- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Virology Journal
(Discontinued)
ISSN: 1874-3579 ― Volume 15, 2021
Development and Field Testing of a Real-Time PCR Assay for Caprine Arthritis-Encephalitis-Virus (CAEV)
Giovanni Brajon*, 1, Daniela Mandas 2, Manuele Liciardi 4, Flavia Taccori 1, Mauro Meloni 2, Franco Corrias 1, Caterina Montaldo 5, Ferdinando Coghe 2, Cristina Casciari 3, Monica Giammarioli 3, Germano Orrù*, 2, 5
Abstract
Caprine arthritis/encephalitis (CAE) of goats and occasionally sheep are persistent virus infections caused by a lentivirus (CAEV). This viral infection results in arthritis in adult animals and encephalitis in kids. Prognosis for the encephalitic form is normally poor, with substantial economic loss for the farm. In this context an early/fast laboratory diagnosis for CAEV infection could be useful for effective prophylactic action. In this work we performed a quantitative real time PCR designed on the CAEV env gene to detect/quantify in goat/sheep samples, viral RNA or proviral DNA forms of CAEV. This procedure was validated in 15 sheep, experimentally infected with CAEV or with a highly correlated lentivirus (visna maedi, MVV); in addition, a total of 37 clinical goat specimens recruited in CAEV positive herds were analyzed and compared using serological analysis (Elisa and AGID). All samples infected with MVV resulted negative. In sheep experimentally infected with CAEV, proviral DNA was detectable 15 days post infection, whereas the serological methods revealed an indicative positivity after 40-60 days.This method showed a sensitivity of 102 env fragments/PCR) with a linear dynamic range of quantitation from 103 to 107env fragments/PCR; the R2 correlation coefficient was 0.98. All subjects with a clinical diagnosis for Caprine Arthritis-Encephalitis (CAE) resulted CAEV DNA positive.
Article Information
Identifiers and Pagination:
Year: 2011Volume: 6
First Page: 82
Last Page: 90
Publisher Id: TOVJ-6-82
DOI: 10.2174/1874357901206010082
Article History:
Received Date: 17/2/2012Revision Received Date: 10/5/2012
Acceptance Date: 14/5/2012
Electronic publication date: 27/7/2011
Collection year: 2012
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http: //creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to these authors at the DSS. Università degli Studi di Cagliari, Via Binaghi 4, 09121 Cagliari, Italy; Tel: 0039 070 537413; Fax: 0039 070 537437; E-mail: orru@unica.it and Istituto Zooprofilattico Sperimentale delle Regioni Lazio e Toscana. Sezione di Firenze, Via Castelpulci 43, 50018 Scandicci, Italy; Tel: 0039 055 721308; Fax: 0039 055 7311323; E-mail: giovanni.brajon@izslt.it
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 17-2-2012 |
Original Manuscript | Development and Field Testing of a Real-Time PCR Assay for Caprine Arthritis-Encephalitis-Virus (CAEV) | |
INTRODUCTION
Among the group of small ruminant lentiviruses (SRLV), Caprine arthritis-encephalitis virus (CAEV) is able to infect goats and occasionally sheep and other related ruminants [1Reina R, Mora MI, Glaria I, et al. Molecular characterization and phylogenetic study of Maedi Visna and Caprine Arthritis Encephalitis viral sequences in sheep and goats from Spain Virus Res 2006; 121(2 ): 189-98., 2Pasick J. Maedi-visna virus and caprine arthritis-encephalitis virus: distinct species or quasispecies and its implications for laboratory diagnosis Can J Vet Res 1998; 62(4 ): 241-4.]. The major transmission route of this virus is through the ingestion of virus-infected milk from adult goats by kids via colostrum, by iatrogenic transmission or by lateral transmission through prolonged close contact, with infected animals. All breeds and ages of goats are susceptible to infection, and once established, it persists throughout the animal's life. CAEV infection of goats in newborn kids may lead to encephalitis, interstitial pneumonia and in adult animals to chronic arthritis and interstitial mastitis, and all may contribute to impaired milk production [3Leitner G, Krifucks O, Weisblit L, Lavi Y, Bernstein S, Merin U. The effect of caprine arthritis encephalitis virus infection on production in goats Vet J 2010; 183(3 ): 328-1.]. Infection with CAEV has been identified worldwide [4Bandeira DA, de Castro RS, Azevedo EO, de Souza Seixas Melo L, de Melo CB. Seroprevalence of caprine arthritis-encephalitis virus in goats in the Cariri region, Paraiba state, Brazil Vet J 2009; 180(3 ): 399-401.-8Konishi M, Tsuduku S, Haritani M, et al. An epidemic of caprine arthritis encephalitis in Japan: isolation of the virus J Vet Med Sci 2004; 66(8 ): 911-7.]