- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Hypertension Journal
(Discontinued)
ISSN: 1876-5262 ― Volume 12, 2020
The Potential of Late Gadolinium Enhancement to Serve as a Predictor of Ventricular Arrhythmias in Hypertrophic Cardio-myopathy Patients
Thomas D. Gossios1, *, Georgios K. Efthimiadis1, Theodoros D. Karamitsos1, Thomas Zegkos1, Vasilios G. Athyros2, Haralambos I. Karvounis1
Abstract
Hypertrophic cardiomyopathy, the most common inherited cardiomyopathy is well known to be the leading cause of sudden cardiac death in young people. However, amongst the population of patients, a small subset bears increased risk of sudden cardiac death and would benefit from implantation of a defibrillator, currently recognized utilizing a series of established risk factors. This risk stratification model is hampered by low positive predictive value. Therefore, novel predictors of sudden death are sought. The advent of cardiac magnetic resonance and late gadolinium enhancement has allowed accurate quantification of regional fibrosis, a key element of hypertrophic cardiomyopathy, pathophysiologically linked to increased arrhythmogenicity. We sought to review currently available data on the utility of late gadolinium enhancement to serve as a novel predictor of arrhythmias and sudden death. In conclusion, significantly diverse methodological approaches and subsequent findings between available studies on the topic have hampered such use, highlighting the need for uniformly designed large scale, prospective studies in order to clarify which aspects of myocardial fibrosis could serve as predictors of arrhythmic events.
Article Information
Identifiers and Pagination:
Year: 2016Volume: 8
First Page: 1
Last Page: 11
Publisher Id: TOHYPERJ-8-1
DOI: 10.2174/1876526201608010001
Article History:
Received Date: 23/1/2016Revision Received Date: 25/2/2016
Acceptance Date: 2/3/2016
Electronic publication date: 03/06/2016
Collection year: 2016
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution-Non-Commercial 4.0 International Public License (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/legalcode), which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author the Aristotle University of Thessaloniki, AHEPA University Hospital, 1 Stilponos Kyriakidi Street 54636, Thessaloniki, Greece; Tel: +306978554096; E-mail: thomasgossios@hotmail.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 23-1-2016 |
Original Manuscript | The Potential of Late Gadolinium Enhancement to Serve as a Predictor of Ventricular Arrhythmias in Hypertrophic Cardio-myopathy Patients | |
INTRODUCTION
Hypertrophic cardiomyopathy (HCM) is a common genetic familial disease inherited as an autosomal dominant trait. Its key characteristic is unexplained left ventricular hypertrophy (LVH) associated with non-dilated ventricular chambers in the absence of another cardiac or systemic disease that could explain LVH [1Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 124(24): e783-831.
[http://dx.doi.org/10.1161/CIR.0b013e318223e2bd] [PMID: 22068434] ].
In general population, HCM presents in about 1/500 adults and generally leads a benign course, with the majority of patients having a normal life span and minimal symptoms. However, a small subset of clinically recognized patients with HCM is at increased risk for sudden cardiac death (SCD), with an event rate of about 1% per year [1Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 124(24): e783-831.
[http://dx.doi.org/10.1161/CIR.0b013e318223e2bd] [PMID: 22068434] ]. Therefore, early stratification for the recognition of HCM patients at risk for SCD is imperative. Currently established risk factors for sudden death are maximal wall thickness >30mm, family history of SCD, an abnormal blood pressure response in cardiopulmonary exercise testing, history of syncope and non-sustained ventricular tachycardia (NSVT) on Holter monitoring [1Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 124(24): e783-831.
[http://dx.doi.org/10.1161/CIR.0b013e318223e2bd] [PMID: 22068434] ].
The utility of these factors yields a high negative (87 to 98%) but low positive predictive value (<10 to 28%), allowing for the exclusion of low risk patients rather than identifying those at high risk [2Christiaans I, van Engelen K, van Langen IM, et al. Risk stratification for sudden cardiac death in hypertrophic cardiomyopathy: systematic review of clinical risk markers. Europace 2010; 12(3): 313-21.
[http://dx.doi.org/10.1093/europace/eup431] [PMID: 20118111] ]. Additionally, although risk of SCD would be expected to increase incrementally in patients with multiple risk factors, simply summing up risk
factors appears to have low sensitivity in identifying patients at risk for SCD. Even in patients deemed as high risk based on established risk factors who received an implantable cardioverter defibrillator (ICD) for primary prevention, discharge rates did not exceed 4%/year, subjecting the remainder of patients to the potentially unnecessary and even detrimental burden of an ICD [3Maron BJ, Spirito P, Shen WK, et al. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA 2007; 298(4): 405-12.
[http://dx.doi.org/10.1001/jama.298.4.405] [PMID: 17652294] ]. Moreover, decision making for ICD implantation for primary SCD prevention in patients with none or even one of the aforementioned risk factors, also appears to be a field of continuous debate [4O’Mahony C, Tome-Esteban M, Lambiase PD, et al. A validation study of the 2003 American College of Cardiology/European Society of Cardiology and 2011 American College of Cardiology Foundation/American Heart Association risk stratification and treatment algorithms for sudden cardiac death in patients with hypertrophic cardiomyopathy. Heart 2013; 99(8): 534-41.
[http://dx.doi.org/10.1136/heartjnl-2012-303271] [PMID: 23339826] ]. Interest has shifted in an effort to develop more accurate predictive models, based on existing risk stratification factors [5O'Mahony C, Jichi F, Pavlou M, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J 2014; 35(30): 2010-20.
[PMID: 24126876] ], or to investigate the potential of novel factors in order to determine increased risk in HCM patients, with myocardial fibrosis appearing as a highly promising marker on this regard.
In HCM, mutations result in disoriented or dysfunctional myocytes, which at a histopathological level lead to myocardial disarray intertwined with areas of interstitial fibrosis [6Varnava AM, Elliott PM, Sharma S, McKenna WJ, Davies MJ. Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease. Heart 2000; 84(5): 476-82.
[http://dx.doi.org/10.1136/heart.84.5.476] [PMID: 11040002] ]. These findings, along with small intramural coronary arteriole disease, especially in areas of hypertrophy, provide an ideal arrhythmogenic substrate [7Kwon DH, Smedira NG, Rodriguez ER, et al. Cardiac magnetic resonance detection of myocardial scarring in hypertrophic cardiomyopathy: correlation with histopathology and prevalence of ventricular tachycardia. J Am Coll Cardiol 2009; 54(3): 242-9.
[http://dx.doi.org/10.1016/j.jacc.2009.04.026] [PMID: 19589437] ]. The magnitude of these histopathological findings has been associated with an unfavorable outcome in HCM patients due to SCD [8Varnava AM, Elliott PM, Mahon N, Davies MJ, McKenna WJ. Relation between myocyte disarray and outcome in hypertrophic cardiomyopathy. Am J Cardiol 2001; 88(3): 275-9.
