- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Clinical Trials Journal
(Discontinued)
ISSN: 1876-8210 ― Volume 4, 2013
Flexible Dosing of Trospium Chloride for the Treatment of OAB – Results of a Non-Interventional Study in 4,092 Patients
A Wiedemann1, §, W Kusche2, §, C Neumeister*, 3
Abstract
Introduction& Objectives:
This was a multicenter, non-interventional study performed in urology practices investigating prescribed dosage regimens as well as the efficacy and tolerability of an oral therapy with trospium chloride in patients with overactive bladder (OAB) under routine conditions.
Material and Methods:
Naive or insufficiently treated OAB patients in which oral therapy with trospium chloride1 was prescribed by the physician were eligible to participate. Dosing and duration of treatment were not predetermined. Core symptoms of OAB, a Quality of Life (QoL) score as well as dosage regimens were assessed at the beginning and at two consecutive visits. Adverse events and withdrawals were documented.
Results:
In 4,092 cases pre and post treatment data was available for the evaluation of efficacy; all 4,167 cases were included in the safety analysis. After a mean treatment period of 40 days, all core symptoms of OAB had improved e.g. the median total of micturitions was reduced from 12 to 8 (day) and from 3 to 1 (night). The percentage of incontinent patients dropped from 54.4% to 29.1%. The proportion of patients requiring incontinence pads was reduced from 47.6% to 30.1%. The median QoL score had improved from 3 to 1. Only 20% of the patients were treated with the standard dose of 45mg trospium chloride. Nearly 50% of the patients received 30mg (or less), and 60mg to 90mg were prescribed in 30% of the cases. There were 300 early treatment withdrawals (7.3%) and 54 patients experienced adverse events (1.3%).
Conclusions:
The distinct majority of OAB patients benefit from flexible dosing of trospium chloride, a compound with an equally favorable efficacy and tolerability profile.
Article Information
Identifiers and Pagination:
Year: 2011Volume: 3
First Page: 1
Last Page: 5
Publisher Id: TOCTJ-3-1
DOI: 10.2174/1876821001103010001
Article History:
Received Date: 22/7/2010Revision Received Date: 28/4/2011
Acceptance Date: 05/5/2011
Print publication date: 14/6/2011
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http: //creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Department of Medical Science/Clinical Research, Clinical Research, Dr. R. Pfleger GmbH, D-96045 Bamberg, Germany; Tel: +49 951 6043 161; Fax: +49 951 6043 226; E-mail: Claudia.neumeister@dr-pfleger.de§ These authors contributed equally to this work.Trospium chloride coated tablets (Spasmex® 30mg TC Filmtabletten), Dr.R. Pfleger GmbH, D-96045 Bamberg,Germany
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 22-7-2010 |
Original Manuscript | Flexible Dosing of Trospium Chloride for the Treatment of OAB – Results of a Non-Interventional Study in 4,092 Patients | |
INTRODUCTION
Overactive bladder (OAB) is defined as a syndrome of “urgency with or without urge incontinence, usually with frequency and nocturia” [1]. In 2000 the prevalence of OAB was reported to be 17% [2, 3] while higher rates (20% in women and 44% in men) have been published more recently [4, 5].
Anticholinergics, such as tolterodine or trospium chloride, constitute a first line pharmacological option in the treatment of an isolated overactive bladder syndrome (OAB). Trospium chloride is an anticholinergic compound with a high affinity to all muscarinic receptor-subtypes and with primarily peripheral properties. In the elderly population especially, the use of trospium chloride appears favorable due to its practical absence of central nervous side effects [6].
1Trospium chloride coated tablets (Spasmex® 30mg TC Filmtabletten), Dr. R. Pfleger GmbH, D-96045 Bamberg, Germany
The results of a recent study in patients with OAB and benign prostate syndrome have shown that the prescribed daily dosages of trospium chloride largely deviated from the official standard dose of the product which is 45mg trospium chloride per day [7]. Fixed dosing does not seem to be suitable for all patients. Currently, flexible dosing of antimuscarinic agents has become the strategy of choice to meet the needs of the individual patient [13].
