- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Process Chemistry Journal
(Discontinued)
ISSN: 1875-1806 ― Volume 6, 2014
A Novel Liquid-Liquid Microextraction Based on Solidification of Floating Organic Droplet Method for Determination of Phenols in Aqueous Samples
H Faraji1, *, M Mirzaee 2, F Sorkheh 2, M Helalizadeh 2, A.A Tabrizi 2
Abstract
Pre-concentration and determination of 7 phenolic compounds in water samples has been achieved using a new liquid-liquid microextraction coupled HPLC-DAD. Microextraction efficiency factors have been investigated and optimized: 14 µL 1-undecanol micro drop exposed for 15 min floated on surface of a 20 mL water sample at 55°C, stirred at 1200 rpm, low pH level and saturated salt conditions. Under the selected conditions, limit of detection of 0.5- 3.0 µg L-1 (S/N=3) and linearity range of 0.3-870 µg L-1 have been obtained. A reasonable repeatability (RSD ≤ 10.1%, n=5) with satisfactory linearity (0.9992 ≥ r2 ≥ 0.9973) of results illustrated a good performance of the present method. The relative recovery of different natural water samples was higher than 84%.
Article Information
Identifiers and Pagination:
Year: 2010Volume: 3
First Page: 1
Last Page: 6
Publisher Id: TOCPCJ-3-1
DOI: 10.2174/1875180601003010001
Article History:
Received Date: 31/8/2009Revision Received Date: 12/10/2009
Acceptance Date: 10/11/2009
Electronic publication date: 15/1/2010
Collection year: 2010
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the No 14, Mahdieh 5, Varamin, Iran; P-Code: 3371786837; Tel: +98 292 222 6954; Fax: +98 292 222 4767; E-mail: hakimfaraji@yahoo.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 31-8-2009 |
Original Manuscript | A Novel Liquid-Liquid Microextraction Based on Solidification of Floating Organic Droplet Method for Determination of Phenols in Aqueous Samples | |
1. INTRODUCTION
Phenolic compounds are important water pollutions which are subject to legislation because of their toxicity, even at low concentration. A European Community (EC) directive specifies a legal tolerance level of 0.1 µg L-1 for each phenolic compounds and 0.5 µg L-1 for the sum of all compounds in water intended for human consumption [1, 2]. Phenols are usually determined by many analytical approaches such as high-performance liquid chromatography (HPLC) [3-5], capillary gas chromatography (GC) [6], spectrophotometery [7] and electrochemical methods [8]. GC has been widely used for the analysis of phenols, usually with a derivatization step [9, 10]. However, derivatization increases the sample preparation time and introduces a possible source of errors.
Despite technological advances in instrumentation of chemical analysis, the resultant sensitivities are limited. Anyway the determination of phenolic compounds usually requires sample pre-concentration through liquid-liquid extraction (LLE) [11] or solid-phase extraction (SPE) [12, 13]. These methods are time-consuming and are required large amounts of solvents. Solid phase microextraction (SPME) [14, 15], headspace solid-phase microextraction (HSPME) [16] and stir bar sorptive extraction (SBSE) [17, 18] are solvent-free and fast, however, they are expensive and their sorbents are fragile. Further, they have limited lifetime and also sample carry-over can be a problem. Jeannot and Cantwell developed liquid-liquid microextraction (LLME) system in which extraction was achieved into a single drop [19]. Zhao and Lee reported liquid-phase microextraction (LPME) for extraction of phenols [20].
Recently, a simple, quick and inexpensive LPME sample preparation method has been developed for extraction of analytes from water samples. This technique is based on distribution of the analytes between micro liters volume of the extraction solvent (floated on the surface of the aqueous sample) and the aqueous sample matrix [21].
The goal of this study was to assess the technique suitability for the detection of a group of the phenolic compounds in water samples. The analytes were monitored by liquid chromatography combined with diode array detector. The influence of different experimental parameters on the yield of the sample preparation step is described and discussed. In the end, this recommended method was employed to investigate the levels of the target species in several water samples.
