- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Evaluation of Safety, Antileishmanial, and Chemistry of Ethanolic Leaves Extracts of Seven Medicinal Plants: An In-vitro Study
Nargis Shaheen1, Naveeda Akhter Qureshi1, *, Attiya Iqbal1, Asma Ashraf1, Huma Fatima1
Abstract
Background:
Cutaneous leishmaniasis is a neglected tropical disease that currently affects people among 98 countries and causes significant morbidity and mortality. Current chemotherapeutic intervention is unsatisfactory and has various limitations that highlight the necessity to develop safe and effective therapeutic approaches from natural products.
Objective:
The main objective of current study was the evaluation of the antileishmanial activity along with toxicity assessment of selected plant extracts.
Methods:
The ethanolic leaves extracts of selected plants were evaluated for their qualitative and quantitative phytochemical screening by standard protocols. The antioxidant potential of plant extracts was determined by total antioxidant capacity, ferric reducing power and DPPH radical scavenging assays. The cytotoxicity analysis using brine shrimp lethality assay and in-vitro antileishmanial activity against promastigotes of L. tropica (Accession# MN891719) were also evaluated.
Results:
The preliminary examination of crude extracts revealed that P. armeniaca showed the highest total phenolic and flavonoid content (279.62±5.40µgGAE/mgDW and 205.70 ±2.41µgQA/mgDW, respectively), among others. P. armeniaca showed strongest antioxidants (120.37±4.90 µgAAE/mgDW) and FRP values (278.71±1.03µgAAE/mgDW). All the plant extracts showed cytotoxicity in safety range >1000µg/ml except F. glomerata having LC50 values of 454.34 µg/ml. In the present study, P. communis and P. pashia showed some level of activity (LC50 56.68 and 60.95µg/ml respectively) while P. armeniaca demonstrated the highest antileishmanial activity (LC50 16.18µg/ml).
Conclusion:
The findings are highly encouraging so, further and extensive investigations of P. arminica should be carried out; especially bio guided fractionation to identify the active fraction and further chemical characterization of structure.
Article Information
Identifiers and Pagination:
Year: 2020Volume: 7
First Page: 26
Last Page: 36
Publisher Id: CHEM-7-26
DOI: 10.2174/1874842202007010026
Article History:
Received Date: 22/5/2020Revision Received Date: 18/8/2020
Acceptance Date: 21/8/2020
Electronic publication date: 20/11/2020
Collection year: 2020
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at Department of Zoology, Faculty of Biological Science, Quaid-i-Azam University, Islamabad 45320, Pakistan;
Tel: +92-051-90643201; E-mail: nqureshi@qau.edu.pk
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 22-5-2020 |
Original Manuscript | Evaluation of Safety, Antileishmanial, and Chemistry of Ethanolic Leaves Extracts of Seven Medicinal Plants: An In-vitro Study | |
1. INTRODUCTION
Leishmaniasis is a life-threatening vector born infection caused by a parasite of the genus Leishmania (family Trypanosomatidea) [1Croft, S.L.; Sundar, S.; Fairlamb, A.H. Drug resistance in leishmaniasis. Clin. Microbiol. Rev., 2006, 19(1), 111-126.
[http://dx.doi.org/10.1128/CMR.19.1.111-126.2006] [PMID: 16418526] ]. The female sandfly (by bite) belonging to genera Phlebotomus and Lutzomyia is responsible for Leishmania transmission [2Steverding, D. The history of leishmaniasis. Parasit. Vectors, 2017, 10(1), 82-92.
[http://dx.doi.org/10.1186/s13071-017-2028-5] [PMID: 28202044] ]. The leishmaniasis includes a wide range of clinical manifestations that is determined by the immune response of the human host, and affecting species are divided into three groups: (i) Visceral leishmaniasis, (ii) Mucocutaneous leishmaniasis, and (iii) Cutaneous leishmaniasis [3Zulfiqar, B.; Shelper, T.B.; Avery, V.M. Leishmaniasis drug discovery: recent progress and challenges in assay development. Drug Discov. Today, 2017, 22(10), 1516-1531.
[http://dx.doi.org/10.1016/j.drudis.2017.06.004] [PMID: 28647378] ]. The causative agents of CL belong to various Leishmania spp. i.e., Leishmania major, Leishmania tropica, Leishmania braziliensis, and Leishmania amazonensis. In Pakistan, the most prevalent form of CL is L. tropica [4Brooker, S.; Mohammed, N.; Adil, K.; Agha, S.; Reithinger, R.; Rowland, M.; Ali, I.; Kolaczinski, J. Leishmaniasis in refugee and local Pakistani populations. Emerg. Infect. Dis., 2004, 10(9), 1681-1684.
[http://dx.doi.org/10.3201/eid1009.040179] [PMID: 15498178] ], and it has a huge impact on public health [5Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. RE:view, 2017, 6, 750.]. About 1.5-2 million individuals are affected by this infection in 98 countries, while 350 billion people are breathing in those areas which are prone to leishmaniasis [6de Souza, A.; Marins, D.S.S.; Mathias, S.L.; Monteiro, L.M.; Yukuyama, M.N.; Scarim, C.B.; Löbenberg, R.; Bou-Chacra, N.A. Promising nanotherapy in treating leishmaniasis. Int. J. Pharm., 2018, 547(1-2), 421-431.
[http://dx.doi.org/10.1016/j.ijpharm.2018.06.018] [PMID: 29886097] ]. Approximately 70,000 deaths are recorded due to CL infection annually [7Blum, J.; Desjeux, P.; Schwartz, E.; Beck, B.; Hatz, C. Treatment of cutaneous leishmaniasis among travellers. J. Antimicrob. Chemother., 2004, 53(2), 158-166.
[http://dx.doi.org/10.1093/jac/dkh058] [PMID: 14729756] ]. CL is endemic in the following eight countries; Afghanistan, Iran, Pakistan, Saudi Arabia, Algeria, Peru, Brazil, and Syria [8Hepburn, N.C. Cutaneous leishmaniasis: current and future management. Expert Rev. Anti Infect. Ther., 2003, 1(4), 563-570.
[http://dx.doi.org/10.1586/14787210.1.4.563] [PMID: 15482153] ]. In Pakistan, devastating epidemics of CL has been described in Khyber Pakhtunkhwa, Punjab, Baluchistan, Sindh, and Azad Jammu Kashmir [9Noor, S.M.; Hussain, D. Cutaneous leishmaniasis in Sadda, Kurram agency, Pakistan. J. Pak. Assoc. Dermatol., 2004, 14, 114-117.-12Talat, H.; Attarwala, S.; Saleem, M. Cutaneous leishmaniasis with HIV-Case report. J. Coll. Physicians Surg. Pak., 2014, 24, 93-95.]. The spread of CL increase to non-endemic areas of Pakistan is due to the migration of millions of refugees toward North Western Pakistan [13Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. Leishmaniasis worldwide and global estimates of its incidence. PLoS One, 2012, 7(5)e35671
[http://dx.doi.org/10.1371/journal.pone.0035671] [PMID: 22693548] ]. It is now well documented that CL is one of the important neglected tropical diseases with the utmost disease burden [14González, C.; Wang, O.; Strutz, S.E.; González-Salazar, C. Sánchez- Cordero, V.; Sarkar, S. Climate change and risk of leishmaniasis in NorthAmerica: predictions from ecological nichemodels of vector and reservoir species. PLoS Negl. Trop. Dis., 2010, 4, 585.
[http://dx.doi.org/10.1371/journal.pntd.0000585] , 152016.http://www.who.int/leishmaniasis/en/].
Up to now, there is no effective vaccine available for the treatment of CL [16Bekhit, A.A.; El-Agroudy, E.; Helmy, A.; Ibrahim, T.M.; Shavandi, A.; Bekhit, A.E.A. Leishmania treatment and prevention: Natural and synthesized drugs. Eur. J. Med. Chem., 2018, 160, 229-244.
[http://dx.doi.org/10.1016/j.ejmech.2018.10.022] [PMID: 30342363] ]. Chemotherapy is the main approach for the treatment of CL infection. The 1st line treatment of CL, including sodium stibogluconate (pentostam) and meglumine antimoniate (glucantime) is usually recommended since1959 [17Camacho, Md.; Phillipson, J.D.; Croft, S.L.; Solis, P.N.; Marshall, S.J.; Ghazanfar, S.A. Screening of plant extracts for antiprotozoal and cytotoxic activities. J. Ethnopharmacol., 2003, 89(2-3), 185-191.
[http://dx.doi.org/10.1016/S0378-8741(03)00269-1] [PMID: 14611881] ]. Treatment of CL with the pentavalent antimonials compound has some limitations because it needs intramuscular injection administration, side effects, resistance, and prolonged treatment periods [17Camacho, Md.; Phillipson, J.D.; Croft, S.L.; Solis, P.N.; Marshall, S.J.; Ghazanfar, S.A. Screening of plant extracts for antiprotozoal and cytotoxic activities. J. Ethnopharmacol., 2003, 89(2-3), 185-191.
[http://dx.doi.org/10.1016/S0378-8741(03)00269-1] [PMID: 14611881] , 18Sen, R.; Chatterjee, M. Plant derived therapeutics for the treatment of Leishmaniasis. Phytomedicine, 2011, 18(12), 1056-1069.
[http://dx.doi.org/10.1016/j.phymed.2011.03.004] [PMID: 21596544] ]. Moreover, the 2nd line drugs, i.e., pentamidine and amphotericin b are toxic and expensive [17Camacho, Md.; Phillipson, J.D.; Croft, S.L.; Solis, P.N.; Marshall, S.J.; Ghazanfar, S.A. Screening of plant extracts for antiprotozoal and cytotoxic activities. J. Ethnopharmacol., 2003, 89(2-3), 185-191.
[http://dx.doi.org/10.1016/S0378-8741(03)00269-1] [PMID: 14611881] ]. The pentavalent antimony compound glucantime is the most common commercially available medication used for the treatment of CL in Pakistan, Iran, Afghanistan, and other parts of the world [19Saebi, E. Parasitic diseases in Iran (protozoa)., (9th ed. ), 2005, -22Rashid, U.; Sultana, R.; Shaheen, N.; Hassan, S.F.; Yaqoob, F.; Ahmad, M.J.; Iftikhar, F.; Sultana, N.; Asghar, S.; Yasinzai, M.; Ansari, F.L.; Qureshi, N.A. Structure based medicinal chemistry-driven strategy to design substituted dihydropyrimidines as potential antileishmanial agents. Eur. J. Med. Chem., 2016, 115, 230-244.
[http://dx.doi.org/10.1016/j.ejmech.2016.03.022] [PMID: 27017551] ]. The amphotericin b deoxycholate is used as 1st choice drug to cure the pregnant women and 2nd choice when pentavalent antimonials response is failed [23Brasil, Manual de Vigilância da Leishmaniose Tegumentar Americana., (3rd ed. ), 2010, ]. However, in Brazil, amphotericin b has been associated with severe side effects [24Bennett, J.E. Antimicrobial: antifungal agents.Goodman and Gilman’s the Pharmacological Basis of Therapeutics., (11th ed. ) 2006, , 1225-1242.
]. Liposomal amphotericin b also reported adverse effects [25Solomon, M.; Baum, S.; Barzilai, A.; Scope, A.; Trau, H.; Schwartz, E. Liposomal amphotericin B in comparison to sodium stibogluconate for cutaneous infection due to Leishmania braziliensis. J. Am. Acad. Dermatol., 2007, 56(4), 612-616.
[http://dx.doi.org/10.1016/j.jaad.2006.06.044] [PMID: 17276541] ]. The safety profile of various amphotericin formulations has not been evaluated. Even though liposomal amphotericin b has not yet been approved against leishmaniasis in few countries such as Brazil, the medicine indicated in a case in which other options (amphotericin b deoxycholate, pentamidine, and pentavalent antimonials) are unsuccessful [23Brasil, Manual de Vigilância da Leishmaniose Tegumentar Americana., (3rd ed. ), 2010, ]. All synthetic medicines have side effects that enhance the enzyme secretions such as glucantime, which creates kidney and liver problems [26Hadighi, R.; Mohebali, M.; Boucher, P.; Hajjaran, H.; Khamesipour, A.; Ouellette, M. Unresponsiveness to Glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS Med., 2006, 3(5)e162
[http://dx.doi.org/10.1371/journal.pmed.0030162] [PMID: 16605301] , 27al-Majali, O.; Routh, H.B.; Abuloham, O.; Bhowmik, K.R.; Muhsen, M.; Hebeheba, H. A 2-year study of liquid nitrogen therapy in cutaneous leishmaniasis. Int. J. Dermatol., 1997, 36(6), 460-462.
