The Open Bioactive Compounds Journal
(Discontinued)
ISSN: 1874-8473 ― Volume 9, 2020
Analgesic and Anti-Gastropathic Effects of Salidroside Isolated from Acer tegmentosum Heartwood
Yeong-Min Yooa, e, Jung-Hwan Namb, Min-Young Kima, Jongwon Choic, Kyung-Tae Leed, Hee-Juhn Parka, *
Abstract
The heartwood of Acer tegmentosum(Acereaceae) has been used as a Korean traditional medicinal drug against alcohol poisoning and hepatitis. To find the biologically active substance in A. tegmentosum heartwood, we investigated the protective effects of the heartwood extract and its constituents on pain and gastropathy in mouse. In these experiments, salidroside, a major compound, significantly reduced gastric lesion and pain in mice. Oral administration of salidroside at the 10 and 20 mg/kg doses greatly reduced the gastric lesion induced by HCl/ethanol (inhibitory effect, 51.5 and 68.8%, respectively) and by indomethacin/bethanechol (inhibitory effect, 31.3 and 38.8%, respectively). Salidroside also stabilized pH of gastric juice and the increase of gastric juice secretion and total acid output. Taken together, these results demonstrated that salidroside is the main ingredient of A. tegmentosum heartwood to prevent gastric lesion and pain that can be caused by drinking alcohol.
Article Information
Identifiers and Pagination:
Year: 2009Volume: 2
First Page: 1
Last Page: 7
Publisher Id: TOBCJ-2-1
DOI: 10.2174/1874847300902010001
Article History:
Received Date: 20/5/2008Revision Received Date: 10/10/2008
Acceptance Date: 13/12/2008
Electronic publication date: 26/1/2009
Collection year: 2009
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Department of Pharmaceutical Engineering, Sangji University, Wonju 660, Gangwon-do 220-702, Korea; Tel: +82-33-730-0564; Fax: +82-33-730-0564; E-mail: hjpark@sangji.ac.kr
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 20-5-2008 |
Original Manuscript | Analgesic and Anti-Gastropathic Effects of Salidroside Isolated from Acer tegmentosum Heartwood | |
INTRODUCTION
Gastroesophageal reflux disease and peptic ulcer are the most important and common gastrointestinal disorders [1Pilotto A. Aging and upper gastrointestinal disorders Best Pract Res Clin Gastroenterol 2004; 18: 73-81.
[http://dx.doi.org/10.1016/j.bpg.2004.06.015] [PMID: 15588798] ]. Peptic ulcers have a prevalence of 4-5% in humans and are related to food, stress, genetic and environmental factors [2Aihara T, Nakamura E, Amagase K, et al. Pharmacological control of gastric acid secretion for the treatment of acid-related peptic disease Pharmacol Ther 2003; 98: 109-27.]. Major causative factors of peptic ulcer involve Helicobacter pylori infection, excessive abuse of drugs such as non-steroidal anti-inflammatory drugs (NSAIDs), irregular eating habits, smoking, alcohol consumption, and psychological stresses [3Behrman SW. Management of complicated peptic ulcer disease Arch Surg 2005; 140: 201-8.
[http://dx.doi.org/10.1001/archsurg.140.2.201] [PMID: 15724004] ]. Peptic ulcers occur in tissues destroyed by gastric juice and stomach acid because of an imbalance between aggressive factors (e.g., stomach acid and pepsin) and defensive factors (e.g., mucus, bicarbonate and mucosa blood flow) in the digestive system; this imbalance results in impairment of the mucosa and muscularis mucosa. In addition, reactive oxygen species (ROS) and lipid peroxidation by oxidative stress can lead to gastric mucosal lesions [4Demir S, Yilmaz M, Koseoglu M, Akalin N, Aslan D, Aydin A. Role of free radicals in peptic ulcer and gastritis Turk J Gastroenterol 2003; 14: 39-43.
[PMID: 14593536] ]. Once ROS trigger and maintain ischemic status in the gastric mucosa, hydroxyl radicals generated from superoxide anions cause gastric lesions by virtue of damage to the mucosa microvessels and the subsequent decrease in blood flow [5Perry MA, Wadhwa S, Parks DA, Pickard W, Granger DN. Role of oxygen radicals in ischemia-induced lesions in the cat stomach Gastroenterology 1986; 90: 362-267.
[PMID: 3753593] ].
The leaves and heartwood of Acer tegmentosum (Acereaceae) have been used in Korean traditional medicine for alcohol and liver detoxification. Its heartwood has been used to treat liver diseases including liver cirrhosis and liver cancer. Internal use of heartwood extract prior to drinking has also been known to prevent alcohol poisoning [6Kim TJ. Plant Resources in Korea Publishing Co Seoul Seoul 1996; Vol. III, 7Hur JM, Jun M, Yang EJ, Choi SH, Park JC, Song KS. Isolation of isoprenoidal compounds from the stems of Acer tegmentosum max Kor J Pharmacogn 2007; 38: 67-70.]. In previous studies, diarylheptanoids [8Kubo M, Inoue T, Nagai M. Studies on the constituents of Aceraceae plants. III. Structure of acerogenin B from Acer nikoense Maxim Chem Pharm Bull 1980; 28: 1300-3.