2. EXPERIMENTAL
2.1. Reagents
The phenolic compounds include phenol (PN), 3-chlorophenol (3CP), 2,4-dimethylphenol (24DMP), 2,4-dichlorophenol (24DCP), 2,4,6-trichlorophenol (246TCP), 2,3,4,6-tetrachlorophenol (2346TeCP) and pentachlorophenol (PCP) were obtained from Merck (Darmstadt, Germany). Standard solutions (2000 mg L-1) from each individual compounds were prepared in methanol. The organic solvents (HPLC-grade or suprasolv for chromatography), sodium chloride and potassium chloride (analytical reagent grade) were purchased from Merck (Darmstadt, Germany) and Fluka (Buchs, Switzerland). Ultrapure water was prepared by a Milli-Q system (Bedford, MA, USA). Individual stock standard solution of 1000 mg L-1 for each compound was prepared in methanol and stored at -20 °C. Stock standard mixture of the phenolic compounds was weekly prepared by diluting the stock solutions with methanol. The working standard solutions were daily made by diluting the mixed stock solutions with Milli-Q water to the required concentration. A pH 2 buffer was prepared using 25 mL of 0.2 M KCl and 6.5 mL of 0.2 M HCl in 100 mL of water, and saturated salt solutions were prepared with NaCl.
Tap water sample was freshly collected from our laboratory and mineral water was obtained from supermarket. The tap and mineral water samples were collected in glass bottles. The tap water sample was filtered before the analysis by a 0.45 µm membrane filter (MSI, Westboro, MA, USA). The water samples were stored in refrigerator at 4 °C until their analysis.
2.2. Apparatus
The residual analysis was carried out using an Agilent 1100 HPLC equipped with a manual injector and diode array detector. A Zorbax Eclipse XDB-C18 column (150 mm ( 4.6 mm, 5 µm particle sizes) was used and all injections were performed manually with 20.0 µL sample loop. The operating conditions were as follows: mobile phase, methanol/water, 95:5 v/v; flow rate, 0.8 mL/min; column temperature, 25 ± 1°C; and the wavelength of detector, 220 nm. Screw cap glass test tubes (10 mL) with conical bottoms (used as extraction vessels) were heated at 500 °C in a furnace (model CWF 1200, Carbolite, UK) to remove any organic compounds. Stirring the solution was carried out with a magnetic heater-stirrer (Heidolph MR 3001K, Germany). A simple water bath placed on the heater-stirrer was for controlling the temperature of the sample solutions.
2.3. Extraction Procedure
Extractions were carried out according to the following procedure: (1) a 20-mL aqueous solution containing the phenols was added into the sample vial with a 8 mm × 4 mm magnetic stirring bar; (2) the sample vial was put on a MR 3001K hot plate stirrer (Heidolph, Germany); (3) desired volume of the organic solvent was placed on the surface of the solution using a 25 µL Hamilton syringe (Bondaduz, Switzerland); (4) the vial was sealed and the stirrer turned on; (5) after the extraction, sample vial was transferred into an ice beaker, and the organic solvent was solidified nearly 5 min afterwards; (6) the solidified organic solvent was transferred into the conical vial by a simple spatula where, it started to melt; (7) the whole melted solvent was injected into the HPLC for quantification.
3. RESULTS AND DISCUSSION
3.1. Selection of Extraction Solvent
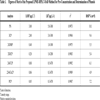
The selection of a proper extraction solvent is of great importance for the optimization of the LPME process. To choose an appropriate organic solvent, the following points should be considered. Firstly, the chosen solvent should illustrate a high boiling point and a low vapor pressure in order to reduce the risk of evaporation [22]. Secondly, it should exhibit a good chromatographic behavior [23] and, thirdly, the partitioning coefficient of the analyte should be high. Furthermore, the solvent must have a good affinity for the target compounds [24] and, finally, it should demonstrate a melting point near the room temperature (in the range of 10–30 °C) [21]. According to these considerations, several extracting solvents, including 1-undecanol, 1-dodecanol, 2-dodecanol and n-hexadecane were considered. Among the tested extracting solvents, 1-undecanol presented the best extraction efficiency, while its chromatographic peak was easily separated from the analyte peaks. Also because of its low vapor pressure at the extraction conditions, the extract was stable at the extraction period (Fig. 1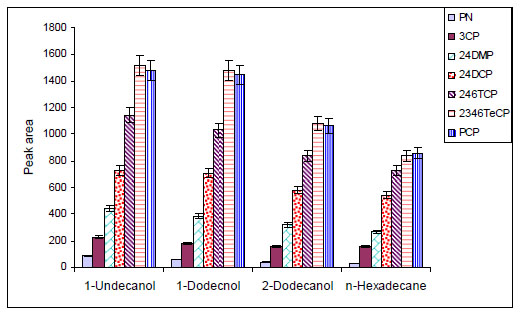 ). Thus, 1-undecanol was chosen as the extracting solvent in this investigation. It is noteworthy that a 20 mL aqueous solution spiked into with the phenols (at the concentration level of 5.0 µg L-1) was used in the extraction studies.