[http://dx.doi.org/10.1046/j.1365-4362.1997.00045.x] [PMID: 9248896] ]. There are many other therapeutic regimens used, but no agreement exists over which is the best one [28Gonzales, U.; Pinart, M.; Reginfo-Pardo, M.; Macaya, A.; Alvar, J.; Tweed, J.A. Interventions for American cutaneous and mucocutaneous leishmaniasis Cochrane Databas. Syst. Rev., 2009, 15, 4-34.]. The drugs, which are effective to some extent, are not economically feasible and are unavailable in the neglected geographical regions. The focus should be on the compounds free of side effects on the liver, kidney and renal artery failures [29Bahmani, M.; Eftekhari, Z. An ethnoveterinary study of medicinal plants in treatment of diseases and syndromes of herd dog in southern regions of Ilam province, Iran. Comp. Clin. Pathol., 2012, 22(3), 403-407.
[http://dx.doi.org/10.1007/s00580-012-1423-8] [PMID: 23667351] -31Bahmani, M.; Rafieian-Kopaei, M.; Avijgan, M.; Hosseini, S.; Golshahi, H. Ethnobotanical studies of medicinal plants used by Kurdish owner’s in south range of Ilam Province, west of Iran. Am.-Eurasian J. Agric. Environ. Sci., 2012, 12, 1128-1133.]. A new alternative effective drug is urgently needed to treat CL infection because new cases of leishmaniasis are increasing now a day [32Brodskyn, C.; de Oliveira, C.I.; Barral, A.; Barral-Netto, M. Vaccines in leishmaniasis: advances in the last five years. Expert Rev. Vaccines, 2003, 2(5), 705-717.
[http://dx.doi.org/10.1586/14760584.2.5.705] [PMID: 14711330] ]. The importance of medicinal plants due to the presence of various medical agents gains more interest worldwide [33Shahbazi, Y. Antibacterial and Antioxidant Properties of Methanolic Extracts of Apple (Malus pumila), Grape (Vitis vinifera), Pomegranate (Punica granatum L.) and Common Fig (Ficus carica L.) Fruits. Pharm. Sci., 2017, 23, 308-315.
[http://dx.doi.org/10.15171/PS.2017.45] ]. Therefore, there is a vital need for the screening of natural plants against leishmaniasis. People in countryside areas prefer traditional medicinal usage for curing health services [34Chan-Bacab, M.J.; Peña-Rodríguez, L.M. Plant natural products with leishmanicidal activity. Nat. Prod. Rep., 2001, 18(6), 674-688.
[http://dx.doi.org/10.1039/b100455g] [PMID: 11820764] ]. The natural products having high antileishmanial activity were used for the discovery of the active compounds. About 250,000 medicinal plants have been reported worldwide. However, only 6% have been evaluated for their biological activities. In clinical trials, only about 1% of therapeutic natural products are investigated [18Sen, R.; Chatterjee, M. Plant derived therapeutics for the treatment of Leishmaniasis. Phytomedicine, 2011, 18(12), 1056-1069.
[http://dx.doi.org/10.1016/j.phymed.2011.03.004] [PMID: 21596544] , 35Jameel, M.; Islamuddin, M.; Ali, A.; Afrin, F.; Ali, M. Isolation, characterization and antimicrobial evaluation of a novel compound N-octacosan 7β ol, from Fumaria parviflora Lam. BMC Complement. Altern. Med., 2014, 14(14), 98.
[http://dx.doi.org/10.1186/1472-6882-14-98] [PMID: 24621260] ]. Approximately 35% of standard drugs developed from semi-synthetic derivatives, while about 30% were based on pharmacophore or natural products. However, it is notable that about 65% of parasitic medications [152016.http://www.who.int/leishmaniasis/en/] isolated from natural products have been accepted by health authorities from 1981-2006 [36Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod., 2012, 75(3), 311-335.
[http://dx.doi.org/10.1021/np200906s] [PMID: 22316239] ]. In Iran, plant-based drugs are inexpensive and are proven to be more effective against different infectious diseases. An important assessment of the clinical data revealed that herbal medication is commonly approved well than synthetic [37Delfan, B.; Bahmani, M.; Hassanzadazar, H.; Saki, K.; Rafieian-Kopaei, M. Identification of medicinal plants affecting on headaches and migraines in Lorestan Province, West of Iran. Asian Pac. J. Trop. Med., 2014, 7S1, S376-S379.
[http://dx.doi.org/10.1016/S1995-7645(14)60261-3] [PMID: 25312153] ].
Kashmir is well known as a global center for the diversity of plants [38Shinwari, M.I.; Shinwari, M.I. Botanical Diversity in Pakistan; Past, Present and Future., 2010, , 85-104.]. The wide topographical variations in plant species ranging from alpine subtropical flora and higher altitude flora plains [39Afshan, N.S.; Iqbal, S.H.; Khalid, A.N.; Niazi, A.R. Some additions to the Uredinales of Azad Jammu and Kashmir, Pakistan. Pak. J. Bot., 2011, 43(2), 1373-1379.]. About 80% of Pakistan’s plant species are confined to Kashmir and the Western Mountains [40Ali, S.I. Significance of flora with special reference to Pakistan. Pak. J. Bot., 2008, 40(30), 967-971.]. Due to vastness and inaccessibility with climate variation, numerous regions of Azad Jammu and Kashmir remain unexplored. Trar Khel was given less attention by taxonomists as an integral part of the Western Himalayan Kashmir. Trar Khel is a mountainous region topographically situated in the humid Himalayan climate. Therefore, it deserves special consideration for the preservation of the environment and also for the suitable development of natural products [41Haq, F.U.; Ahmad, H.; Alam, M.; Ahmad, I. Rahatullah. Species Diversity of Vascular Plants of Nandiar Valley Western Himalaya, Pakistan. Pak. J. Bot., 2010, 42, 213-229.]. In Azad Jammu and Kashmir, there is no report of the test conducted on medicinal plants for antileishmanial activity [40Ali, S.I. Significance of flora with special reference to Pakistan. Pak. J. Bot., 2008, 40(30), 967-971.]. Therefore, this study aims to explore the phytochemical properties, antioxidant activities, and in-vitro antileishmanial activity of Pyrus pashia, Malus pumila, Prunus persica, Pyrus communis, Prunus armeniaca, Ficus glomerata. and Diospyros lotus extracts. If the promising activity against promastigotes of L. tropica of these medicinal plants is evaluated by in-vitro assay, the more effective natural antileishmanial component can be prepared in further studies due to the availability of raw material in high access.
2. MATERIALS AND METHODS
2.1. Study Area
Azad Jammu and Kashmir is a Western Himalayan foothill with a surface area of 13, 269km2 lies in northeastern Pakistan. Trar Khel is one of the tensile of district Sudhnoti Azad Jammu and Kashmir. It is located between 73o41' 9” East longitudes and 33o42'54” North latitude with an elevation range of 1372m. The area of study is mountainous and hilly and can be divided into the temperate, subtropical, and alpine zone. The climate of Trar Khel is with moderate hot summer, and cold winter is predominantly moist temperate to alpine (Fig. 1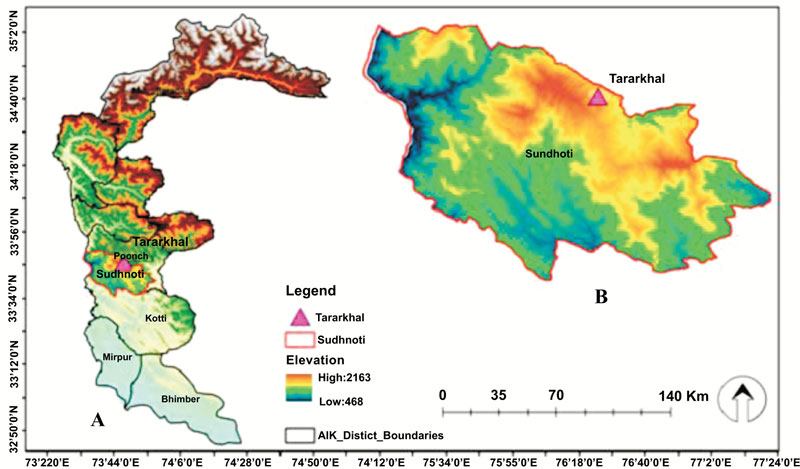 ).
).
2.2. Plant Collection and Identification
The leaves of P. pashia, M. pumila, P. persica, P. communis, P. armeniaca, F. glomerata, and D. lotus were collected from Trar Khel in 2018. The collected plant species were identified through Pakistan flora [42Nasir, E.; Ali, S.; Stewart, R.R. Flora of West Pakistan: an annotated catalogue of the 560 vascular plants of West Pakistan and Kashmir. Fakhri., 1972, 561, 56., 43Nasir, YJ.; Ali, S. Flora of Pakistan. Department of Botany, University of Karachi National Herbarium., 1994-2010, 562], and the voucher specimens were deposited in Herbarium of Pakistan, Quaid-i-Azam University Islamabad. The plant families’ names, common name, and voucher numbers, are presented in Table 1. After collection, the leaves of plants were washed with tap water and shadow dried for three weeks at room temperature (27-37ºC).
2.3. Extracts Preparation
After drying, the collected leaves were crushed in an electric mill (mesh, IKA MF: pore diameter 0.5mm). The powder (30g) obtained was used for extraction in (100%) ethanol solvent (250ml) by using the Soxhlet apparatus (Shanghai Heqi, China) at 40-60oC (6 cycles per hour) for eight hours. The resultant extracts were filtered by Whatman No. 1 filter, and the solvent was removed using a rotary vacuum evaporator (R-300, Rotavapor, Germany). The resultant extracts were stored at 4oC for further analysis.
2.4. Phytochemical Analysis
A standard qualitative phytochemical test was conducted in the current study for the determination of saponins, terpenoids, flavonoids, alkaloids [44Chew, Y.L.; Goh, J.K.; Lim, Y.Y. Assessment of in vitro antioxidant capacity and polyphenolic composition of selected medicinal herbs from Leguminosae family in Peninsular Malaysia. Food Chem., 2009, 119, 373-378.
[http://dx.doi.org/10.1016/j.foodchem.2009.01.091] ], phenols [45Al-Daihan, S.; Al-Faham, M.; Al-Shawi, N.; Almayman, R.; Brnawi, A.; Zargar, S. Antibacterial activity and phytochemical screening of some medicinal plants commonly used in Saudi Arabia against selected pathogenic microorganisms. J King Saud Uni-Sci., 2013, 25, 115-120.
[http://dx.doi.org/10.1016/j.jksus.2012.11.003] ], tannins [46Raaman, N. Phytochemical techniques. Pitam Pura., 2006, , 2022.], and coumarins [47Evans, W. C. Trease and Evans Pharmacognosy, (16th. ) , 22, 24, 25, 28-42.] in selected ethanolic leaves extracts.
2.4.1. Total Phenolic Content
For quantitative phytochemical analysis, the Folin-Ciocalteu (FC) method was used to determine total phenolic content (TPC) in the plant extracts [48Ranalli, A.; Contento, S.; Lucera, L.; Di Febo, M.; Marchegiani, D.; Di Fonzo, V. Factors affecting the contents of iridoid oleuropein in olive leaves (Olea europaea L.). J. Agric. Food Chem., 2006, 54(2), 434-440.
[http://dx.doi.org/10.1021/jf051647b] [PMID: 16417301] ]. Briefly, about 20ul of each crude extract (DMSOmg/ml) was transferred by micropipette to the wells of the 96 well microtiter plate and then added FC reagent (90ul). After incubation at room temperature for 5min, 90ul of Na2CO3 (6% w/v) was added. At 630nm, absorbance was measured by the microplate reader (microplate reader ELX 800, Biotek, USA). The TPC was expressed as µg gallic acid equivalent (GAE)/ mg of dry weight (DW).