[http://dx.doi.org/10.1016/S0002-9149(01)01640-X] [PMID: 11472707] , 9Shirani J, Pick R, Roberts WC, Maron BJ. Morphology and significance of the left ventricular collagen network in young patients with hypertrophic cardiomyopathy and sudden cardiac death. J Am Coll Cardiol 2000; 35(1): 36-44.
[http://dx.doi.org/10.1016/S0735-1097(99)00492-1] [PMID: 10636256] ]. Attempts to quantify myocardial fibrosis in vivo, utilizing circulating serum biomarkers of collagen turnover, have provided evidence of a relationship between myocardial fibrosis and SCD [10Zachariah JP, Colan SD, Lang P, et al. Circulating matrix metalloproteinases in adolescents with hypertrophic cardiomyopathy and ventricular arrhythmia. Circ Heart Fail 2012; 5(4): 462-6.
[http://dx.doi.org/10.1161/CIRCHEARTFAILURE.111.966200] [PMID: 22628530] ]. However, the utility of such markers is hampered by low specificity and most importantly, inability to localize myocardial scar.
Over the past decade, the advent of cardiac magnetic resonance (CMR) has provided the most accurate modality for the assessment of cardiac morphology and hypertrophy distribution [11Pennell DJ, Sechtem UP, Higgins CB, et al. Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Panel report. Eur Heart J 2004; 25(21): 1940-65.
[http://dx.doi.org/10.1016/j.ehj.2004.06.040] [PMID: 15522474] , 12Rickers C, Wilke NM, Jerosch-Herold M, et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation 2005; 112(6): 855-61.
[http://dx.doi.org/10.1161/CIRCULATIONAHA.104.507723] [PMID: 16087809] ]. Moreover, the ability of gadolinium-based contrast agents to identify areas of focal fibrosis, has allowed the in vivo study of myocardial fibrosis in terms of localization, distribution and quantification using the late gadolinium enhancement (LGE) technique [13Choudhury L, Mahrholdt H, Wagner A, et al. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2002; 40(12): 2156-64.
[http://dx.doi.org/10.1016/S0735-1097(02)02602-5] [PMID: 12505229] , 14Moon JC, Reed E, Sheppard MN, et al. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 2004; 43(12): 2260-4.
[http://dx.doi.org/10.1016/j.jacc.2004.03.035] [PMID: 15193690] ]. Recently, the presence of LGE has been shown to be associated with adverse outcomes in HCM.
The aim of this review is to present available data on the prognostic value of LGE in HCM with a particular focus on the prediction of arrhythmic events.
Search Strategy and Study Selection Criteria
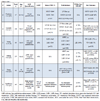
A PubMed/Medline search was performed using the following terms "Cardiomyopathy, Hypertrophic"[Mesh] AND "Tachycardia, Ventricular"[Mesh] OR "Ventricular Fibrillation"[Mesh] OR "Death, Sudden, Cardiac"[Mesh] AND “Late Gadolinium enhancement” OR “Myocardial Scar” OR “Myocardial Fibrosis”. Additionally, a search with the same terminology was performed in the Scopus database. The bibliography of the retrieved articles was manually searched for relevant references. Overall, the search yielded 8 observational studies in which the relationship between LGE and detected arrhythmias was investigated and an additional 7 studies and 2 meta-analyses providing information on SCD and related events as endpoints. Study characteristics and key points are presented in Tables 1 and 2.
Relationship of Fibrosis to Ventricular Arrhythmias
Evidence of an association between risk factors and LGE was provided by Moon et al. in a study of 53 patients that showed a significant association between simultaneous presence of LGE and ≥2 risk factors for SCD, a finding even more pronounced in younger patients [15Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol 2003; 41(9): 1561-7.
[http://dx.doi.org/10.1016/S0735-1097(03)00189-X] [PMID: 12742298] ]. Focusing on the correlation of ventricular arrhythmias and LGE, Dumont et al. enrolled 104 HCM patients who underwent CMR, as per protocol. Ninety six of these patients also underwent a 24 hour Holter recording [16Dumont CA, Monserrat L, Soler R, et al. Clinical significance of late gadolinium enhancement on cardiovascular magnetic resonance in patients with hypertrophic cardiomyopathy. Rev Esp Cardiol 2007; 60(1): 15-23.
[http://dx.doi.org/10.1157/13097921] [PMID: 17288951] ]. The investigators quantified LGE depending on affected segments, utilizing a standardized 17-segment model [17Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002; 105(4): 539-42.
[http://dx.doi.org/10.1161/hc0402.102975] [PMID: 11815441] ]. Non-sustained VT appeared to correlate in a positive manner with increasing number of segments presenting with LGE (p<0.04). In general, patients with larger degrees of LGE, tended to be diagnosed at an earlier age, presented with increased left ventricular (LV) mass and decreased LV ejection fraction (LVEF), overall exhibiting a worse clinical profile.
In another study of 120 HCM patients, increased prevalence of NSVT was found in patients with fibrosis (38% vs 8%, p<0.001) [18Payá E, Marín F, González J, et al. Variables associated with contrast-enhanced cardiovascular magnetic resonance in hypertrophic cardiomyopathy: clinical implications. J Card Fail 2008; 14(5): 414-9.
[http://dx.doi.org/10.1016/j.cardfail.2008.02.006] [PMID: 18514934] ]. On multivariate analysis, a significant, linear correlation between NSVT and presence of LGE was shown (p=0.011). Notably, LGE was associated with more significant LVH and worse systolic function. Additionally, patients with LGE exhibited increased levels of NT pro-BNP which has been strongly associated with worse heart failure status and outcome in HCM patients [19Coats CJ, Gallagher MJ, Foley M, et al. Relation between serum N-terminal pro-brain natriuretic peptide and prognosis in patients with hypertrophic cardiomyopathy. Eur Heart J 2013; 34(32): 2529-37.
[http://dx.doi.org/10.1093/eurheartj/eht070] [PMID: 23455360] ].
From an inverse perspective, i.e. whether patients with NSVT presented with a greater extent of fibrosis on LGE, 47 HCM patients underwent 48 hour Holter monitoring and CMR [20Dimitrow PP, Klimeczek P, Vliegenthart R, et al. Late hyperenhancement in gadolinium-enhanced magnetic resonance imaging: comparison of hypertrophic cardiomyopathy patients with and without nonsustained ventricular tachycardia. Int J Cardiovasc Imaging 2008; 24(1): 77-83.
[http://dx.doi.org/10.1007/s10554-007-9209-9] [PMID: 17624806] ]. Although some degree of LGE (1 g to 76 g) was found in the vast majority (97%) of patients with NSVT, compared to 60% of patients without NSVT (p<0.05), there was no difference regarding its extent between the two groups.
