- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Bioactive Compounds Journal
(Discontinued)
ISSN: 1874-8473 ― Volume 9, 2020
Marine Streptomyces Sp. VITMK1 Derived Pyrrolo [1, 2-A] Pyrazine-1, 4-Dione, Hexahydro-3-(2-Methylpropyl) and Its Free Radical Scavenging Activity
Manickavelu Manimaran, Krishnan Kannabiran*
Abstract
Background:
Free radical generation has been proved to be responsible for various cellular diseases. It is necessary to combat free radicals using antioxidants derived from natural sources.
Objective:
The objective of this study is to evaluate the antioxidant activity of the diketopiperazine compound extracted from Streptomyces sp. VITMK1 isolated from mangrove sediment soil collected from Pichavaram, Tamil Nadu, India.
Methods:
The antioxidant potential of pyrrolo [1, 2-A] pyrazine-1, 4-dione, hexahydro-3-(2-methylpropyl) (diketopiperazine) extracted from Streptomyces sp. VITMK1 was studied using reducing power assay. The scavenging of 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical and nitric oxide (NO) radical by the compound was also studied. The cytotoxic activity of the compound on RAW 264.7 macrophage cell line was studied using MTT cell viability assay.
Results:
The compound exhibited strong DPPH radical scavenging activity (72.48±0.32% at 500 µg/mL) and NO radical scavenging activity (73.03±1.02% at 500 µg/mL). MTT cell viability assay revealed that the compound exhibited concentration-dependent cell viability and was observed to be 92% at 125 µg/mL concentration.
Conclusion:
The antioxidant activity of the diketopiperazine compound extracted from Streptomyces sp. VITMK1 can be probed further to establish its radical scavenging activity.
Article Information
Identifiers and Pagination:
Year: 2017Volume: 5
First Page: 23
Last Page: 30
Publisher Id: TOBCJ-5-23
DOI: 10.2174/1874847301705010023
Article History:
Received Date: 19/06/2017Revision Received Date: 15/08/2017
Acceptance Date: 18/08/2017
Electronic publication date: 26/09/2017
Collection year: 2017
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the VIT University, Department of Biomedical Sciences, Vellore, Tamil Nadu, India; Tel: +91-416-2202477; E-mail: kkb.biomol@gmail.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 19-06-2017 |
Original Manuscript | Marine Streptomyces Sp. VITMK1 Derived Pyrrolo [1, 2-A] Pyrazine-1, 4-Dione, Hexahydro-3-(2-Methylpropyl) and Its Free Radical Scavenging Activity | |
1. INTRODUCTION
Reactive oxygen species (ROS) are a group of free radicals that are derived from oxygen. ROS are produced as a result of cellular metabolism and excessive accumulation of ROS leads to oxidative stress which plays an important role in the pathogenesis of various diseases like cardiovascular diseases, inflammatory diseases, atherosclerosis, cancer [1Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39(1): 44-84.
[http://dx.doi.org/10.1016/j.biocel.2006.07.001] [PMID: 16978905] ] and in many pathological progression in the central nervous system [2Calabrese V, Cornelius C, Mancuso C, Lentile R, Stella AM, Butterfield DA. Redox homeostasis and cellular stress response in aging and neurodegeneration. Methods Mol Biol 2010; 610: 285-308.
[http://dx.doi.org/10.1007/978-1-60327-029-8_17] [PMID: 20013185] ]. Mitochondrial electron transport chain, enzymatic reactions, respiratory burst during phagocytosis and xenobiotic metabolisms (redox cycling) are endogenous sources of free radicals. Exogenous sources include ionizing radiation, UV radiation, ultrasound and chemicals etc. Antioxidants prevent oxidation by donating electrons and thereby combat free radicals and neutralize them. Antioxidants provide protection against various infections and degenerative diseases [3Sharma SK, Gupta VK. In vitro antioxidant studies of Ficus racemosa Linn. root. Pharmacogn Mag 2008; 4: 70-4.]. Human cells have a natural mechanism aided by enzymes superoxide dismutase, catalase and glutathione peroxidase to neutralize the free radicals thereby protecting the cells [4Ferreira IC, Barros L, Soares ME, et al. Antioxidant activity and phenolic contents of Olea europaea L. leaves sprayed with different copper formulations. Food Chem 2007; 103: 188-95.