. CAEV has a tropism for monocytes and macrophages; the infection of host cells involved in the immune system carries the virus throughout the body with multi organ involvement and progression to the disease can take months to years, causing chronic inflammatory diseases. CAEV is closely related to the maedi-visna virus (MVV), but it is a genetically distinct lentivirus. As nucleotide sequence information has increased, it has been shown that MVV and CAEV should not be regarded as sheep-specific and goat-specific pathogens respectively, but rather that they should be more correctly referred to as SRLV [1Reina R, Mora MI, Glaria I, et al. Molecular characterization and phylogenetic study of Maedi Visna and Caprine Arthritis Encephalitis viral sequences in sheep and goats from Spain Virus Res 2006; 121(2 ): 189-98., 9Pisoni G, Bertoni G, Puricelli M, Maccalli M, Moroni P. Demonstration of coinfection with and recombination by caprine arthritis-encephalitis virus and maedi-visna virus in naturally infected goats J Virol 2007; 81(10 ): 4948-55., 10Pisoni G, Quasso A, Moroni P. Phylogenetic analysis of small-ruminant lentivirus subtype B1 in mixed flocks: evidence for natural transmission from goats to sheep Virology 2005; 339(2 ): 147-52.]. In the diagnostic field, serological tests are routinely applied to detect SRLV infections. Although third-generation ELISAs, like those used in this study, are highly sensitive and specific [11Saman E, Van Eynde G, Lujan L, et al. A new sensitive serological assay for detection of lentivirus infections in small ruminants Clin Diagn Lab Immunol 1999; 6(5 ): 734-40.], seroconversion can, however, be relatively slow and variable and there is no simple relationship between the moment of conversion and the initial infection. To be able to prove the availability of actual viral particles in blood and tissues, a PCR test may be used, as in the present study. Theoretically, SRLV can be present in the clinical samples (blood, organs, semen) in three forms: namely (i) incorporated proviral DNA (in macrophages), (ii) as virions (complete virus particles with their ssRNA core and protein coat released by budding from the plasma membrane) and (iii) as free virus (released by cell lysis). In practice, CAEV proviral-DNA and viral mRNA could be detected by PCR base methods. The current most widely used test for caprine arthritis-encephalitis (CAE) is agar gel immunodiffusion (AGID) recommended by the World Animal Health Organization (OIE,2009). However, this seems to underestimate infection incidence especially in animals with delayed sera conversion [12Johnson LK, Meyer AL, Zink MC. Detection of ovine lentivirus in seronegative sheep by in situ hybridization, PCR, and cocultivation with susceptible cells Clin Immunol Immunopathol 1992; 65(3 ): 254-60.]. This can impede early or precise serological diagnosis in animals, which could be an efficient infection source. By direct diagnostic procedures, CAEV-DNA or CAEV-RNA have been detected by PCR or RT-PCR base methods, these procedures required identification by agarose electrophoresis or sometimes by the capillary sequencing method [13Kaba J, Rola M, Materniak M, Kuzmak J, Nowicki M. Isolation and characterization of caprine arthritis encephalitis virus in goats from Poland Pol J Vet Sci 2009; 12(2 ): 183-8.-15Ali Al Ahmad MZ, Fieni F, Martignat L, et al. Proviral DNA of caprine arthritis encephalitis virus (CAEV) is detected in cumulus oophorus cells but not in oocytes from naturally infected goats Theriogenology 2005; 64(7 ): 1656-66.]. Up until now, these molecular tools have required a long time and can be expensive; in addition, they do not allow viral quantization; to overcome these problems, we used a real time PCR method for the detection and quantization of CAEV strains in different goat clinical samples. This could be a useful anti-CAEV prophylactic strategy controller or used in experimental infection studies for monitoring, avoiding possible cross reactions with other strongly related virus such as maedi visna virus (MVV). The purpose of this study was to develop a real-time PCR assay able to detect/quantify CAEV DNA or RNA in goat/sheep clinical samples.
MATERIALS AND METHODOLOGY
Animals and Clinical Samples
Our studies were performed in two distinct Italian native goat populations: (I) Sardinian, (II) Alpine, Central Italy.
A total of thirty-seven goat samples were processed:
Sardinian goat population, 16 subjects from 2 herds, ten of these were culled (Table 1).
- 16 DNA extracts from blood
- 10 DNA extracts from intra-articular fluid
- 10 RNA extracts from intra-articular fluid
Clinical evaluation for CAEV symptoms was observed for these subjects [16Ganter M. [Clinical aspect of caprine arthritis and encephalitis] Tierarztl Prax 1993; 21(6 ): 517-20.].
Alpine goat population, 21 subjects from 1 herd
- 21 DNA extracts from blood
- 21 RNA extracts from serum
These herds occasionally showed serological CAEV positivity by the Agar Gel Immuno diffusion (AGID) technique [17Burgu I, Akca Y, Alkan F, Ozkul A, Karaoglu T, Cabalar M. Antibody prevalence of caprine arthritis encephalitis virus (CAEV) in goats in Turkey Dtsch Tierarztl Wochenschr 1994; 101(10 ): 390-1.], or by ELISA [18Ali Al Ahmad MZ, Chebloune Y, Bouzar BA, et al. Lack of risk of transmission of caprine arthritis-encephalitis virus (CAEV) after an appropriate embryo transfer procedure Theriogenology 2008; 69(4 ): 408-15.] see Tables 1 and 2.
