- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Biomaterials Journal
(Discontinued)
ISSN: 1876-5025 ― Volume 5, 2014
Physical Characterization of Drug Loaded Microcapsules and Controlled In Vitro Release Study
S Jaya*, T.D Durance, R Wang
Abstract
Microencapsulation of model drug, acetylsalicylic acid into bio-based polymer, alginate- pectin matrix and chitosan had been undertaken in this work to characterize the microcapsules based on their composition. Alginate-pectin with the proportion of 40:60 solution was prepared with 0.3 g of drug. This mix was homogenized and atomized using nitrogen gas into 1.0M calcium chloride solution to form sol-gel microcapsules. 0.3 g concentration of the drug solution was prepared with 2% glacial acetic acid and 2 g of chitosan was mixed into that and homogenized using sonicator. Microcapsules were prepared after crosslinking the drug encapsulated chitosan using glutaraldehyde. Drug loaded microcapsules were dried using microwave energy under vacuum at low temperature. Scanning electron microscopy graphs showed that microcapsules have porous and rough surfaces. Fourier Transform Infrared Spectroscopy analysis on the microcapsules confirmed the presence of drug in the polymer matrix. X- ray diffraction pattern showed that the microstructure was more like an amorphous pattern. Drug release of the microcapsules was tested in three different pH levels of 1.2, 7.4 and 8.2. Slow and controlled release of drug was observed at all the pH levels. Over all, the drug release percentage was higher in acidic pH and lower in alkali pH.
Article Information
Identifiers and Pagination:
Year: 2010Volume: 2
First Page: 9
Last Page: 17
Publisher Id: TOBIOMTJ-2-9
DOI: 10.2174/1876502501002010009
Article History:
Received Date: 21/11/2008Revision Received Date: 21/8/2009
Acceptance Date: 22/8/2009
Electronic publication date: 25/3/2010
Collection year: 2010
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http: //creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the University of British Columbia, 2205, East Mall, Vancouver BC, V6T 1Z4, Canada; Tel: 1 (604) 822- 4707; Fax: 1 (604) 822 5143; E-mail: jsundaram3@gmail.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 21-11-2008 |
Original Manuscript | Physical Characterization of Drug Loaded Microcapsules and Controlled In Vitro Release Study | |
INTRODUCTION
Natural polymers provide great advantages in biomedical applications such as drug delivery and tissue engineering (scaffolding). They are biocompatible, non toxic and biodegradable materials. Also, they have good potential to incorporate drugs, enzymes, bioactive components etc. [1, 2]. Alginate has been used by many researchers for controlled delivery of incorporated materials [3-5]. Alginate is a naturally occurring polysaccharide obtained from marine brown algae. It is composed of linear copolymers of 1, 4-linked (-D mannuronic acid and (-L gulcuronic acid. It gels in the presence of divalent cation like calcium. Microspheres produced using alginate has been used for the encapsulation of wide varieties of bioactive materials, proteins, enzymes, micronutrients, antibodies etc. [6-8]. It was proved that calcium-alginate microsphere encapsulated with insulin by extrusion process gave 65% encapsulation efficiency [9]. Bio-adhesive nature of alginate with mucosal membrane will help to get intimate contact between intestinal mucosa and lower sized microspheres [5, 10, 11].
Pectin is another polymer (polysaccharide) found in the cell wall of plants. It has high molecular weight heteropolymers containing a 65% (by weight) of D- galacturonic acid units which is joined to one another in chains by means of α(1 → 4) glycosidic linkages. Galacturonic acid is structurally similar to gulcuronic and mannuronic acids of alginate [12]. Crosslinking of pectin with calcium ions gives gel formation, as in the case of alginate [13-15]. Microcapsules containing only alginate, cross-lined with calcium may not be sufficient to give better drug encapsulation. Therefore, incorporating low methoxy pectin in the polymer matrix is expected to increase the efficiency of encapsulation [16].
Chitosan is a cationic linear polysaccharide. It is non toxic and biodegradable. It is β (1→4) linked biopolymer composed of 2-amino-2-deoxy-β-D glucan combined by glycosidic linkages. Chitosan consists of large number of amine groups which are corresponds with the interaction between chitosan and many other substances. Chitosan gives unique functional, nutritional, and biomedical properties [17-19]. Chitosan has been used for many biomedical and pharmaceutical applications to improve drug delivery as well as for controlled delivery [20, 21]. In case of drug delivery applications chitosan has been employed for preparation of drug loaded microcapsules/microspheres and are used to provide controlled drug release and improve bioavailability of the drugs [22]. Cross linking of chitosan with glutaraldehyde gives three dimensional network and increases the internal surface area for absorption. Glutaraldehyde has been commonly used in many cross linking process of chitosan. In general aldehyde groups are highly active and readily form Schiff bases with amino groups [23].
Several oncology-related experimental studies showed that non steroidal anti-inflammatory drugs (NSAIDS) could be used as cancer chemo-preventive agents [24]. It is soluble in both water and alcohol. Long term use of aspirin, which is metabolized into salicylic acid, has been shown to reduce the risk of colon, breast, prostate, lung and skin cancer [24]. It enables better for prevention and treatment. Research conducted on aspirin, related to cardiovascular disease,
proved that it reduced the risk of cardiovascular diseases for women, when it was used as pain relief medicine [25]. Anti-inflammatory nature of salicylates reduces tumour cell proliferation by inhibiting thymine dimer formation required for DNA replication [26, 27]. Other recent research literatures said that the use of salicylates could prevent skin cancer due to UV exposure in addition to its well known activity as a keratolytic agent [28,29].
