- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Synthesis, Characterization of Mixed Cu(II) Pyridyl Tetrazoles and 1,10-Phenanthroline Complexes - DFT and Biological Activity
Ch. Himasekar1, Sheik Mustafa2, Manabolu S. Babu2, *
Abstract
Background:
Mixed ligand copper complexes with 1,10-phenanthroline show good chemical nuclease activity and anticancer activity. Recently, tetrazole derivatives are also promising candidates for anticancer activity. Hence, it is significant to study the DNA binding and anticancer activity of two active N-donor ligands and their copper complexes.
Objectives:
The main objective of this study was to investigate the regioisomeric mixed ligand copper complexes response with calf thymus DNA binding and anti-toxic activity against MCF-7 cell line.
Methods:
The DNA binding interactions of complexes 1-4 with calf thymus DNA (CT-DNA) were monitored by UV/VIS spectroscopy. The absorption spectra of the Cu complexes are compared with and without CT-DNA at 400-450 nm. The cell proliferation was measured by using the standard 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium- 5-carboxanilide (XTT) assay with four different concentrations of the compounds (5, 10, 50, and 100 mm) and cisplatin (as a positive control) was tested in triplicate for 48 h. The results obtained by the XTTassay are expressed as the average standard deviation of two experiments. The IC50 values of the complexes exhibited differential and dose-dependent inhibitory activities on the growth of MCF-7 cancer cells.
Results:
Based on the elemental analysis, molar conductance, magnetic moments, mass, electronic, ESR and IR spectral data, the copper is coordinated by N-atoms of 1,10- phenanthroline and pyridyl tetrazole with octahedral structure. DFT calculations of HOMO and LUMO studies showed that electron density is localized on pyridyl tetrazole ring and phenanthroline ring. The calculated DNA binding constant (Kb) values of 1-4 complexes are in the range 4.2 - 7.6 x104M-1 (Table 4) with similar binding affinity to reported copper tetrazole derivative complexes. The 1-4 complexes with CT DNA interaction are through planar phenanthroline and pyridyl tetrazole ring likely via π-stacking interactions. The IC50 values of complexes show excellent activity with 24(± 0.5); 18(± 0.5); 20(±0.5); (±0.5) and 38 (±0.8) for 1, 2, 3, 4 and cis platin complexes, respectively. After 72 h of the treatment of 1 on MCF-7 cell, IC50 values hinder the cell growth upto 24(± 0.5) µg/ml at 5 µM concentration range (Fig. 5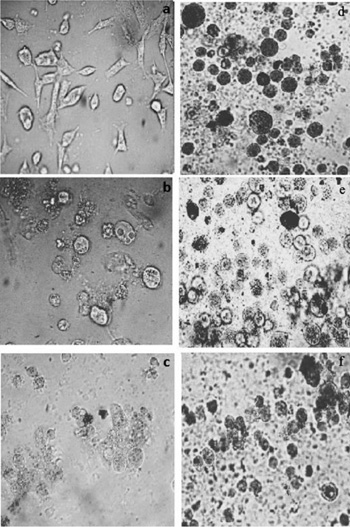 ). It is apparent from IC50 values that the order inhibition is 1 > 3 >2 > 4.
). It is apparent from IC50 values that the order inhibition is 1 > 3 >2 > 4.
Conclusion:
Experimental results are highly encouraging to explore the mixed ligand regio isomeric copper complexes which have shown the parallel result with Cisplatin. By proper structural modification of pyridyl tetrazole ligand, substituent better anticancer agents can be prepared.
Article Information
Identifiers and Pagination:
Year: 2019Volume: 6
First Page: 1
Last Page: 7
Publisher Id: CHEM-6-1
DOI: 10.2174/1874842201906010001
Article History:
Received Date: 20/09/2018Revision Received Date: 12/11/2018
Acceptance Date: 21/11/2018
Electronic publication date: 28/1/2019
Collection year: 2019
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Department of Chemistry, GITAM School of Technology, GITAM University, HTP campus, Rudraram, Medak 502 329, Telangana, India; Tel: 9440975442; E-mails: manabolu@gmail.com, surendrababy.manabolu@gitam.edu
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 20-09-2018 |
Original Manuscript | Synthesis, Characterization of Mixed Cu(II) Pyridyl Tetrazoles and 1,10-Phenanthroline Complexes - DFT and Biological Activity | |
1. INTRODUCTION
A large number of potential ligands compete for metal ions in biofluids [1Maret, W. Analyzing free zinc(II) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics, 2015, 7(2), 202-211.
[http://dx.doi.org/10.1039/C4MT00230J] [PMID: 25362967] ]. The storage and transport of active substances through bio-membranes is related to the specific structure of the complexes [2Rivalta, I.; Brudvig, G.W.; Batista, V.S. Oxomanganese complexes for natural and artificial photosynthesis. Curr. Opin. Chem. Biol., 2012, 16(1-2), 11-18.
[http://dx.doi.org/10.1016/j.cbpa.2012.03.003] [PMID: 22481113] ]. N-donar ligands are biologically important, have a key role in coordinating copper metal and their application as anticancer agents [3Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in copper complexes as anticancer agents. Chem. Rev., 2014, 114(1), 815-862.
[http://dx.doi.org/10.1021/cr400135x] [PMID: 24102434] , 4Nies, D.H. The biological chemistry of the transition metal “transportome” of cupriavidus metallidurans. Metallomics, 2016, 8(5), 481-507.
[http://dx.doi.org/10.1039/C5MT00320B] [PMID: 27065183] ]. Tetrazoles are an important class of N-donor ligands that are metabolically stable substituent for -COOH functional groups; assist in coordination chemistry as ligands, lipophilic spacers [5Sheridan, U.; Gallagher, J.F.; Bjerrum, M.J.; Fleming, A.; Kelleher, F.; McGinley, J. Metal-organic frameworks based on pyridyl-tetrazole ligands containing ester or carboxylate pendant arms. J Inorg Chim Acta, 2014, 421, 200.
