- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Electrocoagulation for the Removal of Copper and Zinc Ions from Water Using Iron Electrodes
Majida K. Ahmad1, Mais A. Mohammed2, Mahmood M. Barbooti2, *
Abstract
Background:
Many methods have been suggested for the removal of heavy metals from water to protect human health and the environment. Methods like precipitation and adsorption were proposed for this purpose.
Objective:
Electrocoagulation involves the generation of coagulant by the action of electricity on two metal electrodes (iron or aluminium) to aid the process of water decontamination.
Methods:
Electrodeposition cell was made with iron electrodes and application of voltage from the power supply (5-25 V) dipped in the working solution (Cu and Zn) at various concentrations (10-50 mg.mL-1) for 30-150 min. Samples were drawn and analysed by atomic absorption spectrophotometry.
Results:
The work indicated efficient removal of the metal ions. The dependence of removal efficiency on the three parameters was studied. The behaviour of the two metal ions was not identical. At low initial concentration, the electrolysis voltage was very important in the removal of Zn and Cu ions. Electrolysis time is essential in the removal process and shows a polynomial dependence of removal efficiency on time. Electrolysis time of 150 min resulted in almost complete removal (94-97%) regardless of the initial concentration. Both co-precipitation and adsorption mechanisms may be involved.
Conclusion:
The removal efficiency was directly dependent on the initial metal ion concentration and electrolysis time. The process gave removal efficiency for copper that is higher than that of the zinc.
Article Information
Identifiers and Pagination:
Year: 2020Volume: 7
First Page: 37
Last Page: 43
Publisher Id: CHEM-7-37
DOI: 10.2174/1874842202007010037
Article History:
Received Date: 12/06/2020Revision Received Date: 21/09/2020
Acceptance Date: 06/10/2020
Electronic publication date: 18/12/2020
Collection year: 2020
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at Department of Applied Science, University of Technology, Baghdad, Iraq; E-mail: 100076@uotechnology.edu.iq
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 12-06-2020 |
Original Manuscript | Electrocoagulation for the Removal of Copper and Zinc Ions from Water Using Iron Electrodes | |
1. INTRODUCTION
The occurrence of heavy metals (HM), in the environment, represents a real challenge. Hence, they must be removed efficiently from surface water to reduce their adverse effects on aquatic life. Any action towards preventing the danger caused by heavy metals to human health and the environment, must involve their removal. This is because they cannot be transformed into another safe species nor being biodegradable. They tend to accumulate in living organisms (Saha and Sanyal, 2010 [1Saha, P.; Sanyal, S.K. Assessment of the removal of cadmium present in wastewater using soil–admixture membrane. Desalination, 2010, 259, 131-139.
[http://dx.doi.org/10.1016/j.desal.2010.04.021] ]). Many methods have been suggested for the removal of heavy metals, depending on the nature and chemistry of the metal ions themselves and their interaction with the environment. The reaction with MgO was suggested to initiate the precipitation reactions and consequently to isolate the heavy metal ions from water in an easily separable species [Barbooti, et al., 2011 [2Barbooti, M.M.; Abid, B.A.; Al-Shuwaiki, N.M. Removal of Heavy Metals Using Chemicals Precipitation. Eng. Technol. J., 2011, 29(3), 595-612.]. Adsorption receives a wide interest where the heavy metal ions attach themselves to a solid through electronic interaction with active centres on the adsorbent particle surfaces. A variety of single and composite materials have been studied and approved as potential adsorbents for the removal of heavy metals from water. Barbooti et al., 2019 [3Barbooti, M.M.; Al-Dabbagh, B.D.; Hilal, R.H. Preparation, characterization and utilization of polyacrylic acid–kaolin composite in the removal of heavy metals from water. Int. J. Environ. Sci. Technol., 2019, 16, 4571-4582.
[http://dx.doi.org/10.1007/s13762-018-2067-2] ] introduced kaolin- polyacrylic acid and polyacrylamide composites as adsorbents for the removal of nickel ions from water. Clay minerals like Kaolin, montmorillonite were also used to adsorb heavy metal ions like Pb, Cr, and Ni from water [3Barbooti, M.M.; Al-Dabbagh, B.D.; Hilal, R.H. Preparation, characterization and utilization of polyacrylic acid–kaolin composite in the removal of heavy metals from water. Int. J. Environ. Sci. Technol., 2019, 16, 4571-4582.
[http://dx.doi.org/10.1007/s13762-018-2067-2] -6Tripathi, A.; Ranjan, M.R. Heavy Metal Removal from Wastewater Using Low Cost Adsorbents, Tripathi and Ranjan. J. Bioremediat. Biodegrad., 2015, 6, 6.
[http://dx.doi.org/10.4172/2155-6199.1000315] ]. Nano-materials were another category of adsorbents that have been utilized for the removal of heavy metals. However, costly adsorbents need to be regenerated and reused to assist the economy of the process [7Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the Removal of Heavy Metals from Wastewater J. Yang, B. Hou, J. Wang, B. Tian. Nanomaterials (Basel), 2019, 9, 424.
[http://dx.doi.org/10.3390/nano9030424] ].
Electrocoagulation, EC, was a recently introduced technique for the purification of water (Barrera-Díaz et al. 2012 [8Barrera-Díaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater., 2012, 223-224, 1-12.
