- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Virology Journal
(Discontinued)
ISSN: 1874-3579 ― Volume 15, 2021
Occurrence of Chicken Parvovirus Infection in Poland
Karolina Tarasiuk*, Grzegorz Woźniakowski, Elżbieta Samorek-Salamonowicz
Abstract
The aim of the foregoing study was the determination of the occurrence of parvovirus in chicken flocks from different regions of Poland during 2002-2011. The material used for this study originated from chickens showing clinical symptoms of stunting and emaciation. For the quick detection of genetic material of the viruses in field samples, real-time PCR was applied. The conducted study implied on the occurrence of parvoviral infections in Poland in approximately 18% of investigated chicken flocks. However, their exact role remains still unknown.
Article Information
Identifiers and Pagination:
Year: 2012Volume: 6
First Page: 7
Last Page: 11
Publisher Id: TOVJ-6-7
DOI: 10.2174/1874357901206010007
Article History:
Received Date: 13/9/2011Revision Received Date: 30/11/2011
Acceptance Date: 15/12/2011
Electronic publication date: 6/2/2012
Collection year: 2012
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http: //creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the National Veterinary Research Insitute, Department of Poultry Viral Diseases, 24-100 Pulawy, Poland; Tel: 0048 81 889 3032; Fax: 0048 81 886 2595; E-mail: ktarasiuk@piwet.pulawy.pl
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 13-9-2011 |
Original Manuscript | Occurrence of Chicken Parvovirus Infection in Poland | |
INTRODUCTION
Parvoviruses are etiological agents of multiple diseases of humans and animals. The study conducted at the beginning of the 80s. of the last century on chickens with symptoms of emaciation and enteropathy has shown the presence of viral particles in their gut. It has been shown that these viruses have a single stranded DNA (ssDNA) with features specific for autonomically replicating parvovirus. Further on these viruses were classified as chicken parvovirus (ChPV). In experimentally infected 1-d-old SPF chickens, the virus caused the occurrence of clinical symptoms resembling runting-stunting syndrome [1 Day M, Zsak L. Determination and analysis of the full-length chicken parvovirus genome Virology 2010; 399(1): 59-64.-3 Palade EA, Kisary J, Benyeda Z, et al. Naturally occurring parvoviral infection in Hungarian broiler flocks Avian Pathol 2011; 40(2): 191-7.].
ChPV virions are non-enveloped particles about 20 nm in diameter with icosahedral symmetry assembled from 32 capsomers. The genome consists of 4-6 kilobase-pair DNA encoding three opened reading frames (ORFs): 5’ORF, 3’ORF and the third small ORF localised between junction of these two ORFs. 5’ORF encodes non-structural protein (NS) taking a part in viral replication while 3’ORF encodes structural proteins of capsid (VP1, VP2 and VP3). The function of the third ORF is not explained so far. ChPV is difficult to propagate in cell clutures and chicken embryos. Meanwhile, in young chickens, ChPV causes runting-stunting syndrome (RSS) frequently described as malabsorbtion syndrome (MS) and rarely as the pale bird syndrome or brittle bone disease. In fact, in young birds the clinical symptoms are noticeable as runting, diarrhoea with subsequent increase in morbidity and mortality with increased factor of fodder uptake [4 Kisary J. Experimental infection of chicken embryos and day-old chickens with parvovirus of chicken origin Avian Pathol 1985a; 14(1): 1-7.-10 Zadori Z, Stefancsik R, Rauch T, Kisary J. Analysis of the complete nucleotide sequences of goose and muscovy duck parvoviruses indicates common ancestral origin with adeno-associated virus 2 Virology 1995; 212(2): 562-73.].
During the last few years, an increase in RSS occurrence is observed in chicken broilers. In the USA, the parvoviral infection was found in 77% flocks of broilers and 78% of turkeys [9Doma??ska-Blicharz K, Minta Z. The role of parvoviruses in enteropathies of poultry Polskie Drobiarstwo 2011; 3(3): 54-.]. The results obtained by Palade et al., [7Zavala G, Sellers H. Runting-stunting syndrome Poult Inform Prof 2005; 85: 1-4.] suggest that all of isolated parvoviruses from poultry fall into two common groups of chicken (ChPV) and turkey (TuPV) parvovirus. Actually in Poland the most common problems in chickens are caused by enteropathy and emaciation. Therefore, the aim of this study was the development and application of real-time PCR method for the detection and determination of the prevalence of ChPV in chicken flocks.
