- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Nanomedicine and Nanotechnology Journal
Formerly: The Open Nanomedicine Journal
(Discontinued)
ISSN: 2666-1500 ― Volume 6, 2020
State of the Art Review on Emerging Applications of Mesoporous Silica
Ajinkya Kailas Pote1, Vishal Vijay Pande1, *
 , Vipul Pralhadbhai Patel1, Mahendra Ashok Giri2, Aniket Uttam Pund3, Nitin Vijay Shelke3
, Vipul Pralhadbhai Patel1, Mahendra Ashok Giri2, Aniket Uttam Pund3, Nitin Vijay Shelke3Abstract
The recent advances in the drug delivery system using a variety of technological platforms have resulted in innovation in the attitude towards diagnosis and therapeutics alike in the present times. Mesoporous Silica possesses favourable chemical properties, thermal stability, and biocompatibility. The unique structure of mesoporous silica makes possible the effective loading of drugs and their subsequent release in a controlled manner at the target site. The properties like pore size, high drug loading, and porosity as well as the surface properties of Mesoporous silica make them a suitable platform for many drug delivery applications. This review focuses on the applications and the advances made in the mesoporous silica to broaden the spectrum of its use especially in the field of medicine. The Mesoporous Silica carrier has proved its use in the field of biosensing, controlled and targeted drug release, gene delivery, water treatment, solubility and bioavailability enhancement and wound healing.
Article Information
Identifiers and Pagination:
Year: 2020Volume: 6
First Page: 12
Last Page: 20
Publisher Id: TONMJ-6-12
DOI: 10.2174/2666150002006010012
Article History:
Received Date: 02/04/2020Revision Received Date: 15/08/2020
Acceptance Date: 16/08/2020
Electronic publication date: 22/10/2020
Collection year: 2020
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Department of Pharmaceutics, Sanjivani College of Pharmaceutical Education and Research, Kopargaon, Maharashtra 423603, India; Tel: 9623443179, E-mail: drvishalpande@gmail.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 02-04-2020 |
Original Manuscript | State of the Art Review on Emerging Applications of Mesoporous Silica | |
1. INTRODUCTION
Modern nanotechnology has evolved as the boon to the medicine and diagnostic sector [1Bharti C, Nagaich U, Pal AK, Gulati N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int J Pharm Investig 2015; 5(3): 124-33.
[http://dx.doi.org/10.4103/2230-973X.160844] [PMID: 26258053] ].
The diagnosis of diseases and their therapy are constantly achieving heights of success due to the application of nanotechnology in the field of biomedicine. Mesoporous Silica carriers have been used for many applications ranging from biosensing and targeted drug delivery to nano adhesives [2Wu H, Li F, Wang S, et al. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 2018; 151: 66-77.
[http://dx.doi.org/10.1016/j.biomaterials.2017.10.018] [PMID: 29078200] ] and solubility enhancement as well as water treatment [3Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI. Mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev 2012; 41(7): 2590-605.
[http://dx.doi.org/10.1039/c1cs15246g] [PMID: 22216418] , 4Zhu W, Wang J, Wu D, et al. Investigating the Heavy Metal Adsorption of Mesoporous Silica Materials Prepared by Microwave Synthesis. Nanoscale Res Lett 2017; 12(1): 323.
[http://dx.doi.org/10.1186/s11671-017-2070-4] [PMID: 28476080] ]. Due to their biocompatible and biodegradable nature, a tremendous amount of research is in progress on Mesoporous Silica Nanoparticles [5Lu MM, Bai J, Shao D, et al. Antibacterial and biodegradable tissue nano-adhesives for rapid wound closure. Int J Nanomedicine 2018; 13: 5849-63.
[http://dx.doi.org/10.2147/IJN.S177109] [PMID: 30310284] ] (MSNs). The synthesis of MSNs is also simple and cost-effective which enables their use in multiple applications. Moreover, these provide uniform and tunable pore size, functionalization of the surface, gating mechanism of the pore opening, thus making these a distinctive and promising drug carrier [6Tang Y, Ke X. Advances of mesoporous silica nanoparticles as drug delivery system. Vol. 43. Zhongguo Yaoke Daxue Xuebao 2012; 567-72.]. The structure of Mesoporous Silica resembles that of the Honeycomb and can be visualised through Transmission Electron Microscopy. Conventional MSNs have the capacity to load a dose of active pharmaceutical moiety up to 200-300 mg which can be extended up to 600 mg/1g of MSNs. However, hollow MSNs with hollow core-mesoporous shell structures are able to achieve a super-high drug loading capacity because these provide more space to load drugs due to the hollow cores, typically >1 g drug/1 g of silica [7Vinu A, Hossain KZ, Ariga K. Recent advances in functionalization of mesoporous silica. J Nanosci Nanotechnol 2005; 5(3): 347-71.
[http://dx.doi.org/10.1166/jnn.2005.089] [PMID: 15913241] ].
The biocompatibility of mesoporous silica depends upon the shape, size, surface charge and porosity. The MSN materials having a size in between 100-200 nm are considered safe and biocompatible. Spherical MSNs are internalized faster by Chinese Hamster Ovarian (CHO) and normal human fibroblast cells than the rod-shaped nanoparticles, possibly due to the lower tendency of the former to form aggregates. MSNs with fewer silanol groups on their cell-contacet surfaces are considered to trigger the haemolysis of RBCs lesser than their nonporous silica counterparts containing a higher density of cell contactable surface silanol groups [8Asefa T, Tao Z. Biocompatibility of Mesoporous Silica Nanoparticles 2012; 25(11): 2265-84.
[http://dx.doi.org/10.1021/tx300166u] , 9Shi Y, Miller ML, Pasqua AJ, Di . Biocompatibility of mesoporous silica nanoparticles 2015; 3594].
Fig. (1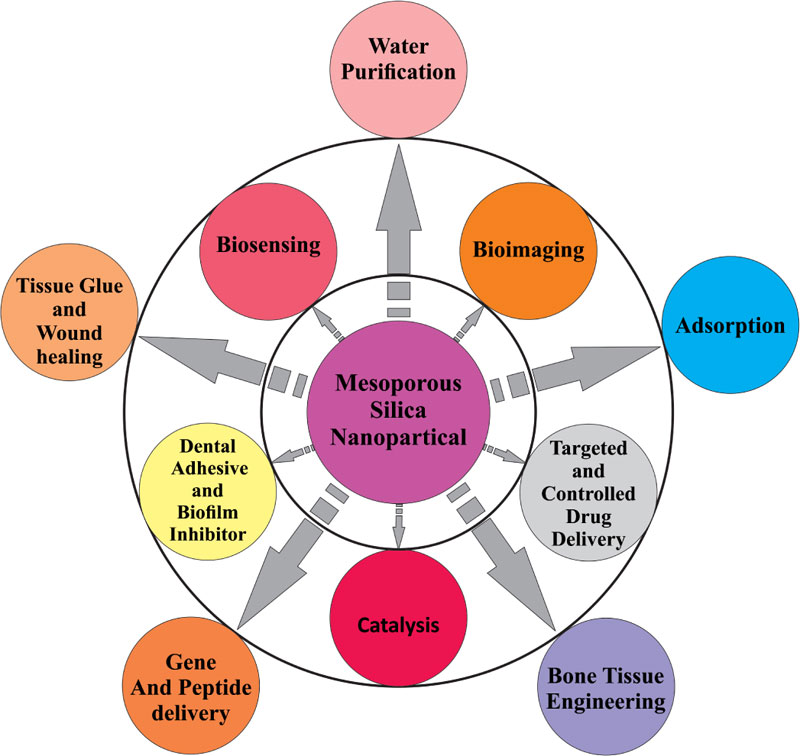 ) shows the multidisciplinary nature of MSNs.
) shows the multidisciplinary nature of MSNs.
Fig. (2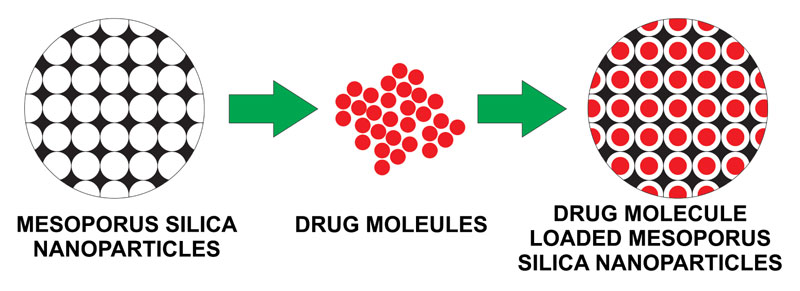 ). depicts the porous nature of mesoporous silica and its capacity to load the drug.
). depicts the porous nature of mesoporous silica and its capacity to load the drug.
