- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Bioactive Compounds Journal
(Discontinued)
ISSN: 1874-8473 ― Volume 9, 2020
Antidiabetic and Antioxidant Potential of GancidinW from Streptomyces Paradoxus VITALK03
Lokesh Ravi, Adhithya Ragunathan, Kannabiran Krishnan*
Abstract
Background:
The aim of the present study was to analyse the antidiabetic and antioxidant potential of GancidinW (GW) extracted from Streptomyces paradoxus VITALK03.
Materials and Methods:
Antidiabetic potential of GW was evaluated by assay of α-amylase and α-glucosidase inhibitory activity; haemoglobin glycosylation and yeast glucose uptake. The antioxidant potential of GW was assessed by 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cation decolorization assay and superoxide assay. The inhibition of α-amylase and α-glucosidase by GW was also studied by in Silico molecular docking analysis.
Results:
GW (1mg/ml) showed 69.32% of α-amylase and 54.04% of α-glucosidase inhibitory activity. GW (1mg/ml) prevented haemoglobin glycosylation up to 30.92% and the glucose uptake by yeast cells was increased up to 64.38%. The binding interaction GW with α-amylase showed the least free binding energy of -6.09Kcal/mol and -7.53Kcal/mol with α-glucosidase by docking studies. GW also demonstrated moderate antioxidant activity in all the antioxidant assays performed.
Conclusion:
The results of this study suggests that the antidiabetic and antioxidant potential of GW can be probed further to develop GW as effective antidiabetic agent.
Article Information
Identifiers and Pagination:
Year: 2017Volume: 05
First Page: 31
Last Page: 42
Publisher Id: TOBCJ-5-31
DOI: 10.2174/1874847301705010031
Article History:
Received Date: 19/06/2017Revision Received Date: 05/09/2017
Acceptance Date: 09/09/2017
Electronic publication date: 31/10/2017
Collection year: 2017
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the School of Biosciences and Technology, VIT University, Vellore-632014, Tamil Nadu, India; Tel: 0416-2202024; E-mail: kkb@vit.ac.in
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 19-06-2017 |
Original Manuscript | Antidiabetic and Antioxidant Potential of GancidinW from Streptomyces Paradoxus VITALK03 | |
1. INTRODUCTION
Actinomycetes are gram positive, saprophytic and filamentous bacteria belonging to the order Actinomycetales and the family Actinomycetaceae [1Abirami M, Khanna VG, Kannabiran K. Antibacterial activity of marine Streptomyces sp. isolated from Andaman & Nicobar Islands, India. Int J Pharma Bio Sci 2013; 4(3): 280-6., 2Krishnan K, Mani A, Jasmine S. Cytotoxic activity of bioactive compound 1, 2- benzene dicarboxylic acid, mono 2- ethylhexyl ester extracted from a marine derived Streptomyces sp. VITSJK8. Int J Mol Cell Med 2014; 3(4): 246-54.
[PMID: 25635251] ]. Among the various prokaryotes, actinomycetes are one of the economically important bacteria and the genus Streptomyces are known for producing several bioactive compounds. Actinomycetes are prolific producers of numerous bioactive compounds and new chemical entities. Out of 23000 bioactive compounds isolated from the microorganisms, more than 10000 bioactive compounds were from actinomycetes, among them, 7600 were isolated from Streptomyces species [3Ravi L, Kannabiran K. Bioactivity-guided extraction and identification of antibacterial compound from marine actinomycetes strains isolated from costal soil samples of Rameswaram and Dhanushkodi, Tamil Nadu, India. Asian J Pharm 2017; 10(4): 505-9.]. Antibacterial, antifungal, antiviral, anticancer and antimalarial activities of bioactive compounds extracted from actinomycetes have already been reported [4Chavan DV, Mulaje SS, Mohalkar R. A review on actinomycetes and their biotechnological application. Int J fo Pharm Sci Res 2013; 10: 1730-42.]. However, in the recent years, the rediscovery of known bioactive compounds in terrestrial actinomycetes have increased and therefore researchers have shown increased interest in exploring marine actinomycetes. The marine ecosystem offers a greater diversity which is vastly different from the terrestrial environment. Despite the recent success with the discovery of novel bioactive compounds from the marine actinomycetes, only 1% of them have been estimated to be identified [5Prudhomme J, McDaniel E, Ponts N, et al. Marine actinomycetes: A new source of compounds against the human malaria parasite. Gregson A, editor. PLoS One.2008.
[http://dx.doi.org/10.1371/journal.pone.0002335] ] and still several compounds yet to be identified. Several pathogens developing resistance to current drugs available in the market; and cancer cells becoming resistant to existing class of cancer drugs are currently considered as a major challenge for scientists worldwide for controlling drug resistance pathogens and cancer. Screening of marine microbes for novel bioactive compounds and new chemical entities is on war footing to combat the microbial drug resistance and drug resistance by cancer cells. Marine actinomycetes isolated from unexplored and underexplored regions can offer new and effective bioactive compounds [6Radhakrishnan M, Suganya S, Balagurunathan R, Kumar V. Preliminary screening for antibacterial and antimycobacterial activity of actinomycetes from less explored ecosystems. World J Microbiol Biotechnol 2010; 26(3): 561-6.
[http://dx.doi.org/10.1007/s11274-009-0198-9] ].
India is one of the 6 countries with leading number of diabetes patients and the number is increasing every year. According to World Health Organization report, India had 69.1 million diabetes cases in 2015. The people living in the urban India, have a high prevalence of diabetes and also, majority of urban population are at the risk of acquiring diabetes, due to their impaired glucose tolerance [7Ramachandran A, Snehalatha C, Kapur A, et al. Diabetes Epidemiology Study Group in India (DESI). High prevalence of diabetes and impaired glucose tolerance in India: National Urban Diabetes Survey. Diabetologia 2001; 44(9): 1094-101.
[http://dx.doi.org/10.1007/s001250100627] [PMID: 11596662] , 8Ramachandran A, Snehalatha C, Vijay V. Burden of type 2 diabetes and its complications - the Indian scenario. Curent Sci 2002; 83: 1471-6.]. It was already reported that diabetes has a great impact on the future of the Indian economy [9Bjork S, Kapur A, King H, Nair J, Ramachandran A. Global policy: aspects of diabetes in India. Health Policy 2003; 66(1): 61-72.
