- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Fuels & Energy Science Journal
(Discontinued)
ISSN: 1876-973X ― Volume 11, 2018
Thermal Stability and Adsorption of Mercury Compounds in Fly Ash
Jun Zhong1, Fangyong Li1, Junhui Fan2, *
Abstract
Fly ashes were collected from five power plants under two loads in China. The ashes were heated at four different temperatures, and mercury speciation was determined based on the release regular pattern of mercury with temperature. The mercury concentration, unburned carbon content and mean ash particle sizes were measured. The correlation of mercury capture and unburned carbon content, mean ash particle sizes were analyzed. Results indicate that the amount of unburned carbon and mercury adsorb is significantly positively correlated in fly ash; the smaller the mean ash particle size, the more mercury particles are captured. There was little HgO and HgSO4, and the main form of mercury compounds in fly ash were HgCl2 and HgS. The high element Cl content can result in high HgCl2 ratio in particular mercury and element S play an important role in adsorbing mercury.
Article Information
Identifiers and Pagination:
Year: 2016Volume: 9
First Page: 114
Last Page: 125
Publisher Id: TOEFJ-9-114
DOI: 10.2174/1876973X01609010114
Article History:
Received Date: 01/06/2016Revision Received Date: 19/08/2016
Acceptance Date: 22/10/2016
Electronic publication date: 07/12/2016
Collection year: 2016
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution-Non-Commercial 4.0 International Public License (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/legalcode), which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the School of Energy and Power Engineering, North China Electric Power University, Baoding, China; Tel: +8613811101294; E-mails: 394890445@qq.com, bianzhugeng04@163.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 01-06-2016 |
Original Manuscript | Thermal Stability and Adsorption of Mercury Compounds in Fly Ash | |
1. INTRODUCTION
The heavy metal Hg is a global pollutant [1Chen, J.J.; Ren, J.L.; Zhong, Y.J.; Luo, Y.Y.; Ye, S.J. Quantum chemistry study of mercury adsorption. J. Chinese Soc. Power Eng., 2010, 30(12), 960-964., 2Seigneur, C.; Vijayaraghavan, K.; Lohman, K.; Karamchandani, P.; Scott, C. Global source attribution for mercury deposition in the United States. Environ. Sci. Technol., 2004, 38(2), 555-569.
[http://dx.doi.org/10.1021/es034109t] [PMID: 14750733] ], mercury emissions from human activities almost are coal combustion and coal-fired plants have been identified as the major anthropogenic source of mercury [3Yin, L.B.; Zhuo, Y.Q.; Xu, Q.S. Mercury emission from coal-fired power plants in China. Proc. Chin. Soc. Electrical. Eng., 2013, 33(29), 1-9.-5Yudovich, Y.E.; Ketris, M.P. Mercury in coal: a review Part 2. Coal use and environmental problems. Int. J. Coal Geol., 2005, 62(3), 135-165.
[http://dx.doi.org/10.1016/j.coal.2004.11.003] ]. The reaction and migration mechanisms of mercury in fly ash is of great significance to the effective control of Hg emissions. In China, the energy supply highly depends on coal combustion, and coal consumption is expected to increase dramatically in the near future [6Zhang, L.; Wong, M.H. Environmental mercury contamination in China: sources and impacts. Environ. Int., 2007, 33(1), 108-121.
[http://dx.doi.org/10.1016/j.envint.2006.06.022] [PMID: 16914205] -8Zhang, L.; Zhuo, Y.; Chen, L.; Xu, X.; Chen, C. Mercury emissions from six coal-fired power plants in China. Fuel Process. Technol., 2008, 89(11), 1033-1040.
[http://dx.doi.org/10.1016/j.fuproc.2008.04.002] ]. Although mercury concentration in unit mass of coal is quite low, extensive utilization of coal inevitably lead to massive emission of Hg. To restrict Hg emission, the governments announced a series of ruling that regulates the Hg emissions from coal-fired power plants in the world.
The three principal forms of Hg in post-combustion flue gas are gas phase elemental mercury (Hg0), gas phase oxidized mercury (Hg2+) and particulate bound mercury (Hgp), respectively [9Galbreath, K.C.; Zygarlicke, C.J. Mercury transformations in coal combustion flue gas. Fuel Process. Technol., 2000, 65-66, 289-310.
[http://dx.doi.org/10.1016/S0378-3820(99)00102-2] -12Agarwal, H.; Romero, C.E.; Rosales, F.H.; Mendoza-Covarrubias, C. A global kinetic mechanism for the prediction of Hg oxidation by a chlorine species. Energ. Sci. Technol., 2012, 4(1), 41-54.]. Hg2+ is water soluble and it can be removed by wet flue gas desulfurization devices in progress of removing sulfur. Elemental Hg0 does not have water solubility and difficult to be removed. Hgp is easily removed by dust control equipment, such as electrostatic precipitator (ESP) or bag house filters. Lopez-Anton et al. [13Lopez-Anton, M.A.; Yuan, Y.; Perry, R.; Maroto-Valer, M.M. Analysis of mercury species present during coal combustion by thermal desorption. Fuel, 2010, 89(3), 629-634.
[http://dx.doi.org/10.1016/j.fuel.2009.08.034] , 14Rumayor, M.; Diaz-Somoano, M.; Lopez-Anton, M.A.; Martinez-Tarazona, M.R. Mercury compounds characterization by thermal desorption. Talanta, 2013, 114, 318-322.
[http://dx.doi.org/10.1016/j.talanta.2013.05.059] [PMID: 23953477] ] demonstrated that HgCl2, HgS, HgO, and HgSO4 were the main Hg species in coal-fired ash. Due to the diverse thermal stabilities of different mercury species, the mercury speciation can be identified via analyzing their thermal stabilities. Presently, the widely-used technology for direct control of mercury in fly ash is adsorption of active carbon [15Vidic, R.D.; Siler, D.P. Vapor-phase elemental mercury adsorption by activated carbon impregnated with chloride and chelating agents. Carbon, 2001, 39(1), 3-14.
[http://dx.doi.org/10.1016/S0008-6223(00)00081-6] -18Fan, L.; Ling, L.; Wang, B.; Zhang, R. The adsorption of mercury species and catalytic oxidation of Hg0 on the metal-loaded activated carbon. Appl. Catal. A Gen., 2016, 520, 13-23.
[http://dx.doi.org/10.1016/j.apcata.2016.03.036] ], nevertheless, there are some crucial drawbacks, like high carbon-to-mercury proportion and high cost, constrain wide application of mercury captured by activated carbon [19Lee, S.H.; Park, Y.O. Gas-phase mercury removal by carbon-based sorbents. Fuel Process. Technol., 2003, 84(1), 197-206.
[http://dx.doi.org/10.1016/S0378-3820(03)00055-9] -21Lopez-Antón, M.A.; Tascón, J.M.; Martínez-Tarazona, M.R. Retention of mercury in activated carbons in coal combustion and gasification flue gases. Fuel Process. Technol., 2002, 77, 353-358.