[http://dx.doi.org/10.1248/cpb.28.1300] ], rhododendrol glycoside [9Kubo M, Nagai M, Inoue T. Studies on the constituents of Aceraceae plants. Carbon-13 nuclear magnetic resonance spectra of
acerogenin A, rhododendrol, and related compounds, and structure
of aceroside from Acer nikoense Chem Pharm Bull 1983; 31: 1917-22.
[http://dx.doi.org/10.1248/cpb.31.1917] ], tannins [10Hatano T, Hattori S, Ikeda Y, Shingu T, Okuda Y. Tannins of Aceraceous plants. Part II. Gallotannins having a 1,5-anhydro-Dglucitol
core and some ellagitannins from Acer species Chem Pharm Bull 1990; 38: 1902-5.
[http://dx.doi.org/10.1248/cpb.38.1902] ] and phenolic glycosides (catechin, fraxin, and derivatives of quercetin, flavones and morin) [11Park KM, Yang MC, Lee KH, Kim KR, Choi SU, Lee KR. Cytotoxic phenolic constituents of Acer tegmentosum maxim Arch Pharm Res 2006; 29: 1086-90.
[http://dx.doi.org/10.1007/BF02969296] [PMID: 17225455] ] were isolated from the genus Acer. However, the phytochemical constituent with biological activity in A. tegmentosum has not been reported. In the course of our studies of biologically active compounds from Korean natural resources, we investigated the protective effect of the extract and constituent of A. tegmentosum heartwoods on mouse models of pain and gastric lesion.
MATERIALS AND METHODS
Instruments and Materials
Melting point was determined on an Electrothermal 9100 melting point apparatus and was uncorrected. The 1H-NMR spectra (δ ppm, J in Hz) was recorded in DMSO-d6 on a Brucker AM-500 spectrometer (500 MHz), while 13C-NMR spectra was recorded in the same solvent on a Brucker AM-500 spectrometer at 125 MHz with tetramethylsilane (TMS) as an internal standard.
Extraction, Fractionation and Isolation
The heartwood of A. tegimentosum was collected in Pyongchang-gun, Gangwon province in Korea and dried. This plant was identified by Dr. Won-Bae Kim of Highland Agriculture Institute, Rural Development Administration of Korea. Heartwoods of A. tegmentosum (2 kg) were extracted with MeOH (each, 3.0 L) for 5 h three times under reflux. The extracted solution was filtered and concentrated under reduced pressure to give a viscous MeOH extract, which was freeze-dried to yield a powdery MeOH extract. A portion (118.7 g) of the MeOH extract was suspended in 800 ml distilled water and partitioned three times with CHCl3 (each, 800 ml). The CHCl3-soluble portion was dried in vacuo to yield the CHCl3 fraction. The H2O layer was successively fractionated with BuOH (each, 800 ml), and the BuOH-soluble portion was dried in vacuo to yield a BuOH fraction. Since BuOH fraction exhibited the most potent anti-nociceptive andti-gastropathic activity, it was chromatographed for isolation.
Ten grams of the BuOH fraction was subjected to silica gel column (SiO2, Art No. 7734, Merck, Germany, 280 g, 5 × 55 cm) chromatography and gradiently eluted with CHCl3-MeOH {from 1 L of 9:1 (v/v) to 2.5L of 6:4 (v/v)}. The eluate was collected to give seven fractions (I – VII) and then monitored by checking TLC. Fraction II was dried in vacuo and crystallized from MeOH to yield a white amorphous powder (2.5 g, compound 1, Fig.1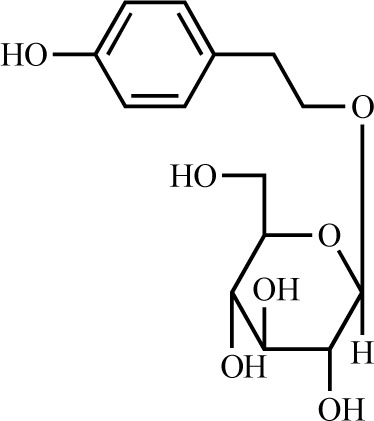 ). Fractions VI and VII were crystallized from MeOH to yield a white amorphous powder that was identified as sucrose by TLC, 1H-NMR and 13C-NMR.
). Fractions VI and VII were crystallized from MeOH to yield a white amorphous powder that was identified as sucrose by TLC, 1H-NMR and 13C-NMR.
 |
Fig. (1) Chemical structure of salidroside. |
Compound 1 – Colorless powder, mp 220(C; 1H-NMR(500 MHz, DMSO-d6) (: 4.17 (1H, d, J=7.5 Hz, H-1′), 6.67 (2H, d, J=8.5 Hz, H-2.6), 7.04 (2H, d, J=3,5 Hz); 13C-NMR (125.5 MHz, DMSO-d6) (: 35.3 (C-7), 61.5(C-6′), 70.3 (C-8), 70.5(C-4′), 73.8 (C-2′), 77.1 (C-3′), 77.3 (C-5′, 103.3 (C-1′), 115.4 (C-2,5), 129.2 (C-1), 130.2 (C-3,5), 155.9 (C-4); FAB-MS m/z 323.2 [M + Na]+.
