- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Bioactive Compounds Journal
(Discontinued)
ISSN: 1874-8473 ― Volume 9, 2020
Identification of Polyphenols and Anti-Oxidant Capacity of Piper aduncum L
Mónica Ramos Escuderoa, D Fernando Ramos Escuderob, Connie M Remsbergc, Jody K Takemotoc, Neal M Daviesc, Jaime A Yáñez*, c
Abstract
Ethanol extracts of Piper aduncum L. leaves from five sub-regions in the tropical Peruvian rainforest were assessed for polyphenol content utilizing liquid chromatography coupled with mass spectrometry with electrospray ionization (LC-MS-ESI). The anti-oxidant capacity was estimated using a DPPH radical scavenging assay. Additionally, the reducing power (capacity to reduce Fe3+ to Fe2+), the capacity to scavenge hydrogen peroxide and the total polyphenol content, were assessed. Analysis led to the identification of gallic acid, catechin, chlorogenic acid, epicatechin, rutin, quercitrin, phloridzin, quercetin, and phloretin, as well as determination of potent anti-oxidant capacities by P. aduncum L.
Article Information
Identifiers and Pagination:
Year: 2008Volume: 1
First Page: 18
Last Page: 21
Publisher Id: TOBCJ-1-18
DOI: 10.2174/1874847300801010018
Article History:
Received Date: 7/4/2008Revision Received Date: 9/5/2008
Acceptance Date: 11/7/2008
Electronic publication date: 15/10/2008
Collection year: 2008
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Schering-Plough Research Institute, Drug Metabolism and Pharmacokinetics, 2015 Galloping Hill Road, Mail Stop K15-3 3700, Kenilworth, NJ 07033, USA; Tel: (908) 487-8777; E-mail: jaimeayanez@gmail.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 7-4-2008 |
Original Manuscript | Identification of Polyphenols and Anti-Oxidant Capacity of Piper aduncum L | |
INTRODUCTION
The identification and characterization of anti-oxidant compounds from plant sources has become an active research area because compounds such as polyphenols act as primary anti-oxidants and free radical scavengers. Polyphenols exhibit multiple pharmacological properties such as anti-microbial, anti-allergenic, anti-ulcerogenic, anti-neo-plastic, and anti-inflammatory activities [1Formica JV, Regelson W. Food Chem Toxicol 1995; 33: 1061.
[http://dx.doi.org/10.1016/0278-6915(95)00077-1] ]. The polyphenol family includes phenolic acids, stilbenes, chalcones, coumarins, cromones, lignans, flavonoids, isoflavonoids, neoflavonoids, and tannins. The polyphenol family has been shown to possess significant anti-oxidant capacities, while maintaining low toxicities [2So FV, Guthrie N, Chambers AF, Moussa M, Carroll KK. Nutr Cancer 1996; 26: 167.
[http://dx.doi.org/10.1080/01635589609514473] [PMID: 8875554] ].
The Piper aduncum L. species (Piperaceae), locally known as “Matico”, has long been used in alternative medicine as an antiseptic for wound healing, and as treatment for haemostasis, dysentery, chronic diarrhea, common cold and gastrointestinal pain in multiple countries [3Morandim AA, Bergamo DCB, Kato MJ, et al. Phytochem Anal 2005; 16: 282.
[PMID: 16042156] ]. Its essential oils have also shown biological activity including strong anti-bacterial, cytotoxic, fungistatic, insecticidal, and molluscidal activities [4Mikich SB, Bianconi GV, Maia BH, Teixeira SD. J Chem
Ecol 2003; 29: 2379.
[http://dx.doi.org/10.1023/A:1026290022642] ]. From a chemical standpoint, a variety of phenolic secondary metabolites such as lignans, chalcones, dihydrochalcones, chromene, and benzoic acid derivatives have been isolated from P. aduncum L. [5Parmar VS, Jain SC, Bisht KS, et al. Phytochemistry 1997; 46: 597.]. Other well known plants in the Piper genus include black pepper (Piper nigrum) and kava-kava (Piper methysticum).
