- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Systems Biology Journal
(Discontinued)
ISSN: 1876-3928 ― Volume 5, 2014
pH is a Neurally Regulated Physiological System. Increased Acidity Alters Protein Conformation and Cell Morphology and is a Significant Factor in the Onset of Diabetes and Other Common Pathologies
Graham Wilfred Ewing*
Abstract
Background:
The body’s physiological stability is maintained by the influence of the autonomic nervous system and the dynamic interaction of the organ systems which regulate all aspects of the body’s function. These physiological systems and their function have been overlooked by the genetic paradigm. A better understanding of these systems may lead to an improved understanding of the fundamental relationship involving sense perception, neural function and organ networks, and cellular and molecular biology. This article follows earlier articles by the author which illustrate that sleep, blood pressure and blood glucose are neurally regulated physiological systems.
Aims:
The purpose of this article is to discuss how Grakov’s mathematical model of the physiological systems (i) influences the contemporary understanding of the processes which regulate the pH in biological systems i.e. that pH is a neurally regulated physiological system; (ii) is a significant factor in the emergence of changes to cell morphology and, ultimately, the onset of pathologies; and (iii) involves environmental factors more commonly associated with phenotype.
Results:
It considers the fundamental role played by pH e.g. regulating the levels and redox state of minerals, protein conformation, the activation of proteins and enzymes, and how this influences metabolic function. It considers the consequences of increased acidity, in particular from alcoholic beverages and acidified soft drinks, and how this influences the processes which regulate the body’s physiological stability.
Conclusions:
The article concludes that the body’s impaired ability to regulate its acidity, exascerbated by the consumption of highly acidic beverages, is a considerably underestimated factor in the subsequent development and onset of many common pathologies e.g. diabetes, cardiovascular diseases, alzheimer’s disease, cancers, etc.
Article Information
Identifiers and Pagination:
Year: 2012Volume: 4
First Page: 8
Last Page: 12
Publisher Id: TOSYSBJ-4-8
DOI: 10.2174/1876392801204010008
Article History:
Received Date: 12/11/2011Revision Received Date: 8/2/2012
Acceptance Date: 17/2/2012
Electronic publication date: 20/4/2012
Collection year: 2012
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Montague Healthcare, Mulberry House, 6 Vine Farm Close, Cotgrave, Nottingham, NG12 3TU, UK; Tel: 00-44-115-9890304; Fax: 00-44-115-9899826; E-mails: graham.ewing@montague-diagnostics.co.uk; graham.ewing@montaguehealthcare.co.uk
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 12-11-2011 |
Original Manuscript | pH is a Neurally Regulated Physiological System. Increased Acidity Alters Protein Conformation and Cell Morphology and is a Significant Factor in the Onset of Diabetes and Other Common Pathologies | |
1. BACKGROUND
The laws of chemistry characterise every chemical reaction. The prevailing reaction conditions influence the rate at which each reaction proceeds. In this article we consider whether the significance of pH has been significantly overlooked in biomedical research.
Changes to the pH of a biochemical system or reaction often influences the structure of chemical components, their level, and subsequently the rate at which these components react. This determines the redox state of minerals; their ability to complex with DNA; their ability to activate enzymatic reactions; and their ability to be filtered and/or eliminated from the body. Altered pH influences protein conformation and the ability of proteins to react. It influences the characteristics of epigenetic reactions such as methylation, histone de-acetylation, acetylation, phosphorylation, etc.
The body’s homeostasis is maintained by neuroregulation of the various organ networks. These multiple physiological systems, their nature and structure, and the factors which influence their function, have been recognised but remain poorly defined. Perhaps the greatest insight into these factors is provided by sports physiologists [1,2] who consider the mechanisms which the body employs to enhance its physical performance. These are at variance with the pharmacological disciplines which are geared towards ameliorating the symptoms associated with morbidity and mortality.
The conventional understanding adopted by biomedical research is that the brain’s function is essentially independent of the way in which the brain reacts to external stimuli. This ignores that a fundamental aspect of the brain’s function is to maintain homeostasis and regulate uncontrolled physical activity; and ignores that sensory signals are converted into biochemical responses which, when experienced as stress, lead to alterations of normal physiology, their manifestation as pathologies and the production of metabolites, and which influence brain function. A better understanding of the physiological systems leads to an understanding of the coordinated function of organs in each neural and organ network i.e. there is a fundamental structural relationship involving sense perception, neural networks, organ networks, and cellular and molecular biology. Extremes of cognitive input, which we perceive as stress, influence cellular and molecular biology [3].
The neuroregulation of pH performs a vital homeostatic function however the prevailing definition of physiological systems makes no reference to acid-base regulation. Contemporary biomedical research focuses almost exclusively on the biochemistries linked to normal protein and enzyme metabolism and often ignores the influence of pH. It focuses almost exclusively on the biochemistries considered to be responsible for the regulation of pH in the brain whilst (i) ignoring that it is the whole function of the brain which regulates all aspects of behaviour and health; (ii) that neural function is influenced by visceral function and feedback; and (iii) that pH influences the level and characteristics of minerals which facilitate enzymatic reactions.