2.4.2. Total Flavonoid Content
Total flavonoid content (TPC) was determined using the method of aluminum chloride colorimetric method previously reported by Bouyahya et al. 2016, 2017 [49Bouyahya, A.; Bensaid, M.; Bakri, Y.; Dakka, N. Phytochemistry and ethnopharmacology of Ficus carica. Int. J. Biochem. Res. Rev., 2016, 14, 1-12.
[http://dx.doi.org/10.9734/IJBCRR/2016/29029] , 50Bouyahya, A.; Abrini, J.; Talbaoui, A.; Et-Touys, A.; Chatoui, K.; Harhar, H.; Bakri, Y.; Dakka, N. Phytochemical screening, antiradical and antibacterial activities of Cistus crispus from Morocco. J. Mater Environ. Sci., 2017, 8, 1560-1566.]. About 20µl of each sample (4mg/ml dimethylsulfoxide), 1M potassium acetate (10ul), 10µl of distilled water (160ul), and 10% (w/v) aluminum chloride were added in the 96 well plates. The absorbance was assessed after 30 minutes at 415nm of incubation. The quercetin 2.5-40ug/ml concentration was used, and resultant TFC was shown to be equal to ug quercetin equivalent (QE) per mg DW. The test was repeated three times.
2.5. Antioxidant Activities Determination
2.5.1. Total Antioxidant Capacity
The antioxidant capacities (TAC) of extracts were determined by phosphomolybdenum assay spectrophoto- metrically using the process reported by Jafri et al. (2014) [51Jafri, L.; Saleem, S. Ihsan ul, H.; Ullah, N.; Mirza, B. In vitro assessment of antioxidant potential and determination of polyphenolic compounds of Hedera nepalensis. Arab. J. Chem., 2017, 10(2), 3699-3707.
[http://dx.doi.org/10.1016/j.arabjc.2014.05.002] ]. This assay is used daily to estimate the TAC of plant extracts, based on acidic molybdenum (VI) reduction to molybdenum (V) by the complex natural extract (antioxidant compound) and green phosphomolybdenum (V) absorption at 695nm [52Prasad, K.N.; Yang, B.; Yang, S.; Chen, Y.; Zhao, M.; Ashraf, M.; Jiang, Y. Identification of phenolic compounds and appraisal of antioxidant and antityrosinase activities from litchi (Litchi sinensis Sonn.) seeds. Food Chem., 2009, 116(1), 1-7.
[http://dx.doi.org/10.1016/j.foodchem.2009.01.079] ]. Briefly, 0.1ml with the final concentration of 100µg/ml of crude extract in methanol was combined with 1ml of reagent solution (28mM sodium phosphate, 4mM ammonium molybdate, and 0.6M sulfuric acid) and 90 minutes of incubation at 95ºC were carried. After incubation, the sample was cooled at room temperature. Mixture absorbance was observed on the UV-spectrophotometer at 695nm against a blank reagent (methanol 0.1ml without plant extract).
2.5.2. Ferric Reducing Power Test
The ferric reducing power (FRP) assay of crude extract was conducted as reported by Zhao et al. (2008) [53Zhao, P.H.; Fan, W.; Dong, J.; Chen, L.; Shan, L.; Lin, Y.; Kong, W. Evaluation of antioxidant activities and total phenolic contents of typical malting barley varieties. Food Chem., 2008, 107(1), 296-304.
[http://dx.doi.org/10.1016/j.foodchem.2007.08.018] ]. In brief, the stock solution extract (500µl) was mixed with Phosphate buffer (500µl) (0.2 M; pH 6.6) and 1% potassium ferricyanide (500µl), and incubation at 50ºC for 20 minutes, followed by the addition of 10% trichloroacetic acid (500µl). The centrifu- gation of the tube was carried for 10min at 10,000rpm. After centrifugation, the upper layer was transferred to a new tube and mixed with the same dH2O volume and 0.1% ferric chloride (100ml). In the presence of crude extract or normal, the FRP operation is based on reducing Fe (III) to Fe (II). At 700nm, the development of Perl’s Prussian blue color indicates Fe (II). The absorbance strength is parallel to power reduction. The results were expressed as equal to ascorbic acid (AAE µg per mg test dry weight of sample).
2.5.3. DPPH Assay
Evaluation of DPPH (2, 2 -diphenyl-1- picrylhydrazyl) free radical scavenging potential was done via the technique already described by Brand Williams et al. (1995) [54Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. (Campinas), 1995, 28(1), 25-30.]. New radical DPPH solution in methanol was prepared before the measurements of absorbance. About 3ml DPPH solution was mixed with plant extract (100µl) at final concentrations of 0.5, 5, 25, 50, and 100µg/ml against blank reagent. The mixture was shaken at room temperature and kept in the dark for 1h. The ability of the resultant extract to donate electron or hydrogen atom was measured, from a purple color to light yellow colored, on a UV spectrophotometer (Bio-Rad, USA) at 517nm. Ascorbic acid was used as a positive control and the experiment was conducted in triplicate.
The DPPH radical percent inhibition was measured by the following formula:
Effect of % DPPH scavenging = [Aº - A1/Aº] X 100
A1 and Aº = Blank reagent and sample absorbance recorded respectively. The antiradical activity was expressed in µg/ml. less IC50 value showed higher antioxidant activity.
2.6. Toxicity Assay
The brine shrimp lethality test was used to predict the toxicity assessment of selected plants. Briefly, the brine shrimp were hatched using brine shrimp eggs at an ambient temperature of 23±1oC in a conically shaped vessel (1L), filled with artificial seawater (38g sea salt/L, pH 8.5 using 1N NAOH) with a constant supply of oxygen for 2 days. After hatching, the nauplii were collected and used for bioassay. The stock solutions of samples were prepared by dissolving the required amount of extracts in a specific volume of 0.5% dimethyl sulfoxide and seawater. Then specific volumes of the samples were shifted from the stock solution to the test tubes to get final sample concentrations of 10, 100, and 1000 ppm (µg/mL) [55Hamidi, M.R.; Jovanova, B.; Panovska, T.K. Toxicоlogical evaluation of the plant products using Brine Shrimp (Artemia salina L.) model Maced. pharm. bull., 2014, 60(1), 9-18.]. The nauplii (10 per vial) were placed in the vial (containing 5ml of brine solution) through glass capillary with samples and maintained at room temperature. After 24 hr, the survived larvae in the sample environment were counted, and tricaine methanesulfonate was used as control. All tests were conducted in triplicates.
2.7. Preparation of Stock Solution and Dilutions for Antileishmanial Activity
The stock solution for the antileishmanial test was prepared by dissolving 1mg/ml of dimethyl sulfoxide in the sterile glass bottles. The stock solution was divided serially from 0.5, 5, 25, 50, and 100µg/ml using dimethyl sulfoxide. The 0.45mg/ml syringes were used to filter all samples [56Eltayeb, A.; Ibrahim, K. Potential antileishmanial effect of three medicinal plants. Indian J. Pharm. Sci., 2012, 74(2), 171-174.
[http://dx.doi.org/10.4103/0250-474X.103856] [PMID: 23326001] ].
2.8. Parasite Culture
The L. tropica (Accession # MN891719) was previously isolated from a patient in AJK. The promastigotes form of L. tropica were cultured in M199 medium with HEPES buffer, 10% fetal calf serum (FSC), penicillin, and streptomycin [57Shah, N.A.; Khan, M.R.; Nadhman, A. Antileishmanial, toxicity, and phytochemical evaluation of medicinal plants collected from Pakistan. BioMed Res. Int., 2014, 2014384204
[http://dx.doi.org/10.1155/2014/384204] [PMID: 24995292] ].
2.9. Evaluation of Anti Promastigotes Activity
The antileishmanial activity assays were done by the methods already described Ogeto et al. (2013) [58Ogeto, T.K.; Odhiambo, R.A.; Shivairo, R.S.; Muleke, C.I.; Osero, B.O.; Anjili, C.; Osuga, I.M. Antileishmanial activity of Aloe secundi flora plant extracts against Leishmania major. Advances in Life Science and Technol, 2013, 13, 2224-2281.] with some modifications. The log phase of promastigotes at 1x106/100µl was used for the current assay. About 90µl of 199 media, 10µl each plant dilution and 50µl promastigotes log phase culture were dispensed to various wells of microtiter plates. Along with amphotericin b as the drug standard, dimethyl sulfoxide as negative control were used. Afterword, the micropipette plate was incubated at 26oC for 72h. The experiment was repeated three times. After incubations, 10µl of all dilution were pipette on the Neubauer chamber and counted under the binocular microscope (Optica, 500 series).
2.10. Statistical Analysis
All triplicate experiments were conducted, and the data analysis was shown as mean±standard deviation (SD). The coefficient associations between the methods of antioxidant activate and total phenolics were confirmed by Microsoft Excel 2010. The lethal concentrations were measured by Probit Analysis at a confidence interval of 95%. When the value of p<0.05, then it is considerd as significant.
3. RESULTS ANS DISCUSSION
3.1. Phytochemical Screening
The natural products are rich sources of selective and new agents for curing imperative tropical diseases caused by protozoan and many other parasites [33Shahbazi, Y. Antibacterial and Antioxidant Properties of Methanolic Extracts of Apple (Malus pumila), Grape (Vitis vinifera), Pomegranate (Punica granatum L.) and Common Fig (Ficus carica L.) Fruits. Pharm. Sci., 2017, 23, 308-315.
[http://dx.doi.org/10.15171/PS.2017.45] ]. The use of herbal medicines becomes a common practice in all developing countries, where basic health services are not accessible worldwide, including AJK. The medicinal flora of AJK has been described as diversified and rich, but limited studies investigated the potential use in the curing of parasitic diseases [38Shinwari, M.I.; Shinwari, M.I. Botanical Diversity in Pakistan; Past, Present and Future., 2010, , 85-104.]. The phytochemical analysis is the first step in bioactive compounds identification. It has been stated that the total flavonoid and phenolic contents are directly correlated with antioxidant activity. Such compounds are referred to as strong antioxidant chain breakers [59Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N.; Boulaaba, M.; Smaoui, A.; Abdelly, C. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. C. R. Biol., 2008, 331(11), 865-873.
[http://dx.doi.org/10.1016/j.crvi.2008.07.024] [PMID: 18940702] ]. In this context, we investigated the raw ethanolic crude extracts of seven selected plant leaves for phytochemical analysis along with antioxidant and in-vitro antileishmanial activities. In the current study, the phyto- chemical screening showed all leaf extracts were rich in phenolics and flavonoids constituents. Similar observations were reported by Saeed et al. (2012) [60Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med., 2012, 12, 221.
[http://dx.doi.org/10.1186/1472-6882-12-221] [PMID: 23153304] ] for Fagonia olivieri and Torilis leptophylla extracts. The alkaloids were absent in D. lotus and P. persica. The absence of alkaloids in P. persica is supported by previous studies in Labia and Jammu Kashmir [61Edrah, S.; Alafid, F.; Kumar, A. Preliminary Phytochemical Screening and Antibacterial Activity of Pistacia atlantica and Prunus persica Plants of Libyan Origin. Inter. J. Sci. Research, 2015, 4(2), 1552-1555., 62Hussain, T.; Baba, I.A.; Jain, S.M. Arif Wani Phytochemical Screening of Methanolic Extract of Prunus persica. IJSR – Inter. J. SCIENTIF. RES., 4(3), 52-53.]. Tannins were only absent in F. glomerata. Terpenoids were present in all extracts expect P. persica and F. glomerata. The plant extracts of P. armeniaca, F. glomerata and D. lotus showed the absence of saponins while present in the remaining all extracts. M. pumila, P. communis, P. pashia, D. lotus, and P. armeniaca showed the presence of steroids except for P. persica and F. glomerata. The quinone was absent in D. lotus and F. glomerata. The coumarins were present in P. armeniaca, among others (Table 2).
