- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Condensed Matter Physics Journal
(Discontinued)
ISSN: 1874-1924 ― Volume 4, 2013
Anomalous Structural Change of Layered Perovskite Manganites La2-2x Sr1+2xMn2O7
Aihua Wang1, 2, Tao Liu3, Yang Liu4, Guohui Cao1, 4, Cheng Dong5, Ning Chen1, 4, Zhaosheng Feng 6, Yang Li*, 7
Abstract
The temperature-dependent structural changes were investigated by using the X-ray diffraction and the Extended X-ray absorption fine spectroscopy (EXAFS) for layered perovskite manganites La2-2xSr1+2xMn2O7 (x=0.33 and 0.4). The anomalous changes of Mn-O bond length and Jahn-Teller (J-T) distortion of MnO6 octahedron were observed, corresponding to ferromagnetic transition temperature (~ 120 K). Around the temperature of 2D short-range magnetic ordering (~ 235 K), there occurs an anomaly of the Jahn-Teller distortions of MnO6. These results indicate that magnetic properties of perovskite manganites La2-2xSr1+2xMn2O7 depend heavily on their microstructure change. The orbital state occupancy is involved in the observed Mn-O bond change.
Article Information
Identifiers and Pagination:
Year: 2009Volume: 2
First Page: 19
Last Page: 24
Publisher Id: TOCMPJ-2-19
DOI: 10.2174/1874186X00902010019
Article History:
Received Date: 8/3/2008Revision Received Date: 8/4/2009
Acceptance Date: 9/4/2009
Electronic publication date: 5/5/2009
Collection year: 2009
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Department of Physics, Beijing University of Science and Technology, Beijing 100083, China; E-mail: ylibp@hotmail.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 8-3-2008 |
Original Manuscript | Anomalous Structural Change of Layered Perovskite Manganites La2-2x Sr1+2xMn2O7 | |
INTRODUCTION
Perovskite manganites Ln1-xAxMnO3 (Ln=La, Nd, Pr; A=Ca, Sr, Ba, Pb) have attracted much attention [1] since the discovery of a colossal magnetoresistance (CMR) effect [2, 3]. The CMR effect of metallic manganites around ferromagnetic transition was generally explained by the double-exchange (DE) model [4]. After investigating magnetic properties of CMR manganites, however, Millis et al., pointed out that some striking characteristics exhibited by perovskite manganites could not be simply explained by the DE model completely [5]. For instance, the ferromagnetic transition temperature (TC) calculated theoretically under the DE model is much higher than the observed value in experiment. Moreover, the resistivity above TCpredicted by the double-exchange theory is significantly smaller than the experimental values by several orders of magnitude. In order to explain this discrepancy, Millis suggested that the electron-phonon interaction induced by the Jahn-Teller (J-T) effect should be taken into account (see ref. [5]). Such electron-phonon interaction would cause the localization of carrier electrons and further reduction of TC. Under this model, thetheoretical TC is even close to the observed value in experiment. Apparently, the study on the J-T effect induced by change of local lattice structure is significant in these manganese materials.
It has been widely accepted that the MnO6 octahedron, as the basic structural constitution in perovskite manganites, can be distorted and modified by substitution for rare earth atoms in Ln1-xAxMnO3 and Ln2-2xA1+2xMn2O7. The distortion of MnO6 depends on elements A and doping concentration x. Moreover, the dynamic structure studies [6] also show that there is a large difference in temperature-dependent lattice distortion for different doping concentrations of x [7, 8]. For the bilayered manganites Ln2-2xA1+2xMn2O7, anisotropy in structure determines distinct characteristics in magnetic properties. Measurements of magnetocaloric properties for the Mn-O bilayer perovskite reveal a broad peak of magnetic entropy change around TC [9]. The layered structure should bias the crystal field and hence lift the degenerate eg orbital [7]. Clearly, the study of the J-T effect corresponding to the local structure will provide very helpful information for understanding peculiarity of CMR.