Neural activity is influenced by sensory input and memory, and can make rapid changes to intercellular and extracellular pH [5]. Lowered intercellular pH influences the levels, redox state and bioavailability of minerals e.g. calcium, magnesium, zinc, etc; and subsequently the action of many proteins and enzymes. Lowered extracellular pH and increased plasma viscosity influences the neural flow of oxygen, blood glucose, the accumulation of metabolic by-products, and the rate at which glucose is metabolised. In addition, the maintenance of normal levels of pH is critical for brain function. The brain receives circa 20% of the body’s total oxygen consumption, and circa 25% of the body’s total supply of glucose [6]. Accordingly the prevailing levels of magnesium, zinc and other minerals, which influence the rate at which insulin (and other processes) are metabolised, are essential for the brain’s normal function.
The expression of proteins and their level (genotype) is considered to be indicative of the degree of progression of a particular disease state however many diseases are now recognised to be multifactorial [7,8] and, in many cases, multi-systemic [4,9]. This places in doubt upon the ability of individual genes or groups of genes, or protein-based biomarkers, to accurately predict the onset or progression of diseases which have complex origins [4]. The levels of proteins expressed; the epigenetic and/or polygenic factors which influence protein expression; protein conformation; the rate at which proteins react with their reactive substrates (phenotype); the level, redox state and bioavailability of minerals; levels of cofactors; the levels and conformation of substrates; and/or the rate at which minerals, substrates and cofactors are absorbed through the visceral membranes into plasma; must all be considered to be factors in the pathological process(es). Modern disease(s) have genetic and environmental origins. Both are significant.
In addition, the levels of proteins expressed and the rate at which these proteins react influences the natural biofluorescent spectra or biophoton emission. This is indicative of the light absorbed as proteins are energised and of the light emitted as proteins decay [10] which influences visual perception. This mechanism provides the data sets for Grakov’s mathematical model which links colour perception to cellular and molecular biochemistry. It incorporates an understanding of the nature and structure of the various physiological systems and how such organ networks function in an apparently coherent or synchronised manner [9].
Contemporary medicine accepts that the study of physiological systems is hugely under-researched yet the lack of suitable tools has hitherto prevented any significant research in this subject. The accepted definition of physiological systems - for example in the Merck Manual - appears to be contradicted by medical practice: (i) the GP’s consultation is largely based upon an assessment of the patient’s systemic stability i.e. of temperature, pH, breathing, digestion, elimination, blood pressure, blood volume, blood glucose, blood cell content, sexual function, posture, sleep; (ii) medical research focuses upon the obvious definitions of physiological systems rather than those which have been accepted by contemporary medical practice e.g. which focuses upon breathing, blood glucose, blood pressure, blood cell content, and blood volume rather than a cardiovascular system; (iii) the contemporary definition of physiological systems ignores functions which are essential for the body’s function e.g. the regulation of temperature, pH, sexual function, etc.
The regulation of pH is perhaps the most complex and sophisticated of all physiological systems (Fig. 1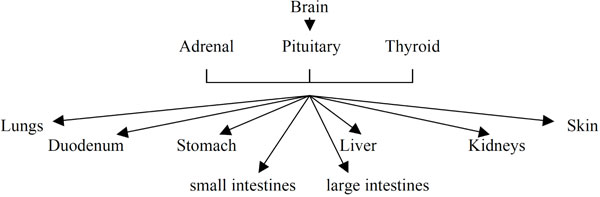 ). It comprises: brain; pituitary, thyroid and adrenal glands; liver; pancreas; blood and peripheral blood vessels; lungs and bronchii; skin; stomach; duodenum; kidneys; small and large intestines (origin: Grakov IG). Although the function of each of these organs influences the regulation of pH there is not yet an acceptance that these organs function as part of a coherent and regulated system.
). It comprises: brain; pituitary, thyroid and adrenal glands; liver; pancreas; blood and peripheral blood vessels; lungs and bronchii; skin; stomach; duodenum; kidneys; small and large intestines (origin: Grakov IG). Although the function of each of these organs influences the regulation of pH there is not yet an acceptance that these organs function as part of a coherent and regulated system.
 |
Fig. (1) pH regulation. |
That molecular biology is influenced by pH is a fundamental aspect of the study of the body’s physiology however it is more difficult to explain how localized variations of pH, at the intracellular and intercellular levels and at different parts of the body can be regulated and moreover that regulated cell function is dependent upon precisely maintained pH in different sub-systems [11,12]. Perhaps this is not surprising when considering that the path of human evolution started as a relatively small group of cells. The cell evolved the ability to handle different environments e.g.
- Light activates proteins. It is fundamental to the growth of all vegetation/plant life and the function of plant cells/photosynthesis.
- Environmental pH including the levels of minerals eg. calcium, magnesium, etc. Life adapted physically, electrochemically and biochemically.
- Temperature e.g. between typically 33-38C (normal 36.8C.) and denaturation of proteins starts to occur at circa 40C.
- Oxygen e.g. typically 21% but has been significantly higher (and lower) in the distant past.
Initially cells evolved and adapted to different environments e.g. to differing levels of pH, temperature and oxygen. This occured in the intracellular environment by diffusion through cell membranes and then at the intercellular level through the subsequent development of physiological structures e.g. the lungs; the ability to metabolise calcium and magnesium and subsequently the development of skeletal structure(s); the development of capilliary structures which enabled the body to deal with the absorption and processing of nutrients; and the process of cell division i.e. of reproduction.