[http://dx.doi.org/10.1016/S0378-3820(02)00054-1] ]. Also, other sorbents had been examined with excellent adsorption capacity to capture mercury [22Ghorishi, S.B.; Sedman, C.B. Low concentration mercury sorption mechanisms and control by calcium-based sorbents: application in coal-fired processes. J. Air Waste Manag. Assoc., 1998, 48(12), 1191-1198.
[http://dx.doi.org/10.1080/10473289.1998.10463752] , 23Ghorishi, S.B.; Singer, C.F.; Jozewicz, W.S.; Sedman, C.B.; Srivastava, R.K. Simultaneous control of Hg0, SO2, and NOx by novel oxidized calcium-based sorbents. J. Air Waste Manag. Assoc., 2002, 52(3), 273-278.
[http://dx.doi.org/10.1080/10473289.2002.10470786] [PMID: 11924858] ], such as calcium-based sorbents and other chemicals as well as adsorption capacity of active carbon [24Vidic, R.D.; Siler, D.P. Vapor-phase elemental mercury adsorption by activated carbon impregnated with chloride and chelating agents. Carbon, 2001, 39(1), 3-14.
[http://dx.doi.org/10.1016/S0008-6223(00)00081-6] ]. In order to control the emission of Hg, lots of scholars have studied the relationship between the composition of fly ash and mercury capture. There is a large amount of literatures about factors that influence mercury capture have emerged in recent years [25Kostova, I.; Vassileva, C.; Dai, S.; Hower, J.C.; Apostolova, D. Influence of surface area properties on mercury capture behaviour of coal fly ashes from some Bulgarian power plants. Int. J. Coal Geol., 2013, 116, 227-235.
[http://dx.doi.org/10.1016/j.coal.2013.03.008] -30Tan, Z.; Xiang, J.; Su, S.; Zeng, H.; Zhou, C.; Sun, L.; Hu, S.; Qiu, J. Enhanced capture of elemental mercury by bamboo-based sorbents. J. Hazard. Mater., 2012, 239-240, 160-166.
[http://dx.doi.org/10.1016/j.jhazmat.2012.08.053] [PMID: 22995206] ], and some common views have been reached: the adsorption of mercury by fly ash is highly correlated with unburned carbon, ash particles, coal type and so on.
To understand with regard to the adsorption of mercury by sorbents, it should figure out the mercury retention mechanisms and stability of particulate mercury in ash, similarly, the identification of mercury species in ash is also important. This paper aims to obtain the mercury species and concentration in fly ash that was collected from five coal-fired power plants in China and clarify the thermal decomposition mechanism of mercury components and factors that influence the adsorption of mercury by ash.
2. MATERIAL AND METHODS
2.1. Sample Collection
Fly ash samples were collected from cold-side ESPs of five boilers under two loads. There are number of electric fields distributed along the direction of fly ash flow in each ESP, ash particles are collected from charged plates in each field. The ash samples were collected from the first and the last field in the direction of gas flow, and the collection took place continuously. The boilers burn pulverized coal of all plants. The fly ash samples were obtained at loads of 100% and 80% from the hoppers of two ESP fields. The collected ashes were labeled according to sampling location and boiler running load and ESP field. For instance, the ash collected from the first ESP field at 100% load in Baoding power plant was labeled BD-100-1. The rest boilers were from Weifang power plant (WF), Zhuhai power plant (ZH), Dongsheng power plant (DS) and Gangu power plant (GG), respectively. All the power plants are equipped with wet flue gas desulfurization system and SCR denitrification.
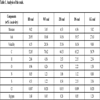
Table 1 shows the compositions of coals burned in five power plants. The content of ash and element Hg and S of BD coal are much higher compared with those of other four coals, conversely, volatile content and element Cl of BD coal are less. The moisture content of WF coal is the least, and the element Cl content is the highest, the element Hg content is slightly higher than the average mercury content of coal in China. The volatile content of ZH coal is the highest, and the ash content, element Cl and Hg are less. The element Cl content of DS coal is the least, ash and volatile contents are relatively less. The volatile content of GG coal is less, relatively, the element Cl and Hg contents are much higher.
2.2. Sample Analysis
The fly ashes had been air-dried prior to the analysis. The proportions of major elements in the coal and ash samples were determined by X-ray fluorescence spectrometry (XRF) using an Axios PW4400X spectrometer. The mean particle size measured by Horiba LA-950 laser scattering particle size distribution analyzer. The unburned carbon content of the fly ash samples was determined through loss-on-ignition tests. The mercury concentrations were determined via using a LUMEX automatic mercury analyzer, the test error was 10%. When determined the mercury content in fly ash samples, the soil (mercury concentration was 200 ng/g) was used as the standard sample, and the actual mercury content was obtained by Equation (1):
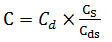 |
(1) |
Where C represents actual mercury content in ash sample in ng/g; Cd represents mercury content in ash sample measured by instrument in ng/g; Cs is actual mercury content in the standard soil sample; Cds is mercury content in the soil standard sample measured by instrument in ng/g. The actual measuring error coefficient of mercury analyzer can be acquired by testing standard soil sample, and practical mercury content in ash samples can be obtained via multiplying by measurement error coefficient and Hg concentration in ash sample which measured by instrument. The measurement error of mercury concentration in ash can be reduced in this way.
2.3. Sample Heating
Lopez-Anton et al. [31Lopez-Anton, M.A.; Perry, R.; Abad-Valle, P.; Díaz-Somoano, M.; Martínez-Tarazona, M.R.; Maroto-Valer, M.M. Speciation of mercury in fly ashes by temperature programmed decomposition. Fuel Process. Technol., 2011, 92(3), 707-711.
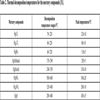
[http://dx.doi.org/10.1016/j.fuproc.2010.12.002] ] stated that the thermal decomposition temperature of HgCl2 was 70 °C. There were two different forms of crystal structure, black cube HgS and red six party HgS, respectively. The black cube HgS decomposed at the temperature of 170 °C, and at the temperature of 240 °C, the red six party HgS began to decompose. The component HgO and HgSO4 decomposed at the temperature of 200 °C and 500 °C, respectively. In this study, the fly ash samples were heated at 80, 180, 210 and 250 °C. The mercury components released at 80 °C was HgCl2, and at 180 °C the HgCl2 and HgS (black) began to decompose. At the temperature of 210 °C, the HgCl2, HgS (black) and HgO decomposed. Almost mercury components would be decomposed at the temperature of 250 °C. To ensure that the mercury compounds had been released completely, the fly ash samples would have been heated three hours at different temperatures. In order to clarify the mercury release rule with heating time at different temperatures, we measured remaining mercury content of ash sample every twenty minutes. Table 2 shows thermal decomposition temperature ranges corresponding to different mercury components.
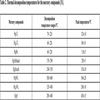
Thermal decomposition temperatures for the mercury compounds [31Lopez-Anton, M.A.; Perry, R.; Abad-Valle, P.; Díaz-Somoano, M.; Martínez-Tarazona, M.R.; Maroto-Valer, M.M. Speciation of mercury in fly ashes by temperature programmed decomposition. Fuel Process. Technol., 2011, 92(3), 707-711.
