- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Clinical Trials Journal
(Discontinued)
ISSN: 1876-8210 ― Volume 4, 2013
Long-Term Follow-Up of Cognitive Function and Activities of Daily Living in Older People: A Feasibility Study in the PROSPER Cohort
Gillian D. Kerr *, 1, Michele Robertson2, David J. Stott1
Abstract
Background:
The Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) considered the benefits of pravastatin therapy and provided insights into cognitive decline/disability in older people but follow-up was short.
Methods:
We performed a feasibility study of 300 PROSPER recruits, 7 years after the trial finished. The subject’s general practitioner provided basic follow-up data. Telephone contact with participants established cognition/functional level. Relatives of those unsuitable for contact were asked to complete postal questionnaires.
Results:
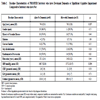
Of 300 participants we established 132 were alive, 135 dead and 33 lost to follow-up. Of 132 survivors data were obtained for 78 participants by telephone, 10 participants with GP diagnosis of dementia, and 3 participants whose relative provided information. Therefore cognitive function was determined in 69% of survivors and functional ability in 61%.
Conclusions:
It was feasible to perform long-term follow-up of cognition/functional ability in the majority of survivors from a large randomised controlled trial.
Article Information
Identifiers and Pagination:
Year: 2011Volume: 3
First Page: 6
Last Page: 12
Publisher Id: TOCTJ-3-6
DOI: 10.2174/1876821001103010006
Article History:
Received Date: 30/5/2011Revision Received Date: 09/7/2011
Acceptance Date: 15/7/2011
Electronic publication date: 05/09/2011
Collection year: 2011
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http: //creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Academic Section of Geriatric Medicine, Walton Building, Glasgow Royal Infirmary, Glasgow G31 2ER, UK; Tel: 0141 211 4976; Fax: 0141 211 4033; E-mail: gilliandkerr@hotmail.co.uk
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 30-5-2011 |
Original Manuscript | Long-Term Follow-Up of Cognitive Function and Activities of Daily Living in Older People: A Feasibility Study in the PROSPER Cohort | |
INTRODUCTION
Cognitive decline and disability in old age are major public health issues, with both having major effects on social functioning and quality of life as well as significant health and social care costs. The prevalence of dementia rises markedly with increasing age, from approximately 1.5% of 65-69 year olds to 30% in those aged over 90 [1]. The risk of physical disability also increases dramatically with advancing age, with approximately 13% of the over-80s categorised as having a ‘severe’ problem in the UK national census [2].
Cognitive decline and disability in older age have many common risk factors. Increasingly it is recognised that vascular disease is an important and potentially preventable contributor to both. The Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) [3] aimed to test the benefits of pravastatin treatment in an elderly cohort of men and women with, or at high risk of developing, cardiovascular disease and stroke. This was a randomised controlled trial of 5804 people aged 70–82 years with a history of, or risk factors for, vascular disease who were assigned to pravastatin or placebo. Those with a mini mental status exam of <24 [4], and therefore likely significant cognitive impairment, were excluded. Pravastatin was stopped at the end of the trial and the average duration of pravastatin therapy was 3.2 years. The primary endpoint was a composite of coronary death, non-fatal myocardial infarction, and fatal or non-fatal stroke and a secondary endpoint of cognitive function and disability. Pravastatin reduced the incidence of the primary endpoint to 408 events compared with 473 on placebo (hazard ratio 0·85, 95% CI 0·74–0·97, p=0·014). However there was no protective effect of pravastatin on cognition or activities of daily living (ADL) but the duration of follow-up may have been too short to demonstrate any benefit.
We aimed to establish a methodology for determining long-term, post trial, cognitive function and ability to perform activities of daily living in PROSPER survivors.
