- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Biogenic Synthesis of Silver Nanoparticle from Mushroom Exopolysaccharides and its Potentials in Water Purification
Adeyemi Ojutalayo Adeeyo*, John Ogony Odiyo
Abstract
Objective:
This study reports a novel eco-friendly biosynthesis of Silver Nanoparticles (AgNPs) from Exopolysaccharides (EPS) of Lentinus edodes after an attempt to optimise the production of EPS through mutagenesis. It further describes some potential application of silver nanoparticles in water treatment.
Methods:
A wild strain of L. edodes was subjected to UV irradiation, a physical mutagen, at 254 nm. The wild and resultant irradiated strains were then assessed for the production of EPS and subsequent application of the crude EPSs for biosynthesis of AgNPs. The particles were characterised by colour pattern and UV-visible spectroscopy. Based on superior EPS production and nanoparticle attributes, nanoparticles obtained from UV irradiated process were further subjected to Scanning Electron Microscopy (SEM). EPS produced was quantified by the phenol-sulphuric acid method and studied by GC-MS.
Results:
Results obtained for EPS productivity indicated the presence of monomer sugars such as arabinose (50.65%), mannose (19.20%), mannitol (15.58%), fructose (7.96%), trehalose (6.49%), and glucuronic acid, xylose, galactose and glucose with low percentages of ≤ 0.11. EPS productivity of wild and mutant strains was obtained as 1.044 and 2.783 mg/ml, respectively, after 7 days of fermentation. The result of EPS production for UV irradiated strain corresponds to a yield improvement of 2.7 fold of the wild-type. UV Spectroscopy and SEM analysis studies on EPS nanoparticle product of the improved (UV irradiated) strain indicated the formation of AgNPs at the absorption band of 421 nm with a size range of 50-100 nm.
Conclusion:
This study, which aimed at eco-friendly synthesis of myco-nanoparticle has established the novel ability of L. edodes’ polysaccharide in silver nanoparticles biosynthesis. It expounded potential frontiers of silver nanoparticles application in the water industry. To the best of the authors’ knowledge, this result represents the first report on the biosynthesis of AgNPs using L. edode’s EPS.
Article Information
Identifiers and Pagination:
Year: 2018Volume: 5
First Page: 64
Last Page: 75
Publisher Id: CHEM-5-64
DOI: 10.2174/1874842201805010064
Article History:
Received Date: 25/4/2018Revision Received Date: 12/6/2018
Acceptance Date: 23/7/2018
Electronic publication date: 28/09/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the School of Environmental Sciences, University of Venda, Private Bag X5050, Thohoyandou 0950, South Africa; Tel: 0723030995; E-mail: firstrebby@gmail.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 25-4-2018 |
Original Manuscript | Biogenic Synthesis of Silver Nanoparticle from Mushroom Exopolysaccharides and its Potentials in Water Purification | |
1. INTRODUCTION
Nanotechnology is the study, construction and utilisation of materials in the physical range of 1-100 nm [1Yürüm, A.; Kocabas-Ataklı, Z.Ö.; Sezen, M.; Semiat, R.; Yürüm, Y. Fast deposition of porous iron oxide on activated carbon by microwave heating and arsenic (V) removal from water. Chem. Eng. J., 2014, 242, 321-332.
[http://dx.doi.org/10.1016/j.cej.2014.01.005] ]. Nanoparticles are regarded as the basic building blocks of nanotechnology and the development of a biological process for the synthesis of nanoparticles is developing into an important branch of nanotechnology [2Amkamwar, B.; Damle, C.; Ahmad, A.; Sastry, M. Biosynthesis of gold and silver nanoparticles using Emblicaofficinalis fruit extract, their phase transfer and transmetallation in an organic solution. J. Nanosci. Nanotechnol., 2005, 5, 1666-1671., 3Cataleya, C. In vitro toxicity assessment of silver nanoparticles in rat alveolar macrophages., Master thesis, Wright state University, USA 2006 pp 1-75.]. In the recent decades, nanoparticles have met a great expansion and serious investigations due to their potentials in a wide spectrum of industrial applications [4Grimsdale, A.C.; Chan, K.L.; Martin, R.E.; Jokisz, P.G.; Holmes, A.B. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev., 2009, 109(3), 897-1091.
[http://dx.doi.org/10.1021/cr000013v] [PMID: 19228015] , 5Geckeler, K.E.; Nishide, H. Advanced nanomaterials., 2010, , 249-295.]. Biologically synthesized nanoparticles have been implicated in the production of antibacterial and antiviral materials, catalysts in biological labeling, biosensors, chemical reactions, detection of genetic disorders, drug delivery, electrical batteries, gene therapy and DNA sequencing, optical receptors [6Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog., 2006, 22(2), 577-583.
[http://dx.doi.org/10.1021/bp0501423] [PMID: 16599579] ].
In order to meet the challenge of water purification, several techniques like adsorption, biosorption, electrochemical treatments, evaporation, flotation, ion exchange, membrane filtration, oxidation, precipitation and reverse osmosis processes are extensively used [7Ivanov, V.; Tay, J.H.; Tay, S.T.L.; Jiang, H.L. Removal of micro-particles by microbial granules used for aerobic wastewater treatment. Water Sci. Technol., 2004, 50(12), 147-154.
[http://dx.doi.org/10.2166/wst.2004.0707] [PMID: 15686015] -9Chen, Y.; Pan, B.; Li, H.; Zhang, W.; Lv, L.; Wu, J. Selective removal of Cu(II) ions by using cation-exchange resin-supported polyethyleneimine (PEI) nanoclusters. Environ. Sci. Technol., 2010, 44(9), 3508-3513.
[http://dx.doi.org/10.1021/es100341x] [PMID: 20373792] ]. Such previous applications have been restricted due to various shortcomings [10Ali, I. New generation adsorbents for water treatment. Chem. Rev., 2012, 112(10), 5073-5091.
[http://dx.doi.org/10.1021/cr300133d] [PMID: 22731247] , 11Ali, ME; Ullah, M; Hamid, SBA Conventional to nano-green adsorbents for water pollution management: A review. Adv Mater Res 2014, 925, 674-678.
[http://dx.doi.org/10.4028/www.scientific.net/AMR.925.674] ]. Research is evolving to use high-tech nanotechnology in water treatment for safe drinking. Nanoparticles are expected to be of vital importance in water treatment technology of the future [12Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir, 2002, 18, 6679-6686.
[http://dx.doi.org/10.1021/la0202374] ] for which research is still at infancy. Developments in nanoscale science and engineering suggest that many of the existing water quality challenges could be resolved or greatly diminished by using bioactive nanoparticles, nanoadsorbents, nanocatalysts, nanostructured catalytic membranes, nanotubes, magnetic nanoparticles and submicron nanopowder. Nanotechnology and its science have been used for detection of algae (e.g. cyanobacterial toxins), antibiotics and biological agents, (like bacteria, parasites and viruses), biological and chemical substances including metals (e.g. cadmium, copper, lead, mercury, nickel, zinc), cyanide organics, nutrients (e.g. phosphate, ammonia, nitrate, nitrite), as well as pesticides [13Nair, A.S.; Pradeep, T. Reactivity of Au and Ag nanoparticles with halocarbons. Appl. Nanosci., 2004, 59-63.]. The large surface area of nanoparticles, catalytic potential and high reactivity, makes them better adsorbing materials than conventional treatment technologies [10Ali, I. New generation adsorbents for water treatment. Chem. Rev., 2012, 112(10), 5073-5091.
[http://dx.doi.org/10.1021/cr300133d] [PMID: 22731247] ]. Some advantages and disadvantages of previous water treatment methods are provided in Ali et al. [11Ali, ME; Ullah, M; Hamid, SBA Conventional to nano-green adsorbents for water pollution management: A review. Adv Mater Res 2014, 925, 674-678.
[http://dx.doi.org/10.4028/www.scientific.net/AMR.925.674] ]
Nanoparticles have been conventionally produced by chemical and physical methods [14Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gramnegative bacteria. Nanomed: Nanotechnol. Biol. Med. (Aligarh), 2012, 8, 37-45.
[PMID: 21703988] ] which involve techniques like heating [15Huang, H.; Yang, X. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: a green method. Carbohydr. Res., 2004, 339(15), 2627-2631.
[http://dx.doi.org/10.1016/j.carres.2004.08.005] [PMID: 15476726] ] and irradiation [16Abid, J.P.; Wark, A.W.; Brevet, P.F.; Girault, H.H. Preparation of silver nanoparticles in solution from a silver salt by laser irradiation. Chem. Commun. (Camb.), 2002, 7(7), 792-793.
[http://dx.doi.org/10.1039/b200272h] [PMID: 12119726] , 17Gasaymeh, S.S.; Radiman, S.; Lee, Y.H.; Saion, E.; Saeed, G.H. Synthesis and characterization of silver/polyvinilpirrolidone (Ag/PVP) nanoparticles using gamma irradiation techniques. Am. J. Appl. Sci., 2010, 7, 892-901.
