The Open Drug Metabolism Journal
(Discontinued)
ISSN: 1874-0731 ― Volume 5, 2011
Pharmacokinetics and Hemodynamic Effects of Diltiazem in Rats Following Single vs Multiple Doses In Vivo#
Pollen K. Yeung*, Angelia Alcos, Jinglan Tang
Abstract
The objective of the study was to compare the pharmacokinetics and hemodynamic effects of diltiazem (DTZ) after single dose and multiple doses using an in vivo rat model. Male SD rats (n = 6 - 8 per group) weighing between 350 - 450 g were used. Each rat received either a single 20mg/kg dose of DTZ or 5mg/kg sc twice daily for 5 doses by subcutaneous (sc) injection. Plasma concentrations of DTZ and its major metabolites were determined by HPLC for up to 8 h. In addition, Systolic Blood Pressure, Diastolic Blood Pressure and Heart Rate were continuously recorded, and analysed using WinNonLin® and considered significant when p < 0.05. The results indicate that after the single 20 mg/kg subcutaneous injection, SBP fell from 138 ± 4 to 125 ± 3 mmHg (-9.4%), DBP from 105 ± 3 to 78 ± 4 mmHg (-26%), and HR from 442 ± 12 to 396 ± 7 bpm (-10%). After 5 mg/kg twice daily for 5 doses, the observed SBP was reduced from 127 ± 5 to 111 ± 7 mmHg (-13%), DBP from 108 ± 6 to 88 ± 7 mmgHg (-19%), and HR from 458 ± 11 to 407 ± 22 bpm (-11%). The pharmacokinetics and hemodynamic data were characterized by an Inhibitory Emax model, which showed a similar profile following the single and multiple doses. Multiple regression analyses of the data predicted that the metabolites in particular deacetyl diltiazem (M1) contributed significantly to the blood pressure lowering effects following multiple doses, but the effects of metabolite were minimal after single dose.
Article Information
Identifiers and Pagination:
Year: 2009Volume: 3
First Page: 56
Last Page: 62
Publisher Id: TODMJ-3-56
DOI: 10.2174/1874073100903010056
Article History:
Received Date: 20/3/2009Revision Received Date: 23/5/2009
Acceptance Date: 25/5/2009
Electronic publication date: 30/6/2009
Collection year: 2009
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http: //creativecommons.org/licenses/by-nc/3.0/ which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Pharmacokinetics & Metabolism Laboratory, College of Pharmacy & Department of Medicine, 5968 College Street, Burbidge Building, Dalhousie University, Halifax, Nova Scotia, B3H 3J5, Canada; Tel: (902) 494-3845; Fax: (902) 494-1396; E-mail: Pollen.Yeung@Dal.Ca# The paper was presented in part at the 107th Annual Meeting of the American Society for Clinical Pharmacology and Therapeutics, Baltimore, MD, USA (March 8 – 11, 2006).
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 20-3-2009 |
Original Manuscript | Pharmacokinetics and Hemodynamic Effects of Diltiazem in Rats Following Single vs Multiple Doses In Vivo# | |
INTRODUCTION
Diltiazem (DTZ) is a calcium antagonist (CCB) widely used in the treatment of angina and hypertension, and may have potential preventing stroke and ischemic heart disease [1, 2]. It is extensively metabolized in humans by CYP450s yielding a host of metabolites some of which have potent pharmacological activities which may contribute to the anti-hypertensive and anti-ischemic properties of the parent DTZ in clinical therapy [3-6]. It has been shown in humans that plasma concentrations of DTZ increased after multiple doses, which was accompanied by a greater increase of plasma concentrations of its active metabolites such as N-monodesmethyl DTZ (MA) and deacetyl DTZ (M1) [7, 8]. The reason could be attributed to inhibition of hepatic and intestinal CYP450 isozymes (e.g. CYP34A and CYP2D6) by DTZ and its metabolites [9-7]. More recently, we have shown that repeated administration of DTZ reduced systemic clearance of DTZ. The concentrations of the active metabolites such as MA, M1 and deacetyl-N-monodesmethy DTZ (M2) were similar to or higher than the concentrations after single dose [18]. Thus, it is unlikely the reduced DTZ clearance is attributed to inhibition of the P-450s catalyzing formation of these active metabolites.
It is known that after normal therapeutic doses, DTZ lowers systolic and diastolic blood pressures (SBP and DBP) and heart rate (HR) in both healthy volunteers and in patients with hypertension, although the blood pressure lowering effect especially on DBP are greater than the effect on the HR [2, 19, 20]. In pre-clinical studies using rabbits and rodents following a single dose, the blood pressure lowering effects were also greater than the effects on HR [6, 21]. It is not clear if the pharmacokinetics and hemodynamic effects of DTZ after a single dose would be different at steady state. To address this question, the current study employs a rodent model to compare if repeated administration of DTZ would produce greater hemodynamic effects than single dose using pharmacokinetic and pharmacodynamic data modeling.