If this is feasible we aim to follow-up all the PROSPER survivors. The primary objective of this large scale study would be to examine the long term effect of Pravastatin. The WOSCOPS long-term follow-up [5] demonstrated carry-over benefit from 5 years of treatment with pravastatin as primary prevention for middle-aged men. These benefits were still noted 10 years after study completion but it is unknown if similar long-term benefits would be seen in the elderly.
Additionally the observational data from PROSPER has shed light on the pathophysiology of cognitive and function decline including roles of haemostasis and thrombosis, inflammation, and lifestyle issues such as alcohol [6-8]. However the utility of these observations are limited due to the relatively short period of follow-up and small decrements in cognition and function. Extending follow-up beyond the study gives opportunity to study predictors of more severe cognitive deficit and functional impairment.
MATERIALS AND METHODOLOGY
We performed a pilot study of 300 Scottish PROSPER recruits to establish feasibility of establishing cognitive function and activities of daily living in PROSPER survivors.
A random sample of 300/2,520 of the original Scottish PROSPER cohort was selected. This was on average 6 year and 10 months after the end of the original PROSPER study. This sample was selected using computer generated random numbers and was done by the Robertson Centre for Biostatistics, Glasgow University, independent of the clinical research team. The Robertson Centre for Biostatistics also identified participants who died during the original study, or who had been identified in record linkage (19th June 2006) as deceased after the randomised observation period of the study.
The subject’s general practitioner (GP) was contacted by letter asking them to confirm the subject was alive and suitable for contact. It was felt unsuitable to contact elderly participants directly because of potential distress to relatives in the case of a subject’s death since the last record linkage or subject distress because of a pre-existing diagnosis of dementia. If the subject could not be contacted the GP was asked to say why (e.g. dementia, dysphasia, subject preference, withdrawal of consent from PROSPER study) and if there was a relative or caregiver who could be contacted instead. The GP was also asked if they considered the subject had dementia. Lastly the GP was asked to provide contact details for the subject or relative and details of current medication. If there was no response from the GP within 2 weeks this was followed up by a single reminder telephone call to the general practice.
If the subject was suitable for review a letter was sent to the subject offering a telephone interview in one week’s time. A contact telephone number was provided to allow participants to opt out if they wish, or change the date or time of the telephone interview.
Telephone contact with the subject involved initial verbal consent for the study. The subject was then asked about their residence, cohabittees and any formal home care support. Current drug treatment was recorded, including use of statins. Disability was then assessed by telephone administration of the Barthel index [9] and short Instrumental Activities of Daily Living questionnaire (IADL) [10]. The Barthel index is a commonly used measure of disability and Korner-Bitensky at al demonstrated a intraclass correlation coefficient of 0.89 when comparing a telephone and home assessment of the Barthel index in 366 individuals [11]. The IADL is made of six sections and the intraclass correlation coefficient for each section ranges from 0.66 to 0.87. The modified Telephone Interview of Cognitive Status (TICSm) was also administered to assess cognition. This is a 21-item questionnaire with a maximum score of 40 [12;13] and it is considered further in the discussion.
When a subject was not suitable for contact and a relative was available a postal questionnaire was sent to the relative. This consisted of the short form of the Informant Questionnaire on Cognitive decline in the elderly (IQCODE) [14], the Barthel index [9] and short IADL questionnaires [10]. The IQCODE has been found to have high internal reliability in a general population sample (alpha = 0.95) and reasonably high test-retest reliability over one year in a sample of those with dementia (r = 0.75) [15]. There were also questions on the type of residence, formal home care support, and any cohabittees. Caregivers or relatives who did not return the postal questionnaire within 2 weeks were reminded by a single telephone call.
DATA ANALYSIS AND STATISTICAL METHODS
It was estimated, based on Scottish mortality rates, that approximately 50% of participants would still be alive at 7 years after the trial finished. Our previous study experience of telephone contact suggested that it should be possible to gather cognitive data on around 74% of available participants [16]. Therefore we estimated obtaining information on 97 in our pilot of 300 PROSPER participants.