The present review especially emphasizes on following applications:
- Biosensing Applications
- Targeted and Controlled Drug Delivery
- Solubility Enhancement
- Gene Delivery
- Wound Healing
2. BIOSENSING APPLICATIONS
The surface to volume ratio of NPs is quite high which allows the incorporation of abundant functional ligands, and also enables multivalency on NP surface which increases the interactions with targets. Capping and gating of MSN derivatives are frequently done to exploit their applications in Controlled-release Systems (CRS). Different detection technologies have been coupled with the CRS to develop diverse biosensors. Zhonghui Chen and his colleagues developed a simple, low cost and highly sensitive Cocaine Biosensor based on Chemiluminescence (CL) system of luminol/H2O2. Controlled released mesoporous silica had been coupled with a chemiluminescent detection technique to develop a sensitive biosensor for the target which does not cause an effect on the CL system itself. Initially, MSNs are loaded with glucose, then positively charged MSN reacts with the aptamer Cocaine which is negative in charge and closes the mesopores of MSNs. In the presence of the target, cocaine binds with its aptamer with high affinity; the flexible linear aptamer structure undergoes non-Watson & Crick interaction and gets converted to branched stems which lead to the release of glucose into the solution. The released glucose reacts with the dissolved oxygen to produce gluconic acid and H2O2 in the presence of Glucose Oxidase (GOx), which further enhances the CL of luminol in the NaOH solution. The increased chemiluminescence intensity is directly related to cocaine concentration. The present method successfully detected cocaine in serum with high selectivity [10Chen Z, Tan Y, Xu K, et al. Stimulus-response mesoporous silica nanoparticle-based chemiluminescence biosensor for cocaine determination. Biosens Bioelectron 2016; 75: 8-14.
[http://dx.doi.org/10.1016/j.bios.2015.08.006] [PMID: 26278045] ]. Zhu and co-workers developed an ATP biosensor that used aptamer-modified Au nanoparticle and closed the pores of MSN; these pores opened in the presence of adenosine triphosphate (ATP) through the competitive binding and the cargo was released. This study demonstrated that the aptamer-target interaction could be used as a stimuli-responsive mechanism in controlled-release systems. As a broad range of targets have been exploited to obtain the aptamers including several cancer biomarkers, so it can be concluded that this aptamer-based controlled-release system should have an equally broad spectrum of applications [11Zhu CL, Lu CH, Song XY, Yang HH, Wang XR. Bioresponsive controlled release using mesoporous silica nanoparticles capped with aptamer-based molecular gate. J Am Chem Soc 2011; 133(5): 1278-81.
[http://dx.doi.org/10.1021/ja110094g] [PMID: 21214180] ]. Zhang Xueao and other Co-workers, in 2009, developed a Biosensor based on acetylcholinesterase immobilized on mesoporous silica thin films. The sensor properties of the biosensor were investigated by using acetylthiocholine iodide as the substrate and Cyt c as the electron transfer mediator. The inhibition versus the logarithm of concentration was found to be linear to organophosphorus pesticide dichlorvos [12Zhang XA, Jia HH, Wang XF, et al. Biosensors based on acetylcholinesterase immobilized on mesoporous silica thin films. Chin Sci Bull 2009; 54(17): 3023-8.
[http://dx.doi.org/10.1007/s11434-009-0441-7] ].
3. TARGETED AND CONTROLLED DRUG DELIVERY
Fig. (3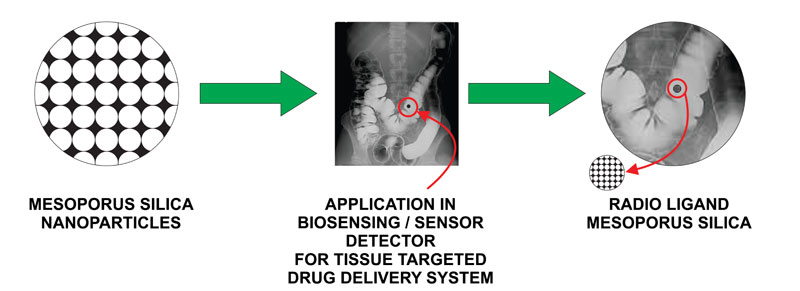 ). illustrates the ability of MSN to target and offer the diagnostic application in the form of bioimaging.
). illustrates the ability of MSN to target and offer the diagnostic application in the form of bioimaging.
Different functionalization and conjugations are done with MSNs to provide smart drug delivery systems [13Yang P, Gai S, Lin J. Functionalized mesoporous silica materials for controlled drug delivery. Chem Soc Rev 2012; 41(9): 3679-98.
[http://dx.doi.org/10.1039/c2cs15308d] [PMID: 22441299] ]. The drug release rate can be retarded by modifying the functionalization or surface charge. It also depends on the pore size and loading capacity. Moreover, drug targeting can be facilitated by suitable surface functionalization; e.g. Folic acid conjugation for cancer cell targeting. Properties like spherical shape prove to be more efficacious in targeting cells compared to the rod shape. The properties like pore size are highly important in case of targeting larger molecules [14Peretti E, Miletto I, Stella B, Rocco F, Berlier G, Arpicco S. Strategies to obtain encapsulation and controlled release of pentamidine in mesoporous silica nanoparticles. Pharmaceutics 2018; 10(4): 11-4.
[http://dx.doi.org/10.3390/pharmaceutics10040195] [PMID: 30347763] ]. Capping and gating associated with mesoporous silica are responsible for target-specific activity and controlled release of the drug [13Yang P, Gai S, Lin J. Functionalized mesoporous silica materials for controlled drug delivery. Chem Soc Rev 2012; 41(9): 3679-98.
[http://dx.doi.org/10.1039/c2cs15308d] [PMID: 22441299] , 15Iturrioz-Rodríguez N, Correa-Duarte MA, Fanarraga ML. Controlled drug delivery systems for cancer based on mesoporous silica nanoparticles. Int J Nanomedicine 2019; 14: 3389-401.
[http://dx.doi.org/10.2147/IJN.S198848] [PMID: 31190798] , 16Pande VV, Borawake DD, Halnor VV. Fabrication and characterization of gemcitabine hydrochloride loaded mesoporous silica nanoparticles as theranostics platform for pancreatic cancer. Mater Technol 2018; 33(13): 815-24.
[http://dx.doi.org/10.1080/10667857.2018.1512782] ]. In the case of cancer which requires the destruction of tumor without harming host cells can be achieved with the help of different gated MSNs [17Narayan R, Nayak UY, Raichur AM, Garg S. Mesoporous silica nanoparticles: A comprehensive review on synthesis and recent advances. Pharmaceutics 2018; 10(3): 1-49.
[http://dx.doi.org/10.3390/pharmaceutics10030118] [PMID: 30082647] ].Various gates are attached to MSNs which are responsive to different stimuli, including pH, light, enzymatic activity and temperature, etc. The tumor microenvironments having different pH and temperature conditions are employed while designing gated mesoporous silica nanoparticles [18Luo Z, Cai K, Hu Y, et al. Mesoporous silica nanoparticles end-capped with collagen: redox-responsive nanoreservoirs for targeted drug delivery. Angew Chem Int Ed Engl 2011; 50(3): 640-3.
[http://dx.doi.org/10.1002/anie.201005061] [PMID: 21226142] ]. The gating not only provides site-specific release but also protects the external environment [19Chen C, Yao W, Sun W, et al. A self-targeting and controllable drug delivery system constituting mesoporous silica nanoparticles fabricated with a multi-stimuli responsive chitosan-based thin film layer. Int J Biol Macromol 2019; 122: 1090-9.
[http://dx.doi.org/10.1016/j.ijbiomac.2018.09.058] [PMID: 30219514] ]. Folic acid conjugations add to the target specificity of the MSNs in cancer therapy [16Pande VV, Borawake DD, Halnor VV. Fabrication and characterization of gemcitabine hydrochloride loaded mesoporous silica nanoparticles as theranostics platform for pancreatic cancer. Mater Technol 2018; 33(13): 815-24.
[http://dx.doi.org/10.1080/10667857.2018.1512782] ]. The therapeutic efficacy of the drug also gets enhanced as the MSNs enhance the solubility and bioavailability of the drug [20Niemelä E, Desai D, Nkizinkiko Y, Eriksson JE, Rosenholm JM. Sugar-decorated mesoporous silica nanoparticles as delivery vehicles for the poorly soluble drug celastrol enables targeted induction of apoptosis in cancer cells. Eur J Pharm Biopharm 2015; 96: 11-21.
[http://dx.doi.org/10.1016/j.ejpb.2015.07.009] [PMID: 26184689] ]. Photodynamic therapy can also be employed to cure cancer and other infectious diseases by passive targeting [21Bayir S, Barras A, Boukherroub R, et al. Mesoporous silica nanoparticles in recent photodynamic therapy applications. Photochem Photobiol Sci 2018; 17(11): 1651-74.
[http://dx.doi.org/10.1039/C8PP00143J] [PMID: 30022180] ]. Vishal Pande and colleagues, in 2018, developed a Gemcitabine loaded, dye loaded, folic acid conjugated MSNs platform for the treatment of pancreatic cancer. They found out that the drug uptake of Gemcitabine in malignant cells was enhanced from the platform as compared to the plain drug. Moreover, dye loaded MSNs could be visualized which made the platform an excellent diagnostic agent and sustained release of the drug took place which was confirmed by in-vitro dissolution testing [16Pande VV, Borawake DD, Halnor VV. Fabrication and characterization of gemcitabine hydrochloride loaded mesoporous silica nanoparticles as theranostics platform for pancreatic cancer. Mater Technol 2018; 33(13): 815-24.