P. aduncum L. is one of the 429 species that belong to the Piperaceae family that have been identified in Peru alone [6Mostacero J, Mejia F, Gamarra O. Consejo Nacional de Ciencia, Tecnología e Innovacion Tecnologica; CONCYTEC. 2002; 1-2: p. 232.]. It is widespread in north and central eastern Peru and it remains as one of the most widely employed phytotherapy agents by local communities. The local herbal market also widely employs it in multiple compositions. Although used widely, no anti-oxidant assessment has been performed and little ethnobotanical or phytochemical research on this species has been conducted. In the present work, liquid chromatography coupled with mass spectrometry and electrospray ionization (LC-MS-ESI) was employed to identify individual polyphenols of ethanol extracts from leaves of P. aduncum L. collected from five sub-regions of the tropical Peruvian rainforest. The DPPH radical and hydrogen peroxide scavenging activity, reducing power, and total polyphenol content, were also assessed.
MATERIALS AND METHODS
Plant Material
Piper aduncum L. leaves were collected during July 2004 by Mónica Ramos Escudero from five sub-regions (Marona Baja, Tingo Maria, Tulumayo, San Isidro, and Honolulo) of the Huallaga river valley in the Peruvian tropical rainforest. Voucher specimens (Table 1) were deposited at the Museo de Ciencia Natural (Natural Science Museum) of the Universidad Nacional Mayor de San Marcos (UNMSM) in Lima, Peru. The specimens were identified by Hamiltón Beltrán, C. Biol. based on the leaf characteristics [7Cronquist A. An Integrated System of Classification of Flowering
Plants. New York: Columbia University Press 1981.
[PMID: 6117027] ].
Extraction and Isolation
The powdered air-dried leaves (1 kg) were extracted with 95% ethanol at room temperature under constant shaking for 2 hours. The extract was centrifuged, the supernatant collected and the insoluble material discarded. The extract (supernatant) was dried to completion and the plant material obtained (percentage yield) was between 14.3% and 16.9% from the initial dry weight. The total ethanol extracts were diluted to different concentrations (as specified under each experimental section) and were tested for radical activity by DPPH assay, hydrogen peroxide scavenging activity, and reducing power. For LC-MS-ESI analysis, the ethanol extracts were dried under a constant flow of compressed nitrogen gas, and reconstituted in mobile phase before injection.
LC-MS-ESI Analysis
The chromatographic conditions previously described [8Schieber A, Keller P, Carle R. J Chromatogr A 2001; 910: 265.
[http://dx.doi.org/10.1016/S0021-9673(00)01217-6] ] were slightly modified. The modifications included using a Phenomenex Luna (2) C18 column (250 x 4.60 mm, i.d. 5 µm particle size, Torrance CA, USA), and a guard Phenomenex Luna (2) C18 column (30 x 4.60 mm, i.d. 3 µm particle size, Torrance CA, USA). Additionally, the mobile phase was altered to consist of 2% (v/v) acetic acid in water (eluent A) and of 0.5% acetic acid in water and acetonitrile (50:50, v/v; eluent B). The gradient was as follows: 10% B to 55% B (70 min), 55% B to 100% B (10 min), 100% B to 10% B (10 min). The flow rate was as follows: 0.8 mL/min (55 min), 1 mL/min (45 min). In order to verify the presence of each compound standard curves (100 ng/mL to 50 μg/mL) for each compound were prepared for calibration. Each eluted peak was confirmed by its corresponding m/z ratio using a Shimadzu LCMS-2010 EV liquid chromatograph mass spectrometer system (Kyoto, Japan) connected to the LC portion consisting of two LC-10AD pumps, a SIL-10AD VP auto injector, a SPD-10A VP UV detector, and a SCL-10A VP system controller. The mass spectrometer conditions consisted of a curved desolvation line (CDL) temperature of 200°C and a block temperature of 200°C. The CDL, interface, and detector voltages were -20.0 V, 4.5 kV, and 1.2 kV, respectively. Vacuum was maintained by an Edwards® E2M30 rotary vacuum pump (Edwards, UK). Liquid nitrogen (Washington State University Central Stores) was used as a source of nebulizer gas (1.5 L/min). In the negative-specific ion mode (SIM) the single plot transitions (m/z) of each compound were monitored. The injection volume for all samples was 30 μL, and the internal standard employed was daidzein. Good linearity (R2 > 0.998) for each compound was observed (based on individual standard curves). Recovery for each compound was > 95%.