All-cause mortality was calculated for those not lost to follow-up. Cognitive impairment was calculated and defined as subject scoring <21 on the TICSm or relative/caregiver giving a score of ≥3.38 on the IQCODE or subject who the GP deemed to have dementia. The cut-off for the IQCDOE are validated and established [14]. The cut-off for the TICSm is less established but is considered in the discussion.
There is no simple cut-off to diagnose disability, therefore the change in Barthel and IADL from study baseline to pilot study follow-up were analysed as continuous variables. Decline in disability was compared between placebo and pravastatin groups using linear models adjusting for the baseline measure of the variable. A further model was fitted adjusting for the known risk factors above. Adjusted least square means and standard errors are reported for each treatment group and mean differences with 95% confidence intervals and p-values are also given.
Baseline characteristics were reported comparing the PROSPER survivors who developed dementia to survivors who did not develop dementia. Continuous variables are presented as mean and standard deviation and categorical variables are summarised as number and percentages. Continuous variables are analysed by two sample t-test and categorical variables by Chi-square test or Fisher’s exact test as appropriate. The distributions of the baseline variables interleukin-6, C-reactive protein and D-dimer were markedly skewed and therefore log-transformed. The summary results presented for these variables are therefore their geometric mean and standard deviation, and the log transformed variables were analysed.
ETHICAL CONSIDERATIONS
The PROSPER study participants had consented to long term follow-up during the original study and telephone contact with the subject involved initial verbal consent for the study. The institutional ethics review boards of all centres approved the original PROSPER protocol, and all participants gave written informed consent. The protocol was consistent with the Declaration of Helsinki.
RESULTS
A flow chart of recruitment and data collection is given as Fig. (1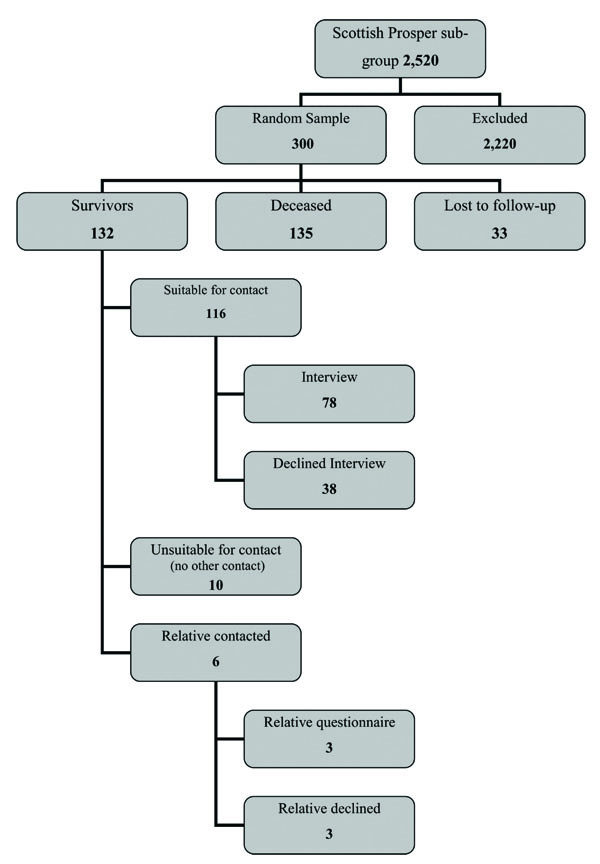 ). Of the 300 Scottish PROSPER participants randomly selected, 135 had died and 132 were known to be alive at the time of review, at an average of 6yrs 10 months after study completion.