[http://dx.doi.org/10.1016/j.ica.2014.05.028] ]. Introducing tetrazole ring into the molecule will reduce the toxic properties of a drug, because it exhibits stronger resistance to in vivo metabolization than the carboxylate group, causing the drug to stay longer (bioavailability) in blood [6Bekhit, A.A.; El-Sayed, O.A.; Al-Allaf, T.A.K.; Aboul-Enein, H.Y.; Kunhi, M.; Pulicat, S.M.; Al-Hussain, K.; Al-Khodairy, F.; Arif, J. Synthesis, characterization and cytotoxicity evaluation of some new platinum(II) complexes of tetrazolo[1,5-a]quinolines. Eur. J. Med. Chem., 2004, 39(6), 499-505.
[http://dx.doi.org/10.1016/j.ejmech.2004.03.003] [PMID: 15183908] , 7Herr, R.J. 5-Substituted-1H-tetrazoles as carboxylic acid isosteres: Medicinal chemistry and synthetic methods Bioorg. Med. Chem., 2002, 10, 3379.(b) Wittenberger, S. J. Recent developments in tetrazole chemistry. A review.. Org. Prep. Proced. Int., 1994, 26, 499.]. A large number of pharmacological applications of tetrazoles with anticonvulsant, antihypertensive, anti-inflammatory, antibacterial, antifungal, anticancer, glycosidase inhibitory, antidiabetic, antiulcer and antitubercular activities are reported [6Bekhit, A.A.; El-Sayed, O.A.; Al-Allaf, T.A.K.; Aboul-Enein, H.Y.; Kunhi, M.; Pulicat, S.M.; Al-Hussain, K.; Al-Khodairy, F.; Arif, J. Synthesis, characterization and cytotoxicity evaluation of some new platinum(II) complexes of tetrazolo[1,5-a]quinolines. Eur. J. Med. Chem., 2004, 39(6), 499-505.
[http://dx.doi.org/10.1016/j.ejmech.2004.03.003] [PMID: 15183908] -9George, S.; Varma, P.S.; Suresh, I.; Shanmugapandiyan, P. Synthesis, antimicrobial and anti-inflammatory activities of 3-(1-Substituted Phenyl-1h-Tetrazol-5-Yl) pyridine derivatives. Asian J Pharm Clin. Res., 2012, 5, 81-85.]. Recently, Yang et al. and others invented tetrazole derivatives as promising candidates for anticancer activity [10Yang, R-Y.; Ali, S.M.; Ashwell, M.A.; Kelleher, E.; Palma, R.; Westlund, N. US Patent, 2009, 0130117A1.(b) Bhaskar, V. H.; Mohite, P. B. J. Optoelectron. Biomed. Mater., 2010, 2, 249., 11Mautner, F.A.; Gspan, C.; Gatterer, K.; Goher, M.A.S.; Abu-Youssef, M.A.M.; Bucher, E.; Sitte, W. Synthesis and characterization of three 5-(4-pyridyl)tetrazolato complexes obtained by reaction of 4-cyanopyridine with metal azides from aqueous solutions. Polyhedron, 2004, 23, 1217-1224.
[http://dx.doi.org/10.1016/j.poly.2004.02.001] ]. Anticancer and DNA binding studies of tetrazole complexes are studied rarely [12Gagnon, A.; Landry, S.; Coulombe, R.; Jakalian, A.; Guse, I.; Thavonekham, B.; Bonneau, P.R.; Yoakim, C.; Simoneau, B. Investigation on the role of the tetrazole in the binding of thiotetrazolylacetanilides with HIV-1 wild type and K103N/Y181C double mutant reverse transcriptases. Bioorg. Med. Chem. Lett., 2009, 19(4), 1199-1205.
[http://dx.doi.org/10.1016/j.bmcl.2008.12.074] [PMID: 19138518] , 13Akhlaghinia, B.; Rezazadeh, S. A novel approach for the synthesis of 5-substituted-1H-tetrazoles. J. Braz. Chem. Soc., 2012, 23, 2197-2203.
[http://dx.doi.org/10.1590/S0103-50532013005000005] ].
While N-donar ligand, such as 1,10-phenanthroline, their copper complexes have been reported as anticancer agents since the initial discovery of the nuclease activity of Cu(phen)2 by Sigman and co-workers. Their action relies mainly on oxygen activation and DNA oxidation [14Sigman, D.S.; Graham, D.R.; D’Aurora, V.; Stern, A.M. Oxygen-dependent cleavage of DNA by the 1,10-phenanthroline. cuprous complex. Inhibition of Escherichia coli DNA polymerase I. J. Biol. Chem., 1979, 254(24), 12269-12272.
[PMID: 387784] , 15Sigman, D.S.; Mazumder, A.; Perrin, D.M. Chemical nucleases. Chem. Rev., 1993, 93(6), 2295-2316.
[http://dx.doi.org/10.1021/cr00022a011] ]. Mixed ligand copper complexes with 1,10-phenanthroline have shown good chemical nuclease activity, preferably in the presence of molecular oxygen and a reducing agent on double-stranded DNA [16Rajarajeswari, C.; Ganeshpandian, M.; Palaniandavar, M.; Riyasdeen, A.; Akbarsha, M.A. Mixed ligand copper(II) complexes of 1,10-phenanthroline with tridentate phenolate/pyridyl/(benz)imidazolyl Schiff base ligands: covalent vs non-covalent DNA binding, DNA cleavage and cytotoxicity. J. Inorg. Biochem., 2014, 140, 255-268.