The significance of what appears to be minor or insignificant changes to biochemistry are often ignored however there are examples in the human and animal world where apparently minor physiological changes can have profound effects e.g.
- Women taking the contraceptive pill might find that their natural selection of a partner might be adversely influenced [13]
- Clownfish lose their hearing in water which is more acidic than the norm [14] and become attracted to their predators [15].
- The migration of birds and animals, and the mating cycle of ruminants, are triggered by minute changes of light upon the pineal gland.
2. WHICH ORGANS DOES THE BODY USE TO REGULATE PH?
The body maintains, at least in the Caucasian, a plasma pH of 7.364. Homeostatic Limits: if plasma pH drops below 6.8 or rises above 7.8, death is likely. The total acid intake has to be balanced by bicarbonate and other buffers e.g. PO4. Imbalance leads to acidosis or, less commonly, alkalosis.
The lungs remove excess CO2, the waste product of glucose metabolism, from the blood. The digestion of food, in particular of proteins, and drink creates acidity to which the pancreas responds by releasing secretin and also by the release of bicarbonate which neutralizes the acidic chyme which enters the duodenum from the stomach. The digestive tract ranges from strongly acidic in the stomach, to slightly acidic (at a pH of typically 5.7-6.7 in the small intestine), pH 7/neutral in the colon and typically pH 7-9 in the large intestine. The stomach releases pepsin, other digestive enzymes and hydrochloric acid (maintains the stomach at a pH of typically 2); thereafter food is churned into chyme by muscular contractions in the stomach. The gallbladder delivers alkaline bile to the duodenum in order to neutralise excess acid and dissolve fatty acids in chyme. The large intestine delivers bicarbonate to maintain the alkaline pH which is required to sustain the function of over 700 bacteria. The kidneys strive to maintain pH of urine of typically 6.4. The pH of the skin is maintained at a pH of typically 4.5-6.0. pH 5.5 is considered to be the optimum pH required by the skin [16]. The Extracellular fluid can act as an acid reservoir. Blood plasma (circa 20%) and interstitial fluid (circa 80%) make up the extracellular fluid (ECF). This 20/80 balance is balanced by the prevailing osmotic pressure. The bones of the skeletal structures can release calcium and magnesium in order to buffer excess acidity [17,18]. This illustrates that the body’s regulation of pH exhibits the characteristics of a physiologically regulated system within defined operating limits.
3. HOW DOES PH INFLUENCE THE BODY’S PHYSIOLOGY?
pH influences the body’s physiology in many ways e.g.
3.1. All physical activity whether at rest or in motion creates CO2 which the body must expel in order to sustain its function and physiological stability [19]. The inability to expel CO2 and to maintain homeostasis progressively destabilises the body’s function.
3.2. pH Influences endocrine function and metabolic rate. Lowered pH influences endocrine function and consequently lowers metabolic rate [20]. It influences the chemical spectrum of hormones emitted [21] which influences metabolic rate [22]. It also reduces the absorption of iodine upon which the thyroid depends.
3.3. pH is an environmental factor (phenotype) which influences the function of most proteins and enzymes (see Fig. 2 ). It alters protein conformation and the prevailing levels of minerals which are often essential for protein conformation and reaction e.g. the ability of zinc to complex with insulin. In particular the prevailing level of pH influences the levels of Sodium and Calcium [23,24], which facilitate the flow of ions across neuronal membranes. This influences the action potentials, rate of neuronal firing and ultimately the fixation of memories. The prevailing pH influences the absorption and bioavailability of Chromium [25-29] and the balance of iron as FeII/Fe III.
). It alters protein conformation and the prevailing levels of minerals which are often essential for protein conformation and reaction e.g. the ability of zinc to complex with insulin. In particular the prevailing level of pH influences the levels of Sodium and Calcium [23,24], which facilitate the flow of ions across neuronal membranes. This influences the action potentials, rate of neuronal firing and ultimately the fixation of memories. The prevailing pH influences the absorption and bioavailability of Chromium [25-29] and the balance of iron as FeII/Fe III.
 |
Fig. (2) |
3.4. The acidity of water and drink
3.4.1. The quality of drinking water may influence the onset of type 1 diabetes [30] although the mechanisms by which water acidity or mineral content may be involved in the etiology of type 1 diabetes remain unknown. The quality of the water supply, in particular the acidity of drinking water, is implicated in diabetes etiology [30].
3.4.2. The intake of acidic and alcoholic drinks alter the natural metabolic processes. Different enzymes (isozymes) metabolise the alcohol at lower pH. This alters lipid metabolism and contributes to weight gain [31].
3.4.3. The incidence of Alzheimer’s disease (AD) was higher in localities with relatively high levels of aluminium [32] but was not associated with higher pH e.g. in drinking water [33] or regular use of antacids [34]. This is significant because many with AD are also diabetic [35].
3.5. The absorption and release of bone calcium buffers the system against increased acidity [36]. Demineralisation of bone is the consequence of significant increases in extracellular acidity [37]. In addition, the influence of acidosis on bone metabolism is more significant in children than adults i.e. when bone structures are growing. Bone metabolism and immunity are interlinked. Immunity is largely a product of bone and spleen metabolism. pH influences immune function [38,39] and hence influences predisposition to viral and bacterial infections.
