2166/wst.1997.0516] |
| [3] | Fortunate, P.S.; Misael, S.N. Removal of Methyl Orange (MO) from Water by adsorption onto Modified Local Clay (Kaolinite). Physical Chemistry., 2016, 6(2), 39-48. |
| [4] | Gonçalves, M.; Guerreiro, M.C.; Ramos, P.H.; de Oliveira, L.C.; Sapag, K. Activated carbon prepared from coffee pulp: potential adsorbent of organic contaminants in aqueous solution. Water Sci. Technol., 2013, 68(5), 1085-1090. [http://dx.doi.org/10.2166/wst.2013.349] [PMID: 24037160] |
| [5] | Kaur, M.; Datta, M. Adsorption Characteristics of Acid Orange 10 from Aqueous Solutions onto Montmorillonite Clay. Adsorpt. Sci. Technol., 2011, 29(3), 301-318. [http://dx.doi.org/10.1260/0263-6174.29.3.301] |
| [6] | Wu, F.C.; Tseng, R.L.; Juang, R.S. A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J. Environ. Manage., 2010, 91(4), 798-806. [http://dx.doi.org/10.1016/j.jenvman.2009.10.018] [PMID: 19917518] |
| [7] | Kaya, I.; Bilici, A.; Gul, M. Schiff base substitute polyphenol and its metal complexes derived from o-vanillin with 2,3-diaminopyridine: synthesis, characterization, thermal, and conductivity properties. Polym. Adv. Technol., 2008, 19(4), 1154-1163. [http://dx.doi.org/10.1002/pat.1073] |
| [8] | Alabbad, E. Estimation the sorption capacity of chemically modified chitosan toward cadmium ion in wastewater effluents. Orient. J. Chem., 2019, 35(2), 757-765. [http://dx.doi.org/10.13005/ojc/350236] |
| [9] | Ma, J.; Yu, F.; Zhou, L.; Jin, L.; Yang, M.; Luan, J.; Tang, Y.; Fan, H.; Yuan, Z.; Chen, J. Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes. ACS Appl. Mater. Interfaces, 2012, 4(11), 5749-5760. [http://dx.doi.org/10.1021/am301053m] [PMID: 23062571] |
| [10] | Suciu, N.A.; Ferrari, T.; Ferrari, F.; Trevisan, M.; Capri, E. Pesticide removal from waste spray-tank water by organoclay adsorption after field application: An approach for a formulation of cyprodinil containing antifoaming/defoaming agents. Environ. Sci. Pollut. Res. Int., 2012, 19(4), 1229-1236. [http://dx.doi.org/10.1007/s11356-011-0643-9] [PMID: 22057850] |
| [11] | Bhatnagar, A.; Jain, A.K. A comparative adsorption study with different industrial wastes as adsorbents for the removal of cationic dyes from water. J. Colloid Interface Sci., 2005, 281(1), 49-55. [http://dx.doi.org/10.1016/j.jcis.2004.08.076] [PMID: 15567379] |
| [12] | Wang, S.; Boyjoo, Y.; Choueib, A.; Zhu, Z.H. Removal of dyes from aqueous solution using fly ash and red mud. Water Res., 2005, 39(1), 129-138. [http://dx.doi.org/10.1016/j.watres.2004.09.011] [PMID: 15607172] |
| [13] | Alakhras, F.; Al-Shahrani, H.; Al-Abbad, E.; Al-Rimawi, F.; Ouerfelli, N. Removal of Pb(II) metal ions from aqueous solutions using chitosan–vanillin derivatives chelating polymers. Pol. J. Environ. Stud., 2019, 28(3), 1523-1534. [http://dx.doi.org/10.15244/pjoes/89545] |
| [14] | Ravi Kumar, M.N.V. A review of chitin and chitosan application. React. Funct. Polym., 2000, 46(1), 1-27. [http://dx.doi.org/10.1016/S1381-5148(00)00038-9] |
| [15] | Shahidi, F.; Kamil, J.; Jeon, Y.; Kim, S. Antioxidant role of chitosan in a cooked cod (GADUS MORHUA) model system. J. Food Lipids, 2002, 9(1), 57-64. [http://dx.doi.org/10.1111/j.1745-4522.2002.tb00208.x] |
| [16] | Shahidi, F.; Abuzaytoun, R. Chitin, chitosan, and co-products: chemistry, production, applications, and health effects. Adv. Food Nutr. Res., 2005, 49, 93-135. [http://dx.doi.org/10.1016/S1043-4526(05)49003-8] [PMID: 15797344] |