[http://dx.doi.org/10.1016/j.jinorgbio.2014.07.016] [PMID: 25199844] , 17Ramakrishnan, S.; Palaniandavar, M. Mixed-ligand copper(II) complexes of dipicolylamine and 1,10-phenanthrolines: The role of diimines in the interaction of the complexes with DNA. J. Chem. Sci., 2005, 117, 179-186.
[http://dx.doi.org/10.1007/BF03356114] ]. In continuation of our search for better chemical nucleases [18Mustafa, S.; Rao, B. U.; Surendrababu, M. S.; Raju, K.K.; Rao, G.N. Synthesis, characterization, and biological activities of pendant arm-pyridyltetrazole copper(ii) complexes: DNA binding/cleavage activity and cytotoxic studies. Chem. Biodiv., 2015, 12, 1516-1534.], newly mixed ligand copper complexes with phenanthroline and pyridyl tetrazoles groups investigated their DNA binding studies and anticancer activity on MCF cell lines.
2. MATERIALS AND METHODS
All chemicals were purchased from Sigma–Aldrich of reagent grade and distilled solvents were used in the synthesis of the ligands and metal complexes. Agarose, calf thymus DNA and plasmid pBR322 were purchased from Genie Biolabs, Bangalore, India. The elemental analyses of complexes were performed by Perkin Elmer CHNS analyzer. Molar conductance was measured using a Digisun conductivity metre in DMF solvent. Mass spectral data is acquired by Q1MSQ1/ auto-injection method on PeSciex API 2000 eV spectrometer. Mass spectra were obtained on a PeSciex API 2000 eV spectrometer and Q1MSQ1/auto-injection mass spectra IR spectra were recorded on Alpha T OPUS spectrometer instrument. PAR model-155 vibrating sample magnetometer is used for magnetic moments studies in the polycrystalline state at a field strength of 2-8 kg with Ni metal as standard. EPR spectra were recorded on a Varian E-122 X-band spectrometer at liquid nitrogen temperature in DMF. TGA/DTA measurements were performed TA Instruments, Model SDT 2960 analyzer with a heating rate of 5οC min–1 in range of 25–500οC, under nitrogen flow on about 10 mg of sample in an aluminum crucible. DNA binding studies and cytotoxic studies were studied as per literature and our previous paper [18Mustafa, S.; Rao, B. U.; Surendrababu, M. S.; Raju, K.K.; Rao, G.N. Synthesis, characterization, and biological activities of pendant arm-pyridyltetrazole copper(ii) complexes: DNA binding/cleavage activity and cytotoxic studies. Chem. Biodiv., 2015, 12, 1516-1534., 19Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res., 1988, 48(17), 4827-4833.
[PMID: 3409223] ]
2.1. Synthesis of Complexes
The appropriate ligands (L1-L4) (1.60 mmol) [18Mustafa, S.; Rao, B. U.; Surendrababu, M. S.; Raju, K.K.; Rao, G.N. Synthesis, characterization, and biological activities of pendant arm-pyridyltetrazole copper(ii) complexes: DNA binding/cleavage activity and cytotoxic studies. Chem. Biodiv., 2015, 12, 1516-1534.] were dissolved in ethanol (25 ml) and added to a CuCl2.H2O (1.36 mmol) and phenanthroline(1.36 mmol) in ethanol solution (10 ml) at 120ᵒC in Teflon lined stainless steel reactor for 48hrs by solvothermal method. The resulting green colored powder is filtered, dried and collected.
[Cu(L1)(Phen)]Cl2(1): Green powder - (24 mg, yield 38%). Calcd. for C22H22CuN8Cl2, Mol. Wt. (532): Carbon, 49.58%; Hydrogen, 4.16%; Nitrogen, 21.03% ; Found: Carbon, 49.18%; Hydrogen, 4.04%; Nitrogen, 21.04%.
[Cu(L3)(Phen)]Cl2 (3): Green solid (21 mg, yield 34%). Calcd. for C20H17CuN7OCl2; Mol. Wt.: 491; Carbon, 46.40% ; Hydrogen, 3.07%; Nitrogen, 19.94; Found: Carbon, 46.13% ; Hydrogen, 3.02%; Nitrogen, 19.86%.
3. RESULTS AND DISCUSSION
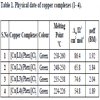
The regioisomers 2-[5-(pyridin-2-yl)-1H-tetrazole]propyl-N,N-dimethylamine] L1, (2-[5-(pyridin-2-yl)-2H-tetrazol-2-yl] propyl-N,N-dimethylamine) L2 and L3 (2-(1-ethanol-1H-tetrazol-5-yl)-pyridine); L4 (2-(2-ethanol-2H-tetrazol-5-yl)py-ridine) were prepared as per our previous paper [18Mustafa, S.; Rao, B. U.; Surendrababu, M. S.; Raju, K.K.; Rao, G.N. Synthesis, characterization, and biological activities of pendant arm-pyridyltetrazole copper(ii) complexes: DNA binding/cleavage activity and cytotoxic studies. Chem. Biodiv., 2015, 12, 1516-1534.]. A clear ethanolic solution of pyridyltetrazole derivatives (L1–L4), 1,10 - phenanthroline and CuCl2.2H2O in 1:1:1 ratio was heated at 120οC in Teflon line steel reactor to give [Cu(L)(phen)] complexes. These complexes are soluble in DMF, DMSO solvent and slightly in ethanol. The molar conductivity of complexes is shown in Table 1 (54.8 - 86.4Ω-1cm2mol-1), suggesting that complexes are highly stable in solution. The magnetic moment values of 1-4 complexes are more than the 1.73 µ (spin-only values), expected for a d9 copper (II) system [20Geary, W.J. The use of conductivity measurement in organic solvents for characterization of coordination compounds. Coord. Chem. Rev., 1971, 7, 81-122.
[http://dx.doi.org/10.1016/S0010-8545(00)80009-0] ].