Experimental Animals
Male ICR mice were purchased from Daehan Bio Link Co. and allowed to adapt to laboratory conditions (temperature: 20 ± 2(C, relative humidity: 40-60%, light/dark cycle: 12 h) for two weeks. Mice weighing 25 ± 2 g were used for experiments. Number of mice was 9 per group. For twenty-four hours before the experiment, the animals were offered only water. Considering that enzyme activities can vary throughout each day, the animals were sacrificed at a fixed time (10:00 A.M.-12:00 P.M.). All experiments were approved by the University of Kyungsung Animal Care and Use Committee. All procedures were conducted in accordance with the “Guide for Care and Use of Laboratory Animals” published by the National Institutes of Health.
Analgesic Experiments
Acetic Acid-Induced Writhing Method:The MeOH extract of A. tegmentosum heartwood, its fractions (100 or 200 mg/kg) or compound 1 (10 or 20 mg/kg) were orally administered to the mice daily for one week, and the mice were then injected intraperitoneally (i.p.) with 10 mg/kg of 0.8% acetic acid (9 mice/experimental group) [12Jung HJ, Choi J, Nam JH, Park HJ. Anti-ulcerogenic effects of effects of the flavonoid-rich fraction from the extract of Orostachys japonicus J Med Food 2007; 10: 702-6.
[http://dx.doi.org/10.1089/jmf.2006.223] [PMID: 18158844] ]. The number of abdominal contractions in a 20-min period was then counted. Aspirin (100 mg/kg, p.o.) was used as a positive control. A significant reduction in the number of abdominal contractions compared to the control was considered a positive analgesic response.
Hot Plate Method: Mice (9 mice/experimental group) were administered the MeOH extract, its fractions (100 or 200 mg/kg daily), or compound 1 (10 or 20 mg/kg daily) for 1 week. One h after the final administration, the mice were placed on a hot-plate (Ugo Basile, Comerio, Italy) maintained at 70°C. The time until each animal licked a fore or hind paw or jumped off the plate was defined as the reaction time [12Jung HJ, Choi J, Nam JH, Park HJ. Anti-ulcerogenic effects of effects of the flavonoid-rich fraction from the extract of Orostachys japonicus J Med Food 2007; 10: 702-6.
[http://dx.doi.org/10.1089/jmf.2006.223] [PMID: 18158844] ]. Morphine (10 mg/kg, p.o.) was used as a reference drug.
Induced Gastric Lesion Experiments
HCl/Ethanol-Induced Gastric Lesions in Mice: After oral administration of the test solution, the mice were fasted for 24 hours prior to the experiment. The mice were then given an oral dose of 0.2 mL of 0.3 M HCl in 60% ethanol. After 24 hours the mice were sacrificed, and their stomachs were opened along the greater curvature and fixed in 2% formalin solution for 10 minutes. After the greater curvature was incised, the extent of gastric damage in the glandular region was defined as the ulcerative lesion index [13Mizui T, Dodeuchi M. Effect of polyamines on acidified ethanol-induced gastric lesions in rats Jpn J Pharmacol 1983; 33: 939-45.
[http://dx.doi.org/10.1254/jjp.33.939] [PMID: 6580476] ].
NSAID-Induced Gastric Lesions in Mice:Test solutions including the MeOH extract, its fractions (100 and 200 mg/kg per day), compound 1 (10 and 20 mg/kg per day) and the cimetidine control (100 mg/kg per day) were orally administered to the mice for 2 weeks. The animals were treated with indomethacin (30 mg/kg, s.c.) and bethanechol (5 mg/kg, i.p.), fasted for 1 hour, and then sacrificed. The stomachs were opened along the greater curvature and fixed in 2% formalin solution for 10 minutes. After the greater curvature was incised, the extent of gastric damage in the glandular region was evaluated according to the ulcerative lesion index [14Rainsford KD. A synergistic interaction between aspirin, or other non-steroidal anti-inflammatory drugs, and stress which produces severe gastric mucosal damage in rats and pigs Agents Actions 1975; 5: 553-8.
[http://dx.doi.org/10.1007/BF01972694] [PMID: 1220559] ].
Measurement of Gastric Secretion in Pylorus-Ligated Mice
Test solutions were administered as previously described and the mice were fasted for 24 hours. Each mouse was then anesthetized with ether. The abdomen of each anesthetized mouse was opened, and the pylorus was ligated; the abdomen was then closed after the sample solutions had been placed in the duodenal tract. Four hours after sealing-up their abdomens, the mice were anesthetized with ether, the stomachs were excised, and gastric juices were collected. These were centrifuged at 2,500 × g, and then the gastric juice volumes and pH values were measured and total acid output was calculated. The total acid output was determined by titration versus 0.05 N NaOH using phenolphthalein as an indicator [15Dai S, Ogle CW. A simple method for the production of peptic ulceration in the rat Life Sci 1973; 12: 505-12.
