- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Synthesis and Characterization of a Series of 1-Aryl-4-[aryldiazenyl]- piperazines. Part I. Isomers of N-(2,3-Dimethylphenyl)-N’-(Aryldiazenyl)- Piperazines
Chenguang Fan, Keith Vaughan*
Abstract
This paper describes the synthesis of several new series of 1-(2-aryldiazen-1-yl-) 4-arylpiperazines and 1-(2- aryldiazen-1-yl-)4-arylalkylpiperazines by using diazonium coupling between arenediazonium ions with the appropriate 1-arylpiperazine or the 1-arylalkylpiperazine. The new compounds have a common thread in that they are isomers of the series of N-(2,3-dimethylphenyl)- N’-(aryldiazenyl)-piperazines. The new triazenes have been characterized by IR and NMR spectroscopy and mass spectrometry.
Article Information
Identifiers and Pagination:
Year: 2015Volume: 2
First Page: 20
Last Page: 35
Publisher Id: CHEM-2-20
DOI: 10.2174/1874842201502010020
Article History:
Received Date: 2/2/2015Revision Received Date: 6/4/2015
Acceptance Date: 7/4/2015
Electronic publication date: 30/10/2015
open-access license: This is an open access article licensed under the terms of the (https://creativecommons.org/licenses/by/4.0/legalcode), which permits unrestricted, noncommercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Department of Chemistry, Saint Mary’s University, Halifax, Nova Scotia, B3H 3C3, Canada; Tel: 902-420-5650; E-mail: keith.vaughan@smu.ca
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 2-2-2015 |
Original Manuscript | Synthesis and Characterization of a Series of 1-Aryl-4-[aryldiazenyl]- piperazines. Part I. Isomers of N-(2,3-Dimethylphenyl)-N’-(Aryldiazenyl)- Piperazines | |
INTRODUCTION
Previous work in this laboratory has described the synthesis of the 1,4-bis-(2-aryl-diazen-1-yl-)piperazines (1)[1Little, V.R.; Tingley, R.; Vaughan, K. Triazene derivatives of [1,x]-diaza-cycloalkanes. Part III. Synthesis and characterization of a series of 1,4-di[2-aryl-1-diazenyl]piperazines Can. J. Chem, 2005, 83, 471-476.
[http://dx.doi.org/10.1139/v05-064] , 2Hunter, N.; Vaughan, K. Triazene derivatives of [1,x]-diazacycloalkanes. Part IX. Synthesis and characterization of a series of 1,4-di[2-aryl-1-diazenyl]-2,6-dimethylpiperazines Can. J. Chem, 2010, 88, 344-351.
[http://dx.doi.org/10.1139/V10-008] ] using the bis-diazonium coupling reaction with piperazine itself (equation (i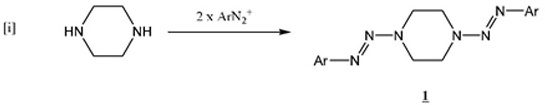 )):
)):
 |
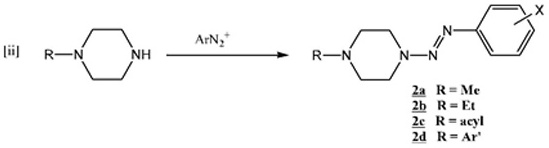 |
In a similar fashion, diazonium coupling with 1-methylpiperazine afforded a series of 1-(2-aryldiazen-1-yl-)4-methylpiperazines (2a) [3Little, V.R.; Vaughan, K. Synthesis and Characterization of a series of 1-methyl-4-[2-aryl-1-diazenyl]piperazines and a series of ethyl 4-[2-aryl-1-diazenyl]-1-piperazinecarboxylates Can. J. Chem, 2004, 82, 1294-1303.
[http://dx.doi.org/10.1139/v04-081] ](equation ii ):
):
Subsequently, this work was extended to the synthesis of the 1-(2-aryldiazen-1-yl-) 4-ethylpiperazines (2b) [4MacLeod, E.; Vaughan, K. Synthesis and characterization of new compounds in the series 1-alkyl-4-[2-aryl-1-diazenyl]piperazines Open Organ. Chem. J, 2015, 9, 1-8.