[http://dx.doi.org/10.3844/ajassp.2010.892.901] ]. However, such practices are costly, toxic and hazardous [18Mohamed, A.G.T. Stachybotrys chartarum: a novel biological agent for the extracellular synthesis of silver nanoparticles and their antimicrobial activity. Indones. J. Biotechnol., 2013, 18, 75-82.
[http://dx.doi.org/10.22146/ijbiotech.7871] ] though such methods have been used to achieve various desired results including size modification [19Zielińsk, A.; Skwarek, E.; Zaleska, A.; Hupka, M.G. Preparation of silver nanoparticles with controlled particle size. Procedia Chem., 2009, 1, 1560-1566.
[http://dx.doi.org/10.1016/j.proche.2009.11.004] ]. Hence the need for alternative, eco-friendly approaches, based on biological methods. There are documented reports that the techniques of nanoparticles synthesis through biological means make such products more biocompatible and environmentally friendly. Applying biological methods for the production of nanomaterials have received extensive attention because the techniques are not expensive and are eco-friendly; also, biosynthesis of nanoparticles can be carried out in one step [20Otari, S.; Patil, R.; Nadaf, N.; Ghosh, S.; Pawar, S. Green biosynthesis of silver nanoparticles from an actinobacteria Rhodococcus sp. Mater. Lett., 2012, 72, 92-94.
[http://dx.doi.org/10.1016/j.matlet.2011.12.109] ]. Green synthesis methods utilise miscellaneous biological natural substances such as marine algae, microfluidics, microorganisms like bacteria, fungi, yeasts and diatoms, plant tissues and fruits, plant extracts and whole plants for the reduction and stabilisation of nanoparticles. Synthesis of nanomaterials using extracts of plants and related materials has numerous advantages over other environmentally green synthesis approaches, because plants are easily available, largely distributed, less expensive, readily scalable and safe to handle [21Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv., 2013, 31(2), 346-356.
[http://dx.doi.org/10.1016/j.biotechadv.2013.01.003] [PMID: 23318667] ]. Among various nanoparticles, silver nanoparticles, due to their properties such as potent antimicrobial activity, catalysis and electrochemical conductivity can be used in different applications like agriculture, biomedicine, food chemistry and photo chemicals [22Allafchian, A.R.; Bahramian, H.; Jalali, S.A.H.; Ahmadvand, H. Synthesis, characterization and antibacterial effect of new magnetically core–shell nanocomposites. J. Magn. Magn. Mater., 2015, 394, 318-324. a
[http://dx.doi.org/10.1016/j.jmmm.2015.06.086] , 23Allafchian, A.R.; Jalali, S.A.H. Synthesis, characterization and antibacterial effect of poly (acrylonitrile/maleic acid)–silver nanocomposite. J. Taiwan Inst. Chem. Eng., 2015, 57, 154-159. b
[http://dx.doi.org/10.1016/j.jtice.2015.05.015] ].
Some comparative studies of chemically and biologically synthesised nanoparticles are presented in Table 1 (antibacterial characteristics) and Table 2 (particle size and distribution characteristics). The presented results revealed that biological methods for nanoparticle synthesis, in addition to being eco-friendly, are comparatively similar and sometimes better in performance to chemically synthesised nanomaterials.
Fungi like mushroom are choice biomaterial for nanoparticle synthesis as they are easy to handle, culture and possesses high wall-binding capacity as well as intracellular metal uptake capabilities [26Sanghi, R.; Verma, P. Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour. Technol., 2009, 100(1), 501-504.
[http://dx.doi.org/10.1016/j.biortech.2008.05.048] [PMID: 18625550] ]. These characteristics may therefore enhance processes and performances of myconanoparticles. Various fungi and fungal extracts have been used for biosynthesis of AgNPs [27Gericke, M.; Pinches, A. Biological synthesis of metal nanoparticles. Hydrometallurgy, 2006, 83, 132-140.
[http://dx.doi.org/10.1016/j.hydromet.2006.03.019] , 28Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: technological concepts and future applications. J. Nanopart. Res., 2008, 10, 507-517.
[http://dx.doi.org/10.1007/s11051-007-9275-x] ] which are currently being explored for safe water purification. The interface of mycology and nanotechnology is termed myconanotechnology [29Mukherjee, P.; Senapati, S.; Mandal, D.; Ahmad, A.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporum. ChemBioChem, 2002, 3(5), 461-463.
[http://dx.doi.org/10.1002/1439-7633(20020503)3:5<461::AID-CBIC461>3.0.CO;2-X] [PMID: 12007181] -31Rai, M.; Yadav, A.; Bridge, P.; Gade, A. Myconanotechnology: A new and emerging science. Appl Mycology, 2009, 14, 258-267.].
This paper reports the mutagenesis of L. edodes for EPS yield enhancement and the application of the produced EPS in biological synthesis of silver nanoparticles (AgNPs) as eco-friendly alternative in bionanotechnology. It highlights some application area of nanomaterials in emerging water quality treatment technologies. The EPS produced was characterised by GC-MS while AgNPs was typified by visual colour pattern, UV-Visible spectroscopy, and Scanning electron microscopy.
2. MATERIAL AND METHODS
2.1. Collection of L. Edodes Strain
A pre-typified wild L. edodes was obtained from Mushroom Research Centre, Himachal Pradesh, India. This was reproduced by tissue culturing and maintained by sub-culturing monthly and stored at 4°C on Potato Dextrose Agar (PDA) slants to maintain viability.
2.2. Induction of Physical Mutation
The mutagenesis process was carried out by irradiating 14-day actively growing culture of the wild fungus on PDA plate (90 mm) with ultraviolet irradiation (254 nm, 15 cm) between the ranges of 5 to 90 mins. Mutants were obtained at 5, 10, 15, 20, 25, 30, 45, 60, 75 and 90 mins and were instantly sub-cultured and incubated in the dark at 26 ± 2°C for 2 weeks. Based on improved (EPS) productivity (assessed using the method of Dubois et al. [32Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. Colorimetric method for determination of sugar and related substances. Anal. Chem., 1956, 28, 350-356.
[http://dx.doi.org/10.1021/ac60111a017] ]), a resultant viable mutant obtained at 10 mins of UV irradiation (UV10) was carefully chosen. This culture was incubated at 25 ± 2°C for 70 hours and subsequently re-introduced onto the newly prepared PDA slant, incubated for 2 weeks to obtain fully ramified UV10 strain. The wild and UV10 strains were re-evaluated for quality enhancement and used for the production of EPS using combined methods of Adebayo et al. [33Adebayo, A.E.; Oloke, J.K.; Achana, Y.; Bora, T. Improvement of Laccase production in Pleurotus pulmonarius LAU 09 by mutation. J. Microbiol., 2012, 2, 11-17.
[http://dx.doi.org/10.5923/j.microbiology.20120201.03] ] and Majolagbe et al. [34Majolagbe, ON; Oloke, JK; Adebayo, EA; Adewoyin, AG; Ayandele, A; Bamigboye, C Study on the antimicrobial activity of exopolysaccharides of Lentinus subnudus using swiss albino rats as animal model. Am Eurasian J of Sci Res, 2013, 8, 47-52.].
2.3. EPS Production, Extraction and Quantification
Exactly 14 day old UV10 strain was used to inoculate 50 ml of production media in 250 ml conical flask consisting in g/L of glucose (10.0), KH2PO4 (0.5), MgSO4 (0.5), peptone (2.0) and yeast extract (1.0). Other fermentation conditions include agitation (100 rpm), temperature (27 ± 2oC), and incubation time of 7 days. Crude EPS was obtained as cell free extract after filtration and centrifugation. Extraction of EPS was carried out by mixing cell free supernatant from the respective production medium with 2 volumes of cold absolute ethanol (v/v) and held at 4oC overnight for EPS precipitation. EPS precipitate was collected as crude fraction after centrifugation at 4000 rpm for 15 min (AG 5702, Eppendof, Germany) using the protocol of Majolagbe et al. [34Majolagbe, ON; Oloke, JK; Adebayo, EA; Adewoyin, AG; Ayandele, A; Bamigboye, C Study on the antimicrobial activity of exopolysaccharides of Lentinus subnudus using swiss albino rats as animal model. Am Eurasian J of Sci Res, 2013, 8, 47-52.] and re-suspended in 20 ml of sterilised distilled water. The quantity of EPS produced per millilitre of distilled water was evaluated by UV spectrophotometry. Absorbance at 490 nm of digested EPS was obtained per extract using phenol-sulphuric acid method of Dubois et al. [32Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. Colorimetric method for determination of sugar and related substances. Anal. Chem., 1956, 28, 350-356.
[http://dx.doi.org/10.1021/ac60111a017] ] and calibrating to total sugar contents using glucose as standard. EPS sugar monomer compositions of the obtained polysaccharides were subsequently assessed using GC-MS (GC2010, Shimadzu, Japan) [35Varinder, K; Bera, MB; Panesar, PS; Chopra, HK Production and characterization of exopolysaccharide produced by Alcaligenes faecalis B14 from indigenous soil. Int. J. Biotechnol Bioengineering Res, 2013, 4, 365-374.].