[http://dx.doi.org/10.1016/0024-3205(73)90344-5] ].
Statistical Analysis
Results are expressed as means ± SD (n=9). Statistical analysis was performed with Duncan’s multiple range tests. Differences were considered significant at p < 0.05.
RESULTS
Analgesic Effects of the MeOH Extract of A. tegmentosum Heartwood and its Fractions
The analgesic and anti-gastropathic effects of the MeOH extract of A. tegmentosum heartwood were evaluated in mice since A. tegmentosum has been used to prevent or treat alcohol poisoning in the folkloric medicinal society of Korea. Excessive drinking often causes headache, abdominal pain and gastric disorders. In addition, phytochemical isolation was also performed to identify the biologically active substance in the heartwood.
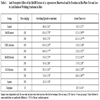
As shown in Table 1, the MeOH extract of A. tegmentosum heartwoods alleviated pain and had gastropathic action, indicative of why it has been used to treat alcohol poisoning. Among the MeOH extract and its CHCl3-, BuOH- and H2O fractions, the BuOH fraction exhibited the most potent effect. Administration of 100 or 200 mg/kg of BuOH fraction significantly lengthened the jumping latency in the hot plate test from 10.3 ± 3.17 seconds for the control group to 15.2 ± 2.41 seconds or 17.9 ± 2.38 seconds, respectively. Control mice had a writhing number of 66.4 ± 2.41 compared to 53.4 ± 2.70 at the 100 mg/kg dose and 45.4 ± 2.88 at the 200 mg/kg dose for the BuOH fraction-treated group. Thus, the A. tegmentosum extracts exhibited central and peripheral analgesic effects in the hot plate test and the writhing test.
Effects of the MeOH Extract of A. tegmentosum Heartwood and its Fractions on Gastropathy
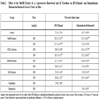
Ulcerative indices were determined in mice with HCl/ethanol- and indomethacin/bethanechol-induced ulcers by measuring ulcerative lesion diameter. As shown in Table 2, treatment with 0.3 M HCl and 60% EtOH caused gastric ulcers (diameter 27.4 ± 2.10 mm); however, pretreatment with the MeOH extract (100 and 200 mg/kg, p.o.) for 2 weeks reduced the ulcerative index compared to the control group. Indomethacin, a non-steroidal anti-inflammatory drug, and bethanechol, a cholinergic drug, were also administered to mice to induce gastric ulcers. Treatment with indomethacin/bethanechol caused gastric ulcers (diameter 14.7 ± 0.93 mm), whereas pretreatment with the MeOH extract reduced the ulcerative index compared to the control. Among the CHCl3-, BuOH- and H2O fractions, the BuOH fraction had the most potent anti-gastropathic activity. HCl/EtOH caused gastric ulcers (diameter 27.4 ± 2.10 mm), while treatment with the BuOH fraction decreased the diameter to 21.7 ± 3.43 mm and 18.9 ± 2.11 mm at 100 and 200 mg/kg doses, respectively. Furthermore, in the indomethacin/bethanechol-induced gastric ulcer, treatment with the BuOH fraction reduced the ulcerative index from 14.7 ± 0.93 mm in the control group to 10.8 ± 0.39 mm and 9.4 ± 0.50 mm at the 100 and 200 mg/kg doses, respectively.
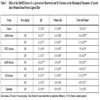
Effects of the MeOH Extract of A. tegmentosum Heartwood and its Fractions on Gastric Juice Secretion
The volume of gastric juice was measured using the method described by Dai and Ogle [15Dai S, Ogle CW. A simple method for the production of peptic ulceration in the rat Life Sci 1973; 12: 505-12.
[http://dx.doi.org/10.1016/0024-3205(73)90344-5] ]. The pH values and total acid output were also measured. The effects of the MeOH extract and its fractions on gastric secretion in pylorus-ligated mice are shown in Table 3. Oral administration of the MeOH extract resulted in an increase of pH and decreases of gastric juice volume and total acid output in the pylorus-ligated mice at the 100 and 200 mg/kg doses. The BuOH fraction exhibited the most potent effects among the MeOH extract and tested fractions, suggesting that the bioactive compound was contained in the BuOH fraction.
Isolation and Analgesic Effects of Salidroside
Phytochemical isolation of the BuOH fraction led to the isolation of compound 1, which was identified as salidroside by comparing its spectroscopic data with the literature [11Park KM, Yang MC, Lee KH, Kim KR, Choi SU, Lee KR. Cytotoxic phenolic constituents of Acer tegmentosum maxim Arch Pharm Res 2006; 29: 1086-90.
