- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Bioactive Compounds Journal
(Discontinued)
ISSN: 1874-8473 ― Volume 9, 2020
Bioactive Alkaloids from South American Psychotria and Related Species
Diogo D. Porto1, Amélia T. Henriques2, Arthur G. Fett-Neto1, 3, *
Abstract
Many important molecules have been discovered from tropical and sub-tropical plant biodiversity. However, the largest part of the chemical profile of such biodiversity remains unknown. Combining ethnopharmacological and chemotaxonomical investigation can be a good strategy in bioactive compound discovery. South American Psychotria species studied by this approach proved to be a rich source of new bioactive alkaloids, some of which bear unique chemical skeletons.
Article Information
Identifiers and Pagination:
Year: 2009Volume: 2
First Page: 29
Last Page: 36
Publisher Id: TOBCJ-2-29
DOI: 10.2174/1874847300902010029
Article History:
Received Date: 3/5/2009Revision Received Date: 15/5/2009
Acceptance Date: 20/5/2009
Electronic publication date: 15 /9/2009
Collection year: 2011
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Center for Biotechnology, Federal University of Rio Grande do Sul, P.O Box 15005, Porto Alegre, RS, Brazil, Postal Code: 91501-970, Brazil; Tel: 55 51 3308 7642; Fax: 55 51 3308 7309; E-mail: fettneto@cbiot.ufrgs.br
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 3-5-2009 |
Original Manuscript | Bioactive Alkaloids from South American Psychotria and Related Species | |
INTRODUCTION
Plants synthesize an extensive array of secondary metabolites, or natural products, and many of them have pharmacological properties. These bioactive compounds can be found in plants traditionally used for medical purposes, i.e. medicinal plants. Extracts, infusions and other preparations from medicinal plants were the sole alternative to human healthcare needs until the nineteenth century [1Gurib-Fakim A. Medicinal plants traditions of yesterday and drugs of tomorrow Mol Aspects Med 2006; 27: 1-93.
[http://dx.doi.org/10.1016/j.mam.2005.07.008] [PMID: 16105678] ]. The isolation of morphine in 1806 is regarded by many as the beginning of phytochemistry [2Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants Life Sci 2005; 78: 431-41.
[http://dx.doi.org/10.1016/j.lfs.2005.09.012] [PMID: 16198377] , 3Hartmann T. From waste products to ecochemicals fifty years research on plant secondary metabolism Phytochemistry 2007; 68: 2831-46.
[http://dx.doi.org/10.1016/j.phytochem.2007.09.017] [PMID: 17980895] ]. From then on, the development of organic chemistry provided standardized medications through synthethic drugs and active compounds isolated from biological material.
Despite being complex and expensive, biodiversity-screening programs remain productive approaches in drug discovery [4Harvey A. Strategies for discovering drugs from previously
unexplored natural products Drug Discov Today 2000; 5(7): 294-300.
[http://dx.doi.org/10.1016/S1359-6446(00)01511-7] ]. Between 1981 and 2006, 36% of all small molecule new chemical entities were natural products or derived from them, usually by semisynthethic modification [5Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years J Nat Prod 2007; 70: 461-77.
[http://dx.doi.org/10.1021/np068054v] [PMID: 17309302] ]. Other 24% were “natural product mimics”, i.e. competitive inhibitors of natural substrates. High throughput screening techniques [6Gassner NC, Tamble CM, Bock JE, et al. Accelerating the discovery of biologically active small molecules using
a high-throughput yeast halo assay J Nat Prod 2007; 70: 383-90.] as well as advancements in biochemistry and biotechnology of secondary metabolism [7Petersen M. Current status of metabolic phytochemistry Phytochemistry 2007; 68: 2847-60.
[http://dx.doi.org/10.1016/j.phytochem.2007.07.029] [PMID: 17881017] -9Pasquali G, Porto DD, Fett-Neto AG. Metabolite engineering of cell cultures versus whole plant complexity in production of
bioactive monoterpene indole alkaloids recent progress related to old dilemma J Biosci Bioeng 2006; 101(4): 287-96.
[http://dx.doi.org/10.1263/jbb.101.287] [PMID: 16716935] ] are major contemporary achievements which are accelerating natural products research.
This review focuses on molecules found in Rubiaceae plants that have been evaluated for their bioactivity. To that end, a systematic search for full length articles was carried out in indexed databases (e.g. Science Citation Index Expanded© ), highlighting alkaloids discovered within the last decade from South American Psychotria species of the Atlantic Forest biome.
ACTIVE ALKALOIDS FROM RUBIACEAE PLANTS
The Rubiaceae, a large plant family with 10,700 species [10Mongrand S, Badoc A, Patouille B, Lacomblez C, Chavent M, Bessoule JJ. Chemotaxonomy of the Rubiaceae family based on leaf fatty acid composition Phytochemistry 2005; 66(5): 549-59.
[http://dx.doi.org/10.1016/j.phytochem.2004.12.021] [PMID: 15721947] ], yielded the most widely used human stimulant, caffeine (reviewed in Ashihara et al. [11Ashihara H, Sano H, Crozier A. Caffeine and related purine alkaloids Biosynthesis catabolism function and genetic engineering Phytochemistry 2008; 69: 841-56.
[http://dx.doi.org/10.1016/j.phytochem.2007.10.029] [PMID: 18068204] ]). Novel active structures, belonging to diverse biochemical classes such as alkaloids [12Deeni YY, Hussain HSN. Screening for antimicrobial activity and for alkaloids of Nauclea latifolia J Ethnopharmacol 1991; 35: 91-6.
[http://dx.doi.org/10.1016/0378-8741(91)90137-3] -15Chan-Blanco Y, Vaillant F, Perez AM, Reynes M, Brillouet J, Brat P. The noni fruit (Morinda citrifolia La review of agricultural research nutritional and therapeutical properties JFood Compos Anal 2006; 19: 645-54.
[http://dx.doi.org/10.1016/j.jfca.2005.10.001] ], anthraquinones [15Chan-Blanco Y, Vaillant F, Perez AM, Reynes M, Brillouet J, Brat P. The noni fruit (Morinda citrifolia La review of agricultural research nutritional and therapeutical properties JFood Compos Anal 2006; 19: 645-54.
[http://dx.doi.org/10.1016/j.jfca.2005.10.001] -17Wu T, Lin D, Shi L, Damu AG, Kuo P, Kuo Y. Cytotoxic anthraquinones from the stems of Rubia wallichiana Decne Chem Pharm Bull 2003; 51(8): 948-50.
[http://dx.doi.org/10.1248/cpb.51.948] ] and peptides [16Tao J, Morikawa T, Ando S, Matsuda H, Yoshikawa M. Bioactive constituents from chinese natural medicines XI.
Inhibitors of NO production and degranulation in RBL-2H3 from Rubia yunnanensis. Structures of Rubianosides II III and IV
Rubianol-g and Rubianthraquinone Chem Pharm Bull 2003; 51(6): 654-62.
[http://dx.doi.org/10.1248/cpb.51.654] , 18Lee J, Hitotsuyanagi Y, Fukaya H, Kondo K, Takeya K. New cytotoxic byciclic hexapeptides RA-XXIII and RA-XXIV from Rubia cordifolia L Chem Pharm Bull 2008; 56(5): 730-3.
[http://dx.doi.org/10.1248/cpb.56.730] , 19Bokesch HR, Pannel LK, Cochran PK, Sowder RC, McKee TC, Boyd MR. A novel anti-HIV macrocyclic peptide from Palicourea condensata J Nat Prod 2001; 64(2): 249-50.] are constantly being discovered in Rubiaceae species. However, two South American species deserve special consideration: Uncaria tomentosa and Cinchona officinalis.
The Uncaria genus covers 34 species and is a particularly rich source of medicinal natural products (reviewed in Heitzman et al. [20Heitzman ME, Neto CC, Winiarz E, Vaisberg AJ, Hammond GB. Ethnobotany phytochemistry and pharmacology of Uncaria (Rubiaceae) Phytochemistry 2005; 66: 5-29.]). Over 150 compounds have been isolated from Uncaria plants, alkaloids being the most prominent of them. Uncaria tomentosa (Willd.)DC. is endemic to central and south-America and is one of the most important medicinal plants to Peruvian populations [20Heitzman ME, Neto CC, Winiarz E, Vaisberg AJ, Hammond GB. Ethnobotany phytochemistry and pharmacology of Uncaria (Rubiaceae) Phytochemistry 2005; 66: 5-29.]. Extracts from U. tomentosa have a plethora of traditional uses. Further investigations of extracts revealed activities such as immunomodulator [21Keplinger K, Laus G, Wurm M, Dierich MP, Teppner H. Uncaria tomentosa (Willd DC Ethnomedicinal use and new pharmacological toxicological and botanical results J Ethnopharmacol 1999; 64: 23-34.
[http://dx.doi.org/10.1016/S0378-8741(98)00096-8] , 22Reis SRIN, Valente LMM, Sampaio AL, et al. Immunomodulating and antiviral activities of Uncaria tomentosa on human monocytes infected with Dengue Virus-2 Int Immunopharmacol 2008; 8: 468-76.], antiviral [22Reis SRIN, Valente LMM, Sampaio AL, et al. Immunomodulating and antiviral activities of Uncaria tomentosa on human monocytes infected with Dengue Virus-2 Int Immunopharmacol 2008; 8: 468-76.], anti-inflammatory [23Amaral S, Mira L, Nogueira JMF, Silva AP, Florêncio MH. Plant extracts with anti-inflammatory properties - A new
approach for characterization of their bioactive compounds and
establishment of structure-antioxidant activity relationships Bioorgan Med Chem 2009; 17: 1876-83.