MATERIALS AND METHODOLOGY
Chemicals
DTZ was received as gift from Biovail Corp. (Mississauga, ON, Canada), and its metabolites generously donated by the Tanabe Seiyaku Co. (Japan). Solvents were HPLC grade (BDH Chem., Halifax, N.S., Canada), and all other chemicals were reagent grade (Fisher Scientific, Ont., Canada).
Study Protocol
The study protocol was approved by the Dalhousie University Committee on Laboratory Animals (UCLA). Male SD rats, weighing between 350 - 450 g were purchased from Charles River Laboratories, Wilmington, MA, USA. Each animal received subcutaneously (sc) either a single injection of 20mg/kg of DTZ or 5mg/kg of DTZ twice daily (bid) for 5 doses (n = 6 – 8 per group). This dosage was shown previously to produce significant hemodynamic effects [22]. Blood samples (0.3 mL each) were obtained from each animal at 0 (before dosing), 1, 2, 3, 4, 5, 6, and 8 h post dose for the multiple dose experiment via an indwelling catheter as described previously [23]. The same sampling schedule, except without collecting the 5-h sample was adopted for the single dose study. Plasma samples were immediately separated by centrifugation (4oC, 1720 x g, 5 min) and stored at -20oC until analysis. Plasma concentrations of DTZ and its major metabolites (MA, M1 and M2) were determined by a previously published HPLC method [24]. Hemodynamic variables (SBP, DBP and HR) were continuously recorded via the intra-vascular catheter throughout the experiments using a Transpac II disposable transducer (Abbott Laboratories, IL, USA) coupled to a Siemens hemodynamic monitor (Sirecust 400) and chart recorder (Siredoc) (Erlangen, FRG) as previously described [21, 22, 25].
Data Analysis
Pharmacokinetic and hemodynamic data were analysed by the Inhibitory Effect Sigmoidal Emax model using WinNonLin( (V.5.2, Pharsight Mountain View, CA, USA). Goodness-of-fit was assessed by graphical inspection and the Akaike Information Criterion (AIC). Simple and multiple regression analysis were performed using Minitab® (Release 15, State College, PA, USA) to assess the contribution of the metabolites following single and multiple doses. Differences between the single dose and multiple dose data were evaluated by ANOVA or Student’s t-test and considered significant when p < 0.05 (Minitab® Inc., Release 14, State College, PA, USA).
RESULTS
While details of the pharmacokinetic data analysis and interpretation have been recently published [18], the hemodynamic effects and their relationships with pharmacokinetics of DTZ and its metabolites have not been reported; therefore only the plasma concentration-time profiles are shown here (Fig. 1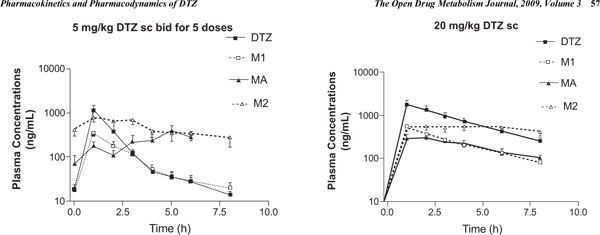 ). As has been reported previously, M2 was the most abundant metabolite in SD rats, followed by M1 and then MA, and M2 and MA accumulated more than DTZ and M1 following repeated administration [18]. Following the single 20 mg/kg subcutaneous injection, the observed SBP fell significantly (p < 0.05) on average from 138 ± 4 to 125 ± 3 mmHg (-9.4%), DBP from 105 ± 3 to 78 ± 4 mmHg (-26%), and HR from 442 ± 12 to 396 ± 7 bpm (-10%). After 5 mg/kg twice daily for 5 doses, the observed SBP over the study period was also reduced significantly (p < 0.05) from 130 ± 5 to 110 ± 8 mmHg (-15%), DBP from 108 ± 6 to 88 ± 7 mmgHg (-19%), and HR from 458 ± 11 to 407 ± 22 bpm (-11%). The maximum hemodynamic effects were attained in less than an hour following the injections in most cases. The BP effects appeared to last for 3 – 4 hours after the smaller multiple doses (5 mg/kg), but longer for 6 – 8 hours after the larger single 20 mg/kg dose. The HR effect was more long lasting than the effect on BP (> 8 h) in the rodent model (Fig. 2
). As has been reported previously, M2 was the most abundant metabolite in SD rats, followed by M1 and then MA, and M2 and MA accumulated more than DTZ and M1 following repeated administration [18]. Following the single 20 mg/kg subcutaneous injection, the observed SBP fell significantly (p < 0.05) on average from 138 ± 4 to 125 ± 3 mmHg (-9.4%), DBP from 105 ± 3 to 78 ± 4 mmHg (-26%), and HR from 442 ± 12 to 396 ± 7 bpm (-10%). After 5 mg/kg twice daily for 5 doses, the observed SBP over the study period was also reduced significantly (p < 0.05) from 130 ± 5 to 110 ± 8 mmHg (-15%), DBP from 108 ± 6 to 88 ± 7 mmgHg (-19%), and HR from 458 ± 11 to 407 ± 22 bpm (-11%). The maximum hemodynamic effects were attained in less than an hour following the injections in most cases. The BP effects appeared to last for 3 – 4 hours after the smaller multiple doses (5 mg/kg), but longer for 6 – 8 hours after the larger single 20 mg/kg dose. The HR effect was more long lasting than the effect on BP (> 8 h) in the rodent model (Fig. 2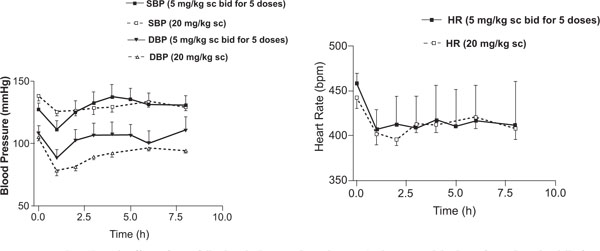 ).