[http://dx.doi.org/10.1016/j.jhazmat.2012.04.054] [PMID: 22608208] ]. The method is based on the co-precipitation and inclusion of the target materials within the in-situ formed coagulants like Al and Fe hydroxides (Naje, et al., 2016 [9Naje, A.S.; Chelliapan, S.; Zakaria, Z.; Ajeel, M.A.; Alaba, P.A. A review of electrocoagulation technology for the treatment of textile wastewater, De Gruyter. Rev. Chem. Eng., 2016, •••, 1-30.]). Roa-Morales, et al. [10Roa-Morales, G.A.; Barrera-Díaz, C.; Balderas-Hernández, P.; Zaldumbide-Ortiz, F.; Perez, H.R.; Bilyeu, B. Removal of color and chemical oxygen demand using a coupled coagulation-electrocoagulation-ozone treatment of industrial wastewater that contains offset printing dyes. J. Mex. Chem. Soc., 2014, 58(3), 362-368.], employed the method for the removal of color and chemical Oxygen Demand by coupling with Ozone treatment. The EC involves the formation of coagulants by electrolytic oxidation of the anode. The emulsion will be broken and the suspension of particulate and pollutants is weakened (Chaturvedi 2013 [11Chaturvedi, S.I. Electrocoagulation: a novel waste water treatment method. Int. J. Mod. Eng. Res., 2013, 3, 93-100.]). Flocs will then form through the coagulation and produces a sludge blanket, which capitulates and bridges the colloidal species in the aqueous phase. The solid oxides, hydroxides, and oxyhydroxides generate the active surfaces for the adsorption of the contaminants.
The potential of iron anode and cathode is based on the production of Fe(III) at the anode and reduction to the Fe(II) at the cathode (Vepsäläinen 2012 [12Vepsäläinen, M. Electrocoagulation in the treatment of industrial waters and wastewaters, Ph. D. Thesis, VTT Technical Research Centre of Finland, 2012.], Moreno et al. (2009 [13Moreno, C.H.A.; Cocke, D.L.; Gomes, J.A.; Morkovsky, P.; Parga, J.; Peterson, E.; Garcia, C. Electrochemical reactions for electrocoagulation using iron electrodes. Ind. Eng. Chem. Res., 2009, 48, 2275-2282.
[http://dx.doi.org/10.1021/ie8013007] ] and Gomes et al. 2007 [14Gomes, J.A.; Daida, P.; Kesmez, M.; Weir, M.; Moreno, H.; Parga, J.R.; Irwin, G.; McWhinney, H.; Grady, T.; Peterson, E.; Cocke, D.L. Arsenic removal by electrocoagulation using combined Al-Fe electrode system and characterization of products. J. Hazard. Mater., 2007, 139(2), 220-231.
[http://dx.doi.org/10.1016/j.jhazmat.2005.11.108] [PMID: 17113227] ]). Neutral to slight alkaline pH medium is typical for the oxidation into Fe(III), as in Equation (1), (Barrera Díaz et al. 2012 [9Naje, A.S.; Chelliapan, S.; Zakaria, Z.; Ajeel, M.A.; Alaba, P.A. A review of electrocoagulation technology for the treatment of textile wastewater, De Gruyter. Rev. Chem. Eng., 2016, •••, 1-30.], Sasson et al. 2009 [15Ben Sasson, M.; Calmano, W.; Adin, A. Iron-oxidation processes in an electroflocculation (electrocoagulation) cell. J. Hazard. Mater., 2009, 171(1-3), 704-709.
[http://dx.doi.org/10.1016/j.jhazmat.2009.06.057] [PMID: 19577360] ].
| Mn+ (aq) + ne- → Me0(s) | (1) |
The amount of metal cations dissolved during the reactions at the anode is calculated using Faraday’s law [Eq. (2)] (Chaturvedi 2013 [11Chaturvedi, S.I. Electrocoagulation: a novel waste water treatment method. Int. J. Mod. Eng. Res., 2013, 3, 93-100.], and Khaled et al. 2015 [16Khaled, B.; Wided, B.; Bechir, H.; Elimame, E.; Mouna, L.; Zied, T. Investigation of electrocoagulation reactor design parameters effect on the removal of cadmium from synthetic and phosphate industrial wastewater. Arab. J. Chem., 2015, 12(8), 1848-1859.
[http://dx.doi.org/10.1016/j.arabjc.2014.12.012] ]):
| M = (ItMw)/zF | (2) |
where I is the current (A), t is the operation time (s), Mw the calculated value according to Faraday’s law under is the molecular weight of the substance (g/mol), F is Faraday’s constant (96,485 C/mol), z is the number of electrons involved in the reaction is 3 for Fe and Al), and m is the quantity of metal dissolved (g). Hydroxyl ions are produced at the cathode (Eq. 3), or by the consumption of the hydronium ions (Eq. 4) (Chaturvedi 2013 [12Vepsäläinen, M. Electrocoagulation in the treatment of industrial waters and wastewaters, Ph. D. Thesis, VTT Technical Research Centre of Finland, 2012.]:
| 2H2O + 2e- → H2 + 2 OH- | (3) |
| 2H+(aq) + 2e- ------ H2(g) | (4) |
Oxygen is formed on the anode (Khaled et al. 2015 [16Khaled, B.; Wided, B.; Bechir, H.; Elimame, E.; Mouna, L.; Zied, T. Investigation of electrocoagulation reactor design parameters effect on the removal of cadmium from synthetic and phosphate industrial wastewater. Arab. J. Chem., 2015, 12(8), 1848-1859.
[http://dx.doi.org/10.1016/j.arabjc.2014.12.012] ]) under typical conditions (electrochemical potential) of EC systems when the anode dissolution follows Faraday’s law (Mouedhen et al. 2008 [17Mouedhen, G.; Feki, M.; Wery, Mde.P.; Ayedi, H.F. Behavior of aluminum electrodes in electrocoagulation process. J. Hazard. Mater., 2008, 150(1), 124-135.
[http://dx.doi.org/10.1016/j.jhazmat.2007.04.090] [PMID: 17537574] ]). The current density directly changes, affecting hydrogen production. In comparison with chemical coagulation, EC surges the pH of the solution if acidic, neutral, or slightly alkaline and declines when it is extremely alkaline. This ultimately affects the speciation of aluminum and iron hydroxides. At highly acidic systems (pH 2), the EC is not adequate to raise the pH of the solution by the formed OH ions. Meanwhile, pH value shows some increase for solutions with pH 3 and higher (Mouedhen et al. 2008 [17Mouedhen, G.; Feki, M.; Wery, Mde.P.; Ayedi, H.F. Behavior of aluminum electrodes in electrocoagulation process. J. Hazard. Mater., 2008, 150(1), 124-135.