[http://dx.doi.org/10.1016/j.bmc.2009.01.045] [PMID: 19201196] ], antioxidant [20Heitzman ME, Neto CC, Winiarz E, Vaisberg AJ, Hammond GB. Ethnobotany phytochemistry and pharmacology of Uncaria (Rubiaceae) Phytochemistry 2005; 66: 5-29., 23Amaral S, Mira L, Nogueira JMF, Silva AP, Florêncio MH. Plant extracts with anti-inflammatory properties - A new
approach for characterization of their bioactive compounds and
establishment of structure-antioxidant activity relationships Bioorgan Med Chem 2009; 17: 1876-83.
[http://dx.doi.org/10.1016/j.bmc.2009.01.045] [PMID: 19201196] ] and cytostatic [24Kurás M, Pilarski R, Nowakowska J, et al. Effect of alkaloid-free and alkaloid-rich preparations from Uncaria tomentosa bark on mitotic activity and chromosome morphology evaluated by Allium test J Ethnopharmacol 2009; 121: 140-7.]. Tetra and pentacyclic alkaloids present in the plant extracts seem to play a major role in some of these effects [21Keplinger K, Laus G, Wurm M, Dierich MP, Teppner H. Uncaria tomentosa (Willd DC Ethnomedicinal use and new pharmacological toxicological and botanical results J Ethnopharmacol 1999; 64: 23-34.
[http://dx.doi.org/10.1016/S0378-8741(98)00096-8] , 24Kurás M, Pilarski R, Nowakowska J, et al. Effect of alkaloid-free and alkaloid-rich preparations from Uncaria tomentosa bark on mitotic activity and chromosome morphology evaluated by Allium test J Ethnopharmacol 2009; 121: 140-7.].
From the discovery of the New World until the mid-19th century, malaria was a major cause of death of European people involved in trade and colonization of tropical lands [25Carter R, Mendis KM. Evolutionary and historical aspects of
the burden of malaria Clin Microbiol Rev 2002; 15(4): 564--594.
[http://dx.doi.org/10.1128/CMR.15.4.564-594.2002] [PMID: 12364370] [PMCID: PMC126857] ]. With the introduction of quinine, an active compound from Cinchona bark, the mortality fell dramatically. Quinine is an alkaloid found in Cinchona officinalis and some other Cinchona species known as “quina quina” by native populations [26Kinsley-Scott TR, Norton SA. Useful plants in dermatology VII Cinchona and antimalarials J Am Acad Dermatol 2003; 49: 499-502.
[http://dx.doi.org/10.1067/S0190-9622(03)01281-7] ]. Other antimalarial alkaloids present in Cinchona bark include quinidine, cinchonine and cinchonidine [27Druilhe P, Brandicourt O, Chongsuphajaisiddhi T, Berthe J. Activity of a combination of three Cinchona bark alkaloids against Plasmodium falciparum in vitro Antimicrob Agents Ch 1988; 32(2): 250-4.
[http://dx.doi.org/10.1128/AAC.32.2.250] ]. In present times, quinine-resistant strains of Plasmodium falciparum have emerged, and new drug leads to address this problem include medicinal plants [28Kvist LP, Christensen SB, Rasmussen HB, Mejia K, Gonzales A. Identification and evaluation of Peruvian plants used to treat malaria and leishmaniasis J Ethnopharmacol 2006; 106: 390-402., 29Bourdy G, Willcox ML, Ginsburg H, Rasoanaivo Ph, Graz B, Deharo E. Ethnopharmacology and malaria new hypothetical leads or old efficient antimalarials Int J Parasitol 2008; 38: 33-41.
[http://dx.doi.org/10.1016/j.ijpara.2007.07.004] [PMID: 17720165] ]. Alkaloids such as cephaeline (Fig. 1a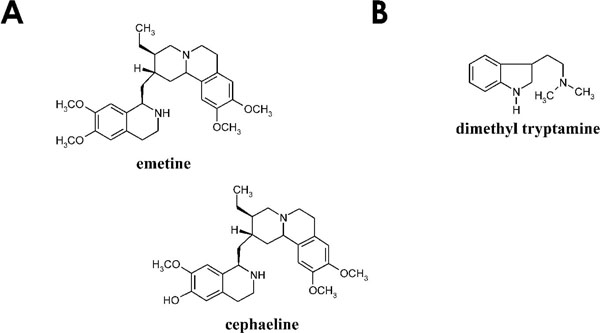 ) and klugine isolated from Psychotria klugii have shown antimalarial and antileishmanial activity [30Muhammad I, Dunbar DC, Khan SI, et al. Antiparasitic alkaloids from Psychotria klugii J Nat Prod 2003; 66: 962-7.
) and klugine isolated from Psychotria klugii have shown antimalarial and antileishmanial activity [30Muhammad I, Dunbar DC, Khan SI, et al. Antiparasitic alkaloids from Psychotria klugii J Nat Prod 2003; 66: 962-7.
[http://dx.doi.org/10.1021/np030086k] [PMID: 12880315] ].
Both Uncaria tomentosa and Cinchona officinalis are examples of medicinal plants discovered within traditional communities. Plants known by these people, who live in the vicinity of rich biodiversity for centuries, are interesting starting points for phytochemical screening through ethnopharmacological surveys [1Gurib-Fakim A. Medicinal plants traditions of yesterday and drugs of tomorrow Mol Aspects Med 2006; 27: 1-93.
[http://dx.doi.org/10.1016/j.mam.2005.07.008] [PMID: 16105678] , 2Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants Life Sci 2005; 78: 431-41.
[http://dx.doi.org/10.1016/j.lfs.2005.09.012] [PMID: 16198377] , 31Soh PN, Benoit-Vical F. Are West African plants a source of future antimalarial drugs J Ethnopharmacol 2007; 114: 130-40.
[http://dx.doi.org/10.1016/j.jep.2007.08.012] [PMID: 17884314] ]. Important new molecules have been discovered from medicinal plants, including the antitumorals vincristine and vinblastine from Catharanthus roseus, the antimalarial artemisin from Artemisia annua and the neuroactive reserpine from Rauwolfia serpentina [1Gurib-Fakim A. Medicinal plants traditions of yesterday and drugs of tomorrow Mol Aspects Med 2006; 27: 1-93.
[http://dx.doi.org/10.1016/j.mam.2005.07.008] [PMID: 16105678] ].
THE PSYCHOTRIA GENUS
The genus Psychotria is one of the largest genera of flowering plants and the largest within Rubiaceae, with estimated 1000 to 1650 species distributed worldwide [32Nepkroeff M, Bremer B, Systma KJ. Reorganization of the genus Psychotria and tribe Psychotrieae (Rubiaceae) inferred from ITS and rbcL sequence data Syst Bot 1999; 24(1): 5-27.]. Psychotria species often accumulate indole alkaloids, and this trait may be important to chemosystematics, since this genus is taxonomically complex due to a lack of conspicuous morphological differentiating features [32Nepkroeff M, Bremer B, Systma KJ. Reorganization of the genus Psychotria and tribe Psychotrieae (Rubiaceae) inferred from ITS and rbcL sequence data Syst Bot 1999; 24(1): 5-27., 33Lopes S, vonPoser GL, Kerber VA, et al. Taxonomic significance of alkaloids and iridoid glucosides in the tribe psychotrieae (Rubiaceae) Biochem Syst Ecol 2004; 32: 1187-95.
[http://dx.doi.org/10.1016/j.bse.2004.04.015] ]. A number of Psychotria species yielded bioactive extracts. Some examples include antibiotic activity in extracts from P. microlabastra [34Khan MR, Kihara M, Omoloso AD. Antimicrobial activity of Psychotria microlabastra Fitoterapia 2001; 72: 818-21.
[http://dx.doi.org/10.1016/S0367-326X(01)00312-4] ] and P. capensis [35McGaw LJ, Jäger AK, vanStaden J. Antibacterial anthel-
mintic and anti-amoebic activity in South African medicinal plants J Ethnopharmacol 2000; 72: 247-63.
[http://dx.doi.org/10.1016/S0378-8741(00)00269-5] ] (Africa), antiviral activity in P. serpens [36Kuo Y, Chen C, Tsai W, Ho Y. Regulation of herpes simplex virus type 1 replication in Vero cells by Psychotria serpens relationship to gene expression DNA replication and protein synthesis Antiviral Res 2001; 51: 95-109.
[http://dx.doi.org/10.1016/S0166-3542(01)00141-3] ] (China) and antiviral/antifungal and antiinflammatory activities found in P. hawaiiensis [37Locher CP, Burch MT, Mower HF, et al. Anti-microbial activity and anti-complement activity of extracts obtained from selected Hawaiian medicinal plants J Ethnopharmacol 1995; 49: 23-32.
[http://dx.doi.org/10.1016/0378-8741(95)01299-0] ]and P. insularum [38Dunstan CA, Noreen Y, Serrano G, Cox PA, Perera P, Bohlin L. Evaluation of some Samoan and Peruvian medicinal plants by prostaglandin biosynthesis and rat ear oedema assays J Ethnopharmacol 1997; 57: 35-56.] (Central America), respectively.
Active molecules produced by Psychotria species include naphtoquinones [39Hayashi T, Smith FT, Lee KH. Antitumor agents 89 Psychorubrin a new cytotoxic naphtoquinone from Psychotria rubra and its structure-activity relationships J Med Chem 1987; 30(11): 2005-8.
[http://dx.doi.org/10.1021/jm00394a013] [PMID: 3669007] ], peptides [40Witherup KM, Bogusky MJ, Anderson PS, et al. Cyclopsychotride A a
biologically active 31-residue cyclic peptide isolated from Psychotria longipes J Nat Prod 1994; 57(12): 1619-25.], benzoquinones [41Solis PN, Lang’at C, Gupta MP, Kirby GC, Warhurst DC, Phillipson JD. Bio-active compounds from Psychotria camponutans Planta Med 1995; 61: 62-5.
[http://dx.doi.org/10.1055/s-2006-958001] [PMID: 7700994] ], pigments [42Glinski JA, David E, Warren TC, et al. Inactivation of cell surface receptors by pheophorbide a a green pigment isolated from Psychotria acuminata Photochem Photobiol 1995; 62(1): 144-50.
