- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Anatomy Journal
(Discontinued)
ISSN: 1877-6094 ― Volume 6, 2014
Anterior Inferior Pancreaticoduodenal Artery Running Between the Dorsal and Ventral Pancreas: Morphological and Embryological Viewpoint
Nozomi Yi1, Shuang-Qin Yi*, 2, Heng-Xiao Wang1, Yuki Ogawa1, Tetsuo Ohta3, Noriyuki Ozaki2, Masahiro Itoh1
Abstract
Objective
To elucidate the configurational relationship of the anterior inferior pancreaticoduodenal artery (AIPD) and embryological ventral/dorsal pancreas anlagen.
Methods
64 cadavers were used for morphological observation by gross dissection of the head and uncinate process of the pancreas as well as its vessels. The localization of pancreatic polypeptide-immunoreactive cells was investigated in 22 cadaveric pancreas samples.
Results
In 73.4% subjects, the AIPD appeared not on the anterior surface of the anterior inferior portion of the pancreatic head, but was covered by pancreatic parenchyme. The islets of Langerhans were irregular in shape, and abundant pancreatic polypeptide-immunoreactive cells were identified in the pancreatic tissues of the posterior region of the AIPD, while in the anterior region of the AIPD, the islets of Langerhans were rounded, and few or no pancreatic polypeptide-immunoreactive cells were identified.
Conclusions
The AIPD may follow the boundary between the dorsal and ventral pancreas, and we can interpret in embryology of the morphological characteristics by which the AIPD is located behind or on the anterior surface of the anterior inferior portion of the pancreatic head.
Article Information
Identifiers and Pagination:
Year: 2010Volume: 2
First Page: 79
Last Page: 85
Publisher Id: TOANATJ-2-79
DOI: 10.2174/1877609401002010079
Article History:
Received Date: 09/4/2010Revision Received Date: 23/4/2010
Acceptance Date: 26/4/2010
Electronic publication date: 2/6/2010
Collection year: 2010
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http: //creativecommons.org/licenses/by-nc/3.0/ which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Department of Anatomy and Neuroembryology, University of Kanazawa, Japan; Tel.: +81-76-265-2494; Fax: +81-76-234-4221; E-mail: yixim@med.kanazawa-u.ac.jp
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 09-4-2010 |
Original Manuscript | Anterior Inferior Pancreaticoduodenal Artery Running Between the Dorsal and Ventral Pancreas: Morphological and Embryological Viewpoint | |
INTRODUCTION
The morphological structure of the pancreas is still an interesting subject for surgeons and anatomists. For surgeons, this is partly because the surgical treatment of pancreatic diseases has generally been considered to be associated with a high incidence of postoperative complications, and has developed to preserve as much of the pancreatic parenchyma as possible. Limited pancreatic resections, such as medial pancreatectomy [1-3], ventral pancreatectomy [4], uncinate process resection [5], superior head resection [6], and inferior head resection [7] have been performed for benign or low-grade malignant neoplasms of the pancreas, such as mucin-producing tumors, and in some cases for pancreatic injury. These procedures allow maximal preservation of normal pancreatic tissue and hence pancreatic endocrine and exocrine functions, and thus result in a better quality of life for patients [8-10]. Therefore surgeons require concrete topographical anatomical knowledge of the head of the pancreas to avoid critical complications; in particular, grasping the morphological structure of the uncinate process and the boundary of the dorsal and ventral pancreas.
The pancreas is composed of dorsal and ventral primordia in embryological view. The ventral primordium, together with the developing bile duct, rotates clockwise behind the duodenum and the dorsal primordium [11]. Consequently, the head of the pancreas can theoretically be divided into two embryological segments: dorsal and ventral primordia. For anatomists, the configurational arrangement and embryological morphology of the pancreas, especially the rotation of the ventral pancreatic primordium and fusion with the dorsal pancreatic primordium, remain uncertain. The vascular anatomy of the pancreaticoduodenal region has been the subject of numerous studies [12, 13]. It has been conjectured that the anterior inferior portion of the pancreatic head may derive from the embryological anlage of the dorsal pancreas [14] and the anterior inferior pancreaticoduodenal artery (AIPD) may follow the boundary between the dorsal and ventral anlage [14-17]; however, this conjecture has not been confirmed in these reports.