Encapsulation techniques have been used extensively to entrap drugs and bioactive compounds and control their release into the gastrointestinal tract [1]. Several techniques were developed to produce encapsulated microspheres. Drug delivery system developed with microsphere might increases the life span of the active ingredients encapsulated inside and control the release. Because of its small size, that have large surface to volume ratio, which is very much suitable for controlled delivery. Microencapsulation offers many advantages that include increased stability, prolonged in vivo half-life, reduction of possible adverse side effects, concentration of the drug resulting in lower required doses, and ease of administration. Many techniques are available for micro encapsulation such as spray drying, spray cooling, extrusion, freeze-drying, co-crystallization etc. In this study, spraying and ultrasonic waves were used to make encapsulation and application of microwave energy under vacuum was attempted to dry the microcapsules. The objectives of this study are:
- to develop a stable gel polymer matrix consisting of alginate-pectin and chitosan microcapsules to encapsulate the selected drug, acetyl salicylic acid (aspirin);
- to characterize the microcapsules using scanning electron microscopy (SEM), X-ray diffraction and Fourier Transform Infra Red Spectroscopy (FTIR) techniques;
- to study the In vitro drug release characterization of the microcapsules at different pH levels of releasing media.
MATERIALS AND METHODS
Materials
Samples of sodium alginate (TICA- algin HG 400) and Pectin (LM 35) in powdered form were received from TIC GUMS, USA. Acetyl salicylic acid (aspirin), chitosan (powder), glutaraldehyde, acetic acid and calcium chloride were purchased from Sigma (USA). Sodium phosphate monobasic (NaH2PO4), Sodium Phosphate Dibasic (Na2HPO4), Sodium Chloride (NaCl), Potassium Chloride (KCl), Potassium Phosphate Monobasic (KH2PO4) and Hydrochloric acid (HCl) were purchased from Fisher chemicals, Canada.
Preparation of Drug Encapsulated Microspheres
Preparation of drug encapsulated microcapsule has been given in Fig. (1a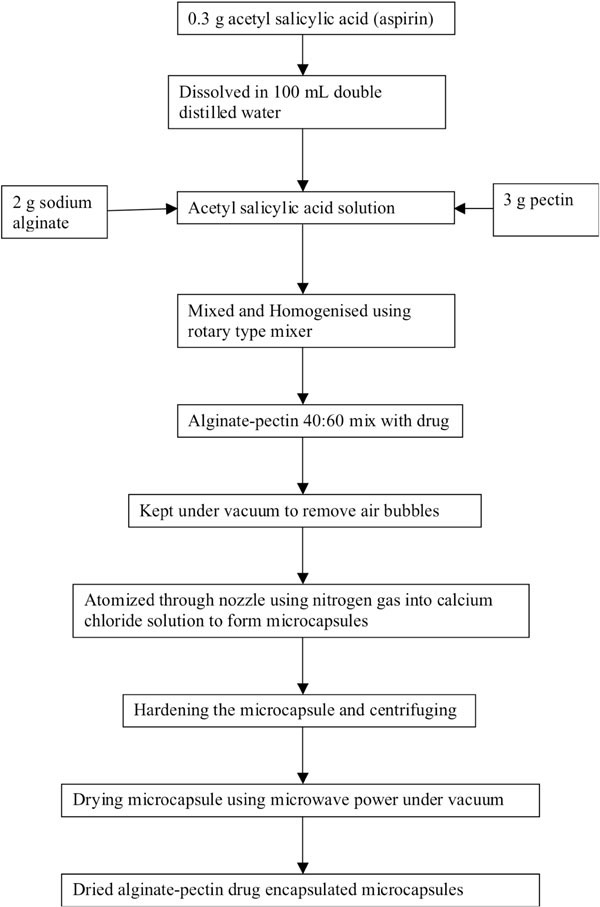 , b
, b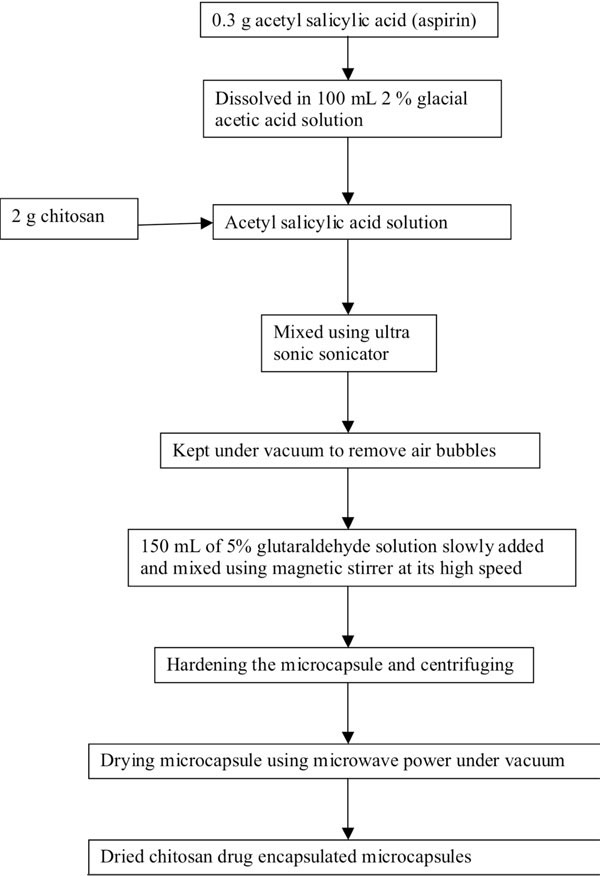 ) as process flow diagrams for alginate-pectin and chitosan respectively. About 0.3 g of acetyl salicylic acid was dissolved in 100 mL double distilled water. After the complete dissolution, 2 g of sodium alginate and 3 g of pectin were mixed and homogenized thoroughly to get alginate-pectin composition of 40:60 on dry basis using rotary type laboratory mixer (Ultra Turrax, T25 basis; IKA Labor technic.). To get chitosan drug loaded microcapsules, about 0.3g of acetyl salicylic acid (aspirin) was dissolved in 100 mL 2% glacial acetic acid solution. After the complete dissolution, 2 g of chitosan was mixed thoroughly using ultrasonic wave sonicator to get encapsulated drug emulsion with chitosan. After mixing the polymer-drug mixture was kept under vacuum to remove all the entrapped air bubbles. Then alginate- pectin-drug mix was atomized through nozzle using nitrogen gas at a pressure of 300 kPa into gently agitated 1.0M calcium chloride solution to ensure covalent cross link among calcium chloride ion, alginate and pectin. It forms discrete aspirin encapsulated microcapsules upon contact with calcium chloride solution. After completing the atomization process, encapsulated particles were allowed to stay inside the calcium chloride solution for 3-4 hours to harden the encapsulated and gelled microcapsules. Into the chitosan- drug emulsion mix 150 mL of 5% glutaraldehyde solution was poured slowly. Glutaraldehyde was acted as a cross linker for chitosan. While pouring, the emulsion mix was stirred using magnetic stirrer at its maximum speed. After pouring all the glutaraldehyde solution the whole mix was continuously stirred for 24 hours to harden the encapsulated and gelled microcapsules. It forms discrete aspirin encapsulated microcapsules upon contact with glutaraldehyde solution. Then the microcapsules were harvested by centrifuging the solution at 5000 rpm for 10 minutes and washed thoroughly using distilled water. Finally the micro-capsules were dried using microwave vacuum drying method.
