- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Virology Journal
(Discontinued)
ISSN: 1874-3579 ― Volume 15, 2021
Iota-Carrageenan as an Antiviral Treatment for the Common Cold
Ronald Eccles1, *
Abstract
Introduction:
The common cold syndrome of acute upper respiratory tract viral infection is the most common disease among mankind and is an extremely common illness in children. There is a great need for a safe and effective antiviral treatment with minimal side effects. The challenge in developing a treatment is the numerous and varied respiratory viruses that cause this common illness and the need for a treatment with good tolerability and safety.
Explanation:
All respiratory viruses must reach the cell surface by passing through respiratory fluid and mucus, and this common feature may allow for the development of antivirals that capture viruses during this transit.
This article discusses how large polyanionic molecules such as iota-carrageenan may trap positively charged respiratory viruses. Iota-carrageenan is a large polysaccharide molecule which is neither absorbed from the respiratory tract nor metabolised. It, therefore, does not have any pharmacological properties. Iota-carrageenan nasal spray has been shown to reduce the titres of respiratory viruses and to reduce the severity of symptoms in placebo-controlled clinical trials, including children and adults. The results of four clinical trials are presented.
Conclusion:
Iota-carrageenan is a good candidate as a safe and effective non-specific antiviral treatment for common cold, and more research is justified on polyanionic molecules like carrageenans as antivirals.
Article Information
Identifiers and Pagination:
Year: 2020Volume: 14
First Page: 9
Last Page: 15
Publisher Id: TOVJ-14-9
DOI: 10.2174/1874357902014010009
Article History:
Received Date: 12/12/2019Revision Received Date: 24/03/2020
Acceptance Date: 04/04/2020
Electronic publication date: 04/05/2020
Collection year: 2020
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Cardiff School of Biosciences, Cardiff University, Cardiff, UK, Tel: 44 (0)2920 844343, Email: eccles@cardiff.ac.uk
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 12-12-2019 |
Original Manuscript | Iota-Carrageenan as an Antiviral Treatment for the Common Cold | |
1. INTRODUCTION
Acute upper respiratory tract viral infections such as common cold are probably the most common viral diseases of mankind. Common cold is caused by a wide range of viruses such as rhinoviruses, coronaviruses, influenza viruses, Respiratory Syncytial Viruses (RSV), parainfluenza viruses, adenoviruses, enteroviruses, metapneumoviruses and unknown viruses which may be responsible for 20-30% of infections [1Heikkinen T, Järvinen A. The common cold. Lancet 2003; 361(9351): 51-9.
[http://dx.doi.org/10.1016/S0140-6736(03)12162-9] [PMID: 12517470] ]. With such a wide range of viruses responsible for the common cold syndrome, it is not surprising that there is a great unmet need for a safe and well-tolerated antiviral treatment for this most common disease.
A major issue in developing an antiviral treatment for the common cold is that focussing on one group of viruses such as rhinoviruses, which may on occasion account for at least 50% of colds [2Turner RB. Rhinovirus: More than just a common cold virus. J Infect Dis 2007; 195(6): 765-6.
[http://dx.doi.org/10.1086/511829] [PMID: 17299703] ], means that the treatment would only be effective in treating 50% of colds, and this would not be acceptable as a freely available treatment to the general public. Antiviral treatments aimed at blocking the viral receptor or host cell receptor are unlikely to be of benefit in treating common cold as they are developed to act specifically on only one group of viruses.
The common cold, therefore, presents a formidable challenge for the development of antiviral treatments because of the diverse viruses responsible for this disease. One common factor shared by all the common cold viruses is that they must somehow reach their specific host cell receptor by moving down through a relatively great depth of respiratory fluid and mucus and that they must achieve this journey without any means of self-propulsion. This article will discuss how common cold viruses may utilise their electrical charge to reach the host cell surface and how this mechanism may be confounded by large polyanionic molecules such as iota-carrageenan.
2. IMPORTANCE OF ELECTRICAL CHARGE ON VIRUSES AND CELLS
Respiratory viruses are trapped and washed away in a protective layer of respiratory mucus as part of the normal respiratory defence against infection by mucociliary clearance. Respiratory mucus and fluid are some 10 micrometres in depth [3Bansil R, Turner BS. The biology of mucus: Composition, synthesis and organization. Adv Drug Deliv Rev 2018; 124: 3-15.
[http://dx.doi.org/10.1016/j.addr.2017.09.023] [PMID: 28970050] ] and compared to the size of respiratory viruses such as a rhinovirus, which is some 30 nanometres in size [4Bella J, Rossmann MG. ICAM-1 receptors and cold viruses. Pharm Acta Helv 2000; 74(2-3): 291-7.
[http://dx.doi.org/10.1016/S0031-6865(99)00056-4] [PMID: 10812972] ]. This presents a formidable barrier to infection. In order to infect cells lining-the respiratory tract, the viruses must contact the host cell and interact with a receptor that triggers penetration of the host cell membrane. The initial movement of the virus towards the cell is dependent on Brownian motion, which knocks the virus in random directions, and eventually, the virus comes close enough to the cell to be attracted to the cell surface by other factors such as the electrostatic attraction of the virus to large charged molecules on the surface of the cell [5Chuck AS, Clarke MF, Palsson BO. Retroviral infection is limited by Brownian motion. Hum Gene Ther 1996; 7(13): 1527-34.
[http://dx.doi.org/10.1089/hum.1996.7.13-1527] [PMID: 8864753] , 6Liu J, Thorp SC. Cell surface heparan sulfate and its roles in assisting viral infections. Med Res Rev 2002; 22(1): 1-25.
[http://dx.doi.org/10.1002/med.1026] [PMID: 11746174] ]. Naked respiratory viruses such as rhinoviruses must attach to a specific receptor on epithelial cells in order to uncoat and invade cells, whereas many enveloped viruses fuse with cellular membranes in order to penetrate the cell [7Fuchs R, Blaas D. Uncoating of human rhinoviruses. Rev Med Virol 2010; 20(5): 281-97.