In the current study, we focus on elucidating the configurational relationship of the AIPD and the embryological ventral/dorsal pancreas anlagen in autopsy cases, and present a schematic concept of development to interpret the morphogenesis course.
SUBJECTS AND METHODS
Cadavers
As shown in Table 1, the study was divided into three experimental groups. The first research (Research 1) was performed on 64 subjects (21 male and 43 female), the ages from 51 to 90 years (median, 85.9 years old), with morphological observation by gross dissection. The data of Research 1 were then employed in the second research (Research 2), and 10 samples from Research 1 were used for an immunohistochemical experiment in Research 2. The third research (Research 3) employed 12 additional subjects (6 male and 4 female) with a mean age of 88.0 years (range, 82–95 years) for immunohistochemistry.
All cadavers were selected from bodies used for the research and practice of anatomy at Tokyo Medical University from 2005 to 2007 and were free from diseases of the pancreas and its surrounding areas.
Morphological Observation
For Research, 1, 64 cadavers were used to dissect the head and uncinate the process of the pancreas as well as its vessels. We focused on the configuration relationship between the arrangement of the anterior inferior border of the pancreatic head and the AIPD. The distances between the AIPD and the anterior inferior border of the head of the pancreas were measured (mm). The distance was 0 mm when the AIPD was located at the same level at the anterior inferior border of the head of the pancreas, and the distance was positive or negative when the AIPD was located behind or on the anterior surface of the anterior inferior border of the head of the pancreas, respectively (Table 2).
Tissue Specimens
For Research 2, after the gross dissection of Research 1 was completed, Among 64 subjects, ten were used randomly in an immunohistochemical experiment. Namely, parts of the pancreatic tissues located in anterior and posterior regions of the AIPD, 0.5–1.0 × 0.5–0.8 × 0.3–0.5 cm3, were removed, respectively. The samples were washed thoroughly for 5 hours under running tap water, and then dehydrated and routinely embedded in paraffin wax. Sections (4 µm) were cut and placed on gelatin-coated glass slides.
For Research 3, employing 12 additional subjects, the head of the pancreas with the superior mesenteric artery was removed. The pancreas samples were realigned (in a transverse plane across the AIPD) in the vertical direction of the AIPD and cut into 5–10 major sections. Each section that included the AIPD was cut into 5- to 7-mm-thick slices, washed thoroughly for 5 hours under running tap water, and then processed as in Research 2.
Both Researches 2 and 3 were used to identify pancreatic polypeptide (PP)-, and insulin-immunoreactive cells in pancreas tissues. The origin of the parenchyma was determined to be the embryologically ventral primordium when the islets of Langerhans had irregular contours and contained abundant PP-positive cells. By contrast, it was determined to be the embryologically dorsal primordium when the surrounding islets were round or oval and PP-positive cells were scarce as compared with the opposite segment, or absent [18-20].
Histological and Immunohistochemical Studies
For the samples in Research 2 and 3, sets of three consecutive sections were stained with hematoxylin and eosin (HE) and immunostained by using antisera to insulin and PP. Immunohistochemical procedures were performed by the avidin-biotin-peroxidase complex (ABC) method according to our previous study [19]. Briefly, the sections were deparaffinized, placed in a glass box filled with 10 mM citrate buffer (pH 6.0) for 8 min at 95°C by two times iterative, for antigen retrieval, pretreated in a temperature-controlled microwave oven. Endogenous peroxidase activity was inhibited by 30-min incubation in methanol containing 0.3% (v/v) hydrogen peroxide. After rinsing in 0.01 M phosphate-buffered saline (PBS), the sections were blocked with normal goat serum for 1 hour at room temperature (RT), incubated with the primary antibodies overnight at 4°C in a humidified chamber, and then with secondary antibodies for 1 hour at RT. Subsequently, the avidin-biotin complex (ABComplex/HRP, DAKO, Denmark) was visualized by incubating the sections for 30 min at RT, after which they were stained for 1 min with 3-3’-diaminobenzidine and 0.005% H2O2, counterstained with Harris’ hematoxylin for 50 s, dehydrated in a graded ethanol series and xylene, and cover-slipped with Entellan neu (Merck, Germany).