[http://dx.doi.org/10.1016/j.foodchem.2006.08.006] ]. Micronutrients from food such as vitamins A, E and β-carotene, selenium and zinc and proteins albumin, ceruloplasmin and transferrin can provide additional protection of cells against cellular damage [5Ostrovidov S, Franck P, Joseph D, et al. Screening of new antioxidant molecules using flow cytometry. J Med Chem 2000; 43(9): 1762-9.
[http://dx.doi.org/10.1021/jm991019j] [PMID: 10794693] ]. Due to the changing life style and growing population, there is a need to use synthetic antioxidants. Butylated hydroxyanisole (BHA), butylated hydroxyltoluene (BHT) and propyl gallate (PG) have been used to control the oxidation process. Their use must be strictly regulated since they cause deleterious effects to human health [6Kaiserová H, Simùnek T, van der Vijgh WJ, et al. Flavonoids as protectors against doxorubicin cardiotoxicity: Role of iron chelation, antioxidant activity, and inhibition of carbonyl reductase. Biochim Biophys Acta 2007; 1772: 1065-74.]. This leads to search for novel antioxidant compounds from natural sources. A lot of research is done on natural antioxidants from plant and microbial sources which serve as safe therapeutics [7Janardhan A, Kumar AP, Viswanath B, Saigopal DV, Narasimha G. Production of bioactive compounds by actinomycetes and their antioxidant properties. Biotechnol Res Int 2014; 2014: 217030.
[http://dx.doi.org/10.1155/2014/217030] [PMID: 24790761] ]. Appreciable quantity of seaweed are consumed on a daily basis globally through food and the seaweed components such as sulfated polysaccharides, phenolic compounds (phlorotannins and bromophenols), and fucoxanthins are reported to have antioxidant properties [8Park EJ, Pezzuto JM. Antioxidant marine products in cancer chemoprevention. Antioxid Redox Signal 2013; 19(2): 115-38.
[http://dx.doi.org/10.1089/ars.2013.5235] [PMID: 23397932] ]. Microorganisms live in varied environmental conditions and thereby are adapted to them by evolutionary process. They would have developed specific defense mechanism to combat oxidative stress in their extreme conditions [9Ishikawa Y. Development of new types of antioxidants from microbial origin. J Jpn Oil Chem Soc 1992; 41: 762-7.
[http://dx.doi.org/10.5650/jos1956.41.762] ]. They could serve as the natural source for novel antioxidants. Among microorganisms, actinomycetes along with marine bacteria have the ability to produce secondary metabolites with diverse chemical structures. Actinomycetes are a group of gram positive unicellular bacteria that have a high G+C content. They are known for their ability to produce secondary metabolites with a broad spectrum of biological activity such as antimicrobial, antitumor, immunosuppressive, antioxidant, enzyme inhibitory and others [10Karthik L, Kumar G, Bhaskara Rao KV. Antioxidant activity of newly discovered lineage of marine actinobacteria. Asian Pac J Trop Med 2013; 6(4): 325-32.
[http://dx.doi.org/10.1016/S1995-7645(13)60065-6] [PMID: 23608337] ]. Each actinomycetes strain has the potential to produce 10-20 secondary metabolites [11Bentley SD, Chater KF, Cerdeño-Tárraga AM, et al. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 2002; 417(6885): 141-7.
[http://dx.doi.org/10.1038/417141a] [PMID: 12000953] ]. Among actinomycetes, Streptomyces have been reported to contribute 70% of the secondary metabolites described under actinomycetes [12Zengler K, Parakar A, Keller M. New methods to access microbial diversity for small molecule discovery. In: Natural products. Humana Press 2005; pp. 275-93.
[http://dx.doi.org/10.1007/978-1-59259-976-9_12] ]. The response of the antioxidant system of Streptomyces growth under oxidative condition is now studied for their antioxidant potential [13Schweder T, Lindequist U, Lalk M. Screening for new metabolites from marine microorganisms. Adv Biochem Eng Biotechnol 2005; 96: 1-48.
[http://dx.doi.org/10.1007/b135781] [PMID: 16566088] ]. This study reports the antioxidant activity of a diketopiperazine compound, Pyrrolo [1, 2-A] pyrazine-1, 4-dione, hexahydro-3-(2-methylpropyl)- extracted from Streptomyces sp. VITMK1 isolated from mangrove soil sediment collected from Pichavaram, Tamil Nadu, India.
