- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Cell Development & Biology Journal
(Discontinued)
ISSN: 1874-0855 ― Volume 3, 2011
HDAC2 Cytoplasmic Sequestration Potentiates Keratinocyte Terminal Differentiation
Robert E. Bakin, Mira O. Jung*
Abstract
A balance of histone acetylation and deacetylation governs the regulation of genes that are involved in the differentiation and stratification of the mammalian epidermis. Class II HDACs (HDAC4, 5, 6, 7, 9, 10) frequently undergo nucleocytoplasmic flux resulting in gene derepression. Of the Class I HDACs (HDAC1, 2, 3, 8), HDAC2 has only been described in a nuclear setting. Here we report that a specific in vivo subpopulation of epidermal keratinocytes undergoing apoptotic-like terminal differentiation demonstrate complete cytoplasmic sequestration of HDAC2, robust Keratin-10 expression, and canonical nuclear fragmentation. Paralleling our in vivo findings, proteosomal degradation of total cellular HDAC2 enhanced Keratin-10 expression in undifferentiated HFK cells. Forced HDAC2 nuclear overexpression and retention results in a partial differentiation block as measured by reduced Keratin-10 expression and delayed chromatin fragmentation. We offer a preliminary model whereby cytoplasmic sequestration of the HDAC2 transcriptional corepressor contributes, in part, to the process of mammalian epidermal differentiation. (words 150)
Article Information
Identifiers and Pagination:
Year: 2008Volume: 1
First Page: 1
Last Page: 9
Publisher Id: TOCBJ-1-1
DOI: 10.2174/1874085500801010001
Article History:
Received Date: 01/10/2007Revision Received Date: 10/12/2007
Acceptance Date: 12/12/2007
Electronic publication date: 09/1/2008
Collection year: 2008
* Address correspondence to this author at the Department of Radiation Medicine, Georgetown University, Lombardi Cancer Center, 3970 Reservoir Road NW, Washington, DC 20057-1482, USA; Tel: 202-687-8352; Fax : 202-687-7529/040; E-mail: jungm@georgetown.edu
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 01-10-2007 |
Original Manuscript | HDAC2 Cytoplasmic Sequestration Potentiates Keratinocyte Terminal Differentiation | |
INTRODUCTION
While genomic DNA ultimately retains the genetic identity of an organism, nuclear protein factors such as histones organize the linear genome into a higher order functional unit known as chromatin. Serving to complement the genetic code, posttranslational histone modifications further regulate developmental gene expression profiles via complex epigenetic mechanisms that are just beginning to be deciphered. Acetylation of N-terminal histone lysine residues is but one of several posttranslational mechanisms by which epigenetic mechanisms are manifest. Reversible histone acetylation levels are ultimately governed by the opposing activities of histone acetyltransferases (HATs) and histone deacetylases (HDACs), often correlated with transcriptional activation and repression, respectively. Attesting to their importance in mammalian embryogenesis, individual genetic disruption of HDAC1 [1] and HDAC7 [2] are embryonic lethal while HDAC5 and 9 deletions lead to cardiac abnormalities in the newborn mouse [3]. HDAC4 has recently been implicated in postnatal murine skeletogenesis [2].
HDAC nuclear retention enhances gene repression while cytoplasmic export results in transcriptional activation [4]. Often dictating HDAC subcellular localization and activity, HDACs are themselves subject to posttranslational modifications including phosphorylation [5], sumoylation [6], ubiquitination [7-9], and proteolysis [10]. Class II HDACs (HDAC4, 5, 6, 7, 9) have the ability to readily undergo cytonuclear [11] and/or cytomitochondrial translocation [12]. Contrarily, Class I HDACs (HDAC1, 2, 3, 8) are generally characterized as nuclear enzymes [11] with few exceptions demonstrating a partial cytoplasmic component for HDAC313, 14] and HDAC8 [15]. To date, only HDAC2 has been characterized as a strictly nuclear protein.