2.4. Calibration Using Reducing Sugar Standard
Exactly 0.1 g of anhydrous glucose, as obtained from Sigma Aldrich (South Africa), was used for preparation of working standard solution and dispensed into Erlenmeyer flask, liquefied in 19 ml of distilled water, and then made up to 100 ml, making dilution factor of 10-2 (1 mg/ml). Then, 2, 3, and 5 ml of the stock solution was diluted to 100 ml to give working standards of 0.02, 0.03, and 0.05 mg per ml respectively. Subsequently, 1 ml was taken from the working solutions into separate 50 ml tube and 1 ml of 5% phenol was added, followed by the addition of 5 ml of concentrated H2SO4 added rapidly and then allowed to cool. The absorbances of admixtures were then read at 490 nm on a UV-visible spectrophotometer (Genesys 10UV scanner, Thermo electron corporation, UK), and used to plot a calibration curve for the determination of sugar content.
2.5. Determination of EPS Contents in Fermentation Broth
Specifically, about 1 ml of the re-dissolved EPS in distilled water (stock) was measured in a test tube and 1 ml of 5% phenol solution was thereafter added, followed by the rapid addition of 5 ml of concentrated sulphuric acid and then allowed to cool. The absorbance for each EPS product of all the UV mutants were therefore obtained at 490 nm and quantity determined using the calibration curve obtained.
2.6. EPS-mediated Synthesis and Characterization of Silver Nanoparticles (AgNPs)
Silver NPs were synthesised by reacting crude EPS of UV10 with 1 milli molar solution of silver nitrate. Exactly 1 ml of EPS solution of the Wild and UV10 was dispensed into 5 ml 1mM AgNO3 in a 15 ml capacity test tube. A control was set up in a third test tube which contained 6 ml of 1 mM AgNO3. The 3 tubes were strongly shaken for about 10 minutes and examined for colour development at room temperature (28 ± 2°C) during intermittent shaking. The formation of colour as a result of AgNPs development was monitored visually and the absorbance spectrum of the reaction solution was measured and recorded on a UV-visible spectrophotometer (Genesys 10 UV, Thermoelectron Corporation, UK). Morphology of the biosynthesised silver nanoparticles was elucidated by Scanning Electron Microscopy (SEM). SEM images were collected using an ASPEX 3020 at an accelerating voltage of 15 kV in bright field mode.
2.7. Comparing EPS and AgNPs UV-Visible Studies of Wild and UV10 Mutants
The exopolysaccharide productivity study was according to the method of Dubois et al. [32Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. Colorimetric method for determination of sugar and related substances. Anal. Chem., 1956, 28, 350-356.
[http://dx.doi.org/10.1021/ac60111a017] ] and Majolagbe et al. [34Majolagbe, ON; Oloke, JK; Adebayo, EA; Adewoyin, AG; Ayandele, A; Bamigboye, C Study on the antimicrobial activity of exopolysaccharides of Lentinus subnudus using swiss albino rats as animal model. Am Eurasian J of Sci Res, 2013, 8, 47-52.]. UV Visible and colour development pattern studies for AgNPs production were carried out to observe the surface plasmon resonance and typical silver nanoparticle colouration respectively. The results obtained were assessed for comparative efficiencies of the resultants EPSs and nanoparticle produced.
3. RESULTS AND DISCUSSION
3.1. EPS Yield and Sugar Component Elucidation
UV10 culture resulting from 10 minutes of exposure to ultraviolet irradiation gave EPS productivity of 2.783 mg/ml at 7 days of fermentation using the sugar calibration curve in Fig. (1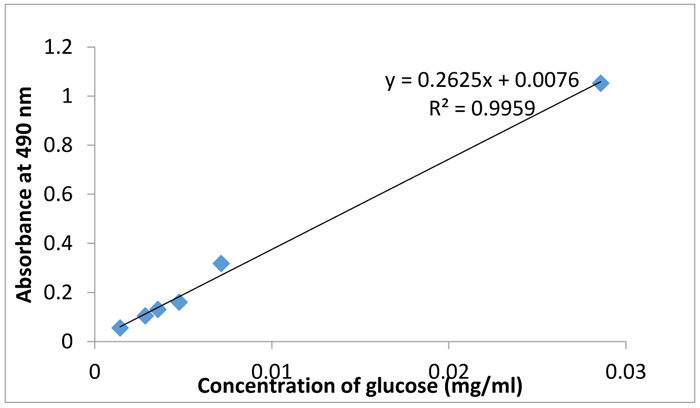 ). This was done by inserting the absorbance reading obtained from UV-vis spectroscopy of UV10 into the regression equation. The monosaccharide sugar composition of the produced EPS was identified as shown in Table 3. The results indicated the presence of monomer sugars that were characteristic of exopolysaccharides including arabinose (50.65%), mannose (19.20%), mannitol (15.58%), fructose (7.96%), trehalose (6.49%), and glucoronic acid, xylose, galactose and glucose with low percentages of ≤ 0.11. The results therefore indicate that the EPS produced is mainly made of arabinose, mannose and mannitol. It confirms the potentials of L. edodes for applications in production of polysaccharides with industrial usefulness. According to Sutherland [36Sutherland, I.W. Structure-function relationships in microbial exopolysaccharides. Biotechnol. Adv., 1994, 12(2), 393-448.
). This was done by inserting the absorbance reading obtained from UV-vis spectroscopy of UV10 into the regression equation. The monosaccharide sugar composition of the produced EPS was identified as shown in Table 3. The results indicated the presence of monomer sugars that were characteristic of exopolysaccharides including arabinose (50.65%), mannose (19.20%), mannitol (15.58%), fructose (7.96%), trehalose (6.49%), and glucoronic acid, xylose, galactose and glucose with low percentages of ≤ 0.11. The results therefore indicate that the EPS produced is mainly made of arabinose, mannose and mannitol. It confirms the potentials of L. edodes for applications in production of polysaccharides with industrial usefulness. According to Sutherland [36Sutherland, I.W. Structure-function relationships in microbial exopolysaccharides. Biotechnol. Adv., 1994, 12(2), 393-448.
[http://dx.doi.org/10.1016/0734-9750(94)90018-3] [PMID: 14545899] ], monosaccharide components most classically found in EPS are sugars such as amino sugars (D-Galactosamine and D-Glucosamine), hexoses (D-Mannose, D-Glucose, D-Galactose, D-Allose, L-Rhamnose, L-Fucose), pentoses (as D-arabinose, D-Ribose, and D-Xylose), or uronic acids (D-Galacturonic acids and D-Glucuronic acids). Thus, these results are mostly in accordance with those previously reported [36Sutherland, I.W. Structure-function relationships in microbial exopolysaccharides. Biotechnol. Adv., 1994, 12(2), 393-448.
[http://dx.doi.org/10.1016/0734-9750(94)90018-3] [PMID: 14545899] -38Carbonero, E.; Gracher, A.; Komura, D.; Marcon, R.; Freitas, C.; Baggio, C.; Santos, A.; Torri, G.; Gorin, P.; Locomini, M. Lentinus edodes heterogalactan: Antinociceptive and anti-inflammatory effects. Food Chem., 2008, 111, 531-537.
[http://dx.doi.org/10.1016/j.foodchem.2008.04.015] ].
 |
Fig. (1) Calibration curve for polysaccharide content determination. |
3.2. Colour Change
Aqueous silver nitrate ions were reduced during exposure to the EPS extract of UV10. The colour of the reaction mixture changed from colourless to purple with observed colour pattern getting stable after 24 hours as shown in Fig. (2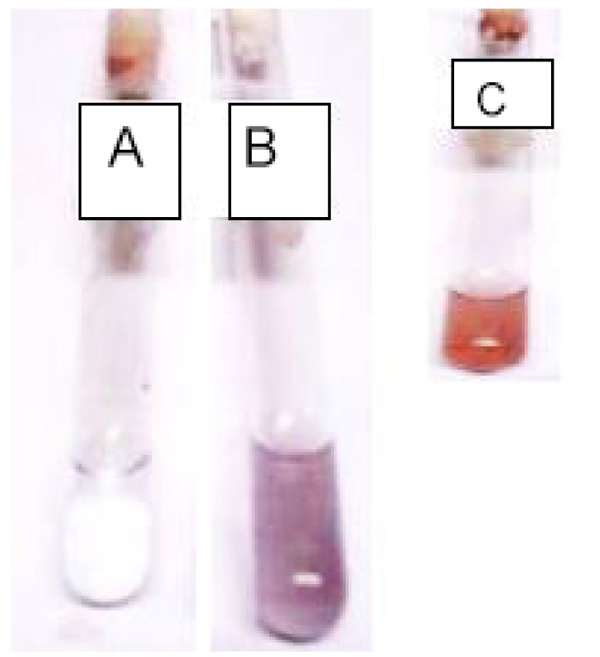 ) (Wild in-set). Purple and yellowish-brown colouration have been reported in relation with nanoparticle synthesis and due to excitation of surface plasmon vibration in metal nanoparticle [39Vanmathi, K.; Sivakumar, T. Isolation and characterization of silver nanoparticles from Fusarium oxysporum. Int. J. Curr. Microbiol. Appl. Sci., 2012, 56-62.]. Characteristic AgNPs colouration earlier reported include yellowish brown [40Paulraj, K.; Seung, T. Synthesis and structural characterization of silver nanoparticles using bacterial exopolysaccharide and its antimicrobial activity against food and multi-drug resistant pathogens. Process Biochem., 2013, 48, 1099-1106.