[http://dx.doi.org/10.1080/10667857.2018.1512782] ]. Nihal Elbialy et al., in 2019, synthesized the smart theranostic platform of PEGylated mesoporous silica nanoparticles loaded-curcumin for the prevention and treatment of cancer. This nanocarrier increased bioavailability of curcumin as well as provided a self-fluorescent system for bioimaging of cancer cells. A sustained pH-triggered drug release in the acidic environment of cancer cells was observed and also it was found to be safe, chemopreventive, therapeutic and diagnostic agent. The in-vitro study proved that the cell cycle arrest at G2/M of liver cancer cell line took place. The in-vivo study indicated that Tumour Chemoprevention Protocol (TCP) exhibited high therapeutic efficacy over Tumour Reduction Protocol (TRP) [22Elbialy NS, Aboushoushah SF, Sofi BF, Noorwali A. Multifunctional curcumin-loaded mesoporous silica nanoparticles for cancer chemoprevention and therapy. Microporous Mesoporous Mater 2019.
[http://dx.doi.org/10.1016/j.micromeso.2019.06.002] ]. Chen and other investigators, in 2018, developed a pH-responsive Doxorubicin loaded Hyaluronic Acid (HA) capped mesoporous silica nano reservoir for targeted drug delivery. HA served as a targeting agent which inhibited the premature drug release and facilitated the release in an acidic environment only. The in-vitro anticancer study of the carrier showed the targeting toward CD-44 overexpressing cells. The platform proved to be safe for other host cells and successfully targeted the cancer cells [23Chen C, Sun W, Wang X, Wang Y, Wang P. pH-responsive nanoreservoirs based on hyaluronic acid end-capped mesoporous silica nanoparticles for targeted drug delivery. Int J Biol Macromol 2018; 111: 1106-15.
[http://dx.doi.org/10.1016/j.ijbiomac.2018.01.093] [PMID: 29357289] ]. Chen and other scientists, in 2018, developed a self-targeting and controllable drug delivery system by fabricating chitosan film formed over doxorubicin-loaded MSNs and functionalized with folic acid having multi-stimuli responsive drug release for cancer treatment. They formed a layer of chitosan crosslinked by disulfide bond on drug-loaded mesoporous silica which was susceptible to pH and GSH stimulated drug release. Morever, folic acid conjugation targeted the platform to cancer cells. In-vitro study on HepG-2 cancer cell line showed folate-receptor mediated endocytosis to occur successfully. It increased the cellular intake of the nanoparticle and showed antitumor activity toward malignant cells and provided safe controlled release and targeted delivery of the anticancer drug [19Chen C, Yao W, Sun W, et al. A self-targeting and controllable drug delivery system constituting mesoporous silica nanoparticles fabricated with a multi-stimuli responsive chitosan-based thin film layer. Int J Biol Macromol 2019; 122: 1090-9.
[http://dx.doi.org/10.1016/j.ijbiomac.2018.09.058] [PMID: 30219514] ]. Erik Niemela and other scientists, in 2015, developed a platform for celastrol drug delivery for cancer treatment. They developed sugar decorated mesoporous silica nanoparticles as a vehicle for increasing solubility of celastrol and increasing its anticancer activity. They functionalized the glucose by conjugating directly to the MSN surface or mediated by a hyperbranched poly(ethylene imine, PEI) layer; the latter approach provided an overall positive surface charge which increased the cellular uptake as well as increased the reaction sites for sugar conjugation. The glucose functionalization increased the specificity of drug release in cancer cells and remained non-toxic to other cells. The uptake in HeLa and A549 cells as cancer cell models, as compared to mouse embryonic fibroblasts (MEFs) as representatives for normal cells, proved the target-specific efficacy of the particles. The analysis was done by flow cytometry, confocal microscopy, and spectrophotometer. The analysis concluded that the solubility of the drug increased. Glucose moiety could successfully target the cancer cells. Moreover, the anticancer activity of the drug markedly increased due to the platform; MSNs proved to be an excellent DDS for Celastrol in the cancer treatment [20Niemelä E, Desai D, Nkizinkiko Y, Eriksson JE, Rosenholm JM. Sugar-decorated mesoporous silica nanoparticles as delivery vehicles for the poorly soluble drug celastrol enables targeted induction of apoptosis in cancer cells. Eur J Pharm Biopharm 2015; 96: 11-21.
[http://dx.doi.org/10.1016/j.ejpb.2015.07.009] [PMID: 26184689] ] (Table 1).
4. SOLUBILITY ENHANCEMENT
Fig. (4 ). depicts the ability of MSNs to load the crystalline drug into pores and convert it to amorphous in order to enhance the solubility of the drug.
). depicts the ability of MSNs to load the crystalline drug into pores and convert it to amorphous in order to enhance the solubility of the drug.
 |
Fig. (1) Different Applications of Mesoporous Silica Nanoparticles |
 |
Fig. (2) Drug Loaded Mesoporous Silica Nanoparticles for Different Applications |
 |
Fig. (3) Tissue Targeting and Bioimaging |
 |
Fig. (4) Application of wound healing by Mesoporous Silica |
Mesoporous silica has proved to be advantageous for poorly soluble drugs in increasing its solubility [24McCarthy CA, Ahern RJ, Dontireddy R, Ryan KB, Crean AM. Mesoporous silica formulation strategies for drug dissolution enhancement: a review. Expert Opin Drug Deliv 2016; 13(1): 93-108.
[http://dx.doi.org/10.1517/17425247.2016.1100165] [PMID: 26549623] -26Niu X, Wan L, Hou Z, et al. Mesoporous carbon as a novel drug carrier of fenofibrate for enhancement of the dissolution and oral bioavailability. Int J Pharm 2013; 452(1-2): 382-9.
[http://dx.doi.org/10.1016/j.ijpharm.2013.05.016] [PMID: 23688621] ]. MSNs have a high specific surface area, high pore volume and appropriate pore sizes in the molecular range, ordered pore structures and silanol groups on their surfaces that can interact with a variety of drug molecules [27Laitinen R, Löbmann K, Strachan CJ, Grohganz H, Rades T. Emerging trends in the stabilization of amorphous drugs. Int J Pharm 2013; 453(1): 65-79.
[http://dx.doi.org/10.1016/j.ijpharm.2012.04.066] [PMID: 22569230] ]. The solubility of the drugs markedly increase due to the confinement of the drugs into the tiny pores of MSNs having a size of 3-50 nm range [28Hartono SB, Hadisoewignyo L, Yang Y, Meka AK, Antaresti , Yu C. Amine functionalized cubic mesoporous silica nanoparticles as an oral delivery system for curcumin bioavailability enhancement. Nanotechnology 2016; 27(50): 505605.
[http://dx.doi.org/10.1088/0957-4484/27/50/505605] [PMID: 27875331] ]. The entrapment of the drug in MSNs is done by the solvent impregnation method [16Pande VV, Borawake DD, Halnor VV. Fabrication and characterization of gemcitabine hydrochloride loaded mesoporous silica nanoparticles as theranostics platform for pancreatic cancer. Mater Technol 2018; 33(13): 815-24.
[http://dx.doi.org/10.1080/10667857.2018.1512782] , 29Panage S, Pande V, Patil S, Borbane S. Design and synthesis of mesoporous silica for inclusion of poorly water soluble drug sertaconazole nitrate as a drug delivery platform. Der Pharm Lett 2014; 6(4): 159-68., 30Pande VV, Jadhav KS, Giri MA, Kendre PN, Vibhute SK, Borawake DD. Design and development of paliperidone mesoporous silica template as a platform for surge dose drug delivery system. Mater Technol 2019; 34(3): 117-25.
[http://dx.doi.org/10.1080/10667857.2018.1538186] ]. Not only the drug solubility increases due to entrapment but also the drug can be protected from different destructive environments [31Sanjay C, Ghate VM, Lewis SA. Mesoporous silica particles for dermal drug delivery: A review. International Journal of Applied Pharmaceutics 2018; 10: 23-6.
[http://dx.doi.org/10.22159/ijap.2018v10i6.28633] ]. This nature of the MSNs has proven to be an excellent solubility enhancer and bioavailability enhancer as well [24McCarthy CA, Ahern RJ, Dontireddy R, Ryan KB, Crean AM. Mesoporous silica formulation strategies for drug dissolution enhancement: a review. Expert Opin Drug Deliv 2016; 13(1): 93-108.
[http://dx.doi.org/10.1517/17425247.2016.1100165] [PMID: 26549623] , 32Song Y, Zhu P, Wu Y, et al. Epsilon-poly-l-lysine decorated ordered mesoporous silica contributes to the synergistic antifungal effect and enhanced solubility of a lipophilic drug. Mater Sci Eng C 2019; 99(99): 231-40.
[http://dx.doi.org/10.1016/j.msec.2019.01.077] [PMID: 30889695] ]. The solubility enhancement mechanism of the mesoporous silica is clearly associated with the conversion of unstable crystalline form to stable amorphous form [33Slowing II, Trewyn BG, Giri S, Lin VSY. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv Funct Mater 2007.
