- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Virology Journal
(Discontinued)
ISSN: 1874-3579 ― Volume 15, 2021
Comparison of Immune Responses in Pigs Infected with Chinese Highly Pathogenic PRRS Virus Strain HV and North American Strain NADC-20
X. Li1, A. Galliher-Beckley1, L. Wang1, J. Nietfeld2, W. Feng3, J. Shi1, *
Abstract
Introduction:
Chinese HP-PRRSV characterized by high morbidity and mortality of all ages of pigs emerged since 2006 in China. The immune response of HP-PRRSV was never compared with conventional low pathogenic PRRSV strain.
Objective:
In this study, we compared the immune responses elicited by a Chinese HP-PRRSV strain HV and a North American RRSV strain NADC20 infections.
Result:
Pigs infected with NADC-20 showed significantly higher Ab titers than HV-PRRSV infected pigs at 9 DPI. Infection with HV-PRRSV induced a significantly higher levels of TNF-α and IL-10 in both sera and lung tissues and higher IFN-α and IFN-γ in the serum. Flow cytometry analysis showed that HV-PRRSV infected pigs generated significantly higher frequencies of NK cells in the peripheral blood and Th/memory, CTLs, and T-reg cells in the lung as compared with NADC-20 infected pigs.
Conclusion:
This study demonstrates that different immunity profiles were elicited by HV-PRRSV and NADC-20, and these differences may contribute to the distinct pathogenesis of HV-PRRSV and NADC-20.
Article Information
Identifiers and Pagination:
Year: 2017Volume: 11
Issue: Suppl-1, M5
First Page: 73
Last Page: 82
Publisher Id: TOVJ-11-73
DOI: 10.2174/1874357901711010073
Article History:
Received Date: 06/10/2016Revision Received Date: 01/11/2016
Acceptance Date: 06/02/2017
Electronic publication date: 30/06/2017
Collection year: 2017
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Department of Anatomy and Physiology, College of Veterinary Medicine, Kansas State University, Manhattan, KS, USA; Tel: +785-532-4506; E-mail: jshi@vet.k-state.edu
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 06-10-2016 |
Original Manuscript | Comparison of Immune Responses in Pigs Infected with Chinese Highly Pathogenic PRRS Virus Strain HV and North American Strain NADC-20 | |
1. INTRODUCTION
Highly-pathogenic PRRS virus (HP-PRRSV) belongs to type 2 genotype (North American, prototype strain VR-2332) of PRRSV, which is a member of the genus Arterivirus, family Arteriviridae. HP-PRRSV is characterized by high fever and high rates of morbidity and mortality [1Tian K, Yu X, Zhao T, et al. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS One 2007; 2: e526.
[http://dx.doi.org/10.1371/journal.pone.0000526] ]. This novel and highly virulent variant of PRRSV, which first emerged in China in 2006, has rapidly spread to most countries in South East Asia [2Ni J, Yang S, Bounlom D, et al. Emergence and pathogenicity of highly pathogenic Porcine reproductive and respiratory syndrome virus in Vientiane, Lao People's Democratic Republic. J Vet Diagn Invest 2012; 24: 349-54.
[http://dx.doi.org/10.1177/1040638711434111] ]. HP-PRRSV exhibits more tissue tropism than classic PRRSV [3He Y, Wang G, Liu Y, et al. Characterization of thymus atrophy in piglets infected with highly pathogenic porcine reproductive and respiratory syndrome virus. Vet Microbiol 2012; 160: 455-62.
[http://dx.doi.org/10.1016/j.vetmic.2012.05.040] ]. Besides lymphoid tissues, IHC examination showed that HP-PRRSV antigen can also be detected in other tissues including trachea, esophagus, liver, kidney, cerebellum, stomach, and intestine [4Li L, Zhao Q, Ge X, et al. Chinese highly pathogenic porcine reproductive and respiratory syndrome virus exhibits more extensive tissue tropism for pigs. Virol J 2012; 9: 203.
[http://dx.doi.org/10.1186/1743-422X-9-203] ].
Compared with the prototype of type 2 genotype strain VR-2332, HP-PRRSV can elicit strong immune responses by the evidence of a striking elevation in the level of cytokines associated with both innate and adaptive immunity in HP-PRRSV infected pigs [5Guo B, Lager KM, Henningson JN, et al. Experimental infection of United States swine with a Chinese highly pathogenic strain of porcine reproductive and respiratory syndrome virus. Virology 2013; 435: 372-84.
[http://dx.doi.org/10.1016/j.virol.2012.09.013] ]. In contrast, VR-2332, first isolated in 1987, only leads to mild clinical symptoms and does not circulate in the field any longer [6Collins JE, Benfield DA, Christianson WT, et al. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J Vet Diagn Invest 1992; 4: 117-26.
[http://dx.doi.org/10.1177/104063879200400201] ]. NADC-20 is a virulent North American PRRSV strain, which was first isolated in an “atypical PRRSV abortion storm” in 2001 [7Harms PA, Sorden SD, Halbur PG, et al. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syndrome virus. Vet Pathol 2001; 38: 528-39.
[http://dx.doi.org/10.1354/vp.38-5-528] ]. It has been widely used for viral challenge to evaluate the efficacy of PRRSV vaccines in the U.S [8Calzada-Nova G, Husmann RJ, Schnitzlein WM, Zuckermann FA. Effect of the host cell line on the vaccine efficacy of an attenuated porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol 2012; 148: 116-25.
[http://dx.doi.org/10.1016/j.vetimm.2012.05.008] ]. Compared with the other strains of PRRSV in the U.S., NADC-20 can lead to clinical fever (≥ 40°C) and more robust immune responses after infection of pigs [8Calzada-Nova G, Husmann RJ, Schnitzlein WM, Zuckermann FA. Effect of the host cell line on the vaccine efficacy of an attenuated porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol 2012; 148: 116-25.
[http://dx.doi.org/10.1016/j.vetimm.2012.05.008] ]. Therefore, analysis of the host immune responses elicited by two virulent strains of PRRSV will contribute to better understanding of the pathogenesis of HP-PRRSV and facilitate more effective vaccine development. In this study, 7-week old pigs were infected with the HV-PRRSV (a HP-PRRSV isolate, GenBank accession no. JX317648), or NADC-20 strain of PRRSV and the clinical symptoms and the profiles of host immune response were compared.