[http://dx.doi.org/10.1111/j.1751-1097.1995.tb05250.x] [PMID: 7638258] ] and alkaloids [43Beretz A, Roth-Georger A, Corre G, Kuballa B, Anton R, Cazenave J. Polyindolinic alkaloids from Psychotria forsteriana Potent inhibitors of the aggregation of human platelets Planta Med 1985; 51: 300-3.]. Perhaps the best known compound isolated from Psychotria species is the alkaloid emetine. Emetine (Fig. 1a ) is an isoquinoline alkaloid extracted from P. ipecacuanha (ipecac) bark, a plant used by traditional communities as stimulant and “antidote to opium” [44Giorgetti M, Negri G, Rodrigues E. Brazilian plants with possible action on the central nervous system - A study of historical sources from the 16th to 19th century J Ethnopharmacol 2007; 109: 338-47.
) is an isoquinoline alkaloid extracted from P. ipecacuanha (ipecac) bark, a plant used by traditional communities as stimulant and “antidote to opium” [44Giorgetti M, Negri G, Rodrigues E. Brazilian plants with possible action on the central nervous system - A study of historical sources from the 16th to 19th century J Ethnopharmacol 2007; 109: 338-47.
[http://dx.doi.org/10.1016/j.jep.2006.08.003] [PMID: 16982166] ] and in the treatment of intoxication due to its emetic effect [45Hasegawa M, Sasaki T, Sadakane K, et al. Studies for the emetic mechanism of Ipecac Syrup (TJN-119) and its active components in ferrets involvement of 5-hydroxytryptamine receptors Jpn J Pharmacol 2002; 89: 113-9.]. Synthethic analogs of emetine, which have less adverse effects, are currently used in the treatment of amoebiasis [46Mangaña-García M, Arista-Viveros A. Cutaneous amebiasis in children Pediatr Dermatol 2008; 10(4): 352-5.]. Emetine is cytotoxic, inhibiting protein synthesis, and may have applications in drug-induced apoptosis [47Möller M, Herzer K, Wenger T, Herr I, Wink M. The alkaloid emetine as a promising agent for the induction and enhancement of drug-induced apoptosis in leukemia cells Oncol Rep 2007; 18: 737-44.
[PMID: 17671728] ].
Another well known Psychotria species is the one used in the preparation of the hallucinogenic drink “ayahuasca”, P. viridis. The decoction is prepared using the plant in combination with the vine Banisteriopsis caapi. (Spruce ex Griseb). Morton (Malpighiaceae). Psychotria viridis and B. caapi are rich sources of the proto-alkaloid dimethyltryptamine (DMT) (Fig. 1b ) and the (-carboline harmine, respectively [48Freedland CS, Mansbach RS. Behavioral profile of constituents in ayahuasca an Amazonian psychoactive plant mixture Drug Alcohol Depend 1999; 54: 183-94.
) and the (-carboline harmine, respectively [48Freedland CS, Mansbach RS. Behavioral profile of constituents in ayahuasca an Amazonian psychoactive plant mixture Drug Alcohol Depend 1999; 54: 183-94.
[http://dx.doi.org/10.1016/S0376-8716(98)00154-9] ]. Both substances are psychoactive, and the two have a strong synergism when administered together, possibly due to inhibitory effects of harmine on monoamine oxidase, a DMT detoxifying enzyme [49Buckholtz NS, Boggan WO. Monoamine oxidase inhibition in brain and liver produced by ?-carbolines structure activity relationships and substrate specificity Biochem Pharmacol 1977; 26: 1991-6.
[http://dx.doi.org/10.1016/0006-2952(77)90007-7] ]. The recent popularization of the ayahuasca in the United States and Europe has raised several debates, from mental health issues to conflicts on drug abuse versus religious freedom [50Santos RG, Landeira-Fernandez J, Strassman RJ, Motta V, Cruz APM. Effects of ayahuasca on psychometric measures of anxiety panic-like and hopelessness in Santo Daime members J Ethnopharmacol 2007; 112: 507-13., 51Tupper KW. The globalization of ayahuasca Harm reduction or benefit maximization Int J Drug Policy 2008; 19: 297-303.
[http://dx.doi.org/10.1016/j.drugpo.2006.11.001] [PMID: 18638702] ].
THE ALKALOIDS FROM PSYCHOTRIA COLORATA MÜLL. ARG
Several South American Psychotria species are used as medicinal plants by Amazon native populations [52Sanz-Biset J, Campos-de-la-Cruz J, Epiquién-Rivera MA, Cañigueral S. A first survey on the medicinal plants of the Chazuta valley (Peruvian Amazon) J Ethnopharmacol 2009; 122: 333-62.].An ethnobotanical survey identified species used as painkillers by “caboclos”, traditional rural communities from the state of Pará, Brazil, which comprises a large fraction of the Amazon rainforest. The extracts of P. colorata showed analgesic activity, and preliminary tests pointed to alkaloids as major responsibles for the effect [53Elisabetsky E, Amador TA, Albuquerque RR, Nunes DS, Carvalho ACT. Analgesic activity of Psychotria colorata (Willdex R.& S.) Muell. Arg. alkaloids J Ethnopharmacol 1995; 48: 77-83.]. Further chemical investigations demonstrated the presence of several pyrrolidinoindoline and quinoline alkaloids (Fig. 2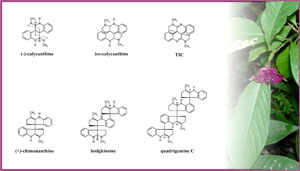 ) [54Verotta L, Pilati T, Tatò M, Elisabetsky E, Amador TA, Nunes DS. Pyrrolidinoindoline alkaloids from Psychotria
colorata J Nat Prod 1998; 61: 392-6.], with hodgkinsine, previously isolated from Hodgkinsonia frutescens F. Muell. (Rubiaceae) [55Fridrichsons J, Mackay MF, Mathieson AM. The absolute molecular structure of hodgkinsine Tetrahedron 1974; 30(1): 85-92.
) [54Verotta L, Pilati T, Tatò M, Elisabetsky E, Amador TA, Nunes DS. Pyrrolidinoindoline alkaloids from Psychotria
colorata J Nat Prod 1998; 61: 392-6.], with hodgkinsine, previously isolated from Hodgkinsonia frutescens F. Muell. (Rubiaceae) [55Fridrichsons J, Mackay MF, Mathieson AM. The absolute molecular structure of hodgkinsine Tetrahedron 1974; 30(1): 85-92.
[http://dx.doi.org/10.1016/S0040-4020(01)97221-7] ], as a major component. Hodgkinsine is a potent analgesic, with results comparable to morphine in murine models using the hot-plate and tail-flick tests [56Kodanko JJ, Hiebert S, Peterson EA, et al. Synthesis of all-low energy stereoisomers of the tris (pyrrolidinoindoline) alkaloid hodgkinsine and preliminary assessment of their antinociceptive activity J Org Chem 2007; 72(21): 7909-14., 57Amador TA, Verotta L, Nunes DS, Elisabetsky E. Anti-
nociceptive profile of hodgkinsine Planta Med 2000; 66: 770-2.
[http://dx.doi.org/10.1055/s-2000-9604] [PMID: 11199142] ].
 |
Fig. (2) Alkaloids isolated from Psychotria colorata. The upper and lower structures are quinoline and pyrrolidinoindoline alkaloids, respectively.TIC: (8-8a),(8´-8´a)-tetradehydroisocalycanthine 3a(R), 3´a(R). Photo modified from [87Zafar S. Royal Botanic Gardens Kew Images http://www.kew.org/science/tropamerica/neotropikey/families/images/Rubiaceae/Psychotria_colorata_5.jpeg.png [Aprl 23 2009];]. |
Besides hodgkinsine, three other P. colorata pyrrolidinoindoline alkaloids, (+)-chimonanthine and the later isolated meso-chimonanthine and psychotridine [58Verotta L, Peterlongo F, Elisabetsky E, Amador TA, Nunes DS. High-performance liquid chromatography - diode array detection - tandem mass spectrometry analyses of the alkaloid extracts of Amazon Psychotria species J Chromatogr A 1999; 841: 165-76.
[http://dx.doi.org/10.1016/S0021-9673(99)00298-8] ], showed analgesic activity [59Verotta L, Orsini F, Sbacchi M, Scheildler MA, Amador TA, Elisabetsky E. Synthesis and antinociceptive activity of chimonanthines and pyrrolidinoindoline-type alkaloids Bioorg Med Chem 2002; 10: 2133-42.
[http://dx.doi.org/10.1016/S0968-0896(02)00078-0] , 60Amador TA, Verotta L, Nunes DS, Elisabetsky E. Involvement of NMDA receptors in the analgesic properties of psychotridine Phytomedicine 2001; 8(3): 202-6.
[http://dx.doi.org/10.1078/0944-7113-00025] [PMID: 11417913] ]. Interestingly, only pyrrolidinoindoline-type alkaloids, and not quinoline, gave positive results to nociceptive tests, suggesting a structure-activity relationship [58Verotta L, Peterlongo F, Elisabetsky E, Amador TA, Nunes DS. High-performance liquid chromatography - diode array detection - tandem mass spectrometry analyses of the alkaloid extracts of Amazon Psychotria species J Chromatogr A 1999; 841: 165-76.
[http://dx.doi.org/10.1016/S0021-9673(99)00298-8] ] (see Fig. 2 ). From the data with capsaicin-induced pain models, it was suggested that the alkaloids act on opioid and glutamate receptors [57Amador TA, Verotta L, Nunes DS, Elisabetsky E. Anti-
nociceptive profile of hodgkinsine Planta Med 2000; 66: 770-2.
). From the data with capsaicin-induced pain models, it was suggested that the alkaloids act on opioid and glutamate receptors [57Amador TA, Verotta L, Nunes DS, Elisabetsky E. Anti-
nociceptive profile of hodgkinsine Planta Med 2000; 66: 770-2.