In contrary, a significant association between the extent of fibrosis and the occurrence of VT/VF was shown by Leonardi et al. [21Leonardi S, Raineri C, De Ferrari GM, et al. Usefulness of cardiac magnetic resonance in assessing the risk of ventricular arrhythmias and sudden death in patients with hypertrophic cardiomyopathy. Eur Heart J 2009; 30(16): 2003-10.
[http://dx.doi.org/10.1093/eurheartj/ehp152] [PMID: 19474054] ]. Fibrosis was visually assessed in 108 patients, utilizing a semi-quantitative method and scoring its extent in each of 17 myocardial segments, [17Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002; 105(4): 539-42.
[http://dx.doi.org/10.1161/hc0402.102975] [PMID: 11815441] ] with 0, 1, 2 and 3 points if 0%, >0-25%, 25-50% and >50% of the segment area presented with LGE respectively. On bivariate analysis LV mass index and total LGE score were significant predictors of arrhythmias, with the latter remaining as the sole predictor on multivariate analysis.
Focusing mainly on the histopathological association between small vessel disease and LGE, 60 patients planned to undergo septal myectomy for symptomatic LVOT obstruction were investigated [7Kwon DH, Smedira NG, Rodriguez ER, et al. Cardiac magnetic resonance detection of myocardial scarring in hypertrophic cardiomyopathy: correlation with histopathology and prevalence of ventricular tachycardia. J Am Coll Cardiol 2009; 54(3): 242-9.
[http://dx.doi.org/10.1016/j.jacc.2009.04.026] [PMID: 19589437] ]. NSVT runs were significantly more frequent on 48 hour Holter recordings in patients with LGE compared to those without. It should be noted that in this study, small vessel disease was also significantly associated with myocardial fibrosis and indirectly linked with NSVT, providing useful insight in arrhythmogenicity in HCM.
In the largest observational study so far, the prevalence and frequency of ventricular arrhythmias, including premature ventricular contractions, couplets and NSVT runs in relation to LGE was studied in 177 patients [22Adabag AS, Maron BJ, Appelbaum E, et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol 2008; 51(14): 1369-74.
[http://dx.doi.org/10.1016/j.jacc.2007.11.071] [PMID: 18387438] ]. All arrhythmic events, but most importantly NSVT presence and frequency of runs, were significantly more pronounced in patients with LGE compared to patients without fibrosis. The presence of LGE yielded a 7-fold increased probability of NSVT, and remained the only predictor of NSVT on multivariate analysis. It should be noted that the latter correlation occurred after adjustment for age and wall thickness as patients with LGE were older and had more pronounced LVH. Notably, there was no significant correlation between the extent of LGE and arrhythmias.
A retrospective observational study in 68 patients showed similar sensitivity and specificity characteristics regarding the ability of LGE to predict ventricular tachyarrhythmias in 48 hour Holter monitoring [23Kwon DH, Setser RM, Popović ZB, et al. Association of myocardial fibrosis, electrocardiography and ventricular tachyarrhythmia in hypertrophic cardiomyopathy: a delayed contrast enhanced MRI study. Int J Cardiovasc Imaging 2008; 24(6): 617-25.
[http://dx.doi.org/10.1007/s10554-008-9292-6] [PMID: 18204915] ]. A cut-off point of 18% was found to be predictive of NSVT episodes with AUC in ROC analysis being 0.71 (95% CI not reported, p<0.01). The presence of LGE yielded a 14-fold probability of ventricular arrhythmias being recorded (p<0.001). In this case, the extent of LGE was significantly associated with the detection of ventricular arrhythmias, either demonstrated as a percentage of myocardial mass 13% (6-19) vs. 6% (0-10), p=0.01 or affected myocardial segments 10 (8-12) vs. 6 (0-12), p<0.05.
Finally, the ability of intermediate-signal intensity LGE 4-6 standard deviations (SD) to predict ventricular arrhythmias (PVCs, ventricular couplets and NSVT on 24h Holter recordings) in comparison to high intensity LGE (>6SD) was investigated in a cohort of 145 patients [24Appelbaum E, Maron BJ, Adabag S, et al. Intermediate-signal-intensity late gadolinium enhancement predicts ventricular tachyarrhythmias in patients with hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 2012; 5(1): 78-85.
[http://dx.doi.org/10.1161/CIRCIMAGING.111.963819] [PMID: 22135401] ]. Patients presenting with arrhythmic events were found to exhibit significantly increased amounts of LGE, regardless of the quantification method used.
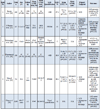
Relationship of Fibrosis to Sudden Cardiac Death
At present, few studies have explored the relationship between fibrosis on LGE-CMR and variable aspects of HCM-related morbidity and mortality, providing data on several distinct arrhythmic endpoints.
A surrogate, although of debatable validity, marker of aborted SCD in HCM patients is appropriate ICD interventions. A study included 87 high risk patients (mean risk factors 1.9±0.8) with an indication for ICD implantation mainly for primary prevention (98%) [25Prinz C, Schwarz M, Ilic I, et al. Myocardial fibrosis severity on cardiac magnetic resonance imaging predicts sustained arrhythmic events in hypertrophic cardiomyopathy. Can J Cardiol 2013; 29(3): 358-63.
[http://dx.doi.org/10.1016/j.cjca.2012.05.004] [PMID: 22749647] ]. Prior to implantation, a CMR study was undertaken and patients were subsequently followed up for a mean of 3.5 years. Fibrosis quantification was based on affected segments and categorized as absent, point-shaped, single segment and multiple segments. During follow-up, patients with severe fibrosis (30% of the cohort) received appropriate ICD interventions, contrary to patients with lesser degrees of LGE who remained free of events. In multivariate analysis, the presence of extensive fibrosis remained the sole predictor of sustained arrhythmic events in this high risk cohort.
A study by Bruder et al. in 220 patients, followed up for a period of 1090 days after undergoing a CMR study, implied that LGE could serve as a better predictor of SCD than the established risk factor model [26Bruder O, Wagner A, Jensen CJ, et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2010; 56(11): 875-87.
[http://dx.doi.org/10.1016/j.jacc.2010.05.007] [PMID: 20667520] ]. LGE presence was associated with a higher LV mass. Systolic function was similar between patients with and without fibrosis. None of the patients without LGE had a history of spontaneous VT versus 8.1% in the LGE group (p=0.05). Patients with 2 risk factors for SCD, deemed as high risk using the current clinical risk stratification model, presented with a mean fibrotic burden of 3.8%. This represented significantly less fibrosis in comparison to 11 patients who died suddenly, with a mean scar burden of 11.8%. Additionally, only 3 of the latter patients had any of the established risk factors for SCD. Although LGE quantity was found to be increased in patients with increasing number of risk factors, this finding was not associated with SCD events. On the other hand, the presence of fibrosis yielded an odds ratio (OR) of 5.14 for SCD, which marginally did not reach statistical significance (p=0.057).
Another study enrolled 217 patients, prospectively followed up for 3.1±1.7 years [27O’Hanlon R, Grasso A, Roughton M, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol 2010; 56(11): 867-74.