).
 |
Fig. (2) Mean hemodynamic effects of DTZ following single 20 mg/kg and repeated subcutaneous injections of 5 mg/kg twice daily for 5 doses of DTZ. Each value represents mean ± SEM. |
Preliminary attempts using other Inhibitory Emax Models for analyses generated highly variable and unrealistic parameter estimates compared to the observed data. Using the Sigmoidal Emax model for inhibitory effect built in WinNonLin(, which was consistently better based on graphical display and the AIC, there were no significant differences (p > 0.05) in the BP and HR lowering effects between the 20mg/kg single dose and 5 mg/kg multiple doses (Table 1). The maximum inhibitory effects (Emin) for SBP and HR as estimated by the model appeared to be greater after the 5 mg/kg multiple dose (Emin = 72 ± 17 vs 93 ± 18 mmHg for SBP; and Emin = 339 ± 50 vs 379 ± 11 bpm for HR), and the effect for DBP was greater after the 20 mg/kg single dose (Emin = 54 ± 9 vs 75 ± 12 mmHg) (Table 1). The effective concentration producing 50% of the maximum inhibitory effect (EC50) for HR, SBP and DBP after the single dose were 908 ± 523, 2971 ± 144, and 4682 ± 1874 ng/ml, respectively. The EC50 after multiple doses were lower for the BP effects, but higher for the HR effect (Table 1). These differences between the two dosages were not statistically significance (p > 0.05), and the data are summarized in Table 1 and Fig. (3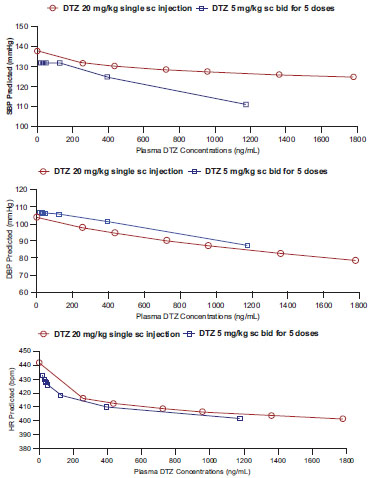 ).
).
In order to estimate the contribution of the effects of metabolites without actually administering the metabolites, the data from each rat were pooled and analysed using multiple regression (Minitab® Inc., Release 15, State College, PA, USA). Following the single dose, there were significant effects (p < 0.05) with HR (a = -0.00963) and DBP (a = -0.00480) response when only plasma concentrations of DTZ were considered. When plasma concentrations of the major metabolites measured (MA, M1 and M2) were also included in the calculation, the multiple regression model predicted that the hemodynamic effects were mainly contributed by the parent DTZ since none of the metabolites showed a significant regression with HR and DBP. In contrary, the metabolite M2 may increase SBP as indicated by a positive regression (+ 0.0219), which may contribute to the lack of significant effect on lowering SBP following the single dose (Table 2). However, after the multiple doses, significant regressions (p < 0.05) were observed for both SBP (a = -0.0157) and DBP (a = -0.0167) when plasma DTZ concentrations alone were included in the evaluation, and regression with HR was no longer significant. When plasma concentrations of the major metabolites were also included in the evaluation, the multiple regression model predicted that the effects of lowering SBP and DBP were mainly attributed to the metabolite M1, and the hemodynamic effects contributed by DTZ were no longer significant (Table 2).