[http://dx.doi.org/10.1002/rmv.654] [PMID: 20629045] ]. The initial encounter between a virus and its host cell is not with a virus-specific receptor but with the ubiquitous glycoprotein attachment factors such as Heparan Sulphate Proteoglycans (HSP) on the cell surface [8Grove J, Marsh M. The cell biology of receptor-mediated virus entry. J Cell Biol 2011; 195(7): 1071-82.
[http://dx.doi.org/10.1083/jcb.201108131] [PMID: 22123832] ]. In general, the surface of mammalian cells presents a negative electric field due to the ubiquitous HSP molecules on the cell surface and this electrical property of cells can be utilised by positively charged viruses to direct the virus towards the cell surface and closer to any specific receptor on the cell surface. Heparan Sulphate Proteoglycans (HSP) are found on the cell surface of all tissues and in the extracellular matrix where they interact with numerous ligands such as cytokines, chemokines, enzymes, and growth factors [9Spillmann D. Heparan sulfate: anchor for viral intruders? Biochimie 2001; 83(8): 811-7.
[http://dx.doi.org/10.1016/S0300-9084(01)01290-1] [PMID: 11530214] , 10Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 2011; 3(7)a004952
[http://dx.doi.org/10.1101/cshperspect.a004952] [PMID: 21690215] ]. HSP are glycoproteins containing one or more covalently attached Heparan Sulphate chains (HS) with 40-300 sugar residues [10Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 2011; 3(7)a004952
[http://dx.doi.org/10.1101/cshperspect.a004952] [PMID: 21690215] ]. The HS chain is made up of linked dimers of sugar molecules, as illustrated in Fig. (1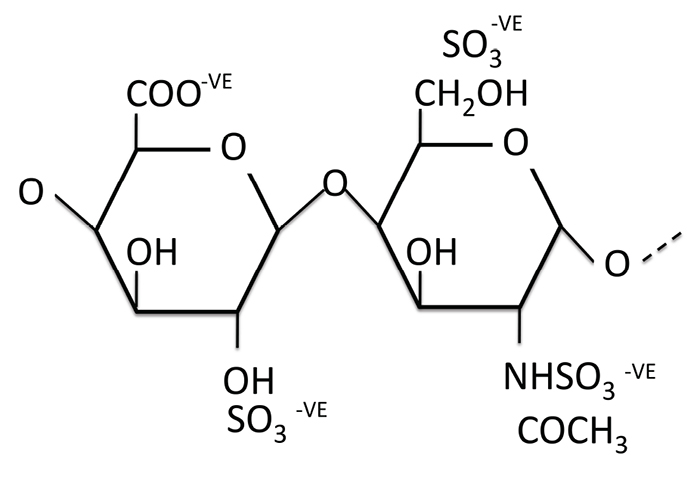 ), and the sulphate and other groups along the chain give HS a high electronegative charge to make it one of the most highly negatively charged polymers in nature [10Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 2011; 3(7)a004952
), and the sulphate and other groups along the chain give HS a high electronegative charge to make it one of the most highly negatively charged polymers in nature [10Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 2011; 3(7)a004952
[http://dx.doi.org/10.1101/cshperspect.a004952] [PMID: 21690215] ].
 |
Fig. (1) Structure of heparan sulphate, illustrating the disaccharide repeating units (dimers) with two negatively charged sulphate groups. (-VE means negatively charged). |
3. CARRAGEENAN
Carrageenan is a general term used to describe sulphated polysaccharides extracted from edible seaweeds. Carrageenan is widely used in the food industry for its thickening and gel-like properties in food products such as ice cream and sauces. It is also used to formulate syrups, and in cosmetics and processed meat [11Necas J, Bartosikova L. Carrageenan: A review. Vet Med (Praha) 2013; 58(4): 187-205.
[http://dx.doi.org/10.17221/6758-VETMED] ]. The in vitro antiviral activity of carrageenan was first described in 1958 when carrageenan was shown to exert a marked inhibitory effect on the growth of influenza B virus and mumps virus in embryonated chicken eggs [12Gerber P, Dutcher JD, Adams EV, Sherman JH. Protective effect of seaweed extracts for chicken embryos infected with influenza B or mumps virus. Proc Soc Exp Biol Med 1958; 99(3): 590-3.
[http://dx.doi.org/10.3181/00379727-99-24429] [PMID: 13614432] ]. Since the discovery of the antiviral activity of carrageenan in 1958 carrageenan has been shown to have in vitro antiviral activity for a wide range of viruses such as hepatitis A virus [13Girond S, Crance JM, Van Cuyck-Gandre H, Renaudet J, Deloince R. Antiviral activity of carrageenan on hepatitis A virus replication in cell culture. Res Virol 1991; 142(4): 261-70.
[http://dx.doi.org/10.1016/0923-2516(91)90011-Q] [PMID: 1665574] ], herpes simplex virus [14Carlucci MJ, Scolaro LA, Noseda MD, Cerezo AS, Damonte EB. Protective effect of a natural carrageenan on genital herpes simplex virus infection in mice. Antiviral Res 2004; 64(2): 137-41.
[http://dx.doi.org/10.1016/j.antiviral.2004.07.001] [PMID: 15498610] ], rhinovirus [15Grassauer A, Weinmuellner R, Meier C, Pretsch A, Prieschl-Grassauer E, Unger H. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol J 2008; 5: 107.
[http://dx.doi.org/10.1186/1743-422X-5-107] [PMID: 18817582] ], papillomavirus [16Buck CB, Thompson CD, Roberts JN, Müller M, Lowy DR, Schiller JT. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog 2006; 2(7)e69
[http://dx.doi.org/10.1371/journal.ppat.0020069] [PMID: 16839203] ], dengue virus [17Talarico LB, Pujol CA, Zibetti RG, et al. The antiviral activity of sulfated polysaccharides against dengue virus is dependent on virus serotype and host cell. Antiviral Res 2005; 66(2-3): 103-10.