For La2-2xSr1+2xMn2O7 system, in the light Sr-doping level, Campbell et al. found that a system of strongly interacting electron-lattice polarons exhibited charge and orbital order at sufficiently high polaron concentrations in La1.2Sr1.8 Mn2O7 [10]. In their study, the structure of short-range polaron correlations in the layered colossal magnetoresistive perovskite manganite La1.2Sr1.8Mn2O7 has been determined by a crystallographic analysis of broad satellite maxima observed in diffuse x-ray and neutron-scattering data. The resulting q ~(0.3,0,±1) modulation is a longitudinal octahedral-stretch mode.
Hur’s group [11] perform measurements of susceptibility and resistivity in order to investigate the charge-ordering behavior in of the layered manganites La2-2xSr1+2xMn2O7 in a wide doping range (0.3 ≤ x ≤ 0.8). The charge-ordering transitions occur in 200 – 340 K and the re-entrant charge-ordering behaviors are exhibited in a doping range (0.47 ≤ x ≤ 0.62).
In addition Wikins et al. investigated La2-2xSr1+2xMn2O7 with heavy Sr-doping level x=0.475 and 0.5 [12]. The x-ray scattering results showed the charge and orbital ordering in the bilayer manganite. By using high-energy x-ray scattering, the structural distortion due to the Jahn-Teller ordering and the charge ordering due to the Mn3+/Mn4+ pattern have been measured. Both the x=0.5 and x=0.475 samples are found to display charge and Jahn-Teller order.
In the heavy doping range 0.54 < x < 0.80, Qiu et al. [13] observed the Jahn-Teller distorted MnO6 octahedra at low temperature by applying atomic pair distribution function (PDF) analysis of neutron powder diffraction data. Their results indicated a gradual change of the local structure with doping rather than an abrupt phase transition as seen in the average structure. The number of J-T distorted octahedral varies smoothly with doping.
The extended X-ray absorption fine spectroscopy (EXAFS) technique which is sensitive to the fine differences in local structure and the short-range ordering [14] is the most desirable to be applied to study the atomic structure of CMR perovskite manganites. The local structure changes with temperature have been investigated for perovskite manganites La2/xCa1/3MnO3 and La0.75Ca0.25MnO3 [15, 16]. Variations of the Mn-O bond length and the octahedral distortion of MnO6 occur near their TC. At low temperatures, six Mn-O bonds of MnO6 with the same length turn into three pairs of Mn-O bonds with different lengths, as the temperature increases above TC. These results indicate that the magnetic properties depend on local structure changes in CMR perovskite manganites. Unlike the ABO3 structure, bilayered CMR manganites show a large anisotropy in structure, which dominates unique magnetic behavior. The Jahn-Teller effect accompanied by local change in structure cannot be simply neglected. It is very significant to study thermal evolution of the local structure of these materials.
In this work, the temperature-dependent local structure of Mn for La2-2xSr1+2xMn2O7 (x = 0.33 and 0.4) was investigated by X-ray diffraction and Mn K-edge extended X-ray absorption fine spectroscopy (EXAFS). We selected x = 0.33 and 0.4 samples because their huge CMR signal in these doping concentrations. In addition, for La2-2xSr1+2xMn2O7 system, in the doping range 0.3-0.4, there is no charge ordering (see ref. [11]). Moreover, in this bilayer system, the presence of rock salt blocker layer has the effect of reducing the dimensionality, and in the x=0.4 doped system there exists a paramagnetic-to-ferromagnetic transition at 126 K with huge accompanying change in CMR [11, 17]. In our experiments, we observed an abnormal variation in term of lattice parameter, bond length of Mn-O, and the distortion ΔJT of MnO6 around ferromagnetic transition temperature. The anomalous behaviors reflect variation of orbital dependent occupancy in the two-dimensional conduction band.