3.2. Total Phenolic and Flavanoid Content
The therapeutic and biological advantages are due to the presence of flavonoids and phenolics constituents in all tested leaf extracts. In Table 3, the ethanolic extract from the selected plants revealed a significant difference in the TPC rate. The highest TPC level was observed in P. armeniaca (279.62±5.40µg GAE/mg DW), followed by P. pashia (241.71±4.27µgGAE/mg DW) and P. communis(180.52 ±4.22 µg GAE/mg DW). M. pumila and P. persica exhibited the TPC in a comparable quantity 54.91±3.90 and 53.32±9.30 µg GAE/mg DW, respectively. The F. glomerata (12.82±6.80 µg GAE/mg DW) and D. lotus (10.91±6.70 µg GAE/mg DW) was found to possess comparatively minor TPC. Moreover, the ethanolic extract of selected plants showed differences in TFC. The TFC level of P. armeniaca remained the highest, and that of M. pumila remained the lowest. P. persica and P. pashia showed a similar quantity (54.90±3.90 and 51.62±9.81µg QA/mg DW, respectively). The P. communis, D. lotus, and F. glomerata exhibited 136.42±2.10, 98.62±1.50, and 106.90±2.90µg QA/mg DW respectively. The current investigations showed that the tested plants contained a distinctive but significant amount of flavonoid and phenolic contents (Table 3). The existence of such compounds explains the use of these plants in folk medicine [63Amarowicz, R.; Pegg, R.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem., 2004, 84(4), 551-562.
[http://dx.doi.org/10.1016/S0308-8146(03)00278-4] ].
3.3. Antioxidant Activities Determination
In Table 4, the TAE of seven selected plants (ethanolic crude extracts) were measured in the presence of antioxidant compounds based on the principle of Mo (V1) reduction to Mo (V). The reduction of Mo (V1) into Mo (V) leads to the formation of a green-colored phosphomolybdenum (V) complex observed at 695nm spectrophotometrically. The TAC of ethanolic extracts was calculated and expressed as ascorbic acid equivalent, i.e., AAE µg/ mgDW. The TAC of ethanolic extracts of P. armeniaca (120.37±4.90µgAAE/mgDW) and P. pashia (100.42±5.20µgAAE/mgDW) were higher than P. persica (94.21±4.30µgAAE/mgDW) and P. communis (91.80±3.90µgAAE/mgDW) respectively. The M. pumila (83.60±3.00µgAAE/mg DW), and F. glomerata (80.60±2.71µgAAE/mgDW) showed high TAC than D. lotus (76.71±3.21 µg AAE/mgDW). These results revealed that ethanolic extracts from all plants contained compounds with high antioxidant potential and association with high levels of flavonoids and phenolic.
A parallel correlation between TAC and FRP was shown in the current investigation. The plant extract from P. armeniaca (120.37±4.90µgAAE/mgDW) showed the highest FRP values (278.71±1.03µgAAE/mgDW). The P. pashia, P. persica, P. communis, F. glomerata, M. pumila, and D. lotus showed 166.58±8.80, 108.21±4.62, 102.61±3.27, 67.62±7.01, 62.20±7.25, and 60.30±6.91µgAAE/mgDW, respectively. The current study showed that the higher the maximum phenolic content of the plant extract, the greatest it will have the reducing power ability (Table 4).
The free radical scavenging (%) of ethanolic extracts were observed at various test concentrations in the following order: P. armeniaca> P. pashia> P. persica> P. communis> M. Pumila> D. lotus> F. glomerata. The DPPH free radical scavenging activity (%) of ethanolic plant extract was greater at the highest extracts concentration. Interestingly, P. armeniaca extract showed the highest (53.94±1.24%) DPPH scavenging activity than other tested plants, which were as compared to other rich in phenolics. However, P. pashia showed maximum antioxidant potential (94.21±4.30%) through scavenging at the highest concentration of 400µg/ml, which showed that P. pashia may contain some compounds that have a higher dose effect. The IC50 for all plants was also observed in following the order: P. armeniaca> P. pashia> P. persica> P. communis> M. pumila> D. Lotus> F. glomerata (Table 5). The antioxidant role of the phenolic compound is well established that attributed their action to scavenge free radicals, give chelated metal ion, electron, or proton [63Amarowicz, R.; Pegg, R.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem., 2004, 84(4), 551-562.
[http://dx.doi.org/10.1016/S0308-8146(03)00278-4] ]. The antioxidant activity of the hydroxyl group number and location to the carboxyl group and as high as the hydrolation increases [64Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process., 2011, 89(3), 217-233.
[http://dx.doi.org/10.1016/j.fbp.2010.04.008] ]. Furthermore, the flavonoid mechanism of action was correlated with free radical scavenging or metal ion chelation [65Kessler, M.; Ubeaud, G.; Jung, L. Anti- and pro-oxidant activity of rutin and quercetin derivatives. J. Pharm. Pharmacol., 2003, 55(1), 131-142.
[http://dx.doi.org/10.1211/002235702559] [PMID: 12625877] ]. This power to scavenge free radicals gives them antioxidant ability [66Sahreen, S.; Khan, M.R.; Khan, R.A. Evaluation of antioxidant activities of various solvent extracts of Carissa opaca fruits. Food Chem., 2010, 122(4), 1205-1211.
[http://dx.doi.org/10.1016/j.foodchem.2010.03.120] ]. In the current investigations, a parallel correlation was observed between TAC and FRP. This parallel correlation was also reported by Kumar and Jain [67Kumar, T.; Jain, V. Appraisal of Total Phenol, Flavonoid Contents, and Antioxidant Potential of Folkloric Lannea coromandelica Using In Vitro and In Vivo Assays. Scientifica (Cairo), 2015, 2015203679
[http://dx.doi.org/10.1155/2015/203679] [PMID: 26457224] ] for Lanneacoro mandelica. TAC (120.37±4.90µgAAE/mgDW) and FRP (278.71±1.03µgAAE/mg DW) of P. armeniaca was highest among other plants extracts. This capacity is because antioxidants are essentially protons or electrons that reduce ferric ion (Fe3+) by electron donation to ferrous ion (Fe2+) [68Shon, M.Y.; Choi, S.D.; Kahng, G.G.; Nam, S.H.; Sung, N.J. Antimutagenic, antioxidant and free radical scavenging activity of ethyl acetate extracts from white, yellow and red onions. Food Chem. Toxicol., 2004, 42(4), 659-666.
[http://dx.doi.org/10.1016/j.fct.2003.12.002] [PMID: 15019191] ]. The present study claimed that the higher the TPC in plant extracts, the more the reduction capacity it will have [69Jafri, L.; Saleem, S.; Ullah, N.; Mirza, B. In vitro assessment of antioxidant potential and determination of polyphenolic compounds of Hedera nepalensis. Arab. J. Chem., 2014, 10, 3699-3706.
[http://dx.doi.org/10.1016/j.arabjc.2014.05.002] ]. The phenolic compounds minimize the risk of health problems because they are immune to reactive oxygen (ROS) damage. Prior investigations stated that the strongest antioxidant compounds were also antileishmanic [70Jain, A.K.; Sharma, S.; Vaidya, A.; Ravichandran, V.; Agrawal, R.K. 1,3,4-thiadiazole and its derivatives: a review on recent progress in biological activities. Chem. Biol. Drug Des., 2013, 81(5), 557-576.
[http://dx.doi.org/10.1111/cbdd.12125] [PMID: 23452185] ]. The DPPH (517nm absorption) is a stable radical capable of antioxidant scavenging [71Lu, Y.; Foo, L.Y. Antioxidant activities of polyphenols from sage (Salvia officinalis). Food Chem., 2001, 75(2), 197-202.
[http://dx.doi.org/10.1016/S0308-8146(01)00198-4] ]. It is widely used to assess the ability of compounds as free radical scavengers and hydrogen donors, as well as the antioxidant activity of important medicinal plant extracts [72Da Porto, C.; Calligaris, S.; Celotti, E.; Nicoli, M.C. Antiradical properties of commercial cognacs assessed by the DPPH(.) test. J. Agric. Food Chem., 2000, 48(9), 4241-4245.
[http://dx.doi.org/10.1021/jf000167b] [PMID: 10995344] ]. In the reaction, DPPH absorbs radical hydrogen or electron from antioxidants and changes its color, which can be calculated by spectrophotometer at 517nm [73Canadanovic-Brunet, J.; Cetkovic, G.; Saponjac, V.T.; Stajcic, S.; Vulić, J.; Djilas, S. Evaluation of phenolic content, antioxidant activity and sensory characteristics of Serbian honey-based product. Ind. Crops Prod., 2014, 62, 1-7.
[http://dx.doi.org/10.1016/j.indcrop.2014.08.009] ].
3.4. Cytotoxicity Assays
Plant pharmacological assessment provides an attractive and good source for safe and novel medical plant growth. It is necessary to check the cytotoxicity of selected plants to improve safety. For the assessment of toxicity determination, brine shrimp toxicity assay is considered as rapid, reliable, and low cost [74Olila, D.; Olwa-Odyek, ; Opuda-Asibo, J. Antibacterial and antifungal activities of extracts of Zanthoxylum chalybeum and Warburgia ugandensis, Ugandan medicinal plants. Afr. Health Sci., 2001, 1(2), 66-72.
[PMID: 12789119] ]. The plant extracts with LC50 values between 100 and 500µg/ml are considered as moderately toxic, and those with <100 are strongly toxic. Values of LC50 >1000 are considered nontoxic [75Nguta, J.M.; Mbaria, J.M.; Gakuya, D.W.; Gathumbi, P.K.; Kabasa, J.D.; Kiama, S.G. Evaluation of acute toxicity of crude plant extracts from Kenyan biodiversity using brine shrimp, Artemia salina L. (Artemiidae). Open Conf. Proc. J., 2012, 3, 30-34.
[http://dx.doi.org/10.2174/2210289201203010030] ]. A total of 7 crude extracts of M. pumila, P. communis, P. persica, P. pashia, P. armeniaca, and D. lotus showed no toxicity with LC50 values >1000µg/ml. The M. pumila, P. communis, and P. persica showed LC50 values of 1283.67, 1411.30, and 1659.90 µg/ml with the significance value of p= 0.003, 0.001, and 0.001, respectively. The extracts of P. pashia, P. armeniaca, and D. lotus showed LC50 values of 1230.66, 1912.31, and 1671.56µg/ml having values of p=0.999, 0.001, and 0.000, respectively. The F. glomerata extract showed moderate toxicity having LC50 values of 454.34µg/ml, having the significance value of p= 0.147. The F. glomerata extract showed moderate toxicity having LC50 values of 454.34µg/ml following the statement of Nguta et al. [75Nguta, J.M.; Mbaria, J.M.; Gakuya, D.W.; Gathumbi, P.K.; Kabasa, J.D.; Kiama, S.G. Evaluation of acute toxicity of crude plant extracts from Kenyan biodiversity using brine shrimp, Artemia salina L. (Artemiidae). Open Conf. Proc. J., 2012, 3, 30-34.
[http://dx.doi.org/10.2174/2210289201203010030] ]. The crude extract of F. glomerata showed moderate activity and is need to not be left as irrelevant because Bussmann et al. (2011) [76Bussmann, R.W.; Malca, G.; Glenn, A.; Sharon, D.; Nilsen, B.; Parris, B.; Dubose, D.; Ruiz, D.; Saleda, J.; Martinez, M.; Carillo, L.; Walker, K.; Kuhlman, A.; Townesmith, A. Toxicity of medicinal plants used in traditional medicine in Northern Peru. J. Ethnopharmacol., 2011, 137(1), 121-140.
[http://dx.doi.org/10.1016/j.jep.2011.04.071] [PMID: 21575699] ] and Nguta et al., (2012) [75Nguta, J.M.; Mbaria, J.M.; Gakuya, D.W.; Gathumbi, P.K.; Kabasa, J.D.; Kiama, S.G. Evaluation of acute toxicity of crude plant extracts from Kenyan biodiversity using brine shrimp, Artemia salina L. (Artemiidae). Open Conf. Proc. J., 2012, 3, 30-34.
[http://dx.doi.org/10.2174/2210289201203010030] ] noticed that toxicity showed variations significantly because of different collection location, the tissue of plants, time of harvest and solvent extraction. Due to this natural variability, the leaves identified LC50 between 100 and 500µg/ml cytotoxicity had served us for more research on biologically active extracts against fleas (Xenopsylla cheopis), mosquitoes (Ades aegypti), ticks (Ixodes scapularis), microbes affecting forest and health of living thing [77Johnston, W.H.; Karchesy, J.J.; Constantine, G.H.; Craig, A.M. Antimicrobial activity of some Pacific Northwest woods against anaerobic bacteria and yeast. Phytother. Res., 2001, 15(7), 586-588.