[http://dx.doi.org/10.1016/j.antiviral.2005.02.001] [PMID: 15911027] ], various enveloped viruses such as cytomegalovirus, vesicular stomatitis virus and human immunodeficiency virus [18Baba M, Snoeck R, Pauwels R, de Clercq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob Agents Chemother 1988; 32(11): 1742-5.
[http://dx.doi.org/10.1128/AAC.32.11.1742] [PMID: 2472775] ], porcine reproductive and respiratory syndrome virus [19Guo C, Zhu Z, Yu P, et al. Inhibitory effect of iota-carrageenan on porcine reproductive and respiratory syndrome virus in vitro. Antivir Ther (Lond) 2019; 24(4): 261-70.
[http://dx.doi.org/10.3851/IMP3295] [PMID: 30747721] ] and influenza A virus [20Wang W, Zhang P, Hao C, Zhang XE, Cui ZQ, Guan HS. In vitro inhibitory effect of carrageenan oligosaccharide on influenza A H1N1 virus. Antiviral Res 2011; 92(2): 237-46.
[http://dx.doi.org/10.1016/j.antiviral.2011.08.010] [PMID: 21867732] ].
The term carrageenan covers a range of sulphated polysaccharides and oligosaccharides, but the three commercially important carrageenans are iota-, kappa- and lambda- carrageenans. These carrageenans are composed of disaccharide repeating units (dimers), with the kappa, iota and lambda dimers having one, two and three sulphate ester groups respectively resulting in correspondent calculated sulphate contents of 20%, 33% and 41% (w/w) [21Campo VL. D.L. K, da Silva Jr DB, Carvalho I. Carrageenans: Biological properties, chemical modifications and structural analysis– A review. Carbohydr Polym 2009; 77: 167-80.
[http://dx.doi.org/10.1016/j.carbpol.2009.01.020] ]. Iota-carrageenan applied as a nasal spray has been shown in human clinical trials to have efficacy as an early treatment for the common cold and to have antiviral activity against common cold and influenza viruses [15Grassauer A, Weinmuellner R, Meier C, Pretsch A, Prieschl-Grassauer E, Unger H. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol J 2008; 5: 107.
[http://dx.doi.org/10.1186/1743-422X-5-107] [PMID: 18817582] , 22Eccles R, Meier C, Jawad M, Weinmüllner R, Grassauer A, Prieschl-Grassauer E. Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold. Respir Res 2010; 11: 108-19.
[http://dx.doi.org/10.1186/1465-9921-11-108] [PMID: 20696083] -25Eccles R, Winther B, Johnston SL, Robinson P, Trampisch M, Koelsch S. Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial. Respir Res 2015; 16: 121.
[http://dx.doi.org/10.1186/s12931-015-0281-8] [PMID: 26438038] ].
4. CARRAGEENAN AS AN ANTIVIRAL FOR HUMAN VIRAL DISEASE
The antiviral activity of a seaweed extract containing carrageenan was first discovered by chance in 1958 by Gerber et al. [12Gerber P, Dutcher JD, Adams EV, Sherman JH. Protective effect of seaweed extracts for chicken embryos infected with influenza B or mumps virus. Proc Soc Exp Biol Med 1958; 99(3): 590-3.
[http://dx.doi.org/10.3181/00379727-99-24429] [PMID: 13614432] ] at the Squibb Institute for Medical Research in New Jersey, USA. The investigators were searching for new antiviral compounds using the chick embryo as a screening model, and influenza B and mumps virus as test viruses. The results of the studies were erratic as they were using 0.25% agar as a vehicle for the viruses and they suspected that the vehicle had more antiviral activity than any of the antivirals they were testing. They went on to study the antiviral effects of carrageenan extracted from seaweeds (agar) and demonstrated that the carrageenan had a marked inhibitory effect on the replication of influenza B and mumps viruses. They demonstrated that significant protection against fatal influenza B infection of the chick embryo was obtained even when the treatment with carrageenan was delayed for as long as 24 hours after the inoculation with the virus [12Gerber P, Dutcher JD, Adams EV, Sherman JH. Protective effect of seaweed extracts for chicken embryos infected with influenza B or mumps virus. Proc Soc Exp Biol Med 1958; 99(3): 590-3.
[http://dx.doi.org/10.3181/00379727-99-24429] [PMID: 13614432] ]. It is surprising that after the clear demonstration of in vitro antiviral activity of carrageenan in 1958, there were no immediate follow-up animal or human studies to develop an antiviral treatment for human use and it was some 50 years later before any human treatment was investigated.
Carrageenan was shown in the years after 1958 to have antiviral activity against a wide range of viruses in vitro and there was increasing interest in the use of carrageenan as a treatment for HPV from 2006 when carrageenan used in sexual lubricants and lubricated condoms was shown to be a potent inhibitor of HPV infection in vitro [16Buck CB, Thompson CD, Roberts JN, Müller M, Lowy DR, Schiller JT. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog 2006; 2(7)e69
[http://dx.doi.org/10.1371/journal.ppat.0020069] [PMID: 16839203] ]. This led to proposals for a human clinical trial in 2010 and the interim analysis of this trial suggests that using a carrageenan-based lubricant gel can reduce the risk of genital HPV infections in women [26Magnan S, Tota JE, El-Zein M, Burchell AN, Schiller JT, Ferenczy A, et al. Efficacy of a Carrageenan gel Against Transmission of Cervical HPV (CATCH): Interim analysis of a randomized, double-blind, placebo-controlled, phase 2B trial. Clin Microbiol Infect 2018.
[PMID: 29684633] ].