) (Wild in-set). Purple and yellowish-brown colouration have been reported in relation with nanoparticle synthesis and due to excitation of surface plasmon vibration in metal nanoparticle [39Vanmathi, K.; Sivakumar, T. Isolation and characterization of silver nanoparticles from Fusarium oxysporum. Int. J. Curr. Microbiol. Appl. Sci., 2012, 56-62.]. Characteristic AgNPs colouration earlier reported include yellowish brown [40Paulraj, K.; Seung, T. Synthesis and structural characterization of silver nanoparticles using bacterial exopolysaccharide and its antimicrobial activity against food and multi-drug resistant pathogens. Process Biochem., 2013, 48, 1099-1106.
[http://dx.doi.org/10.1016/j.procbio.2013.05.011] ], yellow and reddish brown [41Mirunalini, S.; Arulmozhi, V.; Deepalakshmi, K.; Krishnaveni, M. Intracellular biosynthesis and antibacterial activity of silver nanoparticles using edible mushrooms. Not. Sci. Biol., 2012, 4, 55-61.
[http://dx.doi.org/10.15835/nsb448051] ], light-gray [42Chan, Y.; Mat-Don, M. Instantaneous biosynthesis of silver nanoparticles by selected macrofungi. Aust. J. Basic Appl. Sci., 2012, 6, 222-226.], and dark brown [43Karthika, R; Sevarkodiyone, SP Synthesis and characterization of silver nanoparticles using aqueous extract of goat faecal pellets. Int J Current Sci Res, 2015, 1, 2454-5422.]. Observed variation in colour pattern has been attributed to the differences in composition of biomolecules used in nanoparticle synthesis and the excitation of surface Plasmon vibrations in metal nanoparticles [44Mulvaney, P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir, 1996, 12, 788-800.
[http://dx.doi.org/10.1021/la9502711] ]. Time for development of AgNPs reported by some authors include 10 hrs [40Paulraj, K.; Seung, T. Synthesis and structural characterization of silver nanoparticles using bacterial exopolysaccharide and its antimicrobial activity against food and multi-drug resistant pathogens. Process Biochem., 2013, 48, 1099-1106.
[http://dx.doi.org/10.1016/j.procbio.2013.05.011] ], 24 hrs [43Karthika, R; Sevarkodiyone, SP Synthesis and characterization of silver nanoparticles using aqueous extract of goat faecal pellets. Int J Current Sci Res, 2015, 1, 2454-5422.], and 48 hrs [41Mirunalini, S.; Arulmozhi, V.; Deepalakshmi, K.; Krishnaveni, M. Intracellular biosynthesis and antibacterial activity of silver nanoparticles using edible mushrooms. Not. Sci. Biol., 2012, 4, 55-61.
[http://dx.doi.org/10.15835/nsb448051] ]. In this study EPS of L. edodes rapidly reacted with AgNO3 solution to form AgNPs in 24 hrs.
 |
Fig. (2) AgNPs Colour patterns of Control (A) and UV10 mutant (B) after 24 hours. Inset is the colour pattern of AgNPs of Wild (C). |
3.3. UV Visible and SEM Studies of AgNPs Produced by UV10
UV-visible absorption spectroscopy has proved to be a versatile technique in the studies of AgNPs [45Sastry, M.; Mayya, K.S.; Patil, V.; Paranjape, D.V.; Hegde, S.G. Langmuir−Blodgett films of carboxylic acid derivatized silver colloidal particles: Role of subphase pH on degree of cluster incorporation. J. Phys. Chem. B, 1997, 101, 4954-4958.
[http://dx.doi.org/10.1021/jp964087f] ] and provides useful information about morphology, size and stabilisation of AgNPs [46Kanmani, P.; Lim, S.T. Synthesis and structural characterization of silver nanoparticles using bacterial exopolysaccharide and its antimicrobial activity against food and multidrug resistant pathogens. Process Biochem., 2013, 48, 1099-1106.
[http://dx.doi.org/10.1016/j.procbio.2013.05.011] ]. Fig. (3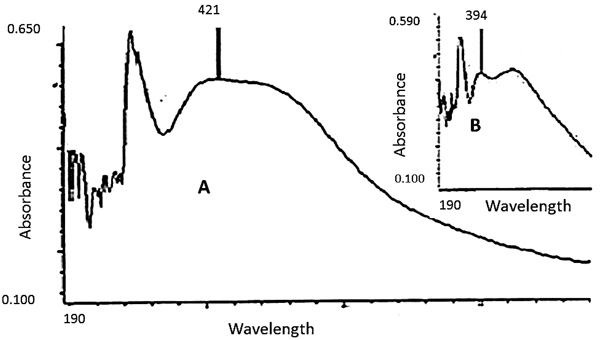 ) illustrates the UV-visible spectra of biosynthesised AgNPs of UV10 strain after 24 hours (Wild inset). The result shows a broad Surface Plasmon Resonance (SPR) with peak attained at 421 nm. The UV spectrophotometry reported in this study falls within the range of a usual and well reported pattern of SPR peaks of silver nanoparticles production [47Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; Kim, Y.K.; Lee, Y.S.; Jeong, D.H.; Cho, M.H. Antimicrobial effects of silver nanoparticles. Nanomedicine (Lond.), 2007, 3(1), 95-101.
) illustrates the UV-visible spectra of biosynthesised AgNPs of UV10 strain after 24 hours (Wild inset). The result shows a broad Surface Plasmon Resonance (SPR) with peak attained at 421 nm. The UV spectrophotometry reported in this study falls within the range of a usual and well reported pattern of SPR peaks of silver nanoparticles production [47Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; Kim, Y.K.; Lee, Y.S.; Jeong, D.H.; Cho, M.H. Antimicrobial effects of silver nanoparticles. Nanomedicine (Lond.), 2007, 3(1), 95-101.
[http://dx.doi.org/10.1016/j.nano.2006.12.001] [PMID: 17379174] -51Kannan, R.R.R.; Arumugam, R.; Ramya, D.; Manivannan, K.; Anantharaman, P. Green synthesis of silver nanoparticles using marine macroalga. Chaetomorphalinum Appl. Nanosci., 2013, 3, 229-233.
[http://dx.doi.org/10.1007/s13204-012-0125-5] ]. These characteristic AgNPs absorption peaks justified the formation of AgNPs. Several authors have also reported peaks on spectra of AgNPs in the range of 391-650 nm [50Thirumurugan, A.; Neethu, A.; Hema, P.; Prakash, P. Biological synthesis of silver nanoparticles by Lantana camara leaf extracts. Int. J. Nanomater. Bioresource, 2011, 1, 22-24.-52Brennan, M.; Armstrong, G; Kelly, J; Whelan, A. Patent publication: Sensors for detecting an analyte using silver nanoparticles. Patent Application Publication, 2006, 1, 1-39.]. According to some established protocol by Brennan et al. [52Brennan, M.; Armstrong, G; Kelly, J; Whelan, A. Patent publication: Sensors for detecting an analyte using silver nanoparticles. Patent Application Publication, 2006, 1, 1-39.], silver particles which displays single absorption band and occur between the range of 410 and 450 nm are spherical in nature. The silver particles produced by UV10 in the wavelength of 421 nm can therefore be presumed to be spherical in nature. Peaks in this range are further supported by other reports to be characteristics of spherical or somewhat spherical shaped nanoparticles [39Vanmathi, K.; Sivakumar, T. Isolation and characterization of silver nanoparticles from Fusarium oxysporum. Int. J. Curr. Microbiol. Appl. Sci., 2012, 56-62., 42Chan, Y.; Mat-Don, M. Instantaneous biosynthesis of silver nanoparticles by selected macrofungi. Aust. J. Basic Appl. Sci., 2012, 6, 222-226., 53Philip, D. Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract. Spectrochim. Acta A Mol. Biomol. Spectrosc., 2009, 73(2), 374-381.
[http://dx.doi.org/10.1016/j.saa.2009.02.037] [PMID: 19324587] ]. These further substantiates the thought that extracts of UV10 produced nanoparticles that are somewhat of spherical morphology under the conditions investigated. The outcome of nanoparticles produced in this work is similar to those previously reported by different authors [21Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv., 2013, 31(2), 346-356.
[http://dx.doi.org/10.1016/j.biotechadv.2013.01.003] [PMID: 23318667] , 42Chan, Y.; Mat-Don, M. Instantaneous biosynthesis of silver nanoparticles by selected macrofungi. Aust. J. Basic Appl. Sci., 2012, 6, 222-226., 53Philip, D. Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract. Spectrochim. Acta A Mol. Biomol. Spectrosc., 2009, 73(2), 374-381.