The present non-interventional study was undertaken in OAB patients focusing on individual trospium chloride doses and their possible adjustments, in order to gain a more complete picture in a larger number of patients.
MATERIALS AND METHODOLOGY
In total, 4,167 patients were entered into this non-interventional prospective study performed at private urology practices in Germany. Patients received an information sheet and signed a consent form regarding data protection. The only inclusion criterion was the presence of OAB symptoms, which was either previously untreated or insufficiently treated.
The study protocol did not contain any dosing instructions regarding trospium chloride, however, the official dosing schemes as well as contraindications were mentioned in the protocol and reference was also made to the Summary of Product Characteristics (SmPC) attached to the protocol. It was further requested that the intended treatment period should not fall below four weeks.
The individual observation forms required to collect the following data at the first visit 1: demography, history, OAB symptoms (micturitions per day/night), and incontinence episodes per week, use of incontinence pads as well as the assessment of the QoL score by asking the patients “How much does your bladder problem interfere with your everyday life?” (5-point ordinal scale ranging from 0 = “none at all” to 4 = “very severe”). Furthermore, details of any previous treatment and its tolerability were to be documented, as well as prescribed dosing details regarding trospium chloride. On visit 2, the following was to be recorded: treatment period and applied dosing scheme of trospium chloride, OAB symptoms, incontinence episodes, use of incontinence pads, QoL score, investigator's and patient's assessment of treatment efficacy and tolerability, and any changes of the therapy regimen. On Visit 3, in addition to the issues to be recorded at visit 2, any adverse events were also to be documented.
Data were entered into the computer and analyzed by SPSS 10 statistical software solely in an exploratory sense.
Prior to study start, the observational study protocol was submitted to an ethics committee (freiburger ethik-kommission GmbH international, Freiburg, Germany). Additionally, in compliance with legal requirements in Germany, the competent authorities were given notice of the study and of participating practices and the official recommendations for the conduct of non-interventional studies were observed[8, 9].
RESULTS
Study Population and Basic Demographic Data
All 4,167 case reports obtained were entered into the safety analysis. According to the study plan, 75 patients with retrospective documentation or an unclear study period were excluded from further analysis, rendering a baseline population of 4,092 patients.
63.3% of the study population were female, 36.5% were male (0.1% missing data). The mean age of the patients was 64.9 years (± 13.7; median 68; min. 14; max. 96) and the mean body weight 77.4 kg (± 13.4; median 77; min. 38; max. 178).
History of OAB Symptoms at Baseline
In the baseline population of 4,092 patients, OAB had been present for “years” in 1,272 of the patients (31.1%) and for “months” in 2,035 patients (49.7%). The rest had been diagnosed shortly before the baseline visit.
In 76.7% of the patients the daily micturition frequency was >8 to 15, and in 17.2% the micturition frequency was >15.
Nightly micturitions were reported by 95.4% of the patients while 4% of the patients were unaffected. In most patients (60%), the frequency of nightly micturitions was 3 - 5.
The majority of patients rated the urgency as “severe” (45.9%) or “medium” (44.3%), while in about 9% of the patient’s urgency was “mild” or not existent.
2,225 patients (54.4%) were affected by incontinence episodes and experienced a mean of 9.7 (± 10.7) per week. 1,946 patients (47.6%) used a mean of 14.6 (± 10) incontinence pads per week.
QoL Score Before Treatment
In the baseline population of 4,092 patients, the median of the QoL score was 3 before therapy start.
Trospium Chloride – Dosing and Treatment Period
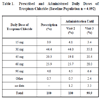
The most frequently prescribed initial daily doses of trospium chloride were 30mg (44.4%), 45mg (20.3%) and 60mg (25.9%), respectively; 4.8% of the patients received a higher dose (90mg/die) and 3.9% of the patients a lower dose (15mg/die).
Consecutive visit 2 and final visit 3 took place 15 days and 32 days (medians) after visit 1, respectively.