DPPH Radical Scavenging Assay
The 1,1-diphenyl-2-picryl-hydrazyl (DPPH) method [9Brand-Williams W, Cuvelier M, Berset C. Lebensmittel Wissenschaft
und Technologie 1995; 28: 25.
[http://dx.doi.org/10.1016/S0023-6438(95)80008-5] ] was modified. Briefly, a 100 μM DPPH solution was freshly prepared in methanol. Various concentrations (0-300 μg/mL) of ethanol extracts (50 μL) were reacted with 950 μL of the DPPH radical solution for 10 minutes at room temperature. Absorbance was measured at 515 nm and results were compared to the standard, α-tocopherol.
Hydrogen Peroxide Scavenging Assay
The H2O2 scavenging assay previously described [10Ruch R, Cheng S, Klauning J. Carcinogenesis 1989; 10: 2123.] was modified. Briefly, a solution of hydrogen peroxide (50 mM) was freshly prepared in phosphate buffered saline (PBS; pH 7.4). Ethanol extracts of P. aduncum L. (400 μL at concentration 100 μg/mL) were reacted with 600 μL of the hydrogen peroxide solution for 30 minutes at room temperature, and absorbance was measured at 230 nm. Ascorbic acid was used as the standard.
Reducing Power
The reducing power assay allows for the quantification of the capacity of a compound or extract to reduce iron from its ferric state (Fe3+) to its ferrous (Fe2+) state. The reducing power assay previously described [11Oyaizu M. Jpn J Nutr 1986; 44: 307.
[http://dx.doi.org/10.5264/eiyogakuzashi.44.307] ] was modified. Briefly, solutions of phosphate buffer (0.2 M, pH 6.6), potassium ferricyanide (1%), stock trichloroacetic acid (TCA) and ferric chloride (FeCl3, 0.1%) were prepared. Ethanol extracts of P. aduncum L. (100 μL at concentration 100 μg/mL) reacted with 2.5 mL of the phosphate buffer solution and 2.5 mL of the potassium ferricyanide solution for 20 minutes at 50°C. Then, the mixture was combined with 2.5 mL of TCA, centrifuged at 3000 rpm for 10 minutes at room temperature, and a 2.5 mL aliquot of the upper layer was obtained. The aliquot was combined with 2.5 mL of distilled water and 0.5 mL of FeCl3 solution, and absorbance was measured at 700 nm. Ascorbic acid was used as the standard and in preparation of calibration curves.
Total Polyphenols Content
The total polyphenol content was measured by the Prussian Blue Assay [12Price ML, Butler LG. J Agric Food Chem 1977; 25: 1268.
[http://dx.doi.org/10.1021/jf60214a034] ], substituting ferric ammonium sulfate for ferric chloride as previously indicated [13Folgarait PJ, Davidson DW. Oikos 1994; 71: 305.
[http://dx.doi.org/10.2307/3546279] ]. Briefly, solutions of ferric chloride (FeCl3, 0.1 M) in 0.1 N HCl, and another solution of potassium ferricyanide (0.008 M) in water were prepared. Ethanol extracts of P. aduncum L. (1 mL at concentration 100 μg/mL) reacted with 3 mL of the FeCl3 solution and 3 mL of the potassium ferricyanide solution for 10 minutes at room temperature, and absorbance was measured at 720 nm. Gallic acid was used to prepare the calibration curves.
Statistical Analysis
Compiled data were presented as mean and standard error of the mean (mean ± SEM). General Linear Model (GLM) Analysis of Variance (ANOVA) with Newman-Keuls multiple comparison test with a p-value < 0.05 been statistically significant (NCSS Statistical and Power Analysis, Kaysville, UT).