[http://dx.doi.org/10.1016/j.fuproc.2010.12.002] ].
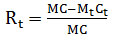 |
(2) |
Where Rt is the Hg release ratio in %; M and Mt are sample masses before and after heating, respectively, at a given temperature in g; C and Ct are the Hg concentrations of the ash samples before and after heating in ng·g-1, respectively. Rt was labeled according to the decomposition temperature of different mercury components, for instance, the HgCl2 decomposes at the temperature of 80 °C, therefore, R80 is the ratio of HgCl2 to total mercury concentration in ash sample.
3. RESULTS AND DISCUSSION
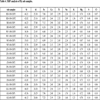
Table 3 presents experimental results of ash samples, including Hg concentration, unburned carbon content and mean ash particle size. Different ash samples have diverse Hg concentration, unburned carbon content and mean ash particle size. The average mercury concentration of BD and GG ash are the highest, and correspond to the highest content of unburned carbon and the minimal particle mean size. ZH ash has the least mercury concentration and unburned carbon content, the particle mean size is relatively maximum.
3.1. Unburned Carbon and Adsorption of Mercury
Unburned carbonaceous fraction in fly ash has been caused for considerable concern in recent years because of its important role in capturing mercury [32Hower, J.C.; Senior, C.L.; Suuberg, E.M.; Hurt, R.H.; Wilcox, J.L.; Olson, E.S. Mercury capture by native fly ash carbons in coal-fired power plants. Pror. Energy Combust. Sci., 2010, 36(4), 510-529.
[http://dx.doi.org/10.1016/j.pecs.2009.12.003] [PMID: 24223466] ]. Unburned carbon in fly ash can both adsorb and oxidize mercury, and mercury adsorption on active carbon sites is a complex phenomenon that depend on temperature, pressure, ambience and so on [33Senior, C.L.; Johnson, S.A. Impact of carbon-in-ash on mercury removal across particulate control devices in coal-fired power plants. Energy Fuels, 2005, 19(3), 859-863.
[http://dx.doi.org/10.1021/ef049861+] ]. There is a great deal of different unburned carbon types that be found in the ash, and the general predominant configuration is char derived from coal pyrolysis. The key features to the potential of unburned carbon to mercury adsorb are its amount, surface area and numbers of active sites. A voluminous literature exists regarding to the amount of unburned carbon in fly ash plays an important role in determining its capacity to adsorb mercury [33Senior, C.L.; Johnson, S.A. Impact of carbon-in-ash on mercury removal across particulate control devices in coal-fired power plants. Energy Fuels, 2005, 19(3), 859-863.
[http://dx.doi.org/10.1021/ef049861+] -35Yang, H.; Xu, Z.; Fan, M.; Bland, A.E.; Judkins, R.R. Adsorbents for capturing mercury in coal-fired boiler flue gas. J. Hazard. Mater., 2007, 146(1-2), 1-11.
[http://dx.doi.org/10.1016/j.jhazmat.2007.04.113] [PMID: 17544578] ].
Fig. (1) shows correlation between unburned carbon content and adsorption of mercury in fly ash. With comparison and analysis, it can be found that the correlation is well between carbon content and mercury adsorb in ZH fly ash sample, the correlation coefficient R2 is 0.97. The correlation is relatively poor of unburned carbon and mercury captured by fly ash in WF ash sample, the correlation coefficient R2 is 0.66. The correlation coefficients of carbon content and mercury capture in other fly ash samples are between 0.66 and 0.97. If all samples are taken into account, the overall correlation coefficient R2 is 0.83, which shows that there is a significantly positive correlation between unburned carbon content and mercury capture in fly ash sample. High carbon content in fly ash means that the ash samples contain more coal chars and unburned carbon particles. There are lots of active carbon sites and oxygen containing functional groups, like carboxyl group, hydroxyl group and alpha oxygen group. These active sites and functional groups can provide adsorption sites for particulate mercury. Therefore, the more carbon particles in fly ash, the more coal char with active sites and oxygen containing functional groups can capture a large amount of mercury particles.
3.2. Ash Particle and Adsorption of Mercury
The influence of mean ash particle size on mercury capture is shown in Fig. (2). Apparently, the correlation between ash size and mercury capture is relatively good, as represented by the wide scatter in data. It can be found that the smaller ash particles, the more mercury grains be captured. Lower triangular represents BD fly ash sample, distribution of these data is more extensive, the correlation coefficient R2 is 0.67. Upper triangular represents WF ash sample, the correlation between mean particle size and mercury capture is good, the correlation coefficient R2 reaches 0.86. Circles represent ZH ash sample, the mean size of these ash particles is much larger, the content of mercury be captured is little, and the correlation coefficient R2 is 0.81. Blocks indicate GG fly ash samples, these ash samples with small particle size, thus, the content of mercury be adsorbed is quite large, the correlation coefficient R2 is 0.83. Cross represent DS ash sample and the correlation coefficient R2 is the largest, it can reach 0.98. Considering all these ash samples, the relation of mean ash particle size and mercury capture does appear positive correlation, the correlation coefficient R2 is 0.60, thus, ash particle size is an important factor to influence the mercury capture. The pores of fly ash change and develop continuously in the process of electrostatic dust removing, the wider the pore distribution of ash, the more favorable to the mercury be captured by fly ash particles. If the mean ash particle size is larger, the distribution of particles is more dispersed and it is not conducive to the mercury captured. On the contrary, the smaller mean ash particles size, the more closer of the ash particles, thus, the specific surface area becomes greater, which can form aggregate with large pores and then capture mercury easily.
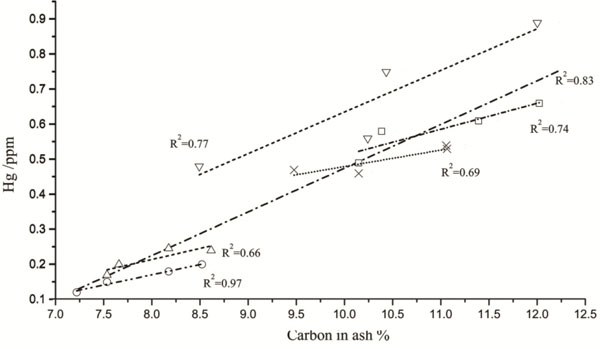 |
Fig. (1) Mercury captured by all of the ashes versus carbon in ash. |
▼BD ash; ▲WF ash; ○ZH ash; ×DS ash; □GG ash
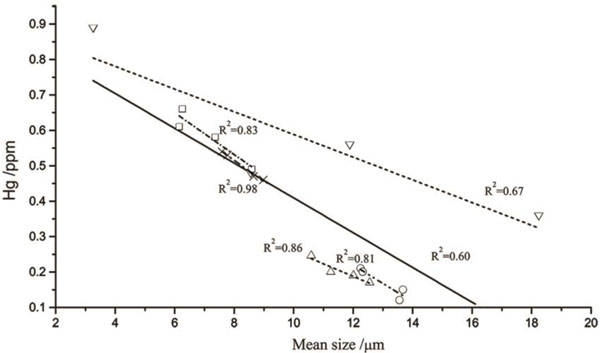 |
Fig. (2) Mercury captured by all of the ashes versus ash particle size. |
▼BD ash;▲WF ash;○ZH ash; ×DS ash;□GG ash
3.3. Coal Rank and Adsorption of Mercury
Coal rank is an important factor in determining the configuration and type on unburned carbon in fly ash and components of gas. Low-rank coal does not undergo thermoplastic transitions, whereas bituminous coal generally expands and undergo thermoplastic transitions when temperature upon 300°C [36Gray, R.J.; DeVanney, K.F. Coke carbon forms: microscopic classification and industrial applications. Int. J. Coal Geol., 1986, 6(3), 277-297.