4. HOW DOES THE METABOLISM OF ALCOHOL INFLUENCE THE BODY’S ACIDITY AND ITS FUNCTION?
4.1. the metabolism of ethyl alcohol creates acidity.
A typical human digestive system produces approximately 3g of ethanol each day. This arises through the normal digestive and fermentation processes. This degradation of ethanol is therefore an essential feature of the life of most living organisms. It is indicative of the normal levels of alcohol which the body is designed to tolerate. Nevertheless Ethyl Alcohol is a poison which, consumed in excess, can increase susceptibility to morbidity and mortality.
Ethanol is first oxidised to acetaldehyde which forms ROS/free radicals. The enzyme alcohol dehydrogenase is dependent upon the availability of zinc and magnesium [40]. Acetaldehyde is subsequently oxidised to acetic acid which is then converted to Acetyl Co-A and subsequently is broken down in the normal citric acid cycle to CO2. The rapid conversion of acetaldehyde is essential in order to avoid the damage which reactive oxygen species can have on the body’s structure and function e.g. to the kidney and liver in the case of chronic alcoholism, and to birth defects (structural, gene-related, behavioural) in the case of foetal alcohol syndrome [41-45]. This leads to the degradation of cells and organs, and ultimately leads to the failure of the mechanisms which regulate the function of the kidney, liver and other visceral organs. It requires the production of anti-oxidants to neutralise the damaging effects of such ROS. The completion of this process produces acetic acid which further lowers extracellular pH before being metabolised in the citric acid cycle to CO2.
4.2. increased acidity influences the levels and bioavailability of Zinc and other Minerals.
Zinc is commonly associated with its role in the metabolism of insulin however it is not solely associated with diabetes. It’s ability to complex with proteins prevents islet amyloid polypeptide from agglomerating and forming fibrils e.g. as found in alzheimer’s disease, diabetes, and other degenerative diseases. [46]. Acid conditions alter the conformation of insulin thereby enhancing the formation of fibrils [47]; the levels and bioavailability of minerals in particular of zinc [48], magnesium, iron, chromium, etc.
Lowered levels of zinc suppress the function of carbonic anhydrase and the processes of respiration; digestion, in particular the function of carboxypeptidase which digests proteins; reproductive function, in particular the growth and function of the reproductive organs; signal transduction; metabolism of RNA and DNA, and gene expression; apoptosis; the function of the kidneys and liver; bone metabolism and hence that of immune function; nervous system; etc. It influences the brain, in particular synaptic plasticity, which is implicated in the processes associated with learning. In excess zinc is a neurotoxin and in deficit it slows metabolic function. It is stored in the metallothioneins.
Zinc is mainly bound to albumin and transferrin. This involves a dynamic relationship between iron and zinc i.e. (i) excess iron reduces zinc levels and vice versa; (ii) increased acidity leads to higher levels of iron and lower levels of zinc. In general, the concentration of zinc stays relatively constant irrespective of zinc intake i.e. at normal pH, however this apparent equilibrium is not maintained during changes of pH. Furthermore increased levels of acidity increase the susceptibility of proteins, particularly of insulin, to changes of conformation [47], dissociation and potentially of aggregation.
In particular, the growth of cancers may be dependent upon levels of iron which are needed for DNA replication [49] e.g. the occurrence of ferritin is five times greater in women with breast cancer [50].
4.3. Increased acidity influences the conformation of DNA and proteins.
- Protein structure is an essential characteristic of the regulatory processes [51,52]. It varies with pH [53,54] and influences the ability of proteins to react with their substrates.
- Genetic modifications, in particular of mutant genes, influence a wide range of medical conditions e.g. proinsulin structure [55], cystic fibrosis [56], cholesterol metabolism, etc.
- DNA is coiled around histones. The characteristics of this coiling influences gene function, and hence the expression of proteins, and is associated with learning and memory [57] i.e. the biochemistries which support the recall of memories are unable to perform in the normal manner. Environmental enrichment has been used to enhance gene expression and re-establish the normal biochemistries necessary for the recall of memories and/or function [58].
- The conformation of DNA is linked to pH [59,60].
- pH is invariably linked to the epigenetic factors which alter the fundamental nature and structure of DNA:
- it influences the strength and integrity of DNA e.g. altering/influencing the degree of cross-linking
- it alters the stability/properties of DNA and results in shortened telomeres [61].
- it alters the rate at which proteins react with their reactive substrates.
5. THE INFLUENCE OF ACIDITY UPON CELL MORPHOLOGY.
Increased acidity creates changes to protein conformation which influence cell conformation. It may alter cellular structure: (i) proteins change shape and character and consequently their spatial orientation within the cell; (ii) it can alter the spatial arrangement of chromosomes in the cell nucleus [62]; (iii) alter the expression of proteins and (iv) influence the rate of occurrence of chromosomal rearrangements.
The long-term exposure to abnormal levels of pH, in particular to higher levels of acidity, exceed the body’s inherent buffering mechanisms and contribute to the early onset of morphological changes which are characteristic of emergent pathologies e.g.