[http://dx.doi.org/10.1055/s-2000-9604] [PMID: 11199142] , 59Verotta L, Orsini F, Sbacchi M, Scheildler MA, Amador TA, Elisabetsky E. Synthesis and antinociceptive activity of chimonanthines and pyrrolidinoindoline-type alkaloids Bioorg Med Chem 2002; 10: 2133-42.
[http://dx.doi.org/10.1016/S0968-0896(02)00078-0] , 60Amador TA, Verotta L, Nunes DS, Elisabetsky E. Involvement of NMDA receptors in the analgesic properties of psychotridine Phytomedicine 2001; 8(3): 202-6.
[http://dx.doi.org/10.1078/0944-7113-00025] [PMID: 11417913] ].
BIOACTIVE ALKALOIDS FROM SOUTHERN BRAZILIAN PSYCHOTRIA SPECIES
The promising results with P. colorata motivated investigations of Southern Brazilian subtropical species. Initial screening of ethanolic extracts from Psychotria plants revealed analgesic activity in six species [61Leal MB. Thesis Universidade Federal do Rio Grande do Sul 1994.] (Fig. 3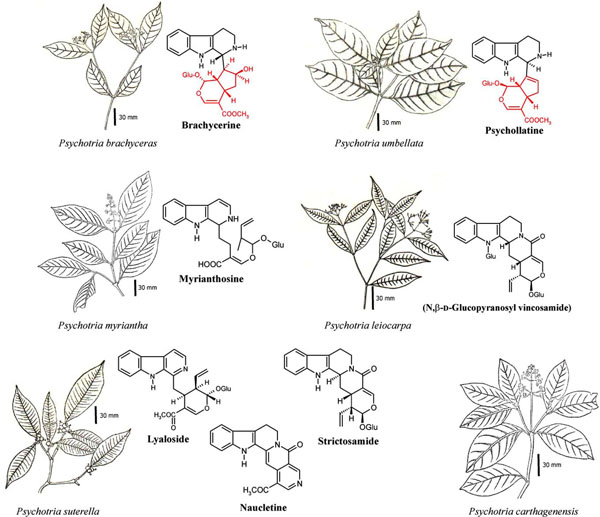 ): P. brachyceras, P. carthagenensis, P. leiocarpa, P. myriantha, P. suterella and P. umbellata. All these species belong to the subgenus Heteropsychotria [33Lopes S, vonPoser GL, Kerber VA, et al. Taxonomic significance of alkaloids and iridoid glucosides in the tribe psychotrieae (Rubiaceae) Biochem Syst Ecol 2004; 32: 1187-95.
): P. brachyceras, P. carthagenensis, P. leiocarpa, P. myriantha, P. suterella and P. umbellata. All these species belong to the subgenus Heteropsychotria [33Lopes S, vonPoser GL, Kerber VA, et al. Taxonomic significance of alkaloids and iridoid glucosides in the tribe psychotrieae (Rubiaceae) Biochem Syst Ecol 2004; 32: 1187-95.
[http://dx.doi.org/10.1016/j.bse.2004.04.015] ]. Among these, only P. carthagenensis from this region did not show positive reaction for alkaloids [62Leal MB, Elisabetsky E. Absence of alkaloids in Psychotria carthagenensis Jacq (Rubiaceae) J Ethnopharmacol 1996; 54: 37-40.
[http://dx.doi.org/10.1016/0378-8741(96)01448-1] ]. Curiously, P. carthagenensis is used in the preparation of ayahuasca brew in substitution to Amazonic P. viridis [62Leal MB, Elisabetsky E. Absence of alkaloids in Psychotria carthagenensis Jacq (Rubiaceae) J Ethnopharmacol 1996; 54: 37-40.
[http://dx.doi.org/10.1016/0378-8741(96)01448-1] ]. However, detailed chemical profiles obtained with adequate analytical facilities on P. carthagenensis from other regions is lacking and generalization of these results must be done with caution.
 |
Fig. (3) Indole alkaloids from Southern Brazilian Psychotria species. The portions in red in brachycerine and psychollatine structures are residues from uncommon terpenoid precursors, which may derive from epiloganine and geniposide, respectively. Plant images adapted from[88Dillenburg CR. Thesis Universidade Federal do Rio Grande do Sul 1978.]. |
Alkaloid extracts from P. myriantha shoots, besides analgesic [63Both FL, Farias FM, Nicoláo LL, Misturini J, Henriques AT, Elisabetsky E. Avaliação da atividade analgésica de extratos alcaloídicos de espécies de Psychotria Rev Bras Plant Med 2002; 5(1): 41-5.], revealed anti-inflammatory activity in chemotaxis assays [64Simões-Pires CA, Farias FM, Marston A, et al. Indole monoterpenes with antichemotactic activity fromPsychotria myriantha Chemotaxonomic significance Nat Prod Comm 2006; 1(12): 1101-6.]. Purification and structural elucidation yielded strictosidinic acid and a new structure, myrianthosine (Fig. 3 ). P. suterella Müll. Arg. leaf extracts, however, did not retain analgesic activity in the alkaloid fractions, which were highly toxic [65Santos LV, Fett-Neto AG, Kerber VA, Elisabetsky E, Quirion JP, Henriques AT. Indole monoterpene alkaloids from leaves of Psychotria suterella Müll Arg (Rubiaceae) Biochem Syst Ecol 2001; 29: 1185-7.]. The alkaloids isolated from this species, lyaloside, strictosamide and naucletine (Fig. 3
). P. suterella Müll. Arg. leaf extracts, however, did not retain analgesic activity in the alkaloid fractions, which were highly toxic [65Santos LV, Fett-Neto AG, Kerber VA, Elisabetsky E, Quirion JP, Henriques AT. Indole monoterpene alkaloids from leaves of Psychotria suterella Müll Arg (Rubiaceae) Biochem Syst Ecol 2001; 29: 1185-7.]. The alkaloids isolated from this species, lyaloside, strictosamide and naucletine (Fig. 3 ), until then had not been reported to thegenus. Recently, strictosamide was found as the major component (about 98%) of the alkaloid extract of leaves of Psychotria nuda (Cham. et Schltdl) Wawra [66Farias FM, Konrath EL, Zuanazzi JA, Henriques AT. Strictosamide from Psychotria nuda (ChamEt Schltdl) Wawra (Rubiaceae) Biochem Syst Ecol 2009; 36: 919- 920.
), until then had not been reported to thegenus. Recently, strictosamide was found as the major component (about 98%) of the alkaloid extract of leaves of Psychotria nuda (Cham. et Schltdl) Wawra [66Farias FM, Konrath EL, Zuanazzi JA, Henriques AT. Strictosamide from Psychotria nuda (ChamEt Schltdl) Wawra (Rubiaceae) Biochem Syst Ecol 2009; 36: 919- 920.
[http://dx.doi.org/10.1016/j.bse.2008.10.002] ]. Strictosamide was also found in Psychotria bahiensis DC. from Trinidad which yielded the bis(monoterpenoid) indole alkaloids bahienosine A and B; these alkaloids incorporate two secologanin units [67Paul JHA, Maxwell AR, Reynolds WF. Novel bis(mono-
terpenoid) indole alkaloids from Psychotria bahiensis J Nat Prod 2003; 66: 752-4.
[http://dx.doi.org/10.1021/np020554a] [PMID: 12828456] ].
P. leiocarpa Cham. et Schlecht is found in Argentina, Paraguay and the Southern Brazilian state of Rio Grande do Sul. The species is dominant in the understorey of subtropical semi-deciduous forests, possibly due to production of allelopathic compounds [68Corrêa LR, Soares GLG, Fett-Neto AG. Allelopathic
potential of Psychotria leiocarpa a understory species of subtro-
pical forests S Afr J Bot 2008; 74(4): 583-90.
[http://dx.doi.org/10.1016/j.sajb.2008.02.006] ]. Ethanolic leaf extracts from P. leiocarpa yielded N, (-d-glucopyranosyl vincosamide (GPV) (Fig. 3 ), an unusual indole alkaloid bearing two glucose residues, as major compound [69Henriques AT, Lopes SO, Paranhos JT, et al. N-?-D-Gluco-pyranosyl vincosamide a light regulated indole alkaloid from
the shoots of Psychotria leiocarpa Phytochemistry 2004; 65: 449-54.]. The indole features a glucose residue attached to the nitrogen, an uncommon configuration never before described for a monoterpenoid indole alkaloid.
), an unusual indole alkaloid bearing two glucose residues, as major compound [69Henriques AT, Lopes SO, Paranhos JT, et al. N-?-D-Gluco-pyranosyl vincosamide a light regulated indole alkaloid from
the shoots of Psychotria leiocarpa Phytochemistry 2004; 65: 449-54.]. The indole features a glucose residue attached to the nitrogen, an uncommon configuration never before described for a monoterpenoid indole alkaloid.
From the extracts of the above six Psychotria species which gave positive results to analgesic activity, only one showed dose-dependence and reversible effect by naloxone, an opioid receptor antagonist [70Kayser V, Guilbaud G. Differential effect of various doses of morphine and naloxone on two nociceptive tests thresholds in
arthritic and normal rats Pain 1990; 41(3): 353-63.
[http://dx.doi.org/10.1016/0304-3959(90)90012-3] ]: Psychotria umbellata Vell. [61Leal MB. Thesis Universidade Federal do Rio Grande do Sul 1994.]. The leaves of P. umbellata accumulate the monoterpene indole alkaloid (MIA) psychollatine (Fig. 3 ), formerly known as umbellatine [71Kerber VA, Passos CS, Verli H, Fett-Neto AG, Quirion
JP, Henriques AT. Psychollatine a glucosidic monoterpene indole alkaloid from Psychotria umbellata J Nat Prod 2008; 71: 697-700.