In 2007 at around the same time that clinical trials were being discussed based on the effects of carrageenan on HPV, interest was also developing at Marinomed Biotech AG (Marinomed), a company in Vienna Austria, on a clinical trial on the efficacy of carrageenan as a nasal spray for the early treatment of a common cold. In vitro studies in Austria conducted by Marinomed, demonstrated that iota-carrageenan was a potent inhibitor of rhinovirus infection of cultured HeLa cells [15Grassauer A, Weinmuellner R, Meier C, Pretsch A, Prieschl-Grassauer E, Unger H. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol J 2008; 5: 107.
[http://dx.doi.org/10.1186/1743-422X-5-107] [PMID: 18817582] ]. Following the positive results of in vitro antiviral studies on rhinovirus, Marinomed contacted the author to conduct the first clinical trial on the antiviral effects of carrageenan at the Common Cold Centre based in Cardiff University, UK. The results of this pilot study were published in 2010 [22Eccles R, Meier C, Jawad M, Weinmüllner R, Grassauer A, Prieschl-Grassauer E. Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold. Respir Res 2010; 11: 108-19.
[http://dx.doi.org/10.1186/1465-9921-11-108] [PMID: 20696083] ] and despite the small number of patients involved in the pilot study (17 in the carrageenan treated group and 18 in the placebo group), the study reported a significant reduction in the symptoms of the common cold (p = 0.046) and a reduction in mixed viral load in nasal lavage (p = 0.009) when iota-carrageenan as an intranasal spray (0.12% solution in saline) was used as an early treatment of common cold (within 48 hours of symptom onset) [22Eccles R, Meier C, Jawad M, Weinmüllner R, Grassauer A, Prieschl-Grassauer E. Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold. Respir Res 2010; 11: 108-19.
[http://dx.doi.org/10.1186/1465-9921-11-108] [PMID: 20696083] ]. The results of this pilot study on iota-carrageenan nasal spray are now supported by three larger studies on the early treatment of common cold involving hundreds of adults and children [24Fazekas T, Eickhoff P, Pruckner N, et al. Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold. BMC Complement Altern Med 2012; 12(1): 147.
[http://dx.doi.org/10.1186/1472-6882-12-147] [PMID: 22950667] , 25Eccles R, Winther B, Johnston SL, Robinson P, Trampisch M, Koelsch S. Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial. Respir Res 2015; 16: 121.
[http://dx.doi.org/10.1186/s12931-015-0281-8] [PMID: 26438038] , 27Ludwig M, Enzenhofer E, Schneider S, et al. Efficacy of a carrageenan nasal spray in patients with common cold: A randomized controlled trial. Respir Res 2013; 14(1): 124.
[http://dx.doi.org/10.1186/1465-9921-14-124] [PMID: 24219370] ].
5. PROPOSED MECHANISM OF ACTION OF IOTA-CARRAGEENAN AS AN ANTIVIRAL
Iota-carrageenan is a highly negatively charged long-chain polysaccharide made up of dimers similar in structure and electrical charge to HS as illustrated in Fig. (2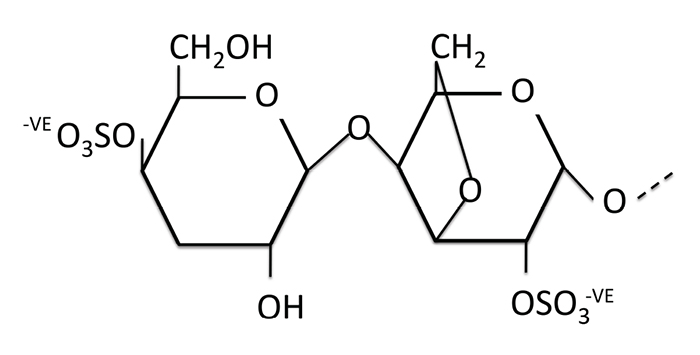 ). Both HS and iota-carrageenan have negatively charged sulphate groups along the chain and these provide most of the dense electrical charge of the polyanionic molecules. Iota-carrageenan mimics the HS that the virus is first attracted to on the surface of the cell and can, therefore, trap the virus and prevent infection, as illustrated in Fig. (3a
). Both HS and iota-carrageenan have negatively charged sulphate groups along the chain and these provide most of the dense electrical charge of the polyanionic molecules. Iota-carrageenan mimics the HS that the virus is first attracted to on the surface of the cell and can, therefore, trap the virus and prevent infection, as illustrated in Fig. (3a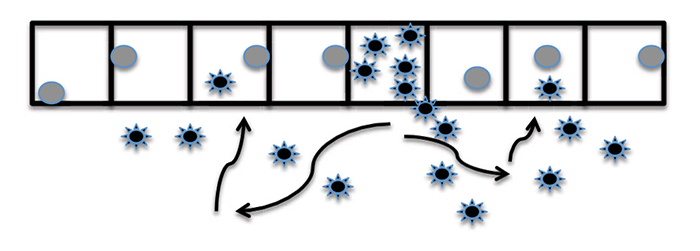 and 3b
and 3b ). In the early stage of infection, newly released viruses infect adjacent epithelial cells and magnify the infection and increase the viral titre as illustrated in Fig. (3a
). In the early stage of infection, newly released viruses infect adjacent epithelial cells and magnify the infection and increase the viral titre as illustrated in Fig. (3a ). When iota-carrageenan is present in the early stages of infection, it acts like airway mucus to trap viruses, as the mucins in airway mucus have a high sialic acid content and this together with the high sulphate content results in a strongly negatively charged surface which may attract any positively charged surface of an airway virus [28Zanin M, Baviskar P, Webster R, Webby R. The Interaction between Respiratory Pathogens and Mucus. Cell Host Microbe 2016; 19(2): 159-68.
). When iota-carrageenan is present in the early stages of infection, it acts like airway mucus to trap viruses, as the mucins in airway mucus have a high sialic acid content and this together with the high sulphate content results in a strongly negatively charged surface which may attract any positively charged surface of an airway virus [28Zanin M, Baviskar P, Webster R, Webby R. The Interaction between Respiratory Pathogens and Mucus. Cell Host Microbe 2016; 19(2): 159-68.