[http://dx.doi.org/10.1016/j.jacc.2010.05.010] [PMID: 20688032] ]. Patients with fibrosis exhibited significantly increased LV mass and reduced LVEF and overall presented with a worse functional status compared to patients without fibrosis. Despite the fact that significantly more patients with LGE received b-blockers and antiarrhythmics, they presented more frequently with a history of documented NSVT 11.8% vs. 3.7% (p=0.04), but not with a history of sustained VT or VF. Additionally, 7.3% of the patients in the LGE group reached the composite arrhythmic end point (sustained VT or VF, appropriate ICD discharge, SCD) versus 2.5% of patients without detected fibrosis, a difference that did not reach statistical significance [hazard ratio (HR): 3.15, 95% CI 0.69 - 14.4; p=0.138]. In this study, both the presence and the amount of LGE served as predictors of arrhythmic events in the univariate analysis, with the latter yielding an HR of 1.30 (95% CI: 1.05 - 1.61; p=0.014).
In a series of 424 patients data available over a period of 43±14 months (range 16 to 94) was retrospectively analyzed [28Rubinshtein R, Glockner JF, Ommen SR, et al. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail 2010; 3(1): 51-8.
[http://dx.doi.org/10.1161/CIRCHEARTFAILURE.109.854026] [PMID: 19850699] ]. In this cohort, significantly increased LV mass and reduced LVEF in the patients with fibrosis was not reflected in differences in NYHA classification. This study provided further evidence on the association between LGE and the detection of self-terminating arrhythmias (PVCs, ventricular couplets, NSVT). The presence of LGE was associated with increased prevalence of NSVT (27% vs. 8.5%, p<0.001), with more frequent runs per patient 4.5±12 (range 1 to 66) vs. 1.1±0.3 (range 1 to 2); p=0.04. Similarly, patients with LGE exhibited significantly more PVCs and episodes of couplets or bigeminy. In terms of sustained arrhythmic events, 4 patients suffered SCD and 4 patients had appropriate ICD discharges over the follow up period. Six out of these patients presented with some degree of fibrosis, while 4 of them showed large degree of LGE, deemed by the investigators as >5% of LV mass. In multivariate analysis, the presence of LGE was considered as a significant predictor of adverse arrhythmic events, together with presence of NSVT. Estimated 6 year event free survival was statistically significantly lower in patients with fibrosis compared to patients without (96% vs. 100%, p=0.01).
In a series of 328 patients, a study sought to investigate whether, apart from the presence of LGE per se and its extent, distribution of fibrosis would be associated with increased risk of SCD or appropriate ICD therapy [29Klopotowski M, Kukula K, Malek LA, et al. The value of cardiac magnetic resonance and distribution of late gadolinium enhancement for risk stratification of sudden cardiac death in patients with hypertrophic cardiomyopathy. J Cardiol 2015; S0914-5087(15)00253-1.
[PMID: 26363820] ]. Patients with fibrosis in this cohort expressed a worse clinical profile, with increased wall thickness and LV mass, more frequent NSVT episodes and abnormal hemodynamic responses to exercise, as well as increased NT-proBNP levels. Therefore they were more actively treated, both medically, in particular with b-blockers, amiodarone and spironolactone, and with more ICD implantations. The study endpoint was reached by 14 patients, all of which exhibited some degree of LGE. Importantly, in all but one of these patients, fibrosis was located away from the interventricular insertion points, where it is most commonly described. This particular distribution of LGE was associated with significantly increased risk of fulfilling the study endpoint on multivariate analysis including the established risk factors for SCD (HR 10.01, 95% CI 1.21-83.86, p=0.033).
Finally, data from two of the largest prospective HCM cohorts yet was recently published, and results appear to be equivocal. Ismail et al recruited 711 patients, who were followed up for a median of 3.5 years after undergoing a CMR scan [30Ismail TF, Jabbour A, Gulati A, et al. Role of late gadolinium enhancement cardiovascular magnetic resonance in the risk stratification of hypertrophic cardiomyopathy. Heart 2014; 100(23): 1851-8.
[http://dx.doi.org/10.1136/heartjnl-2013-305471] [PMID: 24966307] ]. The presence of late enhancement was associated with significantly increased LVH as well as decreased LV systolic function, although this did not correspond to differences in functional status. Patients in this cohort were enrolled as minimally or mildly symptomatic, with 93% of them being classified as NYHA class I/II. In terms of risk stratification, since fibrosis was associated with LVH>30 mm and NSVT, significantly more patients with LGE were deemed as “high risk” for SCD. In line with the higher risk profile, the presence of LGE was associated with increased but not statistically significant SCD rates, aborted or not (HR: 2.69, 95% CI: 0.91-7.97; p=0.073). On the other hand, the amount of detected fibrosis was significantly associated with increased risk of SCD, (HR per 5% increase in LGE 1.24, 95% CI 1.06-1.45; p=0.007). It should be noted that more patients presenting with fibrosis were also on b-blockers. However, on multivariate analysis fibrosis did not maintain its prognostic value and only decreased LVEF was found to be a reliable predictor of SCD events.
Contrary to the results of the aforementioned study, myocardial fibrosis was found to be an independent predictor of SCD in a multicenter cohort of 1293 patients followed up for a median of 3.3 years [31Chan RH, Maron BJ, Olivotto I, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014; 130(6): 484-95.
[http://dx.doi.org/10.1161/CIRCULATIONAHA.113.007094] [PMID: 25092278] ]. The combined endpoint of SCD, aborted cardiac arrest, appropriate ICD discharge occurred in 37 patients, 70% of which exhibited some degree of LGE. Interestingly, 21 of these patients were considered as low risk based on clinical risk stratification. While the absence or even the presence of minimal LGE was associated with minimal estimated risk for SCD (3.4 and 3.5-4.1%/5 years respectively), the amount of detected fibrosis significantly increased risk (up to 15%/5 years in patients with LGE ≥20% of LV mass). In particular, a linear association between LGE extent with risk for SCD was found, with every 10% increase in the former leading to a 40% increase in the latter (adjusted HR 1.46/10% increase in LGE; 95% CI, 1.12–1.92; p=0.002), regardless of confounding factors such as age, systolic function, medication regime and presence of other risk factors. In fact, LGE remained a strong predictor of SCD even in patients who, according to current clinical risk stratification, were deemed as low risk. Additionally, the extent of LGE also served as a predictor of end stage HCM development (adjusted HR, 1.80/10% increase in LGE; 95% CI, 1.40–2.40; p=0.03), without however also predicting heart failure related mortality. Finally, inclusion of LGE% in the currently utilized risk prediction model, greatly increased performance for the prediction of SCD related events AUC increased from 0.71 (95% CI, 0.632–0.788) to 0.741 (95% CI, 0.664–0.818).