- increased blood flow, enlargement of arterial vascular channels, and the development of an inflammatory response; or increased venous outflow (ischaemia)
- cell hyperfunction or hypofunction e.g. in adenal glands. Adrenal hyperfunction is manifest as Cushing’s disease and may be stimulated by caffeine, nicotine, etc. Adrenal hypofunction is manifest as Addisons disease.
- abnormal cell growth or apoptosis/cell death [50]. It has been recognised that adjusting tumour pH has therapeutic potential [63, 64]
6. WHICH ENVIRONMENTAL FACTORS INFLUENCE ACID-BASE HOMEOSTASIS?
Extremes of sensory input influence acid/base homeostasis e.g.
6.1. Stress
6.1.1. Stress in its various manifestations suppresses immune function and influences the autonomic nervous system e.g. raising or lowering blood pressure [65]. It lowers immune function and increases the level of susceptibility to viral infections and a range of conditions including type 1 diabetes mellitus [66] and type 2 diabetes mellitus [67]; heart conditions e.g. myocardial infarction, angina pectoris; etc.
Stress can alter the distribution of acidity in the digestive tract [68,69] thereby leading to digestive reflux, duodenal ulcers and/or ulcerative colitis [70]. The influence of stress alters the normal physiological pathways and triggers the onset of pathologies which include the destabilisation of pH e.g. resulting in asthma [71,72], diabetes mellitus, alzheimer’s disease, heart conditions, incontinence, cancers, etc.
In extremes of stress e.g. of fear, the pH of the amygdala falls and influences the function of the autonomic nervous system thereby stimulating the typical adrenocortical ‘flight or fight’ response [73]. It is the biochemical consequences of impaired function (e.g. of suffocation which raises levels of CO2 in the blood and alters brain pH homeostasis) which we experience as fear, anxiety or panic [74]. The consequences of stress in its many and various manifestations lead to changes to genetic conformation, in particular to our DNA and Chromosomes [75].
6.1.2. Stress influences the genetic pathways. Stress is experienced at different levels. Minor exposures to stress may have a psychological impact, perhaps influencing our future behaviour, but does not significantly influence our physiology. Nevertheless there are examples of DNA methylation which illustrate how absence of fathers in childhood can influence a child’s future demeanor [76]. Stress influences the body’s physiological stability and ultimately leads to changes of DNA [75]. Recent research has illustrated that stress influences the expression of telomerase [77,61] i.e. the length of DNA shortens with exposure to chronic stress thereby influencing longevity.
The enzyme SIRT-6 is encoded the SIRT-6 gene. The protein SIRT6 is implicated in the mechanisms of DNA repair [78]. The absence of the SIRT6 enzyme influences the degree to which chromatin is compacted. Under normal circumstances SIRT6 regulates the levels of the protein HIF1alpha however the absence of SIRT6 relaxes chromatin conformation, activates glycolytic genes and lowers levels of blood glucose. In addition SIRT6 induced changes to chromatin conformation influences the structure and function of telomeres [79]. SIRT6 requires NAD+ as a cofactor. This may be influenced by increased levels of alcohol consumption, and associated devitaminisation, and by increased levels of acidity which will influence the stability and levels of NAD+ and/or NADH.
6.2. Light Influences Intercellular pH
Light acts upon the pituitary gland [80] and subsequently influences the function of other endocrine glands - thyroid, adrenals, etc. It is the medium which transmits cognitive/sensory input. It plays an essential role in the regulation of sleep and hence upon the health of all other physiological systems. Irregular sleep patterns lead to instability in the autonomic nervous system e.g. influencing levels of cortisol, growth hormone, prolactin and vasopressin. Impaired pituitary function can lead to secondary adrenal insufficiency. It influences the function of the HPA-axis leading to primary and secondary adrenal insufficiency e.g. in which the adrenal glands cannot produce sufficient quantities of corticosteroids and hence are unable to properly regulate levels of sodium, potassium and the renal control of absorption/reabsorption of water by the kidneys [81]. This regulation of water is largely controlled by vasopressin which is produced by the hypothalamus but is stored by vesicles within the pituitary gland. In the presence of vasopressin, the kidneys retain water; whilst in the absence of vasopressin the kidneys excrete water. The storage of vasopressin exhibits striking similarities to that of insulin which is complexed in a zinc-based hexamer and may be indicative of a pH-sensitive controlled-release mechanism. In addition, there are indications that light influences the regulation of the intercellular pH-balance [82,83].
6.3. Diet
Diet influences gene structure and the subsequent expression of proteins [84].
- The high carbohydrate diet has increasingly displaced the traditional vegetable-based diet upon which our predecessors were raised. This contributes to increased acidity, demineralisation and devitamination [85]. A high-fat diet, in particular a diet with high levels of palmitic acid (a significant constituent of cocoa butter used in chocolate manufacture) inactivates the PGC-1 gene by epigenetic modification/ methylation. The PGC-1 gene is involved in carbohydrate and lipid metabolism [86]. The inactivation of the PGC-1 gene leads to the inactivation of other genes and influences the level of proteins expressed [87]. Dietary factors influence the function of genes which are linked to the regulation of blood glucose i.e of diabetes, and hence which influence the expression of proteins such as pro-insulin/insulin. Alterations to pH influence the metabolism of fat [88].
- Caffeine and artificial sweeteners have been linked to increases in acidity [89].