[http://dx.doi.org/10.1016/j.chom.2016.01.001] [PMID: 26867175] ]. The long, negatively charged iota-carrageenan molecule attracts and traps the newly released positively charged viruses and prevents them from infecting adjacent nasal epithelial cells, as illustrated in Fig. (3b ). The iota-carrageenan and trapped viruses will be transported by mucociliary clearance to the nasopharynx and then swallowed, and the viruses will be destroyed in the acid environment of the stomach.
). The iota-carrageenan and trapped viruses will be transported by mucociliary clearance to the nasopharynx and then swallowed, and the viruses will be destroyed in the acid environment of the stomach.
The antiviral efficacy of polyanionic molecules such as iota-carrageenan is dependent on early intervention at the first sign of symptoms, in order to prevent the spread and magnification of infection along the respiratory mucosa, and that is why nasal spray treatments, as discussed below, are used in clinical trials within 24-48 hours of the onset of symptoms.
6. WHY IOTA-CARRAGEENAN IS AN IDEAL TREATMENT FOR THE COMMON COLD
Iota-carrageenan nasal spray is an ideal early treatment for the common cold because of several characteristics; safety, tolerability, lack of interaction with any other medications, and a non-specific antiviral action against different groups of viruses.
 |
Fig. (2) Structure of iota-carrageenan, illustrating the disaccharide repeating units (dimers) with two negatively charged sulphate groups. (-VE means negatively charged). |
6.1. Safety
Common cold is the most common diseases of mankind and is especially common in infants and children. Any treatment for common cold must have a good safety profile as the benefits of treating a self-limiting disease of short duration must be balanced against any risks posed by the treatment. Iota-carrageenan is a large polysaccharide molecule which is neither absorbed from the respiratory tract nor metabolised. It works against respiratory viruses by its physical properties and electrical charge, and as a nasal spray is licensed for use as a medical device rather than as a medicine. Iota-carrageenan does not have any pharmacological properties and, therefore, does not have any toxicology or known side effects on treatment. The safety of iota-carrageenan is demonstrated from the results of four placebo-controlled clinical trials which reported that there were no significant differences in adverse events between the verum and placebo-treated groups and those adverse events were mainly related to symptoms of respiratory viral infection [22Eccles R, Meier C, Jawad M, Weinmüllner R, Grassauer A, Prieschl-Grassauer E. Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold. Respir Res 2010; 11: 108-19.
[http://dx.doi.org/10.1186/1465-9921-11-108] [PMID: 20696083] , 24Fazekas T, Eickhoff P, Pruckner N, et al. Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold. BMC Complement Altern Med 2012; 12(1): 147.
[http://dx.doi.org/10.1186/1472-6882-12-147] [PMID: 22950667] , 25Eccles R, Winther B, Johnston SL, Robinson P, Trampisch M, Koelsch S. Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial. Respir Res 2015; 16: 121.
[http://dx.doi.org/10.1186/s12931-015-0281-8] [PMID: 26438038] , 27Ludwig M, Enzenhofer E, Schneider S, et al. Efficacy of a carrageenan nasal spray in patients with common cold: A randomized controlled trial. Respir Res 2013; 14(1): 124.
[http://dx.doi.org/10.1186/1465-9921-14-124] [PMID: 24219370] ]. There are no long-term safety studies on the nasal spray, but it has been marketed across Asia and Europe for several years with no indication of any safety issues. Carrageenans, including iota-carrageenan, are widely used in the food, pharmaceutical and cosmetic industries and are Generally Recognised As Safe (GRAS) [29Hebar A, Koller C, Seifert JM, et al. Non-clinical safety evaluation of intranasal iota-carrageenan. PLoS One 2015; 10(4)e0122911
[http://dx.doi.org/10.1371/journal.pone.0122911] [PMID: 25875737] ]. The safety of iota-carrageenan administered as a nasal spray or nebulised (0.12%) has been studied in rabbits and rats and no absorption of the carrageenan was reported and the data did not show any local intolerance or toxicity of the iota-carrageenan [29Hebar A, Koller C, Seifert JM, et al. Non-clinical safety evaluation of intranasal iota-carrageenan. PLoS One 2015; 10(4)e0122911
[http://dx.doi.org/10.1371/journal.pone.0122911] [PMID: 25875737] ]. It is important to note that the dose of iota-carrageenan given intranasally is very small compared to the large amounts of carrageenans that may be ingested in many foods, and the controversy that surrounds the safety of carrageenans in foods [30David S, Shani Levi C, Fahoum L, et al. Revisiting the carrageenan controversy: do we really understand the digestive fate and safety of carrageenan in our foods? Food Funct 2018; 9(3): 1344-52.
[http://dx.doi.org/10.1039/C7FO01721A] [PMID: 29469913] ] does not apply to the topical use of a nasal spray.
6.2. Tolerability
Common cold is an acute disease that can be treated by self-medication at home. Any treatment for common cold with significant side effects will not be tolerated by the patient. Iota-carrageenan nasal spray does not have any side effects apart from those associated with the use of a nasal spray and it does not cause any nasal irritation or nasal sensation.
6.3. Interaction with Other Medicines and Treatment
Iota-carrageenan works as a medical device by its physical properties and therefore does not have any known interaction with any other concomitant treatments for the common cold or any other disease. If patients are using other nasal treatments such as saline, corticosteroids, bronchodilators, etc., it is best to use the iota-carrageenan nasal spray at least an hour before or after the other treatment, in order to avoid diluting the treatment and any interaction.
6.4. Non-Specific Antiviral Against a Wide Range of Viruses
Common cold is caused by a wide range of RNA and DNA respiratory viruses and any treatment that works specifically against one type of virus is unlikely to be effective in treating the majority of cases of the common cold. In vitro and in vivo studies have shown that iota-carrageenan has efficacy in reducing the viral load of a wide range of RNA and DNA based viruses such as rhinoviruses [15Grassauer A, Weinmuellner R, Meier C, Pretsch A, Prieschl-Grassauer E, Unger H. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol J 2008; 5: 107.