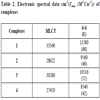
The Electronic molar extinction coefficient data of the copper complexes (1-4) are shown in Table 2. These complexes show charge transfer band in 27,450-33,560-cm-1 region. While single broadband observed due to the 2Eg2T2g transition in the region 8540- 11580 cm-1 is assigned to d-d transition. These transition bands strongly favor a distorted octahedral geometry around the metal ion, which is supported by magnetic susceptibility values [21Hathaway, B.J.; Wilkinson, G.; Gillard, R.D.; Mecleverty, J.A. (Eds.), Comprehensive coordination chemistry, 1987, 5, 81-583.].
I.R. spectra of L1 ligand show peaks at 2955, 1592 and 1450 cm-1 and L3 ligand at 2885, 1575 and 1415 cm-1 for methylene and tetrazole ring, these peaks are shifted to a lower frequency in copper complexes, suggesting the tetrazole group with copper coordination. Additional bands are observed in IR spectra of complexes at 260–220, 245–230 cm-1, apparently due to coordination with metal via nitrogen donor atoms.
The stoichiometry compositions of complexes are established by the FAB mass spectra. The molecular ion peak of complexes 1 were observed at m/z = 534.9 and 2 at m/z = 504 with [Cu(L)(Phen)]Cl2. The ESI-mass studies and elemental analyses are close agreement with the values calculate from molecular formula assigned to these complexes, which is further supported by of representative complexes.
EPR spectra of 1 and 3 were recorded in the X-band region at room temperature in the solid state. The g║ and g┴ values of 1, 3 complexes are 2.264, 2.259 and 2.058, 2.060, respectively. From Table 3, it observed g║ > g┴ > 2.0023, suggesting unpaired electron lies predominantly in the dX2-Y2 orbital.
The axial symmetry parameter (G) values are calculated [22Hathaway, B.J.; Tomlinson, A.A.G. Copper(II) ammonia complexes. Coord. Chem. Rev., 1970, 5, 1-43.
[http://dx.doi.org/10.1016/S0010-8545(00)80073-9] , 23Shebl, M. Synthesis, spectral and magnetic studies of mono- and bi-nuclear metal complexes of a new bis(tridentate NO2) Schiff base ligand derived from 4,6-diacetylresorcinol and ethanolamine. Spectrochim. Acta A Mol. Biomol. Spectrosc., 2009, 73(2), 313-323.
[http://dx.doi.org/10.1016/j.saa.2009.02.030] [PMID: 19345138] ], the values of 1 and 3 complexes are >4 (G = 4.3), suggesting that the local tetragonal axes are aligned in parallel or slightly misaligned, unpaired electron in dX2-Y2 orbital and exchange coupling effects are not operative in these complexes. The shapes of the spectra are consistent with the octahedral geometry around the Cu(II) center in the complexes [24Shebl, M. Synthesis, spectral studies, and antimicrobial activity of binary and ternary Cu(II), Ni(II), and Fe(III) complexes of new hexadentate Schiff bases derived from 4,6-diacetylresorcinol and amino acids. J. Coord. Chem., 2009, 62, 3217-3231.
[http://dx.doi.org/10.1080/00958970903012785] ].
Based on the elemental analysis, molar conductance, magnetic moments, mass, electronic, ESR and IR spectral data tentatively structures of 1-4 complexes are shown in Fig. (1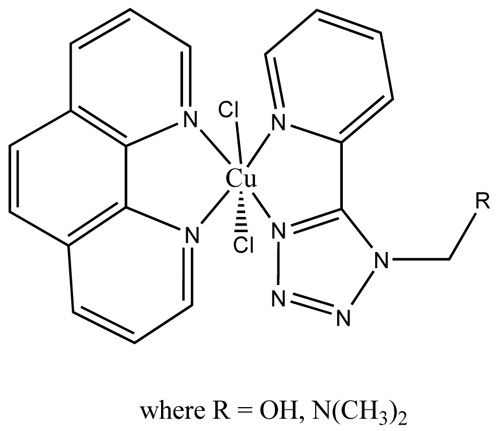 ).
).
3.1. Computational Studies
Density Functional Theory (DFT) calculations at B3LYP level were carried out for geometry optimization of 1 and 3 complexes in their ground state. The geometrical optimized structure and frontier orbital’s of HOMO and LUMO of 1 are shown in Fig (2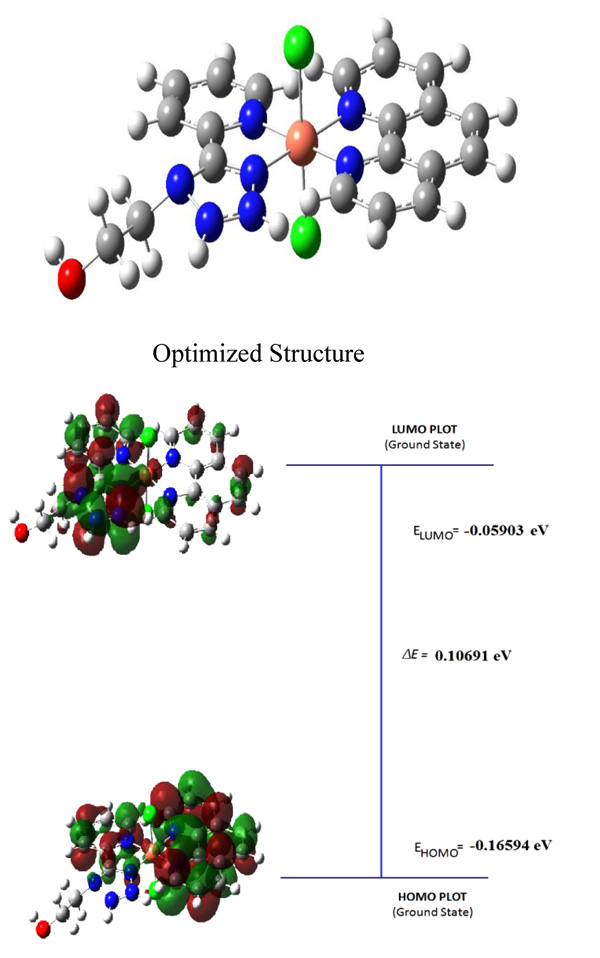 ). The measured bond lengths of Cu-N atoms of phenanthroline are 1.92ᵒA and 1.89ᵒA and Cu-N of pyridyl tetrazole are 1.89ᵒA and 1.87ᵒA, respectively.