The primary antibodies were guinea pig anti-human insulin (polyclonal; Dako, USA), and rabbit anti-human pancreatic polypeptide (polyclonal; YLEM, Italy), diluted at 1:200 and ready-to-use, respectively. The secondary antibody was biotin-conjugated goat anti- guinea pig IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and biotin-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology).
The control experiments consisted of the following: (1) omission of primary antiserum, and (2) substitution of primary antibody with 0.05 M Tris-BSA buffer. These controls were carried out at the same time as treatment with the primary antibody.
Ethics
Written informed consent was obtained from all study subjects. The study protocol conforms to the ethical guidelines of the "World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects" adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, as revised in Tokyo 2004. The study was approved by the institutional ethical review committee.
RESULTS
Morphological Observation for Research 1
Table 2 summarizes the findings of morphological observation. The results were summarized in 3 groups. In group 1, in 47 of 64 cases (73.4%), the AIPD was not on the anterior surface of the anterior inferior region of the head of the pancreas, but was covered by pancreatic parenchyma, called partial pancreatic tissues in the “pancreatic lip” (Fig. 1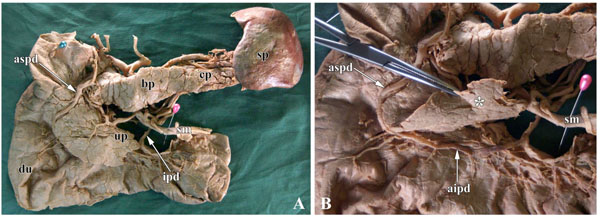 ). In these 47 cases, the distance between the AIPD and the anterior inferior edge of the head of pancreas was 1~33 mm. Moreover, in 3 cases, the parenchyma of the pancreatic lip extended to the region of the uncinate process of pancreas. In group 2, in 2 of 64 cases, the AIPD was located at the same level as the anterior inferior border of the pancreatic lip. In group 3, in 15 of 64 cases, the AIPD was lower than the anterior inferior border of the pancreatic lip, and the distance was 1~5 mm (Table 2), with the AIPD following the anterior surface of the anterior inferior portion of the pancreatic head.
). In these 47 cases, the distance between the AIPD and the anterior inferior edge of the head of pancreas was 1~33 mm. Moreover, in 3 cases, the parenchyma of the pancreatic lip extended to the region of the uncinate process of pancreas. In group 2, in 2 of 64 cases, the AIPD was located at the same level as the anterior inferior border of the pancreatic lip. In group 3, in 15 of 64 cases, the AIPD was lower than the anterior inferior border of the pancreatic lip, and the distance was 1~5 mm (Table 2), with the AIPD following the anterior surface of the anterior inferior portion of the pancreatic head.