RESULTS AND DISCUSSION
The validated LC-MS-ESI method had a limit of quantification (LOQ) of 50 ng/mL for all the compounds and a coefficient of variation (CV %) for the different compounds ranging from 3-7% inter-day and 5-8% intra-day. Standard curves ranged from 100 ng/mL to 50 μg/mL. As shown in Fig. (1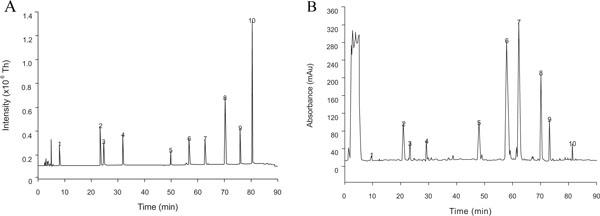 ), baseline separation and resolution was achieved for all the compounds and the total ion chromatograms were recorded in the negative ion mode. It was found that the predominant phenolic constituents in Piper aduncum L. were quercetin, phloridzin, and epicatechin followed by quercetin glycosides (quercitrin and rutin), catechin, benzoic acids (gallic acid and chlorogenic acid), and phloretin.
), baseline separation and resolution was achieved for all the compounds and the total ion chromatograms were recorded in the negative ion mode. It was found that the predominant phenolic constituents in Piper aduncum L. were quercetin, phloridzin, and epicatechin followed by quercetin glycosides (quercitrin and rutin), catechin, benzoic acids (gallic acid and chlorogenic acid), and phloretin.
It was observed that the composition in individual phenolics varied depending on the geographical area. However, the content of catechin, epicatechin, and quercetin-3-rhamnoside (quercitrin) remained constant independent of the geographical area (Table 1). The total sum of individual phenolics from highest to lowest was San Isidro > Honolulo > Tingo María > Tulumayo > Marona Baja, which correlated with the DDPH radical scavenging, H2O2 scavenging activity, reducing power, and total polyphenol content (Table 2). The sub-regions from where P. aduncum L. was sampled are within a 10 mile-ratio, but based on the statistically significant differences in the measured parameters, geographical location plays an important role. This observation correlates with previous reports that demonstrated that the absence or presence of pharmacologically active substances can vary within the same specimen depending on the collection place, weather conditions, soil characteristics, time of the year when the collection is performed, and farming conditions [14Machado LR, Sharapin N. Segundo Congreso Internacional y Segundo Congreso Peruano de Plantas Medicinales y Fitoterapia. Lima, Peru 2003; pp. 13-20.].
DPPH Radical Scavenging Activities, Hydrogen Peroxide (H2O2) Scavenging Activities, Reducing Powers, and Total Polyphenol Content of P. aduncum L. Ethanol Extracts (mean ± SEM, n = 4) from Different Geographical Sub-Regions. All, Except the DDPH Radical Scavenging Assay (Concentration Range 0-300 ug/mL), were Performed at Extract Concentration of 100 µg/mL Including the Controls. AAE = Ascorbic Acid Equivalent, GAE = Gallic Acid Equivalent, NA = Not Applicable
The P. aduncum L. ethanol extracts exhibited DPPH IC50 values of 85-220 μg/mL, which correlate to the values for green tea (100-300 μg/mL) and are slightly lower than the values for black tea (200-800 μg/mL) [15du Toit R, Volsteedt Y, Apostolides Z. Toxicology 2001; 166: 63.
[http://dx.doi.org/10.1016/S0300-483X(01)00446-2] ]. Ability to act as a scavenger may be responsible to some extent for the reported biological activities of P. aduncum L. The different polyphenols detected (most of them for the first time) in P. aduncum L. may be responsible to some extent for its anti-oxidant capacity since gallic acid, chlorogenic acid, catechin, and quercetin have reported IC50 values of 1-8 μg/mL [16Cos P, Ying L, Calomme M, et al. J Nat Prod 1998; 61: 71.
[http://dx.doi.org/10.1021/np970237h] [PMID: 9461655] ]. However, since P. aduncum L. exhibited greater IC50 values than any polyphenolic compound alone, there might be some combinational effects or other factors (e.g. extraction solvent or procedure) yet to be determined. Furthermore, it was observed that P. aduncum L. from all the tested sub-regions contains higher hydrogen peroxide scavenging capacity than cat’s claw (Uncaria tomentosa) [17Goncalves C, Dinis T, Batista MT. Phytochemistry 2005; 66: 89.