Two meta-analyses on the prognostic value of LGE have been published, with contradictory results, leading to further debate on the issue. In order to delineate the effect of LGE on clinical outcomes in HCM, a meta-analysis utilized data from 3 studies, published until 2010, [26Bruder O, Wagner A, Jensen CJ, et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2010; 56(11): 875-87.
[http://dx.doi.org/10.1016/j.jacc.2010.05.007] [PMID: 20667520] -28Rubinshtein R, Glockner JF, Ommen SR, et al. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail 2010; 3(1): 51-8.
[http://dx.doi.org/10.1161/CIRCHEARTFAILURE.109.854026] [PMID: 19850699] ] and additionally a study by Maron et al. [32Maron MS, Appelbaum E, Harrigan CJ, et al. Clinical profile and significance of delayed enhancement in hypertrophic cardiomyopathy. Circ Heart Fail 2008; 1(3): 184-91.
[http://dx.doi.org/10.1161/CIRCHEARTFAILURE.108.768119] [PMID: 19808288] ] that did not explicitly provide data on arrhythmic events and SCD. Pooled data from these 4 studies showed a significant association of the presence of fibrosis with cardiac mortality (pooled OR 2.92, 95% CI: 1.01-8.42; p=0.047), heart failure mortality (pooled OR: 5.68, 95% CI: 1.04-31.07; p=0.045) but only a trend towards SCD related events (pooled OR: 2.39, 95% CI: 0.87-6.58; p=0.091) [33Green JJ, Berger JS, Kramer CM, Salerno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging 2012; 5(4): 370-7.
[http://dx.doi.org/10.1016/j.jcmg.2011.11.021] [PMID: 22498326] ].
Contrary to that, a meta-analysis of 3067 patients [34Briasoulis A, Mallikethi-Reddy S, Palla M, Alesh I, Afonso L. Myocardial fibrosis on cardiac magnetic resonance and cardiac outcomes in hypertrophic cardiomyopathy: a meta-analysis. Heart 2015; 101(17): 1406-11.
[http://dx.doi.org/10.1136/heartjnl-2015-307682] [PMID: 26060120] ], additionally including the large cohorts by Chan [31Chan RH, Maron BJ, Olivotto I, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014; 130(6): 484-95.
[http://dx.doi.org/10.1161/CIRCULATIONAHA.113.007094] [PMID: 25092278] ] and Ismail et al. [30Ismail TF, Jabbour A, Gulati A, et al. Role of late gadolinium enhancement cardiovascular magnetic resonance in the risk stratification of hypertrophic cardiomyopathy. Heart 2014; 100(23): 1851-8.
[http://dx.doi.org/10.1136/heartjnl-2013-305471] [PMID: 24966307] ], found the presence of myocardial fibrosis to be associated with increased rates of SCD (OR 2.52, 95% CI 1.44 - 4.4; p=0.001), cardiac death, all-cause mortality (OR 1.80, 95% CI 1.21 - 2.67; p=0.003) but only a trend towards HF related death (OR 2.47, 95% CI 0.98 - 6.24; p=0.06). Notably, no significant association between the extent of LGE and endpoints was found on meta-regression analysis, probably attributed to the small number of patients with significant amounts of LGE throughout the study population.
DISCUSSION
Over the past decade, research on the utilization of CMR and its role in the assessment of HCM has provided significant insights with regards to cardiac structure and function and subsequently in HCM patient management. Currently, CMR represents the modality of choice in patients with suboptimal or inconclusive echocardiographic studies [35Nagueh SF, Bierig SM, Budoff MJ, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: Endorsed by the American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2011; 24(5): 473-98.
[http://dx.doi.org/10.1016/j.echo.2011.03.006] [PMID: 21514501] ], yielding high sensitivity in detecting LVH [12Rickers C, Wilke NM, Jerosch-Herold M, et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation 2005; 112(6): 855-61.
[http://dx.doi.org/10.1161/CIRCULATIONAHA.104.507723] [PMID: 16087809] ], especially in patients with focal [36Maron MS, Maron BJ, Harrigan C, et al. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J Am Coll Cardiol 2009; 54(3): 220-8.
[http://dx.doi.org/10.1016/j.jacc.2009.05.006] [PMID: 19589434] ], apical [37Moon JC, Fisher NG, McKenna WJ, Pennell DJ. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart 2004; 90(6): 645-9.
[http://dx.doi.org/10.1136/hrt.2003.014969] [PMID: 15145868] ], or right ventricular hypertrophy [38Maron MS, Hauser TH, Dubrow E, et al. Right ventricular involvement in hypertrophic cardiomyopathy. Am J Cardiol 2007; 100(8): 1293-8.
[http://dx.doi.org/10.1016/j.amjcard.2007.05.061] [PMID: 17920373] ]. It has also provided valuable information on the structural characteristics of the mitral valve leaflets [39Maron MS, Olivotto I, Harrigan C, et al. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation 2011; 124(1): 40-7.
[http://dx.doi.org/10.1161/CIRCULATIONAHA.110.985812] [PMID: 21670234] ] and subvalvular apparatus [40Harrigan CJ, Appelbaum E, Maron BJ, et al. Significance of papillary muscle abnormalities identified by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am J Cardiol 2008; 101(5): 668-73.
[http://dx.doi.org/10.1016/j.amjcard.2007.10.032] [PMID: 18308018] ], contributing to the mechanism of LVOT obstruction in HCM.
On the other hand, although the identification of myocardial fibrosis with LGE-CMR has provided important insights in terms of tissue characterization and diagnosis in borderline cases, the available data on its utility as a predictor of SCD remains ambiguous. To date, in available guidelines on HCM, LGE-CMR is not a part of the proposed predictive model for SCD (HCM Risk-SCD) and no recommendation on its use as a risk factor is provided [1Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 124(24): e783-831.
[http://dx.doi.org/10.1161/CIR.0b013e318223e2bd] [PMID: 22068434] , 5O'Mahony C, Jichi F, Pavlou M, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J 2014; 35(30): 2010-20.
[PMID: 24126876] , 41Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014; 35(39): 2733-79.
[http://dx.doi.org/10.1093/eurheartj/ehu284] [PMID: 25173338] ]. Although the vast majority of published studies so far utilized uniform standardized measures in terms of population enrollment, patient characteristics and CMR image acquisition, a number of study aspects such as endpoint definitions or LGE quantification method are largely diverse. Regarding the variability in endpoint selection amongst available studies, the small frequency of SCD events in HCM populations subsequently leads to an inherent inability of studies to provide robust data on hard endpoints; therefore in most cases composite endpoints were utilized. In order to achieve this goal, larger cohorts with more prolonged follow up periods are required. Additionally, although the majority of studies implemented a signal intensity >2 SD above the average of normal myocardium in order to determine the extent of LGE [42Hundley WG, Bluemke D, Bogaert JG, et al. Society for Cardiovascular Magnetic Resonance guidelines for reporting cardiovascular magnetic resonance examinations. J Cardiovasc Magn Reson 2009; 11: 5.