) as process flow diagrams for alginate-pectin and chitosan respectively. About 0.3 g of acetyl salicylic acid was dissolved in 100 mL double distilled water. After the complete dissolution, 2 g of sodium alginate and 3 g of pectin were mixed and homogenized thoroughly to get alginate-pectin composition of 40:60 on dry basis using rotary type laboratory mixer (Ultra Turrax, T25 basis; IKA Labor technic.). To get chitosan drug loaded microcapsules, about 0.3g of acetyl salicylic acid (aspirin) was dissolved in 100 mL 2% glacial acetic acid solution. After the complete dissolution, 2 g of chitosan was mixed thoroughly using ultrasonic wave sonicator to get encapsulated drug emulsion with chitosan. After mixing the polymer-drug mixture was kept under vacuum to remove all the entrapped air bubbles. Then alginate- pectin-drug mix was atomized through nozzle using nitrogen gas at a pressure of 300 kPa into gently agitated 1.0M calcium chloride solution to ensure covalent cross link among calcium chloride ion, alginate and pectin. It forms discrete aspirin encapsulated microcapsules upon contact with calcium chloride solution. After completing the atomization process, encapsulated particles were allowed to stay inside the calcium chloride solution for 3-4 hours to harden the encapsulated and gelled microcapsules. Into the chitosan- drug emulsion mix 150 mL of 5% glutaraldehyde solution was poured slowly. Glutaraldehyde was acted as a cross linker for chitosan. While pouring, the emulsion mix was stirred using magnetic stirrer at its maximum speed. After pouring all the glutaraldehyde solution the whole mix was continuously stirred for 24 hours to harden the encapsulated and gelled microcapsules. It forms discrete aspirin encapsulated microcapsules upon contact with glutaraldehyde solution. Then the microcapsules were harvested by centrifuging the solution at 5000 rpm for 10 minutes and washed thoroughly using distilled water. Finally the micro-capsules were dried using microwave vacuum drying method.
 |
Fig. (1a) Process flow diagram of alginate-pectin microcapsule preparation. |
 |
Fig. (1b) Process flow diagram of chitosan microcapsule preparation. |
Microwave Vacuum Drying
Drying of microcapsules was conducted at 300 watts microwave power in a vacuum microwave dehydrator (Model 1.8, EnWave Corporation, Vancouver, Canada). About 30 g of drug encapsulated microcapsules were spread evenly in a glass Petri dish of diameter 14 cm. This was placed inside the drying chamber. The absolute pressure maintained during the process was 3.3 kPa. After creating this vacuum level, microwave energy was supplied to the sample to assist the drying process. Drying was continued until the sample reached less than 1-2 % (w.b) moisture content. In between, the drying process was stopped and the microcapsules were mixed gently to get even drying. The temperature was maintained below 40oC throughout the drying process.
Morphology Analysis of Dry Microcapsule
Scanning electron microscopic(SEM) graphs of dried microcapsules were obtained using Hitachi S4700 Field emission SEM (Japan) for morphology analysis. The dried sample was placed in an air tight desiccator, which had silica gel to remove moisture. The sample was kept in this condition until they attained a constant weight. Small amount of dried microcapsules were dispersed into alcohol to separate individual particle on previously fixed glass plates on iron stub. The alcohol was allowed to evaporate and then the microcapsules were made electrically conductive by coating, in a vacuum, with a thin layer of gold for 40 s. Images were obtained at an excitation voltage of 20 kV at different magnifications varying from 350 to 6000.
X-Ray Diffraction Analysis
X-ray diffraction patterns of dried alginate-pectin and chitosan microcapsules were obtained at room temperature using a Rigaku Multiflex powder diffractometer (CuKα( radiation generator) operated at a voltage of 40 kV. Finely ground microcapsules were analyzed in two theta angle range of 6-60 and the process parameters were set as: scan step size of 0.02 (2(θ) and scan step time of 0.05 s. It was found that there were no peaks below and above the angles 6 and 60 respectively. So this range was selected for scanning. Same way pure alginate, pectin, chitosan and aspirin samples were also analyzed.
FTIR Spectroscopy Analysis
FTIR spectra of dried alginate-pectin and chitosan microcapsules were obtained using a FTIR spectrometer (Perkin Elmer 2000 Infrared Spectrophotometer). Dried microcapsules and pure raw materials were previously ground and mixed thoroughly with potassium bromide at 1:5 (sample: KBr) ratio, respectively. The transparent KBr discs were prepared by compressing the powders, under the force of 2.8-3 MPa for 3-5 min in a hydraulic press. Fifty scans were obtained at a resolution of 2 cm-1 from 4000 – 400 cm-1 wave number.