[http://dx.doi.org/10.1186/1743-422X-5-107] [PMID: 18817582] ] hepatitis A [13Girond S, Crance JM, Van Cuyck-Gandre H, Renaudet J, Deloince R. Antiviral activity of carrageenan on hepatitis A virus replication in cell culture. Res Virol 1991; 142(4): 261-70.
[http://dx.doi.org/10.1016/0923-2516(91)90011-Q] [PMID: 1665574] ] and influenza viruses [23Leibbrandt A, Meier C, König-Schuster M, et al. Iota-carrageenan is a potent inhibitor of influenza A virus infection. PLoS One 2010; 5(12)e14320
[http://dx.doi.org/10.1371/journal.pone.0014320] [PMID: 21179403] ].
6.5. Efficacy of Iota-Carrageenan Nasal Spray as a Treatment for the Common Cold
Iota-carrageenan nasal spray has been shown to reduce the titres of respiratory viruses and to reduce the severity of symptoms in placebo-controlled clinical trials, including children and adults [22Eccles R, Meier C, Jawad M, Weinmüllner R, Grassauer A, Prieschl-Grassauer E. Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold. Respir Res 2010; 11: 108-19.
[http://dx.doi.org/10.1186/1465-9921-11-108] [PMID: 20696083] , 24Fazekas T, Eickhoff P, Pruckner N, et al. Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold. BMC Complement Altern Med 2012; 12(1): 147.
[http://dx.doi.org/10.1186/1472-6882-12-147] [PMID: 22950667] , 25Eccles R, Winther B, Johnston SL, Robinson P, Trampisch M, Koelsch S. Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial. Respir Res 2015; 16: 121.
[http://dx.doi.org/10.1186/s12931-015-0281-8] [PMID: 26438038] , 27Ludwig M, Enzenhofer E, Schneider S, et al. Efficacy of a carrageenan nasal spray in patients with common cold: A randomized controlled trial. Respir Res 2013; 14(1): 124.
[http://dx.doi.org/10.1186/1465-9921-14-124] [PMID: 24219370] ]. A summary of some of the results of clinical trials is illustrated in Table 1.
 |
Fig. (3a) In the early stage of infection, newly released viruses infect adjacent epithelial cells and magnify the infection and increase the viral titre. |
Eccles et al. (2010) conducted the first double-blind, randomised, placebo-controlled clinical trial on an iota-carrageenan (0.12%) nasal spray in Cardiff, United Kingdom. The study was a small study involving 35 adults (mean age 19.6 years) with early symptoms of the common cold (<48 hours onset of symptoms). The nasal spray was administered into each nasal passage three times a day for four days. Nasal lavage was obtained on day 1 (before treatment) and day 3 or 4 (during treatment). The results reported that the pre-defined primary efficacy variable showed a significant difference (p < 0.046) between placebo and iota-carrageenan treatment groups for the total symptom score (day 2-4) with the iota-carrageenan treatment group having lower symptom scores than placebo. Nasal lavage was analyzed by real-time RT-PCR for the presence of viral genomes. Viral load in the respiratory virus-positive patients increased by almost sixfold, whereas it decreased by 92% in the iota-carrageenan treatment group (p < 0.009).
Fazekas et al. (2012) conducted a double-blind, randomised, placebo-controlled trial similar to the design of the trial conducted by Eccles et al. (2010) on 153 children aged between 1-18 years (mean age 5 years) with early symptoms of the common cold (<36 hours onset of symptoms) in Vienna, Austria. The nasal spray was administered into each nasal passage three times a day for seven days. The primary efficacy variable was the difference in total symptom scores between days 2-7, but this did not reach any statistically significant difference between treatment groups, perhaps because the children and their parents had difficulty in assessing symptom scores due to the young age of the children. However, the trial did report a significant reduction in viral load (p = 0.026), reduced time to clearance of the disease symptoms (7.6 in the iota-carrageenan treatment group versus 9.4 days in the placebo group, p = 0.038).
Ludwig et al. (2013) conducted a double-blind, randomised, placebo-controlled trial on 211 patients (mean age 33 years) with early symptoms of the common cold (< 48 hours onset of symptoms) in Vienna, Austria. The nasal spray was administered into each nasal passage three times a day for seven days. In patients showing a laboratory-confirmed cold virus infection and adherence to the protocol, alleviation of symptoms was 2.1 days faster in the carrageenan group in comparison to placebo (p = 0.037). Viral titers in nasal fluids showed a significantly greater decrease in carrageenan treated patients in the intention-to-treat population (p = 0.024) and in the per-protocol population (p = 0.018) between days 1 and 3/4.
Eccles et al. (2015) conducted a double-blind, randomised, placebo-controlled trial on 200 patients (mean age 20 years) with early symptoms of the common cold (< 48 hours onset of symptoms) in Cardiff, United Kingdom. The nasal spray was administered into each nasal passage four times a day for a mandatory four days, with an option to continue treatment up to a maximum duration of 10 days. The primary end-point was the difference between treatment groups for total symptom scores between days 2-4 and this reached statistical significance (p = 0.0364) when one patient was excluded from analysis due to probably misunderstanding the symptom scoring. Only 45% of the patients tested positive for a respiratory virus, but in these patients, there was a non-significant trend for a reduction in viral titres in the iota-carrageenan group compared to the placebo group.
Clinical trials on patients with the common cold are difficult because of the acute nature of the disease and because patients often self-medicate or present too late for treatment and must be excluded from entry into trials that are studying the early treatment of a common cold. Like any clinical trial, the four clinical trials on iota-carrageenan can be criticised as being either a small pilot study [22Eccles R, Meier C, Jawad M, Weinmüllner R, Grassauer A, Prieschl-Grassauer E. Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold. Respir Res 2010; 11: 108-19.