MATERIALS AND METHODOLOGY
Samples.
Samples were collected from broiler chickens in age ranging from 1 to 6.5-weeks old and layer hens in age from 14 to 37 weeks. These birds originated from 142 farms from different regions of Poland. The birds have shown clinical symptoms of stunting and considerable emaciation. During the autopsy examination, the sections of the gut were taken and homogenised and 20% suspension in PBS was prepared. After triple freezing and thawing procedure and centrifugation for 5 min. at 1400 x g (Micro 22, Hettich centrifuge, Germany), the supernatants were collected and stored at -20°C.
DNA extraction.
Total DNA was extracted using QIAamp Mini Kit (Qiagen, Germany) according to manufacturer’s procedure. Integrity of extracted DNA was confirmed after electrophoresis in 2% agar gel with addition of ethidium bromide (0.5µg/mL, Sigma-Aldrich, Germany) and visualisation under UV light transiluminator (Vilber-Lourmat, Germany). Extracted DNA was stored at -20°C.
PCR.
Amplification of fragment of non-structural NS gene of ChPV was done using the following primers: ChPVF-5’-TTCTAATAACGATATCACTCAAGTTTC-3’, ChPVR-5’ TTTGCGCTTGCGGTGAAGTCTGGCTCG-3’ as previously described [9Doma??ska-Blicharz K, Minta Z. The role of parvoviruses in enteropathies of poultry Polskie Drobiarstwo 2011; 3(3): 54-.]. Reaction programme was as follows: 95°C/5min. (initial denaturation), then 35 cycles of : 94°C/30sec., 60.8°C/1min.(primer anealing), 72°C/1min. (primer elongation), 72°C/3 min (final elongation of products). The reaction was carried out in 25 µL reaction mixture that contained: 2.5 μL 10 x concentrated PCR buffer (EurX, Poland), 1 μL MgCl2 (1.5mM), 1μL dNTP (0.3 μM of each, DNA Gdansk, Poland), 0.5 μL (0.2μM) of each ChPVR and ChPVF primer, 0.5 μL (2.5 U) DNA polymerase, and 18 μL of deionised water. PCR was conducted in T Professional Basic Gradient Thermocycler (Biometra, Germany). PCR products were separated in 2% agarose gel with addition of ethidium bromid (0.5µg/ml, Sigma-Aldrich, Germany) and visualisation under UV light transiluminator (Vilber-Lourmat, Germany). As the positive result, the presence of 561 bp product was considered. DNA extracted from non-infected chicken embryo fibroblasts (CEF SPF) was the negative control. The positive control was DNA extracted from isolated Polish 114/10 field strain, which was identified by PCR product sequencing as ChPV.
Positive control.
Positive control for real-time PCR was pNS114 plasmid containing amplified PCR product about 561 bp long of NS gene from 114/10 strain. The product was purified from gel using QIAquick Gel Purification Kit (Qiagen, Germany) then inserted by ligation into pGEM-T (Promega, USA) according to manufacturers recommendations. The obtained pNS114 was propagated in E. coli DH5α cells (Qiagen, Germany) in Max 4000 Q (Barnstead/Lab-line, USA) for 18 h with shaking 250 g/min., and isolated from E. coli cells containing pNS114 using Plasmid Maxi Kit (Qiagen, Germany) then finally sequenced (Genomed S.A. Warsaw, Poland). The sequence of plasmid was submitted to NCBI GenBank (JF834321) and used as a positive control.
Real-time PCR.