[http://dx.doi.org/10.1016/0166-5162(86)90005-4] ]. Coals of anthracite rank generally do not display thermoplastic properties. Jennifer et al. [37Wilcox, J.; Rupp, E.; Ying, S.C.; Lim, D.H.; Negreira, A.S.; Kirchofer, A.; Lee, K. Mercury adsorption and oxidation in coal combustion and gasification processes. Int. J. Coal Geol., 2012, 90, 4-20.
[http://dx.doi.org/10.1016/j.coal.2011.12.003] ] proposed that carbon from low-rank coals have high mercury capture efficiency. High-rank coals can produce lower carbon-content ash with less oxygen containing functional groups in unburned carbon, whereas high concentration of unburned carbon is usually found in ash produced from low-rank coals. Hower [38Hower, J.C.; Kostova, I.J. Comparative studies of mercury capture by Bulgarian and Kentucky fly ash carbons. Annual Meeting, 2008, , pp. 6-9.] demonstrated that low-carbon Bulgarian fly ash sourced from low-rank coals has a greater tendency to capture Hg than does bituminous-sourced Kentucky fly ashes. Table 1 shows that BD and GG coal rank are lower than others coals, ZH and DS coal are closely ranked, WF coal has the highest rank. Given that BD and GG ashes have high mercury concentration, and ZH ash has the least mercury concentration. Experimental results show that the mercury content level in fly ash corresponds to coal rank, it can also be considered that the lower coal rank, the more mercury may be captured by fly ash. The experimental results are consistent with Hower’s conclusions [38Hower, J.C.; Kostova, I.J. Comparative studies of mercury capture by Bulgarian and Kentucky fly ash carbons. Annual Meeting, 2008, , pp. 6-9.].
3.4. Chemical Composition and Adsorption of Mercury
Adsorption of mercury by fly ash are not only influenced by physical adsorption, but also affected by chemical composition in fly ash. In order to clarify chemical component influence on mercury capture, XRF was applied to measure primary element in ash sample, and the detailed data had been listed in Table 4. There are primary element Si, Al, Fe, Ca, Ti, Na and K in ash samples. Pavlish et al. [39Pavlish, J.H.; Sondreal, E.A.; Mann, M.D.; Olson, E.S.; Galbreath, K.C.; Laudal, D.L.; Benson, S.A. Status review of mercury control options for coal-fired power plants. Fuel Process. Technol., 2003, 82(2), 89-165.
[http://dx.doi.org/10.1016/S0378-3820(03)00059-6] ] suggested that Fe oxides can be used as a catalyst to catalyze the reaction of HCl and Hg. Galbreath [40Galbreath, K.C.; Zygarlicke, C.J. Mercury transformations in coal combustion flue gas. Fuel Process. Technol., 2000, 65, 289-310.
[http://dx.doi.org/10.1016/S0378-3820(99)00102-2] ] demonstrated that element Ca bonded to active carbon can provide high reactivity sites to adsorb particulate mercury. To a certain extent, element S and Cl can promote the adsorption of mercury [41Yao, Y.; Velpari, V.; Economy, J. Design of sulfur treated activated carbon fibers for gas phase elemental mercury removal. Fuel, 2014, 116, 560-565.
[http://dx.doi.org/10.1016/j.fuel.2013.08.063] -43Xu, Y.; Zeng, X.; Luo, G.; Zhang, B.; Xu, P.; Xu, M.; Yao, H. Chlorine-Char composite synthesized by co-pyrolysis of biomass wastes and polyvinyl chloride for elemental mercury removal. Fuel, 2016, 183, 73-79.
[http://dx.doi.org/10.1016/j.fuel.2016.06.024] ]. In Table 4, it can be found that BD and WF ash have similar element Fe, Cl and S content, BD ash has the much higher element Ca and corresponds to high mercury in ash. The element Fe, Ca, S and Cl concentration in ZH ash are higher than that of DS ash, but ZH ash has less mercury content compared with DS ash. The element Fe, Ca and Cl contents in DS ash are similar to GG ash, the element S concentration in DS ash is higher than that of GG ash, but DS ash has less mercury content than that of GG ash. There is no significant correlation between element Fe, Ca, S, Cl concentration and mercury content in fly ash, it indicates that physical adsorption plays a major role in capturing mercury. Although chemical component may promote the adsorption of mercury, it has little influence than physical adsorption.
3.5. Thermal Stability of Mercury Component
Fig. (3) shows the ratios of different Hg compounds in fly ash samples. The major Hg species in all of the ashes are HgCl2, HgS (black, red), and HgO. Hg2SO4 and Hg2Cl2 are unstable, Hg2Cl2 easily break into Hg and HgCl2 with light. Similarly, Hg2SO4 easily decompose to Hg and HgSO4. The content of element bromine is little in coals, thus, HgBr2 can be ignored.
The HgCl2 concentrations in ashes are significantly different. WF coal has the highest Cl content, and the mean HgCl2 ratios of WF coal ashes exceed 30%. By contrast, the Cl content of BD coal is much lower compared with other coal samples. As a result, the HgCl2 ratios of BD coal ashes are the lowest at less than 5.5%. Given the information above, the high Cl content in coal can lead to a high HgCl2 ratio in fly ash. From the Fig. (3) it can be observed that the ratios of HgO are little in all fly ash samples. The ratio of HgO in WF ash sample is about 5%, the highest ratio is ZH fly ash sample and it can reach 13%. The content of HgSO4 is the least and it can be neglected.
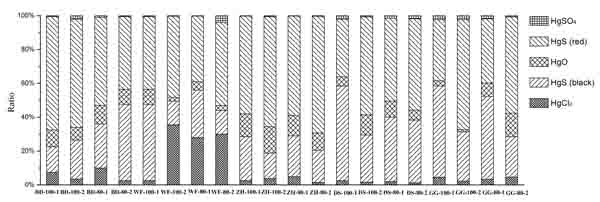 |
Fig. (3) Ratios of different mercury compounds in the ashes. |
Fig. (4a and b) show the release curves of Hg compounds in ten fly ash samples at four different temperatures. All ash samples were collected from the first ESP field under two loads of each power plants.