In addition, 15 other sheep, serologically negative for CAEV and recruited from a herd from central Italy, were used for CAEV/MMV experimental infections (see later).
Viral Strains
Two different viral clinical isolates (CAEV and MMV) were used for sheep experimental infection and as positive controls, in order to check the reliability of the real time PCR procedure described in this paper.
- The first strain, originally isolated by explantation of the synovial membrane from an arthritic goat, (It-Pi1) was identified as a CAEV- like genotype B by traditional PCR procedures [19Reina R, Grego E, Profiti M, et al. Development of specific diagnostic test for small ruminant lentivirus genotype E Vet Microbiol 2009; 138(3-4 ): 251-7., 20Lacerenza D, Giammarioli M, Grego E, et al. Antibody response in sheep experimentally infected with different small ruminant lentivirus genotypes Vet Immunol Immunopathol 2006; 112(3-4 ): 264-71.].
- The second Visna maedi- like strain (It-651), obtained by sheep tissue explantation, was identified as genotype A [20Lacerenza D, Giammarioli M, Grego E, et al. Antibody response in sheep experimentally infected with different small ruminant lentivirus genotypes Vet Immunol Immunopathol 2006; 112(3-4 ): 264-71., 21Shah C, Boni J, Huder JB, et al. Phylogenetic analysis and reclassification of caprine and ovine lentiviruses based on 104 new isolates: evidence for regular sheep-to-goat transmission and worldwide propagation through livestock trade Virology 2004; 319(1 ): 12-26.].
Serological Analysis
Serological analysis was performed by the Agar Gel Immuno diffusion (AGID) technique and by Elisa procedures described by other authors in previous publications [17Burgu I, Akca Y, Alkan F, Ozkul A, Karaoglu T, Cabalar M. Antibody prevalence of caprine arthritis encephalitis virus (CAEV) in goats in Turkey Dtsch Tierarztl Wochenschr 1994; 101(10 ): 390-1., 20Lacerenza D, Giammarioli M, Grego E, et al. Antibody response in sheep experimentally infected with different small ruminant lentivirus genotypes Vet Immunol Immunopathol 2006; 112(3-4 ): 264-71.]. In particular, two commercially available ELISAs were used. One whole virus ELISA (hereafter named WVcomELISA) was from Bommeli Diagnostics (Checkit MVV/CAEV) and a recombinant based ELISA (named RcomELISA) was made by the Institut Pourquier, employing the recombinant capsid antigen and trans membrane protein. Both tests were carried out following the manufacturer’s instructions.
Experimental Infection
Experimental infection was carried out during a previous study at Perugia’s Istituto Zooprofilattico Sperimentale (IZSP) and a set of immunological results with some samples has already been published, Lacerenza et al., 2006 [20Lacerenza D, Giammarioli M, Grego E, et al. Antibody response in sheep experimentally infected with different small ruminant lentivirus genotypes Vet Immunol Immunopathol 2006; 112(3-4 ): 264-71.].
Briefly, 15 sheep from the Appennine population were selected from a long term negative flock and housed in Perugia’s Istituto Zooprofilattico Sperimentale at the age of 8 months. They were kept here for another 6 months before experimental infection and tested twice with negative results for small ruminant lentivirus antibodies. On the day before infection, the subjects were randomly divided into three groups (five sheep per group) each housed in different independent units:
- Group A were uninfected (negative control, receiving 1 ml of mock, consisting of uninfected cell culture medium).
- Group B were infected by intratracheal administration of 1 ml of virus, containing cell culture supernatant (strain It- It-Pi1, 4.6 *103 TCID50/ml, CAEV).
- Group C were infected by intratracheal administration of 1 ml of virus, containing cell culture supernatant (strain It- 561, 4.6*103 TCID50/ml) (MVV).
All sheep were sampled before inoculation on the day of infection, and at regular intervals (every 7 days) until day 71. Serum and blood samples were collected for serological analysis and nucleic acid extraction. Use of these samples was authorized by IZSP.
Viral nucleic Acid Extraction
DNeasy 96 Blood & Tissue Kit (QIAGEN) was used for DNA extraction from blood and intra-articular fluid. RNeasy Mini Kit (QIAGEN) was used for RNA extraction from sera, while QiAamp RNA Blood Mini Kit was used for RNA extraction from fresh whole blood. For a correct procedure we followed the manufacturer’s instructions. The concentration of nucleic acid nucleic suspensions was measured with a Qubit® 2.0 Fluorometer by using a Qubit® RNA Assay Kit and Qubit® dsDNA HS Assay Kit (Invitrogen) following the manufacturer’s instructions for RNA and DNA measurement respectively. These samples were stored at -80°C until use.