- pH influences the levels and bioavailability of minerals, including Chromium. Significant sources of chromium, which assists the body to regulate blood glucose [90] are to be found in brassicas and cocoa butter.
- An alkaline diet influences the elimination of calcium and bone metabolism. Diets which are high in levels of protein and cereals, produce excess acid which increases the rate at which calcium is excreted [91].
- The Influence of Acidified Soft drinks and Alcoholic Beverages
Low levels of minerals and in particular zinc and magnesium are the consequences of increased acidity. They are strongly associated with the onset of diabetes and subsequent secondary effects such as cardiovascular disease(s). Low levels of magnesium are linked to muscular spasms and, in the alcoholic, to delirium tremens. Mineral homeostasis is based on dietary intake, intestinal absorption, and renal regulation [92,93] however it may not be the consumption of ethyl alcohol which is significant but instead the acidity of the alcohol (typically pH: 3.5-4.5) and of its metabolites. This is confirmed by considering the effect which strongly acidic soft drinks have on the body’s function. The consumption of alcoholic beverages lead to progressive devitamination [94-97] i.e. it reduces the levels of vitamin cofactors which are essential for many enzymatic reactions. In addition, platelet stickiness, a classic indicator of sugar chemistries and glycation, is a common feature of the dehydration which follows the consumption of alcohol [98]. It is an indicator of platelet aggregation [99,100] which can potentially lead to the onset of cardiovascular complications.
The pH of diluted phosphoric acid which gives cola drinks their acidity and tang has a pH of typically 2.5-4.5. The pH of regular and diet pops ranges from typically 2.5-3.5 whilst branded colas have a pH of typically 3.5, and diet versions a pH of typically 4.5. Demineralization, or loss of tooth material, begins at a pH of circa 5.5. Healthy people have body fluids that are slightly alkaline, in the range 7.1-7.5 pH, whilst the general population have a pH of 4.5-7.5. All aspects of our function i.e. our behaviour, exercise, drink (fruit juices) and food (proteins, grains and fatty acids), creates some degree of acidity with which the body’s natural buffering mechanisms are designed to cope; however acidified soft drinks and colas in particular provide an estimated 1,000-10,000 more times acidity than that experienced in normal lifestyles. Acidified soft drinks such as colas have been statistically linked to the occurrence of strokes and heart attacks [101], osteoporosis [23], gout [102], complications in pregnancy [103], paralysis [104], bone fractures [105] and an increased risk of developing cancers [106]. This is consistent with the consumption of acidity through beer and alcoholic beverages which significantly enhance the risk and occurrence of cancer(s) [107,108,97].
Acute and Chronic acidosis are linked to lowered levels of potassium, bicarbonate, phosphate, and calcium [109-111]. By contrast alkaline diets [112-115] are associated with improved health and lessened levels of morbidity and mortality.
7. PH INFLUENCES THE STABILITY OF OTHER PHYSIOLOGICAL SYSTEMS
7.1. pH Influences the Levels of Minerals and the Regulation of Blood Glucose
The solubility, redox state and bioavailability of minerals is pH dependent. A lower pH influences protein conformation. Accordingly the prevailing level of acidity (pH) dramatically influences the availability of Zinc and its ability to complex with proteins. Increasing levels of acidity and/or the nature of the mineral salts have a profound effect upon the solubility of zinc e.g.
Salt Solubility
Zinc Carbonate 0.21 gms per litre
Zinc Sulphate 580 gms per litre
Zinc Chloride 4320 gms per litre
Changes of acidity leads to the preferential absorption of some minerals and elimination of others. Increased acidity decreases the levels of zinc, magnesium and potassium. This leads to the preferential absorption or retention of neurotoxins e.g. cadmium, lead and mercury; and alters the balance or relationship involving copper and zinc which is considered to be an important characteristic of homeostasis.
Elevated levels of iron, linked to increased acidity, is a common feature of liver cirrhosis, diabetes, cardiac myopathy, etc.
The availability of zinc influences key physiological systems and processes e.g. zinc is involved in the function of DNA and RNA polymerases and consequently is a critical component in the synthesis of proteins. It is a component of carbonic anhydrase, angiotensin-converting enzyme, and is essential for the synthesis of haem the primary precursor of haemoglobin, and the free radical scavenger superoxide dismutase. The enzymatic synthesis of ATP is zinc-dependent. Lack of zinc is implicated in apoptosis [116]. The regulation of zinc homeostasis may be essential for the body’s stability [117]. Increased acidity influences the ability of zinc to complex with insulin and the consequently low levels of zinc are a feature of diabetes [118]. In vitro studies indicate that the quaternary structure of insulin under physiological conditions (pH 6-7) is hexameric. This quaternary structure is disrupted at low pH levels, where monomeric and dimeric structures are observed. The loss of quaternary structure at low pH is concurrent with a significant reduction in activity. Accordingly, increased acidity influences the controlled release and conformation of insulin and consequently the regulation of blood glucose.
Increased acidity increases the amounts of magnesium, zinc and other minerals, which are excreted in urine. Accordingly, increased acidity of urine is a feature of the body’s failed attempts to regulate its overall pH and results in mineral depletion [119-121]. It is a particular feature of glucose metabolism [122,123], cardiovascular disease [124-127], kidney disease [128], rheumatoid arthritis [129], osteoporosis [130] and cancers [131]. It is also a feature of many solid tumours that they are more significantly acidic than normal tissues.