). Thus, 1-undecanol was chosen as the extracting solvent in this investigation. It is noteworthy that a 20 mL aqueous solution spiked into with the phenols (at the concentration level of 5.0 µg L-1) was used in the extraction studies.
 |
Fig. (1) Effect of organic solvent type on extraction efficiency. |
3.2. Volume of Extraction Solvent
The effect of micro drop volume on the analytical signal was studied in the range of 10.0-20.0 µL. Fig. (2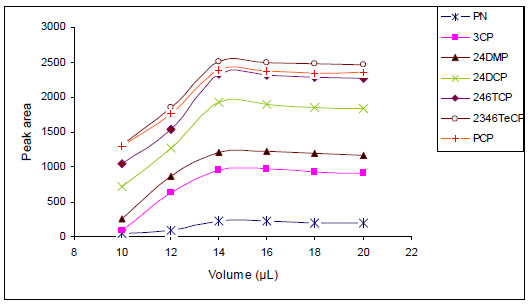 ) shows that there was a corresponding increase in the analytical signal of phenolic compounds from 10.0 until 20.0 µL of solvent volumes. Based on LLE equations, rate of the analytes migration into micro drop is directly related to the surface area between the two liquid phases and is inversely related to the organic-phase volume. Thus, increase in the drop volume raises the interfacial area following the analytical signals. But further increase in the micro drop volume leads to decrease in the analytical signals. Hence, the volume of 14.0 µL was chosen as the optimal solvent volume.
) shows that there was a corresponding increase in the analytical signal of phenolic compounds from 10.0 until 20.0 µL of solvent volumes. Based on LLE equations, rate of the analytes migration into micro drop is directly related to the surface area between the two liquid phases and is inversely related to the organic-phase volume. Thus, increase in the drop volume raises the interfacial area following the analytical signals. But further increase in the micro drop volume leads to decrease in the analytical signals. Hence, the volume of 14.0 µL was chosen as the optimal solvent volume.
 |
Fig. (2) Extraction efficiencies obtained for different organic solvent volume. |
3.3. Stirring Rate
Agitation of the sample solution enhances the rate of extraction. The stirring speed has a direct influence on extraction efficiency in limited times due to increasing mass transfer into the organic drop. In this work, the samples with a volume of 20 mL were stirred at different stirring speed (400, 600, 800, 1000 and 1200 rpm) on a stirrer plate. Based on Fig. (3 ), 1200 rpm was chosen as the suitable stirring speed.
), 1200 rpm was chosen as the suitable stirring speed.
 |
Fig. (3) Effect of stirring rate on the relative peak area. |
3.4. Effect of Ionic Strength and pH
The addition of acid and salt, singularly and in combination, was investigated as a means of enhancing the amount extracted by the solvent. Fig. (4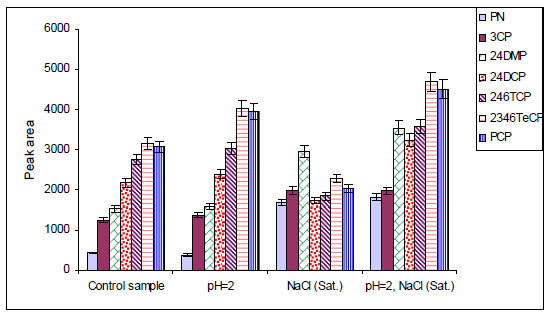 )shows signal intensity obtained for all conditions using a 20 min extraction time, which was also used for control samples of the same concentration at neutral pH and with no salt added. The positive effects of both acid and salt can be realized when they are used in combination. Thus, according to the results, the positive effects of both acid and salt can be realized when they are used in combination. With pH 2 and saturated salt conditions, the amount extracted for every analyte in the mixture was greater than the control sample at pH 7 and with no salt added. Under these conditions, all phenolic compounds are in their neutral form and are salted out of solution and into the solvent.
)shows signal intensity obtained for all conditions using a 20 min extraction time, which was also used for control samples of the same concentration at neutral pH and with no salt added. The positive effects of both acid and salt can be realized when they are used in combination. Thus, according to the results, the positive effects of both acid and salt can be realized when they are used in combination. With pH 2 and saturated salt conditions, the amount extracted for every analyte in the mixture was greater than the control sample at pH 7 and with no salt added. Under these conditions, all phenolic compounds are in their neutral form and are salted out of solution and into the solvent.