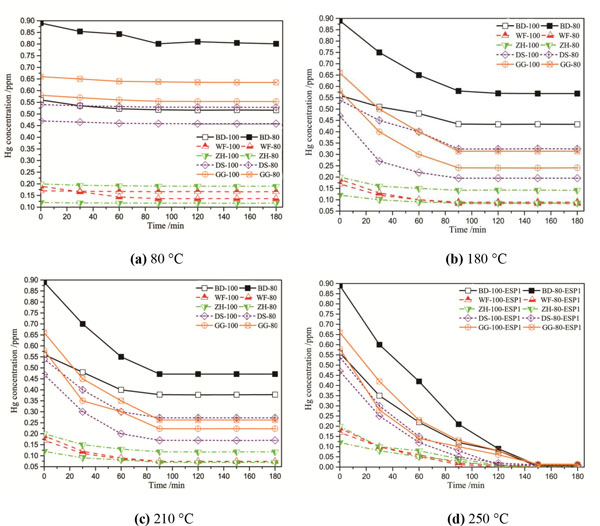 |
Fig. (4) Release of mercury species in fly ash at four temperatures. |
Fig. (4a) shows the release curves of Hg compounds in different ash samples at the temperature of 80 °C. According to Lopez-Anton et al. [31Lopez-Anton, M.A.; Perry, R.; Abad-Valle, P.; Díaz-Somoano, M.; Martínez-Tarazona, M.R.; Maroto-Valer, M.M. Speciation of mercury in fly ashes by temperature programmed decomposition. Fuel Process. Technol., 2011, 92(3), 707-711.
[http://dx.doi.org/10.1016/j.fuproc.2010.12.002] ], the HgCl2 captured by fly ash begins to release at 70 °C. Thus, the capture of HgCl2 by ashes would be detected in the decrease in mercury concentrations when the ashes were heated at 80 °C. After heating 180 minutes, the content of released mercury is still less than 10%, it indicates that the amount of HgCl2 is little in fly ash samples. A large number of studies on the reaction of Hg in flue gas have demonstrate that chlorine is the key element for the oxidation of Hg0 and generation of Hgp, the lower element chlorine content in raw coal leads to low HgCl2 concentration in fly ash. It indicates that the Cl content of raw coal is the key factor to affect HgCl2 content in fly ash. Fig. (4b) shows the release curves of Hg compounds in ash samples at the heating temperature of 180 °C. According to Table 2, black cube HgS begins to release at temperature of 170 °C. Thus, the mercury released at heating temperature of 180 °C should be HgCl2 and HgS (black). It can be found that the content of HgCl2 and black cube HgS are different in diverse samples, these two kinds of mercury components decompose rapidly in the previous half of hour, and release completely after one hour. Finally, the remaining mercury contents in ash samples keep to a constant. Fig. (4c) shows the release curves of Hg compounds at the temperature of 210 °C. According to Table 2, HgO begins to release at temperature of 200 °C. Therefore, the mercury released at heating temperature of 210 °C should be HgCl2, black cube HgS and HgO. In this Figure, it can be observed that nearly half of mercury compounds have be decomposed. The mercury components release rapidly in the previous hour, and after continuously heating for half an hour the mercury contents remain basically constant. The experimental results indicate that HgCl2, black cube HgS and HgO contents represent half of the total mercury concentrations. Fig. (4d) shows the release profiles of Hg compounds in fly ash samples at the heating temperature of 250 °C. According to Table 2, the heating temperature 250 °C is higher than that of the mercury compounds decomposition except for HgSO4. Therefore, when the fly ash samples are heated at 250 °C continuously, almost all of the mercury components will be released in ash samples. In Fig. (4d), all curves close to the X axis at 150 minutes, the amount of mercury compounds close to zero.
Meij et al. [44Meij, R. The distribution of trace elements during the combustion of coal. In: Environmental aspects of trace elements in coal; Dalway, J. S.;Fari, G.; Eds.; Kluwer Academic Publishers: Boston , 1995; pp. 111-127.] considered that the chlorine can influence the adsorption of mercury. Lee [45Lee, S.J.; Seo, Y.C.; Jurng, J.; Lee, T.G. Removal of gas-phase elemental mercury by iodine-and chlorine-impregnated activated carbons. Atmos. Environ., 2004, 38(29), 4887-4893.
[http://dx.doi.org/10.1016/j.atmosenv.2004.05.043] ] proposed that chlorine plays an important role in the sorption of mercury in fly ash. Kellie et al. [46Kellie, S.; Cao, Y.; Duan, Y.; Li, L.; Chu, P.; Mehta, A.; Pan, W.P. Factors affecting mercury speciation in a 100-MW coal-fired boiler with low-NOx burners. Energy Fuels, 2005, 19(3), 800-806.
[http://dx.doi.org/10.1021/ef049769d] ] suggested that high element chlorine in coal, which can generate high HCl concentration in flue gas and can promote formation of Hg2+. HCl is the exclusive form of halogen in flue gas, and it can promote Hg oxidation on the zigzag carbon edge site of the unburned carbon. Reactions of Hg and Cl are included in homogeneous and heterogeneous reaction. Homogeneous reaction mechanisms include reaction (3) to (6) [47Sliger, R.N.; Kramlich, J.C.; Marinov, N.M. Towards the development of a chemical kinetic model for the homogeneous oxidation of mercury by chlorine species. Fuel Process. Technol., 2000, 65, 423-438.
[http://dx.doi.org/10.1016/S0378-3820(99)00108-3] ].
| Hg0 + HCl(g) → HgCl(g) + H | (3) |
| Hg0 + Cl2(g) → HgCl(g) + Cl(g) | (4) |
| HgCl(g) + Cl2(g) → HgCl2(g) + Cl | (5) |
| HgCl(g) + HCl(g) → HgCl2(g) + H | (6) |
Where the (g) represents gaseous phase.
Element mercury can be oxidized to gaseous HgCl by gaseous HCl and Cl2, and then some gaseous HgCl be oxidized thoroughly to gaseous HgCl2. HgCl2 is less volatile and could begin to condense on the surface of fly ash particles when the temperature is not too high (below 140°C) in the ESP [44Meij, R. The distribution of trace elements during the combustion of coal. In: Environmental aspects of trace elements in coal; Dalway, J. S.;Fari, G.; Eds.; Kluwer Academic Publishers: Boston , 1995; pp. 111-127.]. As well as heterogeneous reaction mechanisms include reactions (7) to (10) [48Presto, A.A.; Granite, E.J. Survey of catalysts for oxidation of mercury in flue gas. Environ. Sci. Technol., 2006, 40(18), 5601-5609.
[http://dx.doi.org/10.1021/es060504i] [PMID: 17007115] ].
| Hg(g)→Hg(ad) | (7) |
| Cl(g)→Cl(ad) | (8) |
| Hg(ad)+Cl(ad)→HgCl(ad) | (9) |
| HgCl(ad)→HgCl(g) | (10) |
Where the (g) represents gaseous phase, the (ad) represents reactant be adsorbed to the solid surface.