Histological and Immunohistochemical Research of Research 2 and Research 3
In Research 2, the islets of Langerhans were irregular in shape, and abundant PP-immunoreactive cells were identified in the pancreatic tissues of the posterior region of the AIPD (Fig. 2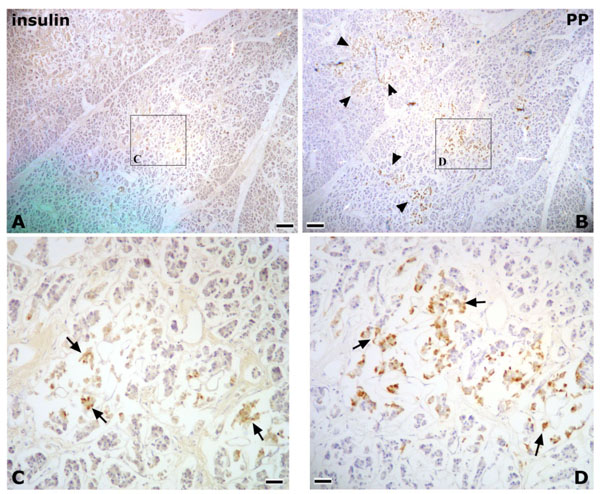 ). On the other hand, in the anterior region of the AIPD, the islets of Langerhans were rounded, and few or no PP-immunoreactive cells were identified (Fig. 3
). On the other hand, in the anterior region of the AIPD, the islets of Langerhans were rounded, and few or no PP-immunoreactive cells were identified (Fig. 3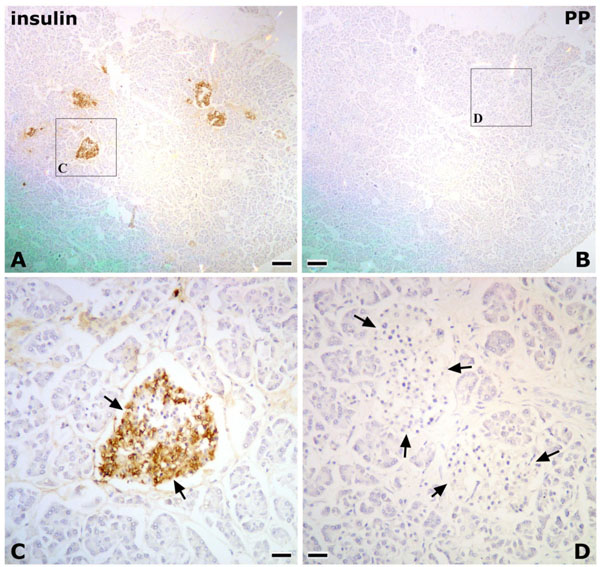 ).
).
In Research 3, en bloc pancreatic tissues containing the AIPD were used to detect the distribution of PP-immunoreactive cells. It was found that abundant PP-positive cells were distributed in the dorsal region of the AIPD and, in that region, the islets of Langerhans were irregular in shape; however, in the ventral region of the AIPD, there were few or no PP-immunoreactive cells, and the islets of Langerhans were rounded (Fig. 4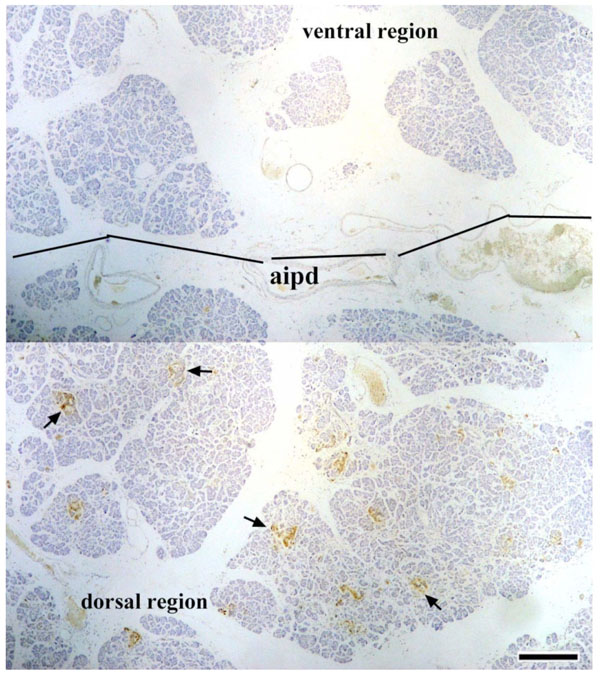 ).
).
DISCUSSION
This study demonstrates that AIPD was located in the boundary of the embryological ventral and dorsal pancreatic primordia, and the course of morphogenesis were first interpreted from a developmental view. To understand this, the configuration relationship of the pancreatic primordium with its vessels needs to be elucidated.
First, the origin of the inferior portion of the head of the pancreas should be located from a developmental view. We do not support the classical description in which the ventral pancreas constitutes most of the heads of the gland, except for the anterior portion of its superior third, which was derived from the dorsal pancreas [21]. In our study, the anterior inferior portion of the pancreatic head showed fewer or no PP-immunoreactive cells, indicating that it arose from the embryological dorsal primordium, whereas the posterior inferior portion showed PP-rich islets, indicating that it was derived from the ventral primordium, and the boundary in the lower portion of the pancreatic head was the AIPD. Our findings are similar to previous reports by Tadokoro et al. [22] and Uchida et al. [8]; however, the relationship between the AIPD and the dorsal/ventral pancreas primordium were not mentioned in these reports.