Drug Release Study
Drug release pattern of each alginate-pectin and chitosan microcapsules were studied at three different pH levels to identify where it could release maximum amount of drug. The aim of this work at this point was focused on to get maximum release. Therefore the buffers with 1.2 (stimulating the gastric pH), 8.2 (stimulating the intestinal pH) and 7.4 pH (neutral pH) levels were prepared to create different release conditions. For pH 1.2: 0.1 M HCl buffer, pH 7.4: PBS (phosphate buffer saline) and pH 8.2: phosphate buffer were used. 0.5 g of dried microcapsules of each composition was taken in 100 mL beaker. About 50 mL of respective buffer solution was added to each of the capsules. The drug release was carried at 37oC for all the buffers. After soaking the microcapsules in respective buffer solutions, at every 30 minutes, 1mL of sample was withdrawn from each and it was replaced with 1mL of respective buffer solutions. The samples were taken for up to 6 hours. To each 1mL of the samples, 10 mL of 1N sodium hydroxide was added. This mixture was heated just to boil and then cooled before reading for the aspirin amount that released from each microcapsules composition. The concentration of aspirin in the sample was read using UV spectrophotometer at 293nm. Each drug release study was repeated for 5 times and the average was presented in the result.
RESULTS AND DISCUSSION
Morphology Analysis of Dried Microcapsule
Fig. (2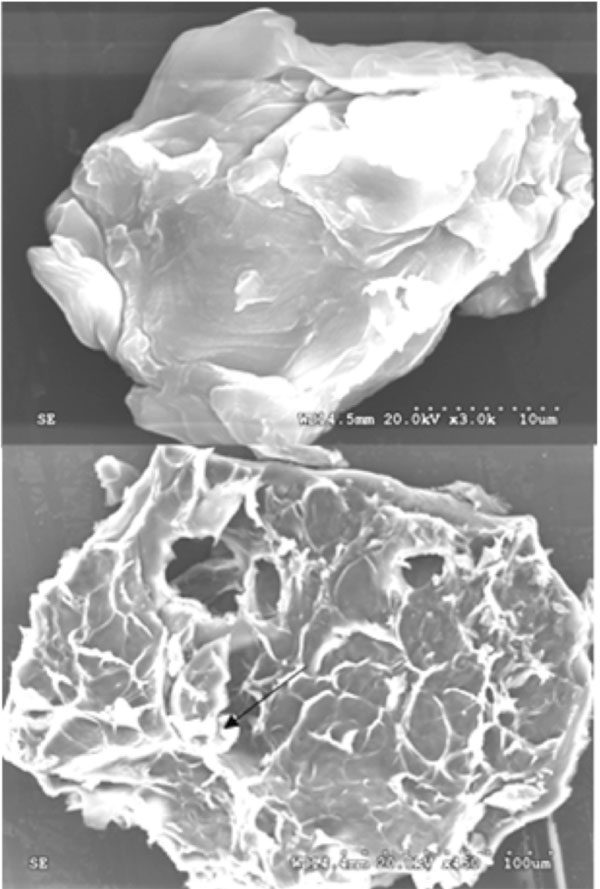 ) shows the morphology of acetyl salicylic acid encapsulated dried alginate – pectin microcapsule. This figure shows both the surface (upper figure) and inner view (bottom figure) of the microcapsule at x3.0k and x450 magnification respectively. It can be observed that inner view shows the encapsulated drug crystals present inside the microcapsule and one such crystal has been shown by arrow mark in the figure. Also it shows that the internal structure of the microcapsule is porous and the pores are interconnected. This kind of structure is helpful for the drug delivery. The surfaces of the microcapsules (upper figure) are appeared to be rough and the particles are not in perfect spherical shape. Pectin contains other neutral sugars of xylose, galactose and arbinose in the structural side chains. These structures lead the pectin molecules with smooth hairy regions. This nature of the pectin molecular structure might be the reason for getting elongated shape microcapsules instead of spherical shape.
) shows the morphology of acetyl salicylic acid encapsulated dried alginate – pectin microcapsule. This figure shows both the surface (upper figure) and inner view (bottom figure) of the microcapsule at x3.0k and x450 magnification respectively. It can be observed that inner view shows the encapsulated drug crystals present inside the microcapsule and one such crystal has been shown by arrow mark in the figure. Also it shows that the internal structure of the microcapsule is porous and the pores are interconnected. This kind of structure is helpful for the drug delivery. The surfaces of the microcapsules (upper figure) are appeared to be rough and the particles are not in perfect spherical shape. Pectin contains other neutral sugars of xylose, galactose and arbinose in the structural side chains. These structures lead the pectin molecules with smooth hairy regions. This nature of the pectin molecular structure might be the reason for getting elongated shape microcapsules instead of spherical shape.