[http://dx.doi.org/10.1016/j.jhazmat.2007.04.090] [PMID: 17537574] ]). The pH change is then affected by the anion concentration in the solution. The electrodes, especially aluminum, may be activated with Mg, In and Zn alloying elements to improve the performance [18Dura, A.; Breslin, C.B. Electrocoagulation using aluminium anodes activated with Mg, In and Zn alloying elements. J. Hazard. Mater., 2019, 366, 39-45.
[http://dx.doi.org/10.1016/j.jhazmat.2018.11.094] [PMID: 30502571] ]. Heidmann and Calmano (2008 [19Heidmann, I.; Calmano, W. Removal of Ni, Cu and Cr from a galvanic wastewater in an electrocoagulation system with Fe-and Al-electrodes. Separ. Purif. Tech., 2010, 71, 308-314.
[http://dx.doi.org/10.1016/j.seppur.2009.12.016] ]) reported that Zn(II), Cu(II), Ni(II), and Ag(I) can be eliminated from the solution by hydrolyzation and co-precipitation. However, Cr(VI) may be reduced to Cr(III), and precipitated as hydroxide. Iron electrodes produced crystalline phases, such as magnetite, and poorly crystalline phases, such as iron oxyhydroxides and lepidocrocite. Un and Aytac (2013 [20Oden, M.K.; Sari-Erkan, H. Treatment of metal plating wastewater using iron electrode by electrocoagulation process: Optimization and process performance. Process Saf. Environ. Prot., 2018, 119, 207-217.
[http://dx.doi.org/10.1016/j.psep.2018.08.001] ]) investigated the sludge produced after EC process with iron electrodes for textile wastewater treatment and described it by X-ray diffraction (XRD) to determine the main elimination pathway in the reactor. Electrode material determines the type of electrochemical reactions taking place in the EC system. Iron dissolves as Fe(II) and is oxidized in bulk solution to Fe(III) in the presence of oxidants like oxygen (Un and Aytac 2013 [21Tezcan Un, U.; Aytac, E. Electrocoagulation in a packed bed reactor-complete treatment of color and cod from real textile wastewater. J. Environ. Manage., 2013, 123, 113-119.
[http://dx.doi.org/10.1016/j.jenvman.2013.03.016] [PMID: 23590945] ], Roopashree and Lokesh 2014 [[21Tezcan Un, U.; Aytac, E. Electrocoagulation in a packed bed reactor-complete treatment of color and cod from real textile wastewater. J. Environ. Manage., 2013, 123, 113-119.
[http://dx.doi.org/10.1016/j.jenvman.2013.03.016] [PMID: 23590945] ]). Fe(II) is a weak coagulant compared to Fe(III) because of the higher solubility of hydroxides and lower positive charge (Un and Ocal 2015) [22Roopashree, A.G.; Lokesh, K.S. Comparative study of electrode material (iron, aluminum and stainless steel) for treatment of textile industry wastewater. Int. J. Environ. Sci., 2014, 4, 519.]. Ideal material selection depends on the type of contaminants and the chemical characteristics of the electrolyte. The method was optimized for the treatment of metal plating wastewater using iron electrodes [23Un, U.T.; Ocal, S.E. Removal of heavy metals (Cd, Cu, Ni) by electrocoagulation. Int. J. Environ. Sci. Dev., 2015, 6, 425.
[http://dx.doi.org/10.7763/IJESD.2015.V6.630] ]. Linares-Hernández et al. (2009 [24Hussin, F.; Abnisa, F.; Issabayeva, G.; Aroua, M.K. Removal of lead by solar-photovoltaic electrocoagulation using novel perforated zinc electrode. J. Clean. Prod., 2017, 147, 206-216.
[http://dx.doi.org/10.1016/j.jclepro.2017.01.096] ]) reported high efficiency in reducing chemical oxygen demand (COD) from industrial wastewater for both color and COD reduction, a combination of iron and aluminum electrodes. Similar results were obtained when paper mill wastewaters were treated with various aluminum and iron electrode combinations (Katal and Pahlavanzadeh 2011 [25Linares-Hernández, I.; Barrera-Díaz, C.; Roa-Morales, G.; Bilyeu, B.; Ureña-Núñez, F. A combined electrocoagulation-sorption process applied to mixed industrial wastewater. J. Hazard. Mater., 2007, 144(1-2), 240-248.
[http://dx.doi.org/10.1016/j.jhazmat.2006.10.015] [PMID: 17118541] ]). Similar results were obtained for copper, chromium and nickel removal from metal plating wastewater (Heidmann and Calmano 2010 [19Heidmann, I.; Calmano, W. Removal of Ni, Cu and Cr from a galvanic wastewater in an electrocoagulation system with Fe-and Al-electrodes. Separ. Purif. Tech., 2010, 71, 308-314.
[http://dx.doi.org/10.1016/j.seppur.2009.12.016] ]). Brahmi, et al., 2019, investigated the electrocoagulation reactor design parameters effect on the removal of cadmium ions from synthetic and phosphate industrial wastewater [26Katal, R.; Pahlavanzadeh, H. Influence of different combinations of aluminum and iron electrode on electrocoagulation efficiency: application to the treatment of paper mill wastewater. Desalination, 2011, 265, 199-205.
[http://dx.doi.org/10.1016/j.desal.2010.07.052] , 27Brahmi, K. W. B. B. Hamrouni, E. Elaloui, M. Loungou, Z. Tlili, Investigation of electrocoagulation reactor design parameters effect on the removal of cadmium from synthetic and phosphate industrial wastewater. Arab. J. Chem., 2019, 12(8), 1848-1859.