[http://dx.doi.org/10.2174/1874095201509010001] ]. Further investigation led to the synthesis of a large number of 1-(2-aryldiazen-1-yl-)4-acylpiperazines (2c); the paper describing these latter results has recently been published [5Little, V.R.; Vaughan, K. Synthesis and Characterization of Several Series of 4-Acyl-1-[2-aryl-1-diazenyl]piperazines Can. J. Chem, 2014, 92, 838-848.
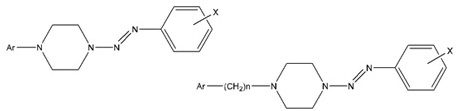
[http://dx.doi.org/10.1139/cjc-2014-0242] ]. The next logical step in the pursuit of new molecules ofthe N-aryldiazenylpiperazines is to investigate the synthesis of the1-aryl-4-[aryldiazenyl] -piperazines (2d) by diazonium salt coupling with a series of 1-arylpiperazines.
In the present work, a series of N-(2,3-dimethylphenyl)-N’-(aryl-diazenyl)-piperazines (3) have been synthesized by diazonium coupling with N-(2,3-dimethylphenyl-)piperazine. These new triazenes represent the first ever piperazine derivatives to be reported with one N-aryl substituent at N4 opposite to the N-aryldiazenyl substituent at N1. The common thread of all the new compounds reported in this paper is that they are isomers of the compounds of series 3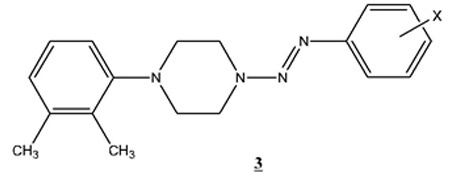 .
.
 |
Isomeric compounds described as 1-(methylphenyl-methyl)-4-(aryldiazenyl)-piperazines (4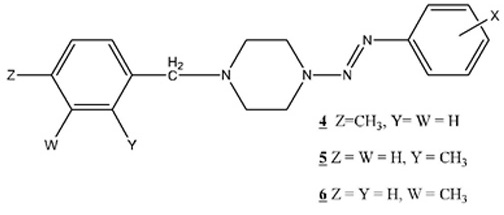 , 5
, 5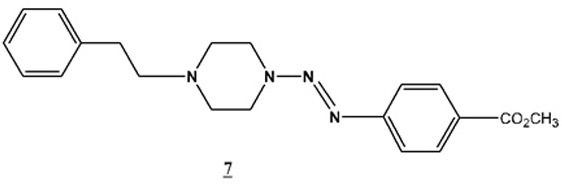 and 6
and 6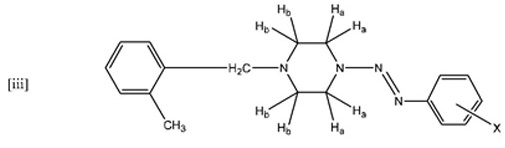 ) have also been prepared and characterized:
) have also been prepared and characterized:
 |
Also the isomer methyl 4-[2Hunter, N.; Vaughan, K. Triazene derivatives of [1,x]-diazacycloalkanes. Part IX. Synthesis and characterization of a series of 1,4-di[2-aryl-1-diazenyl]-2,6-dimethylpiperazines Can. J. Chem, 2010, 88, 344-351.
[http://dx.doi.org/10.1139/V10-008] -1Little, V.R.; Tingley, R.; Vaughan, K. Triazene derivatives of [1,x]-diaza-cycloalkanes. Part III. Synthesis and characterization of a series of 1,4-di[2-aryl-1-diazenyl]piperazines Can. J. Chem, 2005, 83, 471-476.
[http://dx.doi.org/10.1139/v05-064] ]benzoate (7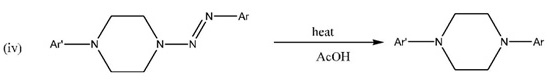 ) has been prepared and characterized.