The mammalian epidermis is a complex and continually differentiating organ providing an essential, life-sustaining barrier to the environment [16]. Proper epidermal differentiation and stratification relies on complex paracrine signaling events emanating from the underlying dermal mesenchyme [17]. Keratinocyte terminal differentiation begins as cells migrate outward from the regenerating multipotent basal cell layer and into the postmitotic spinous cell layer. Keratinocytes ultimately undergo a specialized form of cell death accompanying transcriptional activation of a new array of structural proteins (e.g. keratins), and the formation of the outermost cornified layer [18]. Stratified epithelia of the skin and oral mucosa are examples of the most protected epithelia and are thus developmentally programmed to express specific keratin isoforms (e.g. Keratin-10), but not others (e.g. Keratin-4) [19]. Epithelium not destined to withstand similar mechanical insults lack keratinization [19].
Here we demonstrate the novel finding that among all expressed Class I HDAC isoforms in the developing murine skin, HDAC2 is uniquely sequestered in the cytoplasm of postmitotic, terminally differentiated epidermal spinous cell keratinocytes. The only other occurrence of this phenomenon was in developing murine hair follicles. All other epidermal and dermal cell types fail to express HDAC2 at any timepoint up to and including post-partum day four. Consistent with activation of lineage-specific patterns of gene expression during epidermal differentiation, robust histone acetylation is restricted to the spinous epidermal cell subpopulation undergoing post-mitotic terminal differentiation and HDAC2 cytoplasmic sequestration. Treatment of undifferentiated HFK cells with valproic acid, a compound previously shown to selectively degrade HDAC2 via the proteosomal pathway, resulted in enhanced Keratin-10 protein expression. Transient overexpression of HDAC2 in HFK cells resulted in significant nuclear HDAC2 retention, attenuated expression of keratin-10 as well as inhibition of nuclear condensation and fragmentation. We propose a model whereby cytoplasmic retention of the Class I HDAC2 corepressor contributes, at least in part, to the derepression of lineage-specific patterns of gene expression in the differentiating mammalian epidermis.
MATERIALS AND METHODS
Mouse Paraffin Sectioning
Embryonic day 8 (E8) through 16 (E16) paraffin embedded slides were purchased from Novagen, Inc. Additional serial sections from E15 through E17 embryos and neonates P1 through P5 were obtained from paraffin embedded archival samples. Archived embryonic samples were originally obtained from female mice that were sacrificed on the appropriate day via carbon dioxide asphyxiation. Embryos were placed in neutral buffered formalin for 48 hours, 70% ethanol for 24 hours, and paraffin embedded accordingly.
Antibodies
The following antibodies were purchased from Santa Cruz; HDAC2 rabbit polyclonal (H-54), p63 rabbit polyclonal (H-137), 14-3-3 gamma mouse monoclonal, HDAC-3 rabbit polyclonal (H-99), HDAC3 mouse monoclonal (B-12). Anti-keratin-10 (#PRB-159P) was purchased from Covance, Inc. Histone H3 (05-499) and all anti-acetyl lysine antibodies were purchased from Upstate, Inc. Anti-Flag M2 antibody was purchased from Sigma, Inc.
Expression Vectors
The HDAC2-Flag mammalian expression vector was a generous gift from P. Yao, Duke University. HFK cells were transfected using Lipofectin according to manufacturers protocols.
Cell Fractionation
C4-2 tissue culture cells and E18 murine skin were fractionated using NE-PER Nuclear and Cytoplasmic fractionation reagent according to published protocols (Pierce).
Cell Lines
HFK cells were a generous gift of Dr. Gary Disbrow, Department of Pathology, Georgetown University Medical Center. NHEK cells were obtained from frozen lab stocks. The undifferentiated phenotype was maintained by growth in SFM-keratinocyte medium (Gibco). Keratinocyte differentiation was induced by addition of 10% nFBS to the serum free medium.