[http://dx.doi.org/10.1016/j.arabjc.2014.12.012] ]. The application of the method was further simplified by utilizing solar photovoltaic cells to supply the needed electrical power to feed the process. Khan et al. [28Khan, S.U.; Izharul, D.T.I.; Ayub, H.F.S.; Basheer, F. Hexavalent chromium removal in an electrocoagulation column reactor: Process optimization using CCD, adsorption kinetics and pH modulated sludge formation. Process Saf. Environ. Prot., 2019, 122, 118-130.
[http://dx.doi.org/10.1016/j.psep.2018.11.024] ], employed a column EC reactor to remove hexavalent chromium from water.
In the present work, an electrocoagulation system is constructed with iron electrodes and used in the removal of copper and zinc ions from water. The effect of working parameters are investigated on the removal efficiency of the ions
2. MATERIALS AND METHODS
2.1. Apparatus
The copper and zinc concentrations were measured on AA-7000 supplied from (SHIMADZU) atomic absorption spectrophotometer using the operating conditions recommended by the manufacturer with the proper dilution of the samples as required.
2.2. Chemicals and Reagents
The standard stock solution of zinc (1000 mg.L-1) was prepared by dissolving the analytical grade metal powder from (BDH) in hydrochloric acid and diluted to the mark with nano grade deionized water. For copper, the stock solution (1000 mg.L-1) was prepared by dissolving the analytical grade copper sulfate from (BDH) in deionized and further dilution to the mark of the calibrated flask nano grade deionized water. The preparation of the working solution (10-50 mg.L-1) was done by placing the calculated volume with water to make 500 mL. The electrode material was commercially available steel slabs with dimensions of 3x50x140 mm.
Fig. (1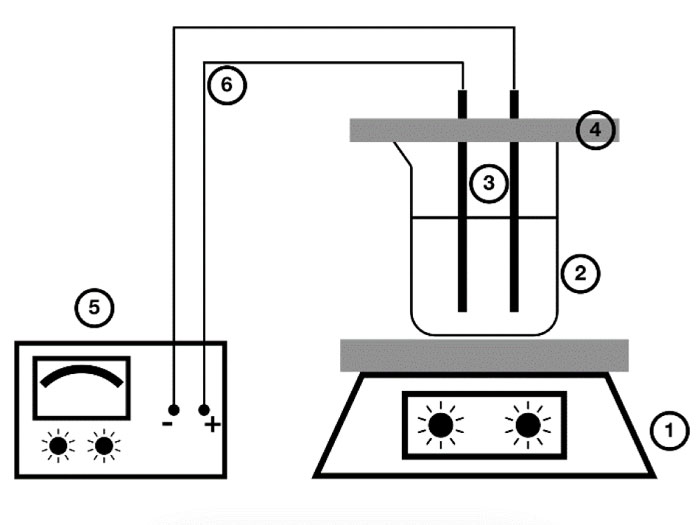 ): Schematic diagram of electrocoagulation set up, 1, Magnetic stirrer, 2, Working solution, 3, Iron electrodes, 4, Plastic bar, 5, Power supply, 6, connection wires.
): Schematic diagram of electrocoagulation set up, 1, Magnetic stirrer, 2, Working solution, 3, Iron electrodes, 4, Plastic bar, 5, Power supply, 6, connection wires.
2.3. Electrocoagulation Setup
2.4. Procedures
Before each treatment, the electrodes (anode and cathode) were rubbed with sandpaper then cleaned with NaOH (2 M) and HCl (2 M) aqueous solutions to avoid a passivation film (Khaled et al., 2015 [16Khaled, B.; Wided, B.; Bechir, H.; Elimame, E.; Mouna, L.; Zied, T. Investigation of electrocoagulation reactor design parameters effect on the removal of cadmium from synthetic and phosphate industrial wastewater. Arab. J. Chem., 2015, 12(8), 1848-1859.
[http://dx.doi.org/10.1016/j.arabjc.2014.12.012] ]). The homogenization of the working solution was done by mixing with a magnetic stirrer to form and float the flocs. Fast stirring is beneficial at the beginning to allow the water component to mix with coagulants and to disperse chemicals in the wastewater stream (Vepsäläinen 2012 [12Vepsäläinen, M. Electrocoagulation in the treatment of industrial waters and wastewaters, Ph. D. Thesis, VTT Technical Research Centre of Finland, 2012.]. The solution was kept without pH adjustment as almost neutral pH gave efficient removal of copper [29Mateen, Q.S.; Khan, S.U.; Islam, D.T.; Khan, N.A.; Farooqi, I.H. Copper (II) removal in a column reactor using electrocoagulation: Parametric optimization by response surface methodology using central composite design, Water Environ., 2020, ]
3. RESULTS AND DISCUSSION
3.1. Effect of Electrolysis Voltage
The effect of electrolysis time on the removal of Cu and Zn from water by electrocoagulation using various voltages was studied, and the residual concentration of the metal ion was measured to estimate the efficiency of removal. Fig. (2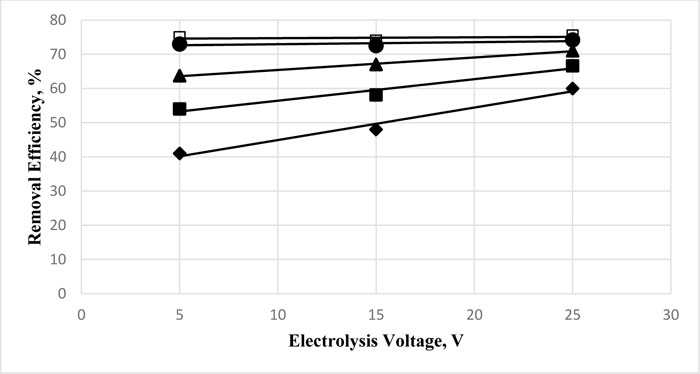 ) shows the removal efficiency of copper as a function of time using three electrolysis voltages (5, 15, and 25 V). At the low time values, there was an appreciable improvement in the removal efficiency (41 to 59%) by the increase of voltage from 5 to 25 V, respectively. For the rest of the electrolysis time values, however, the electrolysis voltage does not seem critical for the removal of copper ions from water, although a slight increase is recorded. Thus, beyond one hour of electrolysis, the effect of voltage was minor on the overall removal efficiency.