Oligonucleotide Primers Design
The PCR target region was identified by multiple sequence alignment programs (ClustalW, and Geneious Biomatters Ltd.) by means of the complete CAEV and visna Maedi proviral genomes, available in the DNA data Bank (GenBank). The env gene sequences, codifying for CAEV viral envelope polyprotein, showed a good conserved region inside the CAEV group, even though a mean difference was observed with the Visna Maedi virus (Fig. 1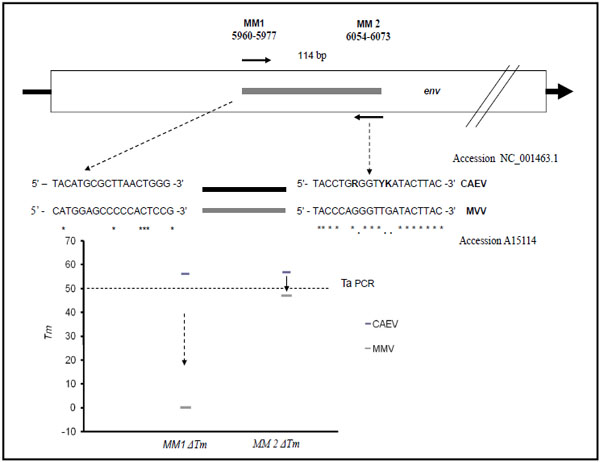 ). The PCR primers, used to perform real time PCR, were designed from a 114 bp fragment, corresponding to nucleotide positions from 5960 to 6073 in the published CAEV genome sequence identified with GenBank accession n. NC_001463. The theoretical melting temperatures of the primers (Tms), the formation of oligonucleotide dimers and self-complementarity were evaluated using Oligo Program version 4 (MedProbe, Oslo, Norway) and by module 1 of the DNA Hybridisation prediction algorithm program, HYTHER http://ozone.chem.wayne.edu/ [22Bommarito S, Peyret N, SantaLucia J Jr. Thermodynamic parameters for DNA sequences with dangling ends Nucleic Acids Res 2000; 28(9
): 1929-34., 23Orru G, Masia G, Romano L, Piras V, Coppola RC. Detection and quantitation of hepatitis E virus in human faeces by real-time quantitative PCR J Virol Methods 2004; 118(2
): 7-82.]. Parameters set as hybridisation conditions were a monovalent cation concentration of 0.05 mol/L, Mg2+ at 0.004 mol/L, a concentration of probe and target of 10-7 mol/L and a hybridisation temperature of 50°C. This fragment was chosen for its high similarity in all CAEV genotypes. The positions of the PCR primers and the comparative matches with the visna maedi (MVV) env region are shown in Fig. (1
). The PCR primers, used to perform real time PCR, were designed from a 114 bp fragment, corresponding to nucleotide positions from 5960 to 6073 in the published CAEV genome sequence identified with GenBank accession n. NC_001463. The theoretical melting temperatures of the primers (Tms), the formation of oligonucleotide dimers and self-complementarity were evaluated using Oligo Program version 4 (MedProbe, Oslo, Norway) and by module 1 of the DNA Hybridisation prediction algorithm program, HYTHER http://ozone.chem.wayne.edu/ [22Bommarito S, Peyret N, SantaLucia J Jr. Thermodynamic parameters for DNA sequences with dangling ends Nucleic Acids Res 2000; 28(9
): 1929-34., 23Orru G, Masia G, Romano L, Piras V, Coppola RC. Detection and quantitation of hepatitis E virus in human faeces by real-time quantitative PCR J Virol Methods 2004; 118(2
): 7-82.]. Parameters set as hybridisation conditions were a monovalent cation concentration of 0.05 mol/L, Mg2+ at 0.004 mol/L, a concentration of probe and target of 10-7 mol/L and a hybridisation temperature of 50°C. This fragment was chosen for its high similarity in all CAEV genotypes. The positions of the PCR primers and the comparative matches with the visna maedi (MVV) env region are shown in Fig. (1 ).
).
Positive Quantitative Standard
A synthetic 114 bp DNA fragment (MM4) was used as a positive control. This fragment, corresponding to the PCR amplicon was synthesized by eurofins MWG/operon (www.eurofinsdna.com). Serial 10 fold dilutions of this target, ranging from 106 molecules/2μl, served as a standard quantification curve.
Optimal Amounts of Template DNA for PCR Reaction
Proviral CAEV DNA is normally represented as the smallest percentage in the sample compared to goat eukaryotic DNA. This aspect could compromise PCR reaction as a false negative result [24Roux KH. Optimization and troubleshooting in PCR Cold Spring Harb Protoc 2009; 2009(4 ): pdb-ip66.]. The clinical sample analyzed in this study showed a total DNA concentration ranging from 5 to 43 ug/ml. For this reason, we performed a real time PCR reaction containing a serial dilution of mixed goat DNA, from 50 to 0.78 µg/ml, with a synthetic CAEV target MM4 env molecules/PCR at a concentration of 1*105 (Table 1). The DNA amount was calculated by a Qubit® 2.0 Fluorometer (Invitrogen) following the manufacturer’s instructions.