2. MATERIALS AND METHODS
2.1. Cells and Virus
MARC-145 cells were maintained in modified Eagle’s medium (MEM) supplemented with 7% fetal bovine serum (FBS) containing 100U penicillin/ml and 100ug streptomycin/ml at 37°C with 5% CO2. For virus infection and titration, MEM supplemented with 2% FBS was used. HV-PRRSV was rescued from HP-PRRSV infectious clone [9Hou J, Wang L, He W, Zhang H, Feng WH. Highly pathogenic porcine reproductive and respiratory syndrome virus impairs LPS- and poly(I:C)-stimulated tumor necrosis factor-alpha release by inhibiting ERK signaling pathway. Virus Res 2012; 167: 106-11.
[http://dx.doi.org/10.1016/j.virusres.2012.03.017] ], and propagated on MARC-145 cells for three passages before use. PRRSV NADC-20 was a kind gift from Dr. Lager Kelly (National Animal Disease Center, USDA-ARS, Ames, IA).
2.2. Experiment Design
All animal experiments were approved by the Institutional Animal Care and Use Committee at Kansas State University. Briefly, fifteen conventional Large White-Duroc crossbred weaned specific-pathogen free piglets (Female, 7 weeks of age) were tested to be PRRSV negative by ELISA and real time RT-PCR and were divided into 3 groups. Five pigs were infected with NADC-20 (2x105 TCID50/pig) and housed for 10 days before the necropsy within the Large Animal Research Center facility (Bio-safety Level 2) at Kansas State University. Another 10 pigs were divided into two groups (n=5/group) and housed in separate rooms within the Biosafety Research Institute (Bio-safety Level 3) at Kansas State University. One group of pigs were infected with HV-PRRV (2x105 TCID50/pig) on day 0, and another group of pigs received MEM medium and served as negative controls throughout the study. Weight measurements and blood samples were collected every 3 days and rectal temperature and clinical signs were monitored daily. All pigs were humanely euthanized at 10 DPI. Thymic and lung tissues were weighed and compared with total body weight to evaluate thymic atrophy and lung inflammation induced by the viral infection. Serum samples were used to measure viral load, PRRSV-specific antibodies and cytokine expression [10Li X, Galliher-Beckley A, Nietfeld JC, Faaberg KS, Shi J, Montanide TM. Gel01 ST adjuvant enhances PRRS modified live vaccine efficacy by regulating porcine humoral and cellular immune responses. World Fournal of Vaccines 2013; 3: 1-9.
[http://dx.doi.org/10.4236/wjv.2013.31001] ].
2.3. Collection of Blood Samples for Analysis
Blood was collected from each pig at 3, 6, and 9 days post PRRSV infection. Serum was separated from clotted blood and preserved at -20°C. Serum was used for evaluation of viral titer and PRRSV-specific ELISA antibody titers (Herdchek Porcine Reproductive and Respiratory Syndrome Antibody test Kit, IDEXX Laboratories) as previously described [10Li X, Galliher-Beckley A, Nietfeld JC, Faaberg KS, Shi J, Montanide TM. Gel01 ST adjuvant enhances PRRS modified live vaccine efficacy by regulating porcine humoral and cellular immune responses. World Fournal of Vaccines 2013; 3: 1-9.
[http://dx.doi.org/10.4236/wjv.2013.31001] ]. Pig serum and the supernatant of lung homogenates were used to cytokine expression analysis as described earlier [11Li X, Galliher-Beckley A, Huang H, Sun X, Shi J. Peptide nanofiber hydrogel adjuvanted live virus vaccine enhances cross-protective immunity to porcine reproductive and respiratory syndrome virus. Vaccine 2013; 31: 4508-15.
[http://dx.doi.org/10.1016/j.vaccine.2013.07.080] ]. IFN-α and IFN-β ELISA kits were purchased from Abcam (Abcam, Cambridge, MA). IL-4, IL-8, IL-10, IFN-γ and TNF-α were purchased from Invitrogen (Life Technologies, Carlsbad, CA). Procedures were performed as per the manufacturer’s instructions. For a given sample, the OD450 was then transformed to concentration by applying a linear regression formula calculated from the results of the standards provided in each kit.
Total RNA was extracted from serum and One-step Taq-Man qPCR was performed to calculate PRRSV RNA copy number in the serum sample according to the brochure of manufacture (EZ-PRRSVTM MPX4.0 Real Time RT-PCR, Tetracore Inc., Rockville, MD). A standard curve was constructed by preparing serial dilutions of an RNA control, supplied in the RT-PCR kit, and virus quantities of unknown samples were determined by linear extrapolation of the Ct value plotted against the standard curve.
Heparinized whole blood was subjected to flow cytometry analysis to determine different lymphocyte populations based on the cell surface marker phenotype: T-helper cells (CD3+CD4+CD8-), cytotoxic T lymphocyte (CD3+CD4-CD8+), Th/memory cells (CD3+CD4+CD8+), T-regulatory cells (CD4+FoxP3+CD25+), NK cells (CD3-CD8+) and γδ T cells (CD8+TcR1N4+) as we described earlier [11Li X, Galliher-Beckley A, Huang H, Sun X, Shi J. Peptide nanofiber hydrogel adjuvanted live virus vaccine enhances cross-protective immunity to porcine reproductive and respiratory syndrome virus. Vaccine 2013; 31: 4508-15.
[http://dx.doi.org/10.1016/j.vaccine.2013.07.080] ]. The mouse anti-pig TcR1N4 antibody was purchased from VMRD (Pullman, WA), and all other antibodies were purchased from BD Biosciences (San Jose, CA). Immuno-stained cells were acquired using a FACS Caliber (BD Biosciences) flow cytometer as previously described [11Li X, Galliher-Beckley A, Huang H, Sun X, Shi J. Peptide nanofiber hydrogel adjuvanted live virus vaccine enhances cross-protective immunity to porcine reproductive and respiratory syndrome virus. Vaccine 2013; 31: 4508-15.
[http://dx.doi.org/10.1016/j.vaccine.2013.07.080] ]. Briefly, PBMC was treated with 2% pig serum to block Fc receptors.
Cells were then stained with an appropriate Ab which was either directly conjugated to a specific fluorochrome or with a purified Ab to pig specific immune cell surface marker (TcR1N4). For cells stained with a purified Ab, labeled cells were treated with anti-species isotype specific secondary Ab conjugated with fluorochrome. Finally, cells were fixed with 1% paraformaldehyde before flow cytometer reading. Percentages of each lymphocyte population were analyzed by 100,000 unique events using FlowJo software (Tree Star, Inc., OR, USA).