). Of the 300 Scottish PROSPER participants randomly selected, 135 had died and 132 were known to be alive at the time of review, at an average of 6yrs 10 months after study completion.
 |
Fig. (1) Study flow chart - Recruitment and data collection for the PROSPER study long-term follow-up, |
Of the 300 participants in our random sample, survivorship was established through record linkage and GP contact in 267 (89%), therefore we had no information on the mortality status of 33 participants. Of the 132 known survivors long-term follow-up cognitive function data were obtained for 78 participants who had telephone interview, 10 participants with a GP diagnosis of dementia but no contact details for a caregiver, and 3 participants unsuitable for personal follow-up for whom their caregiver provided questionnaire data. Two of these 3 participants were unsuitable for telephone follow-up because of severe deafness and one because of dysphasia. The other 3 participants whose relatives were contacted all had a GP diagnosis of dementia but none replied. This gave a total of 91/132 (69%) survivors in whom long-term cognitive outcome could be determined. Functional data was available for the 81 subject who had a telephone interview or for whom their caregiver provided information; this gave a total of 81/132 (61%) survivors in whom both long-term functional and cognitive data could be established.
GPs were reminded after 14 days if the postal questionnaire had not been returned. Many GPs had more than one participant in this follow-up study. The GPs of 99 participants had not responded within 14 days and the GPs of 89 participants were sent a reminder, the others responding before the reminder could be sent out. All but 2 GPs responded (4 participants). Although there was an excellent response from GPs 33 participants remained untraceable as GPs told us 23 were no longer listed with them, 6 had been untraceable at the end of the original PROSPER study and the GPs of 4 participants did not respond despite a reminder.
Thirty eight participants who were suitable for a telephone interview did not have one. Twenty nine declined; 17 did so by phoning in advance, 12 did so on day of interview. Five did not answer their phone despite repeated attempts. We attempted to phone these participants on at least three occasions at varying times of day. One participant had died recently, 1 had no phone, 1 was deaf (although the GP had alerted us to this but felt they could still be contacted) and 1 was away from home throughout the study. Of the 78 participants who did have a telephone interview 8 rearranged their appointment by phone as the original time given did not suit.
Six relatives/caregivers were sent a postal questionnaire. Three replied and after 14 days the other three were reminded by telephone. One told us they did not wish to participate and other two did not reply despite the reminder. Additionally 10 participants were identified as being unsuitable for contact because of dementia but the GP was not aware of the nearest relative/caregiver contact details.
Although this study was largely designed to consider methodology some provisional results were considered. The proportion of deaths, over a mean of 10 years follow-up (including trial duration of 3.2 years and post-trial follow-up period of 6yrs 10 months), was very similar in the original PROSPER study placebo and pravastatin groups (Table 1) at around 50%. The proportion of those with significant cognitive impairment was very similar in the placebo and pravastatin groups at around 31%. The baseline characteristics of the 28 PROSPER survivors who developed significant cognitive impairment were compared with 63 survivors who did not (Table 2). Cognitive impairment associated with older age at baseline (p=0.007). In survivors there was a decline in Barthel by approximately 1 point (20-point scale) over the 10 years from study baseline, and for IADL a decrease by 1.7 points (14-point scale), with no significant differences between the placebo and pravastatin groups (Fig. 2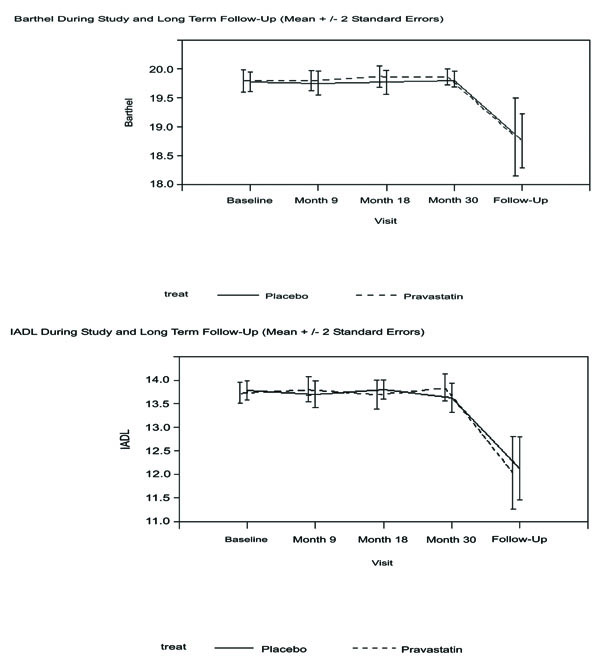 ). This small decline in function may not be of individual clinical significance but will be investigated further in the full follow-up study. However van Exel at al have shown that a 1 point decrease in Barthel after stroke can impact on quality of life [17].