[http://dx.doi.org/10.1186/1532-429X-11-5] [PMID: 19257889] ], some data derived from different thresholds such as 4-6 SD, >6 SD [24Appelbaum E, Maron BJ, Adabag S, et al. Intermediate-signal-intensity late gadolinium enhancement predicts ventricular tachyarrhythmias in patients with hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 2012; 5(1): 78-85.
[http://dx.doi.org/10.1161/CIRCIMAGING.111.963819] [PMID: 22135401] ], or the “full width half maximum” (FWHM) technique [27O’Hanlon R, Grasso A, Roughton M, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol 2010; 56(11): 867-74.
[http://dx.doi.org/10.1016/j.jacc.2010.05.010] [PMID: 20688032] ]. It has been established that different quantification thresholds, yield different results with regards to LGE extent [43Spiewak M, Malek LA, Misko J, et al. Comparison of different quantification methods of late gadolinium enhancement in patients with hypertrophic cardiomyopathy. Eur J Radiol 2010; 74(3): e149-53.
[http://dx.doi.org/10.1016/j.ejrad.2009.05.035] [PMID: 19523780] , 44Aquaro GD, Positano V, Pingitore A, et al. Quantitative analysis of late gadolinium enhancement in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 2010; 12: 21.
[http://dx.doi.org/10.1186/1532-429X-12-21] [PMID: 20374627] ].
Apart from the aforesaid variability in study methodology, deriving results regarding numerous issues have been equivocal. Although LGE is detected along the full spectrum of LV function, almost all available data suggests a strong association between increased LV mass, decreasing LVEF and presence of fibrosis; however, an analogous association with decreasing functional capacity has not been clearly established. Similarly, the degree to which myocardial fibrosis serves as a predisposing factor for the development of end-stage HCM is unknown; although an association has been suggested this has not been uniform across available studies [15Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol 2003; 41(9): 1561-7.
[http://dx.doi.org/10.1016/S0735-1097(03)00189-X] [PMID: 12742298] ]. Whether arrhythmogenicity is a synchronous process with more severe or progressive disease, linearly associated with the development and increment of fibrosis is a hypothesis remaining unanswered. In an akin fashion, no consistent association has been found in available data linking characteristics of fibrosis with different risk factor profiles [15Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol 2003; 41(9): 1561-7.
[http://dx.doi.org/10.1016/S0735-1097(03)00189-X] [PMID: 12742298] , 16Dumont CA, Monserrat L, Soler R, et al. Clinical significance of late gadolinium enhancement on cardiovascular magnetic resonance in patients with hypertrophic cardiomyopathy. Rev Esp Cardiol 2007; 60(1): 15-23.
[http://dx.doi.org/10.1157/13097921] [PMID: 17288951] , 18Payá E, Marín F, González J, et al. Variables associated with contrast-enhanced cardiovascular magnetic resonance in hypertrophic cardiomyopathy: clinical implications. J Card Fail 2008; 14(5): 414-9.
[http://dx.doi.org/10.1016/j.cardfail.2008.02.006] [PMID: 18514934] , 21Leonardi S, Raineri C, De Ferrari GM, et al. Usefulness of cardiac magnetic resonance in assessing the risk of ventricular arrhythmias and sudden death in patients with hypertrophic cardiomyopathy. Eur Heart J 2009; 30(16): 2003-10.
[http://dx.doi.org/10.1093/eurheartj/ehp152] [PMID: 19474054] , 26Bruder O, Wagner A, Jensen CJ, et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2010; 56(11): 875-87.
[http://dx.doi.org/10.1016/j.jacc.2010.05.007] [PMID: 20667520] ]. Notably, LGE has also been found in patients with none of the established risk factors for SCD [45Lyons KS, Dixon LJ, Johnston N, et al. Late gadolinium enhancement is common in patients with hypertrophic cardiomyopathy and no clinical risk factors for sudden cardiac death: A single centre experience. Cardiol J 2014; 21(1): 29-32.
[PMID: 23990187] ].
Whether the presence of fibrosis per se, the topographic distribution of LGE, or the extent of fibrosis is associated with arrhythmogenicity is an issue yet unresolved. Although the extent and not just the presence of LGE has been demonstrated to be the sole predictor of inducibility of VT during electrophysiological testing [46Fluechter S, Kuschyk J, Wolpert C, et al. Extent of late gadolinium enhancement detected by cardiovascular magnetic resonance correlates with the inducibility of ventricular tachyarrhythmia in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 2010; 12: 30.
[http://dx.doi.org/10.1186/1532-429X-12-30] [PMID: 20492668] ], evidence regarding spontaneous arrhythmic events is conflicting.
Finally, currently available data on fibrosis and its relation to arrhythmogenicity and risk of SCD are based solely on the quantification of focal fibrosis by LGE. The advent of novel techniques such as T1 mapping which are able to identify and quantify diffuse myocardial fibrosis appears as a promising tool that has the potential to further advance our knowledge on the relationship between fibrosis and risk for SCD in HCM [47Flett AS, Hayward MP, Ashworth MT, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation 2010; 122(2): 138-44.
[http://dx.doi.org/10.1161/CIRCULATIONAHA.109.930636] [PMID: 20585010] -50Puntmann VO, Voigt T, Chen Z, et al. Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging 2013; 6(4): 475-84.
[http://dx.doi.org/10.1016/j.jcmg.2012.08.019] [PMID: 23498674] ]. Such novel techniques for the detection and quantification of myocardial fibrosis, together with large scale registry based analysis with substantially prolonged follow up periods, such as the HCM registry (NCT01915615) [51Kramer CM. HCMR - Novel Markers of Prognosis in Hypertrophic Cardiomyopathy. ClinicalTrials.gov Identifier: NCT01915615, University of Virginia. Available from: http://hcmregistry.org/ [Accessed Jannuary 29, 2016].], are expected to shed more light onto the crucial matter of precise risk stratification in HCM patients in the future.
CONCLUSION
The value of LGE in the prediction of arrhythmic events has been investigated in a significant number of studies, however with largely inconclusive or even conflicting results. Therefore, this concept still remains under debate, mainly due to methodological discrepancies and inherent low power of available data to provide sound associations with sudden death, deriving from the small number of events. Further large scale, prospective studies, with uniform design in terms of fibrosis definition and adjudication of endpoints are required in order to clarify if LGE could indeed be used as a risk index for sudden cardiac death.