2.4. Histopathological Analysis
Pigs were humanely euthanized at 10 DPI as approved by the Kansas State University Institutional Animal Use and Biosafety Committee. Pathology in the lung was macroscopically and microscopically evaluated and graded as previously described [12Halbur PG, Paul PS, Frey ML, et al. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol 1995; 32: 648-60.
[http://dx.doi.org/10.1177/030098589503200606] ]. Briefly, the dorsal and ventral surfaces of each lung lobe were given a score representing the approximate proportion that was consolidated.
Individual lobe scores were used to determine an overall lung score representing the percentage of lung with macroscopically evident consolidation. Sections of each of the 4 lobes of the right lung were fixed in 10% buffered neutral formalin, paraffin-embedded, sectioned, and stained with hematoxylin and eosin (H&E). Scoring of microscopic lung pathology on a 4 point scale was done in a blinded fashion by a veterinary pathologist in the Kansas State Veterinary Diagnostic Laboratory [12Halbur PG, Paul PS, Frey ML, et al. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol 1995; 32: 648-60.
[http://dx.doi.org/10.1177/030098589503200606] ].
2.5. Statistical Analysis
All data were expressed as the mean value of five pigs ± SEM. The differences in the level of humoral response, body temperature and body weight, viral titer, lung score, cytokine production, and percentage of lymphocyte subpopulations among each group were determined by the one-way analysis of variance (ANOVA) followed by post-hoc Tukey’s test using Sigmaplot 11 software (Systat Software Inc., San Jose, CA). Differences were considered statistically significant when p<0.05.
3. RESULTS
3.1. Pigs Infected with HV-PRRSV had Significantly Higher Fever and Less Body Weight Gain as Compared with NADC-20 Infected Pigs
HP-PRRSV infection is characterized by high fever, high percentage of morbidity and mortality in pigs [1Tian K, Yu X, Zhao T, et al. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS One 2007; 2: e526.
[http://dx.doi.org/10.1371/journal.pone.0000526] ]. Thus, the rectal temperature of pigs was monitored daily. The average body temperature in HV-PRRV infected pigs was above 40°C, the cutoff of clinical fever throughout the study, and it was significantly higher than that in NADC-20 infected pig except at 0 and 7 DPI (Fig. 1A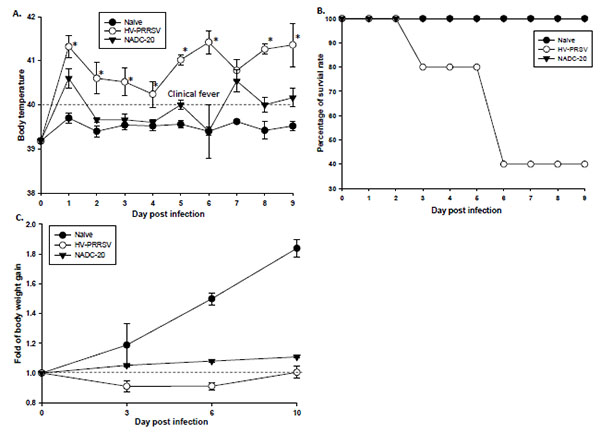 ). The NADC-20 infected pigs developed clinical fever only at 1 and 7 DPI, with the mean body temperature on these two days being 40.5°C.
). The NADC-20 infected pigs developed clinical fever only at 1 and 7 DPI, with the mean body temperature on these two days being 40.5°C.
One pig within the HV-PRRV infection group died at 3 DPI and two other pigs were euthanized due to severe weakness and moribund condition at 6 DPI (Fig. 1B ). The clinical signs of HV-PRRSV-infected pigs included dehydration, respiratory distress, shivering, and inability to bear weight on front limbs. Two of the dead pigs developed cutaneous hemorrhages and cyanotic extremities on the edges of their ears. None of pigs in the NADC-20 infection group or control group were found dead or moribund to warrant euthanasia. Pigs in the NADC-20 infection group showed transient fever, but no other clinical symptoms were observed. HV-PRRSV infected pigs rapidly lost their body weight when compared with the negative control and NADC-20 infected pigs (Fig. 1C
). The clinical signs of HV-PRRSV-infected pigs included dehydration, respiratory distress, shivering, and inability to bear weight on front limbs. Two of the dead pigs developed cutaneous hemorrhages and cyanotic extremities on the edges of their ears. None of pigs in the NADC-20 infection group or control group were found dead or moribund to warrant euthanasia. Pigs in the NADC-20 infection group showed transient fever, but no other clinical symptoms were observed. HV-PRRSV infected pigs rapidly lost their body weight when compared with the negative control and NADC-20 infected pigs (Fig. 1C ).
).
3.2. HV-PRRSV Infection Led to Severe Thymus Atrophy and Lung Inflammation in Pigs
Severe lesions, including marked interstitial pneumonia, lymphadenopathy and thymic atrophy were observed in HV-PRRSV infected pigs. Postmortem findings include pulmonary edema, hematoma, pleural adhesion, peritoneal and pericardial effusions, and renal petchia. Pigs in the HV-PRRSV infection group showed more severe and extensive pneumonia than NADC-20 infected pigs, and the macro- and histopathological lung scores in this group were significantly higher than NADC-20 infected group (Fig. 2A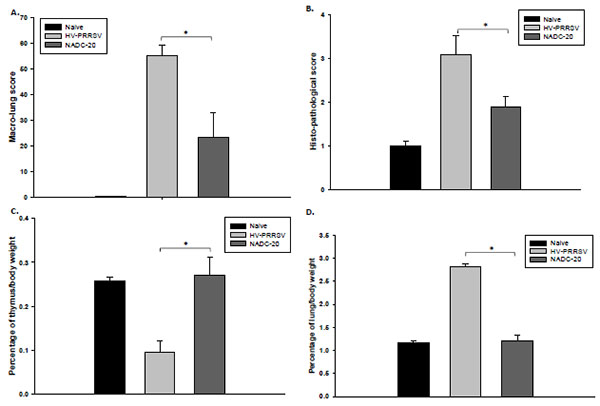 , B
, B ). No pathologic lesions were identified in control pigs.
). No pathologic lesions were identified in control pigs.
HV-PRRSV was previously reported to lead to thymus atrophy [3He Y, Wang G, Liu Y, et al. Characterization of thymus atrophy in piglets infected with highly pathogenic porcine reproductive and respiratory syndrome virus. Vet Microbiol 2012; 160: 455-62.