Proinsulin is expressed by the INS gene [132]. pH influences the conversion of proinsulin to insulin [133, 134], insulin secretion [135], and the conversion of insulin to the zinc hexamer and its storage in the pancreas - the hexamer is a zinc-bound complex which is less active and more stable at relatively neutral pH. This availability of the zinc hexamer serves to ensure a controlled-release supply of insulin in response to glycaemic overload. Lowered extracellular pH shortens the half-life of insulin as it dissociates from its receptor [136] and decreases receptor affinity. Accordingly lowered pH is associated with lowered availability of insulin, lowered ability to regulate blood glucose and increased autonomic instability (Fig. 3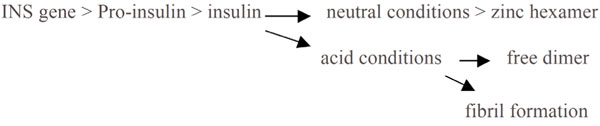 ). Increased acidity influences the availability of calcium and zinc which are required by proteases as part of the process of converting proinsulin to insulin [134], increases the rate at which insulin is released, and hence increases the rate at which insulin is subsequently degraded by the liver and kidneys [137]. Insulin secretion increases and the natural oscillations which regulate pancreatic function i.e the regulation of blood glucose, become increasingly erratic.
). Increased acidity influences the availability of calcium and zinc which are required by proteases as part of the process of converting proinsulin to insulin [134], increases the rate at which insulin is released, and hence increases the rate at which insulin is subsequently degraded by the liver and kidneys [137]. Insulin secretion increases and the natural oscillations which regulate pancreatic function i.e the regulation of blood glucose, become increasingly erratic.
 |
Fig. (3) The influence of pH upon diabetes. |
pH influences the neural regulation of blood glucose [138] which stabilises autonomic influence upon the function of pancreatic beta-cells [139-141]. pH is a mechanism which the brain can employ to reduce metabolic rate and maintain homeostasis or allostasis [142].
Ultimately, the body uses the kidneys to lower pH [92,93] however high urinary pH can lead to problems in the urinary tract e.g. (i) at pH <5.5 uric acid may precipitate and lead to uric acid stone formation, (ii) growth of candida albicans, etc.
7.2. Lowered pH Influences Other Physiological Systems
In addition to its effect upon blood glucose metabolism increased levels of acidity raise blood cell content and blood viscosity. This has the effect of increasing blood pressure. It lowers metabolic rate and body temperature; influences musculoskeletal/ bone metabolism; stimulates the elimination of body fluids; suppresses sexual function and reproduction; etc.
8. HOW DOES THE BODY REGULATE THE ACCUMULATION OF ACIDITY IN BODY TISSUES?
The body’s physiology developed in response to the need to hunt or farm, walk and run, and otherwise conduct the activities required to facilitate and maintain a safe, healthy and active life. It is designed for an active lifestyle. Physical exercise performs the function of regulating the body’s function [143,144]. It influences gene expression [145]. Lack of exercise influences the body’s pH i.e. the ratio of CO2/O2 in the blood, and leads to a ladder of pathologies leading to increasing morbidity and ultimately to premature mortality. It alters the coordinated function of the digestive organs, influences the absorption of essential minerals in the intestines, and influences muscle tone and function in the vasculature in addition to the legs, arms, etc. Increased acidity and lower levels of minerals are features of Diabetes Mellitus and Cardiovascular Disease(s). In periods following regular exercise, improved lung function optimises the elimination of CO2, and hence the regulation of pH, in periods of high and low metabolic requirements. This leads to improved bio-availability of minerals and improved metabolic rate. An exercise program reinvigorates the natural processes which are essential for good health. Without such a program the use of drugs or other therapeutic approaches will only be ‘as-good-as-can-reasonably-be-expected’ i.e. seeking to stabilise the pathology and to prevent its progression. It cannot be possible to re-establish good health in those who are diabetic, overweight, and morbidly obese unless each has an exercise programme which can regulate extracellular pH and enhance the level and bio-availability of minerals required for normal metabolic function.
Measurement of pH e.g. in urine, is a measure of kidney function and of the kidney’s ability to neutralize bile salts. Lack of exercise leads to increased acidity in urine and is an indication of the inability of the buffering systems to deal with excess acidity.
Increased levels of acidity lowers levels of magnesium and hence the function of myocytes (muscle cells) to convert glucose into energy: excess glucose being converted as triglycerides and stored by adipocytes (fat cells). In addition to its role in skeletal function exercise influences smooth muscle function which is essential for the proper function of the skeletal muscles, stomach, intestines, and all visceral organs [146]. Exercise regulates the metabolism of blood glucose [147] and improves platelet aggregation and function [148]. A wide range of genes are implicated including those which express erythropoietin, growth factors, glucose transporters, glycolytic enzymes, etc [149]. As outlined a lack of exercise may lead to the onset of diabetes and alzheimer’s disease [150,151].