ABBREVIATIONS
CONCFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
| [1] | Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 124(24): e783-831. [http://dx.doi.org/10.1161/CIR.0b013e318223e2bd] [PMID: 22068434] |
| [2] | Christiaans I, van Engelen K, van Langen IM, et al. Risk stratification for sudden cardiac death in hypertrophic cardiomyopathy: systematic review of clinical risk markers. Europace 2010; 12(3): 313-21. [http://dx.doi.org/10.1093/europace/eup431] [PMID: 20118111] |
| [3] | Maron BJ, Spirito P, Shen WK, et al. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA 2007; 298(4): 405-12. [http://dx.doi.org/10.1001/jama.298.4.405] [PMID: 17652294] |
| [4] | O’Mahony C, Tome-Esteban M, Lambiase PD, et al. A validation study of the 2003 American College of Cardiology/European Society of Cardiology and 2011 American College of Cardiology Foundation/American Heart Association risk stratification and treatment algorithms for sudden cardiac death in patients with hypertrophic cardiomyopathy. Heart 2013; 99(8): 534-41. [http://dx.doi.org/10.1136/heartjnl-2012-303271] [PMID: 23339826] |
| [5] | O'Mahony C, Jichi F, Pavlou M, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J 2014; 35(30): 2010-20. [PMID: 24126876] |
| [6] | Varnava AM, Elliott PM, Sharma S, McKenna WJ, Davies MJ. Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease. Heart 2000; 84(5): 476-82. [http://dx.doi.org/10.1136/heart.84.5.476] [PMID: 11040002] |
| [7] | Kwon DH, Smedira NG, Rodriguez ER, et al. Cardiac magnetic resonance detection of myocardial scarring in hypertrophic cardiomyopathy: correlation with histopathology and prevalence of ventricular tachycardia. J Am Coll Cardiol 2009; 54(3): 242-9. [http://dx.doi.org/10.1016/j.jacc.2009.04.026] [PMID: 19589437] |
| [8] | Varnava AM, Elliott PM, Mahon N, Davies MJ, McKenna WJ. Relation between myocyte disarray and outcome in hypertrophic cardiomyopathy. Am J Cardiol 2001; 88(3): 275-9. [http://dx.doi.org/10.1016/S0002-9149(01)01640-X] [PMID: 11472707] |
| [9] | Shirani J, Pick R, Roberts WC, Maron BJ. Morphology and significance of the left ventricular collagen network in young patients with hypertrophic cardiomyopathy and sudden cardiac death. J Am Coll Cardiol 2000; 35(1): 36-44. [http://dx.doi.org/10.1016/S0735-1097(99)00492-1] [PMID: 10636256] |
| [10] | Zachariah JP, Colan SD, Lang P, et al. Circulating matrix metalloproteinases in adolescents with hypertrophic cardiomyopathy and ventricular arrhythmia. Circ Heart Fail 2012; 5(4): 462-6. [http://dx.doi.org/10.1161/CIRCHEARTFAILURE.111.966200] [PMID: 22628530] |
| [11] | Pennell DJ, Sechtem UP, Higgins CB, et al. Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Panel report. Eur Heart J 2004; 25(21): 1940-65. [http://dx.doi.org/10.1016/j.ehj.2004.06.040] [PMID: 15522474] |
| [12] | Rickers C, Wilke NM, Jerosch-Herold M, et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation 2005; 112(6): 855-61. [http://dx.doi.org/10.1161/CIRCULATIONAHA.104.507723] [PMID: 16087809] |
| [13] | Choudhury L, Mahrholdt H, Wagner A, et al. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2002; 40(12): 2156-64. [http://dx.doi.org/10.1016/S0735-1097(02)02602-5] [PMID: 12505229] |
| [14] | Moon JC, Reed E, Sheppard MN, et al. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 2004; 43(12): 2260-4. [http://dx.doi.org/10.1016/j.jacc.2004.03.035] [PMID: 15193690] |
| [15] | Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol 2003; 41(9): 1561-7. [http://dx.doi.org/10.1016/S0735-1097(03)00189-X] [PMID: 12742298] |
| [16] | Dumont CA, Monserrat L, Soler R, et al. Clinical significance of late gadolinium enhancement on cardiovascular magnetic resonance in patients with hypertrophic cardiomyopathy. Rev Esp Cardiol 2007; 60(1): 15-23. [http://dx.doi.org/10.1157/13097921] [PMID: 17288951] |
| [17] | Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002; 105(4): 539-42. [http://dx.doi.org/10.1161/hc0402.102975] [PMID: 11815441] |
| [18] | Payá E, Marín F, González J, et al. Variables associated with contrast-enhanced cardiovascular magnetic resonance in hypertrophic cardiomyopathy: clinical implications. J Card Fail 2008; 14(5): 414-9. [http://dx.doi.org/10.1016/j.cardfail.2008.02.006] [PMID: 18514934] |
| [19] | Coats CJ, Gallagher MJ, Foley M, et al. Relation between serum N-terminal pro-brain natriuretic peptide and prognosis in patients with hypertrophic cardiomyopathy. Eur Heart J 2013; 34(32): 2529-37. [http://dx.doi.org/10.1093/eurheartj/eht070] [PMID: 23455360] |
| [20] | Dimitrow PP, Klimeczek P, Vliegenthart R, et al. Late hyperenhancement in gadolinium-enhanced magnetic resonance imaging: comparison of hypertrophic cardiomyopathy patients with and without nonsustained ventricular tachycardia. Int J Cardiovasc Imaging 2008; 24(1): 77-83. [http://dx.doi.org/10.1007/s10554-007-9209-9] [PMID: 17624806] |
| [21] | Leonardi S, Raineri C, De Ferrari GM, et al. Usefulness of cardiac magnetic resonance in assessing the risk of ventricular arrhythmias and sudden death in patients with hypertrophic cardiomyopathy. Eur Heart J 2009; 30(16): 2003-10. [http://dx.doi.org/10.1093/eurheartj/ehp152] [PMID: 19474054] |
| [22] | Adabag AS, Maron BJ, Appelbaum E, et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol 2008; 51(14): 1369-74. [http://dx.doi.org/10.1016/j.jacc.2007.11.071] [PMID: 18387438] |
| [23] | Kwon DH, Setser RM, Popović ZB, et al. Association of myocardial fibrosis, electrocardiography and ventricular tachyarrhythmia in hypertrophic cardiomyopathy: a delayed contrast enhanced MRI study. Int J Cardiovasc Imaging 2008; 24(6): 617-25. [http://dx.doi.org/10.1007/s10554-008-9292-6] [PMID: 18204915] |
| [24] | Appelbaum E, Maron BJ, Adabag S, et al. Intermediate-signal-intensity late gadolinium enhancement predicts ventricular tachyarrhythmias in patients with hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 2012; 5(1): 78-85. [http://dx.doi.org/10.1161/CIRCIMAGING.111.963819] [PMID: 22135401] |
| [25] | Prinz C, Schwarz M, Ilic I, et al. Myocardial fibrosis severity on cardiac magnetic resonance imaging predicts sustained arrhythmic events in hypertrophic cardiomyopathy. Can J Cardiol 2013; 29(3): 358-63. [http://dx.doi.org/10.1016/j.cjca.2012.05.004] [PMID: 22749647] |