[http://dx.doi.org/10.1002/adfm.200601191] ]. Katarina Bukarawhile working with her co-workers in 2016 developed a proof of concept of solubility enhancement in humans using ordered Mesoporous Silica Nanoparticles and Fenofibrate as a model drug. The study was performed as an open-label, randomized, two-way cross-over study, in which 12 healthy human volunteers were made to fast overnight. Fenofibrate formulated with ordered mesoporous silica or a marketed product based on micronized fenofibrate was given as a single dose. Plasma concentrations of fenofibric acid (pharmacologically active metabolite of fenofibrate) were monitored up to 96 h post-dose. The rate (Cmax/dose increased by 77%; tmax reduced by 0.75 h) and extent of absorption (AUC0–24h/dose increased by 54%) of fenofibrate significantly enhanced following administration of the ordered mesoporous silica-based formulation. This proof of concept developed a novel formulation strategy for the delivery of poorly soluble drugs using MSNs [34Bukara K, Schueller L, Rosier J, et al. Ordered mesoporous silica to enhance the bioavailability of poorly water-soluble drugs: Proof of concept in man. Eur J Pharm Biopharm 2016; 108(September): 220-5.
[http://dx.doi.org/10.1016/j.ejpb.2016.08.020] [PMID: 27648957] ]. Nikhil Biswas, in 2017, studied solubility and bioavailability enhancement of a poorly water-soluble drug valsartan using functionalized Mesoporous Silica Nanoparticles. During the study, he developed amine-functionalized mesoporous silica loaded Valasartan [VAL] and coated it with pH-sensitive polymer eudragit L100-55 for pH-dependent sustained release of anionic VAL. During the animal study, he found out that there was a 1.82-fold in bioavailability as compared to the marketed tablet. The blood pressure of rats was under control for 840 minutes as compared to the marketed tablet which lasted for about 360 minutes. From this study, he concluded that the marked increase in solubility and bioavailability was observed due to the amine-functionalized MSNs [35Biswas N. Modified mesoporous silica nanoparticles for enhancing oral bioavailability and antihypertensive activity of poorly water soluble valsartan. Eur J Pharm Sci 2017; 99: 152-60.
[http://dx.doi.org/10.1016/j.ejps.2016.12.015] [PMID: 27993684] ]. Vishal Pande and his colleagues, in 2018, studied the solubility and dissolution enhancement of poorly water-soluble drug Palperidone using MSNs. They synthesized amine-functionalized MSNs and loaded the drug Palperidone with the help of the wet impregnation method. The in-vitro and in-vivo drug releases were studied which were found to be significantly enhanced. The in-vitro drug release in 120 min for MSN loaded drug was 96% while that of the plain drug was 30%. The in-vivo study also confirmed the enhancement of solubility and dissolution of Palperidone [30Pande VV, Jadhav KS, Giri MA, Kendre PN, Vibhute SK, Borawake DD. Design and development of paliperidone mesoporous silica template as a platform for surge dose drug delivery system. Mater Technol 2019; 34(3): 117-25.
[http://dx.doi.org/10.1080/10667857.2018.1538186] ].
5. GENE DELIVERY
The drug delivery applications are most common and have already been reviewed earlier. Various developments have taken place in this context using different functionalizations, different gates, different trigger mechanisms,etc [16Pande VV, Borawake DD, Halnor VV. Fabrication and characterization of gemcitabine hydrochloride loaded mesoporous silica nanoparticles as theranostics platform for pancreatic cancer. Mater Technol 2018; 33(13): 815-24.
[http://dx.doi.org/10.1080/10667857.2018.1512782] , 36García-Fernández A, Aznar E, Martínez-Máñez R, Sancenón F. New advances in in vivo applications of gated mesoporous silica as drug delivery nanocarriers. Small 2020; 16(3): e1902242.
[http://dx.doi.org/10.1002/smll.201902242] [PMID: 31846230] -39Ahmadi Nasab N, Hassani Kumleh H, Beygzadeh M, Teimourian S, Kazemzad M. Delivery of curcumin by a pH-responsive chitosan mesoporous silica nanoparticles for cancer treatment. Artif Cells Nanomed Biotechnol 2018; 46(1): 75-81.
[http://dx.doi.org/10.1080/21691401.2017.1290648] [PMID: 28278578] ]. The study of specific molecules like gene, proteins and peptide is noteworthy [40Torney F, Trewyn BG, Lin VSY, Wang K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat Nanotechnol 2007; 2(5): 295-300.
[http://dx.doi.org/10.1038/nnano.2007.108] [PMID: 18654287] ]. The size of mesoporous silica nanoparticles varies from template to template. Moreover, the pore size can be increased by agents like Trimethyl Benzene [TMB]. The size ranges from 2-40 nm. The size of protein molecules entrapped is up to 100 kDa [41Liu H, Xu P. Smart Mesoporous Silica Nanoparticles for Protein Delivery 2019.
[http://dx.doi.org/10.3390/nano9040511] ]. The target specific delivery [42Yildirim A, Demirel GB, Erdem R, Senturk B, Tekinay T, Bayindir M. Pluronic polymer capped biocompatible mesoporous silica nanocarriers. Chem Commun (Camb) 2013; 49(84): 9782-4.
[http://dx.doi.org/10.1039/c3cc45967e] [PMID: 24026175] ] of the genes to the selected cells is the most important challenge in gene delivery [43Na HK, Kim MH, Park K, et al. Efficient functional delivery of siRNA using mesoporous silica nanoparticles with ultralarge pores. Small 2012; 8(11): 1752-61.
[http://dx.doi.org/10.1002/smll.201200028] [PMID: 22454257] ]. In general, gene delivery vectors can be classified into two categories: viral vectors and non-viral vectors; each of them has been widely reported for gene delivery [44Chao H, Mao L, Bruce AT, Walsh CE. Sustained expression of human factor VIII in mice using a parvovirus-based vector. Blood 2000; 95(5): 1594-9.
[http://dx.doi.org/10.1182/blood.V95.5.1594.005k34_1594_1599] [PMID: 10688813] , 45Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater 2013; 12(11): 967-77.
[http://dx.doi.org/10.1038/nmat3765] [PMID: 24150415] ]. Though viral and non-viral vector systems are available for gene delivery, there are many problems associated with them like biocompatibility, immunogenicity, etc. but as mesoporous silica is an approved biocompatible and biodegradable [5Lu MM, Bai J, Shao D, et al. Antibacterial and biodegradable tissue nano-adhesives for rapid wound closure. Int J Nanomedicine 2018; 13: 5849-63.
[http://dx.doi.org/10.2147/IJN.S177109] [PMID: 30310284] , 46Lin YS, Haynes CL. Synthesis and characterization of biocompatible and size-tunable multifunctional porous silica nanoparticles. Chem Mater 2009.
[http://dx.doi.org/10.1021/cm901259n] ] carrier by the FDA (US), it is suitable for targeted gene delivery [47Li Y, Hei M, Xu Y, Qian X, Zhu W. Ammonium salt modified mesoporous silica nanoparticles for dual intracellular-responsive gene delivery. Int J Pharm 2016; 511(2): 689-702.
[http://dx.doi.org/10.1016/j.ijpharm.2016.07.029] [PMID: 27426108] -49Zarei H, Kazemi Oskuee R, Hanafi-Bojd MY, Gholami L, Ansari L, Malaekeh-Nikouei B. Enhanced gene delivery by polyethyleneimine coated mesoporous silica nanoparticles. Pharm Dev Technol 2019; 24(1): 127-32.
[http://dx.doi.org/10.1080/10837450.2018.1431930] [PMID: 29357725] ]. It is important to note that Mesoporous silica has those three major properties which are required to deliver the gene successfully.
- 1) While there exist nucleases in the bloodstream and intracellular matrices, MSNs can protect the gene from degradation.
- MSNs have the capacity to pass the gene through the plasma membrane, endosome and/or nuclear pore complexes.
- 3) MSNs are nontoxic in nature [49Zarei H, Kazemi Oskuee R, Hanafi-Bojd MY, Gholami L, Ansari L, Malaekeh-Nikouei B. Enhanced gene delivery by polyethyleneimine coated mesoporous silica nanoparticles. Pharm Dev Technol 2019; 24(1): 127-32.
[http://dx.doi.org/10.1080/10837450.2018.1431930] [PMID: 29357725] ]
Flow cytometer study was employed to evaluate the cellular transfection efficiency of hollow MSNs which suggests that a two-fold increase in transfection efficiency was observed due to MSNs [48Zhan Z, Zhang X, Huang J, et al. Improved gene transfer with functionalized hollow mesoporous silica nanoparticles of reduced cytotoxicity. Materials (Basel) 2017; 10(7): 1-11.
[http://dx.doi.org/10.3390/ma10070731] [PMID: 28773087] ]. MSNs have been proven as an excellent gene carrier because of their ability to achieve a positive charge on their surface. The positive charge has the capability to interact with nucleic acids which are negatively charged to form the delivery complex. The rest of the positive charges of the complex are favourable for cell entry. These groups include amine group [50Steinbacher JL, Landry CC. Adsorption and release of siRNA from porous silica. Langmuir 2014; 30(15): 4396-405.
[http://dx.doi.org/10.1021/la402850m] [PMID: 24087929] ] and cationic polymers like PEI [51Li X, Chen Y, Wang M, Ma Y, Xia W, Gu H. A mesoporous silica nanoparticle--PEI--fusogenic peptide system for siRNA delivery in cancer therapy. Biomaterials 2013; 34(4): 1391-401.