9. DISCUSSION
This article highlights the complexity of the body’s function and of the way in which the synchronized function of visceral organs, in particular those which are associated with the regulation of pH, appears to be essential to the body’s function and stability. It supports earlier articles by the author which illustrate how the regulation of blood glucose, blood pressure and sleep are regulated physiological systems. It is further evidence of a complex and regulated network of physiological systems and illustrates that the understanding of the nature and structure of the physiological systems are fundamental to an understanding of the down-stream mechanisms which influence and regulate gene function and cellular and molecular biology.
The brain regulates its physiological stability and hence that of the visceral organs but its function is also influenced by that of the visceral organs [152]. This is a dynamic relationship which enables the body to respond to sensory input - received through the senses, skin and also through nutrition - more commonly referred to as ‘the influence of the environment’ or ‘phenotype’. Extremes of stress increase the body’s level of acidity.
This understanding of physiological systems and their link with cognition and the autonomic nervous system serves to explain the often complex polygenic nature of disease and its many and various causes. It addresses the theoretical deficit between cognition and cellular & molecular biology highlighted by Nobel Laureate Eric Kandel [3] and addresses the limitations of the genetic paradigm identified by Richard C. Strohman [153-155]. It defines the nature of the various environmental factors and their influence upon phenotype; and illustrates that phenotype may be more significant than genotype e.g. explaining how twins which have divergent lifestyles can have distinctly different health outcomes in later life. The genes provide a genetic predisposition however it is lifestyle-related factors which ultimately trigger the onset of pathologies.
It may illustrate that there is no viable alternative to regular exercise and a well balanced diet as a means of preventing the onset and progression of diabetes and obesity. A reasonable level of exercise is essential to maintain the muscular volume and muscle tone, volume of oxygen in blood plasma, and the regulation of pH which are essential for the normal biological processing of blood glucose. It indicates also that regular exercise is an essential pre-requisite for the processes which involve reading, writing, the fixation and recall of memories, and mental computation.
pH influences the rate at which proteins are expressed and also the rate at which such expressed proteins react with their reactive substrates e.g. due to lowered levels of minerals and cofactors.
Scientific research is only able to study effects of known variables i.e. identified variables which are at a significant level. It is increasingly evident that minor changes to pH has a subtle influence upon our lifestyle e.g. how does minor consumption of alcoholic drinks influence our choices of partner? Could such occurrence increase our future susceptibility to conflict, divorce, etc? Whilst the behavioural symptoms and social consequences of excessive alcohol consumption and the elevated levels of systemic acidity are relatively obvious what is less obvious is how apparently minor changes of acidity influence human development and behaviour?
Gene sequencing and profiling illustrates the complexity of the body’s function. It identifies the various genes which may be associated with each medical condition however there is little consideration of the genetic interactions which would be employed e.g. a medical condition can be induced by different groups of genes [156,157]. It is not the individual genes which are important but instead how the genes, and genetic conformation, influence the production of proteins and thereby influence the body’s overall stability.
Each emergent pathology is the consequence of changes to the expression of proteins and also to the factors which influence protein conformation; the ability of proteins to react; the location of proteins in the cell; the prevailing pH, levels of minerals and cofactors; the associated changes to cell morphology; and ultimately of the ability of cells to signal to other cells. Such changes are of phenotype.
There is a strong body of evidence which indicates that the regulation of pH is essential for normal homeostasis. Increased acidity lowers the levels of zinc which is a component of carbonic anhydrase, angiotensin-converting enzyme and is essential for the synthesis of haem, the primary precursor of haemoglobin. Accordingly increased levels of acidity will adversely influence the measurement of diabetes using HbA1c tests.
The greater the deviation from normal pH - the greater will be the rate of onset of pathologies. This highlights the influence of alcoholic drinks and acidified soft beverages which are now considered to be essential components of modern life. It illustrates the overlooked significance of acid-base balance. Apart from the obvious illustration of the effect of alcohol in a child born of a mother who consumes alcohol (foetal alcohol syndrome), perhaps of greatest concern is the legacy which we leave for future generations. The physiological changes which are the consequence of poor lifestyle choice and stress are likely to influence the health of many for future generations [158]. Changes to modern hyperindulgent lifestyles are essential to reduce the prevailing levels of acidity in our diet in order to prevent the onset of Diabetes Mellitus and a ladder of related pathologies.
COMPETING INTERESTS
Graham Ewing is a Director of Montague Healthcare, a company devoted to the commercialisation of Virtual Scanning technology.
ACKNOWLEDGEMENTS
We thank the many researchers who through their work have indirectly contributed to this article.
REFERENCES
Endorsements
Table of Contents
- BACKGROUND
- WHICH ORGANS DOES THE BODY USE TO REGULATE PH?
- HOW DOES PH INFLUENCE THE BODY’S PHYSIOLOGY?
- HOW DOES THE METABOLISM OF ALCOHOL INFLUENCE THE BODY’S ACIDITY AND ITS FUNCTION?
- THE INFLUENCE OF ACIDITY UPON CELL MORPHOLOGY.
- WHICH ENVIRONMENTAL FACTORS INFLUENCE ACID-BASE HOMEOSTASIS?
- PH INFLUENCES THE STABILITY OF OTHER PHYSIOLOGICAL SYSTEMS
- HOW DOES THE BODY REGULATE THE ACCUMULATION OF ACIDITY IN BODY TISSUES?
- DISCUSSION
- COMPETING INTERESTS