EXPERIMENT
Samples of La2-2xSr1+2xMn2O7 (x =0.33, 0.4) were synthesized using the solid-state reaction. Powders of La2-2xSr1+2xMn2O7 were prepared by the sol-gel method. The precursors of nitrates La(NO3)3, Sr(NO3)2 and Mn(NO3)3 were mixed according to the nominal composition of La2-2xSr1+2xMn2O7, then dissolved into pure water and an equal weight of citric acids was added. Stirring the mixture for 24 hours then baking at 120˚C until the liquid-like samples became gel. Heated the gel at 700˚C until nitrates were decomposed and citric acids were removed. The powders were ground and pressed into pellets. A subsequent heat treatment was performed at 1380˚C in air for 5 hours, and then cooled down to room temperature. The pellets were ground to fine powders, pressed again into pellets, and then sintered at the same temperature, the same procedure was repeated 4 times in order to obtain the homogeneous samples.
The as-prepared samples were characterized by X-ray diffraction (Rigaku, Dmax/RC) in the temperature range from 80K to 300K. All patterns show a pure tetragonal Sr3Ti2O7-type phase (space group of I4/mmm, No. 139) in samples. The lattice parameters were analyzed by the PowderX software package [18]. Structural refinement of the powder X-ray diffraction data was carried out using the GSAS software package. The experimental pattern at room temperature is in agreement with the simulated one for the entire 2θ region. R values for the fit are Rwp = 0.03 and Rp = 0.01 for x = 0.33 sample, and Rwp = 0.04 and Rp = 0.02 for x = 0.40 sample.
Magnetization versus temperature of samples was measured in the warming run with a field of 100 Oe after cooling down 5K in zero field using a superconducting quantum interference device (SQUID) magnetometer.
EXAFS data was collected on the 1W1B beam line at the Beijing Synchrotron Radiation Facility (BSRF) by using a Si(111) double-crystal monochromator and ion-chamber as detectors. The storage ring was operated at 2.2 GeV and the injection current was between 80 to 100 mA. In the temperature range 80 - 300K, the Mn K-edge data was collected in transmission mode. La2-2xSr1+2xMn2O7 samples were ground into fine powders with 400 mesh. WinXAS software package [16] was used for data analysis.
RESULTS AND DISCUSSIONS
The lattice parameters a and c of La2-2xSr1+2xMn2O7 (x = 0.33 and 0.4) are presented in Fig. (1a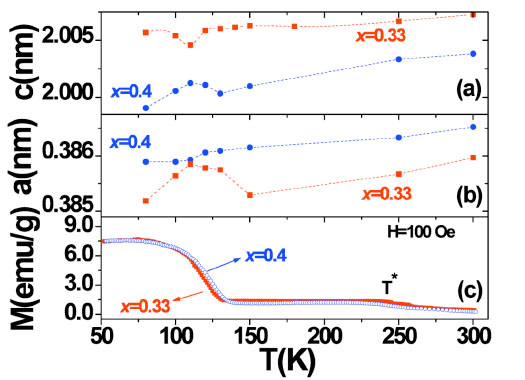 and b
and b ) as a function of temperature T. For both samples, a and c gradually decrease with decreasing temperature at a high temperature range (T > 150K). There occurs an anomalous jump or heave in a and c around the Curie temperature TC. In order to contrast directly temperatures of structural anomaly and magnetic transition, temperature dependent magnetization under H=100 Oe is plotted in Fig. (1c
) as a function of temperature T. For both samples, a and c gradually decrease with decreasing temperature at a high temperature range (T > 150K). There occurs an anomalous jump or heave in a and c around the Curie temperature TC. In order to contrast directly temperatures of structural anomaly and magnetic transition, temperature dependent magnetization under H=100 Oe is plotted in Fig. (1c ). The Curie temperature TC (defined as the temperature of maximum slope in dM/dT) was found to be 118K for x = 0.33 and 125K for x = 0.4, respectively. The temperature width of ferromagnetic transition is about 40K. Comparing with the behavior of single crystals [17, 19], such broadening of magnetic transition for our polycrystalline La2-2xSr1+2xMn2O7 samples is wider than that of the single crystals [20, 21]. We observed an abnormal variation in term of lattice parameter around ferromagnetic transition temperature. The anomalous behaviors reflect variation of orbital dependent occupancy in the two-dimensional conduction band.