Real Time RT-PCR
In this work we used a version 1.2 LightCycler Instrument, (Roche Diagnostics Mannheim, Germany).
Real Time RT-PCR for CAEV RNA
PCR was performed with a LightCycler-RNA Amplifi-cation kit SYBR Green I (Roche Diagnostics Mannheim Germany) according to the manufacturer’s instructions. The 20 μl final volume contained: 2.5 mM MgCl2, 0,5 μM of each primer and 1 U of heat-labile Uracyl-DNA-Glicosilase (UDG, Roche Molecular Biochemicals), 2μl of RNA extract. The PCR program was as follows: (i) an initial reverse transcription at 55°C for 10 min. (ii) denaturation at 95°C for 30 sec. and (iii) 40 cycles of 0 sec at 95°C,10 sec at 50 °C, 24 sec at 72°C and 3 sec at 79 °C (vi) Melting curve: performed for 0 seconds at 95°C, 45°C, 95°C. Transition rates were: 5 °C/s in 72°C segment, 0.1 °C/s in 45° C segment and 20°C/s for another step. Fluorescence was detected at the end of the 79°C segment in the PCR step (single mode) and at the 45°C segment in the melting step (continuous mode) in the F1 channel.
Real Time PCR for CAEV Proviral DNA
PCR was performed with a LightCycler-DNA Amplification kit SYBR Green I (Roche Diagnostics Mannheim Germany) according to the manufacturer’s instructions. The 20 μl final volume contained: 2.5 mM MgCl2, 0,5 μM of each primer, 2μl of DNA extract. The PCR program was the same as described above without the reverse transcription step.The quantitative real time PCR or real time RT-PCR, performed with the LightCycler (Roche Diagnostics Mannheim, Germany), required 30 minutes.
RESULTS
Magnesium Optimization
Magnesium concentration is a critical factor affecting the performance of Taq DNA polymerase. PCR reaction components can affect the amount of free magnesium. In the absence of adequate magnesium, Taq DNA polymerase is ineffective. On the contrary, excess free magnesium reduces enzyme fidelity and may increase the level of nonspecific amplification [24Roux KH. Optimization and troubleshooting in PCR Cold Spring Harb Protoc 2009; 2009(4 ): pdb-ip66.]. For this reason, we prepared a reaction series containing 3-5.5 mM, Mg2+ in 0.4 mM increments by adding 0-0.4-0.8-1,2-1.6-2 µl of a 25 mM MgCl2 stock to 20 µl reactions as described in the “LightCycler® DNA/RNA SYBR Green I” Kit instruction manuals. The most sensitive combination was observed with MgCl2 3.5 mM, with a shift in the PCR threshold cycle (CT) from 32 to 12.
Melting Curve and Procedure Specificity
The melting temperature of a DNA duplex, with SYBR green dye, gives information about DNA fragment length [25Varga A, James D. Real-time RT-PCR and SYBR Green I melting curve analysis for the identification of Plum pox virus strains C, EA, and W: effect of amplicon size, melt rate, and dye translocation J Virol Methods 2006; 132(1-2
): 146-53.], which shows dissociation-characteristics of double-stranded DNA during heating and, normally we can observe two different melting peaks after real time PCR reaction: the first low temperature melting peak represents a possible primer-dimer PCR product; the second (at a higher temperature), represents the signal corresponding to PCR amplicon dissociation. Thus a negative result is represented with the first peak only. RT-PCR, generation of melting curves and fluorescence detection were all performed using: (i) viral strains, (ii) positive clinical strains for MMV and CAEV, (iii) CAEV/MMV negative goat samples. As described in Fig. (2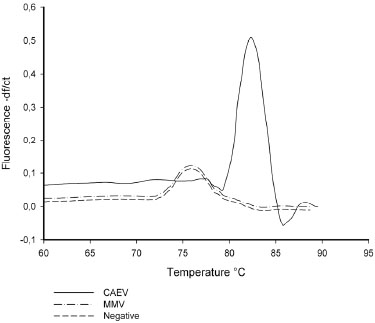 ), a 82°C melting Peak is shown in CAEV samples, even though a Tm peak shift (82-76°C) was detected in the case of MMV samples or in the negative control. These results confirm the prediction obtained by an in silico study described in Fig. (1
), a 82°C melting Peak is shown in CAEV samples, even though a Tm peak shift (82-76°C) was detected in the case of MMV samples or in the negative control. These results confirm the prediction obtained by an in silico study described in Fig. (1 ).
).