Reaction was carried out using primers complementary to NS gene as previously described [9Doma??ska-Blicharz K, Minta Z. The role of parvoviruses in enteropathies of poultry Polskie Drobiarstwo 2011; 3(3): 54-.]. The sequences of the primers were identical with those used for PCR. Reaction was conducted in 96-well plates (Applied Biosystems, USA) in ABI 7500 apparatus with 2.0.1 version software (Applied Biosystems USA). The reaction programme was as follows: 95°C/15 min., then 40 cycles of: 95°C/15 sec., 60°C/30 sec. Next, analysis of melting temperature of obtained products was performed. Reaction volume was 25 µL and contained: 12.5 µL 2x QuantiTect Probe PCR Master Mix, 1 µL (0.4 µM) of each ChPVF and ChPVR primer, 1 µL of SYBR Green dye (Invitrogen, 1 µl/reaction), and 8.5 uL of deionised water. DNA concentration was measured as 40-CT, (cycle treshold), which were proportional to amount of entire DNA of ChPV in the examined samples. The results were analysed using Applied Biosystems 7500 software ver. 2.02 and Microsoft Excel ver. 2007. The sequence of PCR product of NS gene was compared with other sequences accessible in NCBI GenBank.
Sensitivity.
The sensitivity was determined under standard conditions of reaction using eight descending dilutions of pNS114 plasmid from 106 to 101 copies. Reaction sensitivity was the last dilution of plasmid with the presence of fluorescence signal with exact CT value . The results were analysed by the software of ABI 7500 apparatus.
Specificity.
The specificity was validated using DNA of goose parvovirus (GPV) strain Dervac (NVRI, Pulawy, Poland), Marek’s disease virus (MDV) strain HPRS16 (Houghton Laboratory, UK) and DNA isolated from non-infected chicken embryo fibroblasts. The results were analysed as described before.
RESULTS
The first part of the presented study was done for the development and validation of real-time PCR for the detection of ChPV. The sensitivity of new method allowed detecting ChPV DNA in 101 copy dilution of pNS114 plasmid and was specific only for ChPV. The fluorescent curves were detected only in the presence of ChPV and no signal was detected in negative control samples of other examined samples of GPV, MDV and DNA isolated from CEFs (Fig. 1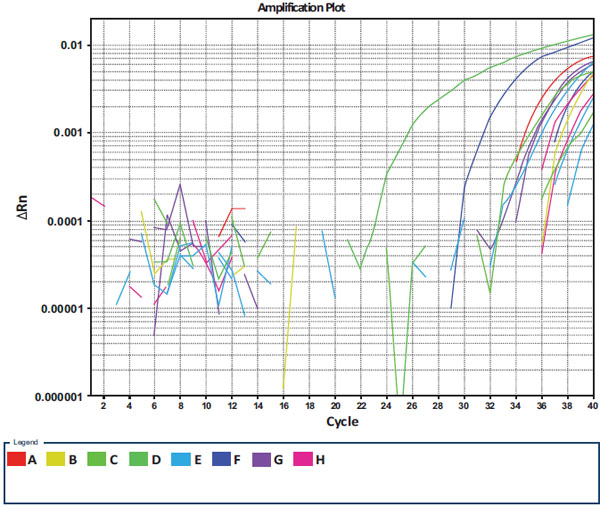 ). The presence of ChPV genetic material was detected in chickens from 25 flocks what presented about 17.6% of overall number of examined flocks. The virus was detected in the gut of layers and broiler chickens. The conducted analysis of melting temperature of real-time PCR products revealed that all of them were specific which was found as a common melting point at 86.6°C. The concentration of DNA of ChPV in the examined samples was expressed as 40- CT value what was shown on Fig. (2
). The presence of ChPV genetic material was detected in chickens from 25 flocks what presented about 17.6% of overall number of examined flocks. The virus was detected in the gut of layers and broiler chickens. The conducted analysis of melting temperature of real-time PCR products revealed that all of them were specific which was found as a common melting point at 86.6°C. The concentration of DNA of ChPV in the examined samples was expressed as 40- CT value what was shown on Fig. (2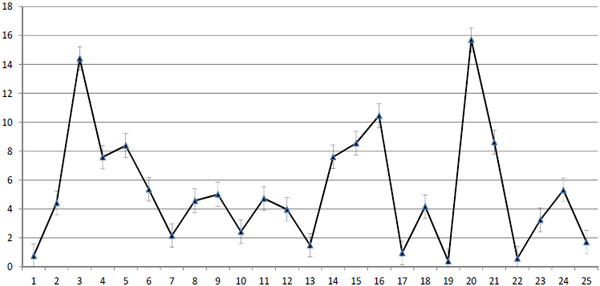 ). The highest concent of viral DNA was observed in the gut of broiler chickens (from CT=25,5 up to CT= 31,6). The age of birds was associated with the concentration of the virus and was the highest among 3-6 week-old broiler chickens and 14 to 21 week-old layers (Fig. 2