Immunofluorescence
Indirect immunofluorescence was performed as described [12] using a Nikon E600 Fluorescence Microscope with the following objectives; Plan Fluor, 20X, N.A. 0.50, WD= 2.1mm for low magnification images and Plan Apo 60X Oil, N.A. 1.40, WD= 0.21mm for hi magnification images. Filter cubes were used as follows; DAPI: (excitation=330-380nm, dichroic=400nm, emission=420nm), FITC: (excitation=480nm, dichroic=505nm, emission=535nm), TRITC: (excitation=540nm, dichroic=565nm, emission=605nm). Digital images were acquired using a Hamamatsu Orca-100, Firewire, 12-bit cooled CCD digital camera with a 1392 x 1040 pixel array and 6.45 x 6.45um pixel size. Computing employed a stand alone Pentium III running Windows XP. 768 MB RAM, 40GB Hard Drive, Zip Drive, writable CD-ROM. MetaMorph (version 6.1.5) imaging analysis software by Universal Imaging Corp. MetaMorph images were cropped in Adobe Photoshop CS when applicable and assembled for publication using Adobe Illustrator CS.
RESULTS
Terminally differentiating keratinocytes demonstrate cytoplasmic HDAC2 sequestration in vivo.
Keratinocytes are the major epithelial cell type of the epidermis and undergo a program of terminal differentiation requiring a balance between proliferation and apoptosis [20]. Postmitotic keratinocytes undergoing terminal differentiation uniquely demonstrate expression of keratin-10 in the epidermal spinous cell layer (Fig. 1A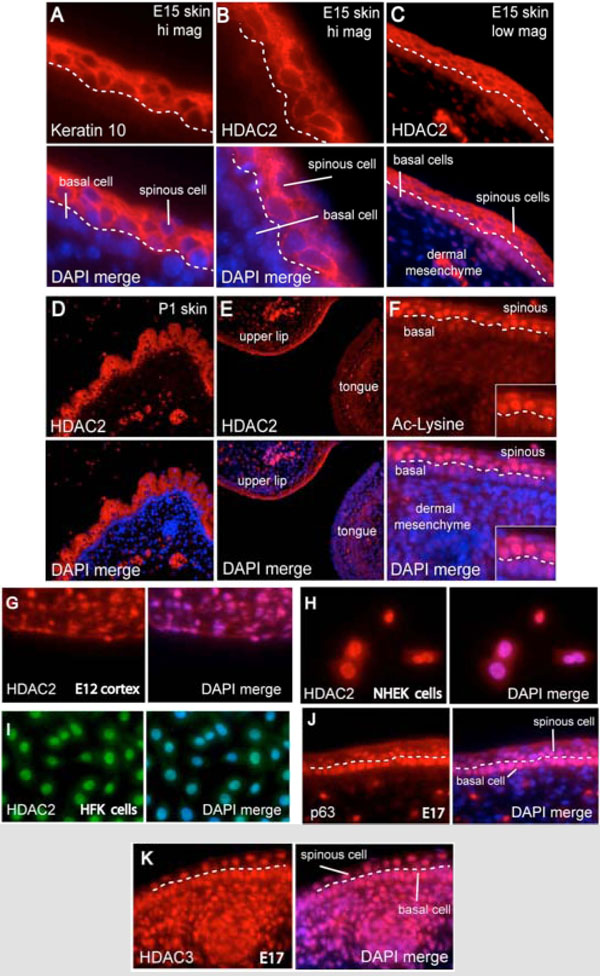 ) [19]. Contrary to current models of strict HDAC2 nuclear localization, we observe robust cytoplasmic HDAC2 (cyto-HDAC2) sequestration in the epidermal spinous cell layer of E15 (Fig. 1B
) [19]. Contrary to current models of strict HDAC2 nuclear localization, we observe robust cytoplasmic HDAC2 (cyto-HDAC2) sequestration in the epidermal spinous cell layer of E15 (Fig. 1B &1C
&1C ) and P1 (Fig. 1D
) and P1 (Fig. 1D ) embryonic murine skin including the keratinizing epidermis of the upper lip (Fig. 1E
) embryonic murine skin including the keratinizing epidermis of the upper lip (Fig. 1E ). Cyto-HDAC2 was specific to differentiated post-mitotic epidermal spinous cells as HDAC2 was not expressed in the adjacent mitotically active basal epithelium or underlying dermal mesenchyme at any timepoint. HDAC2 was observed only in keratinizing epithelium as staining was not detected in E15 tongue (Fig. 1E
). Cyto-HDAC2 was specific to differentiated post-mitotic epidermal spinous cells as HDAC2 was not expressed in the adjacent mitotically active basal epithelium or underlying dermal mesenchyme at any timepoint. HDAC2 was observed only in keratinizing epithelium as staining was not detected in E15 tongue (Fig. 1E ), soft palate, esophagus, or other non-cornifying epithelium (data not shown). We observe strong nuclear histone acetylation only in cyto-HDAC2 positive spinous cells undergoing terminal differentiation (Fig. 1F
), soft palate, esophagus, or other non-cornifying epithelium (data not shown). We observe strong nuclear histone acetylation only in cyto-HDAC2 positive spinous cells undergoing terminal differentiation (Fig. 1F ) that was not present in the underlying basal cells or dermal mesenchyme cells.