[http://dx.doi.org/10.1016/j.saa.2009.02.037] [PMID: 19324587] -55Selvi, K.; Sivakumar, T. Isolation and characterization of silver nanoparticles from Fusarium oxysporum. Int. J. Curr. Microbiol. Appl. Sci., 2012, 1, 56-62.] with respect to the production of silver nanoparticles using whole cells and extracts of mushrooms and other plants.
 |
Fig. (3) UV-Visible spectra of AgNPs of UV10 (A) after 24 hours. Inset, the spectra of UV-Vis spectra of AgNPs of Wild (B). |
3.4. SEM Characterisation of Synthesised Nanoparticles
The scanning electron microscopy implemented to confirm the morphometric physiognomies of shape and size of the bio-nanoparticles showed that nanoparticles produced contained aggregates of particles with spherical morphology (Fig. 4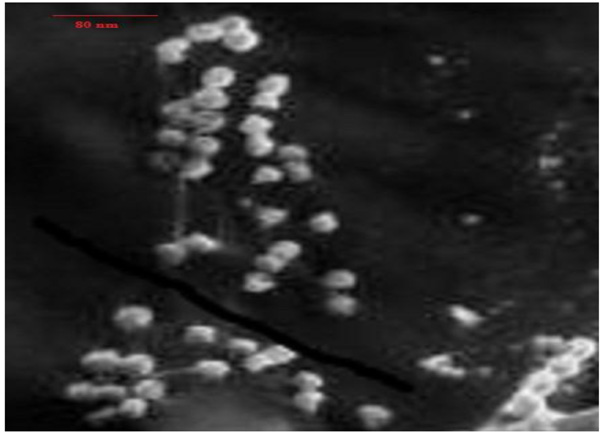 ). The size ranges between 50-100 nm. Spherical shaped AgNPs of varying sizes have been earlier described [51Kannan, R.R.R.; Arumugam, R.; Ramya, D.; Manivannan, K.; Anantharaman, P. Green synthesis of silver nanoparticles using marine macroalga. Chaetomorphalinum Appl. Nanosci., 2013, 3, 229-233.
). The size ranges between 50-100 nm. Spherical shaped AgNPs of varying sizes have been earlier described [51Kannan, R.R.R.; Arumugam, R.; Ramya, D.; Manivannan, K.; Anantharaman, P. Green synthesis of silver nanoparticles using marine macroalga. Chaetomorphalinum Appl. Nanosci., 2013, 3, 229-233.
[http://dx.doi.org/10.1007/s13204-012-0125-5] , 56Lateef, A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Oladipo, I.C.; Beukes, L.S. Cola nitida-Mediated Biogenic Synthesis of Silver Nanoparticles Using Seed and Seed Shell Extracts and Evaluation of Antibacterial Activities. Bionanoscience, 2015, 5, 196-205. a
[http://dx.doi.org/10.1007/s12668-015-0181-x] , 57Lateef, A.; Ojo, S.A.; Akinwale, A.S.; Azeez, L.; Gueguim-Kana, E.B.; Beukes, L.S. Biogenic synthesis of silver nanoparticles using cell-free extract of Bacillus safensis LAU 13: antimicrobial, free radical scavenging and larvicidal activities. Biologia, 2015, 70, 1295-1306. b
[http://dx.doi.org/10.1515/biolog-2015-0164] ]. Nanoparticles have been reported to possess high reduction potentials, good solution chemistry as well as extremely small particle size with large surface area [58Rivero-Huguet, M.; Marshall, W.D. Reduction of hexavalent chromium mediated by micron- and nano-scale zero-valent metallic particles. J. Environ. Monit., 2009, 11(5), 1072-1079.
[http://dx.doi.org/10.1039/b819279k] [PMID: 19436867] ]. The large surface area of nanoparticles as a result of nano-sizes provides a greater number of active sites for pollutants binding. In addition, the particles demonstrate unique characteristics like catalytic potential and high reactivity, which make them better adsorbing materials [10Ali, I. New generation adsorbents for water treatment. Chem. Rev., 2012, 112(10), 5073-5091.
[http://dx.doi.org/10.1021/cr300133d] [PMID: 22731247] ]. Moreover, high density of low coordinated atoms at the surface edges of nanomaterials further makes them very reactive [59Sánchez, A.; Recillas, S.; Font, X.; Casals, E.; González, E.; Puntes, V. Ecotoxicity of, and remediation with, engineered inorganic nanoparticles in the environment. TrAC. Trends Analyt. Chem., 2011, 30, 507-516.
[http://dx.doi.org/10.1016/j.trac.2010.11.011] ]. These distinctive properties can be employed in the adsorption and degradation of water and air pollutants. The successful synthesis of biologically mediated nanomaterial using EPS of L. edodes in this study may therefore herald a simple novel technique of biosynthesis and modulation of nanoparticles of varied sizes and properties. Nanoparticle of varied sizes such as synthensised can be applied in diverse industries, especially, in the field of hydrology as highlighted in this report.
3.5. Comparing EPS Productivity, AgNPs Colour and Spectra pattern of Wild and UV10 Strains
Comparing the EPS productivity of the Wild and UV10 mutant strains of L. edodes, UV10 obtained at 10 minutes of exposure to Ultraviolet irradiation gave the highest exopolysaccharide productivity of 2.783 mg/ml while the Wild strain has a productivity of 1.044 mg/ml after 7 days of fermentation (Table 4). The 10 minutes UV improved strain has an enhanced capacity of 2.7 fold over the Wild strain, which confirms the effectiveness of UV irradiation for EPS productivity enhancement in the mushroom strain. The synthesised nanoparticle of UV10 has a purple colouration and detected around 421 nm while that synthesised by wild strain possesses dark brown colouration with absorbance of 394 nm which are significant nanoparticles formation characteristics [19Zielińsk, A.; Skwarek, E.; Zaleska, A.; Hupka, M.G. Preparation of silver nanoparticles with controlled particle size. Procedia Chem., 2009, 1, 1560-1566.
[http://dx.doi.org/10.1016/j.proche.2009.11.004] ].
 |
Fig. (4) Silver Nanoparticle synthesised from UV10. |
3.6. Potential Water Applications
3.6.1. Water Disinfectants
The antimicrobial activities of silver have been known since ancient times and used efficiently against a wide range of microorganisms [60Kalishwaralal, K.; Deepak, V.; Ram Kumar Pandian, S.; Kottaisamy, M.; BarathmaniKanth, S.; Kartikeyan, B.; Gurunathan, S. Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Coll. Surf. B Biointer., 2010, 77(2), 257-262.
[http://dx.doi.org/10.1016/j.colsurfb.2010.02.007] [PMID: 20197229] ]. Silver nanoparticles (AgNPs) are highly lethal against microorganisms and thus have strong antimicrobial effects against wide spectra of microbes, including bacteria [61Kalhapure, R.S.; Sonawane, S.J.; Sikwal, D.R.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Govender, T. Solid lipid nanoparticles of clotrimazole silver complex: An efficient nano antibacterial against Staphylococcus aureus and MRSA. Coll. Surf. B Biointer., 2015, 136, 651-658.
[http://dx.doi.org/10.1016/j.colsurfb.2015.10.003] [PMID: 26492156] ], fungi [62Krishnaraj, C.; Ramachandran, R.; Mohan, K.; Kalaichelvan, P.T. Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectrochim. Acta A Mol. Biomol. Spectrosc., 2012, 93, 95-99.
[http://dx.doi.org/10.1016/j.saa.2012.03.002] [PMID: 22465774] ] and viruses [63Borrego, B.; Lorenzo, G.; Mota-Morales, J.D.; Almanza-Reyes, H.; Mateos, F.; López-Gil, E.; de la Losa, N.; Burmistrov, V.A.; Pestryakov, A.N.; Brun, A.; Bogdanchikova, N. Potential application of silver nanoparticles to control the infectivity of Rift Valley fever virus in vitro and in vivo. Nanomedicine (Lond.), 2016, 12(5), 1185-1192.
[http://dx.doi.org/10.1016/j.nano.2016.01.021] [PMID: 26970026] ]. Silver nanoparticles are able to adhere to microbial cell wall and consequently penetrate it, causing structural changes of the cell membrane and thus making it more permeable [64Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci., 2004, 275(1), 177-182.
[http://dx.doi.org/10.1016/j.jcis.2004.02.012] [PMID: 15158396] ]. Furthermore, when AgNPs are in contact with microbes, free radicals can be produced which can destroy the cell membrane leading to cell death [65Danilczuk, M.; Lund, A.; Sadlo, J.; Yamada, H.; Michalik, J. Conduction electron spin resonance of small silver particles. Spectrochim. Acta A Mol. Biomol. Spectrosc., 2006, 63(1), 189-191.
[http://dx.doi.org/10.1016/j.saa.2005.05.002] [PMID: 15978868] ]. In addition, as DNA contains abundant sulfur and phosphorus elements, AgNPs may react with those elements and thus destroy them; and this further explains death of cells caused by AgNPs [66Dhanalekshmi, K.I.; Meena, K.S. DNA intercalation studies and antimicrobial activity of Ag@ZrO2 core-shell nanoparticles in vitro. Mater. Sci. Eng. C, 2016, 59, 1063-1068.