[http://dx.doi.org/10.1016/j.vetmic.2012.05.040] ]. To confirm this, the ratio of thymus/total body weight was calculated to evaluate the thymus atrophy at necropsy. The ratio of thymus/total body weight of pigs in HV-PRRSV infection group was significantly lower as compared with NADC-20 infection group (Fig. 2C ), which supports that severe thymus atrophy occurs in HV-PRRSV infected pigs. In contrast, the thymus weights of pigs infected with NADC-20 showed the similar average weight to that of the negative control pigs. The ratio of lung/total body weight was used to evaluate the inflammation status after viral infection. The ratio was significantly higher in HV-PRRSV infected pigs than that in NADC-20 infected pigs (Fig. 2D
), which supports that severe thymus atrophy occurs in HV-PRRSV infected pigs. In contrast, the thymus weights of pigs infected with NADC-20 showed the similar average weight to that of the negative control pigs. The ratio of lung/total body weight was used to evaluate the inflammation status after viral infection. The ratio was significantly higher in HV-PRRSV infected pigs than that in NADC-20 infected pigs (Fig. 2D ), and there was no difference in the ratio between NADC-20 infected pigs with negative control pigs. The above data showed that HV-PRRSV infection lead to significant thymus atrophy and lung inflammation as compared with NADC-20 infection in pigs.
), and there was no difference in the ratio between NADC-20 infected pigs with negative control pigs. The above data showed that HV-PRRSV infection lead to significant thymus atrophy and lung inflammation as compared with NADC-20 infection in pigs.
3.3. HV-PRRSV Infection Showed Enhanced Viral Titers in Pigs but did not Elicit Earlier or Higher PRRSV-Specific IDEXX ELISA Antibodies than NADC-20 Infected Pigs
HP-PRRSV was previously reported to have higher proliferation ability than the classic PRRSV strains [5Guo B, Lager KM, Henningson JN, et al. Experimental infection of United States swine with a Chinese highly pathogenic strain of porcine reproductive and respiratory syndrome virus. Virology 2013; 435: 372-84.
[http://dx.doi.org/10.1016/j.virol.2012.09.013] ]. Indeed, the virus RNA copy number in the serum was higher in HV-PRRSV infected pigs than NADC-20 infected pigs at 3 DPI; however the difference was not significant (Fig. 3A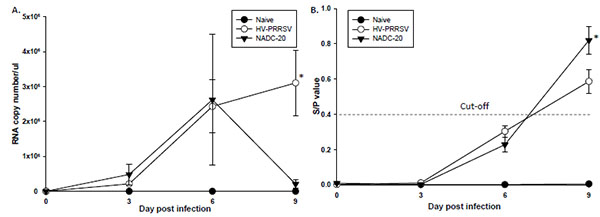 ). At 6 DPI, the viremia in the blood was similar (average 2.5x106 RNA copy number/μl) in both challenge groups. By 9 DPI, the viral titer in NADC-20 infected pigs dropped more than 10 folds, whereas the serum virus copy number of HV-PRRSV infected pigs increased to 3x106 RNA copy number/μl. PRRSV-specific antibodies elicited by the two strains of PRRSV were measured by IDEXX ELISA kit [13Holtkamp DJ, Kliebenstein JB, Neumann EJ, et al. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers, 2013; 21: 72084.]. The high proliferation ability of HP-PRRSV did not elicit earlier or higher titer of PRRSV-specific Ab. At 9 DPI, the average ELISA antibody titer in NADC-20 infected pigs was significantly higher than that in HV-PRRSV infected pigs (Fig. 3B
). At 6 DPI, the viremia in the blood was similar (average 2.5x106 RNA copy number/μl) in both challenge groups. By 9 DPI, the viral titer in NADC-20 infected pigs dropped more than 10 folds, whereas the serum virus copy number of HV-PRRSV infected pigs increased to 3x106 RNA copy number/μl. PRRSV-specific antibodies elicited by the two strains of PRRSV were measured by IDEXX ELISA kit [13Holtkamp DJ, Kliebenstein JB, Neumann EJ, et al. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers, 2013; 21: 72084.]. The high proliferation ability of HP-PRRSV did not elicit earlier or higher titer of PRRSV-specific Ab. At 9 DPI, the average ELISA antibody titer in NADC-20 infected pigs was significantly higher than that in HV-PRRSV infected pigs (Fig. 3B ).
).
3.4. Cytokine Expression in the Serum and Lungs was Up-regulated by HV-PRRSV Infection Compared with NADC-20 Infection
Sera collected at 6 DPI and the supernatant of lung homogenates collected at 10 DPI were analyzed for innate cytokine (TNF-α, IFN-α, IFN-β, and IL-8) and adaptive cytokine (IL-10, IL-4, and IFN-γ) expression. As for the innate cytokines, HV-PRRSV infection induced significantly higher TNF-α level in both serum and lung samples from the pigs (Figs. 4A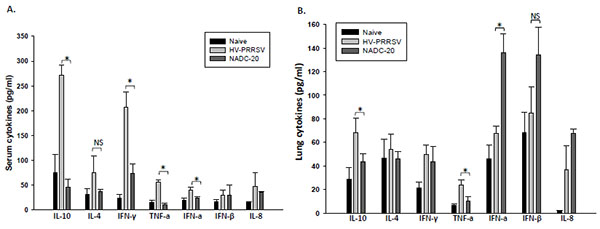 , B
, B ). HV-PRRSV infection also induced significantly higher IFN-α in the serum but significantly lower IFN-α in the lung samples as compared with NADC-20 infected pigs. There was no significant difference between the two infected groups for the expression of IFN-β and IL-8 in serum and lung samples. As for the adaptive cytokines, HV-PRRSV infection elicited significantly higher IL-10 and IFN-γ in the serum of pigs, and significantly higher IL-10 in the lung samples sample as compared with NADC-20 infected pigs Fig. (4A
). HV-PRRSV infection also induced significantly higher IFN-α in the serum but significantly lower IFN-α in the lung samples as compared with NADC-20 infected pigs. There was no significant difference between the two infected groups for the expression of IFN-β and IL-8 in serum and lung samples. As for the adaptive cytokines, HV-PRRSV infection elicited significantly higher IL-10 and IFN-γ in the serum of pigs, and significantly higher IL-10 in the lung samples sample as compared with NADC-20 infected pigs Fig. (4A , B
, B ).
).