[http://dx.doi.org/10.1007/BF02969296] [PMID: 17225455] ] (Fig. 1 ). As shown in Fig. (2
). As shown in Fig. (2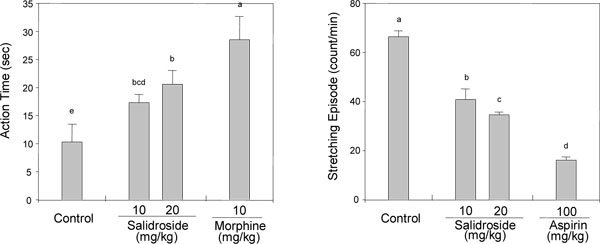 ), salidroside exhibited significant analgesic and anti-gastropathic effects at 10 and 20 mg/kg dosages (p.o.). Oral administration of morphine (positive control, 10 mg/kg, p.o.) lengthened the reaction time in the hot plate test by 176%, while 10 and 20 mg/kg dosages of salidroside prolonged the reaction time by 68.0% and 100%, which suggests that it exerts centrally mediated analgesic action. In the writhing test, 100 mg/kg aspirin, used as the positive control, decreased writhing number by 75.6%, which is indicative of peripherally mediated analgesic action, while administration of 10 and 20 mg/kg salidroside inhibited the writhing number by 38.6% and 47.9%, respectively. These results indicate that salidroside has both centrally and peripherally mediated analgesic activity.
), salidroside exhibited significant analgesic and anti-gastropathic effects at 10 and 20 mg/kg dosages (p.o.). Oral administration of morphine (positive control, 10 mg/kg, p.o.) lengthened the reaction time in the hot plate test by 176%, while 10 and 20 mg/kg dosages of salidroside prolonged the reaction time by 68.0% and 100%, which suggests that it exerts centrally mediated analgesic action. In the writhing test, 100 mg/kg aspirin, used as the positive control, decreased writhing number by 75.6%, which is indicative of peripherally mediated analgesic action, while administration of 10 and 20 mg/kg salidroside inhibited the writhing number by 38.6% and 47.9%, respectively. These results indicate that salidroside has both centrally and peripherally mediated analgesic activity.
Effects of Salidroside on Gastropathy
As shown in Fig. (3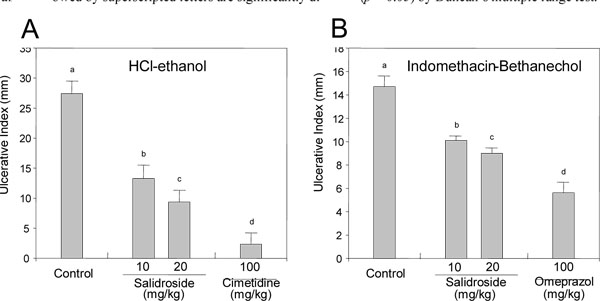 ), cimetidine (100 mg/kg, p.o.), a positive control, inhibited the gastric lesion caused by HCl/EtOH by 91.4%, while administration of salidroside inhibited the gastric lesion diameter by 51.5% and 65.8% at the 10 and 20 mg/kg dosages, respectively. In the indomethacin/bethanechol-induced gastric lesion, administration of 10 and 20 mg/kg of salidroside reduced the ulcerative index by 31.8% and 38.8%, whereas omeprazol, a positive control, decreased the ulcerative index by 61.8%. These results suggest that salidroside has anti-gastropathic activity. This compound also increased the pH of gastric juice and further decreased the volume of gastric juice and the total acid output, although its activities were weaker than those of cimetidine (Fig. 4
), cimetidine (100 mg/kg, p.o.), a positive control, inhibited the gastric lesion caused by HCl/EtOH by 91.4%, while administration of salidroside inhibited the gastric lesion diameter by 51.5% and 65.8% at the 10 and 20 mg/kg dosages, respectively. In the indomethacin/bethanechol-induced gastric lesion, administration of 10 and 20 mg/kg of salidroside reduced the ulcerative index by 31.8% and 38.8%, whereas omeprazol, a positive control, decreased the ulcerative index by 61.8%. These results suggest that salidroside has anti-gastropathic activity. This compound also increased the pH of gastric juice and further decreased the volume of gastric juice and the total acid output, although its activities were weaker than those of cimetidine (Fig. 4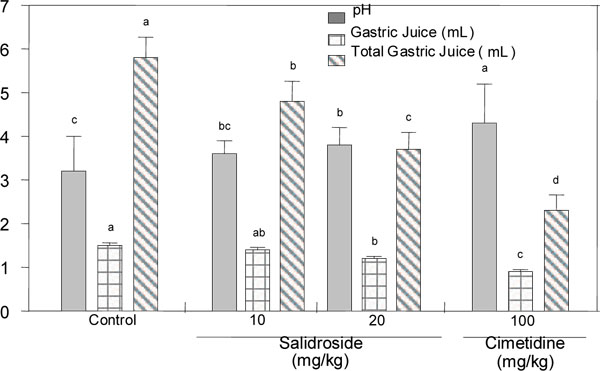 ).
).
DISCUSSION AND CONCLUSION
We sought to find the biologically active substance in A. tegmentosum heartwoods that has analgesic and anti-gastropathic actions in order to increase its pharmacological availability to relieve the symptoms of headache, abdominal pain and vomiting that can follow alcohol consumption. Based on our experimental results, A. tegmentosum heartwood can be used as a therapeutic with analgesic and anti-gastropathic activity before or after drinking alcohol.