In the process of heterogeneous reaction, firstly, gaseous elemental mercury and chlorine adsorb on ash particles, and then reaction occurs between mercury and chlorine via equation (9). Whether adsorbed HgCl releases depend on the bond type and energy between the HgCl and ash particles. Although Cl/Hg rate of WF coal is about 15 times higher than that of BD coal, the mercury concentration in WF ash is one-third of that in BD ash. Similarly, Cl/Hg rate of ZH coal is 14 times higher than that of BD coal, but mercury content in ZH ash is less than that of BD ash. Consequently, it can be found that there does not exist obvious correlation between chlorine content and mercury adsorbed by ash particles. This experimental results consistent with views of Rubel [49Rubel, A.M.; Hower, J.C.; Mardon, S.M.; Zimmerer, M.J. Thermal stability of mercury captured by ash. Fuel, 2006, 85(17-18), 2509-2515.
[http://dx.doi.org/10.1016/j.fuel.2006.05.007] ]. But high content of chlorine in coal can lead to increase of HgCl2 content in ash.
A distinctive feature shown in Fig. (3) is with regard to the high HgS ratios in all of ash samples. The HgS ratios of BD ash samples, WF ash samples, ZH ash samples, DS ash samples and GG ash samples are 83.7% 70.0% 83.8% 88.5% and 88.0% respectively. The formation of HgS requires reduced S; however, the S in coal is supposed to be oxidized to SO2 in an oxidation atmosphere, such as the coal combustion process. The S in coal can only transform into H2S or other species containing reductive S in reductive processes, such as coal pyrolysis. Therefore, HgS can be formed through reactions (11) to (13) [50Morimoto, T.; Wu, S.; Uddin, M.A.; Sasaoka, E. Characteristics of the mercury vapor removal from coal combustion flue gas by activated carbon using H2S. Fuel, 2005, 84(14), 1968-1974.
[http://dx.doi.org/10.1016/j.fuel.2005.04.007] ].
| H2S + 1/2 O2 → H2O + S(ad) | (11) |
| SO2 + 2H2S → 3S(ad) + 2H2O | (12) |
| S(ad)+ Hg0 → HgS | (13) |
Where the (ad) represents elemental sulfur adsorbs on char surface.
H2S can be oxidized to elemental sulfur by oxygen or SO2 on surface of char, and then adsorbed sulfur can capture elemental mercury by forming Hg-S bond. Therefore, elemental sulfur can play a positive role in capturing mercury. Rubel et al. [49Rubel, A.M.; Hower, J.C.; Mardon, S.M.; Zimmerer, M.J. Thermal stability of mercury captured by ash. Fuel, 2006, 85(17-18), 2509-2515.
[http://dx.doi.org/10.1016/j.fuel.2006.05.007] ] demonstrated that the good correlation exists between sulfur and mercury capture. Hsi et al. [51Hsi, H.C.; Chen, S.; Rostam-Abadi, M.; Rood, M.J.; Richardson, C.F.; Carey, T.R.; Chang, R. Preparation and evaluation of coal-derived activated carbons for removal of mercury vapor from simulated coal combustion flue gases. Energy Fuels, 1998, 12(6), 1061-1070.
[http://dx.doi.org/10.1021/ef9801064] ] reported that unburned carbon derived from high sulfur coals have shown to have higher mercury adsorption capacities than those from low sulfur coals. Rostam-Abadi et al. [52Rostam-Abadi, M.; Chen, S.; Hsi, H.C.; Rood, M.J.; Carey, T.R.; Richardson, C.F.; Chang, R. Development and Evaluation of Low-Cost Sorbents for Removal of Mercury Emissions from Coal Combustion Flue Gas; EPRI Report TE-114043, 1998.] suggested that sulfur functional groups can increase the mercury adsorption capacity of active carbons. BD and GG coal have much higher sulfur content compared with other coals, similarly, BD and GG coal ashes have high mercury concentration. ZH coal have the least sulfur content and corresponds to the least mercury concentration in ash. The positive correlation between mercury and sulfur indicated that mercury maybe deposited on ash as a sulfur compound.
CONCLUSION
Promoting the transformation of Hg0 to Hgp helps to control gaseous mercury emission. However, whether the mercury captured by ash still has a potential to pollute the environment depends on thermal and chemical stabilities. The experimental results indicate that there exists a good correlation between mercury capture and carbon particles. The unburned carbon content and adsorption of mercury show a significantly positive correlation. On the contrary, there is a clearly negative correlation between mean ash particle size and mercury capture, the smaller the mean size of ash particles, the more mercury can be captured by ash. Also, the experimental results indicate that the lower the coal rank, the more mercury can be adsorbed on ash particles. Physical adsorption plays a major role in the adsorption of mercury. High Cl content in coal does not absolutely result in capturing more mercury but it can lead to a high HgCl2 ratio in particulate mercury. Element sulfur in coal is an important factor in adsorption of mercury by fly ash. The HgO concentration is less, and HgSO4 ratio is negligible in all of ashes and the main formation of mercury in fly ash are HgCl2 and HgS.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict interest.
ACKNOWLEDGEMENTS
This study was financially supported by the Research project of Southern Power Grid (No. K-GD2014-173) .