) has been prepared and characterized.
 |
EXPERIMENTAL
Materials and Apparatus
The series of aromatic primary amines and the series of 1-arylsubstituted-piperazines were reagent-grade materials purchased from the Aldrich Chemical Co. Ltd., and were used without further purification. Melting points were determined on a Fisher-Johns Melting Point Apparatus and were uncorrected. Infrared spectra were obtained using nujol mulls on a Bruker Vector-22 IR spectrometer. 1H and 13C NMR spectra were obtained on Bruker AC 250MHz and 500MHz spectrometers at the Atlantic Regional Magnetic Resonance Center at Dalhousie University in Halifax, Nova Scotia. Chemical shifts were recorded in CDCl3 solutions at room temperature, and were related to TMS internal standard. The NMR data were interpreted using the TOPSPIN software. The high resolution mass spectra were obtained at Dalhousie University in Halifax, Nova Scotia. Accurate mass measurements were made on a CEC 21-110B mass spectrometer operated at a mass resolution of 8000 (10% valley) by computer-controlled peak matching to appropriate PFK reference ions. Spectra were obtained using electron ionization at 70 volts and a source temperature of 175℃, with samples being introduced by means of a heatable quartz probe. The standard deviation of mass measurement is +/- 0.0008 amu, which is an average of 3.6 ppm over the mass range 100 to 300 amu.
General Procedure
The aromatic primary amine (0.006 mol) was dissolved in 6 mL of 3 mol/L hydrochloric acid, with the aid of heat if necessary, and the resulting solution cooled in an ice salt bath to below 5 ºC. The solution was diazotized with a solution of sodium nitrite (0.006mol) in 3mL water, with the temperature maintained below 5 ºC. Then, the solution was stirred for a further 30 min in the ice bath. A piperazine solution was prepared by mixing the 1-aryl-piperazine (0.005 mol) with 10 mL water; if necessary, a few drops of a dilute hydrochloric acid solution was added to get the arylpiperazine to dissolve. Then, the piperazine solution was added slowly to the diazonium salt solution. After stirring for an additional 30 min, the mixture was neutralized with a saturated sodium bicarbonate solution and then left to stir until precipitation was deemed to be complete (~1 hour). The solid product was filtered under suction, dried, and recrystallized from an appropriate solvent. Physical data (i.e. yield, m.p., recrystallization solvent) and spectroscopic analysis data (i.e. FT-IR, NMR, and high resolution MS data) were collected.
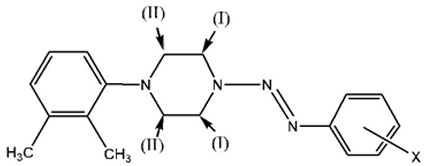
Synthesis of Series of N-(2,3-dimethylphenyl)-N’-(aryldiazenyl)-piperazines (3)
The compounds of series 3a-f were prepared following the general procedure described above. However, during the experiments, there was a problem which may have affected the results. The diazo coupling reaction took place in an aqueous solution. One of the major starting materials, 1-(2,3-Xylyl)-piperazine monohydrochloride was not completely dissolved in water at room temperature even when extra hydrochloric acid was added. It could be dissolved in water with heat, but the reaction system had to be cooled all the time as described in the general procedure. Thus, the low solubility of the 1-(2,3-Xylyl)-piperazine may have limited the completion of the reaction. Nevertheless, the 1-(2,3-Xylyl)-piperazine monohydrochloride in water may have an equilibrium between the dissolved sample and the undissolved sample, when the dissolved 1-(2,3-Xylyl)-piperazine has been used in the reaction, then more of the undissolved piperazine will be dissolved in water. Nevertheless, the longer reaction time may have served to overcome the effect of this factor, as evident in the high yields of compounds of series 3 reported in Table 1.
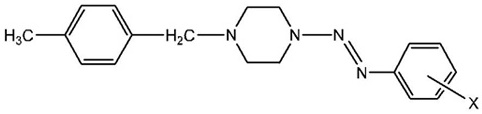
Synthesis of the Series of 1-(4-methylbenzyl)-4-(aryldiazenyl)-piperazines (4)
Following the general procedure described above, the experiments were performed in half scale due to the limit of the amount of starting materials. The same substituents of the aromatic amine were applied; the physical and IR data of the final products for this series are given in Table 2.