[http://dx.doi.org/10.1186/1465-9921-11-108] [PMID: 20696083] ], not achieving statistical significance on the primary outcome measure [25Eccles R, Winther B, Johnston SL, Robinson P, Trampisch M, Koelsch S. Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial. Respir Res 2015; 16: 121.
[http://dx.doi.org/10.1186/s12931-015-0281-8] [PMID: 26438038] ], or only showing effects on viral titre and not on symptoms [24Fazekas T, Eickhoff P, Pruckner N, et al. Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold. BMC Complement Altern Med 2012; 12(1): 147.
[http://dx.doi.org/10.1186/1472-6882-12-147] [PMID: 22950667] , 27Ludwig M, Enzenhofer E, Schneider S, et al. Efficacy of a carrageenan nasal spray in patients with common cold: A randomized controlled trial. Respir Res 2013; 14(1): 124.
[http://dx.doi.org/10.1186/1465-9921-14-124] [PMID: 24219370] ]. But when the four trials are considered together, they provide evidence that an iota-carrageenan nasal spray can reduce viral titres, and also reduce symptom severity in common cold.
7. SOURCE OF IOTA-CARRAGEENAN USED IN NASAL SPRAYS
The iota-carrageenan used in nasal sprays for the treatment of common cold is pharma grade iota-carrageenan, Gelcarin 379NF obtained from DuPont. This source of iota-carrageenan is mainly produced from the seaweed Echeuma denticulatum, which is sometimes named Echeuma spinosum. The polymer contains mainly >90% iota-carrageenan and, to a much lesser extent <10% kappa-carrageenan but does not contain any lambda-carrageenan [31Marinomed Company. 2018.].
CONCLUSION
Common cold is caused by a wide range of respiratory viruses and any antiviral treatment for this common disease must work on some mechanism of viral activity that is common to all of these viruses. All respiratory viruses must pass through the physical barrier of mucus and respiratory fluid in order to reach the cell surface and this article proposes that the viruses may utilise their positive electrical charge in order to reach the negatively charged cell surface. Polyanionic molecules such as iota-carrageenan may present a way of trapping the viruses as they move towards the surface of the cell. The lack of any pharmacological or toxicological activity in large polyanionic molecules such as iota-carrageenan due to their lack of absorption or metabolism makes them a safe topical antiviral treatment. The efficacy and safety of an iota-carrageenan nasal spray have been studied in four clinical trials to date, and the results of these trials provide some support the efficacy and safety of iota-carrageenan as an early treatment for common cold. Further research is needed on polyanionic compounds like iota-carrageenan for the treatment of common cold.
Search Strategy and Selection Criteria
Relevant publications were found by searching PubMed during July2018-January 2020. The main search terms were “carrageenan”, “iota-carrageenan” “common cold”, “URTI”, “heparan sulphate”. The bibliographies of key articles were used to identify other relevant articles. Key articles were used in cited reference searches on the Web of Science database.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICTS OF INTERESTS
The author acts as a consultant to the pharmaceutical industry, including companies involved in marketing carrageenan containing treatments but does not have any financial interest in any company. This writing of this review was not supported by any grant or funds from any organisation. No data from any depository was used in the writing of this review.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
| [1] | Heikkinen T, Järvinen A. The common cold. Lancet 2003; 361(9351): 51-9. [http://dx.doi.org/10.1016/S0140-6736(03)12162-9] [PMID: 12517470] |
| [2] | Turner RB. Rhinovirus: More than just a common cold virus. J Infect Dis 2007; 195(6): 765-6. [http://dx.doi.org/10.1086/511829] [PMID: 17299703] |
| [3] | Bansil R, Turner BS. The biology of mucus: Composition, synthesis and organization. Adv Drug Deliv Rev 2018; 124: 3-15. [http://dx.doi.org/10.1016/j.addr.2017.09.023] [PMID: 28970050] |
| [4] | Bella J, Rossmann MG. ICAM-1 receptors and cold viruses. Pharm Acta Helv 2000; 74(2-3): 291-7. [http://dx.doi.org/10.1016/S0031-6865(99)00056-4] [PMID: 10812972] |
| [5] | Chuck AS, Clarke MF, Palsson BO. Retroviral infection is limited by Brownian motion. Hum Gene Ther 1996; 7(13): 1527-34. [http://dx.doi.org/10.1089/hum.1996.7.13-1527] [PMID: 8864753] |
| [6] | Liu J, Thorp SC. Cell surface heparan sulfate and its roles in assisting viral infections. Med Res Rev 2002; 22(1): 1-25. [http://dx.doi.org/10.1002/med.1026] [PMID: 11746174] |
| [7] | Fuchs R, Blaas D. Uncoating of human rhinoviruses. Rev Med Virol 2010; 20(5): 281-97. [http://dx.doi.org/10.1002/rmv.654] [PMID: 20629045] |
| [8] | Grove J, Marsh M. The cell biology of receptor-mediated virus entry. J Cell Biol 2011; 195(7): 1071-82. [http://dx.doi.org/10.1083/jcb.201108131] [PMID: 22123832] |
| [9] | Spillmann D. Heparan sulfate: anchor for viral intruders? Biochimie 2001; 83(8): 811-7. [http://dx.doi.org/10.1016/S0300-9084(01)01290-1] [PMID: 11530214] |