REFERENCES
| [1] | Chen, J.J.; Ren, J.L.; Zhong, Y.J.; Luo, Y.Y.; Ye, S.J. Quantum chemistry study of mercury adsorption. J. Chinese Soc. Power Eng., 2010, 30(12), 960-964. |
| [2] | Seigneur, C.; Vijayaraghavan, K.; Lohman, K.; Karamchandani, P.; Scott, C. Global source attribution for mercury deposition in the United States. Environ. Sci. Technol., 2004, 38(2), 555-569. [http://dx.doi.org/10.1021/es034109t] [PMID: 14750733] |
| [3] | Yin, L.B.; Zhuo, Y.Q.; Xu, Q.S. Mercury emission from coal-fired power plants in China. Proc. Chin. Soc. Electrical. Eng., 2013, 33(29), 1-9. |
| [4] | Gao, Z.Y.; Zhou, L.M.; Yin, L.B. Effect of calcium addition on formation of particulate mercury in coal combustion process. J. Chin. Soc. Power Eng., 2012, 12(12), 954-989. |
| [5] | Yudovich, Y.E.; Ketris, M.P. Mercury in coal: a review Part 2. Coal use and environmental problems. Int. J. Coal Geol., 2005, 62(3), 135-165. [http://dx.doi.org/10.1016/j.coal.2004.11.003] |
| [6] | Zhang, L.; Wong, M.H. Environmental mercury contamination in China: sources and impacts. Environ. Int., 2007, 33(1), 108-121. [http://dx.doi.org/10.1016/j.envint.2006.06.022] [PMID: 16914205] |
| [7] | You, C.F.; Xu, X.C. Coal combustion and its pollution control in China. Energy, 2010, 35(11), 4467-4472. [http://dx.doi.org/10.1016/j.energy.2009.04.019] |
| [8] | Zhang, L.; Zhuo, Y.; Chen, L.; Xu, X.; Chen, C. Mercury emissions from six coal-fired power plants in China. Fuel Process. Technol., 2008, 89(11), 1033-1040. [http://dx.doi.org/10.1016/j.fuproc.2008.04.002] |
| [9] | Galbreath, K.C.; Zygarlicke, C.J. Mercury transformations in coal combustion flue gas. Fuel Process. Technol., 2000, 65-66, 289-310. [http://dx.doi.org/10.1016/S0378-3820(99)00102-2] |
| [10] | Senior, C.L.; Sarofim, A.F.; Zeng, T.; Helble, J.J.; Mamani-Paco, R. Gas-phase transformations of mercury in coal-fired power plants. Fuel Process. Technol., 2000, 63(2-3), 197-213. [http://dx.doi.org/10.1016/S0378-3820(99)00097-1] |
| [11] | Eswaran, S.; Stenger, H.G. Effect of halogens on mercury conversion in SCR catalysts. Fuel Process. Technol., 2008, 89(11), 1153-1159. [http://dx.doi.org/10.1016/j.fuproc.2008.05.007] |
| [12] | Agarwal, H.; Romero, C.E.; Rosales, F.H.; Mendoza-Covarrubias, C. A global kinetic mechanism for the prediction of Hg oxidation by a chlorine species. Energ. Sci. Technol., 2012, 4(1), 41-54. |
| [13] | Lopez-Anton, M.A.; Yuan, Y.; Perry, R.; Maroto-Valer, M.M. Analysis of mercury species present during coal combustion by thermal desorption. Fuel, 2010, 89(3), 629-634. [http://dx.doi.org/10.1016/j.fuel.2009.08.034] |
| [14] | Rumayor, M.; Diaz-Somoano, M.; Lopez-Anton, M.A.; Martinez-Tarazona, M.R. Mercury compounds characterization by thermal desorption. Talanta, 2013, 114, 318-322. [http://dx.doi.org/10.1016/j.talanta.2013.05.059] [PMID: 23953477] |
| [15] | Vidic, R.D.; Siler, D.P. Vapor-phase elemental mercury adsorption by activated carbon impregnated with chloride and chelating agents. Carbon, 2001, 39(1), 3-14. [http://dx.doi.org/10.1016/S0008-6223(00)00081-6] |
| [16] | Serre, S.D.; Silcox, G.D. Adsorption of elemental mercury on the residual carbon in coal fly ash. Ind. Eng. Chem. Res., 2000, 39(6), 1723-1730. [http://dx.doi.org/10.1021/ie990680i] |
| [17] | Yan, R.; Liang, D.T.; Tsen, L.; Wong, Y.P.; Lee, Y.K. Bench-scale experimental evaluation of carbon performance on mercury vapour adsorption. Fuel, 2004, 83(17), 2401-2409. [http://dx.doi.org/10.1016/j.fuel.2004.06.031] |
| [18] | Fan, L.; Ling, L.; Wang, B.; Zhang, R. The adsorption of mercury species and catalytic oxidation of Hg0 on the metal-loaded activated carbon. Appl. Catal. A Gen., 2016, 520, 13-23. [http://dx.doi.org/10.1016/j.apcata.2016.03.036] |
| [19] | Lee, S.H.; Park, Y.O. Gas-phase mercury removal by carbon-based sorbents. Fuel Process. Technol., 2003, 84(1), 197-206. [http://dx.doi.org/10.1016/S0378-3820(03)00055-9] |
| [20] | Li, Y.H.; Lee, C.W.; Gullett, B.K. Importance of activated carbon's oxygen surface functional groups on elemental mercury adsorption. Fuel, 2003, 82(4), 451-457. [http://dx.doi.org/10.1016/S0016-2361(02)00307-1] |
| [21] | Lopez-Antón, M.A.; Tascón, J.M.; Martínez-Tarazona, M.R. Retention of mercury in activated carbons in coal combustion and gasification flue gases. Fuel Process. Technol., 2002, 77, 353-358. [http://dx.doi.org/10.1016/S0378-3820(02)00054-1] |
| [22] | Ghorishi, S.B.; Sedman, C.B. Low concentration mercury sorption mechanisms and control by calcium-based sorbents: application in coal-fired processes. J. Air Waste Manag. Assoc., 1998, 48(12), 1191-1198. [http://dx.doi.org/10.1080/10473289.1998.10463752] |
| [23] | Ghorishi, S.B.; Singer, C.F.; Jozewicz, W.S.; Sedman, C.B.; Srivastava, R.K. Simultaneous control of Hg0, SO2, and NOx by novel oxidized calcium-based sorbents. J. Air Waste Manag. Assoc., 2002, 52(3), 273-278. [http://dx.doi.org/10.1080/10473289.2002.10470786] [PMID: 11924858] |
| [24] | Vidic, R.D.; Siler, D.P. Vapor-phase elemental mercury adsorption by activated carbon impregnated with chloride and chelating agents. Carbon, 2001, 39(1), 3-14. [http://dx.doi.org/10.1016/S0008-6223(00)00081-6] |
| [25] | Kostova, I.; Vassileva, C.; Dai, S.; Hower, J.C.; Apostolova, D. Influence of surface area properties on mercury capture behaviour of coal fly ashes from some Bulgarian power plants. Int. J. Coal Geol., 2013, 116, 227-235. [http://dx.doi.org/10.1016/j.coal.2013.03.008] |
| [26] | Wiatros-Motyka, M.M.; Sun, C.G.; Stevens, L.A.; Snape, C.E. High capacity co-precipitated manganese oxides sorbents for oxidative mercury capture. Fuel, 2013, 109, 559-562. [http://dx.doi.org/10.1016/j.fuel.2013.03.019] |
| [27] | Saha, A.; Abram, D.N.; Kuhl, K.P.; Paradis, J.; Crawford, J.L.; Sasmaz, E.; Chang, R.; Jaramillo, T.F.; Wilcox, J. An X-ray photoelectron spectroscopy study of surface changes on brominated and sulfur-treated activated carbon sorbents during mercury capture: performance of pellet versus fiber sorbents. Environ. Sci. Technol., 2013, 47(23), 13695-13701. [http://dx.doi.org/10.1021/es403280z] [PMID: 24256554] |