). The Curie temperature TC (defined as the temperature of maximum slope in dM/dT) was found to be 118K for x = 0.33 and 125K for x = 0.4, respectively. The temperature width of ferromagnetic transition is about 40K. Comparing with the behavior of single crystals [17, 19], such broadening of magnetic transition for our polycrystalline La2-2xSr1+2xMn2O7 samples is wider than that of the single crystals [20, 21]. We observed an abnormal variation in term of lattice parameter around ferromagnetic transition temperature. The anomalous behaviors reflect variation of orbital dependent occupancy in the two-dimensional conduction band.
As shown in Fig. (1 ), the lower Sr doping sample (x = 0.33) shows a larger lattice parameter c and smaller lattice parameter a, which implies that two samples have different two-dimensional structural characteristic attributing to Sr doping. In addition, samples with x = 0.33 and x = 0.4 exhibit different anomalous behavior in lattice change around TC. For the sample of x = 0.33, the maximum of a occurs at T = 110 K, and at the same temperature, c reaches its minimum. Unlike x = 0.33, lattice parameter c of sample x = 0.4 shows a maximum at 110 K. There is no significant change for lattice parameter c but a kind of a shoulder appearing at 110 K. Such two samples have different lattice change in trend, which could be accounted for by the different microstructures. As discussed below, the lattice distortions depends on the Sr concentration. Neutron powder diffraction experiments on layered manganites La2-2xSr1+2xMn2O7 (0.32 ≤ x ≤ 0.40) also show that coherent lattice anomalies are observed at the Curie temperature [14]. These behaviors are different from samples with heavy doping level, in which the charge-ordering and antiferromagnetic ordering occur [14]. The magnetocrystalline anisotropy is heavily dependent on the crystallographic structure and composition [7, 22]. It has been widely accepted that the magnetic peculiarity of La2-2xSr1+2xMn2O7 results from structural and magnetic anisotropy [9].
), the lower Sr doping sample (x = 0.33) shows a larger lattice parameter c and smaller lattice parameter a, which implies that two samples have different two-dimensional structural characteristic attributing to Sr doping. In addition, samples with x = 0.33 and x = 0.4 exhibit different anomalous behavior in lattice change around TC. For the sample of x = 0.33, the maximum of a occurs at T = 110 K, and at the same temperature, c reaches its minimum. Unlike x = 0.33, lattice parameter c of sample x = 0.4 shows a maximum at 110 K. There is no significant change for lattice parameter c but a kind of a shoulder appearing at 110 K. Such two samples have different lattice change in trend, which could be accounted for by the different microstructures. As discussed below, the lattice distortions depends on the Sr concentration. Neutron powder diffraction experiments on layered manganites La2-2xSr1+2xMn2O7 (0.32 ≤ x ≤ 0.40) also show that coherent lattice anomalies are observed at the Curie temperature [14]. These behaviors are different from samples with heavy doping level, in which the charge-ordering and antiferromagnetic ordering occur [14]. The magnetocrystalline anisotropy is heavily dependent on the crystallographic structure and composition [7, 22]. It has been widely accepted that the magnetic peculiarity of La2-2xSr1+2xMn2O7 results from structural and magnetic anisotropy [9].
By comparing with the ABO3 type perovskite manganites, there are different magnetic characteristics for La2-2xSr1+2xMn2O7 with double Mn-O layers. First, TC (~120 K) of a bilayered manganite is much lower than that of the ABO3 type manganites with the same doping element and concentration. For instance, TC is about 300 K for La1-xSrxMnO3 (x = 0.3) [22].