[http://dx.doi.org/10.1016/S0168-8510(03)00044-7] [PMID: 14499166] ]. The rapid increase in diabetic population in India is of great concern which needs to be addressed. The continuous increase of diabetic population warrants for the search of new and effective antidiabetic agents. Although several antidiabetic drugs are available in the market, the side effects and cost of these medicines makes it unaffordable and difficult for long-term use. The prevailing situation in India and other countries raise the demand for developing a cost effective antidiabetic drug, with no major side effects, that could benefit the entire diabetic population.
The aim of the work was to study the antidiabetic and antioxidant potential of GW extracted from marine Streptomyces paradoxus sp. VITALK03. The inhibitory potential of GW on α-amylase and α-glucosidase was also validated by molecular docking analysis.
2. MATERIALS AND METHODS
2.1. Isolation of Marine Actinomycetes
Marine actinomycetes were isolated from Rameswaram and Dhanushkodi costal soil samples by serial dilution and spread plate method. All isolates were screened for bioactivity. The chloroform extract prepared from the potential isolate Streptomyces paradoxus sp. VITALK03 was subjected to purification by silica gel column chromatography. The extraction, purification and identification of the active secondary metabolite GW was already been reported [3Ravi L, Kannabiran K. Bioactivity-guided extraction and identification of antibacterial compound from marine actinomycetes strains isolated from costal soil samples of Rameswaram and Dhanushkodi, Tamil Nadu, India. Asian J Pharm 2017; 10(4): 505-9., 10Ravi L, Krishnan K. Extraction and identification antibacterial compound, GancidinW from marine Streptomyces sp. VITLGK012. Indian J Biotechnol 2017. In Press].
2.2. DPPH Assay
DPPH assay (1, 1- diphenyl 2-picryl hydrazyl) was performed based on the method described by Von Gadow et al. (1997) [11von Gadow A, Joubert E, Hansmann CF. Comparison of the antioxidant activity of rooibos tea (Aspalathus linearis) with green, oolong and black tea. Food Chem 1997; 60(1): 73-7.
[http://dx.doi.org/10.1016/S0308-8146(96)00312-3] ]. Two mL of methanolic solution of DPPH (6 ×10-5M) was added to methanolic solution of the test sample (50 µl) (methanolic solution of GW (200, 400, 600, 800 and 1000µg/ml) was prepared and used, Quercetin was used as a standard. The DPPH scavenging effect (decrease in absorbance at 515 nm) of GW (for all the concentrations), quercetin and blank was recorded after 16 min of incubation. The percentage inhibition of the DPPH radicals by GW was calculated according to the formula of Yen and Duh (1994) [12Yen GC, Duh PD. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J Agric Food Chem 1994; (42): 629-32.
[http://dx.doi.org/10.1021/jf00039a005] ].
| Scavenging Activity (%): [(A-B)/A]x100 |
Where ‘A’ is the absorbance of DPPH solution, and ‘B’ is the absorbance of GW [11von Gadow A, Joubert E, Hansmann CF. Comparison of the antioxidant activity of rooibos tea (Aspalathus linearis) with green, oolong and black tea. Food Chem 1997; 60(1): 73-7.
[http://dx.doi.org/10.1016/S0308-8146(96)00312-3] ].
2.3. ABTS Assay
The free radical scavenging potential of GW was tested using 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) radical cation decolorization assay [13Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999; 26(9-10): 1231-7.
[http://dx.doi.org/10.1016/S0891-5849(98)00315-3] [PMID: 10381194] ]. Aqueous solution of ABTS (7 mM) was prepared and ABTS radical cation (ABTS*•+) was produced by reacting ABTS stock solution with 2.45 mM potassium per sulfate (final concentration) and allowing the mixture to stand in the dark at room temperature for 12 h before use. To study the radical scavenging activity of GW, the ABTS•+ solution was diluted with absolute ethanol to get the absorbance of 0.70 (±0.02) at 734 nm and equilibrated at 30°C. After addition of 2.0 mL of diluted ABTS•+ solution (A734 nm = 0.700(±0.02)) to GW (20 µL) (200, 400, 600, 800 and 1000µg/ml), the absorbance was recorded at 30°C exactly 6 min after initial mixing (At). Reagent blank was also used and the reading was taken (A0). Butylated hydroxyl toluene was used as a standard. The percentage inhibition of absorbance at 734 nm was calculated using the above formula and decrease of the absorbance between A0 and At.
| PI = [(AC(0) – AA(t)) /AC(0) ] × 100 |
Where AC(0) is the absorbance of blank at t = 0 min; and AA(t) is the absorbance of GW at t = 6 min [13Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999; 26(9-10): 1231-7.
[http://dx.doi.org/10.1016/S0891-5849(98)00315-3] [PMID: 10381194] ].
2.4. Superoxide Anion Radical Scavenging Activity
Superoxide radicals were generated by the method of Beauchamp and Fridovich (1971) described by Zhishen et al (1999) with slight modification [14Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 1999; 64(4): 555-9.
[http://dx.doi.org/10.1016/S0308-8146(98)00102-2] ]. The superoxide radicals generated from riboflavin and methionine were illuminated and assayed by the reduction of nitroblue tetrazolium (NBT) to form a blue formazan (NBT2+). All solutions were prepared by using 0.05 M phosphate buffer (pH 7.8). The photo-induced reactions were performed using fluorescent lamps (20 W). The total volume of the reaction mixture was 3 mL and the concentrations of the riboflavin, methionine, and NBT were 1.33x10-5, 4.46x10-5 and 8.15x10-8M respectively. The reaction mixture containing different concentrations of the GW (200, 400, 600, 800 and 1000µg/ml) was used to study superoxide radical scavenging activity. The reactant was illuminated at 25°C for 40 min. The photochemically generated superoxide radical from the reaction mixture reduce the NBT to form blue formazan. Tannic acid (15 µg/ mL) was added to the reaction mixture, in which superoxide was scavenged, thereby inhibiting the NBT reduction. The un-illuminated reaction mixture was used as a blank. The absorbance was measured at 560 nm. The decrease in absorbance of the reaction mixture indicates the increased superoxide anion scavenging activity of GW. The percentage inhibition of superoxide anion generation was calculated by using the following formula:
| O2 scavenging effect (%) = (1- AS/AC) x 100 |
Where AC is the absorbance of the GW and AS is the absorbance of tannic acid [14Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 1999; 64(4): 555-9.