[http://dx.doi.org/10.1016/j.biomaterials.2012.10.072] [PMID: 23164421] ], PLL [52Hartono SB, Gu W, Kleitz F, et al. Poly-L-lysine functionalized large pore cubic mesostructured silica nanoparticles as biocompatible carriers for gene delivery. ACS Nano 2012; 6(3): 2104-17.
[http://dx.doi.org/10.1021/nn2039643] [PMID: 22385282] ], PDEAEMA [53Sun JT, Hong CY, Pan CY. Fabrication of PDEAEMA-coated mesoporous silica nanoparticles and pH-responsive controlled release. J Phys Chem C 2010.
[http://dx.doi.org/10.1021/jp103982a] ], PAMAM [54Radu DR, Lai CY, Jeftinija K, Rowe EW, Jeftinija S, Lin VSY. A polyamidoamine dendrimer-capped mesoporous silica nanosphere-based gene transfection reagent. J Am Chem Soc 2004; 126(41): 13216-7.
[http://dx.doi.org/10.1021/ja046275m] [PMID: 15479063] ].
Small amino-functionalized MSNs are far better for the delivery of gene as compared to the cationic polymer grafted MSNs. They have some of the disadvantages like reduced pore volume [55Du X, Xiong L, Dai S, Kleitz F, Zhang Qiao S. Intracellular microenvironment-responsive dendrimer-like mesoporous nanohybrids for traceable, effective, and safe gene delivery. Adv Funct Mater 2014.
[http://dx.doi.org/10.1002/adfm.201402408] ], abundant positive charges on the polymer, thus the negatively charged nucleic acids may hinder the release of the gene [56Eltoukhy AA, Chen D, Alabi CA, Langer R, Anderson DG. Degradable terpolymers with alkyl side chains demonstrate enhanced gene delivery potency and nanoparticle stability. Adv Mater 2013; 25(10): 1487-93.
[http://dx.doi.org/10.1002/adma.201204346] [PMID: 23293063] ]. The large range of properties of MSNs which facilitate efficient loading and release of gene make them a strong alternative and future source for gene delivery. It can be strongly mentioned that efficient, reliable and exact gene delivery can be achieved by MSNs [57Zhou Y, Quan G, Wu Q, et al. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm Sin B 2018; 8(2): 165-77.
[http://dx.doi.org/10.1016/j.apsb.2018.01.007] [PMID: 29719777] ].
6. WOUND HEALING
Fig. (5 ). demonstrates the nano bridging and tissue gluing effect.
). demonstrates the nano bridging and tissue gluing effect.
Currently available options for fast gluing of tissues are fibrin glue, cyanoacrylate adhesives, etc. [58Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med 2008; 26(4): 490-6.
[http://dx.doi.org/10.1016/j.ajem.2007.05.015] [PMID: 18410821] -68Thirupathi Kumara Raja S, Thiruselvi T, Sailakshmi G, Ganesh S, Gnanamani A. Rejoining of cut wounds by engineered gelatin-keratin glue. Biochim Biophys Acta 2013; 1830(8): 4030-9.
[http://dx.doi.org/10.1016/j.bbagen.2013.04.009] [PMID: 23583368] ] The problem associated with the cyanoacrylate adhesives is the immunogenic reaction of severe heat produced at the point of application and the damage of tissue may take place at this site. Also, these may liberate formaldehyde which is severely toxic [5Lu MM, Bai J, Shao D, et al. Antibacterial and biodegradable tissue nano-adhesives for rapid wound closure. Int J Nanomedicine 2018; 13: 5849-63.
[http://dx.doi.org/10.2147/IJN.S177109] [PMID: 30310284] , 69Leggat PA, Smith DR, Kedjarune U. Surgical applications of cyanoacrylate adhesives: a review of toxicity. ANZ J Surg 2007; 77(4): 209-13.
[http://dx.doi.org/10.1111/j.1445-2197.2007.04020.x] [PMID: 17388821] -74Pascual G, Sotomayor S, Rodríguez M, et al. Cytotoxicity of cyanoacrylate-based tissue adhesives and short-term preclinical in vivo biocompatibility in abdominal hernia repair. PLoS One 2016; 11(6)e0157920
[http://dx.doi.org/10.1371/journal.pone.0157920] [PMID: 27322731] ]. Moreover, the surgical stitches and staples are also available but the after marks remain in case of stitches so these are also not acceptable. There should be a platform that may glue the tissues and serve as liquid stitches. Mesoporous silica has found its application in this field as well [2Wu H, Li F, Wang S, et al. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 2018; 151: 66-77.
[http://dx.doi.org/10.1016/j.biomaterials.2017.10.018] [PMID: 29078200] , 5Lu MM, Bai J, Shao D, et al. Antibacterial and biodegradable tissue nano-adhesives for rapid wound closure. Int J Nanomedicine 2018; 13: 5849-63.
[http://dx.doi.org/10.2147/IJN.S177109] [PMID: 30310284] , 75Chen Z, Li F, Liu C, et al. Blood clot initiation by mesoporous silica nanoparticles: dependence on pore size or particle size? J Mater Chem B Mater Biol Med 2016; 4(44): 7146-54.
[http://dx.doi.org/10.1039/C6TB01946C] [PMID: 32263652] ]. Nanoparticles have the ability to glue together the tissues by nano bridging effect [76Gao Y, Han Y, Cui M, Tey HL, Wang L, Xu C. ZnO nanoparticles as an antimicrobial tissue adhesive for skin wound closure. J Mater Chem B Mater Biol Med 2017; 5(23): 4535-41.
[http://dx.doi.org/10.1039/C7TB00664K] [PMID: 32263980] -78Meddahi-Pellé A, Legrand A, Marcellan A, Louedec L, Letourneur D, Leibler L. Organ repair, hemostasis, and in vivo bonding of medical devices by aqueous solutions of nanoparticles. Angew Chem Int Ed Engl 2014; 53(25): 6369-73.
[http://dx.doi.org/10.1002/anie.201401043] [PMID: 24740730] ]. Nanobridging requires a particle size less than 100 nm while the clotting of blood depends on the porosity and the particle size of MSNs. Various metal oxide nanoparticles have already been proven as effective tissue adhesives but it is worthy to know the ability of Mesoporous silica combined with metal oxide nanoparticles to be an effective tissue adhesive and antibacterial platform for wound gluing and healing [76Gao Y, Han Y, Cui M, Tey HL, Wang L, Xu C. ZnO nanoparticles as an antimicrobial tissue adhesive for skin wound closure. J Mater Chem B Mater Biol Med 2017; 5(23): 4535-41.
[http://dx.doi.org/10.1039/C7TB00664K] [PMID: 32263980] ]. As the mesoporous silica is biocompatible, it does not have any toxic effect post-application. It is biodegradable hence it will get degraded to a maximum extent [5Lu MM, Bai J, Shao D, et al. Antibacterial and biodegradable tissue nano-adhesives for rapid wound closure. Int J Nanomedicine 2018; 13: 5849-63.
[http://dx.doi.org/10.2147/IJN.S177109] [PMID: 30310284] , 79Watermann A, Brieger J. Mesoporous silica nanoparticles as drug delivery vehicles in cancer. Nanomaterials (Basel) 2017; 7(7)E189
[http://dx.doi.org/10.3390/nano7070189] [PMID: 28737672] ]. Meng-meng Lu and Co-investigators, in 2018, designed a silver nanoparticle decorated biodegradable mesoporous silica for rapid wound closure. They studied the platform for its wound healing ability in the Wistar rats. They concluded that the wound closed in 30 seconds while it healed in 5 days. Biodegradability was also confirmed which took place completely in 96 hours. It showed excellent antibacterial activity against E coli and the S.aureus which are major wound infecting organisms. MSNs proved to be an excellent nano adhesive and aesthetic wound healer as well [5Lu MM, Bai J, Shao D, et al. Antibacterial and biodegradable tissue nano-adhesives for rapid wound closure. Int J Nanomedicine 2018; 13: 5849-63.
[http://dx.doi.org/10.2147/IJN.S177109] [PMID: 30310284] ]. Wu and other associates, in 2017, developed a Ceria nanocrystal decorated mesoporous silica nanoparticle as a tissue glue for wound healing. They immobilized the ultra-small Ceria nanocrystals on the surface of the MSNs. It not only healed the wound but also significantly inhibited ROS exacerbation mediated deleterious effects, which potentially accelerated the wound healing process. Also, it did not allow to form any scar. Moreover, the platform can be much useful where wound healing and ROS Scavenging activity will be required simultaneously [2Wu H, Li F, Wang S, et al. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 2018; 151: 66-77.
[http://dx.doi.org/10.1016/j.biomaterials.2017.10.018] [PMID: 29078200] ]. It may be interesting to note the haemostatic efficacy of MSN. It could significantly promote the blood clot. There is a direct relationship between pore size and clotting efficiency, while the particle size of MSN has a little influence on the blood clot. The accessibility and diffusion of clotting–promoting proteins to and from the interior surfaces of MSN may be associated with each other as pore size gets directly impacted, and pores on the MSN surface get removed due to the curvature difference caused by the particle size. The ability of MSN to promote cell viability was proved in biocompatibility analysis of MSN where larger pore size resulted in better biocompatibility, but particle size had a negative influence on the cell viability. Rapid haemostasis of MSN in rabbit femoral artery injury testified the superb haemostatic efficiency of MSN [75Chen Z, Li F, Liu C, et al. Blood clot initiation by mesoporous silica nanoparticles: dependence on pore size or particle size? J Mater Chem B Mater Biol Med 2016; 4(44): 7146-54.