[http://dx.doi.org/10.1016/j.msec.2015.11.027] [PMID: 26652465] ]. The dissolution of AgNPs will release antimicrobial Ag+ ions, which may react with the thiol groups of several important enzymes, deactivate them, and interrupt regular functions in the cell [67Prabhu, S.; Poulose, E. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett., 2012, 2, 32-40.
[http://dx.doi.org/10.1186/2228-5326-2-32] ]. As a good antimicrobial agent, silver nanoparticles, under suitable conditions such as effective magnetic separation, would therefore be a good antimicrobial disinfectant in water treatment.
3.6.2. Water Filtration Materials
AgNPs blended with filter materials is considered promising for water purification due to their high antimicrobial activity and low cost. This procedure is expected to overcome the challenge of aggregation in aqueous media that progressively decreases efficacy of nanoparticles during long-term use [68Li, X.; Lenhart, J.J.; Walker, H.W. Aggregation kinetics and dissolution of coated silver nanoparticles. Langmuir, 2012, 28(2), 1095-1104.
[http://dx.doi.org/10.1021/la202328n] [PMID: 22149007] , 69Quang, D.; Sarawade, P.; Jeon, S. Effective water disinfection using silver nanoparticle containing silica beads. Appl. Surf. Sci., 2013, 266, 280-287.
[http://dx.doi.org/10.1016/j.apsusc.2012.11.168] ]. AgNPs deposition on cellulose fibers of an absorbent blotting paper sheet has been reported. The AgNPs sheets showed antibacterial action against suspensions of Enterococcus faecalis and E. coli by inactivating cells of the bacteria strains during filtration through the sheet [70Dankovich, T.A.; Gray, D.G. Bactericidal paper impregnated with silver nanoparticles for point-of-use water treatment. Environ. Sci. Technol., 2011, 45(5), 1992-1998.
[http://dx.doi.org/10.1021/es103302t] [PMID: 21314116] ]. The silver loss from the AgNPs embedded sheets was lesser than the standards for silver in potable water as recommended by the World Health Organization (WHO) and Environmental Protection Agency (EPA) [70Dankovich, T.A.; Gray, D.G. Bactericidal paper impregnated with silver nanoparticles for point-of-use water treatment. Environ. Sci. Technol., 2011, 45(5), 1992-1998.
[http://dx.doi.org/10.1021/es103302t] [PMID: 21314116] ]. In addition, silver nanoparticles incorporated into Polyethersulfone (PES) microfiltration membranes was reported to remarkably inactivate the activity of microbes near the membranes. The PES-AgNPs membranes showed strong antimicrobial characteristic and indicate great potential in the application of silver nanomaterials for the development of potent water filters for safe water processes [71Ferreira, A.; Roque, E.; Fonseca, F.; Borges, C. High flux microfiltration membranes with silver nanoparticles for water disinfection. Desalination Water Treat., 2015, 56, 3590-3598.
[http://dx.doi.org/10.1080/19443994.2014.1000977] ].
3.6.3. Anti-Fouling Agent
Ag nanoparticles on clay materials have drawn significant attention as a result of their anti-fouling properties for domestic (point-of-use) water treatment [72Ren, D.; Smith, J.A. Retention and transport of silver nanoparticles in a ceramic porous medium used for point-of-use water treatment. Environ. Sci. Technol., 2013, 47(8), 3825-3832.
[http://dx.doi.org/10.1021/es4000752] [PMID: 23496137] ]. Silver nanoparticle’s application to ceramic filters fabricated with sawdust and clay have exhibited improved water treatment efficiency in this aspect with filters of higher porosity attaining greater application potentials than those of lesser porosity [73Kallman, E.; Oyanedel-Craver, V.; Smith, J. Ceramic filters impregnated with silver nanoparticles for point-of-use water treatment in rural Guatemala. J. Environ. Eng., 2011, 137, 407-415.
[http://dx.doi.org/10.1061/(ASCE)EE.1943-7870.0000330] ]. Antifouling properties of silver nanoparticles regenerated by TiO2 on forward osmosis membrane have been reported [74Nguyena, A.; Zou, L.; Priest, C. Evaluating the antifouling effects of silver nanoparticles regenerated by TiO2 on forward osmosis membrane. J. Membr. Sci., 2014, 454, 264-271.
[http://dx.doi.org/10.1016/j.memsci.2013.12.024] ] while nano-silica fabricated silver nanoparticles have also been reported as an effective antifouling agent in addition to aiding dye removal and disinfection in polluted water [75Das, S.K.; Khan, M.M.; Parandhaman, T.; Laffir, F.; Guha, A.K.; Sekaran, G.; Mandal, A.B. Nano-silica fabricated with silver nanoparticles: antifouling adsorbent for efficient dye removal, effective water disinfection and biofouling control. Nanoscale, 2013, 5(12), 5549-5560.
[http://dx.doi.org/10.1039/c3nr00856h] [PMID: 23680871] ].
CONCLUSION
In this study, results obtained confirm that an alternative technique for synthesis of silver nanoparticles using biological approach is feasible. To the best of our knowledge, this is the first report of using mutagenesis in biosynthesis for modulation of product of nanoparticles. This procedure may be a useful tool in the production of different types of nanoparticles and may constitute a useful technique in the amplification of eco-friendly and biologically driven processes for safe drinking water treatment and production.
RECOMMENDATION
The potentials of silver nanoparticles in water quality improvement have been highlighted in this work. Biological production and simple UV optimization technique for AgNPs of mushroom origin is hereby established. This cheap method of myco-nanoparticle production and yield improvement can further be extended to the range of underutilised mushrooms, especially, for water research and application purpose.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies in this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Adeyemi Ojutalayo Adeeyo acknowledge University of Venda, South Africa for the privilege of South Africa for the privilege of Research Support Grant (Number SES/17/ERM/13) and Prof A. Lateef for his mentorship role in my training in nanoscience.
REFERENCES
| [1] | Yürüm, A.; Kocabas-Ataklı, Z.Ö.; Sezen, M.; Semiat, R.; Yürüm, Y. Fast deposition of porous iron oxide on activated carbon by microwave heating and arsenic (V) removal from water. Chem. Eng. J., 2014, 242, 321-332. [http://dx.doi.org/10.1016/j.cej.2014.01.005] |
| [2] | Amkamwar, B.; Damle, C.; Ahmad, A.; Sastry, M. Biosynthesis of gold and silver nanoparticles using Emblicaofficinalis fruit extract, their phase transfer and transmetallation in an organic solution. J. Nanosci. Nanotechnol., 2005, 5, 1666-1671. |
| [3] | Cataleya, C. In vitro toxicity assessment of silver nanoparticles in rat alveolar macrophages., Master thesis, Wright state University, USA 2006 pp 1-75. |
| [4] | Grimsdale, A.C.; Chan, K.L.; Martin, R.E.; Jokisz, P.G.; Holmes, A.B. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev., 2009, 109(3), 897-1091. [http://dx.doi.org/10.1021/cr000013v] [PMID: 19228015] |
| [5] | Geckeler, K.E.; Nishide, H. Advanced nanomaterials., 2010, , 249-295. |
| [6] | Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog., 2006, 22(2), 577-583. [http://dx.doi.org/10.1021/bp0501423] [PMID: 16599579] |
| [7] | Ivanov, V.; Tay, J.H.; Tay, S.T.L.; Jiang, H.L. Removal of micro-particles by microbial granules used for aerobic wastewater treatment. Water Sci. Technol., 2004, 50(12), 147-154. [http://dx.doi.org/10.2166/wst.2004.0707] [PMID: 15686015] |
| [8] | Chen, S.; Zou, Y.; Yan, Z.; Shen, W.; Shi, S.; Zhang, X.; Wang, H. Carboxymethylated-bacterial cellulose for copper and lead ion removal. J. Hazard. Mater., 2009, 161(2-3), 1355-1359. [http://dx.doi.org/10.1016/j.jhazmat.2008.04.098] [PMID: 18538922] |