2. MATERIALS AND METHODS
2.1. Isolation of Streptomyces sp. VITMK1 and Extraction of a Diketopiperazine Compound
The strain Streptomyces sp. VITMK1 was isolated from mangrove soil sediment collected from Pichavaram, Tamil Nadu, India. The isolate was subjected to submerged fermentation in tryptone yeast extract medium for 7 days followed by extraction using ethyl acetate (EA). The EA extract was purified using column chromatography resulting in a diketopiperazine compound, Pyrrolo [1, 2-A] pyrazine-1, 4-dione, hexahydro-3-(2-methylpropyl)- [14Manimaran M, Gopal JV, Kannabiran K. Antibacterial activity of Streptomyces sp. VITMK1 isolated from mangrove soil of Pichavaram, Tamil Nadu, India. Proc Natl Acad Sci, India, Sect B Biol Sci 2017; 87: 499-506.
[http://dx.doi.org/10.1007/s40011-015-0619-5] ]. The chemical structure of the compound is given in Fig. (1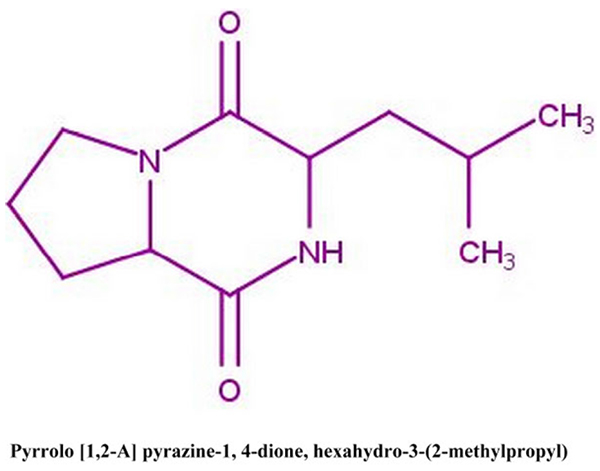 ). The compound was studied for its antioxidant activity.
). The compound was studied for its antioxidant activity.
 |
Fig. (1) Chemical structure of pyrrolo [1, 2-A] pyrazine-1, 4-dione, hexahydro-3-(2-methylpropyl). |
2.2. Reducing Power Assay
The reducing power assay was carried out according to the method described by Oyaizu with minor modifications [15Oyaizu M. Studies on products on browning reaction prepared from glucose amine. Jpn J Nut 1986; 44: 307-15.
[http://dx.doi.org/10.5264/eiyogakuzashi.44.307] ]. 2 ml of the compound at various concentrations (62.5-500 µg/ml) was mixed with 2.5 ml of 200 mM/l of sodium phosphate buffer (pH 6.6) and 2.5 ml of 1% potassium ferricyanide. The mixture was incubated at 50°C for 20 minutes. 2.5 ml of 10% trichloroacetic acid (w/v) was added and the mixture was centrifuged at 650 rpm for 10 minutes. The upper layer was separated and equal volume of deionised water was added with 1 ml of 0.1% ferric chloride and the absorbance was measured at 700 nm. Ascorbic acid was used as a standard.
2.3. Nitric Oxide (NO) Scavenging Assay
The NO scavenging activity was determined according to the method described by Marcocci [16Marcocci L, Maguire JJ, Droy-Lefaix MT, Packer L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem Biophys Res Commun 1994; 201(2): 748-55.
[http://dx.doi.org/10.1006/bbrc.1994.1764] [PMID: 8003011] ]. 1 ml of the compound at various concentrations (62.5-500 µg/ml) was mixed with 1 ml of 5 mM sodium nitroprusside and the mixture was incubated at 25°C for 3 hours. After incubation, 50 µl of griess reagent (1% sulphanilamide, 2% phosphoric acid and 0.1% napthylene diaminedihydrochloride) was added and the absorbance was measured at 540 nm. The NO scavenging activity was calculated by the following equation:
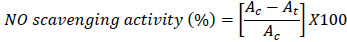 |
where Ac is the absorbance of the control and At is the absorbance of the test sample. Ascorbic acid was used as a standard.
2.4. DPPH Free Radical Scavenging Assay
2,2-diphenyl-1-picrylhydrazyl (DPPH) is a purple colored crystalline powder composed of stable free-radical molecules. The ability to scavenge the free radical (DPPH) was done according to the method described by Hatano [17Hatano T, Kagawa H, Yasuhara T, Okuda T. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. Chem Pharm Bull (Tokyo) 1988; 36(6): 2090-7.