) that was not present in the underlying basal cells or dermal mesenchyme cells.
In agreement with previous studies, HDAC2 demonstrates conventional nuclear localization in E12 murine embryonic cortical neurons (Fig. 1G ), the human epidermal NHEK keratinocyte cell line (Fig. 1H
), the human epidermal NHEK keratinocyte cell line (Fig. 1H ), primary human foreskin keratinocytes (Fig. 1I
), primary human foreskin keratinocytes (Fig. 1I ), as well as all other laboratory cell lines tested (SQ20B, Hs-68, PC3, DU145, LNCaP, MCF-7, MRC5CV1, AT5BIVA, and HeLa, data not shown). Cyto-HDAC2 did not colocalize with the nuclear epithelial stem cell transcription factor p63 [21] (Fig. 1J
), as well as all other laboratory cell lines tested (SQ20B, Hs-68, PC3, DU145, LNCaP, MCF-7, MRC5CV1, AT5BIVA, and HeLa, data not shown). Cyto-HDAC2 did not colocalize with the nuclear epithelial stem cell transcription factor p63 [21] (Fig. 1J ) nor ubiquitously expressed HDAC3 (Fig. 1K
) nor ubiquitously expressed HDAC3 (Fig. 1K ).
).
Hair follicle dermal sheath cells are considered to have stem cell character serving as a renewable source for the regenerating hair follicle. Significantly, the outer root sheath of the hair follicle shares many developmental similarities with the skin epidermis [16, 22]. Dermal sheath hair follicles demonstrate similar cyto-HDAC2 immunoreactivity in post partum mice (Fig. 2A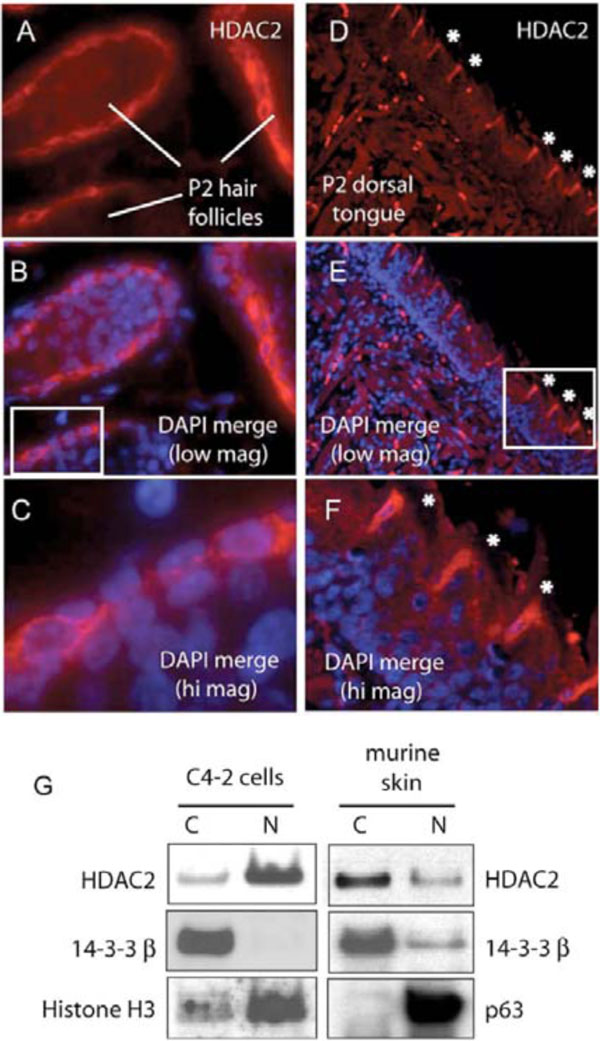 -c
-c ). Cyto-HDAC2 was specific for the dermal papillae layer and was first evident when hair follicles began to display their mature form. Cyto-HDAC2 staining was also observed in a spatially distinct population of keratin positive filiform papillae [23] found specifically on the dorsal surface of the P2 murine tongue (Fig. 2D