[http://dx.doi.org/10.1016/S0308-8146(98)00102-2] ].
2.5. Assay of α-amylase Inhibition
Alpha-amylase hydrolyses alpha-bonds of a large alpha-linked polysaccharide such as glycogen and starch to yield glucose and maltose. Alpha-amylase inhibitory activity was assayed by starch-iodine method [15Vijayalakshmi K. CIS, Sindhu S, Arumugam P. In vitro investigation of antidiabetic potential of selected traditional medicinal plants. Int J Pharmacogn Phytochem Res 2014; 6(4): 856-61., 16Kim JS, Kwon CS, Son KH. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem 2000; 64(11): 2458-61.
[http://dx.doi.org/10.1271/bbb.64.2458] [PMID: 11193416] ] with some modifications. In alpha-amylase inhibition assay 1 ml of substrate- potato starch (1% w/v), 1 ml of GW (250, 500, 750 and 1000 μg/ml), 1 ml of alpha-amylase enzyme (1% w/v) and 2 ml of acetate buffer (0.1 M, 7.2 pH) was added. Acarbose was used as standard and potato starch solution, alpha-amylase solution GW was prepared in 0.5 M acetate buffer. The above mixture was incubated for 1 hr. Then, 0.1 ml iodine-iodide indicator (635 mg iodine and 1 gm potassium iodide in 250 ml distilled water) was added to the mixture. Absorbance was measured at 565 nm in UV-Visible spectrophotometer.
| % inhibition = [(Ac-As)/Ac) x 100 |
Where Ac is the absorbance of the control (containing all reagents except GW), and As is the absorbance of GW [16Kim JS, Kwon CS, Son KH. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem 2000; 64(11): 2458-61.
[http://dx.doi.org/10.1271/bbb.64.2458] [PMID: 11193416] ].
2.6. Assay of α-glucosidase Inhibition
The α-glucosidase inhibitory activity of GW was carried out by the method of Dahlqvist (1964) [17Dahlqvist A. Method for assay of intestinal disaccharidases. Anal Biochem 1964; 7: 18-25.
[http://dx.doi.org/10.1016/0003-2697(64)90115-0] [PMID: 14106916] ] with slight modification [18Thilagam E, Parimaladevi B, Kumarappan C, Mandal SC. α-Glucosidase and α-amylase inhibitory activity of Senna surattensis. J Acupunct Meridian Stud 2013; 6(1): 24-30.
[http://dx.doi.org/10.1016/j.jams.2012.10.005] [PMID: 23433052] ]. The mouse small intestine homogenate was used as a α-glucosidase solution to measure the inhibitory effect. After fasting for 20 h, the small intestine (the portion below the duodenum and above the cecum) was cut, rinsed with ice-cold saline, and homogenized with 12 ml of maleate buffer (100 mM, pH 6.0). The homogenate was used as the source of α-glucosidase solution. The assay mixture consisted of 100 mM maleate buffer (pH 6.0), 2% (w/v) each sugar substrate solution (100 µl), and GW (200–1000 µg/ml). It was preincubated for 5 min at 37°C, and the reaction was initiated by adding the crude α-glucosidase solution (50µl), followed by incubation for 10 min at 37°C. The glucose released in the reaction mixture was determined with the commercially available glucose estimation kit (Span Diagnostic Ltd., Mumbai, India) the rate of carbohydrate decomposition was calculated as the percentage ratio to the amount of glucose obtained when the carbohydrate was completely digested. The rate of inhibition was calculated by the following formula:
Inhibition (%) = [(amount of glucose produced by the positive control) - (amount of glucose produced by the addition of GW) - (glucose production value in blank) / (amount of glucose produced by the positive control)] x 100
2.7. Non-Enzymatic Glycosylation of Haemoglobin
Antidiabetic activity of GW was investigated by estimating the degree of non-enzymatic haemoglobin glycosylation, measured calorimetrically at 520 nm. Glucose (2%), haemoglobin (0.06%) and gentamycin (0.02%) solutions were prepared in phosphate buffer 0.01 M, pH 7.4. The above solutions (1 ml each) were mixed. GW was weighed and dissolved in DMSO to obtain a stock solution of 200-1000 µg/ml and 1 ml of each concentration was added to the above mixture. The above mixture without the GW was also prepared and kept as control. The mixture was incubated in dark at room temperature for 72 hrs. The degree of glycosylation of haemoglobin was measured calorimetrically at 520 nm.
| Inhibition (%) = ((Ac-As)/Ac) x 100. |
Where Ac is the absorbance of control; As is the absorbance of GW.
2.8. Glucose Uptake in Yeast Cells
Yeast cells were prepared as described previously [18Thilagam E, Parimaladevi B, Kumarappan C, Mandal SC. α-Glucosidase and α-amylase inhibitory activity of Senna surattensis. J Acupunct Meridian Stud 2013; 6(1): 24-30.
[http://dx.doi.org/10.1016/j.jams.2012.10.005] [PMID: 23433052] ]. Baker’s yeast was washed with distilled water by repeated centrifugation (3,000×g; 5 min) until the supernatant fluids were clear and finally 10% (v/v) suspension was prepared in distilled water. Different concentrations of GW (1–5 mg) were added to 1 mL of glucose solution (5, 10 and 25 mM) and incubated together for 10 min at 37°C. Reaction was started by adding 100 µl of yeast suspension, vortexed and further incubated at 37°C for 60 min. After 60 min, the tubes were centrifuged (2500 g for 5 min) and glucose was estimated in the supernatant. Metronidazole was taken as a standard drug. The percentage increase in glucose uptake by yeast cells was calculated using the following formula
| Glucose uptake (%) = ((Ac-As)/Ac) x 100. |
Where Ac is the absorbance of control (containing all reagents except GW); As is the absorbance of GW [15Vijayalakshmi K. CIS, Sindhu S, Arumugam P. In vitro investigation of antidiabetic potential of selected traditional medicinal plants. Int J Pharmacogn Phytochem Res 2014; 6(4): 856-61., 18Thilagam E, Parimaladevi B, Kumarappan C, Mandal SC. α-Glucosidase and α-amylase inhibitory activity of Senna surattensis. J Acupunct Meridian Stud 2013; 6(1): 24-30.