As shown in Fig. (1 ), a similar trend of magnetization was observed for the two samples. From room temperature, the magnetization increases slowly with increasing temperature. At about 235 K, there occurs a small plateau of magnetization, which is labeled as T* in Fig. (1
), a similar trend of magnetization was observed for the two samples. From room temperature, the magnetization increases slowly with increasing temperature. At about 235 K, there occurs a small plateau of magnetization, which is labeled as T* in Fig. (1 ). The plateau of the M-T curves can be explained by the appearance of two dimensional (2D) short-range magnetic ordering in the temperature range between TC and T* [23, 24]. With decreasing temperature, three-dimensional long-range magnetic order occurs, leading to a sharp increase of magnetization at TC. A similar phenomenon was previously observed in La2-2xSr1+2xMn2O7 (x= 0.3) [23] and (x = 0.3, 0.35, 0.4) [24].
). The plateau of the M-T curves can be explained by the appearance of two dimensional (2D) short-range magnetic ordering in the temperature range between TC and T* [23, 24]. With decreasing temperature, three-dimensional long-range magnetic order occurs, leading to a sharp increase of magnetization at TC. A similar phenomenon was previously observed in La2-2xSr1+2xMn2O7 (x= 0.3) [23] and (x = 0.3, 0.35, 0.4) [24].
Beside the change in lattice parameters, we are also interested in the change of micro-structure such as the Mn-O bond length around T*, corresponding to 2D short-range magnetic ordering. In order to investigate change of Mn-O local structure further, Mn K-edge extended X-ray absorption fine spectroscopy (EXAFS) was performed. In our distribution model in fitting procedure, Sr occupies La position. We set an average occupancy of 0.33 and 0.4 respectively. We did not give Sr a preferable site to replace the La atom. The absorption spectra were normalized by the pre-edge fit with a linear function and the post-edge fit with a second polynomial. The EXAFS oscillations χ(k) were multiplied by a scaling factor k3, where photoelectron wave vector k ranged from 2.5 to 12.0 Å-1. The radial distribution function of Mn was obtained by the Fourier transform (FT) for k3χ(k). Several Fourier transformed spectra at selected temperatures are shown in Fig. (2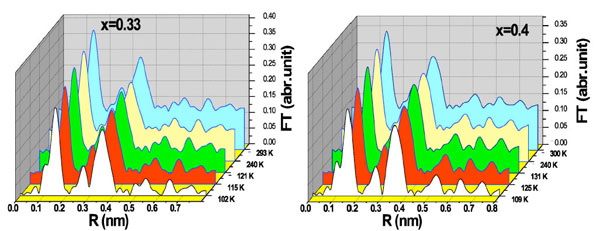 ). According to the structural characteristic of La2-2xSr1+2xMn2O7, the first peak at 0.12 - 0.18 nm in Fig. (2
). According to the structural characteristic of La2-2xSr1+2xMn2O7, the first peak at 0.12 - 0.18 nm in Fig. (2 ) corresponds to the first neighbor coordination of Mn, which forms the MnO6 octahedron as shown in Fig. (3
) corresponds to the first neighbor coordination of Mn, which forms the MnO6 octahedron as shown in Fig. (3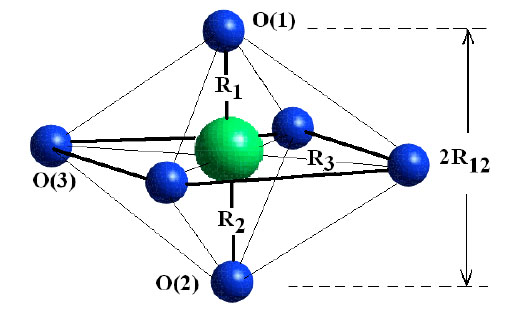 ). The second broad peak locates about 0.24 - 0.28 nm corresponding to Mn-(La/Sr) and Mn-O-Mn pairs. The third big peak around 0.35 - 0.40 nm are assigned with contribution of the second neighbor O atoms in the a-b plane.