| [17] | Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.J. Food Applications of Chitin and Chitosan. Trends Food Sci. Technol., 1999, 10, 37-51. [http://dx.doi.org/10.1016/S0924-2244(99)00017-5] |
| [18] | Shahidi, F.; Synowiecki, J. Isolation and characterization of nutrients and value-added products from snow crab (Chionoecetes opilio) and shrimp (Pandalus borealis) processing discards. J. Agric. Food Chem., 1991, 39(8), 1527-1532. [http://dx.doi.org/10.1021/jf00008a032] |
| [19] | Ajit, P.R.; Namdev, B.S.; Sangamesh, A.P.; Tejraj, M.A. Novel interpenetrating polymer network microspheres of chitosan and methylcellulose for controlled release of theophylline. Carbohydr. Polym., 2007, 69(4), 678-687. [http://dx.doi.org/10.1016/j.carbpol.2007.02.008] |
| [20] | Karathanos, V.T.; Mourtzinos, I.; Yannakopoulou, K.; Andrikopoulos, N.K. Study of the solubility, antioxidant activity and structure of inclusion complex of vanillin with b-cyclodextrin. Food Chem., 2007, 101(2), 652-658. [http://dx.doi.org/10.1016/j.foodchem.2006.01.053] |
| [21] | Sinha, A.K.; Sharma, U.K.; Sharma, N. A comprehensive review on vanilla flavor: extraction, isolation and quantification of vanillin and others constituents. Int. J. Food Sci. Nutr., 2008, 59(4), 299-326. [http://dx.doi.org/10.1080/09687630701539350] [PMID: 17886091] |
| [22] | Mourtzinos, I.; Konteles, S.; Kalogeropoulos, N.; Karathanos, V.T. Thermal oxidation of vanillin affects its antioxidant and antimicrobial properties. Food Chem., 2009, 114(3), 791-797. [http://dx.doi.org/10.1016/j.foodchem.2008.10.014] |
| [23] | Huang, R.; Liu, Q.; Huo, J.; Yang, B. Adsorption of methyl orange onto protonated cross-linked chitosan. Arab. J. Chem., 2017, 10(1), 24-32. [http://dx.doi.org/10.1016/j.arabjc.2013.05.017] |
| [24] | Chiou, M.S.; Li, H.Y. Equilibrium and kinetic modeling of adsorption of reactive dye on cross-linked chitosan beads. J. Hazard. Mater., 2002, 93(2), 233-248. [http://dx.doi.org/10.1016/S0304-3894(02)00030-4] [PMID: 12117469] |
| [25] | Zhu, H.; Jiang, R.; Xiao, L. Adsorption of an anionic azo dye by chitosan/kaolin/c-Fe2O3 composites. Appl. Clay Sci., 2009, 48, 522-526. [http://dx.doi.org/10.1016/j.clay.2010.02.003] |
| [26] | Iqbal, J.; Wattoo, F.H.; Wattoo, M.H.S.; Malik, R.; Tirmizi, S.A.; Imran, R.; Ghangro, A.B. Adsorption of acid yellow dye on flakes of chitosan prepared from fishery wastes. Arab. J. Chem., 2011, 4, 389-395. [http://dx.doi.org/10.1016/j.arabjc.2010.07.007] |
| [27] | Deepika, R.; Venkateshprabhu, M.; Pandimdevi, M. Studies on the behaviour of reactive dyes onto the cross-linked chitosan using adsorption isotherms. Int. J. Environ. Sci., 2013, 4(3), 323-351. |
| [28] | Kyaw, T.T.; Wint, D.S.; Naing, K.M. Studies on the sorption behavior of dyes on cross-linked chitosan beads in acid medium. International Conference on Biomedical Engineering and Technology IPCBEE, 2011, 174-178. |
| [29] | El-Sayed, E.M.; Tamer, T.M.; Omer, A.M.; Mohy Eldin, M.S. Development of novel chitosan schiff base derivatives for cationic dye removal: methyl orange model. Desalination Water Treat., 2016, 57(47), 22632-22645. [http://dx.doi.org/10.1080/19443994.2015.1136694] |
| [30] | Kundu, S.; Gupta, A.K. Arsenic adsorption onto iron oxide-coated cement (IOCC): regression analysis of equilibrium data with several isotherm models and their optimization. Chem. Eng., 2006, 122(1-2), 93-106. [http://dx.doi.org/10.1016/j.cej.2006.06.002] |