), formerly known as umbellatine [71Kerber VA, Passos CS, Verli H, Fett-Neto AG, Quirion
JP, Henriques AT. Psychollatine a glucosidic monoterpene indole alkaloid from Psychotria umbellata J Nat Prod 2008; 71: 697-700.
[http://dx.doi.org/10.1021/np0703951] [PMID: 18288808] ]. Psychollatine has also been recently isolated from Palicourea crocea, a closely related species [72Narine LL, Maxwell AR. Monoterpenoid indole alkaloids from Palicourea crocea Phytochem Lett 2009; 2: 34-6.
[http://dx.doi.org/10.1016/j.phytol.2008.10.007] ] which also produces a stereoisomer of psychollatine, croceaine A [72Narine LL, Maxwell AR. Monoterpenoid indole alkaloids from Palicourea crocea Phytochem Lett 2009; 2: 34-6.
[http://dx.doi.org/10.1016/j.phytol.2008.10.007] , 73Düsman LT, Jorge TCM, Souza MC, et al. Monoterpene indole alkaloids from Palicourea crocea J Nat Prod 2004; 67 (11): 1886-8.]. The alkaloid profile between the two plants may have taxonomic significance. Psychotria and Palicourea are closely related genera (P. umbellata is also referred to as Palicourea brachypoda (Müell. Arg.) L. B. Smith & Downs and as Psychotria brachypoda (Müell. Arg.) Britton). The chemical data from both species supports the suggested fusion between the Psychotria subgenera Heteropsychotria and Palicourea into one genus [32Nepkroeff M, Bremer B, Systma KJ. Reorganization of the genus Psychotria and tribe Psychotrieae (Rubiaceae) inferred from ITS and rbcL sequence data Syst Bot 1999; 24(1): 5-27., 33Lopes S, vonPoser GL, Kerber VA, et al. Taxonomic significance of alkaloids and iridoid glucosides in the tribe psychotrieae (Rubiaceae) Biochem Syst Ecol 2004; 32: 1187-95.
[http://dx.doi.org/10.1016/j.bse.2004.04.015] ].
Psychollatine has analgesic, anxiolytic and antidepressant activities, with amnesic and sedative effects in higher doses (100 mg/kg) [74Both FL, Meneghini L, Kerber VA, Henriques AT, Elisabetsky E. Psychopharmacological profile of the alkaloid psychollatine as a 5HT2A/C serotonin modulator J Nat Prod 2005; 68: 374-80.]. The alkaloid seems to be active on the serotoninergic and opioid systems. Also, both psychollatine and crude methanolic extracts from P. umbellata leaves showed antioxidant and antimutagenic properties in Saccharomyces cerevisae bioassays [75Fragoso V, Nascimento NC, Moura DJ, et al. Antioxidant and antimutagenic properties of the monoterpene indole alkaloid psychollatine and the crude foliar extract of Psychotria umbellata Vell Toxicol in Vitro 2008; 22: 559-66.]. The treatments reduced growth inhibition of wild type and antioxidant-enzyme-defective S. cerevisae strains challenged with hydrogen peroxide and superoxide anion. In addition, both psychollatine and the leaf extract inhibited H2O2-driven mutagenesis in S. cerevisae haploid lines. The alkaloid and crude extract were also effective antioxidants against hydroxyl radical in vitro [75Fragoso V, Nascimento NC, Moura DJ, et al. Antioxidant and antimutagenic properties of the monoterpene indole alkaloid psychollatine and the crude foliar extract of Psychotria umbellata Vell Toxicol in Vitro 2008; 22: 559-66.].
A very similar MIA was isolated from Psychotria brachyceras Müll. Arg. [76Kerber VA, Gregianini TS, Paranhos JT, et al. Brachycerine a novel monoterpene indole alkaloid from Psychotria brachyceras J Nat Prod 2001; 64: 677-9.]. The compound, named brachycerine (Fig. 3e ) is a singlet oxygen [77Gregianini TS, Silveira VC, Porto DD, Kerber VA, Henriques AT, Fett-Neto AG. The alkaloid brachycerine is induced by ultraviolet radiation and is a singlet oxygen quencher Photochem Photobiol 2003; 78(5): 470-4.
) is a singlet oxygen [77Gregianini TS, Silveira VC, Porto DD, Kerber VA, Henriques AT, Fett-Neto AG. The alkaloid brachycerine is induced by ultraviolet radiation and is a singlet oxygen quencher Photochem Photobiol 2003; 78(5): 470-4.
[http://dx.doi.org/10.1562/0031-8655(2003)078<0470:TABIIB>2.0.CO;2] ] and super- oxide (Porto et al., unpublished) quencher, and gave positive results for anti-inflammatory activity in chemotaxis assays (Henriques, A. T., unpublished). Brachycerine, as well as psychollatine, was capable of protecting S. cerevisae strains from oxidative stress, with higher protection against superoxide anion [78Nascimento NC, Fragoso V, Moura DJ, Silva ACR, Fett-Neto AG, Saffi J. Antioxidant and antimutagenic effects of the crude foliar extract and the alkaloid Brachycerine of Psychotria brachyceras Environ Mol Mutagen 2007; 48(9): 728-34.].
Both brachycerine and psychollatine are unusual MIAs. Most alkaloids of this class are derived from strictosidine, which is the product of the condensation of tryptamine and the iridoid secologanin, a reaction catalysed by strictosidine synthase [79Ziegler J, Facchini PJ. Alkaloid biosynthesis metabolism and trafficking Annu Rev Plant Biol 2008; 59: 735-69.
[http://dx.doi.org/10.1146/annurev.arplant.59.032607.092730] [PMID: 18251710] ]. However, brachycerine and psychollatine seem to be products of tryptamine condensation with epiloganin and geniposide derivatives, respectively [71Kerber VA, Passos CS, Verli H, Fett-Neto AG, Quirion
JP, Henriques AT. Psychollatine a glucosidic monoterpene indole alkaloid from Psychotria umbellata J Nat Prod 2008; 71: 697-700.
[http://dx.doi.org/10.1021/np0703951] [PMID: 18288808] , 76Kerber VA, Gregianini TS, Paranhos JT, et al. Brachycerine a novel monoterpene indole alkaloid from Psychotria brachyceras J Nat Prod 2001; 64: 677-9.]. The putative pathway for brachycerine and psychollatine biosynthesis is novel, and may establish a new group of MIAs, together with croceaine A [73Düsman LT, Jorge TCM, Souza MC, et al. Monoterpene indole alkaloids from Palicourea crocea J Nat Prod 2004; 67 (11): 1886-8.].
REGULATION OF ALKALOID PRODUCTION IN PSYCHOTRIA SPECIES
Despite the significant advances achieved in biochemistry, molecular biology and biotechnology of natural products, the production of active compounds in controlled conditions (i.e. cell and tissue culture) often results in economically impracticable yields [9Pasquali G, Porto DD, Fett-Neto AG. Metabolite engineering of cell cultures versus whole plant complexity in production of
bioactive monoterpene indole alkaloids recent progress related to old dilemma J Biosci Bioeng 2006; 101(4): 287-96.
[http://dx.doi.org/10.1263/jbb.101.287] [PMID: 16716935] ]. Chemical synthesis or semi-synthesis of structurally complex active molecules discovered from plants is not always possible. In those cases, industry relies on the plant itself to isolate the active compound in large scale, either from plantations or naturally-occurring specimens. In both cases, isolation from adult plant tissues is often expensive and resulting in low yields [80Rao SR, Ravishankar GA. Plant cell cultures chemical factories of secondary metabolites Biotechnol Adv 2002; 20(2): 101-53.
[http://dx.doi.org/10.1016/S0734-9750(02)00007-1] ], and in the second case, the consequences to the plant natural population and its habitat can be disastrous [81Koo B, Wright BD. The role of biodiversity products as incentives for conserving biological diversity some instructive
examples Sci Total Environ 1999; 240: 21-30.
[http://dx.doi.org/10.1016/S0048-9697(99)00309-5] ].
Molecular biology techniques, along with elicitor-induced accumulation of plant secondary metabolites, may improve bioactive molecule production in plant systems [8Oksman-Caldentey K, Inzé D. Plant cell factories in the post-genomic era new ways to produce designer secondary metabolites Trends Plant Sci 2004; 9(9): 433-40.
[http://dx.doi.org/10.1016/j.tplants.2004.07.006] [PMID: 15337493] , 9Pasquali G, Porto DD, Fett-Neto AG. Metabolite engineering of cell cultures versus whole plant complexity in production of
bioactive monoterpene indole alkaloids recent progress related to old dilemma J Biosci Bioeng 2006; 101(4): 287-96.
[http://dx.doi.org/10.1263/jbb.101.287] [PMID: 16716935] , 82Dixon RA. Engineering of plant natural product pathways Curr Opin Plant Biol 2005; 8: 329-36.
[http://dx.doi.org/10.1016/j.pbi.2005.03.008] [PMID: 15860431] , 83Zhang WJ, Björn LO. The effect of ultraviolet radiation on the accumulation of medicinal compounds in plants Fitoterapia 2009; 80(4): 207-18.
[http://dx.doi.org/10.1016/j.fitote.2009.02.006] [PMID: 19243700] ]. With this in mind, a series of studies were carried out in order to investigate the regulation of Psychotria alkaloids production.
The effect of white light and presence of sucrose in the culture medium on GPV content was assessed in P. leiocarpa seedlings. GPV production was restricted to shoots, strongly promoted by light and inhibited by exogenous sucrose, indicating the importance of photoautotrophic metabolism and photomorphogenic development for alkaloid accumulation. Also, the higher content in older seedlings (150 days) in comparison to younger ones (100 days) showed developmental control of GPV accumulation [69Henriques AT, Lopes SO, Paranhos JT, et al. N-?-D-Gluco-pyranosyl vincosamide a light regulated indole alkaloid from the shoots of Psychotria leiocarpa Phytochemistry 2004; 65: 449-54.].