[http://dx.doi.org/10.1248/cpb.36.2090] [PMID: 3240445] ]. 2 ml of the compound at various concentrations (62.5-500 µg/ml) was mixed with 2 ml of DPPH (0.002% w/v in methanol) and was incubated for 20 minutes in the dark. After incubation, the absorbance was measured at 517 nm. The DPPH scavenging activity was calculated using the same equation that was used to calculate NO scavenging activity. Ascorbic acid was used as a standard.
2.5. MTT Cell Viability Assay
The cytotoxic effect of Pyrrolo [1, 2-A] pyrazine-1, 4-dione, hexahydro-3-(2-methylpropyl)- was assessed on RAW macrophages 264.7 cell line as described by Mosmann [18Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65(1-2): 55-63.
[http://dx.doi.org/10.1016/0022-1759(83)90303-4] [PMID: 6606682] ]. RAW cells (5 × 105 per well) were seeded onto a 96-well plate and was incubated at 37°C 5% CO2 incubator for 24 hours. Following incubation, the cells were treated with various concentration of the compound (15.625 -250 µg/ml) and incubated for 24 hours. Then, 20 µl of MTT (5mg/ml PBS) was added to each well and incubated for 4 hours. The medium was removed and the cells were dissolved in DMSO. The plate was covered in tinfoil and was agitated in a plate rocker for 15 minutes. Optical density at 590 nm was measured using a microplate reader. The percentage of viable cells at each concentration was calculated by the following equation:
 |
2.6. Statistical Analysis
All experiments were carried out in triplicates. The data represented in the figures are mean ± Standard Error (SE) calculated using Microsoft Excel 2007.
3. RESULTS
3.1. Reducing Power Assay
Reducing power is an indication of the compound’s antioxidant potential. The potassium ferricyanide gets reduced to potassium ferrocyanide which then reacts with ferric chloride to form a ferrous ferric complex. The complex had absorption maxima at 700 nm. Increased concentration resulted in increased absorbance values as shown in Fig. (2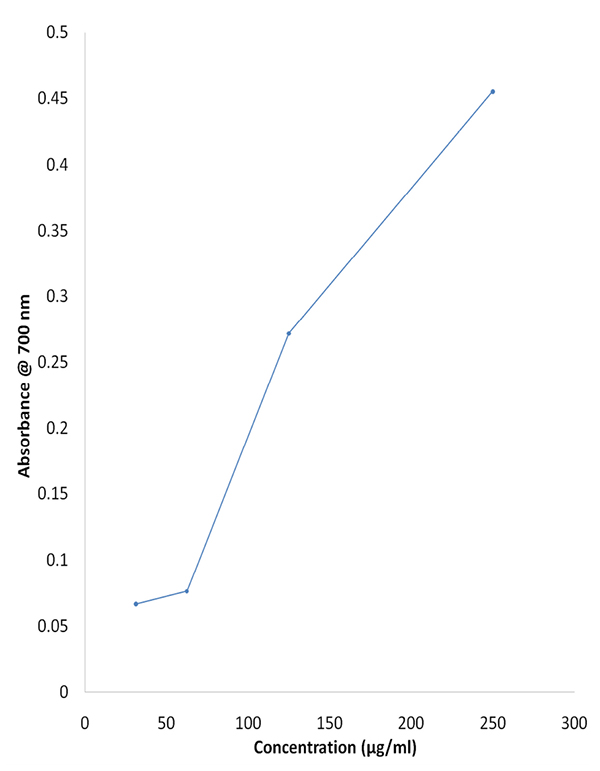 ). This indicated the reducing potential of Pyrrolo [1, 2-A] pyrazine-1, 4-dione, hexahydro-3-(2-methylpropyl)-.
). This indicated the reducing potential of Pyrrolo [1, 2-A] pyrazine-1, 4-dione, hexahydro-3-(2-methylpropyl)-.