[http://dx.doi.org/10.1002/ptr.765] [PMID: 11746838] , 78Dietrich, G.; Dolan, M.C.; Peralta-Cruz, J.; Schmidt, J.; Piesman, J.; Eisen, R.J.; Karchesy, J.J. Repellent activity of fractioned compounds from Chamaecyparis nootkatensis essential oil against nymphal Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol., 2006, 43(5), 957-961.
[http://dx.doi.org/10.1093/jmedent/43.5.957] [PMID: 17017233] ].
3.5. Antileishmanial Activity
About more than 100 plant extracts showed promising activity against various forms of Leishmania parasite [79Rocha, L.G.; Almeida, J.R.; Macêdo, R.O.; Barbosa-Filho, J.M. A review of natural products with antileishmanial activity. Phytomedicine, 2005, 12(6-7), 514-535.
[http://dx.doi.org/10.1016/j.phymed.2003.10.006] [PMID: 16008131] ]. The medical plants Wormwood (Artmisia aucheri Bioss), Purple coneflower (Echinacea purpura), and Marigold (Calendula officinalis) belong to the Asteraceae family showed promising antileishmanial activity against promastigotes of CL in-vitro [80Masbi, N.; Ghafarifar, F.; Bahrami, A.M.; Bastaminejad, S.; Shamsi, M. Evaluation of leishmanicidal effect of watery & ethanolic flowers Calendula officinalis extract on promastigotes of Leishmania major (MRHO/IR/75/ER) in vitro. J Ilam Univ Med Sci, 2010, 18, 28-33., 81Emami, S.A.; Zamanai Taghizadeh Rabe, S.; Ahi, A.; Mahmoudi, M. TaghizadehRabe, S.Z.; Ahi, A.; Mahmoudi, M. Inhibitory activity of eleven Artemisia species from Iran against Leishmania major parasites. Iran. J. Basic Med. Sci., 2012, 15(2), 807-811.
[PMID: 23493354] ]. The Aloe emodin of the family Aloaceae showed decrease promastigotes growth, having IC50 of 52.8µg/ml. The garlic (Allium sativum) inhibits promastigotes growth in 48hrs at a dose of 37µg/ml [82Gharavi, M.; Nobakht, M.; Khademvatan, S.; Fani, F.; Bakhshayesh, M.; Roozbehani, M. The effect of aqueous garlic extract on interleukin-12 and 10 levels in Leishmania major (MRHO/IR/75/ER) infected macrophages. Iran. J. Public Health, 2011, 40(4), 105-111.
[PMID: 23113109] ]. The extracts of Henna (Lawsonia inermis) and Thyme (Zajuria multiflora Bioss) were used for wound healing in CL infection [83Hejazi, S.H.; Shirani-Bidabadi, L.; Zolfaghari-Baghbaderani, A.; Saberi, S.; Nilforoushzadeh, M.A.; Moradi, S.H. Comparison effectiveness of extracts of Thyme, yarrow, henna and garlic on cutaneous leishmaniasis caused by L. major in animal model (Balb/c). Faslnamah-i Giyahan-i Daruyi, 2009, 8, 129-136.]. The crude extract of Aloe vera leaf was assed to test against CL showed a significant effect [84Iqbal, H.; Khattak, B.; Ayaz, S.; Rehman, A.; Ishfaq, M.; Abbas, M.N.; Rehman, H.; Waheed, S.; Wahab, A. Comparative efficacy of Aloe vera and Tamarix aphylla against cutaneous leishmaniasis. Int. J Basic Medi.l Sci. Phar, 2012, 2(2), 42-45.]. Hamarsheh et al. in (2017) [85Hamarsheh, O.; Azmi, K.; Amro, A.; Schultheis, M.; Abdeen, Z.; Firdessa, R.; Sawalha, K.; Rimawi, F.A.; Yaghmour, R.; Moll, H. Antileishmanial potential of crude plant extracts derived from medicinal plants in palestine. Ann Clin Cytol Pathol., 2017, 3(4), 1-7.] stated that Malva sylves tris and Artemisia inculta showed strong antileishmanial activity against CL infection. The grand wormwood (Artemisia absinthium), Yarrow (Achillea millefolium, and Walnut (Jaglana regia) arrest promastigotes growth high as compared to control [86Yektaian, N.; Rafieian, M.; Khalili-Dehkordi, B.; Hejazi, S.H.; Shirani-Bidabadi, L.; Hosseini, V. Effect of combination of Achillea millefolium, Artemisia absinthium & Juglans regia leaves extracts on Leishmania major (MRHO/IR/75/ER), in vitro. Faslnamah-i Giyahan-i Daruyi, 2012, 11, 197-204.]. The Royle (Arnebia euchroma), Sweet wormwood (Artemisia annua), and Jurema (Mimosa tenuiflorw) showed significant antileishmanial activity against promastigotes of L. major as compared to control [87Sozangar, N.; Jeddi, F.; Reaghi, S.; Khorrami, S.; Arzemani, K. Abulkhalsa and yarrow plant effect on Leishmania major in vitro. J. N Khorasan Univ Med Sci., 2012, 4, 329-333.
[http://dx.doi.org/10.29252/jnkums.4.3.329] -89Shamsuddini, S.; Rajab, S. Alian, Mirzayi, M.; Boroufiei M. Efficacy of Mimosa tenuiflora extract on growth of Leishmania protozoa in vitro. Iran J Dermatol., 2006, 9, 175-180.]. The Eucalyptus camaldulensis (Blue gum) showed an inhibitory effect on the parasitic growth of CL, and it also showed a reduction of the lesion size [90L.; Mohebali, M.; NiakanLahiji, M.R.; Tavana, A.M. The therapeutic effect of Eucalyptus, Myrtus, Ferula, Aretmisia, Allium and Urtica extracts against cutaneous leishmaniasis caused by Leishmanaia major in small white mice (outbred). Hakim., 2007, 10, 21-27.]. Similarly, many other medicinal plants have been screened against Leishmania parasites by conducting in-vitro bioassays.
In Table 6, the preliminary evaluation of crude ethanolic extracts of seven plants revealed that among the total crude extracts tested P. communis and P. pashia having moderate antileishmanial activities with IC50 56.68 and 60.95µg/ml values, respectively. These findings may lead to this raw material for other parasitic diseases. Biological activates of P. pashia were also reported by Guven et al. (2006) [91Guven, K.; Yucel, E.; Centintas, F. Antimicrobial activities of fruits of Crataegus and Pyrus species. Pharm. Biol., 2006, 44, 79-83.
[http://dx.doi.org/10.1080/13880200600591253] ]. M. pumila and P. persica, D. lotus and F. glomerata have shown no antileishmanial activity having IC50 >100µg/ml. The M. pumila and P. persica have anti-inflammatory [92Kashyap, D.; Kumar, S.; Dhatwalia, V. Review on phytochemical and pharmacological properties of Prunus persica Linn. Asia-Pac. J. Chem. Eng., 2015, 2, 5-11.], D. lotus used as antiseptic, antitumor, and antidiabetic [93Uddin, G.; Rauf, A.; Siddiqui, B.S.; Shah, S.Q. Preliminary comparative phytochemical screening of Diospyros lotus Stewart. Middle East J. Sci. Res., 2011, 10, 78-81., 94Uddin, G.; Rauf, A.; Siddiqui, B.S.; Muhammad, N.; Khan, A.; Shah, S.U.A. Anti-nociceptive, anti-inflammatory and sedative activities of the extracts and chemical constituents of Diospyros lotus L. Phytomedicine, 2014, 21(7), 954-959.
[http://dx.doi.org/10.1016/j.phymed.2014.03.001] [PMID: 24703326] ], and F. glomerata reported for bacterial infection [95Menezes, C.; Kamath, J.V.; Satyanarayana, D.; Mishra, A. Sandeep; Mishra, A. Antibacterial activity of fruits of ficus glomerata cylma. J. Harmonized Rese Pharmacy, 2013, 2(4), 256-259.]. One prominent extract, P. armeniaca showed the highest antileishmanial activity with IC50 16.18µg/ml, which is less than the control, i.e., 17.72µg/ml. A wide range of pharmacological effects of P. armeniaca has been reported, such as antioxidants [96Guclu, K.; Altun, M.; Ozyurek, M.; Karademir, S.E.; Apak, R. Antioxidant capacity of fresh, sun- and sulphited-driedMalatya apricot (Prunus armeniaca) assayed by CUPRAC, ABTS/TEAC and folin methods. Int. J. Food Sci. Technol., 2006, 41(1), 76-85.
[http://dx.doi.org/10.1111/j.1365-2621.2006.01347.x] ], antimicrobial activities [97Sochor, J.; Zitka, O.; Skutkova, H.; Pavlik, D.; Babula, P.; Krska, B.; Horna, A.; Adam, V.; Provaznik, I.; Kizek, R. Content of phenolic compounds and antioxidant capacity in fruits of apricot genotypes. Molecules, 2010, 15(9), 6285-6305.
[http://dx.doi.org/10.3390/molecules15096285] [PMID: 20877223] ], and anti-asthmatic [98Erdogan, I.O.; Kartal, M. Insights into research on phytochemistry and biological activites of Prunus armeniaca L. apricot). Food Res. Int., 2011, 44, 1238-1243.
[http://dx.doi.org/10.1016/j.foodres.2010.11.014] ]. Minaiyan et al. in 2014 [99Minaiyan, M.; Ghannadi, A. Asadi, M.; Etemad, M.; Mahzouni, P. Antiinflammatory effect of Prunus armeniaca L. (Apricot) extracts ameliorates TNBS induced ulcerative colitis in rats. Res. Pharm. Sci., 2014, 9(4), 225-231.
[PMID: 25657793] ] reported that P. ameniaca have been used in many parasitic diseases. The standard error for positive control and tested plant extracts was calculated with a 95% confidence interval having a significance value of 0.000. The percent mortality recorded was 95.28±0.00 at 100µg/ml of P. armeniaca, while control showed 73.10±0.92. Moderate inhibition of parasitic growth was shown by P. communia and P. pashia at 100µg/ml were 59.76±0.60 and 63.74±0.70. Less than 50% inhibition of parasite growth was shown by P. persica, F. glomerata, and D. lotus at 100µg/ml.
However, pharmacological data of P. armeniaca regarding the antileishmanial activity has not been documented in the literature; some phytochemicals constitute such as phenols, flavonoids, alkaloids, saponins, terpenoids, coumarins, tannins, and quinone was present in this plant in the current study. The coumarian has been already purified from Calophyllum brasiliense (Calophyllaceae) reported antileishmanial activity against L. amazonesis [100Brenzan, M.A.; Nakamura, C.V.; Prado Dias Filho, B.; Ueda-Nakamura, T.; Young, M.C.; Aparício Garcia Cortez, D. Antileishmanial activity of crude extract and coumarin from Calophyllum brasiliense leaves against Leishmania amazonensis. Parasitol. Res., 2007, 101(3), 715-722.
[http://dx.doi.org/10.1007/s00436-007-0542-7] [PMID: 17483964] ]. The alkaloids from the genus Prosopis showed significant in-vitro parasitic activity against L. donovania with compared to the control drug [101Samoylenko, V.; Ashfaq, M.K.; Jacob, M.R.; Tekwani, B.L.; Khan, S.I.; Manly, S.P.; Joshi, V.C.; Walker, L.A.; Muhammad, I. Indolizidine, antiinfective and antiparasitic compounds from Prosopis glandulosa var. glandulosa. J. Nat. Prod., 2009, 72(1), 92-98.
[http://dx.doi.org/10.1021/np800653z] [PMID: 19105653] ]. Some other investigations reported the presence of phenolic [102Bodiwala, H.S.; Singh, G.; Singh, R.; Dey, C.S.; Sharma, S.S.; Bhutani, K.K.; Singh, I.P. Antileishmanial amides and lignans from Piper cubeba and Piper retrofractum. J. Nat. Med., 2007, 61, 418-421.
[http://dx.doi.org/10.1007/s11418-007-0159-2] ] and flavonoids [103Nour, A.M.; Khalid, S.A.; Kaiser, M.; Brun, R.; Abdalla, W.E.; Schmidt, T.J. The antiprotozoal activity of methylated flavonoids from Ageratum conyzoides L. J. Ethnopharmacol., 2010, 129(1), 127-130.