| [9] | Chen, Y.; Pan, B.; Li, H.; Zhang, W.; Lv, L.; Wu, J. Selective removal of Cu(II) ions by using cation-exchange resin-supported polyethyleneimine (PEI) nanoclusters. Environ. Sci. Technol., 2010, 44(9), 3508-3513. [http://dx.doi.org/10.1021/es100341x] [PMID: 20373792] |
| [10] | Ali, I. New generation adsorbents for water treatment. Chem. Rev., 2012, 112(10), 5073-5091. [http://dx.doi.org/10.1021/cr300133d] [PMID: 22731247] |
| [11] | Ali, ME; Ullah, M; Hamid, SBA Conventional to nano-green adsorbents for water pollution management: A review. Adv Mater Res 2014, 925, 674-678. [http://dx.doi.org/10.4028/www.scientific.net/AMR.925.674] |
| [12] | Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir, 2002, 18, 6679-6686. [http://dx.doi.org/10.1021/la0202374] |
| [13] | Nair, A.S.; Pradeep, T. Reactivity of Au and Ag nanoparticles with halocarbons. Appl. Nanosci., 2004, 59-63. |
| [14] | Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gramnegative bacteria. Nanomed: Nanotechnol. Biol. Med. (Aligarh), 2012, 8, 37-45. [PMID: 21703988] |
| [15] | Huang, H.; Yang, X. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: a green method. Carbohydr. Res., 2004, 339(15), 2627-2631. [http://dx.doi.org/10.1016/j.carres.2004.08.005] [PMID: 15476726] |
| [16] | Abid, J.P.; Wark, A.W.; Brevet, P.F.; Girault, H.H. Preparation of silver nanoparticles in solution from a silver salt by laser irradiation. Chem. Commun. (Camb.), 2002, 7(7), 792-793. [http://dx.doi.org/10.1039/b200272h] [PMID: 12119726] |
| [17] | Gasaymeh, S.S.; Radiman, S.; Lee, Y.H.; Saion, E.; Saeed, G.H. Synthesis and characterization of silver/polyvinilpirrolidone (Ag/PVP) nanoparticles using gamma irradiation techniques. Am. J. Appl. Sci., 2010, 7, 892-901. [http://dx.doi.org/10.3844/ajassp.2010.892.901] |
| [18] | Mohamed, A.G.T. Stachybotrys chartarum: a novel biological agent for the extracellular synthesis of silver nanoparticles and their antimicrobial activity. Indones. J. Biotechnol., 2013, 18, 75-82. [http://dx.doi.org/10.22146/ijbiotech.7871] |
| [19] | Zielińsk, A.; Skwarek, E.; Zaleska, A.; Hupka, M.G. Preparation of silver nanoparticles with controlled particle size. Procedia Chem., 2009, 1, 1560-1566. [http://dx.doi.org/10.1016/j.proche.2009.11.004] |
| [20] | Otari, S.; Patil, R.; Nadaf, N.; Ghosh, S.; Pawar, S. Green biosynthesis of silver nanoparticles from an actinobacteria Rhodococcus sp. Mater. Lett., 2012, 72, 92-94. [http://dx.doi.org/10.1016/j.matlet.2011.12.109] |
| [21] | Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv., 2013, 31(2), 346-356. [http://dx.doi.org/10.1016/j.biotechadv.2013.01.003] [PMID: 23318667] |
| [22] | Allafchian, A.R.; Bahramian, H.; Jalali, S.A.H.; Ahmadvand, H. Synthesis, characterization and antibacterial effect of new magnetically core–shell nanocomposites. J. Magn. Magn. Mater., 2015, 394, 318-324. a [http://dx.doi.org/10.1016/j.jmmm.2015.06.086] |
| [23] | Allafchian, A.R.; Jalali, S.A.H. Synthesis, characterization and antibacterial effect of poly (acrylonitrile/maleic acid)–silver nanocomposite. J. Taiwan Inst. Chem. Eng., 2015, 57, 154-159. b [http://dx.doi.org/10.1016/j.jtice.2015.05.015] |
| [24] | Gudikandula, K.; Maringanti, S.C. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci., 2016, 11(9), 714-721. [http://dx.doi.org/10.1080/17458080.2016.1139196] |
| [25] | Kahani, SA; Yagini, Z.A. Comparison between Chemical Synthesis Magnetite Nanoparticles and Biosynthesis Magnetite. Bioinorganic Chem. Appl. , 2014, 1-7. [http://dx.doi.org/10.1155/2014/384984] |
| [26] | Sanghi, R.; Verma, P. Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour. Technol., 2009, 100(1), 501-504. [http://dx.doi.org/10.1016/j.biortech.2008.05.048] [PMID: 18625550] |
| [27] | Gericke, M.; Pinches, A. Biological synthesis of metal nanoparticles. Hydrometallurgy, 2006, 83, 132-140. [http://dx.doi.org/10.1016/j.hydromet.2006.03.019] |
| [28] | Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: technological concepts and future applications. J. Nanopart. Res., 2008, 10, 507-517. [http://dx.doi.org/10.1007/s11051-007-9275-x] |
| [29] | Mukherjee, P.; Senapati, S.; Mandal, D.; Ahmad, A.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporum. ChemBioChem, 2002, 3(5), 461-463. [http://dx.doi.org/10.1002/1439-7633(20020503)3:5<461::AID-CBIC461>3.0.CO;2-X] [PMID: 12007181] |
| [30] | Lloyd, J. Microbial Reduction of Metals and Radionuclides. FEMS Microbial. Rev, 2003, 27, 411-425. |
| [31] | Rai, M.; Yadav, A.; Bridge, P.; Gade, A. Myconanotechnology: A new and emerging science. Appl Mycology, 2009, 14, 258-267. |
| [32] | Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. Colorimetric method for determination of sugar and related substances. Anal. Chem., 1956, 28, 350-356. [http://dx.doi.org/10.1021/ac60111a017] |
| [33] | Adebayo, A.E.; Oloke, J.K.; Achana, Y.; Bora, T. Improvement of Laccase production in Pleurotus pulmonarius LAU 09 by mutation. J. Microbiol., 2012, 2, 11-17. [http://dx.doi.org/10.5923/j.microbiology.20120201.03] |
| [34] | Majolagbe, ON; Oloke, JK; Adebayo, EA; Adewoyin, AG; Ayandele, A; Bamigboye, C Study on the antimicrobial activity of exopolysaccharides of Lentinus subnudus using swiss albino rats as animal model. Am Eurasian J of Sci Res, 2013, 8, 47-52. |
| [35] | Varinder, K; Bera, MB; Panesar, PS; Chopra, HK Production and characterization of exopolysaccharide produced by Alcaligenes faecalis B14 from indigenous soil. Int. J. Biotechnol Bioengineering Res, 2013, 4, 365-374. |
| [36] | Sutherland, I.W. Structure-function relationships in microbial exopolysaccharides. Biotechnol. Adv., 1994, 12(2), 393-448. [http://dx.doi.org/10.1016/0734-9750(94)90018-3] [PMID: 14545899] |
| [37] | Schirm, M.; Arora, S.K.; Verma, A.; Vinogradov, E.; Thibault, P.; Ramphal, R.; Logan, S.M. Structural and genetic characterization of glycosylation of type a flagellin in Pseudomonas aeruginosa. J. Bacteriol., 2004, 186(9), 2523-2531. [http://dx.doi.org/10.1128/JB.186.9.2523-2531.2004] [PMID: 15090491] |
| [38] | Carbonero, E.; Gracher, A.; Komura, D.; Marcon, R.; Freitas, C.; Baggio, C.; Santos, A.; Torri, G.; Gorin, P.; Locomini, M. Lentinus edodes heterogalactan: Antinociceptive and anti-inflammatory effects. Food Chem., 2008, 111, 531-537. [http://dx.doi.org/10.1016/j.foodchem.2008.04.015] |
| [39] | Vanmathi, K.; Sivakumar, T. Isolation and characterization of silver nanoparticles from Fusarium oxysporum. Int. J. Curr. Microbiol. Appl. Sci., 2012, 56-62. |
| [40] | Paulraj, K.; Seung, T. Synthesis and structural characterization of silver nanoparticles using bacterial exopolysaccharide and its antimicrobial activity against food and multi-drug resistant pathogens. Process Biochem., 2013, 48, 1099-1106. [http://dx.doi.org/10.1016/j.procbio.2013.05.011] |
| [41] | Mirunalini, S.; Arulmozhi, V.; Deepalakshmi, K.; Krishnaveni, M. Intracellular biosynthesis and antibacterial activity of silver nanoparticles using edible mushrooms. Not. Sci. Biol., 2012, 4, 55-61. [http://dx.doi.org/10.15835/nsb448051] |
| [42] | Chan, Y.; Mat-Don, M. Instantaneous biosynthesis of silver nanoparticles by selected macrofungi. Aust. J. Basic Appl. Sci., 2012, 6, 222-226. |
| [43] | Karthika, R; Sevarkodiyone, SP Synthesis and characterization of silver nanoparticles using aqueous extract of goat faecal pellets. Int J Current Sci Res, 2015, 1, 2454-5422. |
| [44] | Mulvaney, P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir, 1996, 12, 788-800. [http://dx.doi.org/10.1021/la9502711] |
| [45] | Sastry, M.; Mayya, K.S.; Patil, V.; Paranjape, D.V.; Hegde, S.G. Langmuir−Blodgett films of carboxylic acid derivatized silver colloidal particles: Role of subphase pH on degree of cluster incorporation. J. Phys. Chem. B, 1997, 101, 4954-4958. [http://dx.doi.org/10.1021/jp964087f] |