[http://dx.doi.org/10.1039/C6TB01946C] [PMID: 32263652] ]. So, we can conclude that MSN has a haemostatic effect and it can be successfully implemented in wound healing; furthermore, if it iscombined with other metal oxide nanoparticles, it will enhance its effect and broaden its applicability. Moreover, the nano bridging effect [78Meddahi-Pellé A, Legrand A, Marcellan A, Louedec L, Letourneur D, Leibler L. Organ repair, hemostasis, and in vivo bonding of medical devices by aqueous solutions of nanoparticles. Angew Chem Int Ed Engl 2014; 53(25): 6369-73.
[http://dx.doi.org/10.1002/anie.201401043] [PMID: 24740730] ] of the MSNs could prove itself as a liquid stitches formulation for rapid wound closure.
 |
Fig. (5) Application of wound healing by Mesoporous Silica |
CONCLUSION
Mesoporous Silica possesses tremendous desirable properties. The exploitation of all these properties can lead to benefits and gains in numerous applications. The pore size and loading capacity facilitate the controlled release of the drug; moreover different functionalizations of MSNs can be used to target drugs at specific sites. The release of these drugs can be monitored by ultrasonic waves, light, pH, magnetic properties, etc. The crystalline drugs are converted to amorphous by entrapment into MSNs which provide enhanced solubility and dissolution rate. The nano bridging effect associated with MSNs is utilised by combining with drug or metal nanoparticles, which has proved to be a significant tissue adhesive and an excellent wound healer. Maximum porosity and the adsorption capacity of MSNs make their use feasible in the loading of drugs, NPs, etc. and in drug therapy. MSNs can also be used as a bioimaging tool by combining them with an MRI active agent.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
| [1] | Bharti C, Nagaich U, Pal AK, Gulati N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int J Pharm Investig 2015; 5(3): 124-33. [http://dx.doi.org/10.4103/2230-973X.160844] [PMID: 26258053] |
| [2] | Wu H, Li F, Wang S, et al. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 2018; 151: 66-77. [http://dx.doi.org/10.1016/j.biomaterials.2017.10.018] [PMID: 29078200] |
| [3] | Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI. Mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev 2012; 41(7): 2590-605. [http://dx.doi.org/10.1039/c1cs15246g] [PMID: 22216418] |
| [4] | Zhu W, Wang J, Wu D, et al. Investigating the Heavy Metal Adsorption of Mesoporous Silica Materials Prepared by Microwave Synthesis. Nanoscale Res Lett 2017; 12(1): 323. [http://dx.doi.org/10.1186/s11671-017-2070-4] [PMID: 28476080] |
| [5] | Lu MM, Bai J, Shao D, et al. Antibacterial and biodegradable tissue nano-adhesives for rapid wound closure. Int J Nanomedicine 2018; 13: 5849-63. [http://dx.doi.org/10.2147/IJN.S177109] [PMID: 30310284] |
| [6] | Tang Y, Ke X. Advances of mesoporous silica nanoparticles as drug delivery system. Vol. 43. Zhongguo Yaoke Daxue Xuebao 2012; 567-72. |
| [7] | Vinu A, Hossain KZ, Ariga K. Recent advances in functionalization of mesoporous silica. J Nanosci Nanotechnol 2005; 5(3): 347-71. [http://dx.doi.org/10.1166/jnn.2005.089] [PMID: 15913241] |
| [8] | Asefa T, Tao Z. Biocompatibility of Mesoporous Silica Nanoparticles 2012; 25(11): 2265-84. [http://dx.doi.org/10.1021/tx300166u] |
| [9] | Shi Y, Miller ML, Pasqua AJ, Di . Biocompatibility of mesoporous silica nanoparticles 2015; 3594 |
| [10] | Chen Z, Tan Y, Xu K, et al. Stimulus-response mesoporous silica nanoparticle-based chemiluminescence biosensor for cocaine determination. Biosens Bioelectron 2016; 75: 8-14. [http://dx.doi.org/10.1016/j.bios.2015.08.006] [PMID: 26278045] |
| [11] | Zhu CL, Lu CH, Song XY, Yang HH, Wang XR. Bioresponsive controlled release using mesoporous silica nanoparticles capped with aptamer-based molecular gate. J Am Chem Soc 2011; 133(5): 1278-81. [http://dx.doi.org/10.1021/ja110094g] [PMID: 21214180] |
| [12] | Zhang XA, Jia HH, Wang XF, et al. Biosensors based on acetylcholinesterase immobilized on mesoporous silica thin films. Chin Sci Bull 2009; 54(17): 3023-8. [http://dx.doi.org/10.1007/s11434-009-0441-7] |
| [13] | Yang P, Gai S, Lin J. Functionalized mesoporous silica materials for controlled drug delivery. Chem Soc Rev 2012; 41(9): 3679-98. [http://dx.doi.org/10.1039/c2cs15308d] [PMID: 22441299] |
| [14] | Peretti E, Miletto I, Stella B, Rocco F, Berlier G, Arpicco S. Strategies to obtain encapsulation and controlled release of pentamidine in mesoporous silica nanoparticles. Pharmaceutics 2018; 10(4): 11-4. [http://dx.doi.org/10.3390/pharmaceutics10040195] [PMID: 30347763] |
| [15] | Iturrioz-Rodríguez N, Correa-Duarte MA, Fanarraga ML. Controlled drug delivery systems for cancer based on mesoporous silica nanoparticles. Int J Nanomedicine 2019; 14: 3389-401. [http://dx.doi.org/10.2147/IJN.S198848] [PMID: 31190798] |
| [16] | Pande VV, Borawake DD, Halnor VV. Fabrication and characterization of gemcitabine hydrochloride loaded mesoporous silica nanoparticles as theranostics platform for pancreatic cancer. Mater Technol 2018; 33(13): 815-24. [http://dx.doi.org/10.1080/10667857.2018.1512782] |
| [17] | Narayan R, Nayak UY, Raichur AM, Garg S. Mesoporous silica nanoparticles: A comprehensive review on synthesis and recent advances. Pharmaceutics 2018; 10(3): 1-49. [http://dx.doi.org/10.3390/pharmaceutics10030118] [PMID: 30082647] |
| [18] | Luo Z, Cai K, Hu Y, et al. Mesoporous silica nanoparticles end-capped with collagen: redox-responsive nanoreservoirs for targeted drug delivery. Angew Chem Int Ed Engl 2011; 50(3): 640-3. [http://dx.doi.org/10.1002/anie.201005061] [PMID: 21226142] |
| [19] | Chen C, Yao W, Sun W, et al. A self-targeting and controllable drug delivery system constituting mesoporous silica nanoparticles fabricated with a multi-stimuli responsive chitosan-based thin film layer. Int J Biol Macromol 2019; 122: 1090-9. [http://dx.doi.org/10.1016/j.ijbiomac.2018.09.058] [PMID: 30219514] |
| [20] | Niemelä E, Desai D, Nkizinkiko Y, Eriksson JE, Rosenholm JM. Sugar-decorated mesoporous silica nanoparticles as delivery vehicles for the poorly soluble drug celastrol enables targeted induction of apoptosis in cancer cells. Eur J Pharm Biopharm 2015; 96: 11-21. [http://dx.doi.org/10.1016/j.ejpb.2015.07.009] [PMID: 26184689] |
| [21] | Bayir S, Barras A, Boukherroub R, et al. Mesoporous silica nanoparticles in recent photodynamic therapy applications. Photochem Photobiol Sci 2018; 17(11): 1651-74. [http://dx.doi.org/10.1039/C8PP00143J] [PMID: 30022180] |
| [22] | Elbialy NS, Aboushoushah SF, Sofi BF, Noorwali A. Multifunctional curcumin-loaded mesoporous silica nanoparticles for cancer chemoprevention and therapy. Microporous Mesoporous Mater 2019. [http://dx.doi.org/10.1016/j.micromeso.2019.06.002] |
| [23] | Chen C, Sun W, Wang X, Wang Y, Wang P. pH-responsive nanoreservoirs based on hyaluronic acid end-capped mesoporous silica nanoparticles for targeted drug delivery. Int J Biol Macromol 2018; 111: 1106-15. [http://dx.doi.org/10.1016/j.ijbiomac.2018.01.093] [PMID: 29357289] |
| [24] | McCarthy CA, Ahern RJ, Dontireddy R, Ryan KB, Crean AM. Mesoporous silica formulation strategies for drug dissolution enhancement: a review. Expert Opin Drug Deliv 2016; 13(1): 93-108. [http://dx.doi.org/10.1517/17425247.2016.1100165] [PMID: 26549623] |