). The second broad peak locates about 0.24 - 0.28 nm corresponding to Mn-(La/Sr) and Mn-O-Mn pairs. The third big peak around 0.35 - 0.40 nm are assigned with contribution of the second neighbor O atoms in the a-b plane.
 |
Fig. (2) Magnitude of the Fourier transform, |FT k3χ(k) | at different temperature for x = 0.33 and x = 0.4 samples. |
The MnO6 octahedron as the basic structural constitution in perovskite manganites is shown in Fig. (3 ). Mn has six O atoms as its first neighbors to form three kinds of Mn-O bonds; A) Mn-O(1) (upper apical oxygen) with bond length R1, B) Mn-O(2) (bottom apical oxygen) with bond length R2 along the c axis, and C) a-b planar Mn-O(3) with bond length R3. The EXAFS oscillations k3χ(k) from the first coordination shell of Mn-O were filtered out and fit by using scattering amplitudes and phase-shifts extracted from the standard MnO2. The a-b planar bond length R3 of Mn-O(3) was fitted with coordination number 4. In order to decrease analysis error for Mn-O bond length along the c axis, the average bond length of R1 and R2, i.e. R12=(R1+R2)/2, was fitted with a fixed coordination number 2.
). Mn has six O atoms as its first neighbors to form three kinds of Mn-O bonds; A) Mn-O(1) (upper apical oxygen) with bond length R1, B) Mn-O(2) (bottom apical oxygen) with bond length R2 along the c axis, and C) a-b planar Mn-O(3) with bond length R3. The EXAFS oscillations k3χ(k) from the first coordination shell of Mn-O were filtered out and fit by using scattering amplitudes and phase-shifts extracted from the standard MnO2. The a-b planar bond length R3 of Mn-O(3) was fitted with coordination number 4. In order to decrease analysis error for Mn-O bond length along the c axis, the average bond length of R1 and R2, i.e. R12=(R1+R2)/2, was fitted with a fixed coordination number 2.
In Fig. (4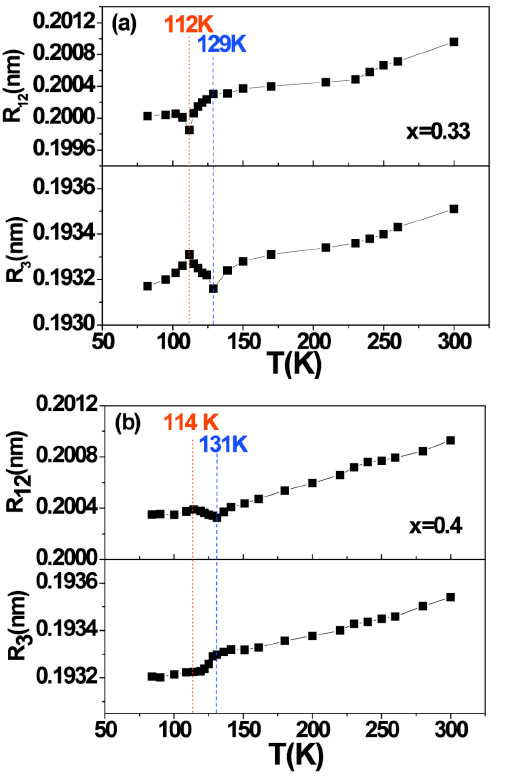 ), the EXAFS analysis display details of local structure change. The temperature dependence of the Mn-O bond length, R3 and R12 are shown in Fig. (4
), the EXAFS analysis display details of local structure change. The temperature dependence of the Mn-O bond length, R3 and R12 are shown in Fig. (4 ). Both samples have a similar Mn-O bond length in the a-b plane. However the x = 0.33 sample as a whole has a larger Mn-O bond length in the direction of c-axis. As shown in Fig. (4
). Both samples have a similar Mn-O bond length in the a-b plane. However the x = 0.33 sample as a whole has a larger Mn-O bond length in the direction of c-axis. As shown in Fig. (4 ), both samples show an anomalous change of bond length within the ferromagnetic transition temperature range. It is worth mentioning that bond lengths in the a-b plane and out of the a-b plane exhibit opposite changes for x = 0.33 and x = 0.4.