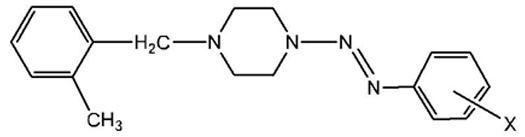
Synthesis of the Series of 1-(2-methylbenzyl)-4-(aryldiazenyl)-piperazines (5)
The procedure follows the general procedure in the first several steps, until the mixture was neutralized with saturated sodium bicarbonate solution. After the neutralization, a sticky precipitate formed, which was isolated by filtration. The mother-liquor was stored in a fridge. In some cases, a further batch of solid product was isolated from the mother liquor after several days; the second batch was combined with the first batch for the purification. The oily sticky solid was dissolved in dichloromethane and transferred into an Erlenmeyer flask which then was placed in a fume hood for overnight or longer to allow the solvent to evaporate completely. The resulting sticky solid was dissolved in a minimum volume of the appropriate hot solvent (e.g. Ethanol) and then cooled slowly. The crystalline solid was precipitated, then filtered under suction and air dried. Physical data (i.e. yield, m.p., recrystallization solvent) and IR spectroscopic data are given in Table 3.
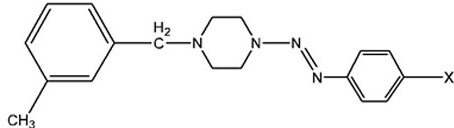
Synthesis of Series of 1-(3-methylbenzyl)-4-(aryldiazenyl)-piperazines (6)
Following the general procedure described above for series 3, the synthesis of the compounds of series 6 were performed in half scale due to the limit of the amount of starting materials. The same substituents of the aromatic amine were applied; the physical and IR data of the final products for this series are given in Table 4.
Synthesis of Methyl 4-[2Hunter, N.; Vaughan, K. Triazene derivatives of [1,x]-diazacycloalkanes. Part IX. Synthesis and characterization of a series of 1,4-di[2-aryl-1-diazenyl]-2,6-dimethylpiperazines Can. J. Chem, 2010, 88, 344-351.
[http://dx.doi.org/10.1139/V10-008] -1Little, V.R.; Tingley, R.; Vaughan, K. Triazene derivatives of [1,x]-diaza-cycloalkanes. Part III. Synthesis and characterization of a series of 1,4-di[2-aryl-1-diazenyl]piperazines Can. J. Chem, 2005, 83, 471-476.
[http://dx.doi.org/10.1139/v05-064] ]benzoate (7)
The synthetic procedure follows the general procedure described above. Due to the limited amount of the 1-(2-phenylethyl)-piperazine available, only one substituted aromatic amine (p-CO2CH3) was applied to the synthesis. The physical data is shown in Table 5.
RESULTS AND DISCUSSION
Synthesis
The N-aryl-N’-(2-aryl-1-diazenyl-)piperazines (3 to 7) were synthesized by diazotization of an aromatic primary amine (ArNH2) in hydrochloric acid, followed by coupling of the diazonium ion with the appropriate N-arylpiperazine or N-arylalkylpiperazines. Most of the crude products were obtained in good yield (57-100%) with the exception of compound 5c. The compounds were purified by recrystallization in high recovery.
Infrared Spectral Analysis
The significant diagnostic results of the Infrared spectra of the new compounds are shown in Table 1 to Table 5. All of the compounds show out-of-plane bending vibration modes of the substituted benzene rings. The para-disubstituted benzene ring, which is present in most of the compounds, except 3f, 4f, and 5e, show out-of-plane bending vibration modes in the range of 825-859 cm-1. Compounds 3f, 4f, and 5e involve a monosubstituted benzene ring, which show two out-of-plane bending vibration modes in the ranges of 693-697 cm-1 and 761-765 cm-1. The N-aryl group, which is attached on the piperazine ring in 3a-f, is a 1,2,3-trisubstituted benzene ring system. Predictably this ring system shows two out-of-plane bending vibration modes in the ranges of 719-724 cm-1 and 780-788 cm-1. The aryl group, which is linked to the piperazine ring in 4a-f, is a para-disubstituted benzene ring, which shows one out-of-plane bending vibration band in the range of 784-801 cm-1. The aryl group, which is connected to the piperazine ring in 5a-e, is an ortho-substituted benzene ring. Compounds 5a-e show one ortho-substituted out-of-plane bending vibration band in the range of 743-746 cm-1. The monosubstituted benzene ring, which joined with the piperazine ring in 7a, shows two out-of-plane bending vibration bands at 699 and 744 cm-1. The carbonyl group of the ester group, which is present in compounds 3b, 4b, 5b, 6b and 7a, shows a stretching vibration band in the range of 1707-1722 cm-1. In the same compounds, carbon oxygen single bond stretching bands of the ester groups were in the range of 1274-1278 cm-1. Nitrile group stretching vibration bands were observed in 3a, 4a, 5a and 6a in the range of 2218-2225 cm-1. Nitro groups, which were contained in 3e, 4e, 5d and 6e, show symmetric stretching vibration modes in the range of 1341-1379 cm-1 and asymmetric stretching vibration modes in the range of 1507-1509 cm-1.