[http://dx.doi.org/10.1016/j.jams.2012.10.005] [PMID: 23433052] ].
3. RESULTS
3.1. Antioxidant Activity of GW
3.1.1. DPPH Assay
GW (1000 µg/ml) demonstrated a moderate (34%) antioxidant activity (free radical inhibitory activity) in DPPH assay. The results of DPPH radical scavenging activity of GW in comparison with the quercetin (standard) is shown in Fig. (1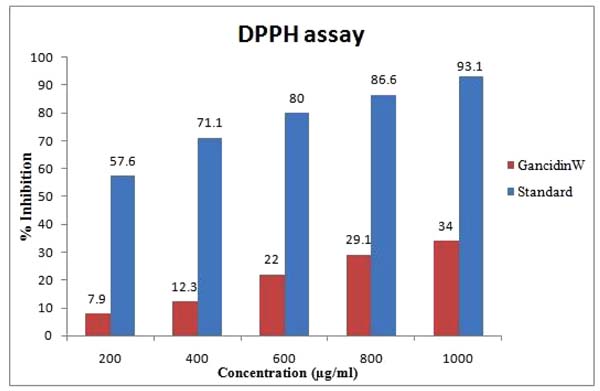 ).
).
 |
Fig. (1) DPPH (1, 1- diphenyl 2-picryl hydrazyl) scavenging activity of GW from Streptomyces paradoxus VITALK03. |
3.1.2. ABTS Assay
GW (1000µg/ml) demonstrated a moderate (48%) antioxidant activity in ABTS assay. The ABTS radical cation decolorization activity of GW in comparison with butylated hydroxyl toluene (standard) is shown in Fig. (2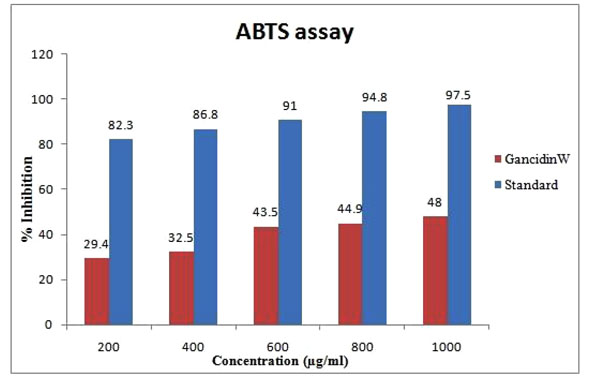 ).
).
 |
Fig. (2) ABTS (2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) free radical scavenging activity of GancidinW. |
3.1.3. Superoxide Assay
GW (1000µg/ml) demonstrated a significant (61.5%) antioxidant activity (free radical inhibition) in superoxide assay. The results of superoxide assay for GW in comparison with tannic acid (standard) is shown in Fig. (3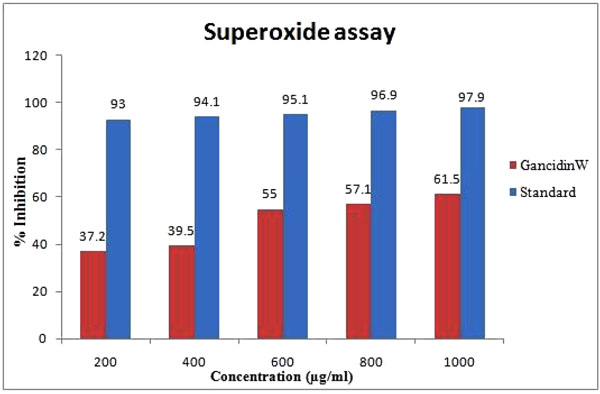 ).
).
 |
Fig. (3) Superoxide radical scavenging activity of GancidinW. |
3.2. Antidiabetic Activity of GW
3.2.1. α-amylase Inhibition
GW (1000µg/ml) showed a significant (69.32%) inhibition of alpha-amylase activity. The alpha-amylase inhibiory activity at various concentrations of GW is shown in Fig. (4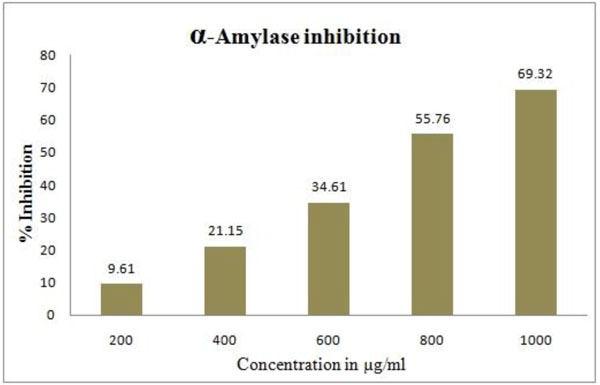 ).
).
 |
Fig. (4) Alpha-amylase inhibitiory activity of GancidinW. |
3.2.2. α-glucosidase Inhibition
GW (1000µg/ml) showed significant (54.05%) alpha-glucosidase inhibitory activity. The alpha-glucosidase inhibitory activity at various concentrations of GW is shown in Fig. (5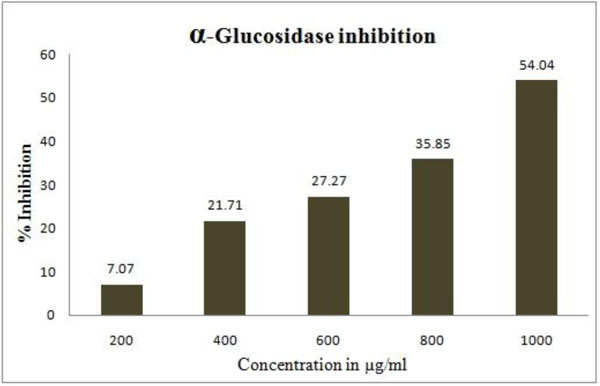 ).