Sensitivity
Real-time PCR, as evaluated here, has been shown to have a detection limit of 102 synthetic MM4 fragment/PCR. The wide linear range (107-103 MM4/PCR) as illustrated in Fig. (3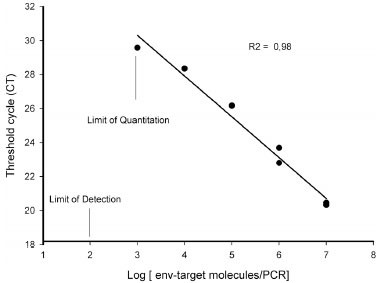 ), Standard curves showed a good correlation regression coefficient R2 of 0.98 [26Koenig M, Reynolds KS, Aldous W, Hickman M. Comparison of Light-Cycler PCR, enzyme immunoassay, and tissue culture for detection of herpes simplex virus Diagn Microbiol Infect Dis 2001; 40(3
): 107-.].
), Standard curves showed a good correlation regression coefficient R2 of 0.98 [26Koenig M, Reynolds KS, Aldous W, Hickman M. Comparison of Light-Cycler PCR, enzyme immunoassay, and tissue culture for detection of herpes simplex virus Diagn Microbiol Infect Dis 2001; 40(3
): 107-.].
Optimal Amount of Total DNA Concentration
The PCR reaction was performable at all serial dilutions of goat DNA tested. Early CT values were around 18 and they were observed under 12.5 µg/ml of total DNA, corresponding to the samples diluted ¼ fold, (data not shown).
Infected Sheep
As described in the Materials and Methods section: a cohort of three different groups of sheep were used, with 5 subjects each of which were :
- infected with a CAEV strain (It-Pi1),
- infected with a Visna maedi strain(It-651),
- non- infected.
For serological analysis and nucleic acid extraction, we took serum and blood specimens for each sheep at 4, 8,15,23,28,42,58,71 days after infection. Real time PCR and RT-real time PCR showed that:
MMV infected and non infected sheep resulted PCR negative (data not shown).
Viral RNA in all infected sheep was undetectable by RT real time PCR (data not shown).
As shown in Fig. (4a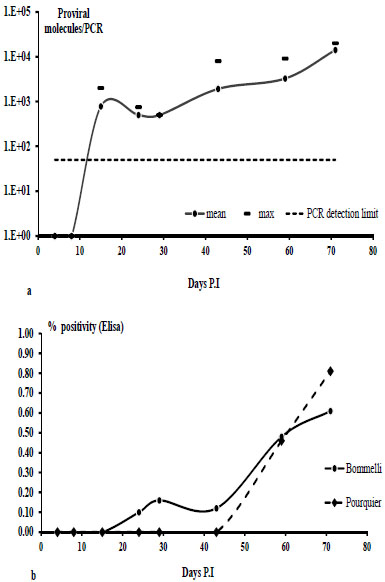 ), proviral DNA was detectable in blood, 15 days after infection for all CAEV infected sheep. The titres (the mean for 5 sheep, calculated as a MM4 CAEV fragment/PCR) ranged from 9*102 (15th day PI) to 1 *104 (71th day PI). 1*103 fragment/PCR, corresponding to about 5*104 virus particles/ml blood.
), proviral DNA was detectable in blood, 15 days after infection for all CAEV infected sheep. The titres (the mean for 5 sheep, calculated as a MM4 CAEV fragment/PCR) ranged from 9*102 (15th day PI) to 1 *104 (71th day PI). 1*103 fragment/PCR, corresponding to about 5*104 virus particles/ml blood.
Fig. (4b ) represents the percentage of positivity of CAEV antigens obtained with two different Elisa manufacturer’s kits (Bommelli and Pourquier). Significant positivity (> 50%) was detected after 60 days PI. The Bommelli kit resulted positive around 20 days PI but it showed a lower positivity percentage (< 20%).
) represents the percentage of positivity of CAEV antigens obtained with two different Elisa manufacturer’s kits (Bommelli and Pourquier). Significant positivity (> 50%) was detected after 60 days PI. The Bommelli kit resulted positive around 20 days PI but it showed a lower positivity percentage (< 20%).
Clinical Samples
A total of thirty seven goat samples were processed. All positive specimens analyzed showed a very low viral titre (≤ 103 C env-DNA copies/ml).
Sardinian Goats
We analyzed a total of 16 subjects from 2 herds. All symptomatic goats (5/16 subjects) and 6 asymptomatic subjects resulted Blood DNA CAEV positive (Table 1), no intrarticular fluid (IAF) resulted positive for CAEV DNA or RNA.
Central Italy Goats (Alpine).
Of the 21 goats analyzed, Table 2, only two samples showed a discrepancy between the serological (Pourquier) and molecular analysis. Sample n. 1 resulted CAEV DNA positive but serologically negative, while sample n. 21 was Elisa positive with CAEV DNA/RNA negative. Considering only the Elisa positive specimens, 77% were Blood CAEV DNA positive and 53% were serum RNA positive. Real time PCR (DNA+RNA) revealed 92% serological positive samples (12/13) CAEV positive and 25% Elisa negative samples.