), both samples show an anomalous change of bond length within the ferromagnetic transition temperature range. It is worth mentioning that bond lengths in the a-b plane and out of the a-b plane exhibit opposite changes for x = 0.33 and x = 0.4.
For the sample of x = 0.33, in the a-b plane, R3 decreases when the temperature decreases, and reaches its minimum at 129K. With further decreasing temperature, R3 anomalously increases and has a maximum at 112K. This change trend is consistent with the changing of the lattice parameter a as shown in Fig. (1 ). In the same temperature range, R12 vertical to the a-b plane exhibits a differently anomalous change; there respectively occurs a minimum at 112 K and a shoulder at 129 K. R12 curvilinear trend is very similar to the lattice parameter c. It is clear that lattice change with temperature depends on the change of Mn-O bond length. Within ferromagnetic transition temperature range, the anomalous changes of R3 and R12 results in a distortion of the MnO6 octahedron. The distortion of MnO6 behaviors anisotropic. For instance, at 112K, MnO6 is compressed along the c-axis and tensioned in the a-b plane.
). In the same temperature range, R12 vertical to the a-b plane exhibits a differently anomalous change; there respectively occurs a minimum at 112 K and a shoulder at 129 K. R12 curvilinear trend is very similar to the lattice parameter c. It is clear that lattice change with temperature depends on the change of Mn-O bond length. Within ferromagnetic transition temperature range, the anomalous changes of R3 and R12 results in a distortion of the MnO6 octahedron. The distortion of MnO6 behaviors anisotropic. For instance, at 112K, MnO6 is compressed along the c-axis and tensioned in the a-b plane.
For the sample of x = 0.4, the variation of the two Mn-O bond lengths R3 and R12 are shown in Fig. (4b ). The temperature corresponding to anomalous change of R3 and R12 shift to a higher temperature by 2K when compared to the sample of x = 0.33 due to its higher TC. In addition, the anomalous trend is different from that of the x = 0.33 sample. For example, there is a R12 maximum and a smaller R3 at T = 114K. The MnO6 octahedron distortion was driven by a tension stress along the c direction and a compressive stress in the a-b plane. The MnO6 octahedron distortions in two samples (x=0.33 and 0.4) have different directions, which implies Sr doping concentration would play an important role. It has been accepted that the proportion of Mn3+/Mn4+ is changed with doping concentration x for bilayered manganite La2-2xSr1+2xMn2O7. Mn4+ component increases when the Sr2+ ion concentration x increases. The difference in the anomalous behaviour in their two samples could be attributed to the different concentration ratio of Mn3+O6vs Mn4+O6 octahedra. Clearly, there are more Mn3+O6 octahedrons in sample of the x = 0.33, while Mn4+O6 certainly manifold in the sample of x = 0.4.
). The temperature corresponding to anomalous change of R3 and R12 shift to a higher temperature by 2K when compared to the sample of x = 0.33 due to its higher TC. In addition, the anomalous trend is different from that of the x = 0.33 sample. For example, there is a R12 maximum and a smaller R3 at T = 114K. The MnO6 octahedron distortion was driven by a tension stress along the c direction and a compressive stress in the a-b plane. The MnO6 octahedron distortions in two samples (x=0.33 and 0.4) have different directions, which implies Sr doping concentration would play an important role. It has been accepted that the proportion of Mn3+/Mn4+ is changed with doping concentration x for bilayered manganite La2-2xSr1+2xMn2O7. Mn4+ component increases when the Sr2+ ion concentration x increases. The difference in the anomalous behaviour in their two samples could be attributed to the different concentration ratio of Mn3+O6vs Mn4+O6 octahedra. Clearly, there are more Mn3+O6 octahedrons in sample of the x = 0.33, while Mn4+O6 certainly manifold in the sample of x = 0.4.