). Cyto-HDAC2 was specific for the dermal papillae layer and was first evident when hair follicles began to display their mature form. Cyto-HDAC2 staining was also observed in a spatially distinct population of keratin positive filiform papillae [23] found specifically on the dorsal surface of the P2 murine tongue (Fig. 2D -F
-F ). Cyto-HDAC2 was not observed at earlier timepoints when dorsal tongue papillae have not yet differentiated nor anywhere on the ventral surface of the tongue at any developmental timepoint. The HDAC2 staining appears to be punctate at the periphery of the cell membrane. HDAC2 was colocalized with β-catenin in other mouse paraffin skin sections and coimmunoprecipitated β-catenin as well (data not shown).
). Cyto-HDAC2 was not observed at earlier timepoints when dorsal tongue papillae have not yet differentiated nor anywhere on the ventral surface of the tongue at any developmental timepoint. The HDAC2 staining appears to be punctate at the periphery of the cell membrane. HDAC2 was colocalized with β-catenin in other mouse paraffin skin sections and coimmunoprecipitated β-catenin as well (data not shown).
To confirm cytoplasmic localization of HDAC2, we compared subcellular fractionations of epithelial cells from E17 murine skin and conventional tissue culture cells. As expected, HDAC2 fractionated into the cytoplasmic component of murine epidermal cells isolated from the skin of intact mice while HDAC2 was nuclear in C4-2 prostate cancer epithelial cells and (Fig. 2G ). 14-3-3beta served as a cytoplasmic marker for both cell populations, while histone H3 and p63 served as nuclear markers for C4-2 and murine skin cells, respectively.
). 14-3-3beta served as a cytoplasmic marker for both cell populations, while histone H3 and p63 served as nuclear markers for C4-2 and murine skin cells, respectively.