Salidroside was the major biologically active substance in A. tegmentosum heartwood and it exhibited distinct analgesic activity and high anti-gastropathic activity at both the 10 and 20 mg/kg doses (p.o.). It also increased the pH of gastric juice and lowered the gastric juice volume, although its activities were weaker than those of cimetidine. Gastropathy is a disease that occurs frequently in modern society. There are many therapeutics to treat gastritis and digestive ulcers such as antacids, anticholinergic agents, H2 antagonists and H+-pump inhibitors and anti-Helicobacter pylori agents, although these medicinal drugs have unwanted side effects and the disease frequently returns [16Sontag S, Graham DY, Belsito A, et al. Cimetidine, cigarette smoking and recurrence of duodenal ulcer N Engl J Med 1994; 311: 689-93.
[http://dx.doi.org/10.1056/NEJM198409133111101] [PMID: 6382004] -19Tames DL, Warren BB, Colleen B, John TF, Brain LS. Hospitaligation and mortality rates from peptic ulcer disease and GI bleeding in the 1990s Am J Gastrol 2002; 97: 2540-9.
[http://dx.doi.org/10.1111/j.1572-0241.2002.06037.x] [PMID: 12385436] ].
There have been several reports of anti-ulcerative natural products such as flavonoids [20Lewis DA, Fields WN, Shaw GP. A natural flavonoid present in unripe plantain banana pulp (Musa sapientum var. paradisiacal) protects the gastric mucosa from aspirin-induced erosions J Ethnopharmacol 1999; 65: 283-8.
[http://dx.doi.org/10.1016/S0378-8741(99)00005-7] ], sesquiterpene lactones [21Yesilada E, Gurbuz I, Bedir E, Tatli I, Khan IA. Isolation of anti-ulcerogenic sesquiterpene lactones from Centaurea solstitialis L. ssp. Solstitialis through bioassay-guided fractionation procedures in rats J Ethnopharmacol 2004; 95: 213-9.
[http://dx.doi.org/10.1016/j.jep.2004.07.021] [PMID: 15507339] ], diterpenes [22Melo PS, Duran ND, Hiruma-Lima CA, Souza-Brito ARM, Haun M. Comparison of the gastroprotective effect of a diterpene lactone isolated from Croton cajucara with its synthetic derivatives J Ethnopharmacol 2003; 87: 169-74.
[http://dx.doi.org/10.1016/S0378-8741(03)00139-9] ] and saponins [23Yesilda E, Takaishi Y. A saponin with anti-ulcerogenic effect from the flowers of Spartium junceum Phytochemistry 1999; 51: 903-8.
[http://dx.doi.org/10.1016/S0031-9422(99)00198-3] , 24Yoshikawa M, Murakami T, Kishi A, Kageura T, Matsuda H. Medicinal flowers. III. Marigold. (1): Hypoglycemic, gastric emptying
inhibitory, and gastroprotective principles and new oleananetype
triterpene oligoglycosides, Calendasaponins A, B, C, and D,
from Egyptian Calendula officinalis Chem Pharm Bull 2001; 49: 863-70.
[http://dx.doi.org/10.1248/cpb.49.863] ]. In addition, the natural products with anti-Helicobacter pylori effects were reported to include alkaloids [25Carroll AR, Ngo AN, Quinn RJ, Redburn J, Hooper NA, Petrosamine B. an inhibitor of the Helicobacter pylori enzyme aspartyl semialdehyde dehydrogenase from the Australian sponge Oceanapia sp J Nat Prod 2005; 68: 804-6.
[http://dx.doi.org/10.1021/np049595s] [PMID: 15921437] ], sesquiterpenes [26Ochi T, Shibata H, Higuti T, Kodama K, Kusumi T, Takaishi Y. Anti-Helicobacter pylori compounds from Santalum album J Nat Prod 2005; 68: 819-24.
[http://dx.doi.org/10.1021/np040188q] [PMID: 15974602] ], sesquiterpene lactones [27Konstatinopoulou M, Karioti A, Skaltsas S, Skaltsa H. Sesquiterpene lactones from Anthemis altissima and their anti-Helicobacter pylori J Nat Prod 2003; 66: 699-702.
[http://dx.doi.org/10.1021/np020472m] [PMID: 12762812] ], flavonoids [28Fukai T, Marumo A, Kanda T, Terada S, Nomura T. Anti-Helicobacter pylori flavonoids from licorice extract Life Sci 2002; 71: 1449-63.
[http://dx.doi.org/10.1016/S0024-3205(02)01864-7] ] and isoflavonoids [29Takashima J, Chiba N, Yoneda K, Ohsaki A. Derrisin, a new rotenoid from Derris malaccensis plain and anti-Helicobacter pylori activity of its related constituents J Nat Prod 2002; 65: 611-3.
[http://dx.doi.org/10.1021/np010126p] [PMID: 11975515] ]. Crude drugs with anti-ulcerative/anti-inflammatory activities [30Guardia T, Juarez AO, Guarreiro E, Guzman JA, Pelze L. Antiinflammatory activity and effect on gastric acid secretion of dehydroleucodine isolated from Artemisia douglasiana J Ethnopharmacol 2003; 88: 195-8.