). This small decline in function may not be of individual clinical significance but will be investigated further in the full follow-up study. However van Exel at al have shown that a 1 point decrease in Barthel after stroke can impact on quality of life [17].
With further time elapsed, and continuing deaths of around 8% per annum, we anticipateat 9 years after study completion, around 42% (n=2438) of the original cohort of 5804 will be available for review. If cognitive function can be established in 69% and basic and instrumental activities of daily living in 61% of these survivors, as in the feasibility study, this would give results for 1680 for cognition and 1487 for activities of daily living. With these numbers we would have >80% power (at p=0.05, 2 tailed) to detect a difference in proportion of 7% (31 versus 38%) with cognitive impairment, between pravastatin and placebo groups. We would have the same power to detect 0.14 of a SD in change in Barthel or IADL between pravastatin and placebo groups. The most conservative estimates of SD of change in these variables are with adjustment for baseline, age, gender, education, history of vascular disease, diabetes mellitus, smoking status, alcohol intake and systolic blood pressure; with these adjustments we found the SD of change of Barthel to be 0.56 units and for IADL 0.73 units. These calculations were done using G*Power 3:1 [18].
DISCUSSION
We have traced (through the GP) and recontacted a subgroup of 300 Scottish participants of the PROSPER study, at an average of 6yrs 10 months after completion of the randomised controlled trial phase of PROSPER. This time-point is at 10 years after entry to PROSPER and measurement of study baselines. We found approximately 50% of the original study sample are now deceased. This is in line with our pre-study estimates and if all of the Scottish PROSPER participants were followed-up this would equate to a cohort of approximately 1,100. We were unable to trace 33/300 (11%) of our study sample. While any loss to follow-up is disappointing this level of case-ascertainment demonstrates that it is possible, using record linkage and GP contact, to ascertain long-term cognitive and ADL outcomes for the majority of participants in PROSPER. The long-term follow-up of the West of Scotland Coronary Prevention Study [19], another large randomised control trial of statin therapy, was able to obtain details of cardiac morbidity and mortality in 91% of participants but this was from hospital/national records only and the participants were not approached.
The GPs were invariably very helpful in responding to our contact although a 14 day reminder phone call was needed for 89/300 participant’s GPs. More up-to-date record linkage with the General Register Office of Scotland might reduce loss to follow-up and it may be possible to use record linkage to establish current GP/address. It is our intention to have complete up-to-date record linkage for the full follow-up study. This will also allow further consideration of mortality including the cause and timing. We did have some information on four non-survivors who had dementia recorded on their death certificate but as there was only partial record linkage in this study we did not include this information. We accept that a GP’s criteria for a diagnosis of dementia may vary. We had aimed to assess those given a GP diagnosis of dementia via postal IQCDOE but for most participants in this category a caregiver was not available and none of the three caregivers approached responded to our questionnaire.
Our previous study experience of telephone contact suggested that it should be possible to gather cognitive data on around 74% of available participants [16]. The acceptance rate in this study for telephone interview was similar to our previous experience but we are considering how this percentage might be increased without pressurising participants. In this study we were able to establish data on cognition in 69% of those participants who were know to be alive and functional ability for 61%. This would equate to the ability to follow-up the cognition/function of 670 Scottish PROSPER participants and about 1,600 participants from the full PROSPER cohort. No other long-term follow-up of a large randomised control trial has attempted to gather data directly from very elderly participants, so these data are unique and may be useful in planning long-term follow-up of other similar cohorts.