 |
Fig. (2) Scanning electron microscopic view of drug loaded Alginate-pectin (40:60) microcapsule. |
Fig. (3 ) shows a sample of SEM micrographs of acetyl salicylic acid encapsulated dried chitosan microcapsules. These micrographs were obtained to investigate the surface morphology of the microcapsules. Chitosan microcapsules were prepared in the size ranges 40 -100 micrometers. Both upper and bottom figure show the surface features of the chitosan microcapsules at 1.5k and 600 magnifications. The surfaces of the microcapsules are appeared to be rough. Also they are not in perfect spherical shape like alginate-pectin microcapsules. Sphericity and surface smoothness of chitosan capsules might be affected by stirring rate of the dispersion medium during cross linking and mixing during the drying process to obtain uniform drying. In general covalently cross-linked chitosan gives porous structure [30]. Due to this porous nature they are considered for drug delivery by means of drug diffusion. When aqueous solution diffuses into interior of the microcapsules by capillary, the drug might be dissolved and released though the same path. Presence of drug crystals inside the chitosan microcapsules could not be confirmed from this surface morphology. However, cross section of the alginate-pectin microcapsule shows the presence of drug crystals of 1- 3 micron in Fig. (1
) shows a sample of SEM micrographs of acetyl salicylic acid encapsulated dried chitosan microcapsules. These micrographs were obtained to investigate the surface morphology of the microcapsules. Chitosan microcapsules were prepared in the size ranges 40 -100 micrometers. Both upper and bottom figure show the surface features of the chitosan microcapsules at 1.5k and 600 magnifications. The surfaces of the microcapsules are appeared to be rough. Also they are not in perfect spherical shape like alginate-pectin microcapsules. Sphericity and surface smoothness of chitosan capsules might be affected by stirring rate of the dispersion medium during cross linking and mixing during the drying process to obtain uniform drying. In general covalently cross-linked chitosan gives porous structure [30]. Due to this porous nature they are considered for drug delivery by means of drug diffusion. When aqueous solution diffuses into interior of the microcapsules by capillary, the drug might be dissolved and released though the same path. Presence of drug crystals inside the chitosan microcapsules could not be confirmed from this surface morphology. However, cross section of the alginate-pectin microcapsule shows the presence of drug crystals of 1- 3 micron in Fig. (1 , bottom) with arrow mark. All the microcapsules both chitosan and alginate-pectin exhibit a folded structure, wrinkle type of crevices and crack like porous structure on its surface. Since the sphericity of microcapsules was lost during gelation and drying, microcapsules with holes could be formed; it could not seen by SEM. This may allows the encapsulated drug to leak.
, bottom) with arrow mark. All the microcapsules both chitosan and alginate-pectin exhibit a folded structure, wrinkle type of crevices and crack like porous structure on its surface. Since the sphericity of microcapsules was lost during gelation and drying, microcapsules with holes could be formed; it could not seen by SEM. This may allows the encapsulated drug to leak.
 |
Fig. (3) Scanning electron microscopic view of drug loaded chitosan microcapsule. |
X-Ray Diffraction Analysis
Fig. (4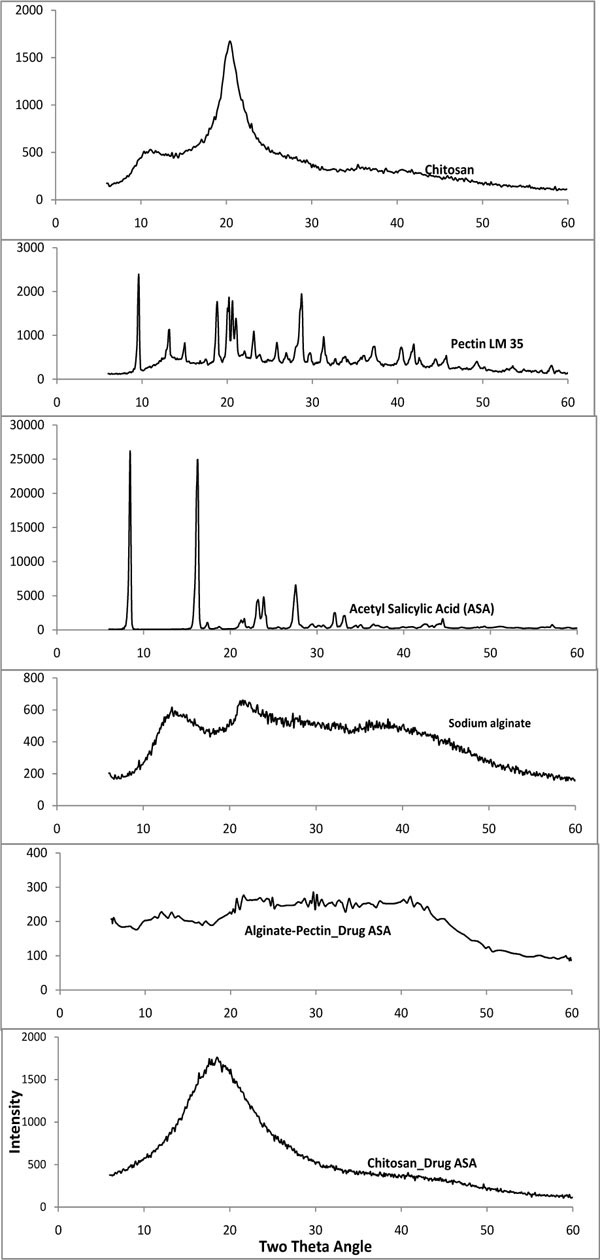 ) shows the X- ray diffraction pattern of drug encapsulated microcapsules with alginate – pectin and chitosan also pure pectin, alginate, chitosan and acetyl salicylic acid. Drug loaded microcapsules show the mix of crystalline and amorphous pattern. Pure sodium alginate has crystalline peaks at 13 and 21o two theta angle. Pure chitosan has characteristic peaks at 20o two theta angle. Pure aspirin has strong peaks at 8o and 16o two theta angles. However, the microcapsules with both chitosan and alginate-pectin show more like amorphous pattern than like crystalline. Alginate-pectin patterns show the crystallinity of pectin but there is a marked reduction compared to the parent pectin peaks. Similarly salicylic acid also shows reduction in crystallinity. Characteristic peak of chitosan became wider. Since the percentage of chitosan was very high compared to the drug percentage encapsulated, the crystalline nature of the drug was diminished in the X-ray diffraction pattern. However the presence of drug was proved by conducting FT-IR analysis. Overall the microcapsules showed the amorphous nature after processing. This might reduce the stability of the drug. Further in depth study on this regard is necessary.