DISCUSSION AND CONCLUSION
Caprine arthritis-encephalitis (CAE) is a disease in goats and sheep caused by a retrovirus (CAEV). This is a persistent lentivirus infection and is often grouped with the Maedi visna (MVV) virus which is a small ruminant lentivirus, SRLV [27Russo P. [Caprine arthritis-encephalitis virus (CAEV). Short review] Ann Rech Vet 1984; 15(1 ): 3-6.]. It is an emerging problem throughout Europe, particularly in the Mediterranean regions. The disease tends to spread widely if left uncontrolled and most affected animals are culled because these animals are sources of infection and their clinical signs worsen over time, causing high economic loss. At the present time, there are no biological products such as vaccines for CAEV prophylaxis; in fact, the only choice is infection prevention by early identification and isolation of CAEV-infected subjects. Most infected subjects possess detectable specific antibodies and a number of serological test are available for CAEV infection diagnosis. The two most commonly used are the agar gel immuno diffusion test (AGID) and the enzyme-linked immunosorbent assay, ELISA [11Saman E, Van Eynde G, Lujan L, et al. A new sensitive serological assay for detection of lentivirus infections in small ruminants Clin Diagn Lab Immunol 1999; 6(5 ): 734-40., 28Knowles DP Jr, Evermann JF, Shropshire C, et al. Evaluation of agar gel immunodiffusion serology using caprine and ovine lentiviral antigens for detection of antibody to caprine arthritis-encephalitis virus J Clin Microbiol 1994; 32(1 ): 243-5.]. In a previous work, Adams et al. [29Adams DS, Crawford TB, Banks KL, McGuire TC, Perryman LE. Immune responses of goats persistently infected with caprine arthritis-encephalitis virus Infect Immun 1980; 28(2 ): 421-7.] demonstrated that Virus-inoculated goats produced a CAEV- specific antibody that reached a maximum level between 49 and 77 days post inoculation. In this work, we also observed that a significant positivity (>50%) could be detected after around 60 days PI in infected sheep. Following other literature data, the time required for seroconversion following infection can be relatively prolonged and unpredictable, measured in months rather than in weeks [17Burgu I, Akca Y, Alkan F, Ozkul A, Karaoglu T, Cabalar M. Antibody prevalence of caprine arthritis encephalitis virus (CAEV) in goats in Turkey Dtsch Tierarztl Wochenschr 1994; 101(10 ): 390-1., 30Crawford TB, Adams DS, Cheevers WP, Cork LC. Chronic arthritis in goats caused by a retrovirus Science 1980; 207(4434 ): 997-.-32Rowe JD, East NE. Risk factors for transmission and methods for control of caprine arthritis-encephalitis virus infection Vet Clin North Am Food Anim Pract 1997; 13(1 ): 35-53.]; this “window period” could cause prevention strategies to fail with a subsequent spread of infection. Direct virus isolation by co-cultivating peripheral blood or milk leukocytes with appropriate caprine cell cultures, such as synovial membrane cellisolation and subsequent confirmation by immunolabelling and electron microscopy is very specific but showed variable sensitivities [33Lerondelle C, Godet M, Mornex JF. Infection of primary cultures of mammary epithelial cells by small ruminant lentiviruses Vet Res 1999; 30(5 ): 467-74.]; in addition, this method requires specialised laboratories.
Direct laboratory diagnosis by CAEV nucleic acid recognition for detecting CAEV virus or provirus has already been described [6Daltabuit Test M, de la Concha-Bermejillo A, Espinosa LE, Loza Rubio E, Aguilar Setien A. Isolation of caprine arthritis encephalitis virus from goats in Mexico Can J Vet Res 1999; 63(3 ): 212-5., 13Kaba J, Rola M, Materniak M, Kuzmak J, Nowicki M. Isolation and characterization of caprine arthritis encephalitis virus in goats from Poland Pol J Vet Sci 2009; 12(2 ): 183-8.-15Ali Al Ahmad MZ, Fieni F, Martignat L, et al. Proviral DNA of caprine arthritis encephalitis virus (CAEV) is detected in cumulus oophorus cells but not in oocytes from naturally infected goats Theriogenology 2005; 64(7 ): 1656-66., 34Gil A, Rola M, Kuzmak J. Application of PCR technique in diagnosis of small ruminant lentivirus infection in sheep and goats Pol J Vet Sci 2006; 9(4 ): 213-7.-40Reddy PG, Sapp WJ, Heneine W. Detection of caprine arthritis-encephalitis virus by polymerase chain reaction J Clin Microbiol 1993; 31(11 ): 3042-.]. These procedures represent in situ hybridization methods or PCR (polymerase chain reaction) methods. PCR base methods are now routinely being used in different laboratories, and their specificity is confirmed by amplicons or cloning products.
Traditional PCR is inexpensive, easy to use but current molecular methods do not allow simultaneous CAEV detection/quantization. We have described a fast molecular procedure (around 1.5-2 hours) with good sensitivity and specificity, able to detect and quantify CAEV in the viral or proviral form.