).
 |
Fig. (5) Alpha-glucosidase inhibitiory activity of GancidinW. |
3.2.3. Glycosylation of Haemoglobin
GW (1000µg/ml) prevented the haemoglobin glycosylation by (30.9%). Inhibition of haemoglobin glycosylation at various concentrations of GW is shown in Fig. (6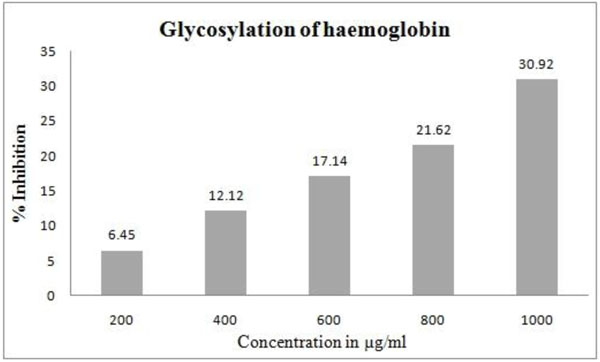 ).
).
 |
Fig. (6) Effect of Gancidin W on haemoglobin glycosylation. |
3.2.4. Glucose Uptake by Yeast Cells
GW (1000µg/ml) significantly increased (64.3%) the uptake of glucose by yeast cells. Increase in glucose uptake by yeast cells at various concentrations of GW is illustrated in Fig. (7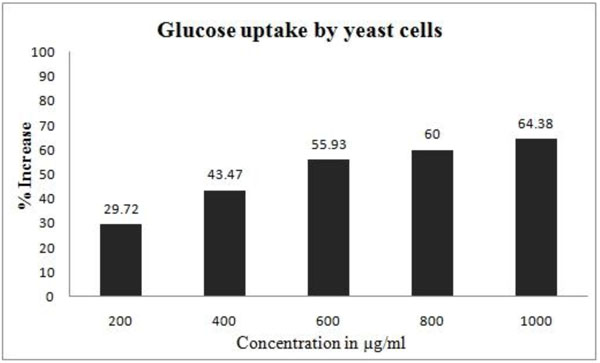 ).
).
 |
Fig. (7) Effect of Gancidin W on glucose uptake by yeast cells. |
3.3. In Silico Protein-Ligand Docking Analysis
The ability of GW to inhibit α-amylase and α-glucosidase were studied by in Silico protein-ligand docking analysis using AutoDock 4.2. GW interaction with α-amylase showed a least free binding energy of -6.09 Kcal/mol and inhibition constant (Ki) of 34.63 µM and formed 1 hydrogen bond with LYS-200 (2.72Å). Interaction of GW with α-amylase is shown in Fig. (8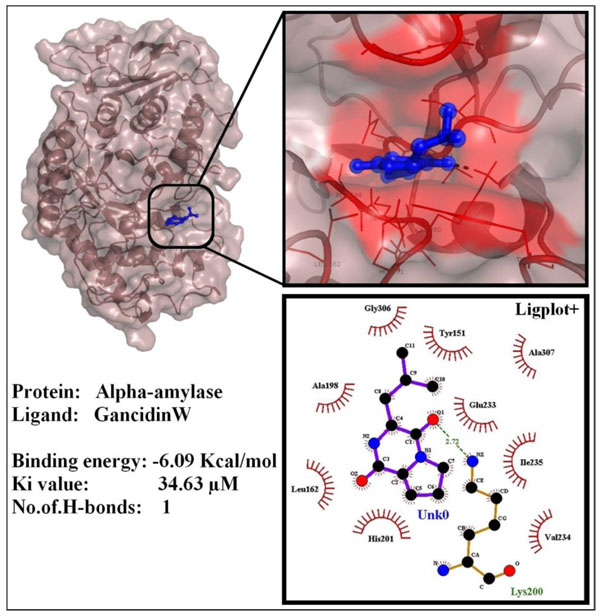 ).
).
 |
Fig. (8) Interactions of GancidinW with alpha-amylase enzyme. |
In Silico analysis revealed the interaction between GW and α-glucosidase enzyme. It showed the least free binding energy of -7.53 Kcal/mol and Ki value of 3.04 µM and formed 2 hydrogen bonds with ILE-13 (2.71Å) and TYR-46 (3.20Å). Interaction of GW and α-glucosidase is shown in Fig. (9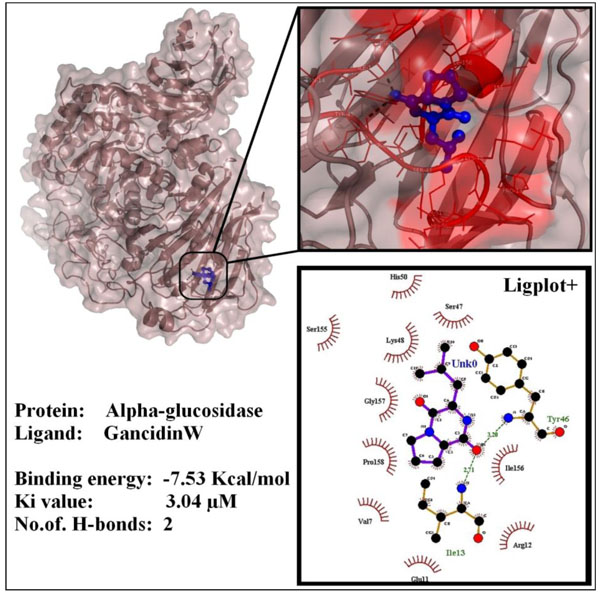 ).
).
 |
Fig. (9) Interactions of GancidinW with alpha-glucosidase enzyme. |
4. DISCUSSION
GW extracted from Streptomyces paradoxus sp. VITALK03 showed significant antidiabetic and antioxidant activity. Antioxidant property helps to reduce the oxidative stress in the host, generated due to the metabolic disorder or the drug interactions within the host. The antioxidant property of compounds helps to control the oxidative stress generated by non-specific reactions. The observed free radical scavenging activity of GW would be helpful to reduce the oxidative stress generated during non-specific interactions. Since GW demonstrated moderate antioxidant activity with high percentage of superoxide radical scavenging activity, it can be used as potential antioxidant agent.
Diabetes mellitus is one of the major health disorders in developing countries affects people of all age groups. Till date there is no effective remedy available for effective control and management of diabetes mellitus except administration required dose of insulin. Several enzymes are involved in carbohydrate metabolism, which serves as the target for controlling hyperglycemia [18Thilagam E, Parimaladevi B, Kumarappan C, Mandal SC. α-Glucosidase and α-amylase inhibitory activity of Senna surattensis. J Acupunct Meridian Stud 2013; 6(1): 24-30.