HDAC2 Nuclear Exclusion Coincides with Keratinocyte Terminal Differentiation In Vitro
In addition to enhanced keratin-10 expression, keratinocyte terminal differentiation incorporates several aspects of apoptotic cell death such as exit from the cell cycle, chromatin condensation, and DNA fragmentation. We next asked whether undifferentiated keratinocytes allowed to undergo in vitro terminal differentiation would demonstrate cytoplasmic HDAC2 sequestration like their in vivo counterparts. Undifferentiated HFK cells were either maintained in defined keratinocyte serum free media (KSFM) or grown in serum-supplemented medium for 72 hours to induce differentiation. Representative indirect immunofluorescence demonstrates a near total relocalization (approx. 99%) of HDAC2 from the nuclei of undifferentiated keratinocytes to the cytoplasm of differentiating HFK cells (Fig. 3A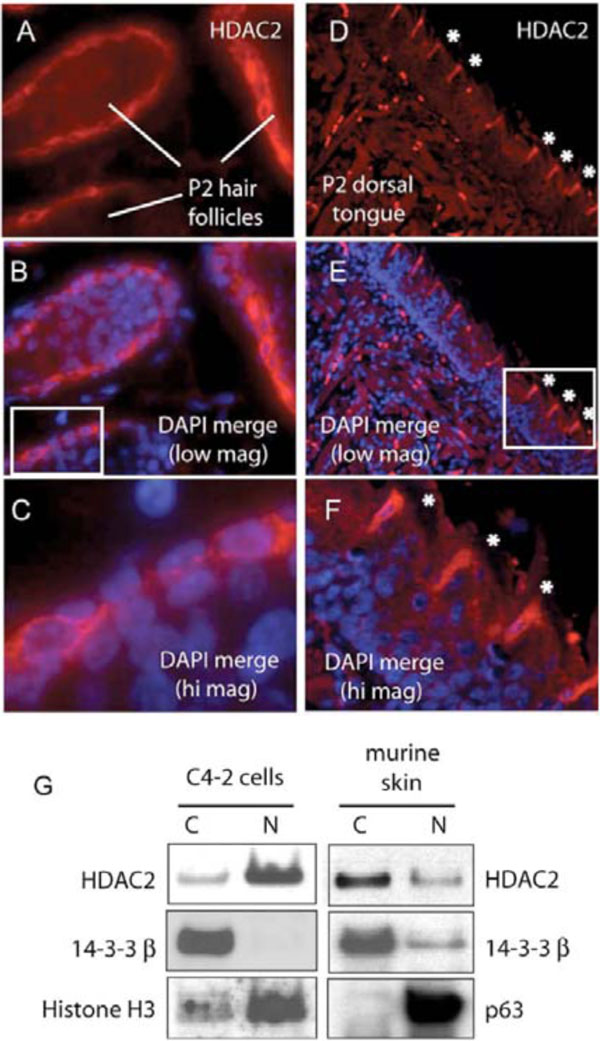 ). Significantly, cells demonstrating cytoplasmic HDAC2 redistribution also show upregulated keratin-10 expression, cell flattening, and chromatin condensation and fragmentation.
). Significantly, cells demonstrating cytoplasmic HDAC2 redistribution also show upregulated keratin-10 expression, cell flattening, and chromatin condensation and fragmentation.
According to our model, selective proteosomal degradation of HDAC2 would be expected to function similarly to cytoplasmic sequestration with respect to derepression of nuclear genes. We next asked whether a targeted reduction in HDAC2 protein levels would facilitate keratinocyte terminal differentiation by enhancing keratin-10 expression. Valproic acid has previously been reported to selectively reduce HDAC2 protein levels via the proteosomal pathway [8, 24, 25]. Four-day administration of 2 mM valproic acid to serum free medium both enhanced keratin-10 expression and reduced HDAC2 protein levels in HFK cells (Fig. 3B ).
).