 |
Fig. (2) Reducing power assay of the diketopiperazine compound extracted from Streptomyces sp. VITMK1. |
3.2. Nitric Oxide (NO) Scavenging Assay
Sodium nitroprusside in aqueous solution at biological pH spontaneously generate nitrite oxide which reacts with oxygen forming nitrite ions. Nitrite ions in the presence of griess reagent will have absorption maxima at 550 nm. As can be seen from Fig. (3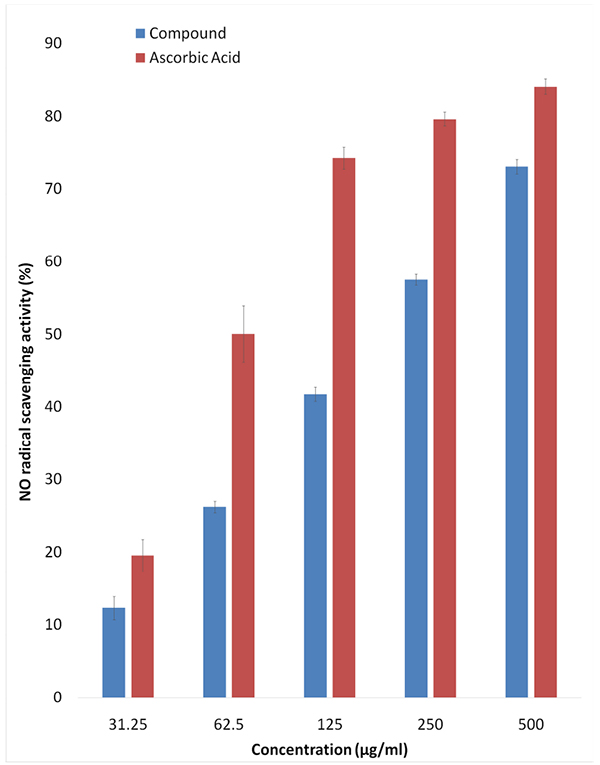 ), the compound at increasing concentration showed higher NO scavenging activity as follows: 12.90±1.58% at 31.25 µg/mL, 12.22±0.77% at 62.5 µg/mL, 41.72±0.97% at 125 µg/mL. 57.48±0.74% at 250 µg/mL and 73.03±1.02% at 500 µg/mL.
), the compound at increasing concentration showed higher NO scavenging activity as follows: 12.90±1.58% at 31.25 µg/mL, 12.22±0.77% at 62.5 µg/mL, 41.72±0.97% at 125 µg/mL. 57.48±0.74% at 250 µg/mL and 73.03±1.02% at 500 µg/mL.
 |
Fig. (3) Nitric Oxide radical scavenging activity of the diketopiperazine compound extracted from Streptomyces sp. VITMK1. |
3.3. DPPH Free Radical Scavenging Assay
After the assay, colour change from purple to yellow was observed. The DPPH free radical has absorption maxima at 517 nm. The decrease in the absorbance corresponds to the percentage of DPPH free radical scavenging capacity. As can be seen from Fig. (4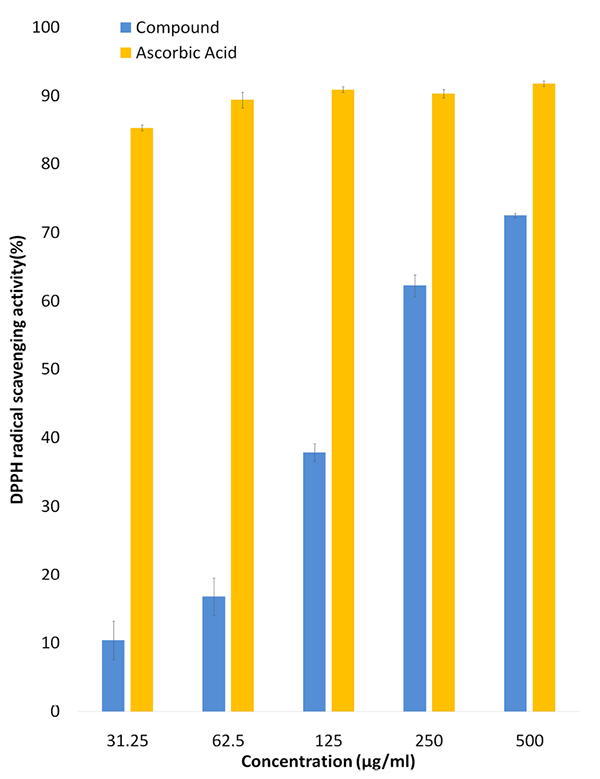 ) the compound at increasing concentration showed higher DPPH free radical scavenging activity as follows: 10.40±2.79% at 31.25 µg/mL, 16.76±2.75% at 62.5 µg/mL, 37.85±1.28% at 125 µg/mL. 62.27±1.58% at 250 µg/mL and 72.48±0.32% at 500 µg/mL.