). The measured bond lengths of Cu-N atoms of phenanthroline are 1.92ᵒA and 1.89ᵒA and Cu-N of pyridyl tetrazole are 1.89ᵒA and 1.87ᵒA, respectively.
It is clear from Fig. (3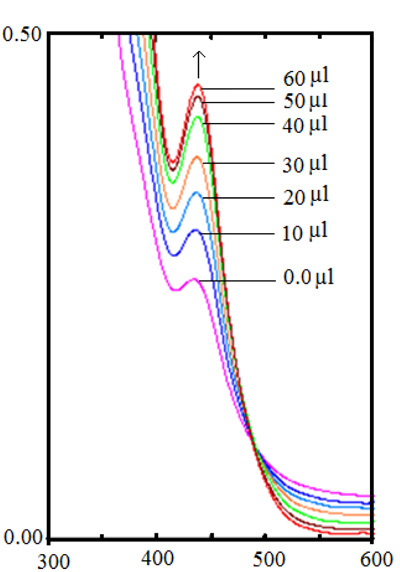 ) that the electron density of HOMO is localized on pyridyl tetrazole ring, while electron density in LUMO is largely on phenanthroline ring. The energy gap between HOMO and LUMO of 1 and 3 complexes is -0.16594 eV and -0.05903 eV which are not influenced by excitation. The electronic density of HOMO and LUMO in singlet state suggests that the absorption transition is due to the Intra-Ligand Charge Transfer (ILCT or π → π*) and partially due to ligand-to-metal charger transfer, metal-to-ligand charger transfer and Metal Centered (MC) transitions which are supported by UV-V absorption spectra.
) that the electron density of HOMO is localized on pyridyl tetrazole ring, while electron density in LUMO is largely on phenanthroline ring. The energy gap between HOMO and LUMO of 1 and 3 complexes is -0.16594 eV and -0.05903 eV which are not influenced by excitation. The electronic density of HOMO and LUMO in singlet state suggests that the absorption transition is due to the Intra-Ligand Charge Transfer (ILCT or π → π*) and partially due to ligand-to-metal charger transfer, metal-to-ligand charger transfer and Metal Centered (MC) transitions which are supported by UV-V absorption spectra.
3.2. Thermal Studies
TGA and DTA analyses were carried out to investigate the thermal stability of the 1 and 3 complexes in static air at a temperature range 30 to 630οC with a heating rate of 10οC min−1. TGA and DTA curves show a stable with no weight loss up to 130οC temperature for complex 1 (Fig. 4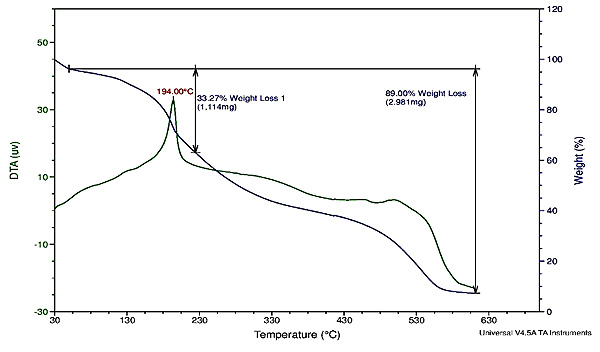 ). The initial degradation is due to the loss of phenanthroline and -N-(CH2)2(OH) moieties in complex (1), -N-(CH2)3(NH2) in complex (3) with a practical weight loss of 32.77% (Calc.31.92%) and 33.27% (Calc.31.42%). The exothermic peak of DTA curve exhibits at 171.9 and 194.0οC for 1 and 3, respectively. Further degradation in complex 1 at 365οC and 500οC is due to loss of tetrazole species and other organic moieties with a weight loss of 48.93% (Calc. 48.37%). Degradation in complex 3 at 330οC and 530οC is by the loss of tetrazole and organic moieties with a weight loss of 47.7% (Calc. 47.56%) with final residual weight to cupric oxide.
). The initial degradation is due to the loss of phenanthroline and -N-(CH2)2(OH) moieties in complex (1), -N-(CH2)3(NH2) in complex (3) with a practical weight loss of 32.77% (Calc.31.92%) and 33.27% (Calc.31.42%). The exothermic peak of DTA curve exhibits at 171.9 and 194.0οC for 1 and 3, respectively. Further degradation in complex 1 at 365οC and 500οC is due to loss of tetrazole species and other organic moieties with a weight loss of 48.93% (Calc. 48.37%). Degradation in complex 3 at 330οC and 530οC is by the loss of tetrazole and organic moieties with a weight loss of 47.7% (Calc. 47.56%) with final residual weight to cupric oxide.
 |
Fig. (1) Tentative assigned structure for copper complexes. |
 |
Fig. (2) Optimized structure, HOMO and LUMO orbital of complex 1. |
3.3. DNA Binding Studies
Complexes Vs CT-DNA binding studies were investigated by UV-Vis spectroscopy. The absorption spectra of the copper complexes were compared with and without CT-DNA at 400 nm (Fig. 3 ) and the binding constants of 1-4 complexes are given in Table 4. All the complexes (1-4) show a bathochromic and hyperchromic shift with an increase in absorbance on the addition of increasing amounts of CT-DNA with respect to control (without CT DNA). These absorbance values were used to calculate the intrinsic binding constant Kb (Table 4). There is no change in absorption studies of complexes in Tris buffer solution, suggesting that these are stable [25Nagaj, J.; Stokowa-Sołtys, K.; Kurowska, E.; Frączyk, T.; Jeżowska-Bojczuk, M.; Bal, W. Revised coordination model and stability constants of Cu(II) complexes of tris buffer. Inorg. Chem., 2013, 52(24), 13927-13933.