). The highest concent of viral DNA was observed in the gut of broiler chickens (from CT=25,5 up to CT= 31,6). The age of birds was associated with the concentration of the virus and was the highest among 3-6 week-old broiler chickens and 14 to 21 week-old layers (Fig. 2 ). In case of four ChPV strains (48/08, 42/09, 8/11 and 18/11) (Table 1), the CT value was close to 40. Therefore, these results were treated as doubtful. However, the coincidental contamination of reaction mixture was excluded by set of negative controls. The comparison of obtained sequence of PCR product specific for NS of 114/10 strain has shown 93% similarity to field ChPV strains isolated in Hungary (Fig. 3
). In case of four ChPV strains (48/08, 42/09, 8/11 and 18/11) (Table 1), the CT value was close to 40. Therefore, these results were treated as doubtful. However, the coincidental contamination of reaction mixture was excluded by set of negative controls. The comparison of obtained sequence of PCR product specific for NS of 114/10 strain has shown 93% similarity to field ChPV strains isolated in Hungary (Fig. 3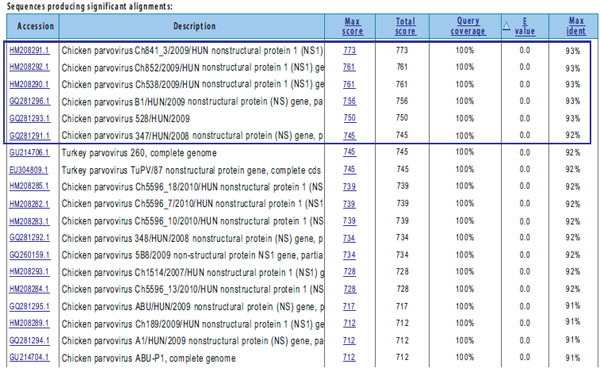 ).
).
DISCUSSION
Primary occurrence of parvoviruses was found in chicken and turkey flocks in the United States of America [9Doma??ska-Blicharz K, Minta Z. The role of parvoviruses in enteropathies of poultry Polskie Drobiarstwo 2011; 3(3): 54-.], Hungary [7Zavala G, Sellers H. Runting-stunting syndrome Poult Inform Prof 2005; 85: 1-4.], and Poland [11 Kisary J, Nagy B, Bitay Z. Presence of parvoviruses in the intestine of chickens showing stunting syndrome Avian Pathol 1984; 13(2): 339-43.]. Previously, it has been shown that both chicken and turkey parvoviruses share high similarity and homology of DNA sequence [9Doma??ska-Blicharz K, Minta Z. The role of parvoviruses in enteropathies of poultry Polskie Drobiarstwo 2011; 3(3): 54-., 10 Zadori Z, Stefancsik R, Rauch T, Kisary J. Analysis of the complete nucleotide sequences of goose and muscovy duck parvoviruses indicates common ancestral origin with adeno-associated virus 2 Virology 1995; 212(2): 562-73.]. Therefore, these viruses fall into common Parvovirus genus. Meanwhile, the other well-known parvovirus of geese (GPV) and Muscovy ducks (MDPV), which causes Derzsy’s disease, belongs to Dependovirus genus [12 Zsak L, Strother KO, Day M. Development of a polymerase chain reaction procedure for detection of chicken and turkey parvoviruses Avian Dis 2009; 53(1): 83-.]. GPV and MDPV are distinct from poultry parvoviruses and have no common antigenic features. Due to the lack of fast diagnostic methods, which allow identifying poultry parvoviruses, Zsak et al., [9Doma??ska-Blicharz K, Minta Z. The role of parvoviruses in enteropathies of poultry Polskie Drobiarstwo 2011; 3(3): 54-.] developed PCR with application of hexamers, which consist of random three sets of primers complementary to sequence of poultry parvoviruses. In the presented study, we developed and conducted real-time PCR method as the more sensitive and less laborious alternative technique in comparison to previously described PCR. This advantagable technique was not previously used for the identification of chicken or turkey parvoviruses. The method was developed with use of primers