) shows the X- ray diffraction pattern of drug encapsulated microcapsules with alginate – pectin and chitosan also pure pectin, alginate, chitosan and acetyl salicylic acid. Drug loaded microcapsules show the mix of crystalline and amorphous pattern. Pure sodium alginate has crystalline peaks at 13 and 21o two theta angle. Pure chitosan has characteristic peaks at 20o two theta angle. Pure aspirin has strong peaks at 8o and 16o two theta angles. However, the microcapsules with both chitosan and alginate-pectin show more like amorphous pattern than like crystalline. Alginate-pectin patterns show the crystallinity of pectin but there is a marked reduction compared to the parent pectin peaks. Similarly salicylic acid also shows reduction in crystallinity. Characteristic peak of chitosan became wider. Since the percentage of chitosan was very high compared to the drug percentage encapsulated, the crystalline nature of the drug was diminished in the X-ray diffraction pattern. However the presence of drug was proved by conducting FT-IR analysis. Overall the microcapsules showed the amorphous nature after processing. This might reduce the stability of the drug. Further in depth study on this regard is necessary.
 |
Fig. (4) X-ray diffraction analysis of drug loaded chitosan and alginate-pectin microcapsules. |
FTIR Spectroscopy Analysis
Fig. (5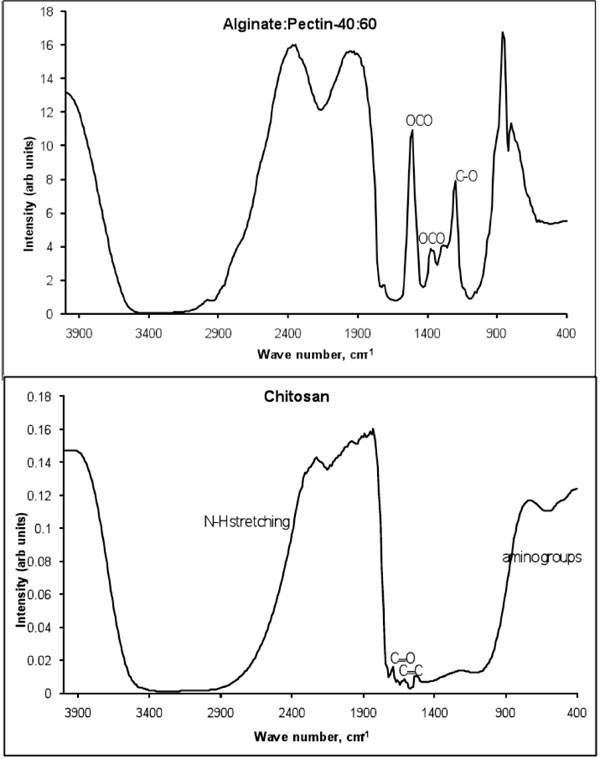 ) shows the FTIR spectroscopy spectrum of drug loaded alginate-pectin and chitosan microcapsules. The bands at 1620 and 1420 cm-1 present in the IR spectrum of sodium alginate are assigned to symmetric and symmetric stretching peaks of carboxylate salt groups. In addition, the bands around 1320 cm-1 (C–O stretching), 1120 cm-1 (C–C stretching), 1090 cm-1 (C–O stretching), 1020 cm-1 (C–O–C stretching), and 950 cm-1 (C–O stretching) are attributed to its saccharide structure [31]. In the FTIR spectrum of pectin, bands related to C-O stretching of carboxyl group could be observed at 1619 cm-1 [32]. FTIR spectrum of drug loaded microsphere confirms the presence of acetyl salicylic acid. Several strong vibrations are presented at 1770 (C=O stretch), 1600 (C=C stretch), 1580 (OCO anti symmetric stretch), 1400 (OCO symmetric stretch) and 1238, 1212, and 1184 cm-1 (C-O and C-C stretching modes) in the spectrum of the pure aspirin. Similar peaks are appeared in the spectrum of drug loaded-microcapsules.
) shows the FTIR spectroscopy spectrum of drug loaded alginate-pectin and chitosan microcapsules. The bands at 1620 and 1420 cm-1 present in the IR spectrum of sodium alginate are assigned to symmetric and symmetric stretching peaks of carboxylate salt groups. In addition, the bands around 1320 cm-1 (C–O stretching), 1120 cm-1 (C–C stretching), 1090 cm-1 (C–O stretching), 1020 cm-1 (C–O–C stretching), and 950 cm-1 (C–O stretching) are attributed to its saccharide structure [31]. In the FTIR spectrum of pectin, bands related to C-O stretching of carboxyl group could be observed at 1619 cm-1 [32]. FTIR spectrum of drug loaded microsphere confirms the presence of acetyl salicylic acid. Several strong vibrations are presented at 1770 (C=O stretch), 1600 (C=C stretch), 1580 (OCO anti symmetric stretch), 1400 (OCO symmetric stretch) and 1238, 1212, and 1184 cm-1 (C-O and C-C stretching modes) in the spectrum of the pure aspirin. Similar peaks are appeared in the spectrum of drug loaded-microcapsules.
 |
Fig. (5) FTIR spectra of drug leaded chitosan and alginate-pectin microcapsules. |
The main characteristic bands of chitosan amine groups are at 1540 cm-1 and the carbonyl groups are at 1650 cm-1. Amines are bases, and their corresponding conjugate acid "onium" salts are often formed. These derivatives show strong, broad N-H stretching absorptions in the 2250 to 3000 cm-1 region. The aldehyde groups form covalent imine bonds with the amino groups of chitosan via a Schiff reaction. The broad band at 3500-3400 cm-1 was due to the stretching vibration of –NH2 and –OH groups. Also –NH2 (scissoring) N-H (wagging) of amine groups present in the chitosan are occurred at 1550-1650 cm-1 and 900-660 cm-1. The bands at 1100-1000 cm-1 are due to the saccharide structure of the chitosan. The aldehyde bond of C=O is appeared at 1700 cm-1. Also the broad absorption band between 4000-3000 cm-1 is obtained due to the glutaraldehyde cross linking solution. Several strong vibrations at 1600 (C=C stretch), 1580 (OCO anti symmetric stretch), 1210-1320cm-1 (O-C) and 1184 cm-1 (C-C stretching modes) are the characteristics spectrum of the pure aspirin.