Brachycerine accumulation, in P. brachyceras, was restricted to shoots. The highest concentration occurred in inflorescences (0.3%), followed by fully expanded leaves (0.18%), stems (0.13%), young leaves (0.12%) and fruit pulp (0.04%) [84Gregianini TS, Porto DD, Nascimento NC, Fett JP, Henriques AT, Fett-Neto AG. Environmental and ontogenetic control of accumulation of brachycerine a bioactive indole alkaloid from Psychotria brachyceras J Chem Ecol 2004; 30(10): 2023-36.]. The leaf alkaloid content in field-grown trees showed seasonal variation in some years, with lower concentrations in the summer. In vitro cultured seedlings 14 days after radicle emission accumulated brachycerine contents comparable to those of adult plants [84Gregianini TS, Porto DD, Nascimento NC, Fett JP, Henriques AT, Fett-Neto AG. Environmental and ontogenetic control of accumulation of brachycerine a bioactive indole alkaloid from Psychotria brachyceras J Chem Ecol 2004; 30(10): 2023-36.].
Leaf brachycerine content in cuttings was responsive to several stimuli. Mechanical damage or jasmonic acid, an herbivory-related hormone [85Wasternack C. Jasmonates an update in biosynthesis signal transduction and action in plant stress response growth and development Ann Bot-(Lond) 2007; 100: 681-97.], induced brachycerine accumulation (Gregianini et al., 2004) [84Gregianini TS, Porto DD, Nascimento NC, Fett JP, Henriques AT, Fett-Neto AG. Environmental and ontogenetic control of accumulation of brachycerine a bioactive indole alkaloid from Psychotria brachyceras J Chem Ecol 2004; 30(10): 2023-36.], suggesting a deterrent role for the alkaloid. However, no toxic effect was detected in generalist herbivore bioassays (Porto et al., unpublished data). Ultraviolet (UV) radiation, both in UV-C (254 nm, germicidal lamp) and UV-B (280-315 nm) ranges, induced up to 10-fold brachycerine accumulation in leaves of cuttings [77Gregianini TS, Silveira VC, Porto DD, Kerber VA, Henriques AT, Fett-Neto AG. The alkaloid brachycerine is induced by ultraviolet radiation and is a singlet oxygen quencher Photochem Photobiol 2003; 78(5): 470-4.
[http://dx.doi.org/10.1562/0031-8655(2003)078<0470:TABIIB>2.0.CO;2] ]. The antioxidant properties of brachycerine and its strong UV-regulation indicate a protective role for the alkaloid in planta.
Differently from brachycerine, psychollatine accumulation in P. umbellata cuttings is rather insensitive to the treatments described. However, the plant naturally accumulates a relatively high amount of psychollatine (1-4% of dry weight) [9Pasquali G, Porto DD, Fett-Neto AG. Metabolite engineering of cell cultures versus whole plant complexity in production of
bioactive monoterpene indole alkaloids recent progress related to old dilemma J Biosci Bioeng 2006; 101(4): 287-96.
[http://dx.doi.org/10.1263/jbb.101.287] [PMID: 16716935] ]. An in vitro somatic embryogenesis protocol was established from cultured rhizogenic callus, and, after three months of acclimatization, psychollatine-accumulating plants were obtained with alkaloid contents similar to those found in adult plants; in addition, the capacity to produce the alkaloid was closely associated with the differentiation of somatic embryo shoots [86Paranhos JT, Fragoso V, Henriques AT, Ferreira AG, Fett-Neto AG. Regeneration of Psychotria umbellata and production of the analgesic indole alkaloid umbellatine Tree Physiol 2005; 25: 251-5.
[http://dx.doi.org/10.1093/treephys/25.2.251] [PMID: 15574407] ].
The alkaloids of Psychotria species from Southern Brazil represent relatively primitive structures compared to the bisindolic alkaloids of the best studied species in monoterpene indole alkaloid metabolism, Catharanthus roseus. However, these simpler Psychotria alkaloids also display interesting pharmacological properties. The presence of new structures, such as the N-glycosylated GPV and the non-secologanin derived terpene moieties of brachycerine and psychollatine highlight the prominent chemical diversity in this genus. Other features that are common to most of the described alkaloids of Psychotria include the retention of glucose residues (which may favor solubility in aqueous environments), antioxidant capacity, the requirement of differentiated shoots for active alkaloid production, and the accumulation in reproductive structures.
CONCLUDING REMARKS
The alkaloids from Psychotria colorata (Fig. 2 ) [87Zafar S. Royal Botanic Gardens Kew Images http://www.kew.org/science/tropamerica/neotropikey/families/images/Rubiaceae/Psychotria_colorata_5.jpeg.png [Aprl 23 2009];] were a direct result of ethnopharmacological surveys in the Amazonian region. The simple expansion of chemical investigations to include related species from a different biome (chemotaxonomical survey of Psychotria species of Southern Brazil, [88Dillenburg CR. Thesis Universidade Federal do Rio Grande do Sul 1978.]), involving plants that even lack popular names, revealed several other bioactive alkaloids. Further research on the biological activity of these new compounds, elucidation of their biosynthetic origins and regulation of production, along with studies on plant propagation, may convert relatively unknown species in viable sources of new drugs.
) [87Zafar S. Royal Botanic Gardens Kew Images http://www.kew.org/science/tropamerica/neotropikey/families/images/Rubiaceae/Psychotria_colorata_5.jpeg.png [Aprl 23 2009];] were a direct result of ethnopharmacological surveys in the Amazonian region. The simple expansion of chemical investigations to include related species from a different biome (chemotaxonomical survey of Psychotria species of Southern Brazil, [88Dillenburg CR. Thesis Universidade Federal do Rio Grande do Sul 1978.]), involving plants that even lack popular names, revealed several other bioactive alkaloids. Further research on the biological activity of these new compounds, elucidation of their biosynthetic origins and regulation of production, along with studies on plant propagation, may convert relatively unknown species in viable sources of new drugs.
REFERENCES
| [1] | Gurib-Fakim A. Medicinal plants traditions of yesterday and drugs of tomorrow Mol Aspects Med 2006; 27: 1-93. [http://dx.doi.org/10.1016/j.mam.2005.07.008] [PMID: 16105678] |
| [2] | Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants Life Sci 2005; 78: 431-41. [http://dx.doi.org/10.1016/j.lfs.2005.09.012] [PMID: 16198377] |
| [3] | Hartmann T. From waste products to ecochemicals fifty years research on plant secondary metabolism Phytochemistry 2007; 68: 2831-46. [http://dx.doi.org/10.1016/j.phytochem.2007.09.017] [PMID: 17980895] |
| [4] | Harvey A. Strategies for discovering drugs from previously
unexplored natural products Drug Discov Today 2000; 5(7): 294-300. [http://dx.doi.org/10.1016/S1359-6446(00)01511-7] |
| [5] | Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years J Nat Prod 2007; 70: 461-77. [http://dx.doi.org/10.1021/np068054v] [PMID: 17309302] |
| [6] | Gassner NC, Tamble CM, Bock JE, et al. Accelerating the discovery of biologically active small molecules using a high-throughput yeast halo assay J Nat Prod 2007; 70: 383-90. |
| [7] | Petersen M. Current status of metabolic phytochemistry Phytochemistry 2007; 68: 2847-60. [http://dx.doi.org/10.1016/j.phytochem.2007.07.029] [PMID: 17881017] |
| [8] | Oksman-Caldentey K, Inzé D. Plant cell factories in the post-genomic era new ways to produce designer secondary metabolites Trends Plant Sci 2004; 9(9): 433-40. [http://dx.doi.org/10.1016/j.tplants.2004.07.006] [PMID: 15337493] |
| [9] | Pasquali G, Porto DD, Fett-Neto AG. Metabolite engineering of cell cultures versus whole plant complexity in production of
bioactive monoterpene indole alkaloids recent progress related to old dilemma J Biosci Bioeng 2006; 101(4): 287-96. [http://dx.doi.org/10.1263/jbb.101.287] [PMID: 16716935] |
| [10] | Mongrand S, Badoc A, Patouille B, Lacomblez C, Chavent M, Bessoule JJ. Chemotaxonomy of the Rubiaceae family based on leaf fatty acid composition Phytochemistry 2005; 66(5): 549-59. [http://dx.doi.org/10.1016/j.phytochem.2004.12.021] [PMID: 15721947] |
| [11] | Ashihara H, Sano H, Crozier A. Caffeine and related purine alkaloids Biosynthesis catabolism function and genetic engineering Phytochemistry 2008; 69: 841-56. [http://dx.doi.org/10.1016/j.phytochem.2007.10.029] [PMID: 18068204] |
| [12] | Deeni YY, Hussain HSN. Screening for antimicrobial activity and for alkaloids of Nauclea latifolia J Ethnopharmacol 1991; 35: 91-6. [http://dx.doi.org/10.1016/0378-8741(91)90137-3] |
| [13] | Cardoso CL, Castro-Gamboa I, Silva DHS, et al. Indole glucoalkaloids from Chimarrhis turbinata and their evaluation as antioxidant agents and acetylcholinesterase inhibitors J Nat Prod 2004; 67(11): 1882-5. |
| [14] | Takayama H. Chemistry and pharmacology of analgesic indole alkaloids from the Rubiaceous plant, Mytragina speciosa Chem Pharm Bull 2004; 52(8): 916-28. [http://dx.doi.org/10.1248/cpb.52.916] |
| [15] | Chan-Blanco Y, Vaillant F, Perez AM, Reynes M, Brillouet J, Brat P. The noni fruit (Morinda citrifolia La review of agricultural research nutritional and therapeutical properties JFood Compos Anal 2006; 19: 645-54. [http://dx.doi.org/10.1016/j.jfca.2005.10.001] |
| [16] | Tao J, Morikawa T, Ando S, Matsuda H, Yoshikawa M. Bioactive constituents from chinese natural medicines XI.