) shows the removal efficiency of copper as a function of time using three electrolysis voltages (5, 15, and 25 V). At the low time values, there was an appreciable improvement in the removal efficiency (41 to 59%) by the increase of voltage from 5 to 25 V, respectively. For the rest of the electrolysis time values, however, the electrolysis voltage does not seem critical for the removal of copper ions from water, although a slight increase is recorded. Thus, beyond one hour of electrolysis, the effect of voltage was minor on the overall removal efficiency.
Linear correlation can be observed for the increase of efficiency with voltage. The slope of the straight line decreases with the increase of electrolysis time, and rather, constant removal efficiency is established for the various voltages at the highest electrolysis time (150 min.).
For zinc removal, the results of the effect of time on the removal efficiency are given in Fig. (3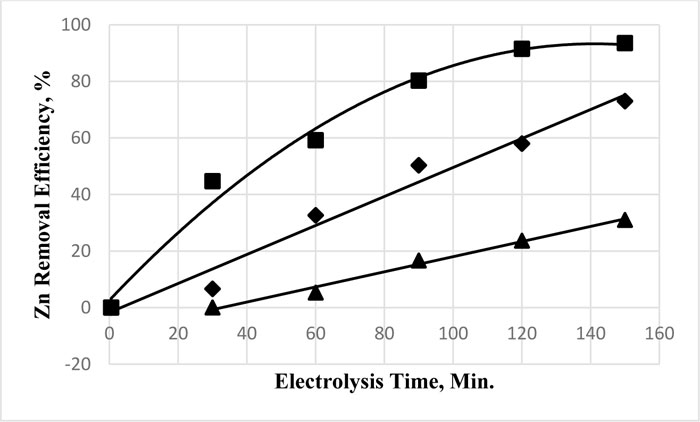 ) for various electrolysis voltages. There was no appreciable removal prior to one hr of electrolysis when the voltage was 5V. Higher electrolysis voltage resulted in good removal from the first 5 min of electrolysis. The removal efficiency exhibited a linear increase with the electrolysis time at 15 V. The efficiency increase with time at 25 V followed a polynomial relation. The higher voltage of electrolysis and, thus, the resultant current density will be higher. Such a condition improves the removal efficiency of Zn and Cu, as reported by Chen et al., 2018 [30Chen, F.; Li, X.; Luo, Z.; Ma, J.Zh.Q.; Zhang, S. Advanced treatment of copper smelting wastewater by the combination of internal micro-electrolysis and electrocoagulation. Sep. Sci. Technol., 2018, 53(16), 2639-2646.
) for various electrolysis voltages. There was no appreciable removal prior to one hr of electrolysis when the voltage was 5V. Higher electrolysis voltage resulted in good removal from the first 5 min of electrolysis. The removal efficiency exhibited a linear increase with the electrolysis time at 15 V. The efficiency increase with time at 25 V followed a polynomial relation. The higher voltage of electrolysis and, thus, the resultant current density will be higher. Such a condition improves the removal efficiency of Zn and Cu, as reported by Chen et al., 2018 [30Chen, F.; Li, X.; Luo, Z.; Ma, J.Zh.Q.; Zhang, S. Advanced treatment of copper smelting wastewater by the combination of internal micro-electrolysis and electrocoagulation. Sep. Sci. Technol., 2018, 53(16), 2639-2646.
[http://dx.doi.org/10.1080/01496395.2018.1463265] ]. The difference in the behavior of zinc and copper lies in the electrochemical properties of the two metal ions in the solution.
The low voltage (5 V) did not cause any removal prior to one hour of electrolysis. Identical efficiency values were obtainable at 5 V after 30 and 60 min. The removal efficiency at the longest electrolysis time (150 min) at 5 V could be achieved after 60 min at 15 volts. The efficiency at 15 Volts after 2 hr was almost identical to that recorded after one hour of electrolysis at 25 volts. The removal efficiency linearly increases with the increase of the applied voltage for electrolysis times of 60-120 min. The increase follows a polynomial relation for 30 and 150 minutes of electrolysis time.
 |
Fig. (2) Effect of electrolysis voltage on the removal efficiency of copper of 30 mg.L-1 initial concentration after electrolysis for 30, ♦, 60, ■, 90, ▲, 120, ☻, and 150 min, □, at neutral medium. |
 |
Fig. (3) Effect of electrolysis time on the removal efficiency of zinc of 30 mg. L-1 at electrolysis voltage of 5 V (▲); 15 V (♦) and 25 V (■), at neutral medium. |
3.2. Effect of Electrolysis Time
Fig. (4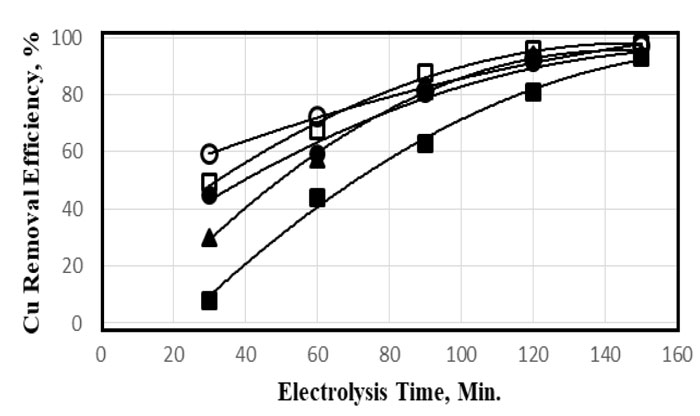 ) shows the dependence of copper removal efficiency of the electrolysis time at electrolysis voltage of 25 V using various initial zinc concentrations. The removal efficiency as a function of time followed a logarithmic relation. Within the first 5 minutes, almost 50% of the total removal was performed for 40 and 50 mg.L-1. The rest of the removal proceeded linearly with the increase of time. However, the voltage was an essential factor in the determination of removal efficiency. The increase of voltage from 15 to 25 V resulted in an increase in efficiency by 20%. At the early stages of the electrolysis, the initial concentration plays an important role in the removal of copper ions.