| [10] | Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 2011; 3(7)a004952 [http://dx.doi.org/10.1101/cshperspect.a004952] [PMID: 21690215] |
| [11] | Necas J, Bartosikova L. Carrageenan: A review. Vet Med (Praha) 2013; 58(4): 187-205. [http://dx.doi.org/10.17221/6758-VETMED] |
| [12] | Gerber P, Dutcher JD, Adams EV, Sherman JH. Protective effect of seaweed extracts for chicken embryos infected with influenza B or mumps virus. Proc Soc Exp Biol Med 1958; 99(3): 590-3. [http://dx.doi.org/10.3181/00379727-99-24429] [PMID: 13614432] |
| [13] | Girond S, Crance JM, Van Cuyck-Gandre H, Renaudet J, Deloince R. Antiviral activity of carrageenan on hepatitis A virus replication in cell culture. Res Virol 1991; 142(4): 261-70. [http://dx.doi.org/10.1016/0923-2516(91)90011-Q] [PMID: 1665574] |
| [14] | Carlucci MJ, Scolaro LA, Noseda MD, Cerezo AS, Damonte EB. Protective effect of a natural carrageenan on genital herpes simplex virus infection in mice. Antiviral Res 2004; 64(2): 137-41. [http://dx.doi.org/10.1016/j.antiviral.2004.07.001] [PMID: 15498610] |
| [15] | Grassauer A, Weinmuellner R, Meier C, Pretsch A, Prieschl-Grassauer E, Unger H. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol J 2008; 5: 107. [http://dx.doi.org/10.1186/1743-422X-5-107] [PMID: 18817582] |
| [16] | Buck CB, Thompson CD, Roberts JN, Müller M, Lowy DR, Schiller JT. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog 2006; 2(7)e69 [http://dx.doi.org/10.1371/journal.ppat.0020069] [PMID: 16839203] |
| [17] | Talarico LB, Pujol CA, Zibetti RG, et al. The antiviral activity of sulfated polysaccharides against dengue virus is dependent on virus serotype and host cell. Antiviral Res 2005; 66(2-3): 103-10. [http://dx.doi.org/10.1016/j.antiviral.2005.02.001] [PMID: 15911027] |
| [18] | Baba M, Snoeck R, Pauwels R, de Clercq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob Agents Chemother 1988; 32(11): 1742-5. [http://dx.doi.org/10.1128/AAC.32.11.1742] [PMID: 2472775] |
| [19] | Guo C, Zhu Z, Yu P, et al. Inhibitory effect of iota-carrageenan on porcine reproductive and respiratory syndrome virus in vitro. Antivir Ther (Lond) 2019; 24(4): 261-70. [http://dx.doi.org/10.3851/IMP3295] [PMID: 30747721] |
| [20] | Wang W, Zhang P, Hao C, Zhang XE, Cui ZQ, Guan HS. In vitro inhibitory effect of carrageenan oligosaccharide on influenza A H1N1 virus. Antiviral Res 2011; 92(2): 237-46. [http://dx.doi.org/10.1016/j.antiviral.2011.08.010] [PMID: 21867732] |
| [21] | Campo VL. D.L. K, da Silva Jr DB, Carvalho I. Carrageenans: Biological properties, chemical modifications and structural analysis– A review. Carbohydr Polym 2009; 77: 167-80. [http://dx.doi.org/10.1016/j.carbpol.2009.01.020] |
| [22] | Eccles R, Meier C, Jawad M, Weinmüllner R, Grassauer A, Prieschl-Grassauer E. Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold. Respir Res 2010; 11: 108-19. [http://dx.doi.org/10.1186/1465-9921-11-108] [PMID: 20696083] |
| [23] | Leibbrandt A, Meier C, König-Schuster M, et al. Iota-carrageenan is a potent inhibitor of influenza A virus infection. PLoS One 2010; 5(12)e14320 [http://dx.doi.org/10.1371/journal.pone.0014320] [PMID: 21179403] |
| [24] | Fazekas T, Eickhoff P, Pruckner N, et al. Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold. BMC Complement Altern Med 2012; 12(1): 147. [http://dx.doi.org/10.1186/1472-6882-12-147] [PMID: 22950667] |
| [25] | Eccles R, Winther B, Johnston SL, Robinson P, Trampisch M, Koelsch S. Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial. Respir Res 2015; 16: 121. [http://dx.doi.org/10.1186/s12931-015-0281-8] [PMID: 26438038] |
| [26] | Magnan S, Tota JE, El-Zein M, Burchell AN, Schiller JT, Ferenczy A, et al. Efficacy of a Carrageenan gel Against Transmission of Cervical HPV (CATCH): Interim analysis of a randomized, double-blind, placebo-controlled, phase 2B trial. Clin Microbiol Infect 2018. [PMID: 29684633] |
| [27] | Ludwig M, Enzenhofer E, Schneider S, et al. Efficacy of a carrageenan nasal spray in patients with common cold: A randomized controlled trial. Respir Res 2013; 14(1): 124. [http://dx.doi.org/10.1186/1465-9921-14-124] [PMID: 24219370] |
| [28] | Zanin M, Baviskar P, Webster R, Webby R. The Interaction between Respiratory Pathogens and Mucus. Cell Host Microbe 2016; 19(2): 159-68. [http://dx.doi.org/10.1016/j.chom.2016.01.001] [PMID: 26867175] |
| [29] | Hebar A, Koller C, Seifert JM, et al. Non-clinical safety evaluation of intranasal iota-carrageenan. PLoS One 2015; 10(4)e0122911 [http://dx.doi.org/10.1371/journal.pone.0122911] [PMID: 25875737] |
| [30] | David S, Shani Levi C, Fahoum L, et al. Revisiting the carrageenan controversy: do we really understand the digestive fate and safety of carrageenan in our foods? Food Funct 2018; 9(3): 1344-52. [http://dx.doi.org/10.1039/C7FO01721A] [PMID: 29469913] |
| [31] | Marinomed Company. 2018. |
Endorsements
Browse Contents
Table of Contents
- INTRODUCTION
- IMPORTANCE OF ELECTRICAL CHARGE ON VIRUSES AND CELLS
- CARRAGEENAN
- CARRAGEENAN AS AN ANTIVIRAL FOR HUMAN VIRAL DISEASE
- PROPOSED MECHANISM OF ACTION OF IOTA-CARRAGEENAN AS AN ANTIVIRAL
- WHY IOTA-CARRAGEENAN IS AN IDEAL TREATMENT FOR THE COMMON COLD
- SOURCE OF IOTA-CARRAGEENAN USED IN NASAL SPRAYS
- CONCLUSION