Furthermore, the uncinate process of the pancreas is generally described as having an exclusive embryonic origin from the ventral primordium [23, 24], but our findings showed that the uncinate process is of double embryonic origin, the ventral and dorsal pancreas. Uchida et al. [8] also reported that the uncinate process consisted of both the ventral (upper two-thirds) and dorsal primordia in five of eight autopsy specimens. In Fig. (5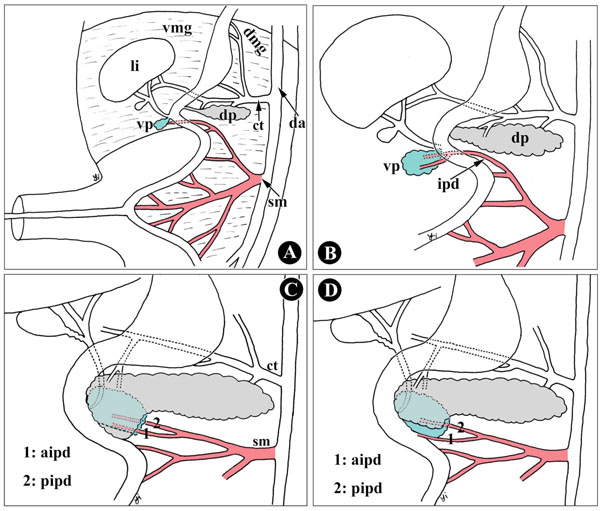 ), during development, the size of the ventral and dorsal primordia influences the inclination of the ventral and dorsal pancreas in the uncinate process. The uncinate process is therefore not derived solely from the primordium, dorsal or ventral.
), during development, the size of the ventral and dorsal primordia influences the inclination of the ventral and dorsal pancreas in the uncinate process. The uncinate process is therefore not derived solely from the primordium, dorsal or ventral.
Second is the course of the AIPD, along the anterior surface or behind the lower portion of the pancreatic head. In current textbooks and atlases of anatomy, it has been described or shown that the AIPD runs along the anterior surface of the lower portion of the pancreatic head [25, 26]; however, in the present study, the AIPD ran not with the anterior surface, but behind the lower portion of the pancreatic head in 73.4% of subjects in our investigation. Similar results were reported by Nagai [8] and the lower portion was called the “mentum” or “chin” of the pancreas. Nagai [8] conjectured that the anterior inferior portion of the pancreatic head may derive from the embryological anlage of the dorsal pancreas; however, the conjecture was not confirmed in this report. According to our results, PP-poor islets are distributed in the anterior inferior portion of the pancreatic head; therefore, the “mentum” of the pancreas was proven to derive from the embryological anlage of the dorsal pancreas by immunohistochemistry.
Third, it was a very important discovery that the embryological ventral and dorsal pancreatic anlagen are irrigated by the superior mesenteric artery and celiac trunk, respectively. Experimental animal, house musk shrew (Suncus murinus), which are common insectivores, has shown morphological characteristic very similar to those of humans than other species, i.e., mice, rats, and is useful as a model of human physiology and pathophysiology [20, 27-29]. According to our previous studies, the pancreases do not fuse as in humans or other species, and the two pancreases are located on the right and left of the common bile duct in Suncus murinus. The right and left pancreases are supplied by the superior mesenteric artery and celiac trunk, respectively [27], and the distribution of PP immunoreactive cells is limited to the islets of Langerhans of the right pancreas, but not the left pancreas [20]. It is suggested that the right pancreas originates from the embryological anlage of the ventral pancreas (PP-rich islets); hence, the ventral pancreatic anlage is irrigated by the superior mesenteric artery, and the dorsal anlage is irrigated by the celiac trunk in Suncus murinus. Baetens et al. [18] also reported that, in rats, the pancreatic regions that provided the two different types of islets have been shown to be irrigated by different arterial systems, the celiac artery for the dorsal region and splenic lobes and the superior mesenteric artery for the ventral region and the duodenal lobe, which were demonstrated to have PP-rich islets.