) and the binding constants of 1-4 complexes are given in Table 4. All the complexes (1-4) show a bathochromic and hyperchromic shift with an increase in absorbance on the addition of increasing amounts of CT-DNA with respect to control (without CT DNA). These absorbance values were used to calculate the intrinsic binding constant Kb (Table 4). There is no change in absorption studies of complexes in Tris buffer solution, suggesting that these are stable [25Nagaj, J.; Stokowa-Sołtys, K.; Kurowska, E.; Frączyk, T.; Jeżowska-Bojczuk, M.; Bal, W. Revised coordination model and stability constants of Cu(II) complexes of tris buffer. Inorg. Chem., 2013, 52(24), 13927-13933.
[http://dx.doi.org/10.1021/ic401451s] [PMID: 24304384] ]. The calculated Kb values of 1-4 complexes are in the range of 4.2 - 7.6 x 104 M-1 (Table 4) with similar binding affinity to reported copper tetrazole derivative complexes [26Haleel, A.; Arthi, P.; Reddy, N.D.; Veena, V.; Sakthivel, N.; Arun, Y.; Perumal, P.T.; Rahiman, A.K. DNA binding, molecular docking and apoptotic inducing activity of nickel(II), copper(II) and zinc(II) complexes of pyridine-based tetrazolo[1,5-a]pyrimidine ligands. RSC Adv., 2014, 4, 60816-60830.
[http://dx.doi.org/10.1039/C4RA11197D] ]. The 1-4 complexes with CT DNA interaction are through planar phenanthroline and pyridyl tetrazole ring likely via π-stacking interactions. As per the literature shift and Kb values of 1-4 complexes is similar to cis-platin, suggesting binding of 1-4 complexes to CT-DNA by electrostatic or groove binding [27Arjmand, F.; Sayeed, F.; Muddassir, M. Synthesis of new chiral heterocyclic Schiff base modulated Cu(II)/Zn(II) complexes: Their comparative binding studies with CT-DNA, mononucleotides and cleavage activity. J. Photochem. Photobiol. B, 2011, 103(2), 166-179.
[http://dx.doi.org/10.1016/j.jphotobiol.2011.03.001] [PMID: 21459607] , 28Skyrianou, K.C.; Perdih, F.; Turel, I.; Kessissoglou, D.P.; Psomas, G. Nickel-quinolones interaction. Part 2--interaction of nickel(II) with the antibacterial drug oxolinic acid. J. Inorg. Biochem., 2010, 104(2), 161-170.
[http://dx.doi.org/10.1016/j.jinorgbio.2009.10.017] [PMID: 19939458] ].
 |
Fig. (3) Absorption spectra of complex 1 with increasing concentration of CT-DNA. |
 |
Fig. (4) TGA and DTA curve of complex 1. |
4. THERMAL DENATURATION STUDIES
The Thermal Denaturation studies of 1-4 complexes with CT DNA are carried as per our previous studies and literature [29Halliwell, B.; Gutteridge, J.M.; Aruoma, O.I. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem., 1987, 165(1), 215-219.
[http://dx.doi.org/10.1016/0003-2697(87)90222-3] [PMID: 3120621] ]. The transition temperature (Tm) of CT DNA from helix form to coil from is monitored by absorbance studies at 260 nm with concentrations at [DNA]/[complex] = 25 of DNA bases. The Tm of free CT-DNA is observed at 60.1οC under employed experimental conditions. Under similar conditions, the addition of complexes 1-4 to CT DNA increased Tm values by 4, 3, 3 and 3οC respectively, indicating that 1-4 complexes stabilize the double helix of DNA.
5. CYTOTOXICITY STUDIES
XTT assay (2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl)-2 H-tetrazolium-5-carboxanilide) was used to measure the cell proliferation activity at different concentrations of 1-4 complexes (5, 10, 50 and 100 µM) and tested in triplicate for 48 h [30Hangan, A.; Bodoki, A.; Oprean, L.; Alzuet, G.; Liu-Gonz, M.; Borr, J. Synthesis, crystallographic and spectroscopic characterization and magnetic properties of dimer and monomer ternary copper(II) complexes with sulfonamide derivatives and 1,10-phenanthroline. Nuclease activity by the oxidative mechanism. Polyhedron, 2010, 29, 1305-1313.
[http://dx.doi.org/10.1016/j.poly.2009.12.030] , 31Roehm, N.W.; Rodgers, G.H.; Hatfield, S.M.; Glasebrook, A.L. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J. Immunol. Methods, 1991, 142(2), 257-265.
[http://dx.doi.org/10.1016/0022-1759(91)90114-U] [PMID: 1919029] ]. These results are expressed in average-standard deviation of two experiments obtained and Cis platin was used as a standard control. The IC50 values of the 1-4 complexes show dose-dependent effective inhibition on the growth of MCF-7 cells growth. The IC50 values of complexes show excellent activity with 24(± 0.5); 18(± 0.5); 20(±0.5); (±0.5) and 38 (±0.8) for 1, 2, 3, 4 and cis platin complexes, respectively. After 72 h of the treatment of 1 on MCF-7 cell, the IC50 values hinder the cell growth upto 24(± 0.5) µg/ml at 5 µM concentration range (Fig. 5 ). It is apparent from IC50 values that the order inhibition is 1 > 3 >2 > 4.