Inhibitors of NO production and degranulation in RBL-2H3 from Rubia yunnanensis. Structures of Rubianosides II III and IV
Rubianol-g and Rubianthraquinone Chem Pharm Bull 2003; 51(6): 654-62. [http://dx.doi.org/10.1248/cpb.51.654] |
| [17] | Wu T, Lin D, Shi L, Damu AG, Kuo P, Kuo Y. Cytotoxic anthraquinones from the stems of Rubia wallichiana Decne Chem Pharm Bull 2003; 51(8): 948-50. [http://dx.doi.org/10.1248/cpb.51.948] |
| [18] | Lee J, Hitotsuyanagi Y, Fukaya H, Kondo K, Takeya K. New cytotoxic byciclic hexapeptides RA-XXIII and RA-XXIV from Rubia cordifolia L Chem Pharm Bull 2008; 56(5): 730-3. [http://dx.doi.org/10.1248/cpb.56.730] |
| [19] | Bokesch HR, Pannel LK, Cochran PK, Sowder RC, McKee TC, Boyd MR. A novel anti-HIV macrocyclic peptide from Palicourea condensata J Nat Prod 2001; 64(2): 249-50. |
| [20] | Heitzman ME, Neto CC, Winiarz E, Vaisberg AJ, Hammond GB. Ethnobotany phytochemistry and pharmacology of Uncaria (Rubiaceae) Phytochemistry 2005; 66: 5-29. |
| [21] | Keplinger K, Laus G, Wurm M, Dierich MP, Teppner H. Uncaria tomentosa (Willd DC Ethnomedicinal use and new pharmacological toxicological and botanical results J Ethnopharmacol 1999; 64: 23-34. [http://dx.doi.org/10.1016/S0378-8741(98)00096-8] |
| [22] | Reis SRIN, Valente LMM, Sampaio AL, et al. Immunomodulating and antiviral activities of Uncaria tomentosa on human monocytes infected with Dengue Virus-2 Int Immunopharmacol 2008; 8: 468-76. |
| [23] | Amaral S, Mira L, Nogueira JMF, Silva AP, Florêncio MH. Plant extracts with anti-inflammatory properties - A new
approach for characterization of their bioactive compounds and
establishment of structure-antioxidant activity relationships Bioorgan Med Chem 2009; 17: 1876-83. [http://dx.doi.org/10.1016/j.bmc.2009.01.045] [PMID: 19201196] |
| [24] | Kurás M, Pilarski R, Nowakowska J, et al. Effect of alkaloid-free and alkaloid-rich preparations from Uncaria tomentosa bark on mitotic activity and chromosome morphology evaluated by Allium test J Ethnopharmacol 2009; 121: 140-7. |
| [25] | Carter R, Mendis KM. Evolutionary and historical aspects of
the burden of malaria Clin Microbiol Rev 2002; 15(4): 564--594. [http://dx.doi.org/10.1128/CMR.15.4.564-594.2002] [PMID: 12364370] [PMCID: PMC126857] |
| [26] | Kinsley-Scott TR, Norton SA. Useful plants in dermatology VII Cinchona and antimalarials J Am Acad Dermatol 2003; 49: 499-502. [http://dx.doi.org/10.1067/S0190-9622(03)01281-7] |
| [27] | Druilhe P, Brandicourt O, Chongsuphajaisiddhi T, Berthe J. Activity of a combination of three Cinchona bark alkaloids against Plasmodium falciparum in vitro Antimicrob Agents Ch 1988; 32(2): 250-4. [http://dx.doi.org/10.1128/AAC.32.2.250] |
| [28] | Kvist LP, Christensen SB, Rasmussen HB, Mejia K, Gonzales A. Identification and evaluation of Peruvian plants used to treat malaria and leishmaniasis J Ethnopharmacol 2006; 106: 390-402. |
| [29] | Bourdy G, Willcox ML, Ginsburg H, Rasoanaivo Ph, Graz B, Deharo E. Ethnopharmacology and malaria new hypothetical leads or old efficient antimalarials Int J Parasitol 2008; 38: 33-41. [http://dx.doi.org/10.1016/j.ijpara.2007.07.004] [PMID: 17720165] |
| [30] | Muhammad I, Dunbar DC, Khan SI, et al. Antiparasitic alkaloids from Psychotria klugii J Nat Prod 2003; 66: 962-7. [http://dx.doi.org/10.1021/np030086k] [PMID: 12880315] |
| [31] | Soh PN, Benoit-Vical F. Are West African plants a source of future antimalarial drugs J Ethnopharmacol 2007; 114: 130-40. [http://dx.doi.org/10.1016/j.jep.2007.08.012] [PMID: 17884314] |
| [32] | Nepkroeff M, Bremer B, Systma KJ. Reorganization of the genus Psychotria and tribe Psychotrieae (Rubiaceae) inferred from ITS and rbcL sequence data Syst Bot 1999; 24(1): 5-27. |
| [33] | Lopes S, vonPoser GL, Kerber VA, et al. Taxonomic significance of alkaloids and iridoid glucosides in the tribe psychotrieae (Rubiaceae) Biochem Syst Ecol 2004; 32: 1187-95. [http://dx.doi.org/10.1016/j.bse.2004.04.015] |
| [34] | Khan MR, Kihara M, Omoloso AD. Antimicrobial activity of Psychotria microlabastra Fitoterapia 2001; 72: 818-21. [http://dx.doi.org/10.1016/S0367-326X(01)00312-4] |
| [35] | McGaw LJ, Jäger AK, vanStaden J. Antibacterial anthel-
mintic and anti-amoebic activity in South African medicinal plants J Ethnopharmacol 2000; 72: 247-63. [http://dx.doi.org/10.1016/S0378-8741(00)00269-5] |
| [36] | Kuo Y, Chen C, Tsai W, Ho Y. Regulation of herpes simplex virus type 1 replication in Vero cells by Psychotria serpens relationship to gene expression DNA replication and protein synthesis Antiviral Res 2001; 51: 95-109. [http://dx.doi.org/10.1016/S0166-3542(01)00141-3] |
| [37] | Locher CP, Burch MT, Mower HF, et al. Anti-microbial activity and anti-complement activity of extracts obtained from selected Hawaiian medicinal plants J Ethnopharmacol 1995; 49: 23-32. [http://dx.doi.org/10.1016/0378-8741(95)01299-0] |
| [38] | Dunstan CA, Noreen Y, Serrano G, Cox PA, Perera P, Bohlin L. Evaluation of some Samoan and Peruvian medicinal plants by prostaglandin biosynthesis and rat ear oedema assays J Ethnopharmacol 1997; 57: 35-56. |
| [39] | Hayashi T, Smith FT, Lee KH. Antitumor agents 89 Psychorubrin a new cytotoxic naphtoquinone from Psychotria rubra and its structure-activity relationships J Med Chem 1987; 30(11): 2005-8. [http://dx.doi.org/10.1021/jm00394a013] [PMID: 3669007] |
| [40] | Witherup KM, Bogusky MJ, Anderson PS, et al. Cyclopsychotride A a biologically active 31-residue cyclic peptide isolated from Psychotria longipes J Nat Prod 1994; 57(12): 1619-25. |
| [41] | Solis PN, Lang’at C, Gupta MP, Kirby GC, Warhurst DC, Phillipson JD. Bio-active compounds from Psychotria camponutans Planta Med 1995; 61: 62-5. [http://dx.doi.org/10.1055/s-2006-958001] [PMID: 7700994] |
| [42] | Glinski JA, David E, Warren TC, et al. Inactivation of cell surface receptors by pheophorbide a a green pigment isolated from Psychotria acuminata Photochem Photobiol 1995; 62(1): 144-50. [http://dx.doi.org/10.1111/j.1751-1097.1995.tb05250.x] [PMID: 7638258] |
| [43] | Beretz A, Roth-Georger A, Corre G, Kuballa B, Anton R, Cazenave J. Polyindolinic alkaloids from Psychotria forsteriana Potent inhibitors of the aggregation of human platelets Planta Med 1985; 51: 300-3. |
| [44] | Giorgetti M, Negri G, Rodrigues E. Brazilian plants with possible action on the central nervous system - A study of historical sources from the 16th to 19th century J Ethnopharmacol 2007; 109: 338-47. [http://dx.doi.org/10.1016/j.jep.2006.08.003] [PMID: 16982166] |
| [45] | Hasegawa M, Sasaki T, Sadakane K, et al. Studies for the emetic mechanism of Ipecac Syrup (TJN-119) and its active components in ferrets involvement of 5-hydroxytryptamine receptors Jpn J Pharmacol 2002; 89: 113-9. |
| [46] | Mangaña-García M, Arista-Viveros A. Cutaneous amebiasis in children Pediatr Dermatol 2008; 10(4): 352-5. |
| [47] | Möller M, Herzer K, Wenger T, Herr I, Wink M. The alkaloid emetine as a promising agent for the induction and enhancement of drug-induced apoptosis in leukemia cells Oncol Rep 2007; 18: 737-44. [PMID: 17671728] |
| [48] | Freedland CS, Mansbach RS. Behavioral profile of constituents in ayahuasca an Amazonian psychoactive plant mixture Drug Alcohol Depend 1999; 54: 183-94. [http://dx.doi.org/10.1016/S0376-8716(98)00154-9] |
| [49] | Buckholtz NS, Boggan WO. Monoamine oxidase inhibition in brain and liver produced by ?-carbolines structure activity relationships and substrate specificity Biochem Pharmacol 1977; 26: 1991-6. [http://dx.doi.org/10.1016/0006-2952(77)90007-7] |
| [50] | Santos RG, Landeira-Fernandez J, Strassman RJ, Motta V, Cruz APM. Effects of ayahuasca on psychometric measures of anxiety panic-like and hopelessness in Santo Daime members J Ethnopharmacol 2007; 112: 507-13. |