[http://dx.doi.org/10.1016/j.jams.2012.10.005] [PMID: 23433052] ]. Alpha-amylase and glucosidase enzymes play a key role in the conversion of complex sugars in to simple glucose that gets absorbed into the blood. Thus, these enzymes are responsible for increasing the blood sugar level. Under diabetic condition, the sugar level was not controlled by insulin, and thus, the blood sugar level stays high (hyperglycemia) for a prolonged period of time leading to cell damage in specific tissues [16Kim JS, Kwon CS, Son KH. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem 2000; 64(11): 2458-61.
[http://dx.doi.org/10.1271/bbb.64.2458] [PMID: 11193416] -21Paul DK, Dutta S. Evaluation of the antioxidant activity of the roots and rhizomes of Cyperus rotundus L. Indian J Pharm Sci 2006; 68(2): 256-8.
[http://dx.doi.org/10.4103/0250-474X.25731] ]. Therefore, inhibition of α-amylase and α-glucosidase enzymes is of great importance in diabetic patients that will directly control the blood sugar level. In our study the protein-ligand docking analysis showed that GW has high affinity towards α-glucosidase and α-amylase enzymes. This suggests that the observed inhibitory activity of GW over α-glucosidase and α-amylase is due to its interaction with these enzymes.
The ethyl acetate (EA) extract (500 µg/ml) of Streptomyces sp.VITPK9 showed significant (69%) inhibitory activity against α-amylase [22Sanjenbam P, Thenmozhi M, Krishnan K. Screening of glycolytic enzyme inhibitory activity of streptomyces isolates from brine spring and marine sediments of India. J Pharma Res Rev 2013; 2(2): 5-11.]. The EA extract (500 µg/ml) from Streptomyces sp.VITSTK7 inhibited α-glucosidase activity by 64% [20Said O, Fulder S, Khalil K, Azaizeh H, Kassis E, Saad B. Maintaining a physiological blood glucose level with ‘glucolevel’, a combination of four anti-diabetes plants used in the traditional arab herbal medicine. Evid Based Complement Alternat Med 2008; 5(4): 421-8.
[http://dx.doi.org/10.1093/ecam/nem047] [PMID: 18955212] ]. Streptomyces corchorusii has been reported to possess amylase inhibition activity [24Revathy T, Jayasri MA, Suthindhiran K. Anti-oxidant and enzyme-inhibitory potential of marine Streptomyces. Am J Biochem Biotechnol 2013; 9(3): 282-90.
[http://dx.doi.org/10.3844/ajbbsp.2013.282.290] ]. Rhodomarinus, the sub sp. of Streptomyces corchorusii showed α-amylase inhibitor activity [23Imada C. Enzyme inhibitors and other bioactive compounds from marine actinomycetes. Antonie van Leeuwenhoek 2005; 87(1): 59-63.
[http://dx.doi.org/10.1007/s10482-004-6544-x] [PMID: 15726292] ]. Antioxidant and antidiabetic activity of Streptomyces sp. VITMSS05 was reported by Revathy et al., 2013 [24Revathy T, Jayasri MA, Suthindhiran K. Anti-oxidant and enzyme-inhibitory potential of marine Streptomyces. Am J Biochem Biotechnol 2013; 9(3): 282-90.
[http://dx.doi.org/10.3844/ajbbsp.2013.282.290] ]. Significant antioxidant activity of the ethyl acetate extract of Streptomyces sp. VITMSS05 was observed with an IC50 value of 92 µg mL−1. The extract showed 64% inhibition on α-amylase and 91.5% inhibition on α-glucosidase at 100 µg mL−1 with an IC50 value of 385 and 43 µg mL−1. Endophytic actinomycetes Streptomyces olivochromogenes has been reported to possess α-glucosidase inhibiting ability [25Pujiyanto S, Lestari Y, Suwanto A, Budiarti S, Darusman LK. Alpha-glucosidase inhibitor activity and characterization of endophytic actinomycetes isolated from some Indonesian diabetic medicinal plants. Int J Pharm Pharm Sci 2012; 4: 327-33.].
Several compounds extracted from Streptomyces species has been reported to possess significant antidiabetic activity [26Lauritano C, Ianora A. Marine organisms with anti-diabetes properties. Mar Drugs 2016; 14(12): 220.
[http://dx.doi.org/10.3390/md14120220] [PMID: 27916864] , 27Jadon R, Singh V, Chaudhary HS. Update on bioactive molecules of actinomycetes. Biosci Biotechnol Res Asia 2014; 11(2): 705-14.
[http://dx.doi.org/10.13005/bbra/1325] ]. Bioactive antidiabetic compounds voglibose, acarbose, valienamine, adiposin-1, and trestatin-B were reported from Streptomyces hygroscopicus-limoneus [28Kameda Y, Asano N, Yoshikawa M, et al. Valiolamine, a new alpha-glucosidase inhibiting aminocyclitol produced by Streptomyces hygroscopicus. J Antibiot 1984; 37(11): 1301-7.
[http://dx.doi.org/10.7164/antibiotics.37.1301] [PMID: 6392268] , 29De Melo E, Gomes AD, Carvalho I. α-amylase and α-glucosidase inhibitors: chemical structure and biological activity. Tetrahedron Lett 2006; 62: 10277-302.
[http://dx.doi.org/10.1016/j.tet.2006.08.055] ], Actinoplanes utahensis [30Schmidt DD, Frommer W, Junge B, et al. α-Glucosidase inhibitors. New complex oligosaccharides of microbial origin. Naturwissenschaften 1977; 64(10): 535-6.
[http://dx.doi.org/10.1007/BF00483561] [PMID: 337162] ], S. calvus [31Mahmud T. The C7N aminocyclitol family of natural products. Nat Prod Rep 2003; 20(1): 137-66.
[http://dx.doi.org/10.1039/b205561a] [PMID: 12636088] ] and S. dimorphogenes [32Yokose K, Ogawa M, Ogawa K. New α-amylase inhibitor, trestatins. III. Structure determination of new trestatin components Ro 09-0766, Ro 09-0767 and Ro 09-0768. J Antibiot 1984; 37(2): 182-6.