3.5. Higher-frequency of NK cells, Th/memory, CTLs and Treg cells, but Reduced Total T Cells were Observed in HV-PRRSV Infected as Compared with NADC-20 Infected Pigs
The frequency of various lymphocyte populations after infection was monitored by flow cytometry. In blood, the frequency of total T cells and NK cells in HV-PRRSV infected pigs were significantly higher than the NADC-20 infected pigs Fig. (5A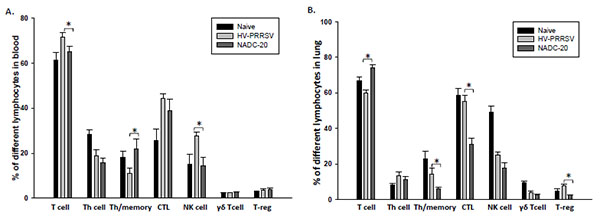 ). In contrast, HV-PRRSV infection significantly decreased Th/memory cell population in blood samples of pigs as compared with NADC-20 infected pigs. There were no significant differences among the groups for all other cell populations assayed. The frequency of Th/memory, CTLs, and T-reg cells in the lung of HV-PRRSV infected pigs were significantly higher than that in NADC-20 infected pigs Fig. (5B
). In contrast, HV-PRRSV infection significantly decreased Th/memory cell population in blood samples of pigs as compared with NADC-20 infected pigs. There were no significant differences among the groups for all other cell populations assayed. The frequency of Th/memory, CTLs, and T-reg cells in the lung of HV-PRRSV infected pigs were significantly higher than that in NADC-20 infected pigs Fig. (5B ). However, the total T cell population in HV-PRRSV infected pigs was significantly lower than NADC-20 infected pigs. There was no difference for the percentage of T-helper cells and γδ T cells in the lung between the two infected groups.
). However, the total T cell population in HV-PRRSV infected pigs was significantly lower than NADC-20 infected pigs. There was no difference for the percentage of T-helper cells and γδ T cells in the lung between the two infected groups.
4. DISCUSSION
The HP-PRRSV has been reported to induce high fever, loss of body weight, severe respiratory symptoms and high mortality. In our study, body temperature in HV-PRRSV infected pigs was higher than 40°C during the duration of the infection, which may partially contribute to dehydration and respiratory distress Fig. (1A ). The HV-PRRSV infection led to significant pig body weight loss as compared with NADC-20 infected pigs. The HV-PRRSV infected pigs lost an average of 10% of their body weight at 3 and 6 DPI, however, body weight returned to original weight by 10 DPI Fig. (1C
). The HV-PRRSV infection led to significant pig body weight loss as compared with NADC-20 infected pigs. The HV-PRRSV infected pigs lost an average of 10% of their body weight at 3 and 6 DPI, however, body weight returned to original weight by 10 DPI Fig. (1C ). The body weight of NADC-20 infected pigs increased consistently after infection, although it was significantly lower when compared with the control pigs at 6 and 10 DPI (Fig. 1C
). The body weight of NADC-20 infected pigs increased consistently after infection, although it was significantly lower when compared with the control pigs at 6 and 10 DPI (Fig. 1C ). Consistent with a previous report [14Hu SP, Zhang Z, Liu YG, et al. Pathogenicity and distribution of highly pathogenic porcine reproductive and respiratory syndrome virus in pigs. Transbound Emerg Dis 2013; 60: 351-9.
). Consistent with a previous report [14Hu SP, Zhang Z, Liu YG, et al. Pathogenicity and distribution of highly pathogenic porcine reproductive and respiratory syndrome virus in pigs. Transbound Emerg Dis 2013; 60: 351-9.
[http://dx.doi.org/10.1111/j.1865-1682.2012.01354.x] , 15Wang G, Song T, Yu Y, et al. Immune responses in piglets infected with highly pathogenic porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol 2011; 142: 170-8.
[http://dx.doi.org/10.1016/j.vetimm.2011.05.004] ], HV-PRRSV infected pigs also showed more severe clinical symptoms including cutaneous hemorrhages and cyanotic extremities on the edge of ears (“blue ear”) and higher mortality rate (3/5 pigs died).
HV-PRRSV led to significant thymus atrophy compared with NADC-20 infection. The ratio of thymus/total body weight was significantly lower in HV-PRRSV infected pigs as compared with NADC-20 infected pigs (Fig. 1C ). Thymus is the primary lymphoid tissue, in which T-lymphocytes mature and constitute the peripheral T-cell repertoire responsible for directing many facets of the adaptive immune responses. The malfunction/atrophy of thymus leads to the depletion of T lymphocytes, which was consistent with the significant loss of total T lymphocytes in the lung analyzed by flow cytometry. In contrast, the ratio of lung/total body weight was significantly higher in HV-PRRSV infected pigs as compared with NADC-20 infected pigs (Fig. 2
). Thymus is the primary lymphoid tissue, in which T-lymphocytes mature and constitute the peripheral T-cell repertoire responsible for directing many facets of the adaptive immune responses. The malfunction/atrophy of thymus leads to the depletion of T lymphocytes, which was consistent with the significant loss of total T lymphocytes in the lung analyzed by flow cytometry. In contrast, the ratio of lung/total body weight was significantly higher in HV-PRRSV infected pigs as compared with NADC-20 infected pigs (Fig. 2 ), which indicated more inflammatory responses after HV-PRRSV infection. Several T cell subpopulations which exert cytotoxic functions, such as CTLs (CD3+CD4-CD8+) and Th/memory (CD3+CD4+CD8+), were significantly higher after HV-PRRSV infection when compared with NADC-20 infection.
), which indicated more inflammatory responses after HV-PRRSV infection. Several T cell subpopulations which exert cytotoxic functions, such as CTLs (CD3+CD4-CD8+) and Th/memory (CD3+CD4+CD8+), were significantly higher after HV-PRRSV infection when compared with NADC-20 infection.
It has been reported that HP-PRRSV has higher proliferation ability than classic PRRSV both in vitro and in vivo [5Guo B, Lager KM, Henningson JN, et al. Experimental infection of United States swine with a Chinese highly pathogenic strain of porcine reproductive and respiratory syndrome virus. Virology 2013; 435: 372-84.
[http://dx.doi.org/10.1016/j.virol.2012.09.013] ]. In this study, both HV-PRRSV and NADC-20 showed similar proliferation ability within the first 6 DPI. Interestingly, by 9 DPI the viremia in NADC-20 infected pigs declined while the viremia of HV-PRRSV infected pigs was still increasing (Fig. 3A ). In a study by Guo et al. [5Guo B, Lager KM, Henningson JN, et al. Experimental infection of United States swine with a Chinese highly pathogenic strain of porcine reproductive and respiratory syndrome virus. Virology 2013; 435: 372-84.
). In a study by Guo et al. [5Guo B, Lager KM, Henningson JN, et al. Experimental infection of United States swine with a Chinese highly pathogenic strain of porcine reproductive and respiratory syndrome virus. Virology 2013; 435: 372-84.