| [26] | Bruder O, Wagner A, Jensen CJ, et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2010; 56(11): 875-87. [http://dx.doi.org/10.1016/j.jacc.2010.05.007] [PMID: 20667520] |
| [27] | O’Hanlon R, Grasso A, Roughton M, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol 2010; 56(11): 867-74. [http://dx.doi.org/10.1016/j.jacc.2010.05.010] [PMID: 20688032] |
| [28] | Rubinshtein R, Glockner JF, Ommen SR, et al. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail 2010; 3(1): 51-8. [http://dx.doi.org/10.1161/CIRCHEARTFAILURE.109.854026] [PMID: 19850699] |
| [29] | Klopotowski M, Kukula K, Malek LA, et al. The value of cardiac magnetic resonance and distribution of late gadolinium enhancement for risk stratification of sudden cardiac death in patients with hypertrophic cardiomyopathy. J Cardiol 2015; S0914-5087(15)00253-1. [PMID: 26363820] |
| [30] | Ismail TF, Jabbour A, Gulati A, et al. Role of late gadolinium enhancement cardiovascular magnetic resonance in the risk stratification of hypertrophic cardiomyopathy. Heart 2014; 100(23): 1851-8. [http://dx.doi.org/10.1136/heartjnl-2013-305471] [PMID: 24966307] |
| [31] | Chan RH, Maron BJ, Olivotto I, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014; 130(6): 484-95. [http://dx.doi.org/10.1161/CIRCULATIONAHA.113.007094] [PMID: 25092278] |
| [32] | Maron MS, Appelbaum E, Harrigan CJ, et al. Clinical profile and significance of delayed enhancement in hypertrophic cardiomyopathy. Circ Heart Fail 2008; 1(3): 184-91. [http://dx.doi.org/10.1161/CIRCHEARTFAILURE.108.768119] [PMID: 19808288] |
| [33] | Green JJ, Berger JS, Kramer CM, Salerno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging 2012; 5(4): 370-7. [http://dx.doi.org/10.1016/j.jcmg.2011.11.021] [PMID: 22498326] |
| [34] | Briasoulis A, Mallikethi-Reddy S, Palla M, Alesh I, Afonso L. Myocardial fibrosis on cardiac magnetic resonance and cardiac outcomes in hypertrophic cardiomyopathy: a meta-analysis. Heart 2015; 101(17): 1406-11. [http://dx.doi.org/10.1136/heartjnl-2015-307682] [PMID: 26060120] |
| [35] | Nagueh SF, Bierig SM, Budoff MJ, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: Endorsed by the American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2011; 24(5): 473-98. [http://dx.doi.org/10.1016/j.echo.2011.03.006] [PMID: 21514501] |
| [36] | Maron MS, Maron BJ, Harrigan C, et al. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J Am Coll Cardiol 2009; 54(3): 220-8. [http://dx.doi.org/10.1016/j.jacc.2009.05.006] [PMID: 19589434] |
| [37] | Moon JC, Fisher NG, McKenna WJ, Pennell DJ. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart 2004; 90(6): 645-9. [http://dx.doi.org/10.1136/hrt.2003.014969] [PMID: 15145868] |
| [38] | Maron MS, Hauser TH, Dubrow E, et al. Right ventricular involvement in hypertrophic cardiomyopathy. Am J Cardiol 2007; 100(8): 1293-8. [http://dx.doi.org/10.1016/j.amjcard.2007.05.061] [PMID: 17920373] |
| [39] | Maron MS, Olivotto I, Harrigan C, et al. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation 2011; 124(1): 40-7. [http://dx.doi.org/10.1161/CIRCULATIONAHA.110.985812] [PMID: 21670234] |
| [40] | Harrigan CJ, Appelbaum E, Maron BJ, et al. Significance of papillary muscle abnormalities identified by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am J Cardiol 2008; 101(5): 668-73. [http://dx.doi.org/10.1016/j.amjcard.2007.10.032] [PMID: 18308018] |
| [41] | Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014; 35(39): 2733-79. [http://dx.doi.org/10.1093/eurheartj/ehu284] [PMID: 25173338] |
| [42] | Hundley WG, Bluemke D, Bogaert JG, et al. Society for Cardiovascular Magnetic Resonance guidelines for reporting cardiovascular magnetic resonance examinations. J Cardiovasc Magn Reson 2009; 11: 5. [http://dx.doi.org/10.1186/1532-429X-11-5] [PMID: 19257889] |
| [43] | Spiewak M, Malek LA, Misko J, et al. Comparison of different quantification methods of late gadolinium enhancement in patients with hypertrophic cardiomyopathy. Eur J Radiol 2010; 74(3): e149-53. [http://dx.doi.org/10.1016/j.ejrad.2009.05.035] [PMID: 19523780] |
| [44] | Aquaro GD, Positano V, Pingitore A, et al. Quantitative analysis of late gadolinium enhancement in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 2010; 12: 21. [http://dx.doi.org/10.1186/1532-429X-12-21] [PMID: 20374627] |
| [45] | Lyons KS, Dixon LJ, Johnston N, et al. Late gadolinium enhancement is common in patients with hypertrophic cardiomyopathy and no clinical risk factors for sudden cardiac death: A single centre experience. Cardiol J 2014; 21(1): 29-32. [PMID: 23990187] |
| [46] | Fluechter S, Kuschyk J, Wolpert C, et al. Extent of late gadolinium enhancement detected by cardiovascular magnetic resonance correlates with the inducibility of ventricular tachyarrhythmia in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 2010; 12: 30. [http://dx.doi.org/10.1186/1532-429X-12-30] [PMID: 20492668] |
| [47] | Flett AS, Hayward MP, Ashworth MT, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation 2010; 122(2): 138-44. [http://dx.doi.org/10.1161/CIRCULATIONAHA.109.930636] [PMID: 20585010] |
| [48] | Dass S, Suttie JJ, Piechnik SK, et al. Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circ Cardiovasc Imaging 2012; 5(6): 726-33. [http://dx.doi.org/10.1161/CIRCIMAGING.112.976738] [PMID: 23071146] |
| [49] | Ellims AH, Iles LM, Ling LH, Hare JL, Kaye DM, Taylor AJ. Diffuse myocardial fibrosis in hypertrophic cardiomyopathy can be identified by cardiovascular magnetic resonance, and is associated with left ventricular diastolic dysfunction. J Cardiovasc Magn Reson 2012; 14: 76. [http://dx.doi.org/10.1186/1532-429X-14-76] [PMID: 23107451] |
| [50] | Puntmann VO, Voigt T, Chen Z, et al. Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging 2013; 6(4): 475-84. [http://dx.doi.org/10.1016/j.jcmg.2012.08.019] [PMID: 23498674] |
| [51] | Kramer CM. HCMR - Novel Markers of Prognosis in Hypertrophic Cardiomyopathy. ClinicalTrials.gov Identifier: NCT01915615, University of Virginia. Available from: http://hcmregistry.org/ [Accessed Jannuary 29, 2016]. |