1H NMR Spectroscopic Analysis
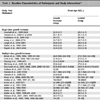
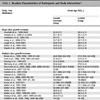
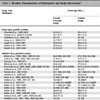
The 1H NMR results are shown in Table 6 to Table 10. These results are expressed as chemical shift in ppm relative to TMS(1%) at room temperature in CDCl3. All of the compounds contained two benzene rings; the aromatic signals appeared in the range of δ 8.23-6.90 with a coupling constant (J) in the range of 7.0-9.0 Hz. One of the two benzene rings was initially from aniline derivatives, which are most of the para-substituted anilines except 3f, 4f, and 5e; such that they show two doublet peaks characteristic of an AA’BB’ system in the range of aromatic protons. The highest chemical shift peaks are always due to the two equivalent aromatic protons which are close to the triazene subunit. Some aromatic protons were not resolved due to overlapping peaks in the range of δ 7.20-6.90. The starting material, the aryl piperazine, was studied by NMR spectroscopy as a model compound to distinguish the protons in the two aryl groups, and the protons on the aryl group were matched with the same proton in the final triazene product in an approximate range.
 |
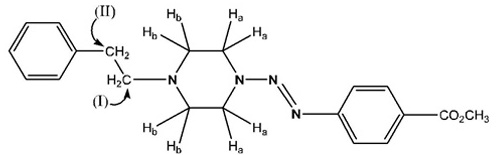
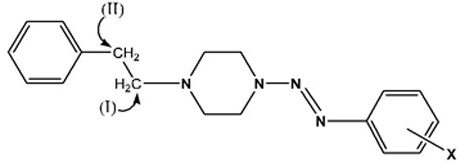
The most significant peaks are the methylene protons on the piperazine ring protons Ha and Hb (see schematic [iii] above). As shown in tables 6 to 10, Ha has a higher chemical shift than that of Hb. due to the closer proximity of Ha to the triazene subunit. In general, the methylene protons are represented by two triplet peaks each integrating for 4H. However, some of the triplet peaks were not resolved resulting in broad peaks, especially for Ha. Such observation was due to the restricted rotation of the nitrogen-nitrogen single bond in the triazene subunit [6Barra, M.; Srivastava, S.; Brockman, E. Substituent and solvent effects on the N2-N3 hindered rotation of cis-1,3-diphenyltriazenes J. Phys. Org. Chem, 2004, 17, 1057-1060.
[http://dx.doi.org/10.1002/poc.824] ]. The Ha protons have representative peaks in the range of δ 4.07-3.75 with integration for 4H and coupling constants in the range of 4.8-5.3 Hz for the resolving triplet peaks. The Hb protons have representative peaks in the range of δ 3.07-2.55 with coupling constants in the range of 5.1-6.3 Hz for the resolving triplet peaks. Signals arising from the substituents on the benzene ring are consistent with the substituents present, such as the O-methyl protons arising as singlets in the range δ 3.08 -3.92 in the spectra of 3b, 4b, 5b, 6b and 7a. The ethylene bridge linking the piperazine and aryl rings in compound 7a is manifested by two 2H triplet peaks at δ 2.68 ppm and δ 2.83 ppm with a coupling constant J = 8.0 Hz. The full analysis of the 1H spectrum of 7a was complicated by the coincidence of the signals of Ha and the O-methyl protons at 3.89 ppm and the (considerable overlap of the triplets of CH2 (II) and proton Hb.