[http://dx.doi.org/10.1016/S0378-8741(03)00211-3] ] or with anti-ulcerative/anti-nociceptive activities [31Gonzalez FG, Stasi LCD. Antiulcerogenic and analgesic activities of the leaves of Wilbrandia ebracteata in mice Phytomedicine 2002; 9: 125-34.
[http://dx.doi.org/10.1078/0944-7113-00096] [PMID: 11995945] ] were also reported, suggesting that these medicinal herbal drugs have their effects via immunological or antioxidative mechanisms. Therefore, the anti-gastropathic activity of salidroside elucidated in the present study should be studied further to determine its mechanism of action. Based on the high anti-gastropathic activity of salidroside, it could be used to develop a therapeutic agent against gastric disease. For example, since 4-hydroxyphenylethyl alcohol, an aglycone of salidroside, has a simple structure and can be chemically synthesized, it could be developed as a new therapeutic agent using the chemical structure of salidroside.
ACKNOWLEDGMENT
This work was supported by a grant (Code # 20050401- 034-695-183-01-00) from the BioGreen 21 Program, Rural Development Administration, Republic of Korea.
REFERENCES
| [1] | Pilotto A. Aging and upper gastrointestinal disorders Best Pract Res Clin Gastroenterol 2004; 18: 73-81. [http://dx.doi.org/10.1016/j.bpg.2004.06.015] [PMID: 15588798] |
| [2] | Aihara T, Nakamura E, Amagase K, et al. Pharmacological control of gastric acid secretion for the treatment of acid-related peptic disease Pharmacol Ther 2003; 98: 109-27. |
| [3] | Behrman SW. Management of complicated peptic ulcer disease Arch Surg 2005; 140: 201-8. [http://dx.doi.org/10.1001/archsurg.140.2.201] [PMID: 15724004] |
| [4] | Demir S, Yilmaz M, Koseoglu M, Akalin N, Aslan D, Aydin A. Role of free radicals in peptic ulcer and gastritis Turk J Gastroenterol 2003; 14: 39-43. [PMID: 14593536] |
| [5] | Perry MA, Wadhwa S, Parks DA, Pickard W, Granger DN. Role of oxygen radicals in ischemia-induced lesions in the cat stomach Gastroenterology 1986; 90: 362-267. [PMID: 3753593] |
| [6] | Kim TJ. Plant Resources in Korea Publishing Co Seoul Seoul 1996; Vol. III |
| [7] | Hur JM, Jun M, Yang EJ, Choi SH, Park JC, Song KS. Isolation of isoprenoidal compounds from the stems of Acer tegmentosum max Kor J Pharmacogn 2007; 38: 67-70. |
| [8] | Kubo M, Inoue T, Nagai M. Studies on the constituents of Aceraceae plants. III. Structure of acerogenin B from Acer nikoense Maxim Chem Pharm Bull 1980; 28: 1300-3. [http://dx.doi.org/10.1248/cpb.28.1300] |
| [9] | Kubo M, Nagai M, Inoue T. Studies on the constituents of Aceraceae plants. Carbon-13 nuclear magnetic resonance spectra of
acerogenin A, rhododendrol, and related compounds, and structure
of aceroside from Acer nikoense Chem Pharm Bull 1983; 31: 1917-22. [http://dx.doi.org/10.1248/cpb.31.1917] |
| [10] | Hatano T, Hattori S, Ikeda Y, Shingu T, Okuda Y. Tannins of Aceraceous plants. Part II. Gallotannins having a 1,5-anhydro-Dglucitol
core and some ellagitannins from Acer species Chem Pharm Bull 1990; 38: 1902-5. [http://dx.doi.org/10.1248/cpb.38.1902] |
| [11] | Park KM, Yang MC, Lee KH, Kim KR, Choi SU, Lee KR. Cytotoxic phenolic constituents of Acer tegmentosum maxim Arch Pharm Res 2006; 29: 1086-90. [http://dx.doi.org/10.1007/BF02969296] [PMID: 17225455] |
| [12] | Jung HJ, Choi J, Nam JH, Park HJ. Anti-ulcerogenic effects of effects of the flavonoid-rich fraction from the extract of Orostachys japonicus J Med Food 2007; 10: 702-6. [http://dx.doi.org/10.1089/jmf.2006.223] [PMID: 18158844] |
| [13] | Mizui T, Dodeuchi M. Effect of polyamines on acidified ethanol-induced gastric lesions in rats Jpn J Pharmacol 1983; 33: 939-45. [http://dx.doi.org/10.1254/jjp.33.939] [PMID: 6580476] |
| [14] | Rainsford KD. A synergistic interaction between aspirin, or other non-steroidal anti-inflammatory drugs, and stress which produces severe gastric mucosal damage in rats and pigs Agents Actions 1975; 5: 553-8. [http://dx.doi.org/10.1007/BF01972694] [PMID: 1220559] |
| [15] | Dai S, Ogle CW. A simple method for the production of peptic ulceration in the rat Life Sci 1973; 12: 505-12. [http://dx.doi.org/10.1016/0024-3205(73)90344-5] |