) the compound at increasing concentration showed higher DPPH free radical scavenging activity as follows: 10.40±2.79% at 31.25 µg/mL, 16.76±2.75% at 62.5 µg/mL, 37.85±1.28% at 125 µg/mL. 62.27±1.58% at 250 µg/mL and 72.48±0.32% at 500 µg/mL.
 |
Fig. (4) DPPH radical scavenging activity of the diketopiperazine compound extracted from Streptomyces sp. VITMK1. |
3.4. MTT Cell Viability Assay
It was observed that at 31.25 µg/mL, the compound did not affect the cells and cell viability of 94.31±0.03% and 91.95±0.06% were registered at concentration of 62.5 µg/mL and at 125 µg/mL. The compound exhibited concentration dependent cell viability on RAW macrophages 264.7 cell line.
4. DISCUSSION
It is a widely known fact that the free radicals have a critical role in various diseases these days [19Chen AF, Chen DD, Daiber A, et al. Free radical biology of the cardiovascular system. Clin Sci 2012; 123(2): 73-91.
[http://dx.doi.org/10.1042/CS20110562] [PMID: 22455351] , 20Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med 2010; 49(11): 1603-16.
[http://dx.doi.org/10.1016/j.freeradbiomed.2010.09.006] [PMID: 20840865] ]. ROS and reactive nitrogen species (RNS) have been reported to cause DNA damage [21Yen G, Lai H, Chou H. Nitric oxide-scavenging and antioxidant effects of Uraria crinita root. Food Chem 2001; 74: 471-8.
[http://dx.doi.org/10.1016/S0308-8146(01)00165-0] ]. It necessitated the investigation of potential antioxidant compounds from natural sources. A wide spectrum of sources is available for natural antioxidants, among which actinomycetes occupy a key position. There have been earlier reports of antioxidant activity reported from actinomycetes. Kim reported the isolation and identification of actinomycetes from marine source and the antioxidative effects of its culture supernatant. Initially the DPPH scavenging activity of the isolate was 60%. When the conditions were optimized the DPPH radical scavenging activity increased to 88% [22Kim M, Lee J, Kim D, et al. Isolation and identification of antioxidant producing marine-source actinomycetes and optimal medium conditions. Food Sci Biotechnol 2014; 23: 1629-35.
[http://dx.doi.org/10.1007/s10068-014-0222-1] ]. This suggests that the optimization of conditions to obtain optimal cell growth and antioxidant activity would improve the antioxidant activity of the actinomycete isolate and the compounds that they produce. Intracellular and extracellular extracts of Streptomyces species VITTK3 were assessed for antioxidant activity and it was found that the extracellular extract showed 96% and the intracellular extract showed 22% at a concentration of 5 mg/mL [23Thenmozhi M, Sindhura S, Kannabiran K. Characterization of antioxidant activity of Streptomyces species VITTK3 isolated form Puducherry Coast, India. J Adv Sci Res 2010; 1: 46-52.]. The DPPH radical scavenging activity of our study was 72.48±0.32% at 500 µg/mL. The concentration with which we worked was ten times less that the concentration used by Thenmozhi et al. Diketopiperazines isolated from other sources have been evaluated for their antioxidant potential. A diketopiperazine isolated from the bacterium Pseudoalteromonas haloplanktis TAC125 was evaluated for its antioxidant activity by DPPH free-radical scavenging assay. It showed 72% activity at 10 mmol and 51% activity at 1 mmol when compared to hydroquinone which had 100% activity [24Mitova M, Tutino ML, Infusini G, Marino G, De Rosa S. Exocellular peptides from pntarctic psychrophile Pseudoalteromonas haloplanktis. Mar Biotechnol (NY) 2005; 7(5): 523-31.
[http://dx.doi.org/10.1007/s10126-004-5098-2] [PMID: 15988629] ]. The potential antioxidant activity of three diketopiperazine compounds cyclo(prolyl-valyl), cyclo(prolyl-leucyl) and cyclo (prolyl-ioslleucyl) isolated from the mushroom Sarcodon aspratus showed DPPH free radical scavenging activity. The EC50 of the three compounds were reported to be 0.15, 0.17, 0.18 mM respectively [25Kim JM, Moon BS, Park YM, Yoo NH, Ryoo IJ, Chinht NT, et al. Structures and antioxidant activity of diketopiperazines isolated from the mushroom Sarcodon aspratus. J Korean Soc Appl Biol Chem 2005; 48: 93-7.]. The reducing capacity of a compound serves as a significant indicator of its potential antioxidant activity. In order to study the reducing ability of the compound, we studied the transformation of Fe3+ to Fe2+ in the presence of the extracted compound. The ferrous ferric complex formed was read at 700 nm for absorption using a UV Spectrophotometer. The results observed from the reducing power assay showed that the diketopiperazine compound is a strong antioxidant. It is essential to make use of a free radical scavenging assay to evaluate the antioxidant activity of the compound. The DPPH assay is one of the most common and relatively quick methods used for testing radical scavenging activity of bioactive metabolites [26Elmastas M, Isildak O, Turkekul I, et al. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. J Food Compos Anal 2007; 20: 337-45.