13C NMR
The structures of all new compounds have been investigated by 13C NMR spectroscopy (see Tables 11 to 15). For compounds 3a-f, there are ten magnetically non-equivalent aromatic carbon atoms in each molecule. The aromatic carbons resonate in the range of δ 116-155 ppm. The mono- or para-substituted benzene ring has four magnetically non-equivalent carbon atoms, since two carbon atoms on the ortho position are magnetically equivalent and the same goes for the two carbon atoms on the meta position. The 1,2,3-trisubstituted benzene ring has six carbon atoms that are all magnetically non-equivalent. The carbon atoms in the piperazine ring were often difficult to resolve due to the dynamic equilibrium in the triazene moiety. The carbon atoms in the piperazine ring are represented by the two peaks (both are usually broad peaks, if observed) in the range of δ 52.4-43.0 ppm. The two methyl groups on the 1,2,3-trisubstituted benzene ring were represented by the two peaks in the ranges of δ 20.6-20.3 ppm and δ 13.9-13.5 ppm. The nitrile group carbons resonate at δ 108.6 ppm. The carbonyl carbon in the ester subunit was represented by a peak at δ 167.0 ppm; the methyl group in the ester group was represented by a peak at δ 51.9 ppm. In 3d, the p-tolyl methyl group resonates at δ 21.0 ppm.
 |
For the compounds 4a-f, there are two benzene rings, one mono-substituted and one para-disubstituted. Therefore, there are eight magnetically non-equivalent aromatic carbons in each compound, which are represented by eight peaks in the range of δ 155.6-119.0 ppm. For the piperazine carbons and the substituents on the aniline derivatives, chemical shifts similar to those seen previously in compounds 3a-f were observed. The two carbons linked to the aryl group, in which the aryl group is attached to the piperazine ring, is represented by two peaks in the ranges of δ 62.6-62.1 ppm and δ 21.1-20.7 ppm. The carbons of the methylene groups between the benzene ring and the piperazine ring resonate in higher frequency (δ 62.6-62.1 ppm).
For the compounds 5a-e, there are ten magnetically non-equivalent aromatic carbons for each compound, which include six carbons from the ortho substituted benzene ring and four carbons from the para- or mono-substituted benzene ring. The ten aromatic carbons resonated in the range of δ 155.6-118.7 ppm. All other carbons located in the piperazine ring and the substituents have similar chemical shifts as described previously for compounds 3a-f.
For the compounds 6a-e, there are ten magnetically non-equivalent aromatic carbons for each compound, which include six carbons from the meta substituted benzene ring and four carbons from the para- or mono-substituted benzene ring. The ten aromatic carbons resonated in the range of δ 155.6-118.7 ppm. All other carbons located in the piperazine ring and the substituents have similar chemical shifts as described previously for compounds 3a-f.
For the compound 7a, eight magnetically non-equivalent aromatic carbons were involved in each compound, due to the fact that the two benzene rings were para- and mono-substituted rings. The two methylene groups between the benzene ring and the piperazine ring were represented by two peaks at δ 59.6 and δ 51.4 ppm. The higher chemical shift accounts for the methylene group linked to the piperazine ring. All the other carbons in the molecule resonated in the reasonable range as described previously for compounds 3a-f.
MASS SPECTRAL ANALYSIS
The high resolution mass spectrometry (MS) results are shown for all new compounds in Tables 16 to 20. Mass spectroscopy (MS) is an important tool in synthetic organic chemistry, especially high resolution MS. Although MS can be used to predict the conformation of the molecule by matching the peaks to the small fragments of the molecule, the MS results can not be used because it is not capable of distinguishing the isomers in this project. The main use of the MS is to provide the evidence of the formation of the target molecule by matching the actual molecular weight with the experimental molecular ion mass. In the high resolution MS, the results were obtained with the standard deviation of +/-0.0008 amu. According to the MS results, all the molecular ions have been found in the spectra and matched with the actual mass of the molecule within the standard deviation.
CONCLUSION
The series of isomers of N-(2,3-dimethylphenyl)-N’-(aryldiazenyl)-piperazines were prepared in this study. The physical data of all the compounds were measured. All the compounds were extensively characterized through infrared, NMR, and mass spectral analysis. It is always interesting to speculate on the potential applications of new compounds like those reported in this paper. The new 1-aryl-4-[aryldiazenyl]-piperazines (2d) have the potential to undergo thermal cleavage under appropriate conditions to provide a route to unsymmetrically substituted N-aryl-N’-arylpiperazines (8). In a previous report [7Yanarates, E.; Disli, A.; Yildirir, Y.; New, N. N’-bis-(substitutedphenylazo)-piperazines and their cleavage reactions in acetic acid Org. Prep. Proced. Int, 1999, 31, 429-433.