In contrast obtaining contact details for relatives/caregivers and then getting a response proved difficult, and this line of enquiry yielded only limited additional information. It may be possible to get additional information from GPs for those participants who are not contactable rather then try to get this through relatives/caregivers.
We found a prevalence of significant cognitive impairment in around 31% of traceable participants. This is higher than expected; the EURODEM analysis [1] gives a prevalence of dementia at age 80-85 of approximately 11% for men and 12.6% for women. This is despite the fact that those with known dementia were prevented from entering the original PROSPER study. However we have had to use a pragmatic definition of significant cognitive impairment that included a simple cut-off on the TICSm. We accept that cognitive impairment is a spectrum and handling the TICSm as a continuous variable may have benefits in improving statistical power. However the dichotomisation of scales, such as the MMSE, is widely used to simplify the presentation and analysis of data in research, and in clinical practice to aid diagnosis and ascertain suitability for treatment. Our initial plan was to use a cut-off of <27 for the TICSm as one of our criteria for dementia. However this led to an implausibly high prevalence of dementia in survivors. We therefore reviewed our criteria for dementia, in particular the cut-off of TICSm to be applied. The TICSm is a modification of a validated telephone memory screen; it shows a strong correlation (r = 0.94) to the Mini Mental Status Examination [12]. When using a 27 point cut-off score, the TICS (unmodified) has been claimed to have 99% sensitivity and 86% specificity for a dementia diagnosis [13]. However there is precedence for considering other cut-points; the MRC Heart Protection Study used a cut-off of <22 as indicative of clinically significant cognitive impairment [20]. Validation work in the context of cerebrovascular disease found a cut-off of <21 for the TICSm produced a sensitivity of 92% and a specificity of 80% [21] for diagnosis of dementia. Ideally we would have used a combination of the TICSm and proxy questionnaire in all participants; such combined data have been claimed to have 100% sensitivity and 83% specificity for dementia diagnosis [22]. However we found that obtaining proxy information on the PROSPER survivors was not feasible. Given the above literature, we suggest that the best estimates of dementia risk are based on the most conservative criteria that include the cut-off of <21 on TICSm. While these figures may still seem high, it is likely that this group of participants, with a high burden of vascular disease and vascular risk factors, are at particularly high risk of developing both Alzheimer disease and vascular dementia [23].
This feasibility study has generated the necessary data to enable informed power calculations of the implications of revisiting the whole cohort. It appears that with long-term follow-up we would have adequate statistical power to expect to detect a moderate absolute and relative difference between pravastatin and placebo groups, in the risk of clinically significant cognitive impairment at 7% and 18% respectively; we would also be adequately powered to detect <0.1 point difference in change in both Barthel and IADL between the pravastatin and placebo groups, equivalent approximately to the decline in Barthel seen with 1 year of ageing, and the decline in IADL seen with 6 months of ageing in this population.
These methods do have a number of limitations. In particular loss to follow-up and potential attrition bias are a concern. It is possible that we have underestimated the rate of dementia and significant cognitive impairment as the participants who have been lost to follow-up are perhaps even more likely to have been affected.
We were able to define groups of PROSPER survivors with and without major cognitive impairment. The small number in this feasibility study restrict what further exploration can be made for risk factors for cognitive impairment; although our data showed an expected association of older age at baseline as a risk factor for cognitive impairment, other established risk factors did not.
CONCLUSIONS
We found that it was feasible to follow-up cognitive function and ADL in elderly survivors from the PROSPER study, using GP contact followed by telephone subject interview. Postal questionnaires to caregivers proved less productive. Telephone follow-up of the whole cohort of PROSPER participants appears feasible and may determine new and novel risk factors for cognitive decline and disability, including the long-term effect of a period of treatment with pravastatin.
ACKNOWLEDGEMENT
Grant Support: Chief Scientist Office grant reference number CZG/2/377.