). It is apparent from IC50 values that the order inhibition is 1 > 3 >2 > 4.
 |
Fig. (5) Cytotoxic activities of a)–c) Cis platin(control), d)–f) complex 1 and after 12, 24, and 48 h, respectively. |
6. INHIBITORY EFFECTS OF COMPLEXES ON THE SURVIVAL OF MCF-7 AT DIFFERENT CONCENTR- ATIONS
Survival studies of MCF -7 at different concentrations were carried out by incubating cells with complexes 1-4 constantly and washed to remove the copper complex. The cell survival was determined at the complex concentration of 5, 10, 50 and 100 µM. At these concentration, complexes 1 was able to kill 78%; 65%; 48% and 46%, respectively. All copper complexes show better toxicity at low concentration 5 µg/ml with 55%, 38% 28%; and 24%; for complex 1-4, respectively.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise
ACKNOWLEDGEMENT
Declared none.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers Website along with the published article.
REFERENCES
| [1] | Maret, W. Analyzing free zinc(II) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics, 2015, 7(2), 202-211. [http://dx.doi.org/10.1039/C4MT00230J] [PMID: 25362967] |
| [2] | Rivalta, I.; Brudvig, G.W.; Batista, V.S. Oxomanganese complexes for natural and artificial photosynthesis. Curr. Opin. Chem. Biol., 2012, 16(1-2), 11-18. [http://dx.doi.org/10.1016/j.cbpa.2012.03.003] [PMID: 22481113] |
| [3] | Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in copper complexes as anticancer agents. Chem. Rev., 2014, 114(1), 815-862. [http://dx.doi.org/10.1021/cr400135x] [PMID: 24102434] |
| [4] | Nies, D.H. The biological chemistry of the transition metal “transportome” of cupriavidus metallidurans. Metallomics, 2016, 8(5), 481-507. [http://dx.doi.org/10.1039/C5MT00320B] [PMID: 27065183] |
| [5] | Sheridan, U.; Gallagher, J.F.; Bjerrum, M.J.; Fleming, A.; Kelleher, F.; McGinley, J. Metal-organic frameworks based on pyridyl-tetrazole ligands containing ester or carboxylate pendant arms. J Inorg Chim Acta, 2014, 421, 200. [http://dx.doi.org/10.1016/j.ica.2014.05.028] |
| [6] | Bekhit, A.A.; El-Sayed, O.A.; Al-Allaf, T.A.K.; Aboul-Enein, H.Y.; Kunhi, M.; Pulicat, S.M.; Al-Hussain, K.; Al-Khodairy, F.; Arif, J. Synthesis, characterization and cytotoxicity evaluation of some new platinum(II) complexes of tetrazolo[1,5-a]quinolines. Eur. J. Med. Chem., 2004, 39(6), 499-505. [http://dx.doi.org/10.1016/j.ejmech.2004.03.003] [PMID: 15183908] |
| [7] | Herr, R.J. 5-Substituted-1H-tetrazoles as carboxylic acid isosteres: Medicinal chemistry and synthetic methods Bioorg. Med. Chem., 2002, 10, 3379.(b) Wittenberger, S. J. Recent developments in tetrazole chemistry. A review.. Org. Prep. Proced. Int., 1994, 26, 499. |
| [8] | Popova, E.A.; Trifonov, R.E.; Ostrovskii, V.A. Advances in synthesis of tetrazoles coordinated to metal ions. ARKIVO, 2012, 1, 45-65. |
| [9] | George, S.; Varma, P.S.; Suresh, I.; Shanmugapandiyan, P. Synthesis, antimicrobial and anti-inflammatory activities of 3-(1-Substituted Phenyl-1h-Tetrazol-5-Yl) pyridine derivatives. Asian J Pharm Clin. Res., 2012, 5, 81-85. |
| [10] | Yang, R-Y.; Ali, S.M.; Ashwell, M.A.; Kelleher, E.; Palma, R.; Westlund, N. US Patent, 2009, 0130117A1.(b) Bhaskar, V. H.; Mohite, P. B. J. Optoelectron. Biomed. Mater., 2010, 2, 249. |
| [11] | Mautner, F.A.; Gspan, C.; Gatterer, K.; Goher, M.A.S.; Abu-Youssef, M.A.M.; Bucher, E.; Sitte, W. Synthesis and characterization of three 5-(4-pyridyl)tetrazolato complexes obtained by reaction of 4-cyanopyridine with metal azides from aqueous solutions. Polyhedron, 2004, 23, 1217-1224. [http://dx.doi.org/10.1016/j.poly.2004.02.001] |
| [12] | Gagnon, A.; Landry, S.; Coulombe, R.; Jakalian, A.; Guse, I.; Thavonekham, B.; Bonneau, P.R.; Yoakim, C.; Simoneau, B. Investigation on the role of the tetrazole in the binding of thiotetrazolylacetanilides with HIV-1 wild type and K103N/Y181C double mutant reverse transcriptases. Bioorg. Med. Chem. Lett., 2009, 19(4), 1199-1205. [http://dx.doi.org/10.1016/j.bmcl.2008.12.074] [PMID: 19138518] |
| [13] | Akhlaghinia, B.; Rezazadeh, S. A novel approach for the synthesis of 5-substituted-1H-tetrazoles. J. Braz. Chem. Soc., 2012, 23, 2197-2203. [http://dx.doi.org/10.1590/S0103-50532013005000005] |
| [14] | Sigman, D.S.; Graham, D.R.; D’Aurora, V.; Stern, A.M. Oxygen-dependent cleavage of DNA by the 1,10-phenanthroline. cuprous complex. Inhibition of Escherichia coli DNA polymerase I. J. Biol. Chem., 1979, 254(24), 12269-12272. [PMID: 387784] |