| [31] | Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon. Part I. Two-parameter models and equations allowing determination of thermodynamic parameters. J. Hazard. Mater., 2007, 147(1-2), 381-394. [http://dx.doi.org/10.1016/j.jhazmat.2007.01.021] [PMID: 17276594] |
| [32] | Saha, T.K.; Bhounil, N.C.; Karmaker, S.; Ahmed, M.G.; Ichikawa, H.; Fukumori, Y. Adsorption of methyl orange noto chitosan from aqueous solution. J. Water Resource Prot., 2010, 2, 898-906. [http://dx.doi.org/10.4236/jwarp.2010.210107] |
| [33] | Kang, S.; Qin, L.; Zhao, Y.; Wang, W.; Zhang, T.; Yang, L.; Rao, F.; Song, S. Enhanced removal of methyl orange on exfoliated montmorillonite/chitosan gel in presence of methylene blue. Chemosphere, 2020, 238,124693. [http://dx.doi.org/10.1016/j.chemosphere.2019.124693] [PMID: 31524627] |
| [34] | Wong, V.L.; Tay, S.Y.; Lim, S.S. Enhanced removal of methyl orange from aqueous solution by chitosan-CaCl2 beads. Mater. Sci. Eng. J., 2020, 736, 1-14. [http://dx.doi.org/10.1088/1757-899X/736/2/022049] |
| [35] | Huang, R.; Liu, Q.; Huo, J.; Yang, B. Adsorption of methyl orange onto protonated cross- linked chitosan. Arab. J. Chem., 2017, 10, 24-32. [http://dx.doi.org/10.1016/j.arabjc.2013.05.017] |
| [36] | Allouche, F.; Yassaa, N.; Lonuici, H. Sorption of methyl orange from aqueous solution on chitosan biomass. Procedia Earth Planet. Sci., 2015, 15, 596-601. [http://dx.doi.org/10.1016/j.proeps.2015.08.109] |
| [37] | Maleki, A.; Pajootan, E.; Hayati, B. Ethyl acrylate grafted chitosan for heavy metal removal from wastewater: Equilibrium, kinetic and thermodynamic studies. J. Taiwan Inst. Chem. Eng., 2015, 51, 127-134. [http://dx.doi.org/10.1016/j.jtice.2015.01.004] |
| [38] | Gibbs, G.; Tobin, J.M.; Guibal, E. Sorption of acid green 25 on chitosan: influence of experimental parameters on uptake kinetics and sorption isotherms. J. Appl. Polym. Sci., 2003, 90(4), 1073-1080. [http://dx.doi.org/10.1002/app.12761] |
| [39] | Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical models of sorption kinetics including a surface reaction mechanism: A review. Adv. Colloid Interface Sci., 2009, 152(1-2), 2-13. [http://dx.doi.org/10.1016/j.cis.2009.07.009] [PMID: 19735907] |
| [40] | Habiba, U.; Siddique, T.A.; Joo, T.C.; Salleh, A.; Ang, B.C.; Afifi, A.M. Synthesis of chitosan/polyvinyl alcohol/zeolite composite for removal of methyl orange, Congo red and chromium(VI) by flocculation/adsorption. Carbohydr. Polym., 2017, 157, 1568-1576. [http://dx.doi.org/10.1016/j.carbpol.2016.11.037] [PMID: 27987870] |
| [41] | Theydan, S.K. Effect of Process Variables, Adsorption kinetics and equilibrium studies of hexavalent chromium removal from aqueous solution by date seeds and its activated carbon by ZnCl2. Iraqi Academic Sci. J., 2018, 19(1), 1-12. |
| [42] | Alqaragully, M.B. Removal of textile dyes (maxilon blue, and methyl orange) by date stones activated carbon. Int. J. Adv. Res. Chem. Sci., 2014, 1(1), 48-59. |
| [43] | Li, Q.; Yue, Q.Y.; Su, Y.; Gao, B.Y.; Sun, H.J. Equilibrium, thermodynamics and process design to minimize adsorbent amount for the adsorption of acid dyes onto cationic polymer-loaded bentonite. Chem. Eng., 2010, 158(3), 489-497. [http://dx.doi.org/10.1016/j.cej.2010.01.033] |
| [44] | Kora, A.J.; Rastogi, L. Catalytic degradation of anthropogenic dye pollutants using palladium nanoparticles synthesized by gum olibanum, a glucuronoarabinogalactan biopolymer. Ind. Crops Prod., 2016, 81, 1-10. [http://dx.doi.org/10.1016/j.indcrop.2015.11.055] |