[http://dx.doi.org/10.1016/j.virol.2012.09.013] ], the virus titer and virus load in the serum were significantly higher after rJXwn6 HP-PRRSV infection as compared with VR-2332 infection from 2 to 11 DPI. The discrepancy of the viremia level could be due to the different strains of PRRSV used in each study, and the NADC-20 used in our study is more virulent than VR-2332. However, the high proliferation ability of HV-PRRSV did not correlate with higher titer of PRRSV-specific IDEXX ELISA antibody response, in that the average antibody titer in NADC-20 infected pigs was significantly higher than HV-PRRSV infected pigs at 9 DPI (Fig. 3B ). The IDEXX ELISA measures the antibody response against N proteins of PRRSV, which has no protective ability to the PRRSV infection although it has been widely used for field diagnosis [16Thanawongnuwech R, Suradhat S. Taming PRRSV: Revisiting the control strategies and vaccine design. Virus Res 2010; 154: 133-40.
). The IDEXX ELISA measures the antibody response against N proteins of PRRSV, which has no protective ability to the PRRSV infection although it has been widely used for field diagnosis [16Thanawongnuwech R, Suradhat S. Taming PRRSV: Revisiting the control strategies and vaccine design. Virus Res 2010; 154: 133-40.
[http://dx.doi.org/10.1016/j.virusres.2010.09.003] ]. The different abilities to induce PRRSV IDEXX ELISA Ab between HP-PRRSV and classic PRRSV may contribute to the pathogenesis of viruses, and need further exploration.
TNF-α is a pro-inflammatory cytokine, which plays a very important role in regulation of immune responses, fever development (inflammation), and cell apoptosis [17Salek-Ardakani S, Croft M. Tumor necrosis factor receptor/tumor necrosis factor family members in antiviral CD8 T-cell immunity. J Interferon Cytokine Res 2010; 30: 205-18.
[http://dx.doi.org/10.1089/jir.2010.0026] ]. Several studies showed that PRRSV down-regulated TNF-α production in the early stage of infection, which may be used by virus to circumvent infected cell apoptosis [18Lopez-Fuertes L, Campos E, Domenech N, et al. Porcine reproductive and respiratory syndrome (PRRS) virus down-modulates TNF-alpha production in infected macrophages. Virus Res 2000; 69: 41-6.
[http://dx.doi.org/10.1016/S0168-1702(00)00172-6] , 19Ait-Ali T, Wilson AD, Westcott DG, et al. Innate immune responses to replication of porcine reproductive and respiratory syndrome virus in isolated Swine alveolar macrophages. Viral Immunol 2007; 20: 105-18.
[http://dx.doi.org/10.1089/vim.2006.0078] ]. At the late stage of PRRSV infection, the peak of both apoptotic cells and viral antigen expression were observed in lymph nodes and tonsils of infected animals [20Gomez-Laguna J, Salguero FJ, Fernandez de Marco M, et al. Type 2 porcine reproductive and respiratory syndrome virus infection mediated apoptosis in B- and T-cell areas in lymphoid organs of experimentally infected pigs. Transbound Emerg Dis 2013; 60: 273-8.
[http://dx.doi.org/10.1111/j.1865-1682.2012.01338.x] ]. In our study, HV-PRRSV induced significantly higher TNF-α in both serum and lung samples at 6 DPI, and the high level of TNF-α expression correlates with the high level of viremia. The coincidence between high expression of TNF-α and high level of viremia at the late stage of PRRSV infection may indicate that PRRSV induces TNF-α mediated cell apoptosis to release virion progeny to infect other vulnerable cells.
Previous studies have shown that infection with several classic strains of PRRSV virus induced delayed or failed production of detectable serum IFN-α [21Diaz I, Darwich L, Pappaterra G, Pujols J, Mateu E. Immune responses of pigs after experimental infection with a European strain of Porcine reproductive and respiratory syndrome virus. J Gen Virol 2005; 86: 1943-51.
[http://dx.doi.org/10.1099/vir.0.80959-0] -23Gomez-Laguna J, Salguero FJ, Pallares FJ, et al. Acute phase response in porcine reproductive and respiratory syndrome virus infection. Comp Immunol Microbiol Infect Dis 2010; 33: e51-8.
[http://dx.doi.org/10.1016/j.cimid.2009.11.003] ]. In contrast, HV-PRRSV infection induced significantly higher IFN-α in the serum of pigs but significantly lower levels in the lung samples. Working as a potent antiviral molecule, IFN-α was reported to significantly inhibit PRRSV replication and enhance cellular-mediated immunity (IFN-γ responses) [24Royaee AR, Husmann RJ, Dawson HD, et al. Deciphering the involvement of innate immune factors in the development of the host response to PRRSV vaccination. Vet Immunol Immunopathol 2004; 102: 199-216.
[http://dx.doi.org/10.1016/j.vetimm.2004.09.018] , 25Albina E, Carrat C, Charley B. Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syndrome virus. J Interferon Cytokine Res 1998; 18: 485-90.
[http://dx.doi.org/10.1089/jir.1998.18.485] ]. However, the elevated serum IFN-α has no effect on virus clearance by the evidence of high level of viremia in HV-PRRSV infected pigs at 9 DPI (Figs. 3A and 4A
and 4A ). Also, the low level of IFN-α expression in the lung tissue after HV-PRRSV infection did not lead to decreased IFN-γ production as compared with NADC-20 infected pigs. Therefore, the role of IFN-α in the pathogenesis of PRRSV and host immunity to combat PRRSV needs to be further explored.
). Also, the low level of IFN-α expression in the lung tissue after HV-PRRSV infection did not lead to decreased IFN-γ production as compared with NADC-20 infected pigs. Therefore, the role of IFN-α in the pathogenesis of PRRSV and host immunity to combat PRRSV needs to be further explored.
HV-PRRSV also elicited a significant elevation of adaptive immunity cytokines in the serum samples, such as IL-10 and IFN-γ, and significantly higher IL-10 in the lung samples (Figs. 4A , B
, B ). Induction of IL-10 following PRRSV infection is believed to be a focal mechanism leading to the unique immunological outcomes and interference of PRRSV vaccine efficacy. The production of IL-10 in the early stage of PRRSV infection is associated with a wide array of PRRSV-induced immunomodulatory activities [24Royaee AR, Husmann RJ, Dawson HD, et al. Deciphering the involvement of innate immune factors in the development of the host response to PRRSV vaccination. Vet Immunol Immunopathol 2004; 102: 199-216.