designed by Zsak et al., [9Doma??ska-Blicharz K, Minta Z. The role of parvoviruses in enteropathies of poultry Polskie Drobiarstwo 2011; 3(3): 54-.], SYBR Green dye and optimisation of specific reaction conditions. Real-time PCR allowed detecting ChPV in chicken gut with the sensitivity equal to 101 copies of pNS114 plasmid. According to previous reports from Hungary and USA described by Palade et al., [7Zavala G, Sellers H. Runting-stunting syndrome Poult Inform Prof 2005; 85: 1-4.] and Zsak et al., [9Doma??ska-Blicharz K, Minta Z. The role of parvoviruses in enteropathies of poultry Polskie Drobiarstwo 2011; 3(3): 54-.], chicken parvoviruses have been isolated from the gut of birds with and without intestinal disorders. In our study, we have shown that infection with ChPV in frequently observed during the last weeks of broiler chicken life. Among these chickens no clinical symptoms were noted except emaciation and stunting. The highest concentration of ChPV expressed as CT value was found in 2.5-week old broilers and 18-week old layers (Table 1). However, in layers older than 22 weeks from two flocks, the presence of parvovirus was negligible since the CT value were close to 40 cycle. Infection among these birds were asymptomatic as well. Meanwhile, a considerable correlation was found between stunting of chickens and concentration of the virus in their gut because the CT value reached 25.5 cycle.
Our results on the occurrence of parvoviral infection mostly in young chickens confirm previously published data [3 Palade EA, Kisary J, Benyeda Z, et al. Naturally occurring parvoviral infection in Hungarian broiler flocks Avian Pathol 2011; 40(2): 191-7., 7Zavala G, Sellers H. Runting-stunting syndrome Poult Inform Prof 2005; 85: 1-4., 9Doma??ska-Blicharz K, Minta Z. The role of parvoviruses in enteropathies of poultry Polskie Drobiarstwo 2011; 3(3): 54-.]. This may indicate on vertical transmission of the infection. This hypothesis is supported by the occurrence of RSS symptoms in hatched nestlings infected by in ovo route [1 Day M, Zsak L. Determination and analysis of the full-length chicken parvovirus genome Virology 2010; 399(1): 59-64.]. Similarly, the study conducted by Domańska –Blicharz et al., [11 Kisary J, Nagy B, Bitay Z. Presence of parvoviruses in the intestine of chickens showing stunting syndrome Avian Pathol 1984; 13(2): 339-43.] has shown the higher incidence of parvoviruses in poults from 3 to 7 weeks of age than in older birds. They revealed TuPV infection in 26% of turkey flocks. Additionally results described by Palade et al., [7Zavala G, Sellers H. Runting-stunting syndrome Poult Inform Prof 2005; 85: 1-4.] suggest all poultry parvoviruses isolated in Hungary fall into two groups of ChPV and TuPV
CONCLUSION
The occurrence of ChPV was detected in almost 18% of examined strains. Supporting the reports about raising incidence of TuPV and ChPV and their close similarity the conducted analysis of nucleotide sequence of NS gene fragment of 114/10 strain has shown 93% of homology with field isolated from Hungary. This may imply on spreading of ChPV in South and Eastern Europe. In summary, the conducted study indicates on the occurrence of ChPV in Polish chicken flocks but their exact role in enteropathies and asymptomatic infections is not understood. This will be investigated in the future.
ACKNOWLEDGEMENT
Declared None.
CONFLICT OF INTEREST
Declared None.