HDAC2 Nuclear Retention Attenuates Keratinocyte Terminal Differentiation
Our results suggest that forced nuclear retention of HDAC2 may prevent or delay some features of keratinocyte terminal differentiation. Nuclear HDAC2-Flag was over-expressed under non-differentiating culture conditions for two days, then released from terminal differentiation block by the addition of 10% nFBS. We demonstrate that after three days subsequent growth in serum containing medium, overexpressed HDAC2 is still retained in the nucleus to a considerable extent. In nearly all cells (approx. 99%) demonstrating robust nuclear HDAC2-Flag, keratin-10 expression is near background and nuclear condensation and fragmentation is prevented (Fig. 4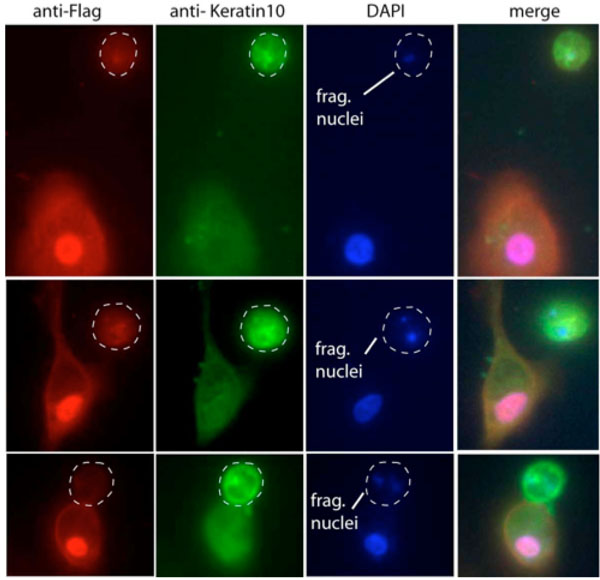 ). On the other hand, adjacent nontransfected cells continue to express keratin-10 and display fragmented apoptotic nuclei. Parallel experimentation revealed that HDAC2-Flag overexpressing cells display a faint endogenous HDAC2 cytoplasmic component upon differentiation (data not shown). We conclude that forced nuclear overexpression of HDAC2 can attenuate at least some aspects of keratinocyte terminal differentiation.
). On the other hand, adjacent nontransfected cells continue to express keratin-10 and display fragmented apoptotic nuclei. Parallel experimentation revealed that HDAC2-Flag overexpressing cells display a faint endogenous HDAC2 cytoplasmic component upon differentiation (data not shown). We conclude that forced nuclear overexpression of HDAC2 can attenuate at least some aspects of keratinocyte terminal differentiation.
In this report, we characterize the novel in vivo observation of cytoplasmic sequestration of HDAC2 in a specific murine epidermal cell population destined for keratinization and terminal differentiation. We further show in vitro that terminally differentiating human foreskin keratinocytes (HFK) similarly demonstrate cytoplasmic sequestration of HDAC2 and elevated keratin-10 expression. Selective proteosomal degradation of endogenous HDAC2 levels in HFK cells promotes the terminally differentiated phenotype by elevating keratin-10 expression. Exogenous overexpression of nuclear HDAC2 delays both the expression of keratin-10 and nuclear fragmentation in HFK cells released from terminal differentiation block. This report offers initial insight into a novel mechanism of embryonic epidermal differentiation whereby the cytoplasmic sequestration of a Class I HDAC isoform contributes, at least in part, to the process of keratinocyte terminal differentiation.
DISCUSSION
A recent report has implicated that a loss of HDAC2 expression in two colorectal cell lines confers selective resistance to the hydroxyamic acid HDAC inhibitor TSA, but not the carboxylic acid HDAC inhibitors valproate or butyrate [26]. To date, HDAC2 has been observed in a strictly nuclear setting [11, 27]. In agreement with such reports, we observe robust nuclear HDAC2 localization in undifferentiated primary cultures of human foreskin keratinocytes (HFKs), normal human keratinocytes (NHEKs), and all other mammalian cell lines tested. Here we report the unexpected finding of near total HDAC2 cytoplasmic sequestration in a specific subpopulation of postmitotic murine keratinocytes destined for terminal differentiation. Our data demonstrated that HDAC2-Flag overexpression resulted in nuclear accumulation in a significant population of transfected HFK cells. As HDAC2 has no reported nuclear import or export motifs, we can only speculate as to the reason for nuclear accumulation of overexpressed HDAC2-Flag. Despite our inability to determine the underlying mechanism responsible for nuclear HDAC2-Flag accumulation, all HFK cells overexpressing nuclear HDAC2-Flag failed to demonstrate both fragmented nuclei and robust Keratin-10 expression after four days in differentiation medium. Significantly, these phenotypes are characteristic of at least a partial keratinocyte differentiation block.