Drug Release Study
Fig. (6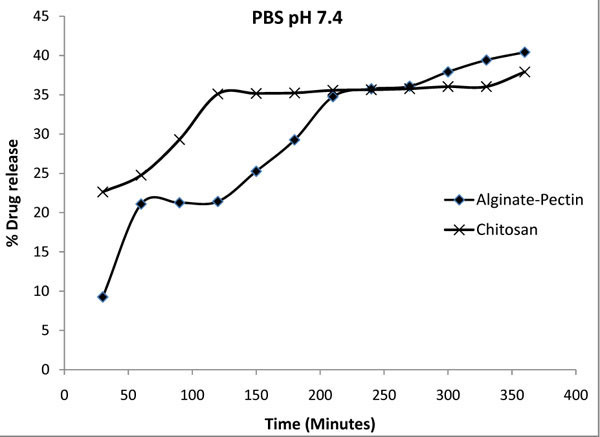 ) shows the percentage of drug released from the chitosan and alginate pectin microcapsules at pH 7.4 in PBS buffer. The data in this figure representing the average of the release performed for 5 times. The standard deviations obtained for the alginate-pectin and chitosan release study were 0.42 and 0.26 respectively. It shows that maximum release of 40% was obtained from the alginate pectin and 38 % from chitosan microcapsules. In general both the microcapsules showed controlled release of drug and it increased gradually with the time.
) shows the percentage of drug released from the chitosan and alginate pectin microcapsules at pH 7.4 in PBS buffer. The data in this figure representing the average of the release performed for 5 times. The standard deviations obtained for the alginate-pectin and chitosan release study were 0.42 and 0.26 respectively. It shows that maximum release of 40% was obtained from the alginate pectin and 38 % from chitosan microcapsules. In general both the microcapsules showed controlled release of drug and it increased gradually with the time.
 |
Fig. (6) Drug release profile of alginate-pectin and chitosan microcapsules in PBS solution at pH 7.4. |
Fig. (7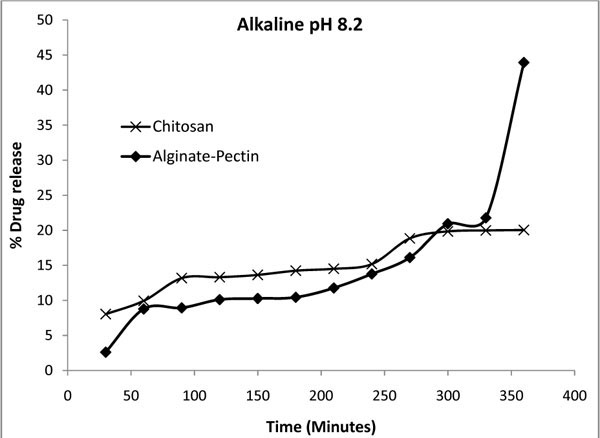 ) shows the percentage of drug released from the microcapsules at pH 8.2 (intestinal pH) in phosphate buffer. The standard deviations of five times repeated release study were 0.51 and 0.36 for the alginate-pectin and chitosan microcapsules respectively. Alginate pectin combination shows 44 % of drug released in 360 minutes, where as chitosan shows only 20 %. The overall drug release process was influenced by the physical and mechanical properties of the gel barrier that formed around the capsules. Increased in amount of pectin caused the gel barrier poor and increased the drug release percentage. To get more control over the drug delivery the current polymer matrix could be improved in such way by increasing its gelation time and then the gel strength. Overall, compared to pH 7.4 the percentage of drug released in alkaline pH is less for chitosan and more for alginate-pectin microcapsules. It showed that the chitosan has strong gel barrier against alkaline pH. Fig. (7) also shows that the release profile of chitosan microcapsule in alkaline pH was very irregular. In practical the drug release from covalently cross linked chitosan is controlled by cross-linking density. Higher the density, lower the release rate and vice versa. Inter chains formed during cross-linking and gave more interactions and inhibited swelling. Thus, cross-linked chitosan exhibits pH sensitive nature for its swelling and drug release. Poor drug release profile could also be obtained if most of the amino groups involved in the cross-linking. When the chitosan microcapsules were soaked in the alkali pH there might be a chance of further cross-linking of amino groups present in the chitosan. This might be the reason for lower drug release rate at alkaline pH.
) shows the percentage of drug released from the microcapsules at pH 8.2 (intestinal pH) in phosphate buffer. The standard deviations of five times repeated release study were 0.51 and 0.36 for the alginate-pectin and chitosan microcapsules respectively. Alginate pectin combination shows 44 % of drug released in 360 minutes, where as chitosan shows only 20 %. The overall drug release process was influenced by the physical and mechanical properties of the gel barrier that formed around the capsules. Increased in amount of pectin caused the gel barrier poor and increased the drug release percentage. To get more control over the drug delivery the current polymer matrix could be improved in such way by increasing its gelation time and then the gel strength. Overall, compared to pH 7.4 the percentage of drug released in alkaline pH is less for chitosan and more for alginate-pectin microcapsules. It showed that the chitosan has strong gel barrier against alkaline pH. Fig. (7) also shows that the release profile of chitosan microcapsule in alkaline pH was very irregular. In practical the drug release from covalently cross linked chitosan is controlled by cross-linking density. Higher the density, lower the release rate and vice versa. Inter chains formed during cross-linking and gave more interactions and inhibited swelling. Thus, cross-linked chitosan exhibits pH sensitive nature for its swelling and drug release. Poor drug release profile could also be obtained if most of the amino groups involved in the cross-linking. When the chitosan microcapsules were soaked in the alkali pH there might be a chance of further cross-linking of amino groups present in the chitosan. This might be the reason for lower drug release rate at alkaline pH.