As depicted in Table 1, administered doses at visit 2 were practically in agreement with the prescription at visit 1. At visit 3, administration data showed a decreased proportion of the 30mg dose while proportions of higher doses (60mg and 90mg) had increased.
Concomitant Medication
For the treatment of urological symptoms (e.g. benign prostate syndrome), 16.1% of the patients took alpha-receptor blockers, 4.3% of the patients received 5-αlpha- reductase inhibitors, and 3.5% of the patients used phytopharmaceuticals, in addition to trospium chloride.
The most common concomitant medications for the treatment of basic diseases (e.g. obesity, cardiovascular diseases, Parkinson’s disease) were beta-receptor blockers in 25.3% of the patients, tricyclic antidepressants in 4.6% of the patients, and anti-parkinson drugs in 3.6% of the patients.
OAB Symptoms During Treatment with Trospium Chloride
At visit 2, a significant improvement of all symptoms was evident, and a further change for the better was achieved by visit 3.
At visit 3, the proportion of patients rating the sensation of urgency as “severe” was reduced from 45.9% to 2.3%. Concomitantly, the percentage of patients with only “mild” urgency rose from 8.8% to 51.5%, and the fraction of patients without urgency problems increased from 0.6% to 14.6% (Fig. 1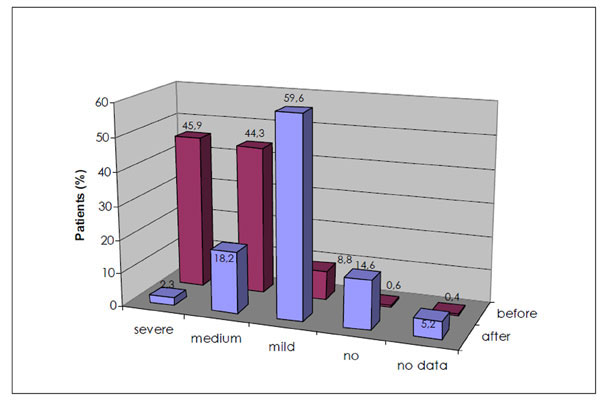 ).
).
 |
Fig. (1) Degree of urgency before and after therapy with trospium chloride (n=4,092). |
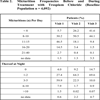
Concordantly, a clear reduction in micturition frequencies (day and night) was observed. The proportion of patients with more than 10 daily micturitions dropped from 63.7% to 11.0%, while the fraction of patients with up to 10 daily micturitions rose from 35.9% to 85.7%. Further, a clear reduction of nightly micturition frequencies was observed (Table 2).
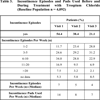
Furthermore, the proportion of patients with incontinence episodes was more than halved from 54.4% to 21.1%. However, patients who were still affected profited substantially from treatment with trospium chloride as the median of incontinence episodes was reduced from 7 to 4 events per week. Consequently, the average number (median) of incontinence pads used per week was halved by 50% from 14 pads at baseline to 7 at visit 3 (Table 3).
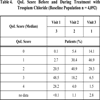
QoL Score
The positive development of OAB symptoms under treatment with trospium chloride were also reflected by distinct changes in the QoL score (median) which improved during treatment from 3 at baseline to 1 at visit 3. Details of changes are shown in Table 4.
Rating of Treatment Efficacy
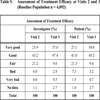
The general assessment of treatment efficacy by investigators and patients at visits 2 and 3 is shown in Table 5. The ratings reflect a fair degree of satisfaction already at visit 2 after 15 days of treatment. At visit 3, in 84.4% of the cases the investigators rated the therapy outcome as “very good” or “good”, and only in 3.3% as “poor”. The patients' ratings were comparable.
Rating of Treatment Tolerability
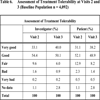
At visit 3, general tolerability of oral treatment with trospium chloride was rated as “very good” or “good” by 90.2% of the investigators and 87.1% of the patients (Table 6).