[http://dx.doi.org/10.1016/j.jep.2010.02.015] [PMID: 20219663] ] from Ageratum conyzoides (Asteraceae) possess antileishmanial activities against promastigotes of L. donovania. Torres Santos et al. (2004) [104Torres-Santos, E.C.; Lopes, D.; Oliveira, R.R.; Carauta, J.P.; Falcao, C.A.; Kaplan, M.A.; Rossi-Bergmann, B. Antileishmanial activity of isolated triterpenoids from Pourouma guianensis. Phytomedicine, 2004, 11(2-3), 114-120.
[http://dx.doi.org/10.1078/0944-7113-00381] [PMID: 15070160] ] has reported the terpenoids from Pourouma guianensis (Moraceae) as an antileishmanial agent against L. amazonesis. The antileishmanial activity against L. donovania and L.major was demonstrated by quinone from Zhumeria majdae (Labiateae) [105Moein, M.R.; Pawar, R.S.; Khan, S.I.; Tekwani, B.L.; Khan, I.A. Antileishmanial, antiplasmodial and cytotoxic activities of 12,16-dideoxy aegyptinone B from Zhumeria majdae Rech.f. & Wendelbo. Phytother. Res., 2008, 22(3), 283-285.
[http://dx.doi.org/10.1002/ptr.2305] [PMID: 17886231] ] and Perovskia abrotanoides (Lamiaceae) [106Sairafianpour, M.; Christensen, J.; Staerk, D.; Budnik, B.A.; Kharazmi, A.; Bagherzadeh, K.; Jaroszewski, J.W. Leishmanicidal, antiplasmodial, and cytotoxic activity of novel diterpenoid 1,2-quinones from Perovskia abrotanoides: new source of tanshinones. J. Nat. Prod., 2001, 64(11), 1398-1403.
[http://dx.doi.org/10.1021/np010032f] [PMID: 11720520] ]. Paolini et al. (2004) [107Paolini, V.; Fouraste, I.; Hoste, H. In vitro effects of three woody plant and sainfoin extracts on two parasitic stage of 3 parasitic nematode species. Parsitology, 2004, 129, 69-77.
[http://dx.doi.org/10.1017/S0031182004005268] [PMID: 15267113] ] verified the anti-parasitic activity of tannins.
CONCLUSION
It is concluded from the current investigation that plant extracts could be an effective alternative for synthetic drugs against L. tropica. The plant P. arminiaca contains chemical compounds that may lead to the development of an affordable and effective antileishmanial drug against cutaneous leishmaniasis (L. tropica). In most developing countries, these results provide a different way to used natural plant-based remedies that might be less toxic, safer, and cheaper than available recommended medicines. Azad Jammu and Kashmir is an area rich in possibilities, and world flora represents an enormous source of material for testing. Therefore, extensive studies are needed, particularly bio-guided fractionation for the identification of active fraction and more chemical characterization.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The present study was conducted with the approval and assigned a protocol (#BEC-FBS-QAU-152) by the bioethical committee of Quaid-i-Azam University Islamabad, Pakistan.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
ACKNOWLEDGEMENTS
The authors are grateful to the patient who participated in this study. The authors are also thankful to the Department of Zoology Quaid-i-Azam University Islamabad for facilitating us to carry out our research work.
REFERENCES
| [1] | Croft, S.L.; Sundar, S.; Fairlamb, A.H. Drug resistance in leishmaniasis. Clin. Microbiol. Rev., 2006, 19(1), 111-126. [http://dx.doi.org/10.1128/CMR.19.1.111-126.2006] [PMID: 16418526] |
| [2] | Steverding, D. The history of leishmaniasis. Parasit. Vectors, 2017, 10(1), 82-92. [http://dx.doi.org/10.1186/s13071-017-2028-5] [PMID: 28202044] |
| [3] | Zulfiqar, B.; Shelper, T.B.; Avery, V.M. Leishmaniasis drug discovery: recent progress and challenges in assay development. Drug Discov. Today, 2017, 22(10), 1516-1531. [http://dx.doi.org/10.1016/j.drudis.2017.06.004] [PMID: 28647378] |
| [4] | Brooker, S.; Mohammed, N.; Adil, K.; Agha, S.; Reithinger, R.; Rowland, M.; Ali, I.; Kolaczinski, J. Leishmaniasis in refugee and local Pakistani populations. Emerg. Infect. Dis., 2004, 10(9), 1681-1684. [http://dx.doi.org/10.3201/eid1009.040179] [PMID: 15498178] |
| [5] | Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. RE:view, 2017, 6, 750. |
| [6] | de Souza, A.; Marins, D.S.S.; Mathias, S.L.; Monteiro, L.M.; Yukuyama, M.N.; Scarim, C.B.; Löbenberg, R.; Bou-Chacra, N.A. Promising nanotherapy in treating leishmaniasis. Int. J. Pharm., 2018, 547(1-2), 421-431. [http://dx.doi.org/10.1016/j.ijpharm.2018.06.018] [PMID: 29886097] |
| [7] | Blum, J.; Desjeux, P.; Schwartz, E.; Beck, B.; Hatz, C. Treatment of cutaneous leishmaniasis among travellers. J. Antimicrob. Chemother., 2004, 53(2), 158-166. [http://dx.doi.org/10.1093/jac/dkh058] [PMID: 14729756] |
| [8] | Hepburn, N.C. Cutaneous leishmaniasis: current and future management. Expert Rev. Anti Infect. Ther., 2003, 1(4), 563-570. [http://dx.doi.org/10.1586/14787210.1.4.563] [PMID: 15482153] |
| [9] | Noor, S.M.; Hussain, D. Cutaneous leishmaniasis in Sadda, Kurram agency, Pakistan. J. Pak. Assoc. Dermatol., 2004, 14, 114-117. |
| [10] | Kakarsulemankhel, J.K. Kaal Dana” (Cutaneous Leishmaniasis) In South-West Pakistan: A Preliminary Study, Turkey. parasioloji Dergisi., 2004, 28, 5-11. |
| [11] | Bhutto, A.M.; Soomro, F.R.; Baloch, J.H.; Matsumoto, J.; Uezato, H.; Hashiguchi, Y.; Katakura, K. Cutaneous leishmaniasis caused by Leishmania (L.) major infection in Sindh province, Pakistan. Acta Trop., 2009, 111(3), 295-298. [http://dx.doi.org/10.1016/j.actatropica.2009.05.009] [PMID: 19467219] |
| [12] | Talat, H.; Attarwala, S.; Saleem, M. Cutaneous leishmaniasis with HIV-Case report. J. Coll. Physicians Surg. Pak., 2014, 24, 93-95. |
| [13] | Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. Leishmaniasis worldwide and global estimates of its incidence. PLoS One, 2012, 7(5)e35671 [http://dx.doi.org/10.1371/journal.pone.0035671] [PMID: 22693548] |
| [14] | González, C.; Wang, O.; Strutz, S.E.; González-Salazar, C. Sánchez- Cordero, V.; Sarkar, S. Climate change and risk of leishmaniasis in NorthAmerica: predictions from ecological nichemodels of vector and reservoir species. PLoS Negl. Trop. Dis., 2010, 4, 585. [http://dx.doi.org/10.1371/journal.pntd.0000585] |
| [15] | 2016.http://www.who.int/leishmaniasis/en/ |
| [16] | Bekhit, A.A.; El-Agroudy, E.; Helmy, A.; Ibrahim, T.M.; Shavandi, A.; Bekhit, A.E.A. Leishmania treatment and prevention: Natural and synthesized drugs. Eur. J. Med. Chem., 2018, 160, 229-244. [http://dx.doi.org/10.1016/j.ejmech.2018.10.022] [PMID: 30342363] |
| [17] | Camacho, Md.; Phillipson, J.D.; Croft, S.L.; Solis, P.N.; Marshall, S.J.; Ghazanfar, S.A. Screening of plant extracts for antiprotozoal and cytotoxic activities. J. Ethnopharmacol., 2003, 89(2-3), 185-191. [http://dx.doi.org/10.1016/S0378-8741(03)00269-1] [PMID: 14611881] |
| [18] | Sen, R.; Chatterjee, M. Plant derived therapeutics for the treatment of Leishmaniasis. Phytomedicine, 2011, 18(12), 1056-1069. [http://dx.doi.org/10.1016/j.phymed.2011.03.004] [PMID: 21596544] |
| [19] | Saebi, E. Parasitic diseases in Iran (protozoa)., (9th ed. ), 2005, |
| [20] | Kheirandish, F.; Delfan, B.; Farhadi, S.; Ezatpour Khamesipour, A.; Kazemi, B.; Ebrahimzade, F.; Rashidipour, M. The effect of Satureja khuzestanica essential oil on the lesions induced by Leishmania major in BALB/c mice. Afr. J. Pharm. Pharmacol., 2011, 5(5), 648-653. [http://dx.doi.org/10.5897/AJPP11.130] |
| [21] | Mahmoudvand, H.; Tavakoli, R.; Sharififar, F.; Minaie, K.; Ezatpour, B.; Jahanbakhsh, S.; Sharifi, I. Leishmanicidal and cytotoxic activities of Nigella sativa and its active principle, thymoquinone. Pharm. Biol., 2015, 53(7), 1052-1057. [http://dx.doi.org/10.3109/13880209.2014.957784] [PMID: 25471014] |
| [22] | Rashid, U.; Sultana, R.; Shaheen, N.; Hassan, S.F.; Yaqoob, F.; Ahmad, M.J.; Iftikhar, F.; Sultana, N.; Asghar, S.; Yasinzai, M.; Ansari, F.L.; Qureshi, N.A. Structure based medicinal chemistry-driven strategy to design substituted dihydropyrimidines as potential antileishmanial agents. Eur. J. Med. Chem., 2016, 115, 230-244. [http://dx.doi.org/10.1016/j.ejmech.2016.03.022] [PMID: 27017551] |
| [23] | Brasil, Manual de Vigilância da Leishmaniose Tegumentar Americana., (3rd ed. ), 2010, |
| [24] | Bennett, J.E. Antimicrobial: antifungal agents.Goodman and Gilman’s the Pharmacological Basis of Therapeutics., (11th ed. ) 2006, , 1225-1242. |
| [25] | Solomon, M.; Baum, S.; Barzilai, A.; Scope, A.; Trau, H.; Schwartz, E. Liposomal amphotericin B in comparison to sodium stibogluconate for cutaneous infection due to Leishmania braziliensis. J. Am. Acad. Dermatol., 2007, 56(4), 612-616. [http://dx.doi.org/10.1016/j.jaad.2006.06.044] [PMID: 17276541] |
| [26] | Hadighi, R.; Mohebali, M.; Boucher, P.; Hajjaran, H.; Khamesipour, A.; Ouellette, M. Unresponsiveness to Glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS Med., 2006, 3(5)e162 [http://dx.doi.org/10.1371/journal.pmed.0030162] [PMID: 16605301] |
| [27] | al-Majali, O.; Routh, H.B.; Abuloham, O.; Bhowmik, K.R.; Muhsen, M.; Hebeheba, H. A 2-year study of liquid nitrogen therapy in cutaneous leishmaniasis. Int. J. Dermatol., 1997, 36(6), 460-462. [http://dx.doi.org/10.1046/j.1365-4362.1997.00045.x] [PMID: 9248896] |
| [28] | Gonzales, U.; Pinart, M.; Reginfo-Pardo, M.; Macaya, A.; Alvar, J.; Tweed, J.A. Interventions for American cutaneous and mucocutaneous leishmaniasis Cochrane Databas. Syst. Rev., 2009, 15, 4-34. |
| [29] | Bahmani, M.; Eftekhari, Z. An ethnoveterinary study of medicinal plants in treatment of diseases and syndromes of herd dog in southern regions of Ilam province, Iran. Comp. Clin. Pathol., 2012, 22(3), 403-407. [http://dx.doi.org/10.1007/s00580-012-1423-8] [PMID: 23667351] |