We designed a real time PCR protocol by using a primer couple flanking the env gene region representative for all CAEV sequences deposited in the GeneBank but containing many mismatch regions, in comparison with the Visna Maedi (MVV) isolate. With MVV env sequences, simulation analysis in silico by Hyther and mfold servers showed an increase in Gibbs energy (delta G), with a subsequent Tm decrement positioned from 7 to 50 °C for upper and lower primers respectively (Fig. 1 ). This observation was confirmed by a reconstruction experiment using a Visna maedi strain (It-651) as a DNA/RNA direct template for PCR real time or in experimentally infected sheep; these results suggest an absence of false CAEV positive results with samples containing MVV, demonstrating the optimal specificity of this molecular approach.
). This observation was confirmed by a reconstruction experiment using a Visna maedi strain (It-651) as a DNA/RNA direct template for PCR real time or in experimentally infected sheep; these results suggest an absence of false CAEV positive results with samples containing MVV, demonstrating the optimal specificity of this molecular approach.
In this work we used an intercaling dye as a fluorescent probe (Sybr Green I). This procedure required a melting curve at the end of the amplification cycle to determine if the product is pure (amplicon only) or if there are non specific products like primer–dimers [41Wilhelm J, Pingoud A. Real-time polymerase chain reaction Chembiochem 2003; 4(11
): 1120-8.]. In our experiment, shown in Fig. (2 ), melting curves are localized on the graph in two distinct areas: (i) a 78°C peak region due to primer dimer products in negative samples (such as MVV positive samples), (ii) a 83° C peak region in positive CAEV samples; for this reason we positioned the fluorescence detention point at 80°C when the aspecific product signal is the lowest (for PCR and RT PCR), thereby avoiding specificity errors due to inappropriate fluorescence signals (Fig. 2
), melting curves are localized on the graph in two distinct areas: (i) a 78°C peak region due to primer dimer products in negative samples (such as MVV positive samples), (ii) a 83° C peak region in positive CAEV samples; for this reason we positioned the fluorescence detention point at 80°C when the aspecific product signal is the lowest (for PCR and RT PCR), thereby avoiding specificity errors due to inappropriate fluorescence signals (Fig. 2 ).
).
By using a synthetic env gene fragment reproducing the PCR amplicon, this real time PCR showed a 5 log linearity range, from 102 to 107env fragments/PCR with a regression factor (R2) of 0.98. Viral enumeration using the present procedure in clinical or laboratory samples (such as virus or provirus) could be useful for viraemia or proviral evaluation during anti-CAEV prophylactic measures or in laboratory experiments (such as cell culture infection). A comparative evaluation between serological and molecular procedures is shown by experimental infections and by biological samples recruited in subjects coming from infected areas.
First of all, experimental infections suggested a complete absence of fluorescence signals in samples recruited in sheep infected with MVV, despite the fact that a mean of 103 proviral molecules PCR is detectable in CAEV infected sheep at 15 day PI, a correspective serological positive signal is detected around 40-60 day PI, this datum suggests that with this real time PCR we could “gain a time” of around 25-45 days in diagnostic procedures, which (as discussed above) could be useful in an early prophylactic anti CAEV procedure. CAEV RNA was not detectable in these sheep until 71 days PI; this result could be due to low viraemia during this period. In clinical samples, only 7 Alpine goats were CAEV RNA positive, and their simultaneous positivity for CAEV DNA could indicate a very old infection (Table 2). All symptomatic goats (5 subjects) resulted proviral real time PCR positive (Table 1). The prognostic effect of this molecular approach on diagnosis for CAE is represented by Sardinian goats monitored serologically by the AGID procedure and the Alpine type monitored by Elisa. Two serologically negative samples resulted PCR positive (Tables 1 and 2), these subjects could be in a “window period”. However some discrepancies were revealed in these specimens, i.e. sample 396 (Table 2) and A02 and K133 where serological positivity did not correspond to the molecular signal This could be due to different causes: (I) specificity mistakes with serological methods, (ii) PCR sensitivity mistakes (target < 102 env/PCR or PCR inhibitors, in this case an internal amplification control (i.e. on 18S rRNA gene) could be useful. The blood for proviral detection represented the best procedure in laboratory diagnosis for CAEV infection in goats/sheep.
In conclusion, we tested and validated the feasibility of a real time PCR such as PCR or RT PCR as a method for the detection and simultaneous quantisation of Caprine Arthritis-Encephalitis Virus in naturally-infected goats or in experimentally infected sheep. This method showed a good sensitivity (102 env fragment/PCR) and a good specificity (100%) when this protocol was applied in experimentally infected subjects or goats with a clinical diagnosis of Caprine Arthritis-Encephalitis (CAE).
ACKNOWLEDGEMENTS
This work was supported by the Italian Ministry of Health, Ricerca Finalizzata, art-12 bis D.L.229/99, (DIAG-NOVA).
CONFLICT OF INTEREST
The authors declares that they have no financial/commer-cial conflicts of interest.