). Induction of IL-10 following PRRSV infection is believed to be a focal mechanism leading to the unique immunological outcomes and interference of PRRSV vaccine efficacy. The production of IL-10 in the early stage of PRRSV infection is associated with a wide array of PRRSV-induced immunomodulatory activities [24Royaee AR, Husmann RJ, Dawson HD, et al. Deciphering the involvement of innate immune factors in the development of the host response to PRRSV vaccination. Vet Immunol Immunopathol 2004; 102: 199-216.
[http://dx.doi.org/10.1016/j.vetimm.2004.09.018] , 26Suradhat S, Thanawongnuwech R, Poovorawan Y. Upregulation of IL-10 gene expression in porcine peripheral blood mononuclear cells by porcine reproductive and respiratory syndrome virus. J Gen Virol 2003; 84: 453-9.
[http://dx.doi.org/10.1099/vir.0.18698-0] ]. Consistent with previous studies, the expression of IL-10 in the serum and lung samples was significantly higher in HV-PRRSV infected pigs when compared with NADC-20 infected pigs [5Guo B, Lager KM, Henningson JN, et al. Experimental infection of United States swine with a Chinese highly pathogenic strain of porcine reproductive and respiratory syndrome virus. Virology 2013; 435: 372-84.
[http://dx.doi.org/10.1016/j.virol.2012.09.013] ]. The high level expression of IL-10 correlates with high titer of viremia in this study and PRRSV antigen gene expression in the lungs and tonsils of PRRSV infected pigs in previous studies [22Gomez-Laguna J, Salguero FJ, Barranco I, et al. Cytokine expression by macrophages in the lung of pigs infected with the porcine reproductive and respiratory syndrome virus. J Comp Pathol 2010; 142: 51-60.
[http://dx.doi.org/10.1016/j.jcpa.2009.07.004] ]. Some strains of modified live PRRSV vaccines also induced IL-10 production in vaccinated pigs, which may partially contribute to the failure of PRRSV vaccination [24Royaee AR, Husmann RJ, Dawson HD, et al. Deciphering the involvement of innate immune factors in the development of the host response to PRRSV vaccination. Vet Immunol Immunopathol 2004; 102: 199-216.
[http://dx.doi.org/10.1016/j.vetimm.2004.09.018] ]. Therefore, circumventing the inhibitory effect of IL-10 in the early stage of PRRVS vaccination/infection could be a challenge for PRRSV vaccine development.
IFN-γ is a key cytokine associated with host cell-mediated immunity (CMI) response, and is secreted by natural killer cells and several different T cell subpopulations. Significantly higher levels of IFN-γ in the serum was found in pigs infected with HV-PRRSV as compared with NADC-20 infected pigs (Fig. 4A ), which was associated with a significantly higher percentage of NK cells in the blood (Fig. 5A
), which was associated with a significantly higher percentage of NK cells in the blood (Fig. 5A ). The coincidence of high levels of IFN-γ expression and the high percentage of NK cells may indicate that the production of IFN-γ at this stage might be a result of the innate immune response, most likely from antigen-stimulated NK cells [27Wesley RD, Lager KM, Kehrli ME Jr. Infection with Porcine reproductive and respiratory syndrome virus stimulates an early gamma interferon response in the serum of pigs. Can J Vet Res 2006; 70: 176-82.]. However, the higher level of IFN-γ in the serum did not lead to lower level of viremia in these pigs. The level of viremia in HV-PRRSV infected pigs was significantly higher than that in NADC-20 infected pigs. Therefore, the role IFN-γ plays in the protection to PRRSV infection at this stage is questionable.
). The coincidence of high levels of IFN-γ expression and the high percentage of NK cells may indicate that the production of IFN-γ at this stage might be a result of the innate immune response, most likely from antigen-stimulated NK cells [27Wesley RD, Lager KM, Kehrli ME Jr. Infection with Porcine reproductive and respiratory syndrome virus stimulates an early gamma interferon response in the serum of pigs. Can J Vet Res 2006; 70: 176-82.]. However, the higher level of IFN-γ in the serum did not lead to lower level of viremia in these pigs. The level of viremia in HV-PRRSV infected pigs was significantly higher than that in NADC-20 infected pigs. Therefore, the role IFN-γ plays in the protection to PRRSV infection at this stage is questionable.
CONCLUSION
In conclusion, by comparing immune responses in pigs infected with two virulent PRRSV strains: Chinese HV-PRRSV and U.S. NADC-20 strain, we found that the high proliferation ability of HV-PRRSV did not enhance the early production of PRRSV-specific ELISA antibodies (Ab). HV-PRRSV induced higher levels of TNF-α and IL-10 in both serum and the lung and higher levels of IFN-α and IFN-γ in the serum than did NADC-20. Flow cytometry analysis showed that HV-PRRSV infected pigs generated significantly higher frequencies of NK cells in the peripheral blood and Th/memory, CTLs, and T-reg cells in the lung tissue as compared with NADC-20 infected pigs. Thus, this study demonstrates that different immunity profiles were elicited by HV-PRRSV and NADC-20, and these differences may contribute to the better understanding of the pathogenesis of HP-PRRSV.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors report no potential conflict of interests.
ACKNOWLEDGEMENTS
We thank all BRI staff at Kansas State University who were involved in this project for their veterinarian help. We also thank Dr. Brooke Bloomberg for her technical help. This research was supported in part by KBA-CBRI 611310, NIH R21 AI085416, an award from the National Bio and Agro-defense facility Transition Fund, and a research grant from Kansas State University Research Foundation.