Therapy Withdrawals and Side Effects
Treatment with trospium chloride was prematurely terminated in 300 of 4,092 patients (7.3%), thereof in 111 cases (37%) due to lacking efficacy, in 94 cases (31.3%) due to lack of acceptance by the patient, and in 37 cases (12.3%) due to adverse effects.
All in all, evidence of adverse effects was found in 54 cases (1.4%) in the observation forms, and were mostly known side effects of trospium chloride such as dry mouth, diarrhea, constipation, gastrointestinal disorders and urinary retention.
DISCUSSION
Anticholinergic agents are the most common and the most effective drugs to date for the treatment of OAB [8] and other forms of urinary incontinence [9-2]. Once-daily formulations have been developed for several anticholinergic agents in order to improve patient compliance and tolerability [16]; however the negative side of such formulations is that they only allow for fixed dosing. Nowadays, results of randomized, controlled clinical trials as well as open label, flexible-dose studies indicate the advantages of adaptable dosing, which allow a more personalized treatment [13-15, 17]. This approach considers the individual sensitivity of the patient to the drug as well as the willingness to tolerate a certain degree of side effects. For the selection of an effective dose with minimal side effects, different drug doses should be made applicable [17].
The results of this observational study are in line with the recent developments concerning an individualized drug therapy for patients suffering from overactive bladder. In only 20% of the cases the daily standard dose of 45mg trospium chloride was used. In almost 50% of the cases, 30mg (or less) were prescribed, but about 30% of the patients received 60 to 90mg/die, which is above the actual official dose range in Germany. Interestingly, in Austria the regulatory authority has already allowed dose adjustment of trospium chloride2 up to 90mg/die after considering the individual efficacy and tolerability.
For trospium chloride, several immediate-release formulations with 5 different doses (5mg, 10mg, 15mg, 20mg, 30mg) and 1 extended release formulation (60mg) are available on the German market, therefore providing various possibilities for dose titration. Additionally, the 30mg formulation presented here can easily be halved.
Individual adjustment of doses, including the prescription of the higher doses is (a) evidence for the extensive experience with trospium chloride with regard to both efficacy and tolerability, and (b) also in accordance with the recommendations for a personalized drug therapy [9].
Furthermore, the results of this non-interventional investigation in 4,092 OAB patients confirm the recognized therapeutic value of the anticholinergic trospium chloride. The majority of patients noted a meaningful reduction in all OAB symptoms (less frequency day and night, less urgency, less urge incontinence). Moreover, in 61% of patients with incontinence symptoms before treatment, complete continence was achieved. It is noteworthy that the aforementioned very positive results in this study were obtained after an average observation period of only 32 days. Therefore, an even higher responder rate may be expected in long-term therapy. Even at higher doses, the generally good tolerability of trospium chloride was not impaired which supports earlier results [7].
CONCLUSION
The results of this study confirm the therapeutic value of trospium chloride in patients with OAB symptoms. Individual dosing is commonly used in everyday clinical practice as it was shown to take place in just under 80% of the observed patients.
CONFLICT OF INTEREST
Wiedemann A – Consultant to Sponsor
Kusche W – Employee of A.CRO GmbH, the contract research organization commissioned with data management and analysis
Neumeister C – Employee of Sponsor
NOTES
1 Trospium chloride coated tablets (Spasmex® 30mg TC Filmtabletten), Dr. R. Pfleger GmbH, D-96045 Bamberg, Germany
2 Trospium chloride coated tablets (Inkontan® 15/30 mg Filmtabletten) Pharm. Fabrik Montavit Ges.m.b.H., A-6060 Absam/Tirol, Austria
ACKNOWLEDGEMENTS
The authors wish to thank all contributing urologists in Germany for their valuable cooperation. We thank Anna Wolf for proofreading the manuscript.
2Trospium chloride coated tablets (Inkontan® 15/30 mg Filmtabletten) Pharm. Fabrik Montavit Ges.m.b.H., A-6060 Absam/Tirol, Austria