 |
Fig. (4) Effect of acid and salt on extraction |
3.5. Sample Temperature
The effect of sample temperature on extraction efficiency was also investigated between 26 and 55 ºC. Generally, by increasing temperature, higher enrichment factors can be obtained in LPME experiments. This process facilitates mass transfer of the analytes from the sample into the organic solvent and thus increases the efficiency of the extraction. Results showed that by increasing the temperature, the extraction efficiency was increased (Fig. 5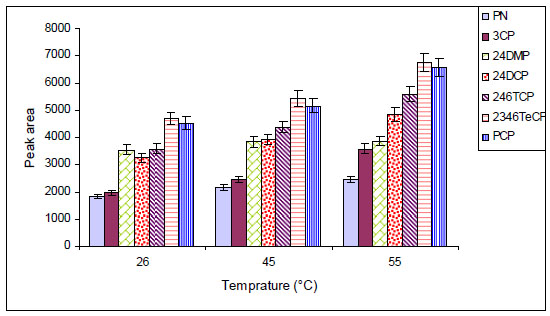 ). At high temperatures (>55 °C), the over-pressurization of the sample vial made the extraction system unstable. Thus, in further experiments the sample vial temperature was held at 55 °C.
). At high temperatures (>55 °C), the over-pressurization of the sample vial made the extraction system unstable. Thus, in further experiments the sample vial temperature was held at 55 °C.
 |
Fig. (5) Influence of temperature on the extraction efficiencies. |
3.6. Extraction Time
To increase the precision and sensitivity of the LPME method, it is necessary to select an exposure time that guarantees the equilibrium between the aqueous and organic phases. Therefore, the extraction time plays a very essential role in the whole process. The range of extraction time investigated was 5-30 min with other extraction conditions being constant. Fig. (6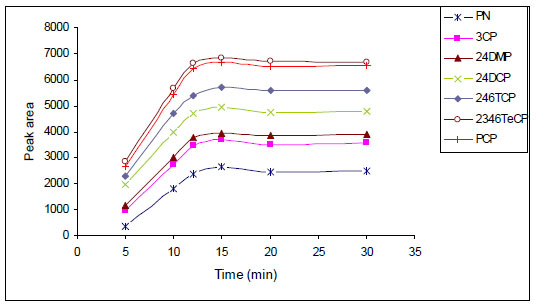 ) shows that there was a corresponding increase in analytical signal from 5 to 15 min, followed by a period of stability. An extraction time of 15 min was selected as a reasonable compromise between enrichment factor and analysis time.
) shows that there was a corresponding increase in analytical signal from 5 to 15 min, followed by a period of stability. An extraction time of 15 min was selected as a reasonable compromise between enrichment factor and analysis time.
 |
Fig. (6) Time profiles obtained for the studied analytes. |
3.7. Quantitative Evaluation and Real Samples
Quantitative parameters of the purposed method, such as linearity ranges (LR) and limit of detections (LOD) were calculated by pre-concentration 20 mL of water sample, spiked by standard solution of phenolic compounds as summarized in Table 1. As it is illustrated, that the LR were obtained at concentration range of 0.3-870 µg L-1 for phenolic compounds, (RSD < 10.1%). The coefficient of determinations (r2) was satisfactory in the range of 0.9973-0.9992 for all the analytes studied. Limit of detections, based on a signal to noise ratio of S/N=3 [25], were ranged from 0.05 to 3.0 µg L-1.
3.8. Analysis of Real Sample
In order to evaluate efficiency of the proposed method in monitoring of low levels of phenols, their levels in tap and mineral water were investigated. Results are summarized in Table 2. The chromatograms obtained by HPLC-DAD of unspiked mineral water and that spiked at two concentrations of each analyte after the developed method at optimum conditions is shown in Fig. (7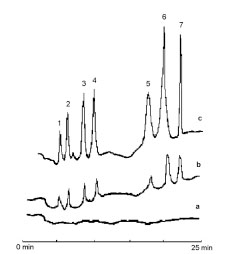 ).
).
 |
Fig. (7) Chromatograms of mineral water sample, (a) unspiked; (b)spiked at 4.0 µg L-1and (c) spiked at 10.0 µg L-1 phenols. (1) PN,(2) 3CP, (3) 24DMP, (4) 24DCP, (5) 234TCP, (6) 2346TeCP, (7)PCP. |
4. CONCLUSION
A novel LPME and in situ derivatization method coupled with HPLC-DAD for pre-concentration and determination of seven phenolic compounds in water samples has been developed. The present method in comparison with other sample preparation methods is simple in operation, has good repeatability, lower analysis cost and relative high recoveries. In this technique sample preparation time as well as the consumption of toxic organic solvents has been minimized without affecting the sensitivity of technique. Different water samples showed no matrix influence in this method.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the financial support provided by the Research Council of the Islamic Azad University of Varamin to carryout this work.