REFERENCES
| [1] | Tian K, Yu X, Zhao T, et al. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS One 2007; 2: e526. [http://dx.doi.org/10.1371/journal.pone.0000526] |
| [2] | Ni J, Yang S, Bounlom D, et al. Emergence and pathogenicity of highly pathogenic Porcine reproductive and respiratory syndrome virus in Vientiane, Lao People's Democratic Republic. J Vet Diagn Invest 2012; 24: 349-54. [http://dx.doi.org/10.1177/1040638711434111] |
| [3] | He Y, Wang G, Liu Y, et al. Characterization of thymus atrophy in piglets infected with highly pathogenic porcine reproductive and respiratory syndrome virus. Vet Microbiol 2012; 160: 455-62. [http://dx.doi.org/10.1016/j.vetmic.2012.05.040] |
| [4] | Li L, Zhao Q, Ge X, et al. Chinese highly pathogenic porcine reproductive and respiratory syndrome virus exhibits more extensive tissue tropism for pigs. Virol J 2012; 9: 203. [http://dx.doi.org/10.1186/1743-422X-9-203] |
| [5] | Guo B, Lager KM, Henningson JN, et al. Experimental infection of United States swine with a Chinese highly pathogenic strain of porcine reproductive and respiratory syndrome virus. Virology 2013; 435: 372-84. [http://dx.doi.org/10.1016/j.virol.2012.09.013] |
| [6] | Collins JE, Benfield DA, Christianson WT, et al. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J Vet Diagn Invest 1992; 4: 117-26. [http://dx.doi.org/10.1177/104063879200400201] |
| [7] | Harms PA, Sorden SD, Halbur PG, et al. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syndrome virus. Vet Pathol 2001; 38: 528-39. [http://dx.doi.org/10.1354/vp.38-5-528] |
| [8] | Calzada-Nova G, Husmann RJ, Schnitzlein WM, Zuckermann FA. Effect of the host cell line on the vaccine efficacy of an attenuated porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol 2012; 148: 116-25. [http://dx.doi.org/10.1016/j.vetimm.2012.05.008] |
| [9] | Hou J, Wang L, He W, Zhang H, Feng WH. Highly pathogenic porcine reproductive and respiratory syndrome virus impairs LPS- and poly(I:C)-stimulated tumor necrosis factor-alpha release by inhibiting ERK signaling pathway. Virus Res 2012; 167: 106-11. [http://dx.doi.org/10.1016/j.virusres.2012.03.017] |
| [10] | Li X, Galliher-Beckley A, Nietfeld JC, Faaberg KS, Shi J, Montanide TM. Gel01 ST adjuvant enhances PRRS modified live vaccine efficacy by regulating porcine humoral and cellular immune responses. World Fournal of Vaccines 2013; 3: 1-9. [http://dx.doi.org/10.4236/wjv.2013.31001] |
| [11] | Li X, Galliher-Beckley A, Huang H, Sun X, Shi J. Peptide nanofiber hydrogel adjuvanted live virus vaccine enhances cross-protective immunity to porcine reproductive and respiratory syndrome virus. Vaccine 2013; 31: 4508-15. [http://dx.doi.org/10.1016/j.vaccine.2013.07.080] |
| [12] | Halbur PG, Paul PS, Frey ML, et al. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol 1995; 32: 648-60. [http://dx.doi.org/10.1177/030098589503200606] |
| [13] | Holtkamp DJ, Kliebenstein JB, Neumann EJ, et al. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers, 2013; 21: 72084. |
| [14] | Hu SP, Zhang Z, Liu YG, et al. Pathogenicity and distribution of highly pathogenic porcine reproductive and respiratory syndrome virus in pigs. Transbound Emerg Dis 2013; 60: 351-9. [http://dx.doi.org/10.1111/j.1865-1682.2012.01354.x] |
| [15] | Wang G, Song T, Yu Y, et al. Immune responses in piglets infected with highly pathogenic porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol 2011; 142: 170-8. [http://dx.doi.org/10.1016/j.vetimm.2011.05.004] |
| [16] | Thanawongnuwech R, Suradhat S. Taming PRRSV: Revisiting the control strategies and vaccine design. Virus Res 2010; 154: 133-40. [http://dx.doi.org/10.1016/j.virusres.2010.09.003] |
| [17] | Salek-Ardakani S, Croft M. Tumor necrosis factor receptor/tumor necrosis factor family members in antiviral CD8 T-cell immunity. J Interferon Cytokine Res 2010; 30: 205-18. [http://dx.doi.org/10.1089/jir.2010.0026] |
| [18] | Lopez-Fuertes L, Campos E, Domenech N, et al. Porcine reproductive and respiratory syndrome (PRRS) virus down-modulates TNF-alpha production in infected macrophages. Virus Res 2000; 69: 41-6. [http://dx.doi.org/10.1016/S0168-1702(00)00172-6] |
| [19] | Ait-Ali T, Wilson AD, Westcott DG, et al. Innate immune responses to replication of porcine reproductive and respiratory syndrome virus in isolated Swine alveolar macrophages. Viral Immunol 2007; 20: 105-18. [http://dx.doi.org/10.1089/vim.2006.0078] |
| [20] | Gomez-Laguna J, Salguero FJ, Fernandez de Marco M, et al. Type 2 porcine reproductive and respiratory syndrome virus infection mediated apoptosis in B- and T-cell areas in lymphoid organs of experimentally infected pigs. Transbound Emerg Dis 2013; 60: 273-8. [http://dx.doi.org/10.1111/j.1865-1682.2012.01338.x] |
| [21] | Diaz I, Darwich L, Pappaterra G, Pujols J, Mateu E. Immune responses of pigs after experimental infection with a European strain of Porcine reproductive and respiratory syndrome virus. J Gen Virol 2005; 86: 1943-51. [http://dx.doi.org/10.1099/vir.0.80959-0] |
| [22] | Gomez-Laguna J, Salguero FJ, Barranco I, et al. Cytokine expression by macrophages in the lung of pigs infected with the porcine reproductive and respiratory syndrome virus. J Comp Pathol 2010; 142: 51-60. [http://dx.doi.org/10.1016/j.jcpa.2009.07.004] |
| [23] | Gomez-Laguna J, Salguero FJ, Pallares FJ, et al. Acute phase response in porcine reproductive and respiratory syndrome virus infection. Comp Immunol Microbiol Infect Dis 2010; 33: e51-8. [http://dx.doi.org/10.1016/j.cimid.2009.11.003] |
| [24] | Royaee AR, Husmann RJ, Dawson HD, et al. Deciphering the involvement of innate immune factors in the development of the host response to PRRSV vaccination. Vet Immunol Immunopathol 2004; 102: 199-216. [http://dx.doi.org/10.1016/j.vetimm.2004.09.018] |
| [25] | Albina E, Carrat C, Charley B. Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syndrome virus. J Interferon Cytokine Res 1998; 18: 485-90. [http://dx.doi.org/10.1089/jir.1998.18.485] |
| [26] | Suradhat S, Thanawongnuwech R, Poovorawan Y. Upregulation of IL-10 gene expression in porcine peripheral blood mononuclear cells by porcine reproductive and respiratory syndrome virus. J Gen Virol 2003; 84: 453-9. [http://dx.doi.org/10.1099/vir.0.18698-0] |
| [27] | Wesley RD, Lager KM, Kehrli ME Jr. Infection with Porcine reproductive and respiratory syndrome virus stimulates an early gamma interferon response in the serum of pigs. Can J Vet Res 2006; 70: 176-82. |