 |
Fig. (7) Drug release profile of alginate-pectin and chitosan microcapsules in alkaline solution at pH 8.2. |
Fig. (8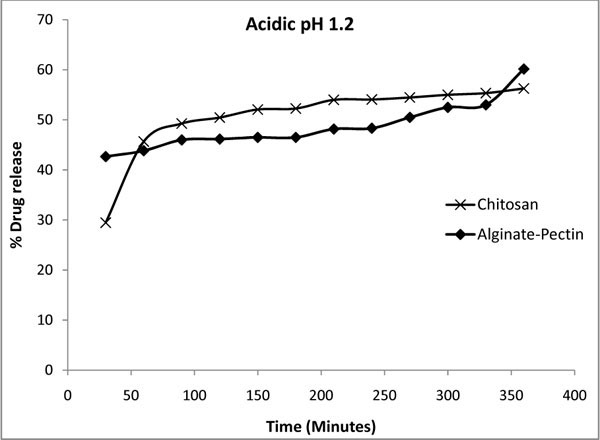 ) shows the percentage of drug released from microcapsules at pH 1.2 (gastric pH) in 0.1 M HCl buffer. The data plotted were an average of 5 sets of release obtained with standard deviation of 0.23 and 0.57 for the alginate-pectin and chitosan microcapsules respectively. Here the maximum 60 % of drug release was obtained for alginate: pectin and 56 % for chitosan microcapsule. Both the microcapsules showed more release percentage in acidic pH than in alkaline and neutral pH. This result is inconsistent with the results of slower release from alginate-pectin capsules in acidic solution than in alkaline reported by Liu and Krishnan [33] and Bodmeier and Paeratakul [34]. However Østberg and others [35] reported that faster release in acidic solution, which is similar to this report. This kind of variation could be possible due to the difference in chemical composition of commercially available alginate and pectin in terms of guluronic/mannuronic acid ratios and the level of free acid groups. In general the drug release process was influenced by the physical and mechanical properties of the gel barrier that formed around the capsules and its interaction with the drug. Stronger the interaction slower the drug release. At neutral and alkaline pH, drug release from hard capsules containing alginate-pectin was controlled by the formation of a hydrated viscous layer around the capsules, which acted as a barrier to drug release by opposing the penetration of water into the matrices and the movement of dissolved solutes out of the matrices. Chitosan and its formulations have the property to float and swell gradually in acid medium [36]. Therefore, at acidic pH the chitosan microcapsules had more swelling than in alkali pH, and released more drugs. If the drug delivery system has sensitive swelling in an acidic environment it controls the delivery and can able to make the system more useful to deliver in the gastrointestinal track. It could be possible by changing the cross linking density by introducing additional polymer in the inter chain between the chitosan and cross-linker. The results suggested that the structure of the capsules in different pH levels was varying each other to give different release percentage. The percentage of drug released from the microcapsules was dependent on the summative effect of the physicochemical properties of the drug and the composition of microcapsule matrix.
) shows the percentage of drug released from microcapsules at pH 1.2 (gastric pH) in 0.1 M HCl buffer. The data plotted were an average of 5 sets of release obtained with standard deviation of 0.23 and 0.57 for the alginate-pectin and chitosan microcapsules respectively. Here the maximum 60 % of drug release was obtained for alginate: pectin and 56 % for chitosan microcapsule. Both the microcapsules showed more release percentage in acidic pH than in alkaline and neutral pH. This result is inconsistent with the results of slower release from alginate-pectin capsules in acidic solution than in alkaline reported by Liu and Krishnan [33] and Bodmeier and Paeratakul [34]. However Østberg and others [35] reported that faster release in acidic solution, which is similar to this report. This kind of variation could be possible due to the difference in chemical composition of commercially available alginate and pectin in terms of guluronic/mannuronic acid ratios and the level of free acid groups. In general the drug release process was influenced by the physical and mechanical properties of the gel barrier that formed around the capsules and its interaction with the drug. Stronger the interaction slower the drug release. At neutral and alkaline pH, drug release from hard capsules containing alginate-pectin was controlled by the formation of a hydrated viscous layer around the capsules, which acted as a barrier to drug release by opposing the penetration of water into the matrices and the movement of dissolved solutes out of the matrices. Chitosan and its formulations have the property to float and swell gradually in acid medium [36]. Therefore, at acidic pH the chitosan microcapsules had more swelling than in alkali pH, and released more drugs. If the drug delivery system has sensitive swelling in an acidic environment it controls the delivery and can able to make the system more useful to deliver in the gastrointestinal track. It could be possible by changing the cross linking density by introducing additional polymer in the inter chain between the chitosan and cross-linker. The results suggested that the structure of the capsules in different pH levels was varying each other to give different release percentage. The percentage of drug released from the microcapsules was dependent on the summative effect of the physicochemical properties of the drug and the composition of microcapsule matrix.
 |
Fig. (8) Drug release profile of alginate-pectin and chitosan microcapsules in acidic solution at pH 1.2. |
CONCLUSIONS
Alginate–pectin and chitosan microcapsules were successfully prepared using internal gelation method. The drug release was affected by release medium condition as well as the properties of the polymer, polymer cross-linking density and drug. This work showed that more drug release was obtained for acidic pH environment. The contribution of each release mechanism to the overall drug release process was influenced by the physical and mechanical properties of the gel barrier that formed around the capsules. Morphology of alginate-pectin and chitosan microcapsules showed folded structure, wrinkle type of crevices and crack like porous structure on its surface. Micrographs confirmed the presence of drug crystals. FTIR spectrum result also confirmed the same. Overall, the molecular structures of the microcapsules were amorphous. In this study, controlled release of drug was achieved up to 360 minutes by the gradual increase from time to time for three pH levels. The structure of the microcapsules in different pH levels was varying each other to give different drug release percentage. Based on the above results, it may be concluded that alginate-pectin is more suitable for targeted drug release at intestine than chitosan. Over all alginate-pectin gave more release percentage in all tested release medium compared to chitosan.
ACKNOWLEDGEMENT
The financial support provided by Natural Science and Engineering Research Council of Canada (NSERC) for conducting this research is gratefully acknowledged.