[http://dx.doi.org/10.1016/j.phytochem.2004.10.025] [PMID: 15649515] ] and greater total polyphenol content than garlic (Allium sativum L.) [18Tapsell LC, Hemphill I, Cobiac L, et al. Med J Aust 2006; 185: S4.], which are two well characterized medicinal plants.
CONCLUSION
This is the first report that has quantified various well-characterized polyphenols in P. aduncum L. and the first study in assessing its anti-oxidant capacity, reducing power, and capacity to scavenge hydrogen peroxide, and reducing power, which may be a rational explanation for the therapeutic application of the herb in Latin American medicine.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the Consejo Nacional de Ciencia y Tecnología (CONCYTEC) for a research grant to MRE, a research grant from the Organic Center to NMD, and a research fellowship from the Organic Center to JAY and an equipment grant from Shimadzu to NMD. CMR is the recipient of a United States Pharmacopeia fellowship.
REFERENCES
| [1] | Formica JV, Regelson W. Food Chem Toxicol 1995; 33: 1061. [http://dx.doi.org/10.1016/0278-6915(95)00077-1] |
| [2] | So FV, Guthrie N, Chambers AF, Moussa M, Carroll KK. Nutr Cancer 1996; 26: 167. [http://dx.doi.org/10.1080/01635589609514473] [PMID: 8875554] |
| [3] | Morandim AA, Bergamo DCB, Kato MJ, et al. Phytochem Anal 2005; 16: 282. [PMID: 16042156] |
| [4] | Mikich SB, Bianconi GV, Maia BH, Teixeira SD. J Chem
Ecol 2003; 29: 2379. [http://dx.doi.org/10.1023/A:1026290022642] |
| [5] | Parmar VS, Jain SC, Bisht KS, et al. Phytochemistry 1997; 46: 597. |
| [6] | Mostacero J, Mejia F, Gamarra O. Consejo Nacional de Ciencia, Tecnología e Innovacion Tecnologica; CONCYTEC. 2002; 1-2: p. 232. |
| [7] | Cronquist A. An Integrated System of Classification of Flowering
Plants. New York: Columbia University Press 1981. [PMID: 6117027] |
| [8] | Schieber A, Keller P, Carle R. J Chromatogr A 2001; 910: 265. [http://dx.doi.org/10.1016/S0021-9673(00)01217-6] |
| [9] | Brand-Williams W, Cuvelier M, Berset C. Lebensmittel Wissenschaft
und Technologie 1995; 28: 25. [http://dx.doi.org/10.1016/S0023-6438(95)80008-5] |
| [10] | Ruch R, Cheng S, Klauning J. Carcinogenesis 1989; 10: 2123. |
| [11] | Oyaizu M. Jpn J Nutr 1986; 44: 307. [http://dx.doi.org/10.5264/eiyogakuzashi.44.307] |
| [12] | Price ML, Butler LG. J Agric Food Chem 1977; 25: 1268. [http://dx.doi.org/10.1021/jf60214a034] |
| [13] | Folgarait PJ, Davidson DW. Oikos 1994; 71: 305. [http://dx.doi.org/10.2307/3546279] |
| [14] | Machado LR, Sharapin N. Segundo Congreso Internacional y Segundo Congreso Peruano de Plantas Medicinales y Fitoterapia. Lima, Peru 2003; pp. 13-20. |
| [15] | du Toit R, Volsteedt Y, Apostolides Z. Toxicology 2001; 166: 63. [http://dx.doi.org/10.1016/S0300-483X(01)00446-2] |
| [16] | Cos P, Ying L, Calomme M, et al. J Nat Prod 1998; 61: 71. [http://dx.doi.org/10.1021/np970237h] [PMID: 9461655] |
| [17] | Goncalves C, Dinis T, Batista MT. Phytochemistry 2005; 66: 89. [http://dx.doi.org/10.1016/j.phytochem.2004.10.025] [PMID: 15649515] |
| [18] | Tapsell LC, Hemphill I, Cobiac L, et al. Med J Aust 2006; 185: S4. |