DISCUSSION
Although DTZ has been in clinical use for over 20 years, it is still widely used clinically and it is also one of the drugs most frequently used as a probe to demonstrate fundamental pharmacokinetic and pharmacodynamics principles. As has been shown previously in both rats and rabbits following single dose, DTZ has greater effect on the DBP, than on SBP or HR [6 , 21 , 22]. A single 20 mg/kg dose given by intravascular injection lowered DBP by > 50% in normotensive SD rats, whereas only a decrease of 25% was observed for the SBP. The hemodynamic effects were much smaller (< 10%) following oral administration [21]. We have found in the current study a decrease of 26% in DBP and only 9% reduction for SBP following the same 20mg/kg dose given by subcutaneous injection. Similarly based on the estimated Emax and Emin calculated from the Inhibitory Emax model (Table 1), the reduction for DBP was 50% vs 33 % for the SBP. The much greater hemodynamic effects observed previously were attributed mainly to the 5 times higher maximum plasma concentrations of DTZ attained after the direct intra-arterial injections [18, 21].
When smaller doses were given repeatedly as described in the current study (twice daily for 5 doses), DTZ produced similar hemodynamic effects as the single larger dose (p > 0.05), and that the effect was also greater for DBP than SBP or HR (Table 1). The plasma concentrations required to produce half of the maximum effect (EC50) appeared to be lower after the multiple doses although the differences were not statistically significant (Table 1). To our knowledge, the current study is the first to report a direct comparison of the pharmacokinetics and hemodynamic effects of DTZ between a single and multiple doses in an animal model.
The similar hemodynamic effects are likely attributed mainly to pharmacokinetic factors such that plasma concentrations of DTZ and particularly its pharmacologically active metabolites MA, M1 and M2 are similar or even higher after the 5 mg/kg multiple doses compared to the single 20 mg/kg dose (Fig. 1 ) [18]. The predicted Emin (maximum inhibitory effect) for HR and SBP were greater following the multiple doses even though plasma concentrations of DTZ were lower compared to single dose (Fig. 3
) [18]. The predicted Emin (maximum inhibitory effect) for HR and SBP were greater following the multiple doses even though plasma concentrations of DTZ were lower compared to single dose (Fig. 3 ). The results of our current study also indicated that the metabolites in particular M1 are significant contributors to the hemodynamic effects of DTZ in rats following multiple doses (Table 2). It is possible that there are also potential interactions between DTZ and its metabolites as indicated by their + vs – regression coefficients, but these interactions are difficult to interpret under the current model (Table 2). While the reliability of the model to predict metabolite effect will need to be validated, the results support the thesis that the metabolites may contribute significantly to the therapeutic effects of DTZ at steady state in patients after chronic treatment [26 , 27], and that the metabolite effects may be assessed without administering the metabolites separately using a similar approach with multiple regression model as described in the paper. While we have previously shown that in rabbits M1 has comparable blood pressure lowering effect as DTZ, and greater effect than M2 when administered separately [6], it is not clear if it is also more potent in the rodent model. Further validation of the rat model such as by administering the metabolites separately may be warranted as it would facilitate clinical development of new cardiovascular agents and novel drug therapy.
). The results of our current study also indicated that the metabolites in particular M1 are significant contributors to the hemodynamic effects of DTZ in rats following multiple doses (Table 2). It is possible that there are also potential interactions between DTZ and its metabolites as indicated by their + vs – regression coefficients, but these interactions are difficult to interpret under the current model (Table 2). While the reliability of the model to predict metabolite effect will need to be validated, the results support the thesis that the metabolites may contribute significantly to the therapeutic effects of DTZ at steady state in patients after chronic treatment [26 , 27], and that the metabolite effects may be assessed without administering the metabolites separately using a similar approach with multiple regression model as described in the paper. While we have previously shown that in rabbits M1 has comparable blood pressure lowering effect as DTZ, and greater effect than M2 when administered separately [6], it is not clear if it is also more potent in the rodent model. Further validation of the rat model such as by administering the metabolites separately may be warranted as it would facilitate clinical development of new cardiovascular agents and novel drug therapy.
In summary, the current study has shown that the hemodynamic effects of DTZ following a single 20 mg/kg injection are similar to those after 5 mg/kg given twice daily for 5 doses, and could be characterized by a similar Inhibitory Effect Sigmoidal Emax model. The results also suggest that the metabolites may play an important role for the hemodynamic effects of DTZ at steady state. In particular, M1 may be the main metabolite contributing to the BP lowering effects of DTZ following chronic administration in the rat model.
ACKNOWLEDGEMENTS
The research described in this paper was supported in part by an Operating Grant from the Canadian Institute of Health Research Regional Partnership Funding Program (CIHR/NSHRF/PEF No. 63070) and a Research Development Grant from the Dalhousie University Faculty of Health Professions. There is no conflict of interest from the authors that would bias interpretation of the experimental data described in this manuscript.