[http://dx.doi.org/10.1016/j.jfca.2006.07.003] ]. Griess assay used to determine the level of over produced cellular nitric oxide induced by cytokines and endotoxin was the choice to evaluate the nitric oxide radical scavenging assay [27Dirsch VM, Stuppner H, Vollmar AM. The Griess assay: suitable for a bio-guided fractionation of anti-inflammatory plant extracts? Planta Med 1998; 64(5): 423-6.
[http://dx.doi.org/10.1055/s-2006-957473] [PMID: 9690344] ]. The compound was observed to exhibit strong DPPH free radical scavenging activity and NO radical scavenging activity. In all the assays ascorbic acid was used as the standard and it can be seen that the compound exhibits similar antioxidant activity at higher concentrations. The MTT assay results on the RAW 264.7 macrophage cell line suggest that the compound pyrrolo [1, 2-A] pyrazine-1, 4-dione, hexahydro-3-(2-methylpropyl)- was nontoxic to RAW macrophages 264.7 cell line.
CONCLUSION
The diketopiperazine compound pyrrolo [1, 2-A] pyrazine-1, 4-dione, hexahydro-3-(2-methylpropyl)– extracted from Streptomyces sp. VITMK1 is a nontoxic potential antioxidant agent capable of scavenging free radicals. The study also emphasizes that actinomycetes are an inexhaustible source of secondary metabolites with varied biological activity.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to thank the management of VIT University for providing necessary facilities to carrry out this work.
REFERENCES
| [1] | Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39(1): 44-84. [http://dx.doi.org/10.1016/j.biocel.2006.07.001] [PMID: 16978905] |
| [2] | Calabrese V, Cornelius C, Mancuso C, Lentile R, Stella AM, Butterfield DA. Redox homeostasis and cellular stress response in aging and neurodegeneration. Methods Mol Biol 2010; 610: 285-308. [http://dx.doi.org/10.1007/978-1-60327-029-8_17] [PMID: 20013185] |
| [3] | Sharma SK, Gupta VK. In vitro antioxidant studies of Ficus racemosa Linn. root. Pharmacogn Mag 2008; 4: 70-4. |
| [4] | Ferreira IC, Barros L, Soares ME, et al. Antioxidant activity and phenolic contents of Olea europaea L. leaves sprayed with different copper formulations. Food Chem 2007; 103: 188-95. [http://dx.doi.org/10.1016/j.foodchem.2006.08.006] |
| [5] | Ostrovidov S, Franck P, Joseph D, et al. Screening of new antioxidant molecules using flow cytometry. J Med Chem 2000; 43(9): 1762-9. [http://dx.doi.org/10.1021/jm991019j] [PMID: 10794693] |
| [6] | Kaiserová H, Simùnek T, van der Vijgh WJ, et al. Flavonoids as protectors against doxorubicin cardiotoxicity: Role of iron chelation, antioxidant activity, and inhibition of carbonyl reductase. Biochim Biophys Acta 2007; 1772: 1065-74. |
| [7] | Janardhan A, Kumar AP, Viswanath B, Saigopal DV, Narasimha G. Production of bioactive compounds by actinomycetes and their antioxidant properties. Biotechnol Res Int 2014; 2014: 217030. [http://dx.doi.org/10.1155/2014/217030] [PMID: 24790761] |
| [8] | Park EJ, Pezzuto JM. Antioxidant marine products in cancer chemoprevention. Antioxid Redox Signal 2013; 19(2): 115-38. [http://dx.doi.org/10.1089/ars.2013.5235] [PMID: 23397932] |
| [9] | Ishikawa Y. Development of new types of antioxidants from microbial origin. J Jpn Oil Chem Soc 1992; 41: 762-7. [http://dx.doi.org/10.5650/jos1956.41.762] |
| [10] | Karthik L, Kumar G, Bhaskara Rao KV. Antioxidant activity of newly discovered lineage of marine actinobacteria. Asian Pac J Trop Med 2013; 6(4): 325-32. [http://dx.doi.org/10.1016/S1995-7645(13)60065-6] [PMID: 23608337] |