Furthermore, our recent study indicated that carcinomas of the ventral and dorsal pancreas exhibit different patterns of lymphatic spread. Namely, when the tumor is confined to the ventral pancreas domain, lymph node metastases were limited to areas with the superior mesenteric artery besides peripancreatic lymph nodes. When the tumor was in the dorsal pancreas domain, the lymph node metastases were limited to areas along the common hepatic artery and the hepatoduodenal ligament besides peripancreatic lymph nodes [30]; therefore, it is suggested that, during embryonic development, the ventral and dorsal pancreatic anlagen are irrigated by the superior mesenteric artery and celiac trunk, respectively.
Consequently, it is not difficult to understand the mechanism of the AIPD course along the boundary between the dorsal and ventral anlagen, and why it is located behind or on the anterior surface of the lower portion of the pancreatic head. As shown in Fig. (5 ), during embryonic development, the dorsal and ventral pancreatic primordia arise from ventral and dorsal mesogastrium, irrigated by the superior mesenteric artery and celiac trunk (Fig. 5A
), during embryonic development, the dorsal and ventral pancreatic primordia arise from ventral and dorsal mesogastrium, irrigated by the superior mesenteric artery and celiac trunk (Fig. 5A , B
, B ). In this fusion process of two primordia, the ventral primordium has, together with the developing common bile duct, rotates clockwise behind the duodenum and the dorsal primordium. Thus, the topographic position of the two primordia has inverted in the adult pancreas: the present anterior segment originates from the embryologically dorsal primordium, while the posterior segment from the ventral primordium [31]; however, the overlap pattern of the ventral and dorsal pancreas has not been unified. The factors influencing the patterns include the size of the ventral pancreas, rotating angles and directions on the ventral pancreas, and the cranio-caudal distance (with upper and lower) of the ventral and dorsal pancreas or the distance of the orifice of Santorini’s duct and Wirsung’s duct, that is to say, both the ventral pancreas and its vessels, including AIPD, might be covered by the dorsal pancreas when the ventral pancreas is smaller, or the distance between the orifice of Santorini’s duct and Wirsung’s duct is relatively distant, or the rotating direction turns slightly cephalad (Fig. 5C
). In this fusion process of two primordia, the ventral primordium has, together with the developing common bile duct, rotates clockwise behind the duodenum and the dorsal primordium. Thus, the topographic position of the two primordia has inverted in the adult pancreas: the present anterior segment originates from the embryologically dorsal primordium, while the posterior segment from the ventral primordium [31]; however, the overlap pattern of the ventral and dorsal pancreas has not been unified. The factors influencing the patterns include the size of the ventral pancreas, rotating angles and directions on the ventral pancreas, and the cranio-caudal distance (with upper and lower) of the ventral and dorsal pancreas or the distance of the orifice of Santorini’s duct and Wirsung’s duct, that is to say, both the ventral pancreas and its vessels, including AIPD, might be covered by the dorsal pancreas when the ventral pancreas is smaller, or the distance between the orifice of Santorini’s duct and Wirsung’s duct is relatively distant, or the rotating direction turns slightly cephalad (Fig. 5C ). In contrast, the AIPD appears on the anterior surface of the anterior inferior portion of the pancreatic head (Fig. 5D
). In contrast, the AIPD appears on the anterior surface of the anterior inferior portion of the pancreatic head (Fig. 5D ).
).
In summary, the current study adds further insight into and knowledge of the embryonic development and anatomy of the ventral and dorsal pancreas. The AIPD may follow the boundary between the dorsal and ventral pancreatic anlagen, and we have interpreted embryologically why the AIPD is located on the anterior surface or behind the anterior inferior portion of the pancreatic head. Our conclusion may be of direct or indirect relevance to the pancreatic surgeon.
ACKNOWLEDGEMENTS
This study was supported in part by Grant 17590155 from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
ABBREVIATIONS
| AIPD | = Anterior inferior pancreaticoduodenal artery |
| PBS | = Phosphate-buffered saline |
| PP | = Pancreatic polypeptide |
| RT | = Room temperature |