| [25] | Khanfar M, Fares MM, Salem MS, Qandil AM. Mesoporous silica based macromolecules for dissolution enhancement of Irbesartan drug using pre-adjusted pH method. Microporous Mesoporous Mater 2013; 173: 22-8. [http://dx.doi.org/10.1016/j.micromeso.2013.02.007] |
| [26] | Niu X, Wan L, Hou Z, et al. Mesoporous carbon as a novel drug carrier of fenofibrate for enhancement of the dissolution and oral bioavailability. Int J Pharm 2013; 452(1-2): 382-9. [http://dx.doi.org/10.1016/j.ijpharm.2013.05.016] [PMID: 23688621] |
| [27] | Laitinen R, Löbmann K, Strachan CJ, Grohganz H, Rades T. Emerging trends in the stabilization of amorphous drugs. Int J Pharm 2013; 453(1): 65-79. [http://dx.doi.org/10.1016/j.ijpharm.2012.04.066] [PMID: 22569230] |
| [28] | Hartono SB, Hadisoewignyo L, Yang Y, Meka AK, Antaresti , Yu C. Amine functionalized cubic mesoporous silica nanoparticles as an oral delivery system for curcumin bioavailability enhancement. Nanotechnology 2016; 27(50): 505605. [http://dx.doi.org/10.1088/0957-4484/27/50/505605] [PMID: 27875331] |
| [29] | Panage S, Pande V, Patil S, Borbane S. Design and synthesis of mesoporous silica for inclusion of poorly water soluble drug sertaconazole nitrate as a drug delivery platform. Der Pharm Lett 2014; 6(4): 159-68. |
| [30] | Pande VV, Jadhav KS, Giri MA, Kendre PN, Vibhute SK, Borawake DD. Design and development of paliperidone mesoporous silica template as a platform for surge dose drug delivery system. Mater Technol 2019; 34(3): 117-25. [http://dx.doi.org/10.1080/10667857.2018.1538186] |
| [31] | Sanjay C, Ghate VM, Lewis SA. Mesoporous silica particles for dermal drug delivery: A review. International Journal of Applied Pharmaceutics 2018; 10: 23-6. [http://dx.doi.org/10.22159/ijap.2018v10i6.28633] |
| [32] | Song Y, Zhu P, Wu Y, et al. Epsilon-poly-l-lysine decorated ordered mesoporous silica contributes to the synergistic antifungal effect and enhanced solubility of a lipophilic drug. Mater Sci Eng C 2019; 99(99): 231-40. [http://dx.doi.org/10.1016/j.msec.2019.01.077] [PMID: 30889695] |
| [33] | Slowing II, Trewyn BG, Giri S, Lin VSY. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv Funct Mater 2007. [http://dx.doi.org/10.1002/adfm.200601191] |
| [34] | Bukara K, Schueller L, Rosier J, et al. Ordered mesoporous silica to enhance the bioavailability of poorly water-soluble drugs: Proof of concept in man. Eur J Pharm Biopharm 2016; 108(September): 220-5. [http://dx.doi.org/10.1016/j.ejpb.2016.08.020] [PMID: 27648957] |
| [35] | Biswas N. Modified mesoporous silica nanoparticles for enhancing oral bioavailability and antihypertensive activity of poorly water soluble valsartan. Eur J Pharm Sci 2017; 99: 152-60. [http://dx.doi.org/10.1016/j.ejps.2016.12.015] [PMID: 27993684] |
| [36] | García-Fernández A, Aznar E, Martínez-Máñez R, Sancenón F. New advances in in vivo applications of gated mesoporous silica as drug delivery nanocarriers. Small 2020; 16(3): e1902242. [http://dx.doi.org/10.1002/smll.201902242] [PMID: 31846230] |
| [37] | Zhang Z, Wang L, Wang J, et al. Mesoporous silica-coated gold nanorods as a light-mediated multifunctional theranostic platform for cancer treatment. Adv Mater 2012; 24(11): 1418-23. [http://dx.doi.org/10.1002/adma.201104714] [PMID: 22318874] |
| [38] | Lungare S, Hallam K, Badhan RKS. Phytochemical-loaded mesoporous silica nanoparticles for nose-to-brain olfactory drug delivery. Int J Pharm 2016; 513(1-2): 280-93. [http://dx.doi.org/10.1016/j.ijpharm.2016.09.042] [PMID: 27633279] |
| [39] | Ahmadi Nasab N, Hassani Kumleh H, Beygzadeh M, Teimourian S, Kazemzad M. Delivery of curcumin by a pH-responsive chitosan mesoporous silica nanoparticles for cancer treatment. Artif Cells Nanomed Biotechnol 2018; 46(1): 75-81. [http://dx.doi.org/10.1080/21691401.2017.1290648] [PMID: 28278578] |
| [40] | Torney F, Trewyn BG, Lin VSY, Wang K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat Nanotechnol 2007; 2(5): 295-300. [http://dx.doi.org/10.1038/nnano.2007.108] [PMID: 18654287] |
| [41] | Liu H, Xu P. Smart Mesoporous Silica Nanoparticles for Protein Delivery 2019. [http://dx.doi.org/10.3390/nano9040511] |
| [42] | Yildirim A, Demirel GB, Erdem R, Senturk B, Tekinay T, Bayindir M. Pluronic polymer capped biocompatible mesoporous silica nanocarriers. Chem Commun (Camb) 2013; 49(84): 9782-4. [http://dx.doi.org/10.1039/c3cc45967e] [PMID: 24026175] |
| [43] | Na HK, Kim MH, Park K, et al. Efficient functional delivery of siRNA using mesoporous silica nanoparticles with ultralarge pores. Small 2012; 8(11): 1752-61. [http://dx.doi.org/10.1002/smll.201200028] [PMID: 22454257] |
| [44] | Chao H, Mao L, Bruce AT, Walsh CE. Sustained expression of human factor VIII in mice using a parvovirus-based vector. Blood 2000; 95(5): 1594-9. [http://dx.doi.org/10.1182/blood.V95.5.1594.005k34_1594_1599] [PMID: 10688813] |
| [45] | Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater 2013; 12(11): 967-77. [http://dx.doi.org/10.1038/nmat3765] [PMID: 24150415] |
| [46] | Lin YS, Haynes CL. Synthesis and characterization of biocompatible and size-tunable multifunctional porous silica nanoparticles. Chem Mater 2009. [http://dx.doi.org/10.1021/cm901259n] |
| [47] | Li Y, Hei M, Xu Y, Qian X, Zhu W. Ammonium salt modified mesoporous silica nanoparticles for dual intracellular-responsive gene delivery. Int J Pharm 2016; 511(2): 689-702. [http://dx.doi.org/10.1016/j.ijpharm.2016.07.029] [PMID: 27426108] |
| [48] | Zhan Z, Zhang X, Huang J, et al. Improved gene transfer with functionalized hollow mesoporous silica nanoparticles of reduced cytotoxicity. Materials (Basel) 2017; 10(7): 1-11. [http://dx.doi.org/10.3390/ma10070731] [PMID: 28773087] |
| [49] | Zarei H, Kazemi Oskuee R, Hanafi-Bojd MY, Gholami L, Ansari L, Malaekeh-Nikouei B. Enhanced gene delivery by polyethyleneimine coated mesoporous silica nanoparticles. Pharm Dev Technol 2019; 24(1): 127-32. [http://dx.doi.org/10.1080/10837450.2018.1431930] [PMID: 29357725] |
| [50] | Steinbacher JL, Landry CC. Adsorption and release of siRNA from porous silica. Langmuir 2014; 30(15): 4396-405. [http://dx.doi.org/10.1021/la402850m] [PMID: 24087929] |
| [51] | Li X, Chen Y, Wang M, Ma Y, Xia W, Gu H. A mesoporous silica nanoparticle--PEI--fusogenic peptide system for siRNA delivery in cancer therapy. Biomaterials 2013; 34(4): 1391-401. [http://dx.doi.org/10.1016/j.biomaterials.2012.10.072] [PMID: 23164421] |
| [52] | Hartono SB, Gu W, Kleitz F, et al. Poly-L-lysine functionalized large pore cubic mesostructured silica nanoparticles as biocompatible carriers for gene delivery. ACS Nano 2012; 6(3): 2104-17. [http://dx.doi.org/10.1021/nn2039643] [PMID: 22385282] |
| [53] | Sun JT, Hong CY, Pan CY. Fabrication of PDEAEMA-coated mesoporous silica nanoparticles and pH-responsive controlled release. J Phys Chem C 2010. [http://dx.doi.org/10.1021/jp103982a] |
| [54] | Radu DR, Lai CY, Jeftinija K, Rowe EW, Jeftinija S, Lin VSY. A polyamidoamine dendrimer-capped mesoporous silica nanosphere-based gene transfection reagent. J Am Chem Soc 2004; 126(41): 13216-7. [http://dx.doi.org/10.1021/ja046275m] [PMID: 15479063] |
| [55] | Du X, Xiong L, Dai S, Kleitz F, Zhang Qiao S. Intracellular microenvironment-responsive dendrimer-like mesoporous nanohybrids for traceable, effective, and safe gene delivery. Adv Funct Mater 2014. [http://dx.doi.org/10.1002/adfm.201402408] |