| [15] | Sigman, D.S.; Mazumder, A.; Perrin, D.M. Chemical nucleases. Chem. Rev., 1993, 93(6), 2295-2316. [http://dx.doi.org/10.1021/cr00022a011] |
| [16] | Rajarajeswari, C.; Ganeshpandian, M.; Palaniandavar, M.; Riyasdeen, A.; Akbarsha, M.A. Mixed ligand copper(II) complexes of 1,10-phenanthroline with tridentate phenolate/pyridyl/(benz)imidazolyl Schiff base ligands: covalent vs non-covalent DNA binding, DNA cleavage and cytotoxicity. J. Inorg. Biochem., 2014, 140, 255-268. [http://dx.doi.org/10.1016/j.jinorgbio.2014.07.016] [PMID: 25199844] |
| [17] | Ramakrishnan, S.; Palaniandavar, M. Mixed-ligand copper(II) complexes of dipicolylamine and 1,10-phenanthrolines: The role of diimines in the interaction of the complexes with DNA. J. Chem. Sci., 2005, 117, 179-186. [http://dx.doi.org/10.1007/BF03356114] |
| [18] | Mustafa, S.; Rao, B. U.; Surendrababu, M. S.; Raju, K.K.; Rao, G.N. Synthesis, characterization, and biological activities of pendant arm-pyridyltetrazole copper(ii) complexes: DNA binding/cleavage activity and cytotoxic studies. Chem. Biodiv., 2015, 12, 1516-1534. |
| [19] | Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res., 1988, 48(17), 4827-4833. [PMID: 3409223] |
| [20] | Geary, W.J. The use of conductivity measurement in organic solvents for characterization of coordination compounds. Coord. Chem. Rev., 1971, 7, 81-122. [http://dx.doi.org/10.1016/S0010-8545(00)80009-0] |
| [21] | Hathaway, B.J.; Wilkinson, G.; Gillard, R.D.; Mecleverty, J.A. (Eds.), Comprehensive coordination chemistry, 1987, 5, 81-583. |
| [22] | Hathaway, B.J.; Tomlinson, A.A.G. Copper(II) ammonia complexes. Coord. Chem. Rev., 1970, 5, 1-43. [http://dx.doi.org/10.1016/S0010-8545(00)80073-9] |
| [23] | Shebl, M. Synthesis, spectral and magnetic studies of mono- and bi-nuclear metal complexes of a new bis(tridentate NO2) Schiff base ligand derived from 4,6-diacetylresorcinol and ethanolamine. Spectrochim. Acta A Mol. Biomol. Spectrosc., 2009, 73(2), 313-323. [http://dx.doi.org/10.1016/j.saa.2009.02.030] [PMID: 19345138] |
| [24] | Shebl, M. Synthesis, spectral studies, and antimicrobial activity of binary and ternary Cu(II), Ni(II), and Fe(III) complexes of new hexadentate Schiff bases derived from 4,6-diacetylresorcinol and amino acids. J. Coord. Chem., 2009, 62, 3217-3231. [http://dx.doi.org/10.1080/00958970903012785] |
| [25] | Nagaj, J.; Stokowa-Sołtys, K.; Kurowska, E.; Frączyk, T.; Jeżowska-Bojczuk, M.; Bal, W. Revised coordination model and stability constants of Cu(II) complexes of tris buffer. Inorg. Chem., 2013, 52(24), 13927-13933. [http://dx.doi.org/10.1021/ic401451s] [PMID: 24304384] |
| [26] | Haleel, A.; Arthi, P.; Reddy, N.D.; Veena, V.; Sakthivel, N.; Arun, Y.; Perumal, P.T.; Rahiman, A.K. DNA binding, molecular docking and apoptotic inducing activity of nickel(II), copper(II) and zinc(II) complexes of pyridine-based tetrazolo[1,5-a]pyrimidine ligands. RSC Adv., 2014, 4, 60816-60830. [http://dx.doi.org/10.1039/C4RA11197D] |
| [27] | Arjmand, F.; Sayeed, F.; Muddassir, M. Synthesis of new chiral heterocyclic Schiff base modulated Cu(II)/Zn(II) complexes: Their comparative binding studies with CT-DNA, mononucleotides and cleavage activity. J. Photochem. Photobiol. B, 2011, 103(2), 166-179. [http://dx.doi.org/10.1016/j.jphotobiol.2011.03.001] [PMID: 21459607] |
| [28] | Skyrianou, K.C.; Perdih, F.; Turel, I.; Kessissoglou, D.P.; Psomas, G. Nickel-quinolones interaction. Part 2--interaction of nickel(II) with the antibacterial drug oxolinic acid. J. Inorg. Biochem., 2010, 104(2), 161-170. [http://dx.doi.org/10.1016/j.jinorgbio.2009.10.017] [PMID: 19939458] |
| [29] | Halliwell, B.; Gutteridge, J.M.; Aruoma, O.I. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem., 1987, 165(1), 215-219. [http://dx.doi.org/10.1016/0003-2697(87)90222-3] [PMID: 3120621] |
| [30] | Hangan, A.; Bodoki, A.; Oprean, L.; Alzuet, G.; Liu-Gonz, M.; Borr, J. Synthesis, crystallographic and spectroscopic characterization and magnetic properties of dimer and monomer ternary copper(II) complexes with sulfonamide derivatives and 1,10-phenanthroline. Nuclease activity by the oxidative mechanism. Polyhedron, 2010, 29, 1305-1313. [http://dx.doi.org/10.1016/j.poly.2009.12.030] |
| [31] | Roehm, N.W.; Rodgers, G.H.; Hatfield, S.M.; Glasebrook, A.L. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J. Immunol. Methods, 1991, 142(2), 257-265. [http://dx.doi.org/10.1016/0022-1759(91)90114-U] [PMID: 1919029] |