| [28] | Lopez-Anton, M.A.; Rumayor, M.; Díaz-Somoano, M.; Martínez-Tarazona, M.R. Influence of a CO2-enriched flue gas on mercury capture by activated carbons. Chem. Eng. J., 2015, 262, 1237-1243. [http://dx.doi.org/10.1016/j.cej.2014.10.088] |
| [29] | Fuente-Cuesta, A.; Diaz-Somoano, M.; Lopez-Anton, M.A.; Cieplik, M.; Fierro, J.L.; Martínez-Tarazona, M.R. Biomass gasification chars for mercury capture from a simulated flue gas of coal combustion. J. Environ. Manage., 2012, 98, 23-28. [http://dx.doi.org/10.1016/j.jenvman.2011.12.013] [PMID: 22325640] |
| [30] | Tan, Z.; Xiang, J.; Su, S.; Zeng, H.; Zhou, C.; Sun, L.; Hu, S.; Qiu, J. Enhanced capture of elemental mercury by bamboo-based sorbents. J. Hazard. Mater., 2012, 239-240, 160-166. [http://dx.doi.org/10.1016/j.jhazmat.2012.08.053] [PMID: 22995206] |
| [31] | Lopez-Anton, M.A.; Perry, R.; Abad-Valle, P.; Díaz-Somoano, M.; Martínez-Tarazona, M.R.; Maroto-Valer, M.M. Speciation of mercury in fly ashes by temperature programmed decomposition. Fuel Process. Technol., 2011, 92(3), 707-711. [http://dx.doi.org/10.1016/j.fuproc.2010.12.002] |
| [32] | Hower, J.C.; Senior, C.L.; Suuberg, E.M.; Hurt, R.H.; Wilcox, J.L.; Olson, E.S. Mercury capture by native fly ash carbons in coal-fired power plants. Pror. Energy Combust. Sci., 2010, 36(4), 510-529. [http://dx.doi.org/10.1016/j.pecs.2009.12.003] [PMID: 24223466] |
| [33] | Senior, C.L.; Johnson, S.A. Impact of carbon-in-ash on mercury removal across particulate control devices in coal-fired power plants. Energy Fuels, 2005, 19(3), 859-863. [http://dx.doi.org/10.1021/ef049861+] |
| [34] | Hower, J.C.; Maroto-Valer, M.M.; Taulbee, D.N.; Sakulpitakphon, T. Mercury capture by distinct fly ash carbon forms. Energy Fuels, 2000, 14(1), 224-226. [http://dx.doi.org/10.1021/ef990192n] |
| [35] | Yang, H.; Xu, Z.; Fan, M.; Bland, A.E.; Judkins, R.R. Adsorbents for capturing mercury in coal-fired boiler flue gas. J. Hazard. Mater., 2007, 146(1-2), 1-11. [http://dx.doi.org/10.1016/j.jhazmat.2007.04.113] [PMID: 17544578] |
| [36] | Gray, R.J.; DeVanney, K.F. Coke carbon forms: microscopic classification and industrial applications. Int. J. Coal Geol., 1986, 6(3), 277-297. [http://dx.doi.org/10.1016/0166-5162(86)90005-4] |
| [37] | Wilcox, J.; Rupp, E.; Ying, S.C.; Lim, D.H.; Negreira, A.S.; Kirchofer, A.; Lee, K. Mercury adsorption and oxidation in coal combustion and gasification processes. Int. J. Coal Geol., 2012, 90, 4-20. [http://dx.doi.org/10.1016/j.coal.2011.12.003] |
| [38] | Hower, J.C.; Kostova, I.J. Comparative studies of mercury capture by Bulgarian and Kentucky fly ash carbons. Annual Meeting, 2008, , pp. 6-9. |
| [39] | Pavlish, J.H.; Sondreal, E.A.; Mann, M.D.; Olson, E.S.; Galbreath, K.C.; Laudal, D.L.; Benson, S.A. Status review of mercury control options for coal-fired power plants. Fuel Process. Technol., 2003, 82(2), 89-165. [http://dx.doi.org/10.1016/S0378-3820(03)00059-6] |
| [40] | Galbreath, K.C.; Zygarlicke, C.J. Mercury transformations in coal combustion flue gas. Fuel Process. Technol., 2000, 65, 289-310. [http://dx.doi.org/10.1016/S0378-3820(99)00102-2] |
| [41] | Yao, Y.; Velpari, V.; Economy, J. Design of sulfur treated activated carbon fibers for gas phase elemental mercury removal. Fuel, 2014, 116, 560-565. [http://dx.doi.org/10.1016/j.fuel.2013.08.063] |
| [42] | Sowlat, M.H.; Abdollahi, M.; Gharibi, H.; Yunesian, M.; Rastkari, N. Removal of vapor-phase elemental mercury from stack emissions with sulfur-impregnated activated carbon; Springer International Publishing, 2014, pp. pp. 1-34. |
| [43] | Xu, Y.; Zeng, X.; Luo, G.; Zhang, B.; Xu, P.; Xu, M.; Yao, H. Chlorine-Char composite synthesized by co-pyrolysis of biomass wastes and polyvinyl chloride for elemental mercury removal. Fuel, 2016, 183, 73-79. [http://dx.doi.org/10.1016/j.fuel.2016.06.024] |
| [44] | Meij, R. The distribution of trace elements during the combustion of coal. In: Environmental aspects of trace elements in coal; Dalway, J. S.;Fari, G.; Eds.; Kluwer Academic Publishers: Boston , 1995; pp. 111-127. |
| [45] | Lee, S.J.; Seo, Y.C.; Jurng, J.; Lee, T.G. Removal of gas-phase elemental mercury by iodine-and chlorine-impregnated activated carbons. Atmos. Environ., 2004, 38(29), 4887-4893. [http://dx.doi.org/10.1016/j.atmosenv.2004.05.043] |
| [46] | Kellie, S.; Cao, Y.; Duan, Y.; Li, L.; Chu, P.; Mehta, A.; Pan, W.P. Factors affecting mercury speciation in a 100-MW coal-fired boiler with low-NOx burners. Energy Fuels, 2005, 19(3), 800-806. [http://dx.doi.org/10.1021/ef049769d] |
| [47] | Sliger, R.N.; Kramlich, J.C.; Marinov, N.M. Towards the development of a chemical kinetic model for the homogeneous oxidation of mercury by chlorine species. Fuel Process. Technol., 2000, 65, 423-438. [http://dx.doi.org/10.1016/S0378-3820(99)00108-3] |
| [48] | Presto, A.A.; Granite, E.J. Survey of catalysts for oxidation of mercury in flue gas. Environ. Sci. Technol., 2006, 40(18), 5601-5609. [http://dx.doi.org/10.1021/es060504i] [PMID: 17007115] |
| [49] | Rubel, A.M.; Hower, J.C.; Mardon, S.M.; Zimmerer, M.J. Thermal stability of mercury captured by ash. Fuel, 2006, 85(17-18), 2509-2515. [http://dx.doi.org/10.1016/j.fuel.2006.05.007] |
| [50] | Morimoto, T.; Wu, S.; Uddin, M.A.; Sasaoka, E. Characteristics of the mercury vapor removal from coal combustion flue gas by activated carbon using H2S. Fuel, 2005, 84(14), 1968-1974. [http://dx.doi.org/10.1016/j.fuel.2005.04.007] |
| [51] | Hsi, H.C.; Chen, S.; Rostam-Abadi, M.; Rood, M.J.; Richardson, C.F.; Carey, T.R.; Chang, R. Preparation and evaluation of coal-derived activated carbons for removal of mercury vapor from simulated coal combustion flue gases. Energy Fuels, 1998, 12(6), 1061-1070. [http://dx.doi.org/10.1021/ef9801064] |
| [52] | Rostam-Abadi, M.; Chen, S.; Hsi, H.C.; Rood, M.J.; Carey, T.R.; Richardson, C.F.; Chang, R. Development and Evaluation of Low-Cost Sorbents for Removal of Mercury Emissions from Coal Combustion Flue Gas; EPRI Report TE-114043, 1998. |