| [11] | Bentley SD, Chater KF, Cerdeño-Tárraga AM, et al. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 2002; 417(6885): 141-7. [http://dx.doi.org/10.1038/417141a] [PMID: 12000953] |
| [12] | Zengler K, Parakar A, Keller M. New methods to access microbial diversity for small molecule discovery. In: Natural products. Humana Press 2005; pp. 275-93. [http://dx.doi.org/10.1007/978-1-59259-976-9_12] |
| [13] | Schweder T, Lindequist U, Lalk M. Screening for new metabolites from marine microorganisms. Adv Biochem Eng Biotechnol 2005; 96: 1-48. [http://dx.doi.org/10.1007/b135781] [PMID: 16566088] |
| [14] | Manimaran M, Gopal JV, Kannabiran K. Antibacterial activity of Streptomyces sp. VITMK1 isolated from mangrove soil of Pichavaram, Tamil Nadu, India. Proc Natl Acad Sci, India, Sect B Biol Sci 2017; 87: 499-506. [http://dx.doi.org/10.1007/s40011-015-0619-5] |
| [15] | Oyaizu M. Studies on products on browning reaction prepared from glucose amine. Jpn J Nut 1986; 44: 307-15. [http://dx.doi.org/10.5264/eiyogakuzashi.44.307] |
| [16] | Marcocci L, Maguire JJ, Droy-Lefaix MT, Packer L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem Biophys Res Commun 1994; 201(2): 748-55. [http://dx.doi.org/10.1006/bbrc.1994.1764] [PMID: 8003011] |
| [17] | Hatano T, Kagawa H, Yasuhara T, Okuda T. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. Chem Pharm Bull (Tokyo) 1988; 36(6): 2090-7. [http://dx.doi.org/10.1248/cpb.36.2090] [PMID: 3240445] |
| [18] | Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65(1-2): 55-63. [http://dx.doi.org/10.1016/0022-1759(83)90303-4] [PMID: 6606682] |
| [19] | Chen AF, Chen DD, Daiber A, et al. Free radical biology of the cardiovascular system. Clin Sci 2012; 123(2): 73-91. [http://dx.doi.org/10.1042/CS20110562] [PMID: 22455351] |
| [20] | Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med 2010; 49(11): 1603-16. [http://dx.doi.org/10.1016/j.freeradbiomed.2010.09.006] [PMID: 20840865] |
| [21] | Yen G, Lai H, Chou H. Nitric oxide-scavenging and antioxidant effects of Uraria crinita root. Food Chem 2001; 74: 471-8. [http://dx.doi.org/10.1016/S0308-8146(01)00165-0] |
| [22] | Kim M, Lee J, Kim D, et al. Isolation and identification of antioxidant producing marine-source actinomycetes and optimal medium conditions. Food Sci Biotechnol 2014; 23: 1629-35. [http://dx.doi.org/10.1007/s10068-014-0222-1] |
| [23] | Thenmozhi M, Sindhura S, Kannabiran K. Characterization of antioxidant activity of Streptomyces species VITTK3 isolated form Puducherry Coast, India. J Adv Sci Res 2010; 1: 46-52. |
| [24] | Mitova M, Tutino ML, Infusini G, Marino G, De Rosa S. Exocellular peptides from pntarctic psychrophile Pseudoalteromonas haloplanktis. Mar Biotechnol (NY) 2005; 7(5): 523-31. [http://dx.doi.org/10.1007/s10126-004-5098-2] [PMID: 15988629] |
| [25] | Kim JM, Moon BS, Park YM, Yoo NH, Ryoo IJ, Chinht NT, et al. Structures and antioxidant activity of diketopiperazines isolated from the mushroom Sarcodon aspratus. J Korean Soc Appl Biol Chem 2005; 48: 93-7. |
| [26] | Elmastas M, Isildak O, Turkekul I, et al. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. J Food Compos Anal 2007; 20: 337-45. [http://dx.doi.org/10.1016/j.jfca.2006.07.003] |
| [27] | Dirsch VM, Stuppner H, Vollmar AM. The Griess assay: suitable for a bio-guided fractionation of anti-inflammatory plant extracts? Planta Med 1998; 64(5): 423-6. [http://dx.doi.org/10.1055/s-2006-957473] [PMID: 9690344] |