[http://dx.doi.org/10.7164/antibiotics.37.182] [PMID: 6608512] ] respectively. Lowering of the post prandial blood glucose level by Voglibose through the inhibition of α-glucosidase in diabetic people has been reported. Voglibose is an oral alpha-glucosidase and alpha-amylase inhibitor, first launched by Bayer in Switzerland in 1989 for the oral treatment of type-2 diabetes mellitus, Acarbose was used as α-glucosidase and α-amylase inhibitor [30Schmidt DD, Frommer W, Junge B, et al. α-Glucosidase inhibitors. New complex oligosaccharides of microbial origin. Naturwissenschaften 1977; 64(10): 535-6.
[http://dx.doi.org/10.1007/BF00483561] [PMID: 337162] ]. Potent α-glucosidase activity of aminocyclitol, isolated from the fermentation broth of Streptomyces hygroscopicus subspecies limoneus has been reported. Pyrostatins A and B, the novel compounds extracted from Streptomyces sp. has been reported to exhibit specific inhibitory activity against N-acetyl-glucosaminidase (Imada, 2005). The novel antidiabetic compound NFAT-133 extracted from Streptomyces strain PM0324667 was reported to induce glucose uptake in L6 skeletal muscle cells [33Kulkarni-Almeida AA, Brahma MK, Padmanabhan P, et al. Fermentation, Isolation, Structure, and antidiabetic activity of NFAT-133 produced by Streptomyces strain PM0324667. AMB Express 2011; 1(1): 42.
[http://dx.doi.org/10.1186/2191-0855-1-42] [PMID: 22104600] ]. NIFTA has been reported to be effectively reduced systemic glucose levels in diabetic animals.
GW prevents 30% of haemoglobin glycosylation suggests that it is effective in preventing the interaction of glucose with RBCs in blood and it is one of the essential feature of any antidiabetic agent. Increased haemoglobin-glucose level is a serious problem in diabetic patients (hyperglycemia). Glucose forms a covalent bond with haemoglobin, resulting in increased blood plasma sugar level causes damage to insulin-independent organs like kidney, blood vessels, eye lense, etc,. Preventing the increase of haemoglobin-glucose level greatly reduces the risk of developing hyperglycemia associated damage. Non-enzymatic glycosylation of haemoglobin is an oxidation reaction and an antioxidant can counter this reaction, thereby preventing the process of glycosylation. Since GW demonstrated significant antioxidant potential, it can prevent haemoglobin glycosylation as well [15Vijayalakshmi K. CIS, Sindhu S, Arumugam P. In vitro investigation of antidiabetic potential of selected traditional medicinal plants. Int J Pharmacogn Phytochem Res 2014; 6(4): 856-61., 21Paul DK, Dutta S. Evaluation of the antioxidant activity of the roots and rhizomes of Cyperus rotundus L. Indian J Pharm Sci 2006; 68(2): 256-8.
[http://dx.doi.org/10.4103/0250-474X.25731] ].
Glucose uptake assay by yeast cells showed 64% increase in glucose uptake when treated with GW. Increased uptake in yeast cells will occur only when intracellular glucose level is reduced. GW treatment effectively reduces glucose concentration in the yeast cells, thereby causing the cells to take up increased concentrations of glucose [15Vijayalakshmi K. CIS, Sindhu S, Arumugam P. In vitro investigation of antidiabetic potential of selected traditional medicinal plants. Int J Pharmacogn Phytochem Res 2014; 6(4): 856-61.].
CONCLUSION
GW exhibits antidiabetic activity by inhibiting α-glucosidase and α-amylase enzymes, and prevents haemoglobin glycosylation. It also exhibits free radical scavenging antioxidant activity. The antidiabetic and antioxidant potential of GW can be explored further to develop as an effective antidiabetic agent.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Authors thank the management of VIT University for providing facilities to carry out this study.
REFERENCES
| [1] | Abirami M, Khanna VG, Kannabiran K. Antibacterial activity of marine Streptomyces sp. isolated from Andaman & Nicobar Islands, India. Int J Pharma Bio Sci 2013; 4(3): 280-6. |
| [2] | Krishnan K, Mani A, Jasmine S. Cytotoxic activity of bioactive compound 1, 2- benzene dicarboxylic acid, mono 2- ethylhexyl ester extracted from a marine derived Streptomyces sp. VITSJK8. Int J Mol Cell Med 2014; 3(4): 246-54. [PMID: 25635251] |
| [3] | Ravi L, Kannabiran K. Bioactivity-guided extraction and identification of antibacterial compound from marine actinomycetes strains isolated from costal soil samples of Rameswaram and Dhanushkodi, Tamil Nadu, India. Asian J Pharm 2017; 10(4): 505-9. |
| [4] | Chavan DV, Mulaje SS, Mohalkar R. A review on actinomycetes and their biotechnological application. Int J fo Pharm Sci Res 2013; 10: 1730-42. |
| [5] | Prudhomme J, McDaniel E, Ponts N, et al. Marine actinomycetes: A new source of compounds against the human malaria parasite. Gregson A, editor. PLoS One.2008. [http://dx.doi.org/10.1371/journal.pone.0002335] |
| [6] | Radhakrishnan M, Suganya S, Balagurunathan R, Kumar V. Preliminary screening for antibacterial and antimycobacterial activity of actinomycetes from less explored ecosystems. World J Microbiol Biotechnol 2010; 26(3): 561-6. [http://dx.doi.org/10.1007/s11274-009-0198-9] |
| [7] | Ramachandran A, Snehalatha C, Kapur A, et al. Diabetes Epidemiology Study Group in India (DESI). High prevalence of diabetes and impaired glucose tolerance in India: National Urban Diabetes Survey. Diabetologia 2001; 44(9): 1094-101. [http://dx.doi.org/10.1007/s001250100627] [PMID: 11596662] |