| [30] | Ghasemi Pirbalouti, A.; Momeni, M.; Bahmani, M. Ethnobotanical study of medicinal plants used by Kurd tribe in Dehloran and Abdanan Districts, Ilam Province, Iran. Afr. J. Tradit. Complement. Altern. Med., 2012, 10(2), 368-385. [PMID: 24146463] |
| [31] | Bahmani, M.; Rafieian-Kopaei, M.; Avijgan, M.; Hosseini, S.; Golshahi, H. Ethnobotanical studies of medicinal plants used by Kurdish owner’s in south range of Ilam Province, west of Iran. Am.-Eurasian J. Agric. Environ. Sci., 2012, 12, 1128-1133. |
| [32] | Brodskyn, C.; de Oliveira, C.I.; Barral, A.; Barral-Netto, M. Vaccines in leishmaniasis: advances in the last five years. Expert Rev. Vaccines, 2003, 2(5), 705-717. [http://dx.doi.org/10.1586/14760584.2.5.705] [PMID: 14711330] |
| [33] | Shahbazi, Y. Antibacterial and Antioxidant Properties of Methanolic Extracts of Apple (Malus pumila), Grape (Vitis vinifera), Pomegranate (Punica granatum L.) and Common Fig (Ficus carica L.) Fruits. Pharm. Sci., 2017, 23, 308-315. [http://dx.doi.org/10.15171/PS.2017.45] |
| [34] | Chan-Bacab, M.J.; Peña-Rodríguez, L.M. Plant natural products with leishmanicidal activity. Nat. Prod. Rep., 2001, 18(6), 674-688. [http://dx.doi.org/10.1039/b100455g] [PMID: 11820764] |
| [35] | Jameel, M.; Islamuddin, M.; Ali, A.; Afrin, F.; Ali, M. Isolation, characterization and antimicrobial evaluation of a novel compound N-octacosan 7β ol, from Fumaria parviflora Lam. BMC Complement. Altern. Med., 2014, 14(14), 98. [http://dx.doi.org/10.1186/1472-6882-14-98] [PMID: 24621260] |
| [36] | Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod., 2012, 75(3), 311-335. [http://dx.doi.org/10.1021/np200906s] [PMID: 22316239] |
| [37] | Delfan, B.; Bahmani, M.; Hassanzadazar, H.; Saki, K.; Rafieian-Kopaei, M. Identification of medicinal plants affecting on headaches and migraines in Lorestan Province, West of Iran. Asian Pac. J. Trop. Med., 2014, 7S1, S376-S379. [http://dx.doi.org/10.1016/S1995-7645(14)60261-3] [PMID: 25312153] |
| [38] | Shinwari, M.I.; Shinwari, M.I. Botanical Diversity in Pakistan; Past, Present and Future., 2010, , 85-104. |
| [39] | Afshan, N.S.; Iqbal, S.H.; Khalid, A.N.; Niazi, A.R. Some additions to the Uredinales of Azad Jammu and Kashmir, Pakistan. Pak. J. Bot., 2011, 43(2), 1373-1379. |
| [40] | Ali, S.I. Significance of flora with special reference to Pakistan. Pak. J. Bot., 2008, 40(30), 967-971. |
| [41] | Haq, F.U.; Ahmad, H.; Alam, M.; Ahmad, I. Rahatullah. Species Diversity of Vascular Plants of Nandiar Valley Western Himalaya, Pakistan. Pak. J. Bot., 2010, 42, 213-229. |
| [42] | Nasir, E.; Ali, S.; Stewart, R.R. Flora of West Pakistan: an annotated catalogue of the 560 vascular plants of West Pakistan and Kashmir. Fakhri., 1972, 561, 56. |
| [43] | Nasir, YJ.; Ali, S. Flora of Pakistan. Department of Botany, University of Karachi National Herbarium., 1994-2010, 562 |
| [44] | Chew, Y.L.; Goh, J.K.; Lim, Y.Y. Assessment of in vitro antioxidant capacity and polyphenolic composition of selected medicinal herbs from Leguminosae family in Peninsular Malaysia. Food Chem., 2009, 119, 373-378. [http://dx.doi.org/10.1016/j.foodchem.2009.01.091] |
| [45] | Al-Daihan, S.; Al-Faham, M.; Al-Shawi, N.; Almayman, R.; Brnawi, A.; Zargar, S. Antibacterial activity and phytochemical screening of some medicinal plants commonly used in Saudi Arabia against selected pathogenic microorganisms. J King Saud Uni-Sci., 2013, 25, 115-120. [http://dx.doi.org/10.1016/j.jksus.2012.11.003] |
| [46] | Raaman, N. Phytochemical techniques. Pitam Pura., 2006, , 2022. |
| [47] | Evans, W. C. Trease and Evans Pharmacognosy, (16th. ) , 22, 24, 25, 28-42. |
| [48] | Ranalli, A.; Contento, S.; Lucera, L.; Di Febo, M.; Marchegiani, D.; Di Fonzo, V. Factors affecting the contents of iridoid oleuropein in olive leaves (Olea europaea L.). J. Agric. Food Chem., 2006, 54(2), 434-440. [http://dx.doi.org/10.1021/jf051647b] [PMID: 16417301] |
| [49] | Bouyahya, A.; Bensaid, M.; Bakri, Y.; Dakka, N. Phytochemistry and ethnopharmacology of Ficus carica. Int. J. Biochem. Res. Rev., 2016, 14, 1-12. [http://dx.doi.org/10.9734/IJBCRR/2016/29029] |
| [50] | Bouyahya, A.; Abrini, J.; Talbaoui, A.; Et-Touys, A.; Chatoui, K.; Harhar, H.; Bakri, Y.; Dakka, N. Phytochemical screening, antiradical and antibacterial activities of Cistus crispus from Morocco. J. Mater Environ. Sci., 2017, 8, 1560-1566. |
| [51] | Jafri, L.; Saleem, S. Ihsan ul, H.; Ullah, N.; Mirza, B. In vitro assessment of antioxidant potential and determination of polyphenolic compounds of Hedera nepalensis. Arab. J. Chem., 2017, 10(2), 3699-3707. [http://dx.doi.org/10.1016/j.arabjc.2014.05.002] |
| [52] | Prasad, K.N.; Yang, B.; Yang, S.; Chen, Y.; Zhao, M.; Ashraf, M.; Jiang, Y. Identification of phenolic compounds and appraisal of antioxidant and antityrosinase activities from litchi (Litchi sinensis Sonn.) seeds. Food Chem., 2009, 116(1), 1-7. [http://dx.doi.org/10.1016/j.foodchem.2009.01.079] |
| [53] | Zhao, P.H.; Fan, W.; Dong, J.; Chen, L.; Shan, L.; Lin, Y.; Kong, W. Evaluation of antioxidant activities and total phenolic contents of typical malting barley varieties. Food Chem., 2008, 107(1), 296-304. [http://dx.doi.org/10.1016/j.foodchem.2007.08.018] |
| [54] | Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. (Campinas), 1995, 28(1), 25-30. |
| [55] | Hamidi, M.R.; Jovanova, B.; Panovska, T.K. Toxicоlogical evaluation of the plant products using Brine Shrimp (Artemia salina L.) model Maced. pharm. bull., 2014, 60(1), 9-18. |
| [56] | Eltayeb, A.; Ibrahim, K. Potential antileishmanial effect of three medicinal plants. Indian J. Pharm. Sci., 2012, 74(2), 171-174. [http://dx.doi.org/10.4103/0250-474X.103856] [PMID: 23326001] |
| [57] | Shah, N.A.; Khan, M.R.; Nadhman, A. Antileishmanial, toxicity, and phytochemical evaluation of medicinal plants collected from Pakistan. BioMed Res. Int., 2014, 2014384204 [http://dx.doi.org/10.1155/2014/384204] [PMID: 24995292] |
| [58] | Ogeto, T.K.; Odhiambo, R.A.; Shivairo, R.S.; Muleke, C.I.; Osero, B.O.; Anjili, C.; Osuga, I.M. Antileishmanial activity of Aloe secundi flora plant extracts against Leishmania major. Advances in Life Science and Technol, 2013, 13, 2224-2281. |
| [59] | Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N.; Boulaaba, M.; Smaoui, A.; Abdelly, C. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. C. R. Biol., 2008, 331(11), 865-873. [http://dx.doi.org/10.1016/j.crvi.2008.07.024] [PMID: 18940702] |
| [60] | Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med., 2012, 12, 221. [http://dx.doi.org/10.1186/1472-6882-12-221] [PMID: 23153304] |
| [61] | Edrah, S.; Alafid, F.; Kumar, A. Preliminary Phytochemical Screening and Antibacterial Activity of Pistacia atlantica and Prunus persica Plants of Libyan Origin. Inter. J. Sci. Research, 2015, 4(2), 1552-1555. |
| [62] | Hussain, T.; Baba, I.A.; Jain, S.M. Arif Wani Phytochemical Screening of Methanolic Extract of Prunus persica. IJSR – Inter. J. SCIENTIF. RES., 4(3), 52-53. |
| [63] | Amarowicz, R.; Pegg, R.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem., 2004, 84(4), 551-562. [http://dx.doi.org/10.1016/S0308-8146(03)00278-4] |
| [64] | Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process., 2011, 89(3), 217-233. [http://dx.doi.org/10.1016/j.fbp.2010.04.008] |
| [65] | Kessler, M.; Ubeaud, G.; Jung, L. Anti- and pro-oxidant activity of rutin and quercetin derivatives. J. Pharm. Pharmacol., 2003, 55(1), 131-142. [http://dx.doi.org/10.1211/002235702559] [PMID: 12625877] |
| [66] | Sahreen, S.; Khan, M.R.; Khan, R.A. Evaluation of antioxidant activities of various solvent extracts of Carissa opaca fruits. Food Chem., 2010, 122(4), 1205-1211. [http://dx.doi.org/10.1016/j.foodchem.2010.03.120] |
| [67] | Kumar, T.; Jain, V. Appraisal of Total Phenol, Flavonoid Contents, and Antioxidant Potential of Folkloric Lannea coromandelica Using In Vitro and In Vivo Assays. Scientifica (Cairo), 2015, 2015203679 [http://dx.doi.org/10.1155/2015/203679] [PMID: 26457224] |
| [68] | Shon, M.Y.; Choi, S.D.; Kahng, G.G.; Nam, S.H.; Sung, N.J. Antimutagenic, antioxidant and free radical scavenging activity of ethyl acetate extracts from white, yellow and red onions. Food Chem. Toxicol., 2004, 42(4), 659-666. [http://dx.doi.org/10.1016/j.fct.2003.12.002] [PMID: 15019191] |
| [69] | Jafri, L.; Saleem, S.; Ullah, N.; Mirza, B. In vitro assessment of antioxidant potential and determination of polyphenolic compounds of Hedera nepalensis. Arab. J. Chem., 2014, 10, 3699-3706. [http://dx.doi.org/10.1016/j.arabjc.2014.05.002] |
| [70] | Jain, A.K.; Sharma, S.; Vaidya, A.; Ravichandran, V.; Agrawal, R.K. 1,3,4-thiadiazole and its derivatives: a review on recent progress in biological activities. Chem. Biol. Drug Des., 2013, 81(5), 557-576. [http://dx.doi.org/10.1111/cbdd.12125] [PMID: 23452185] |
| [71] | Lu, Y.; Foo, L.Y. Antioxidant activities of polyphenols from sage (Salvia officinalis). Food Chem., 2001, 75(2), 197-202. [http://dx.doi.org/10.1016/S0308-8146(01)00198-4] |
| [72] | Da Porto, C.; Calligaris, S.; Celotti, E.; Nicoli, M.C. Antiradical properties of commercial cognacs assessed by the DPPH(.) test. J. Agric. Food Chem., 2000, 48(9), 4241-4245. [http://dx.doi.org/10.1021/jf000167b] [PMID: 10995344] |