) shows the dependence of copper removal efficiency of the electrolysis time at electrolysis voltage of 25 V using various initial zinc concentrations. The removal efficiency as a function of time followed a logarithmic relation. Within the first 5 minutes, almost 50% of the total removal was performed for 40 and 50 mg.L-1. The rest of the removal proceeded linearly with the increase of time. However, the voltage was an essential factor in the determination of removal efficiency. The increase of voltage from 15 to 25 V resulted in an increase in efficiency by 20%. At the early stages of the electrolysis, the initial concentration plays an important role in the removal of copper ions.
For zinc removal ions from water, the efficiency increases with time following a polynomial function for various initial concentrations (Fig. 5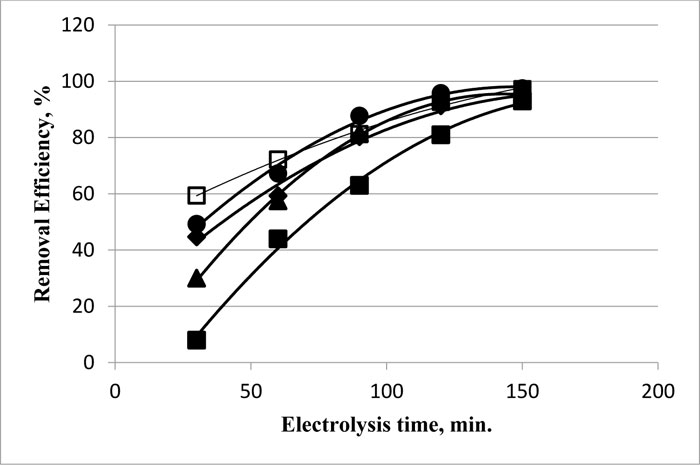 ). At short electrolysis time (30 min), the initial concentration was very effective, and many differences in the removal efficiency were recorded, being 8% at 10 ppm and 60% at the 50 ppm-level. At longer electrolysis time, the efficiency attained values ranging from 93 to 97.5% for the 10 and 50 ppm initial concentrations, respectively. There is a general increase in efficiency with the increase of initial concentration, especially at short electrolysis time (30 and 60 min). The process showed perfection as the initial concentration increases. However, the removal efficiency at the long electrolysis time values (150 min.) was almost independent of the concentration, and attained values in the range 95 to 98% for the 10 and 50 mg.L-1 after two hours of electrolysis.
). At short electrolysis time (30 min), the initial concentration was very effective, and many differences in the removal efficiency were recorded, being 8% at 10 ppm and 60% at the 50 ppm-level. At longer electrolysis time, the efficiency attained values ranging from 93 to 97.5% for the 10 and 50 ppm initial concentrations, respectively. There is a general increase in efficiency with the increase of initial concentration, especially at short electrolysis time (30 and 60 min). The process showed perfection as the initial concentration increases. However, the removal efficiency at the long electrolysis time values (150 min.) was almost independent of the concentration, and attained values in the range 95 to 98% for the 10 and 50 mg.L-1 after two hours of electrolysis.
3.3. Effect of Initial Concentration
Fig. (6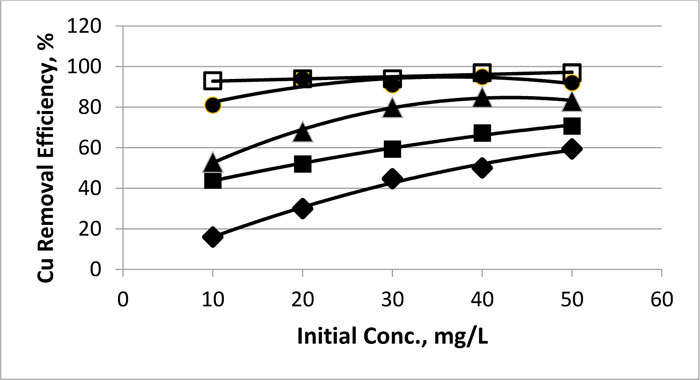 ) shows the dependence of removal efficiency of copper on the initial concentration for various electrolysis times using electrolysis voltage of 25 V. At the early stages of the electrolysis, the initial concentration plays an important role in the removal of copper ions, when almost 50% of the total removal was performed within the first 5 minutes. At the low concentration range (10 and 20 mg.L-1), the efficiency was highly dependent on the electrolysis time being higher with longer electrolysis time. The 10 mg.L-1 values of 16% were recorded for 30 minutes and 93% after 150 mins. Respectively. The effect was lower for the high initial concentration (50 mg.L-1), being 59.4 and 97% for 30 and 150 mins, respectively. The efficiency was almost independent of the initial concentration when 150 min time was used for the process.
) shows the dependence of removal efficiency of copper on the initial concentration for various electrolysis times using electrolysis voltage of 25 V. At the early stages of the electrolysis, the initial concentration plays an important role in the removal of copper ions, when almost 50% of the total removal was performed within the first 5 minutes. At the low concentration range (10 and 20 mg.L-1), the efficiency was highly dependent on the electrolysis time being higher with longer electrolysis time. The 10 mg.L-1 values of 16% were recorded for 30 minutes and 93% after 150 mins. Respectively. The effect was lower for the high initial concentration (50 mg.L-1), being 59.4 and 97% for 30 and 150 mins, respectively. The efficiency was almost independent of the initial concentration when 150 min time was used for the process.