[http://dx.doi.org/10.1080/00304949909355733] ], it was shown that the synmmetrical 1,4-bis-(2-aryldiazen-1-yl-)piperazines (1) undergo thermal cleavage in acetic acid to afford symmetrical N,N’-diarylpiperazines. The analogous reaction of 2d would afford the unsymmetricaly substituted piperazines (8) thus:
Further work in this laboratory will be undertaken to try to make this idea a reality.
Aryldiazenylpiperazines have also been utilized as a means to the immobilization of a diazonium ion by covalent linkage to piperazine attached to a (Merrifield-)resin. The resin bound 1-aryldiazenyl)piperazine was used as a substrate for a Wallach reaction with hydrogen [18F]fluoride to produce radio-labelled 2-fluorophenyl phenyl ether [8Riss, P.J.; Kuschel, S. Aigbirhio. No carrier added nucleophilic aromatic radiofluorination using solid phase supported arene diazonium sulfonates and 10(aryldiazenyl-)piperazines Tet. Letts, 2012, 53(14), 1717-1719.
[http://dx.doi.org/10.1016/j.tetlet.2012.01.082] ].
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The authors are grateful to the Natural Sciences and Engineering Research Council of Canada (NSERC) for a Discovery Grant to the principal author (KV). We are also grateful to the Faculty of Graduate Studies and Research at Saint Mary’s University for a Summer Research Award to Chenguang Fan. We are also grateful to the Atlantic Region Magnetic Resonance Centre at Dalhousie University for providing NMR spectra, and to Dalhousie University for providing mass spectral data. In particular, we would like to thank Dr. Mike Lumsden for assistance with the NMR spectral data, and Mr. Xiao Feng for assistance with mass spectra.
REFERENCES
| [1] | Little, V.R.; Tingley, R.; Vaughan, K. Triazene derivatives of [1,x]-diaza-cycloalkanes. Part III. Synthesis and characterization of a series of 1,4-di[2-aryl-1-diazenyl]piperazines Can. J. Chem, 2005, 83, 471-476. [http://dx.doi.org/10.1139/v05-064] |
| [2] | Hunter, N.; Vaughan, K. Triazene derivatives of [1,x]-diazacycloalkanes. Part IX. Synthesis and characterization of a series of 1,4-di[2-aryl-1-diazenyl]-2,6-dimethylpiperazines Can. J. Chem, 2010, 88, 344-351. [http://dx.doi.org/10.1139/V10-008] |
| [3] | Little, V.R.; Vaughan, K. Synthesis and Characterization of a series of 1-methyl-4-[2-aryl-1-diazenyl]piperazines and a series of ethyl 4-[2-aryl-1-diazenyl]-1-piperazinecarboxylates Can. J. Chem, 2004, 82, 1294-1303. [http://dx.doi.org/10.1139/v04-081] |
| [4] | MacLeod, E.; Vaughan, K. Synthesis and characterization of new compounds in the series 1-alkyl-4-[2-aryl-1-diazenyl]piperazines Open Organ. Chem. J, 2015, 9, 1-8. [http://dx.doi.org/10.2174/1874095201509010001] |
| [5] | Little, V.R.; Vaughan, K. Synthesis and Characterization of Several Series of 4-Acyl-1-[2-aryl-1-diazenyl]piperazines Can. J. Chem, 2014, 92, 838-848. [http://dx.doi.org/10.1139/cjc-2014-0242] |
| [6] | Barra, M.; Srivastava, S.; Brockman, E. Substituent and solvent effects on the N2-N3 hindered rotation of cis-1,3-diphenyltriazenes J. Phys. Org. Chem, 2004, 17, 1057-1060. [http://dx.doi.org/10.1002/poc.824] |
| [7] | Yanarates, E.; Disli, A.; Yildirir, Y.; New, N. N’-bis-(substitutedphenylazo)-piperazines and their cleavage reactions in acetic acid Org. Prep. Proced. Int, 1999, 31, 429-433. [http://dx.doi.org/10.1080/00304949909355733] |
| [8] | Riss, P.J.; Kuschel, S. Aigbirhio. No carrier added nucleophilic aromatic radiofluorination using solid phase supported arene diazonium sulfonates and 10(aryldiazenyl-)piperazines Tet. Letts, 2012, 53(14), 1717-1719. [http://dx.doi.org/10.1016/j.tetlet.2012.01.082] |