| [16] | Sontag S, Graham DY, Belsito A, et al. Cimetidine, cigarette smoking and recurrence of duodenal ulcer N Engl J Med 1994; 311: 689-93. [http://dx.doi.org/10.1056/NEJM198409133111101] [PMID: 6382004] |
| [17] | Thomxon AB, Mayai S, Berk JE. Medical management of uncomplicated peptic ulcer disease Bochus Gastroenterology Berk JE; EB Sanders Co USA 1985; 1116-9. |
| [18] | Hentschel E, Brandstaffer G, Pragosics B, et al. Effect of radnitidine and amoxicillin plus metronidazole of the eradication of H. pylori and the recurrence of duodenal ulcer N Eng J Med 1993; 328: 308-12. [http://dx.doi.org/10.1056/NEJM199302043280503] [PMID: 8419816] |
| [19] | Tames DL, Warren BB, Colleen B, John TF, Brain LS. Hospitaligation and mortality rates from peptic ulcer disease and GI bleeding in the 1990s Am J Gastrol 2002; 97: 2540-9. [http://dx.doi.org/10.1111/j.1572-0241.2002.06037.x] [PMID: 12385436] |
| [20] | Lewis DA, Fields WN, Shaw GP. A natural flavonoid present in unripe plantain banana pulp (Musa sapientum var. paradisiacal) protects the gastric mucosa from aspirin-induced erosions J Ethnopharmacol 1999; 65: 283-8. [http://dx.doi.org/10.1016/S0378-8741(99)00005-7] |
| [21] | Yesilada E, Gurbuz I, Bedir E, Tatli I, Khan IA. Isolation of anti-ulcerogenic sesquiterpene lactones from Centaurea solstitialis L. ssp. Solstitialis through bioassay-guided fractionation procedures in rats J Ethnopharmacol 2004; 95: 213-9. [http://dx.doi.org/10.1016/j.jep.2004.07.021] [PMID: 15507339] |
| [22] | Melo PS, Duran ND, Hiruma-Lima CA, Souza-Brito ARM, Haun M. Comparison of the gastroprotective effect of a diterpene lactone isolated from Croton cajucara with its synthetic derivatives J Ethnopharmacol 2003; 87: 169-74. [http://dx.doi.org/10.1016/S0378-8741(03)00139-9] |
| [23] | Yesilda E, Takaishi Y. A saponin with anti-ulcerogenic effect from the flowers of Spartium junceum Phytochemistry 1999; 51: 903-8. [http://dx.doi.org/10.1016/S0031-9422(99)00198-3] |
| [24] | Yoshikawa M, Murakami T, Kishi A, Kageura T, Matsuda H. Medicinal flowers. III. Marigold. (1): Hypoglycemic, gastric emptying
inhibitory, and gastroprotective principles and new oleananetype
triterpene oligoglycosides, Calendasaponins A, B, C, and D,
from Egyptian Calendula officinalis Chem Pharm Bull 2001; 49: 863-70. [http://dx.doi.org/10.1248/cpb.49.863] |
| [25] | Carroll AR, Ngo AN, Quinn RJ, Redburn J, Hooper NA, Petrosamine B. an inhibitor of the Helicobacter pylori enzyme aspartyl semialdehyde dehydrogenase from the Australian sponge Oceanapia sp J Nat Prod 2005; 68: 804-6. [http://dx.doi.org/10.1021/np049595s] [PMID: 15921437] |
| [26] | Ochi T, Shibata H, Higuti T, Kodama K, Kusumi T, Takaishi Y. Anti-Helicobacter pylori compounds from Santalum album J Nat Prod 2005; 68: 819-24. [http://dx.doi.org/10.1021/np040188q] [PMID: 15974602] |
| [27] | Konstatinopoulou M, Karioti A, Skaltsas S, Skaltsa H. Sesquiterpene lactones from Anthemis altissima and their anti-Helicobacter pylori J Nat Prod 2003; 66: 699-702. [http://dx.doi.org/10.1021/np020472m] [PMID: 12762812] |
| [28] | Fukai T, Marumo A, Kanda T, Terada S, Nomura T. Anti-Helicobacter pylori flavonoids from licorice extract Life Sci 2002; 71: 1449-63. [http://dx.doi.org/10.1016/S0024-3205(02)01864-7] |
| [29] | Takashima J, Chiba N, Yoneda K, Ohsaki A. Derrisin, a new rotenoid from Derris malaccensis plain and anti-Helicobacter pylori activity of its related constituents J Nat Prod 2002; 65: 611-3. [http://dx.doi.org/10.1021/np010126p] [PMID: 11975515] |
| [30] | Guardia T, Juarez AO, Guarreiro E, Guzman JA, Pelze L. Antiinflammatory activity and effect on gastric acid secretion of dehydroleucodine isolated from Artemisia douglasiana J Ethnopharmacol 2003; 88: 195-8. [http://dx.doi.org/10.1016/S0378-8741(03)00211-3] |
| [31] | Gonzalez FG, Stasi LCD. Antiulcerogenic and analgesic activities of the leaves of Wilbrandia ebracteata in mice Phytomedicine 2002; 9: 125-34. [http://dx.doi.org/10.1078/0944-7113-00096] [PMID: 11995945] |