 |
Fig. (4) Dependence of removal efficiency of Cu on the electrolysis time for various initial concentrations 10 (■), 20 (▲), 30 (☻), 40 (□), and 50 ppm (○) using a voltage of 25 V, at neutral medium. |
 |
Fig. (5) Dependence of removal efficiency of Zn on the electrolysis time for various initial concentrations 10 (■), 20 (▲), 30 (♦), 40 (☻), and 50 ppm (□) using a voltage of 25 V. |
 |
Fig. (6) The dependence of removal efficiency of copper on the initial concentrations for various electrolysis time at 25 V electrolysis voltage. |
Fig. (7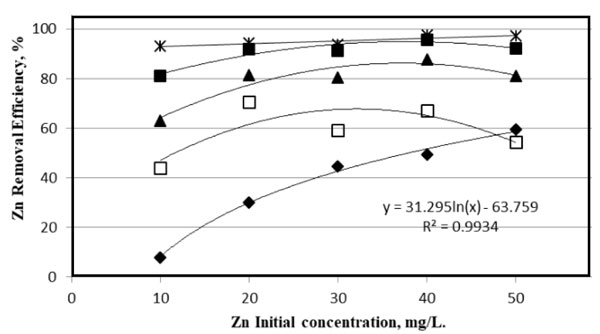 ) displays the dependence of removal efficiency of zinc with the initial concentrations for various electrolysis time at 25 V electrolysis voltage. At a short electrolysis duration (30 min), the initial concentration of zinc is highly effective on the removal efficiency. It follows a polynomial relationship with a reasonable correlation coefficient of (R2 = 0.9924) as follows.
) displays the dependence of removal efficiency of zinc with the initial concentrations for various electrolysis time at 25 V electrolysis voltage. At a short electrolysis duration (30 min), the initial concentration of zinc is highly effective on the removal efficiency. It follows a polynomial relationship with a reasonable correlation coefficient of (R2 = 0.9924) as follows.
| Reff = - 0.0132C2 + 1.8623C - 1.2933 | (5) |
Where Reff is the removal efficiency and C is the initial concentration of zinc in the working solution. For other electrolysis time intervals, the effect of the concentration was minor, and the efficiency gets higher as the electrolysis time increases. The Reff ranged from 93-97% for all concentrations when the electrolysis time was 150 min.
CONCLUSION
Electrocoagulation can be recommended as a simple and rather cheap method for the removal of heavy metals from water. The removal efficiency is dependent on the initial concentration only at short electrolysis times. Electrolysis voltages of 15 V and higher are preferred to give remarkable removal efficiency values regardless of the initial concentration for durations of 90 min and longer. At 25 V, the removal efficiency ranged from 93-97% for all concentrations when the electrolysis time was 150 min. Regarding the cost of the process, there are two main criteria for comparison of the available method with the addition of alum to the water. The first is the omission of purchase of the alum. The second is the reduction in the equipment of the process.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are grateful for the support of the technical workshop staff for their help.
REFERENCES
| [1] | Saha, P.; Sanyal, S.K. Assessment of the removal of cadmium present in wastewater using soil–admixture membrane. Desalination, 2010, 259, 131-139. [http://dx.doi.org/10.1016/j.desal.2010.04.021] |
| [2] | Barbooti, M.M.; Abid, B.A.; Al-Shuwaiki, N.M. Removal of Heavy Metals Using Chemicals Precipitation. Eng. Technol. J., 2011, 29(3), 595-612. |
| [3] | Barbooti, M.M.; Al-Dabbagh, B.D.; Hilal, R.H. Preparation, characterization and utilization of polyacrylic acid–kaolin composite in the removal of heavy metals from water. Int. J. Environ. Sci. Technol., 2019, 16, 4571-4582. [http://dx.doi.org/10.1007/s13762-018-2067-2] |
| [4] | Ugwu, I.; Igbokwe, O.A. Sorption of Heavy Metals on Clay Minerals and Oxides: A Review In Advanced Sorption Process Applications, 2019. [http://dx.doi.org/10.5772/ intechopen. 80989] |
| [5] | Barbooti, M.M. Simultaneous removal of chromium and lead from water by sorption on iraqi montmorillonite. J. Environ. Prot. (Irvine Calif.), 2015, 6, 236-247. [http://dx.doi.org/10.4236/jep.2015.63024] |
| [6] | Tripathi, A.; Ranjan, M.R. Heavy Metal Removal from Wastewater Using Low Cost Adsorbents, Tripathi and Ranjan. J. Bioremediat. Biodegrad., 2015, 6, 6. [http://dx.doi.org/10.4172/2155-6199.1000315] |
| [7] | Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the Removal of Heavy Metals from Wastewater J. Yang, B. Hou, J. Wang, B. Tian. Nanomaterials (Basel), 2019, 9, 424. [http://dx.doi.org/10.3390/nano9030424] |
| [8] | Barrera-Díaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater., 2012, 223-224, 1-12. [http://dx.doi.org/10.1016/j.jhazmat.2012.04.054] [PMID: 22608208] |
| [9] | Naje, A.S.; Chelliapan, S.; Zakaria, Z.; Ajeel, M.A.; Alaba, P.A. A review of electrocoagulation technology for the treatment of textile wastewater, De Gruyter. Rev. Chem. Eng., 2016, •••, 1-30. |
| [10] | Roa-Morales, G.A.; Barrera-Díaz, C.; Balderas-Hernández, P.; Zaldumbide-Ortiz, F.; Perez, H.R.; Bilyeu, B. Removal of color and chemical oxygen demand using a coupled coagulation-electrocoagulation-ozone treatment of industrial wastewater that contains offset printing dyes. J. Mex. Chem. Soc., 2014, 58(3), 362-368. |
| [11] | Chaturvedi, S.I. Electrocoagulation: a novel waste water treatment method. Int. J. Mod. Eng. Res., 2013, 3, 93-100. |