Considering the Mn electronic structure, eg electron occupancy of conduction band 3dx2 - y2 and 3d3z2 - r2 is primarily governed by the doping level x [7]. The lower Sr-doping sample is with a larger out-of-plane Mn-O length and with lower hole concentration x. The different layered structure should bias the crystal field and hence lift the degeneracy of the eg orbital states [9]. The different lattice distortion can be understood in terms of the eg orbital state occupancy. Considering the different MnO6 distortions in samples of x = 0.33 and 0.40, the orbital state occupancy of the doped electrons prefer to occupy the 3d3z2 - r2 band for x = 0.33, while electrons dominantly occupy 3dx2 - y2 band for x = 0.4.
In addition, as shown in Fig. (4 ) there is a perceptible change of R3 and R12 corresponding to the 2D short-range magnetic ordering temperature T*, which attributes to a slight MnO6 octahedron distortion.
) there is a perceptible change of R3 and R12 corresponding to the 2D short-range magnetic ordering temperature T*, which attributes to a slight MnO6 octahedron distortion.
In order to highlight the local structure change and to discuss conveniently the Jahn-Teller effect in La2-2xSr1+2xMn2O7, the J-T distortion ΔJT of x = 0.33 and x = 0.4 samples at different temperatures are shown in Fig. (5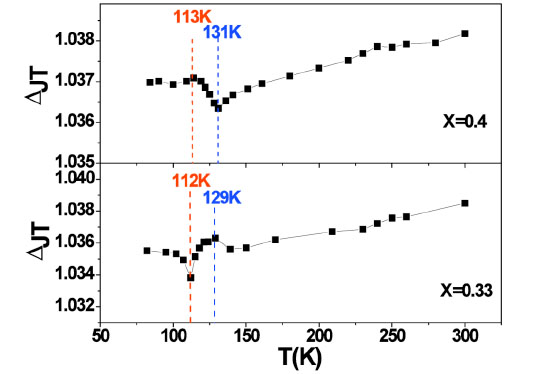 ). The J-T distortion is defined as the ratio of bond length out of the a-b plane and in the a-b plane, ΔJT=(R1+R2)/2R3 [11]. Both samples show their anomalous behavior in J-T distortion ΔJT around TC. Particularly the opposite change trend of J-T distortion ΔJT for x = 0.33 and 0.4 implies that the eg orbital state occupancy is involved in the observed Mn-O bond change.
). The J-T distortion is defined as the ratio of bond length out of the a-b plane and in the a-b plane, ΔJT=(R1+R2)/2R3 [11]. Both samples show their anomalous behavior in J-T distortion ΔJT around TC. Particularly the opposite change trend of J-T distortion ΔJT for x = 0.33 and 0.4 implies that the eg orbital state occupancy is involved in the observed Mn-O bond change.
 |
Fig. (5) Temperature dependence of the value of MnO6 Jahn-Teller distortion ΔJT for x = 0.33 and x = 0.4 samples. |
In summary, we performed XRD and EXAFS measurements to investigate the temperature dependence of local structure change in the bilayered manganite La2-2xSr1+2x Mn2O7 (x = 0.33 and x = 0.4). We observed an abnormal variation in term of lattice a (and c), bond length of Mn-O, and the distortion ΔJT of MnO6 at ferromagnetic transition temperature. Moreover, there is a detectable anomalous change in Mn-O bond length and J-T distortion around 2D short-range ferromagnetic ordering temperature T*. The different change trend of Mn-O reflects the orbital state occupancy in the 2D conduction band for different Sr doping.
ACKNOWLEDGEMENTS
This work was supported by the Beijing Natural Science Foundation (Grant No. 1072007), National Natural Science Foundation of China (Grant No.50372005) and National Science Foundation (Grant No. DMR-0821284).