| [56] | Eltoukhy AA, Chen D, Alabi CA, Langer R, Anderson DG. Degradable terpolymers with alkyl side chains demonstrate enhanced gene delivery potency and nanoparticle stability. Adv Mater 2013; 25(10): 1487-93. [http://dx.doi.org/10.1002/adma.201204346] [PMID: 23293063] |
| [57] | Zhou Y, Quan G, Wu Q, et al. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm Sin B 2018; 8(2): 165-77. [http://dx.doi.org/10.1016/j.apsb.2018.01.007] [PMID: 29719777] |
| [58] | Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med 2008; 26(4): 490-6. [http://dx.doi.org/10.1016/j.ajem.2007.05.015] [PMID: 18410821] |
| [59] | Toriumi DM, O’Grady K, Desai D, Bagal A. Use of octyl-2-cyanoacrylate for skin closure in facial plastic surgery. Plast Reconstr Surg 1998; 102(6): 2209-19. [http://dx.doi.org/10.1097/00006534-199811000-00062] [PMID: 9811023] |
| [60] | Panda A, Kumar S, Kumar A, Bansal R, Bhartiya S. Fibrin glue in ophthalmology. Indian J Ophthalmol 2009; 57(5): 371-9. [http://dx.doi.org/10.4103/0301-4738.55079] [PMID: 19700876] |
| [61] | Lauto A, Mawad D, Foster LJR. Adhesive biomaterials for tissue reconstruction. J Chem Technol Biotechnol 2008. [http://dx.doi.org/10.1002/jctb.1771] |
| [62] | Shapiro AJ, Dinsmore RC, North JH Jr. Tensile strength of wound closure with cyanoacrylate glue. Am Surg 2001; 67(11): 1113-5. [PMID: 11730233] |
| [63] | Robertson FP, Magill LJ, Davidson C, Mitchell H, Davidson BR. Cyanoacrylate tissue glues for cutaneous wound closure. In: Wound Healing Biomaterials 2016. [http://dx.doi.org/10.1016/B978-1-78242-456-7.00008-8] |
| [64] | Brennan M. Fibrin glue. Blood Rev 1991; 5(4): 240-4. [http://dx.doi.org/10.1016/0268-960X(91)90015-5] [PMID: 1782483] |
| [65] | Jankowitz BT, Atteberry DS, Gerszten PC, et al. Effect of fibrin glue on the prevention of persistent cerebral spinal fluid leakage after incidental durotomy during lumbar spinal surgery. Eur Spine J 2009; 18(8): 1169-74. [http://dx.doi.org/10.1007/s00586-009-0928-6] [PMID: 19283413] |
| [66] | Ong CCP, Jacobsen AS, Joseph VT. Comparing wound closure using tissue glue versus subcuticular suture for pediatric surgical incisions: a prospective, randomised trial. Pediatr Surg Int 2002; 18(5-6): 553-5. [http://dx.doi.org/10.1007/s00383-002-0728-0] [PMID: 12415411] |
| [67] | Rushbrook JL, White G, Kidger L, Marsh P, Taggart TFO. The antibacterial effect of 2-octyl cyanoacrylate (Dermabond®) skin adhesive. J Infect Prev 2014; 15(6): 236-9. [http://dx.doi.org/10.1177/1757177414551562] [PMID: 28989390] |
| [68] | Thirupathi Kumara Raja S, Thiruselvi T, Sailakshmi G, Ganesh S, Gnanamani A. Rejoining of cut wounds by engineered gelatin-keratin glue. Biochim Biophys Acta 2013; 1830(8): 4030-9. [http://dx.doi.org/10.1016/j.bbagen.2013.04.009] [PMID: 23583368] |
| [69] | Leggat PA, Smith DR, Kedjarune U. Surgical applications of cyanoacrylate adhesives: a review of toxicity. ANZ J Surg 2007; 77(4): 209-13. [http://dx.doi.org/10.1111/j.1445-2197.2007.04020.x] [PMID: 17388821] |
| [70] | Landegren T, Risling M, Persson JKE, Sondén A. Cyanoacrylate in nerve repair: transient cytotoxic effect. Int J Oral Maxillofac Surg 2010; 39(7): 705-12. [http://dx.doi.org/10.1016/j.ijom.2010.03.008] [PMID: 20434310] |
| [71] | Leggat PA, Kedjarune U, Smith DR. Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health 2004; 42(2): 207-11. [http://dx.doi.org/10.2486/indhealth.42.207] [PMID: 15128170] |
| [72] | Sulheim E, Iversen TG, To Nakstad V, et al. Cytotoxicity of poly(Alkyl cyanoacrylate) nanoparticles. Int J Mol Sci 2017; 18(11)E2454 [http://dx.doi.org/10.3390/ijms18112454] [PMID: 29156588] |
| [73] | Hida T, Sheta SM, Proia AD, McCuen BW II. Retinal toxicity of cyanoacrylate tissue adhesive in the rabbit. Retina 1988; 8(2): 148-53. [http://dx.doi.org/10.1097/00006982-198808020-00013] [PMID: 3420314] |
| [74] | Pascual G, Sotomayor S, Rodríguez M, et al. Cytotoxicity of cyanoacrylate-based tissue adhesives and short-term preclinical in vivo biocompatibility in abdominal hernia repair. PLoS One 2016; 11(6)e0157920 [http://dx.doi.org/10.1371/journal.pone.0157920] [PMID: 27322731] |
| [75] | Chen Z, Li F, Liu C, et al. Blood clot initiation by mesoporous silica nanoparticles: dependence on pore size or particle size? J Mater Chem B Mater Biol Med 2016; 4(44): 7146-54. [http://dx.doi.org/10.1039/C6TB01946C] [PMID: 32263652] |
| [76] | Gao Y, Han Y, Cui M, Tey HL, Wang L, Xu C. ZnO nanoparticles as an antimicrobial tissue adhesive for skin wound closure. J Mater Chem B Mater Biol Med 2017; 5(23): 4535-41. [http://dx.doi.org/10.1039/C7TB00664K] [PMID: 32263980] |
| [77] | Kim JH, Kim H, Choi Y, Lee DS, Kim J, Yi GR. Colloidal mesoporous silica nanoparticles as strong adhesives for hydrogels and biological tissues. ACS Appl Mater Interfaces 2017; 9(37): 31469-77. [http://dx.doi.org/10.1021/acsami.7b09083] [PMID: 28836756] |
| [78] | Meddahi-Pellé A, Legrand A, Marcellan A, Louedec L, Letourneur D, Leibler L. Organ repair, hemostasis, and in vivo bonding of medical devices by aqueous solutions of nanoparticles. Angew Chem Int Ed Engl 2014; 53(25): 6369-73. [http://dx.doi.org/10.1002/anie.201401043] [PMID: 24740730] |
| [79] | Watermann A, Brieger J. Mesoporous silica nanoparticles as drug delivery vehicles in cancer. Nanomaterials (Basel) 2017; 7(7)E189 [http://dx.doi.org/10.3390/nano7070189] [PMID: 28737672] |
| [80] | Zhu Y, Shi J, Chen H, Shen W, Dong X. A facile method to synthesize novel hollow mesoporous silica spheres and advanced storage property. Microporous Mesoporous Mater 2005; 84(1-3): 218-22. [http://dx.doi.org/10.1016/j.micromeso.2005.05.001] |
| [81] | Song S-W, Hidajat K, Kawi S. Functionalized SBA-15 materials as carriers for controlled drug delivery: influence of surface properties on matrix-drug interactions. Langmuir 2005; 21(21): 9568-75. [http://dx.doi.org/10.1021/la051167e] [PMID: 16207037] |
| [82] | Hillerström A, Andersson M, Samuelsson J, van Stam J. Solvent strategies for loading and release in mesoporous silica. Colloid and Interface Sci Commun 2014; 3: 5-8. [http://dx.doi.org/10.1016/j.colcom.2015.01.001] |
| [83] | Doadrio JC, Sousa EM, Izquierdo-Barba I, Doadrio AL, Perez-Pariente J, Vallet-Regí M. Functionalization of mesoporous materials with long alkyl chains as a strategy for controlling drug delivery pattern. J Mater Chem 2006; 16(5): 462-6. [http://dx.doi.org/10.1039/B510101H] |
| [84] | Qu F, Zhu G, Huang S, Li S, Qiu S. Effective controlled release of captopril by silylation of mesoporous MCM-41. ChemPhysChem 2006; 7(2): 400-6. [http://dx.doi.org/10.1002/cphc.200500294] [PMID: 16411260] |
| [85] | Kwon S, Singh RK, Perez RA, Abou Neel EA, Kim H-W, Chrzanowski W. Silica-based mesoporous nanoparticles for controlled drug delivery. J Tissue Eng 2013; 42041731413503357 [http://dx.doi.org/10.1177/2041731413503357] [PMID: 24020012] |
| [86] | Pourjavadi A, Tehrani ZM. Mesoporous silica nanoparticles (MCM-41) coated PEGylated chitosan as a pH-responsive nanocarrier for triggered release of erythromycin. Int J Polym Mater Polym Biomater 2014; 63(13): 692-7. [http://dx.doi.org/10.1080/00914037.2013.862534] |
| [87] | Pande VV, Khedkar PV, Giri MA. Fabrication and Characterisation of gemcitabine hydrochloride loaded magnetically responsive mesoporous silica nanocomposites as smart hybrid theranostic platform for treatment of pancreatic cancer. Mater Technol 2020; 00(00): 1-8. [http://dx.doi.org/10.1080/10667857.2020.1734729] |