| [12] | Vepsäläinen, M. Electrocoagulation in the treatment of industrial waters and wastewaters, Ph. D. Thesis, VTT Technical Research Centre of Finland, 2012. |
| [13] | Moreno, C.H.A.; Cocke, D.L.; Gomes, J.A.; Morkovsky, P.; Parga, J.; Peterson, E.; Garcia, C. Electrochemical reactions for electrocoagulation using iron electrodes. Ind. Eng. Chem. Res., 2009, 48, 2275-2282. [http://dx.doi.org/10.1021/ie8013007] |
| [14] | Gomes, J.A.; Daida, P.; Kesmez, M.; Weir, M.; Moreno, H.; Parga, J.R.; Irwin, G.; McWhinney, H.; Grady, T.; Peterson, E.; Cocke, D.L. Arsenic removal by electrocoagulation using combined Al-Fe electrode system and characterization of products. J. Hazard. Mater., 2007, 139(2), 220-231. [http://dx.doi.org/10.1016/j.jhazmat.2005.11.108] [PMID: 17113227] |
| [15] | Ben Sasson, M.; Calmano, W.; Adin, A. Iron-oxidation processes in an electroflocculation (electrocoagulation) cell. J. Hazard. Mater., 2009, 171(1-3), 704-709. [http://dx.doi.org/10.1016/j.jhazmat.2009.06.057] [PMID: 19577360] |
| [16] | Khaled, B.; Wided, B.; Bechir, H.; Elimame, E.; Mouna, L.; Zied, T. Investigation of electrocoagulation reactor design parameters effect on the removal of cadmium from synthetic and phosphate industrial wastewater. Arab. J. Chem., 2015, 12(8), 1848-1859. [http://dx.doi.org/10.1016/j.arabjc.2014.12.012] |
| [17] | Mouedhen, G.; Feki, M.; Wery, Mde.P.; Ayedi, H.F. Behavior of aluminum electrodes in electrocoagulation process. J. Hazard. Mater., 2008, 150(1), 124-135. [http://dx.doi.org/10.1016/j.jhazmat.2007.04.090] [PMID: 17537574] |
| [18] | Dura, A.; Breslin, C.B. Electrocoagulation using aluminium anodes activated with Mg, In and Zn alloying elements. J. Hazard. Mater., 2019, 366, 39-45. [http://dx.doi.org/10.1016/j.jhazmat.2018.11.094] [PMID: 30502571] |
| [19] | Heidmann, I.; Calmano, W. Removal of Ni, Cu and Cr from a galvanic wastewater in an electrocoagulation system with Fe-and Al-electrodes. Separ. Purif. Tech., 2010, 71, 308-314. [http://dx.doi.org/10.1016/j.seppur.2009.12.016] |
| [20] | Oden, M.K.; Sari-Erkan, H. Treatment of metal plating wastewater using iron electrode by electrocoagulation process: Optimization and process performance. Process Saf. Environ. Prot., 2018, 119, 207-217. [http://dx.doi.org/10.1016/j.psep.2018.08.001] |
| [21] | Tezcan Un, U.; Aytac, E. Electrocoagulation in a packed bed reactor-complete treatment of color and cod from real textile wastewater. J. Environ. Manage., 2013, 123, 113-119. [http://dx.doi.org/10.1016/j.jenvman.2013.03.016] [PMID: 23590945] |
| [22] | Roopashree, A.G.; Lokesh, K.S. Comparative study of electrode material (iron, aluminum and stainless steel) for treatment of textile industry wastewater. Int. J. Environ. Sci., 2014, 4, 519. |
| [23] | Un, U.T.; Ocal, S.E. Removal of heavy metals (Cd, Cu, Ni) by electrocoagulation. Int. J. Environ. Sci. Dev., 2015, 6, 425. [http://dx.doi.org/10.7763/IJESD.2015.V6.630] |
| [24] | Hussin, F.; Abnisa, F.; Issabayeva, G.; Aroua, M.K. Removal of lead by solar-photovoltaic electrocoagulation using novel perforated zinc electrode. J. Clean. Prod., 2017, 147, 206-216. [http://dx.doi.org/10.1016/j.jclepro.2017.01.096] |
| [25] | Linares-Hernández, I.; Barrera-Díaz, C.; Roa-Morales, G.; Bilyeu, B.; Ureña-Núñez, F. A combined electrocoagulation-sorption process applied to mixed industrial wastewater. J. Hazard. Mater., 2007, 144(1-2), 240-248. [http://dx.doi.org/10.1016/j.jhazmat.2006.10.015] [PMID: 17118541] |
| [26] | Katal, R.; Pahlavanzadeh, H. Influence of different combinations of aluminum and iron electrode on electrocoagulation efficiency: application to the treatment of paper mill wastewater. Desalination, 2011, 265, 199-205. [http://dx.doi.org/10.1016/j.desal.2010.07.052] |
| [27] | Brahmi, K. W. B. B. Hamrouni, E. Elaloui, M. Loungou, Z. Tlili, Investigation of electrocoagulation reactor design parameters effect on the removal of cadmium from synthetic and phosphate industrial wastewater. Arab. J. Chem., 2019, 12(8), 1848-1859. [http://dx.doi.org/10.1016/j.arabjc.2014.12.012] |
| [28] | Khan, S.U.; Izharul, D.T.I.; Ayub, H.F.S.; Basheer, F. Hexavalent chromium removal in an electrocoagulation column reactor: Process optimization using CCD, adsorption kinetics and pH modulated sludge formation. Process Saf. Environ. Prot., 2019, 122, 118-130. [http://dx.doi.org/10.1016/j.psep.2018.11.024] |
| [29] | Mateen, Q.S.; Khan, S.U.; Islam, D.T.; Khan, N.A.; Farooqi, I.H. Copper (II) removal in a column reactor using electrocoagulation: Parametric optimization by response surface methodology using central composite design, Water Environ., 2020, |
| [30] | Chen, F.; Li, X.; Luo, Z.; Ma, J.Zh.Q.; Zhang, S. Advanced treatment of copper smelting wastewater by the combination of internal micro-electrolysis and electrocoagulation. Sep. Sci. Technol., 2018, 53(16), 2639-2646. [http://dx.doi.org/10.1080/01496395.2018.1463265] |