| [8] | Ramachandran A, Snehalatha C, Vijay V. Burden of type 2 diabetes and its complications - the Indian scenario. Curent Sci 2002; 83: 1471-6. |
| [9] | Bjork S, Kapur A, King H, Nair J, Ramachandran A. Global policy: aspects of diabetes in India. Health Policy 2003; 66(1): 61-72. [http://dx.doi.org/10.1016/S0168-8510(03)00044-7] [PMID: 14499166] |
| [10] | Ravi L, Krishnan K. Extraction and identification antibacterial compound, GancidinW from marine Streptomyces sp. VITLGK012. Indian J Biotechnol 2017. In Press |
| [11] | von Gadow A, Joubert E, Hansmann CF. Comparison of the antioxidant activity of rooibos tea (Aspalathus linearis) with green, oolong and black tea. Food Chem 1997; 60(1): 73-7. [http://dx.doi.org/10.1016/S0308-8146(96)00312-3] |
| [12] | Yen GC, Duh PD. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J Agric Food Chem 1994; (42): 629-32. [http://dx.doi.org/10.1021/jf00039a005] |
| [13] | Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999; 26(9-10): 1231-7. [http://dx.doi.org/10.1016/S0891-5849(98)00315-3] [PMID: 10381194] |
| [14] | Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 1999; 64(4): 555-9. [http://dx.doi.org/10.1016/S0308-8146(98)00102-2] |
| [15] | Vijayalakshmi K. CIS, Sindhu S, Arumugam P. In vitro investigation of antidiabetic potential of selected traditional medicinal plants. Int J Pharmacogn Phytochem Res 2014; 6(4): 856-61. |
| [16] | Kim JS, Kwon CS, Son KH. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem 2000; 64(11): 2458-61. [http://dx.doi.org/10.1271/bbb.64.2458] [PMID: 11193416] |
| [17] | Dahlqvist A. Method for assay of intestinal disaccharidases. Anal Biochem 1964; 7: 18-25. [http://dx.doi.org/10.1016/0003-2697(64)90115-0] [PMID: 14106916] |
| [18] | Thilagam E, Parimaladevi B, Kumarappan C, Mandal SC. α-Glucosidase and α-amylase inhibitory activity of Senna surattensis. J Acupunct Meridian Stud 2013; 6(1): 24-30. [http://dx.doi.org/10.1016/j.jams.2012.10.005] [PMID: 23433052] |
| [19] | Pari L, Amarnath Satheesh M. Antidiabetic activity of Boerhaavia diffusa L.: effect on hepatic key enzymes in experimental diabetes. J Ethnopharmacol 2004; 91(1): 109-13. [http://dx.doi.org/10.1016/j.jep.2003.12.013] [PMID: 15036478] |
| [20] | Said O, Fulder S, Khalil K, Azaizeh H, Kassis E, Saad B. Maintaining a physiological blood glucose level with ‘glucolevel’, a combination of four anti-diabetes plants used in the traditional arab herbal medicine. Evid Based Complement Alternat Med 2008; 5(4): 421-8. [http://dx.doi.org/10.1093/ecam/nem047] [PMID: 18955212] |
| [21] | Paul DK, Dutta S. Evaluation of the antioxidant activity of the roots and rhizomes of Cyperus rotundus L. Indian J Pharm Sci 2006; 68(2): 256-8. [http://dx.doi.org/10.4103/0250-474X.25731] |
| [22] | Sanjenbam P, Thenmozhi M, Krishnan K. Screening of glycolytic enzyme inhibitory activity of streptomyces isolates from brine spring and marine sediments of India. J Pharma Res Rev 2013; 2(2): 5-11. |
| [23] | Imada C. Enzyme inhibitors and other bioactive compounds from marine actinomycetes. Antonie van Leeuwenhoek 2005; 87(1): 59-63. [http://dx.doi.org/10.1007/s10482-004-6544-x] [PMID: 15726292] |
| [24] | Revathy T, Jayasri MA, Suthindhiran K. Anti-oxidant and enzyme-inhibitory potential of marine Streptomyces. Am J Biochem Biotechnol 2013; 9(3): 282-90. [http://dx.doi.org/10.3844/ajbbsp.2013.282.290] |
| [25] | Pujiyanto S, Lestari Y, Suwanto A, Budiarti S, Darusman LK. Alpha-glucosidase inhibitor activity and characterization of endophytic actinomycetes isolated from some Indonesian diabetic medicinal plants. Int J Pharm Pharm Sci 2012; 4: 327-33. |
| [26] | Lauritano C, Ianora A. Marine organisms with anti-diabetes properties. Mar Drugs 2016; 14(12): 220. [http://dx.doi.org/10.3390/md14120220] [PMID: 27916864] |
| [27] | Jadon R, Singh V, Chaudhary HS. Update on bioactive molecules of actinomycetes. Biosci Biotechnol Res Asia 2014; 11(2): 705-14. [http://dx.doi.org/10.13005/bbra/1325] |
| [28] | Kameda Y, Asano N, Yoshikawa M, et al. Valiolamine, a new alpha-glucosidase inhibiting aminocyclitol produced by Streptomyces hygroscopicus. J Antibiot 1984; 37(11): 1301-7. [http://dx.doi.org/10.7164/antibiotics.37.1301] [PMID: 6392268] |
| [29] | De Melo E, Gomes AD, Carvalho I. α-amylase and α-glucosidase inhibitors: chemical structure and biological activity. Tetrahedron Lett 2006; 62: 10277-302. [http://dx.doi.org/10.1016/j.tet.2006.08.055] |
| [30] | Schmidt DD, Frommer W, Junge B, et al. α-Glucosidase inhibitors. New complex oligosaccharides of microbial origin. Naturwissenschaften 1977; 64(10): 535-6. [http://dx.doi.org/10.1007/BF00483561] [PMID: 337162] |
| [31] | Mahmud T. The C7N aminocyclitol family of natural products. Nat Prod Rep 2003; 20(1): 137-66. [http://dx.doi.org/10.1039/b205561a] [PMID: 12636088] |
| [32] | Yokose K, Ogawa M, Ogawa K. New α-amylase inhibitor, trestatins. III. Structure determination of new trestatin components Ro 09-0766, Ro 09-0767 and Ro 09-0768. J Antibiot 1984; 37(2): 182-6. [http://dx.doi.org/10.7164/antibiotics.37.182] [PMID: 6608512] |
| [33] | Kulkarni-Almeida AA, Brahma MK, Padmanabhan P, et al. Fermentation, Isolation, Structure, and antidiabetic activity of NFAT-133 produced by Streptomyces strain PM0324667. AMB Express 2011; 1(1): 42. [http://dx.doi.org/10.1186/2191-0855-1-42] [PMID: 22104600] |