From a transcriptional standpoint, it seems reasonable to conclude that nuclear histones cannot be deacetylated by HDAC2 when it resides in the cytoplasm. Ostensibly, this would favor transcriptional derepression of HDAC2 governed gene promoters during murine keratinocyte terminal differentiation. We suggest that cytoplasmic HDAC2 sequestration is a transcriptionally positive event similar to, but qualitatively different from the HDAC2 protein reduction model demonstrated by Tou et al. [24]. In this study it was shown that Class I HDAC protein levels naturally decline during murine epithelial gut development resulting in subsequent derepression of distinct developmental gene expression profiles. Our results offer another developmental mechanism of nuclear gene activation, namely, cytoplasmic sequestration of a HDAC nuclear corepressor. Unlike our in vitro model system (e.g. HFK cells), it appears unlikely that in vivo HDAC2 ever existed in a nuclear setting as we routinely fail to observe nuclear HDAC2 in murine keratinocytes even at earlier embryonic time-points when the epidermis first begins to stratify (data not shown). For this reason, we speculate that HDAC2 nuclear activity may additionally be regulated, not via protein degradation [24] or elevated nuclear export per se, but instead by a developmentally regulated block of nuclear import, possibly involving posttranslational modification of HDAC2 [7,28-32]. This hypothesis gains strength when one considers that the embryonic epidermis likely can employ alternative negative transcriptional regulatory mechanisms of attenuating HDAC2 nuclear activity besides cytoplasmic sequestration. Indeed, the epidermal basal cells simply fail to express HDAC2. As we observe cytoplasmic HDAC2 interaction with p65/RelA (data not shown), we speculate that HDAC2 cytoplasmic sequestration may have an additional cytoplasmic role in keratinocyte differentiation besides mere nuclear exclusion and transcriptional derepression. Interestingly, p65/RelA is itself reversibly acetylated [33] and has been previously shown to interact with HDAC2 [34]. The suggestion that cyto-HDAC2 functions to modify as yet unidentified non-nuclear factors during keratinocyte differentiation remains a compelling prospect as the number of non-histone targets of HDACs continues to expand [35].
Differential HDAC Cellular Localization – Insight into Isoform Function
HDAC inhibitors are increasingly gaining relevance as robust anti-proliferative and pro-differentiating agents. Recently, the isoform nonspecific HDAC inhibitor SAHA (Zolinza, Merck, Inc.) was approved for treatment of cutaneous t-cell lymphoma (CTCL) [36]. While at least ten other HDAC inhibitors are currently in clinical trials, the lack of isoform-specific HDAC inhibitors contributes to the limited knowledge of HDAC biology. Histone deacetylases likely play a significant role in mammalian biology as HDACs have been shown to modify not only nuclear, but cytoplasmic client proteins as well. As a stepping stone into understanding the biology of HDAC isoforms, experimental manipulation of HDAC cellular localization (e.g. nuclear vs. cytoplasmic) may shed light onto the specific roles of individual HDAC isoforms. Considerable knowledge of nuclear localization sequences and nuclear export would aid in this endeavor.
Valproic acid, long used to treat epilepsy, promotes selective proteasomal degradation of HDAC2 [8] and cytodifferentiation [37, 38]. Suggestively, HDAC inhibitors have been shown to promote differentiation and simultaneously derepress keratin promoters [39-43]. Future in vivo studies are likely necessary to fully characterize the necessity of embryonic HDAC2 cytoplasmic sequestration in the terminal differentiation of postmitotic epidermal keratinocytes. While it seems unlikely that cytoplasmic sequestration of HDAC2 is entirely responsible for keratinocyte terminal differentiation, we suggest that nuclear exclusion of HDAC2 may play at least a supporting role in some transcriptional aspects of the terminal differentiation process.
ACKNOWLEDGMENTS
Invaluable advice and HKF cells were a generous gift from Dr. Gary Disbrow, Department of Pathology This work was supported in part by USMRC grants PC030471 (M. Jung) and PC030019 (R. Bakin) as well as the Lombardi Comprehensive Cancer Center Microscopy and Imaging Shared Resource, U.S. Public Health Service Grant 2P30-CA-51008 and 1S10 RR15768-01. This manuscript is dedicated to Michael Kosmal.