| [46] | Kanmani, P.; Lim, S.T. Synthesis and structural characterization of silver nanoparticles using bacterial exopolysaccharide and its antimicrobial activity against food and multidrug resistant pathogens. Process Biochem., 2013, 48, 1099-1106. [http://dx.doi.org/10.1016/j.procbio.2013.05.011] |
| [47] | Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; Kim, Y.K.; Lee, Y.S.; Jeong, D.H.; Cho, M.H. Antimicrobial effects of silver nanoparticles. Nanomedicine (Lond.), 2007, 3(1), 95-101. [http://dx.doi.org/10.1016/j.nano.2006.12.001] [PMID: 17379174] |
| [48] | Kalimuthu, K.; Suresh Babu, R.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Coll. Surf. B Biointerf., 2008, 65(1), 150-153. [http://dx.doi.org/10.1016/j.colsurfb.2008.02.018] [PMID: 18406112] |
| [49] | Ganesh Babu, M.M.; Gunasekaran, P. Production and structural characterization of crystalline silver nanoparticles from Bacillus cereus isolate. Coll. Surf. B Biointerf., 2009, 74(1), 191-195. [http://dx.doi.org/10.1016/j.colsurfb.2009.07.016] [PMID: 19660920] |
| [50] | Thirumurugan, A.; Neethu, A.; Hema, P.; Prakash, P. Biological synthesis of silver nanoparticles by Lantana camara leaf extracts. Int. J. Nanomater. Bioresource, 2011, 1, 22-24. |
| [51] | Kannan, R.R.R.; Arumugam, R.; Ramya, D.; Manivannan, K.; Anantharaman, P. Green synthesis of silver nanoparticles using marine macroalga. Chaetomorphalinum Appl. Nanosci., 2013, 3, 229-233. [http://dx.doi.org/10.1007/s13204-012-0125-5] |
| [52] | Brennan, M.; Armstrong, G; Kelly, J; Whelan, A. Patent publication: Sensors for detecting an analyte using silver nanoparticles. Patent Application Publication, 2006, 1, 1-39. |
| [53] | Philip, D. Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract. Spectrochim. Acta A Mol. Biomol. Spectrosc., 2009, 73(2), 374-381. [http://dx.doi.org/10.1016/j.saa.2009.02.037] [PMID: 19324587] |
| [54] | Shaligram, N.S.; Singh, S.K.; Singhal, R.S.; Szakacs, G.; Pandey, A. Effect of precultural and nutritional parameters on compactin production by solid-state fermentation. J. Microbiol. Biotechnol., 2009, 19(7), 690-697. [PMID: 19652517] |
| [55] | Selvi, K.; Sivakumar, T. Isolation and characterization of silver nanoparticles from Fusarium oxysporum. Int. J. Curr. Microbiol. Appl. Sci., 2012, 1, 56-62. |
| [56] | Lateef, A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Oladipo, I.C.; Beukes, L.S. Cola nitida-Mediated Biogenic Synthesis of Silver Nanoparticles Using Seed and Seed Shell Extracts and Evaluation of Antibacterial Activities. Bionanoscience, 2015, 5, 196-205. a [http://dx.doi.org/10.1007/s12668-015-0181-x] |
| [57] | Lateef, A.; Ojo, S.A.; Akinwale, A.S.; Azeez, L.; Gueguim-Kana, E.B.; Beukes, L.S. Biogenic synthesis of silver nanoparticles using cell-free extract of Bacillus safensis LAU 13: antimicrobial, free radical scavenging and larvicidal activities. Biologia, 2015, 70, 1295-1306. b [http://dx.doi.org/10.1515/biolog-2015-0164] |
| [58] | Rivero-Huguet, M.; Marshall, W.D. Reduction of hexavalent chromium mediated by micron- and nano-scale zero-valent metallic particles. J. Environ. Monit., 2009, 11(5), 1072-1079. [http://dx.doi.org/10.1039/b819279k] [PMID: 19436867] |
| [59] | Sánchez, A.; Recillas, S.; Font, X.; Casals, E.; González, E.; Puntes, V. Ecotoxicity of, and remediation with, engineered inorganic nanoparticles in the environment. TrAC. Trends Analyt. Chem., 2011, 30, 507-516. [http://dx.doi.org/10.1016/j.trac.2010.11.011] |
| [60] | Kalishwaralal, K.; Deepak, V.; Ram Kumar Pandian, S.; Kottaisamy, M.; BarathmaniKanth, S.; Kartikeyan, B.; Gurunathan, S. Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Coll. Surf. B Biointer., 2010, 77(2), 257-262. [http://dx.doi.org/10.1016/j.colsurfb.2010.02.007] [PMID: 20197229] |
| [61] | Kalhapure, R.S.; Sonawane, S.J.; Sikwal, D.R.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Govender, T. Solid lipid nanoparticles of clotrimazole silver complex: An efficient nano antibacterial against Staphylococcus aureus and MRSA. Coll. Surf. B Biointer., 2015, 136, 651-658. [http://dx.doi.org/10.1016/j.colsurfb.2015.10.003] [PMID: 26492156] |
| [62] | Krishnaraj, C.; Ramachandran, R.; Mohan, K.; Kalaichelvan, P.T. Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectrochim. Acta A Mol. Biomol. Spectrosc., 2012, 93, 95-99. [http://dx.doi.org/10.1016/j.saa.2012.03.002] [PMID: 22465774] |
| [63] | Borrego, B.; Lorenzo, G.; Mota-Morales, J.D.; Almanza-Reyes, H.; Mateos, F.; López-Gil, E.; de la Losa, N.; Burmistrov, V.A.; Pestryakov, A.N.; Brun, A.; Bogdanchikova, N. Potential application of silver nanoparticles to control the infectivity of Rift Valley fever virus in vitro and in vivo. Nanomedicine (Lond.), 2016, 12(5), 1185-1192. [http://dx.doi.org/10.1016/j.nano.2016.01.021] [PMID: 26970026] |
| [64] | Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci., 2004, 275(1), 177-182. [http://dx.doi.org/10.1016/j.jcis.2004.02.012] [PMID: 15158396] |
| [65] | Danilczuk, M.; Lund, A.; Sadlo, J.; Yamada, H.; Michalik, J. Conduction electron spin resonance of small silver particles. Spectrochim. Acta A Mol. Biomol. Spectrosc., 2006, 63(1), 189-191. [http://dx.doi.org/10.1016/j.saa.2005.05.002] [PMID: 15978868] |
| [66] | Dhanalekshmi, K.I.; Meena, K.S. DNA intercalation studies and antimicrobial activity of Ag@ZrO2 core-shell nanoparticles in vitro. Mater. Sci. Eng. C, 2016, 59, 1063-1068. [http://dx.doi.org/10.1016/j.msec.2015.11.027] [PMID: 26652465] |
| [67] | Prabhu, S.; Poulose, E. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett., 2012, 2, 32-40. [http://dx.doi.org/10.1186/2228-5326-2-32] |
| [68] | Li, X.; Lenhart, J.J.; Walker, H.W. Aggregation kinetics and dissolution of coated silver nanoparticles. Langmuir, 2012, 28(2), 1095-1104. [http://dx.doi.org/10.1021/la202328n] [PMID: 22149007] |
| [69] | Quang, D.; Sarawade, P.; Jeon, S. Effective water disinfection using silver nanoparticle containing silica beads. Appl. Surf. Sci., 2013, 266, 280-287. [http://dx.doi.org/10.1016/j.apsusc.2012.11.168] |
| [70] | Dankovich, T.A.; Gray, D.G. Bactericidal paper impregnated with silver nanoparticles for point-of-use water treatment. Environ. Sci. Technol., 2011, 45(5), 1992-1998. [http://dx.doi.org/10.1021/es103302t] [PMID: 21314116] |
| [71] | Ferreira, A.; Roque, E.; Fonseca, F.; Borges, C. High flux microfiltration membranes with silver nanoparticles for water disinfection. Desalination Water Treat., 2015, 56, 3590-3598. [http://dx.doi.org/10.1080/19443994.2014.1000977] |
| [72] | Ren, D.; Smith, J.A. Retention and transport of silver nanoparticles in a ceramic porous medium used for point-of-use water treatment. Environ. Sci. Technol., 2013, 47(8), 3825-3832. [http://dx.doi.org/10.1021/es4000752] [PMID: 23496137] |
| [73] | Kallman, E.; Oyanedel-Craver, V.; Smith, J. Ceramic filters impregnated with silver nanoparticles for point-of-use water treatment in rural Guatemala. J. Environ. Eng., 2011, 137, 407-415. [http://dx.doi.org/10.1061/(ASCE)EE.1943-7870.0000330] |
| [74] | Nguyena, A.; Zou, L.; Priest, C. Evaluating the antifouling effects of silver nanoparticles regenerated by TiO2 on forward osmosis membrane. J. Membr. Sci., 2014, 454, 264-271. [http://dx.doi.org/10.1016/j.memsci.2013.12.024] |
| [75] | Das, S.K.; Khan, M.M.; Parandhaman, T.; Laffir, F.; Guha, A.K.; Sekaran, G.; Mandal, A.B. Nano-silica fabricated with silver nanoparticles: antifouling adsorbent for efficient dye removal, effective water disinfection and biofouling control. Nanoscale, 2013, 5(12), 5549-5560. [http://dx.doi.org/10.1039/c3nr00856h] [PMID: 23680871] |