| [73] | Canadanovic-Brunet, J.; Cetkovic, G.; Saponjac, V.T.; Stajcic, S.; Vulić, J.; Djilas, S. Evaluation of phenolic content, antioxidant activity and sensory characteristics of Serbian honey-based product. Ind. Crops Prod., 2014, 62, 1-7. [http://dx.doi.org/10.1016/j.indcrop.2014.08.009] |
| [74] | Olila, D.; Olwa-Odyek, ; Opuda-Asibo, J. Antibacterial and antifungal activities of extracts of Zanthoxylum chalybeum and Warburgia ugandensis, Ugandan medicinal plants. Afr. Health Sci., 2001, 1(2), 66-72. [PMID: 12789119] |
| [75] | Nguta, J.M.; Mbaria, J.M.; Gakuya, D.W.; Gathumbi, P.K.; Kabasa, J.D.; Kiama, S.G. Evaluation of acute toxicity of crude plant extracts from Kenyan biodiversity using brine shrimp, Artemia salina L. (Artemiidae). Open Conf. Proc. J., 2012, 3, 30-34. [http://dx.doi.org/10.2174/2210289201203010030] |
| [76] | Bussmann, R.W.; Malca, G.; Glenn, A.; Sharon, D.; Nilsen, B.; Parris, B.; Dubose, D.; Ruiz, D.; Saleda, J.; Martinez, M.; Carillo, L.; Walker, K.; Kuhlman, A.; Townesmith, A. Toxicity of medicinal plants used in traditional medicine in Northern Peru. J. Ethnopharmacol., 2011, 137(1), 121-140. [http://dx.doi.org/10.1016/j.jep.2011.04.071] [PMID: 21575699] |
| [77] | Johnston, W.H.; Karchesy, J.J.; Constantine, G.H.; Craig, A.M. Antimicrobial activity of some Pacific Northwest woods against anaerobic bacteria and yeast. Phytother. Res., 2001, 15(7), 586-588. [http://dx.doi.org/10.1002/ptr.765] [PMID: 11746838] |
| [78] | Dietrich, G.; Dolan, M.C.; Peralta-Cruz, J.; Schmidt, J.; Piesman, J.; Eisen, R.J.; Karchesy, J.J. Repellent activity of fractioned compounds from Chamaecyparis nootkatensis essential oil against nymphal Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol., 2006, 43(5), 957-961. [http://dx.doi.org/10.1093/jmedent/43.5.957] [PMID: 17017233] |
| [79] | Rocha, L.G.; Almeida, J.R.; Macêdo, R.O.; Barbosa-Filho, J.M. A review of natural products with antileishmanial activity. Phytomedicine, 2005, 12(6-7), 514-535. [http://dx.doi.org/10.1016/j.phymed.2003.10.006] [PMID: 16008131] |
| [80] | Masbi, N.; Ghafarifar, F.; Bahrami, A.M.; Bastaminejad, S.; Shamsi, M. Evaluation of leishmanicidal effect of watery & ethanolic flowers Calendula officinalis extract on promastigotes of Leishmania major (MRHO/IR/75/ER) in vitro. J Ilam Univ Med Sci, 2010, 18, 28-33. |
| [81] | Emami, S.A.; Zamanai Taghizadeh Rabe, S.; Ahi, A.; Mahmoudi, M. TaghizadehRabe, S.Z.; Ahi, A.; Mahmoudi, M. Inhibitory activity of eleven Artemisia species from Iran against Leishmania major parasites. Iran. J. Basic Med. Sci., 2012, 15(2), 807-811. [PMID: 23493354] |
| [82] | Gharavi, M.; Nobakht, M.; Khademvatan, S.; Fani, F.; Bakhshayesh, M.; Roozbehani, M. The effect of aqueous garlic extract on interleukin-12 and 10 levels in Leishmania major (MRHO/IR/75/ER) infected macrophages. Iran. J. Public Health, 2011, 40(4), 105-111. [PMID: 23113109] |
| [83] | Hejazi, S.H.; Shirani-Bidabadi, L.; Zolfaghari-Baghbaderani, A.; Saberi, S.; Nilforoushzadeh, M.A.; Moradi, S.H. Comparison effectiveness of extracts of Thyme, yarrow, henna and garlic on cutaneous leishmaniasis caused by L. major in animal model (Balb/c). Faslnamah-i Giyahan-i Daruyi, 2009, 8, 129-136. |
| [84] | Iqbal, H.; Khattak, B.; Ayaz, S.; Rehman, A.; Ishfaq, M.; Abbas, M.N.; Rehman, H.; Waheed, S.; Wahab, A. Comparative efficacy of Aloe vera and Tamarix aphylla against cutaneous leishmaniasis. Int. J Basic Medi.l Sci. Phar, 2012, 2(2), 42-45. |
| [85] | Hamarsheh, O.; Azmi, K.; Amro, A.; Schultheis, M.; Abdeen, Z.; Firdessa, R.; Sawalha, K.; Rimawi, F.A.; Yaghmour, R.; Moll, H. Antileishmanial potential of crude plant extracts derived from medicinal plants in palestine. Ann Clin Cytol Pathol., 2017, 3(4), 1-7. |
| [86] | Yektaian, N.; Rafieian, M.; Khalili-Dehkordi, B.; Hejazi, S.H.; Shirani-Bidabadi, L.; Hosseini, V. Effect of combination of Achillea millefolium, Artemisia absinthium & Juglans regia leaves extracts on Leishmania major (MRHO/IR/75/ER), in vitro. Faslnamah-i Giyahan-i Daruyi, 2012, 11, 197-204. |
| [87] | Sozangar, N.; Jeddi, F.; Reaghi, S.; Khorrami, S.; Arzemani, K. Abulkhalsa and yarrow plant effect on Leishmania major in vitro. J. N Khorasan Univ Med Sci., 2012, 4, 329-333. [http://dx.doi.org/10.29252/jnkums.4.3.329] |
| [88] | Heidari, F.I.; Ghaffarifar, F.; Dalimi, A.; Dehkordi, N.M.; Nikoo, S.G. In vitro study of the effect of Artimisin in on promastigotes and amastigotes of Leishmania major. Modares J Med Sci Pathobiol., 2012, 15, 33-43. |
| [89] | Shamsuddini, S.; Rajab, S. Alian, Mirzayi, M.; Boroufiei M. Efficacy of Mimosa tenuiflora extract on growth of Leishmania protozoa in vitro. Iran J Dermatol., 2006, 9, 175-180. |
| [90] | L.; Mohebali, M.; NiakanLahiji, M.R.; Tavana, A.M. The therapeutic effect of Eucalyptus, Myrtus, Ferula, Aretmisia, Allium and Urtica extracts against cutaneous leishmaniasis caused by Leishmanaia major in small white mice (outbred). Hakim., 2007, 10, 21-27. |
| [91] | Guven, K.; Yucel, E.; Centintas, F. Antimicrobial activities of fruits of Crataegus and Pyrus species. Pharm. Biol., 2006, 44, 79-83. [http://dx.doi.org/10.1080/13880200600591253] |
| [92] | Kashyap, D.; Kumar, S.; Dhatwalia, V. Review on phytochemical and pharmacological properties of Prunus persica Linn. Asia-Pac. J. Chem. Eng., 2015, 2, 5-11. |
| [93] | Uddin, G.; Rauf, A.; Siddiqui, B.S.; Shah, S.Q. Preliminary comparative phytochemical screening of Diospyros lotus Stewart. Middle East J. Sci. Res., 2011, 10, 78-81. |
| [94] | Uddin, G.; Rauf, A.; Siddiqui, B.S.; Muhammad, N.; Khan, A.; Shah, S.U.A. Anti-nociceptive, anti-inflammatory and sedative activities of the extracts and chemical constituents of Diospyros lotus L. Phytomedicine, 2014, 21(7), 954-959. [http://dx.doi.org/10.1016/j.phymed.2014.03.001] [PMID: 24703326] |
| [95] | Menezes, C.; Kamath, J.V.; Satyanarayana, D.; Mishra, A. Sandeep; Mishra, A. Antibacterial activity of fruits of ficus glomerata cylma. J. Harmonized Rese Pharmacy, 2013, 2(4), 256-259. |
| [96] | Guclu, K.; Altun, M.; Ozyurek, M.; Karademir, S.E.; Apak, R. Antioxidant capacity of fresh, sun- and sulphited-driedMalatya apricot (Prunus armeniaca) assayed by CUPRAC, ABTS/TEAC and folin methods. Int. J. Food Sci. Technol., 2006, 41(1), 76-85. [http://dx.doi.org/10.1111/j.1365-2621.2006.01347.x] |
| [97] | Sochor, J.; Zitka, O.; Skutkova, H.; Pavlik, D.; Babula, P.; Krska, B.; Horna, A.; Adam, V.; Provaznik, I.; Kizek, R. Content of phenolic compounds and antioxidant capacity in fruits of apricot genotypes. Molecules, 2010, 15(9), 6285-6305. [http://dx.doi.org/10.3390/molecules15096285] [PMID: 20877223] |
| [98] | Erdogan, I.O.; Kartal, M. Insights into research on phytochemistry and biological activites of Prunus armeniaca L. apricot). Food Res. Int., 2011, 44, 1238-1243. [http://dx.doi.org/10.1016/j.foodres.2010.11.014] |
| [99] | Minaiyan, M.; Ghannadi, A. Asadi, M.; Etemad, M.; Mahzouni, P. Antiinflammatory effect of Prunus armeniaca L. (Apricot) extracts ameliorates TNBS induced ulcerative colitis in rats. Res. Pharm. Sci., 2014, 9(4), 225-231. [PMID: 25657793] |
| [100] | Brenzan, M.A.; Nakamura, C.V.; Prado Dias Filho, B.; Ueda-Nakamura, T.; Young, M.C.; Aparício Garcia Cortez, D. Antileishmanial activity of crude extract and coumarin from Calophyllum brasiliense leaves against Leishmania amazonensis. Parasitol. Res., 2007, 101(3), 715-722. [http://dx.doi.org/10.1007/s00436-007-0542-7] [PMID: 17483964] |
| [101] | Samoylenko, V.; Ashfaq, M.K.; Jacob, M.R.; Tekwani, B.L.; Khan, S.I.; Manly, S.P.; Joshi, V.C.; Walker, L.A.; Muhammad, I. Indolizidine, antiinfective and antiparasitic compounds from Prosopis glandulosa var. glandulosa. J. Nat. Prod., 2009, 72(1), 92-98. [http://dx.doi.org/10.1021/np800653z] [PMID: 19105653] |
| [102] | Bodiwala, H.S.; Singh, G.; Singh, R.; Dey, C.S.; Sharma, S.S.; Bhutani, K.K.; Singh, I.P. Antileishmanial amides and lignans from Piper cubeba and Piper retrofractum. J. Nat. Med., 2007, 61, 418-421. [http://dx.doi.org/10.1007/s11418-007-0159-2] |
| [103] | Nour, A.M.; Khalid, S.A.; Kaiser, M.; Brun, R.; Abdalla, W.E.; Schmidt, T.J. The antiprotozoal activity of methylated flavonoids from Ageratum conyzoides L. J. Ethnopharmacol., 2010, 129(1), 127-130. [http://dx.doi.org/10.1016/j.jep.2010.02.015] [PMID: 20219663] |
| [104] | Torres-Santos, E.C.; Lopes, D.; Oliveira, R.R.; Carauta, J.P.; Falcao, C.A.; Kaplan, M.A.; Rossi-Bergmann, B. Antileishmanial activity of isolated triterpenoids from Pourouma guianensis. Phytomedicine, 2004, 11(2-3), 114-120. [http://dx.doi.org/10.1078/0944-7113-00381] [PMID: 15070160] |
| [105] | Moein, M.R.; Pawar, R.S.; Khan, S.I.; Tekwani, B.L.; Khan, I.A. Antileishmanial, antiplasmodial and cytotoxic activities of 12,16-dideoxy aegyptinone B from Zhumeria majdae Rech.f. & Wendelbo. Phytother. Res., 2008, 22(3), 283-285. [http://dx.doi.org/10.1002/ptr.2305] [PMID: 17886231] |
| [106] | Sairafianpour, M.; Christensen, J.; Staerk, D.; Budnik, B.A.; Kharazmi, A.; Bagherzadeh, K.; Jaroszewski, J.W. Leishmanicidal, antiplasmodial, and cytotoxic activity of novel diterpenoid 1,2-quinones from Perovskia abrotanoides: new source of tanshinones. J. Nat. Prod., 2001, 64(11), 1398-1403. [http://dx.doi.org/10.1021/np010032f] [PMID: 11720520] |
| [107] | Paolini, V.; Fouraste, I.; Hoste, H. In vitro effects of three woody plant and sainfoin extracts on two parasitic stage of 3 parasitic nematode species. Parsitology, 2004, 129, 69-77. [http://dx.doi.org/10.1017/S0031182004005268] [PMID: 15267113] |