| [51] | Tupper KW. The globalization of ayahuasca Harm reduction or benefit maximization Int J Drug Policy 2008; 19: 297-303. [http://dx.doi.org/10.1016/j.drugpo.2006.11.001] [PMID: 18638702] |
| [52] | Sanz-Biset J, Campos-de-la-Cruz J, Epiquién-Rivera MA, Cañigueral S. A first survey on the medicinal plants of the Chazuta valley (Peruvian Amazon) J Ethnopharmacol 2009; 122: 333-62. |
| [53] | Elisabetsky E, Amador TA, Albuquerque RR, Nunes DS, Carvalho ACT. Analgesic activity of Psychotria colorata (Willdex R.& S.) Muell. Arg. alkaloids J Ethnopharmacol 1995; 48: 77-83. |
| [54] | Verotta L, Pilati T, Tatò M, Elisabetsky E, Amador TA, Nunes DS. Pyrrolidinoindoline alkaloids from Psychotria colorata J Nat Prod 1998; 61: 392-6. |
| [55] | Fridrichsons J, Mackay MF, Mathieson AM. The absolute molecular structure of hodgkinsine Tetrahedron 1974; 30(1): 85-92. [http://dx.doi.org/10.1016/S0040-4020(01)97221-7] |
| [56] | Kodanko JJ, Hiebert S, Peterson EA, et al. Synthesis of all-low energy stereoisomers of the tris (pyrrolidinoindoline) alkaloid hodgkinsine and preliminary assessment of their antinociceptive activity J Org Chem 2007; 72(21): 7909-14. |
| [57] | Amador TA, Verotta L, Nunes DS, Elisabetsky E. Anti-
nociceptive profile of hodgkinsine Planta Med 2000; 66: 770-2. [http://dx.doi.org/10.1055/s-2000-9604] [PMID: 11199142] |
| [58] | Verotta L, Peterlongo F, Elisabetsky E, Amador TA, Nunes DS. High-performance liquid chromatography - diode array detection - tandem mass spectrometry analyses of the alkaloid extracts of Amazon Psychotria species J Chromatogr A 1999; 841: 165-76. [http://dx.doi.org/10.1016/S0021-9673(99)00298-8] |
| [59] | Verotta L, Orsini F, Sbacchi M, Scheildler MA, Amador TA, Elisabetsky E. Synthesis and antinociceptive activity of chimonanthines and pyrrolidinoindoline-type alkaloids Bioorg Med Chem 2002; 10: 2133-42. [http://dx.doi.org/10.1016/S0968-0896(02)00078-0] |
| [60] | Amador TA, Verotta L, Nunes DS, Elisabetsky E. Involvement of NMDA receptors in the analgesic properties of psychotridine Phytomedicine 2001; 8(3): 202-6. [http://dx.doi.org/10.1078/0944-7113-00025] [PMID: 11417913] |
| [61] | Leal MB. Thesis Universidade Federal do Rio Grande do Sul 1994. |
| [62] | Leal MB, Elisabetsky E. Absence of alkaloids in Psychotria carthagenensis Jacq (Rubiaceae) J Ethnopharmacol 1996; 54: 37-40. [http://dx.doi.org/10.1016/0378-8741(96)01448-1] |
| [63] | Both FL, Farias FM, Nicoláo LL, Misturini J, Henriques AT, Elisabetsky E. Avaliação da atividade analgésica de extratos alcaloídicos de espécies de Psychotria Rev Bras Plant Med 2002; 5(1): 41-5. |
| [64] | Simões-Pires CA, Farias FM, Marston A, et al. Indole monoterpenes with antichemotactic activity fromPsychotria myriantha Chemotaxonomic significance Nat Prod Comm 2006; 1(12): 1101-6. |
| [65] | Santos LV, Fett-Neto AG, Kerber VA, Elisabetsky E, Quirion JP, Henriques AT. Indole monoterpene alkaloids from leaves of Psychotria suterella Müll Arg (Rubiaceae) Biochem Syst Ecol 2001; 29: 1185-7. |
| [66] | Farias FM, Konrath EL, Zuanazzi JA, Henriques AT. Strictosamide from Psychotria nuda (ChamEt Schltdl) Wawra (Rubiaceae) Biochem Syst Ecol 2009; 36: 919- 920. [http://dx.doi.org/10.1016/j.bse.2008.10.002] |
| [67] | Paul JHA, Maxwell AR, Reynolds WF. Novel bis(mono-
terpenoid) indole alkaloids from Psychotria bahiensis J Nat Prod 2003; 66: 752-4. [http://dx.doi.org/10.1021/np020554a] [PMID: 12828456] |
| [68] | Corrêa LR, Soares GLG, Fett-Neto AG. Allelopathic
potential of Psychotria leiocarpa a understory species of subtro-
pical forests S Afr J Bot 2008; 74(4): 583-90. [http://dx.doi.org/10.1016/j.sajb.2008.02.006] |
| [69] | Henriques AT, Lopes SO, Paranhos JT, et al. N-?-D-Gluco-pyranosyl vincosamide a light regulated indole alkaloid from the shoots of Psychotria leiocarpa Phytochemistry 2004; 65: 449-54. |
| [70] | Kayser V, Guilbaud G. Differential effect of various doses of morphine and naloxone on two nociceptive tests thresholds in
arthritic and normal rats Pain 1990; 41(3): 353-63. [http://dx.doi.org/10.1016/0304-3959(90)90012-3] |
| [71] | Kerber VA, Passos CS, Verli H, Fett-Neto AG, Quirion
JP, Henriques AT. Psychollatine a glucosidic monoterpene indole alkaloid from Psychotria umbellata J Nat Prod 2008; 71: 697-700. [http://dx.doi.org/10.1021/np0703951] [PMID: 18288808] |
| [72] | Narine LL, Maxwell AR. Monoterpenoid indole alkaloids from Palicourea crocea Phytochem Lett 2009; 2: 34-6. [http://dx.doi.org/10.1016/j.phytol.2008.10.007] |
| [73] | Düsman LT, Jorge TCM, Souza MC, et al. Monoterpene indole alkaloids from Palicourea crocea J Nat Prod 2004; 67 (11): 1886-8. |
| [74] | Both FL, Meneghini L, Kerber VA, Henriques AT, Elisabetsky E. Psychopharmacological profile of the alkaloid psychollatine as a 5HT2A/C serotonin modulator J Nat Prod 2005; 68: 374-80. |
| [75] | Fragoso V, Nascimento NC, Moura DJ, et al. Antioxidant and antimutagenic properties of the monoterpene indole alkaloid psychollatine and the crude foliar extract of Psychotria umbellata Vell Toxicol in Vitro 2008; 22: 559-66. |
| [76] | Kerber VA, Gregianini TS, Paranhos JT, et al. Brachycerine a novel monoterpene indole alkaloid from Psychotria brachyceras J Nat Prod 2001; 64: 677-9. |
| [77] | Gregianini TS, Silveira VC, Porto DD, Kerber VA, Henriques AT, Fett-Neto AG. The alkaloid brachycerine is induced by ultraviolet radiation and is a singlet oxygen quencher Photochem Photobiol 2003; 78(5): 470-4. [http://dx.doi.org/10.1562/0031-8655(2003)078<0470:TABIIB>2.0.CO;2] |
| [78] | Nascimento NC, Fragoso V, Moura DJ, Silva ACR, Fett-Neto AG, Saffi J. Antioxidant and antimutagenic effects of the crude foliar extract and the alkaloid Brachycerine of Psychotria brachyceras Environ Mol Mutagen 2007; 48(9): 728-34. |
| [79] | Ziegler J, Facchini PJ. Alkaloid biosynthesis metabolism and trafficking Annu Rev Plant Biol 2008; 59: 735-69. [http://dx.doi.org/10.1146/annurev.arplant.59.032607.092730] [PMID: 18251710] |
| [80] | Rao SR, Ravishankar GA. Plant cell cultures chemical factories of secondary metabolites Biotechnol Adv 2002; 20(2): 101-53. [http://dx.doi.org/10.1016/S0734-9750(02)00007-1] |
| [81] | Koo B, Wright BD. The role of biodiversity products as incentives for conserving biological diversity some instructive
examples Sci Total Environ 1999; 240: 21-30. [http://dx.doi.org/10.1016/S0048-9697(99)00309-5] |
| [82] | Dixon RA. Engineering of plant natural product pathways Curr Opin Plant Biol 2005; 8: 329-36. [http://dx.doi.org/10.1016/j.pbi.2005.03.008] [PMID: 15860431] |
| [83] | Zhang WJ, Björn LO. The effect of ultraviolet radiation on the accumulation of medicinal compounds in plants Fitoterapia 2009; 80(4): 207-18. [http://dx.doi.org/10.1016/j.fitote.2009.02.006] [PMID: 19243700] |
| [84] | Gregianini TS, Porto DD, Nascimento NC, Fett JP, Henriques AT, Fett-Neto AG. Environmental and ontogenetic control of accumulation of brachycerine a bioactive indole alkaloid from Psychotria brachyceras J Chem Ecol 2004; 30(10): 2023-36. |
| [85] | Wasternack C. Jasmonates an update in biosynthesis signal transduction and action in plant stress response growth and development Ann Bot-(Lond) 2007; 100: 681-97. |
| [86] | Paranhos JT, Fragoso V, Henriques AT, Ferreira AG, Fett-Neto AG. Regeneration of Psychotria umbellata and production of the analgesic indole alkaloid umbellatine Tree Physiol 2005; 25: 251-5. [http://dx.doi.org/10.1093/treephys/25.2.251] [PMID: 15574407] |
| [87] | Zafar S. Royal Botanic Gardens Kew Images http://www.kew.org/science/tropamerica/neotropikey/families/images/Rubiaceae/Psychotria_colorata_5.jpeg.png [Aprl 23 2009]; |
| [88] | Dillenburg CR. Thesis Universidade Federal do Rio Grande do Sul 1978. |



