- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Cell Signaling Journal
(Discontinued)
ISSN: 1876-3901 ― Volume 4, 2012
A Prickly Subject: Apoptotic Regulation by Hedgehog Morphogens
Mark Ditzel*
Abstract
Morphogens, as intercellular signalling proteins, provide a non-cell-autonomous mechanism to impart positional information to cells and govern essential cellular processes such as apoptosis. Individual morphogen pathways utilise diverse strategies to regulate the assembly and activity of the pro-apoptotic multi-protein complexes – namely the apoptosome and the death-inducing signalling complex (DISC). This review aims to highlight the apoptotic regulatory mechanisms utilised by the Hedgehog (HH) morphogen pathway – with particular emphasis on a novel Caspase-9 activating complex and the utilisation of a pro-apoptotic autocrine-signalling loop.
Article Information
Identifiers and Pagination:
Year: 2011Volume: 3
First Page: 9
Last Page: 19
Publisher Id: TOCELLSJ-3-9
DOI: 10.2174/1876390101103010009
Article History:
Received Date: 25/1/2010Revision Received Date: 20/9/2010
Acceptance Date: 20/10/2010
Electronic publication date: 14/4/2011
Collection year: 2011
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http: //creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Institute of Genetics and Molecular Medicine, Edinburgh CRUK Research Centre, Cancer Biology, Crewe Road South, Edinburgh EH4 2XR, UK; Tel: +44 (0)131 777 3555; E-mail: mditzel@ed.ac.uk
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 25-1-2010 |
Original Manuscript | A Prickly Subject: Apoptotic Regulation by Hedgehog Morphogens | |
INTRODUCTION
Apoptosis and morphogen signalling exhibit an intimate relationship. Both are essential for creating, and subsequently maintaining, adult animals, while aberrations in their activities are detrimental. At a functional level apoptosis can promote morphogen expression [1] and morphogen signalling can both promote or suppress apoptosis. An ability to modulate apoptotic and cell-survival pathways places them as key regulators of life and death. Although the majority of research has highlighted morphogens' roles in co-ordinating proliferation and differentiation in organismal development, more recent efforts have begun to identify the complex molecular mechanisms associated with morphogen-mediated apoptotic regulation.
1. Apoptosis
The majority of apoptosis occurring throughout development removes unnecessary, but otherwise normal cells [2], while apoptosis in the adult primarily removes damaged, defective [3] or infected cells [4]. Hence, cell death is initiated in response to a large number of different developmental cues and cellular insults. Distinct intrinsic or extrinsic cellular signalling pathways are activated in response to molecular instructions or stress signals received from the cells’ interior or exterior, respectively. The intrinsic pathway is predominantly activated in response to cell-autonomous apoptotic signals generated from within the cell [5] (e.g., DNA damage, anoxia and oncogene-activation). In contrast the extrinsic pathway is activated in response to recognition of extracellular ligands by cell-surface receptors (e.g., Fas [6]). In this manner neighbouring or infiltrating cells produce ligands to induce the death of another cell. However, cells may also autonomously produce, release and react to their own extrinsic death signals [7]. Ligand- mediated receptor activation leads to activation of down-stream signalling pathways that in turn lead to caspases-activation [6]. Therefore, extracellular-based instructions can govern the ultimate fate of a cell within an organ or tissue - a statement that is also applicable to morphogens.
Regardless of the type or source of apoptotic signal, caspases, a group of aspartate-directed proteases, lie at the heart of the cells' core death machinery [8]. Both the intrinsic and extrinsic pathways activate caspases, which in turn protealytically activate pro-apoptotic and disable anti-apoptotic proteins [9]. This caspase-orchestrated cleavage of apoptotic regulators ultimately results in cleavage of essential regulators of cellular and genomic integrity [9]. To prevent an inflammatory response, the cell's remains are packaged into membrane bound apoptotic bodies and subsequently engulfed by macrophages and neighbouring cells [10].
2. Morphogens
At a molecular level morphogens can be described as molecules that emanate from a localised source to form a concentration gradient. While at a functional level they act as core organisers of cell patterning, cell fate and ultimately overall tissue morphology [11]. These morphogen-co-ordinated events, incorporating growth, proliferation and cell death permit the exceptional three-dimensional structural complexity of organs.
Of the known morphogens, Bone Morphogenic Proteins (BMPs) [12], Notch [13], Wnts [14] and Hedgehog [15] family members are evolutionary conserved. All four share an ability to (A) act directly on a cell through, in most cases, receptor engagement to elicit (B) qualitatively distinct cellular outcomes in response to different morphogen concentrations/gradients. Therefore a cell's position within a morphogen concentration gradient determines the state of the cell’s intracellular signalling pathways and gene expression profiles. Intriguingly, through distinct mechanisms, morphogen-associated signalling pathways can instigate, as well as suppress, apoptosis (see following sections). Unsurprisingly, defects in these potent signalling pathways manifest in numerous diseases and developmental defects [16].
The ability of a morphogen to confer distinct cellular fates is effected by the cells’ ability to perceive quantitative differences in the slope as well as the absolute concentration of the ligand [17, 18]. Morphogen receptors, unlike more conventional binary “on/off” receptors, are capable of translating quantitative differences in ligand levels/gradients into distinct cellular outcomes. In most cases, differences in morphogen concentrations affect the activation level/specific activity of key transcriptional effectors. And it is these differences in transcription factor activities that initiate differential patterns of gene expression and determine cellular outcomes. Hence morphogens represent potent and complex intercellular signalling molecules whose message is interpreted in a context-specific manner.
In a developing tissue individual cells encounter combinations of different morphogen gradients. Integration of this information by a cell is further influenced by cross-talk and cross–regulation between different morphogen- and non-morphogen-signalling pathways [19]. Cross-talk between pathways occurs at the level of target genes – for example WNT [20], Notch [21] and Hedgehog [22] pathways promote, while BMP pathways [23] repress MYC expression. Cross-regulation of one morphogen’s expression by another is a frequently used mechanism and the example of Hedgehog-mediated expression of pro-apoptotic BMPs will be discussed later.
Due to the sheer breadth of morphogen research, this review will focus upon the pro and anti-apoptotic signalling functions of the Hedgehog family of morphogens: Drosophila Hedgehog (HH) and vertebrate Sonic-, Desert- and Indian-Hedgehog (SHH, DHH and IHH, respectively). Please note that in the text the HH pathway denotes a generalised Hedgehog-family-member pathway, not just that of the Drosophila HH. Overall, this review aims to focus on HH’s ability to influence multiple apoptotic signalling pathways and its relevance to disease and development.
3. Canonical HH Signalling Pathway
The canonical HH-pathway governs the majority of Hedgehog's mitogenic and morphogenic effects [16] (Fig. 1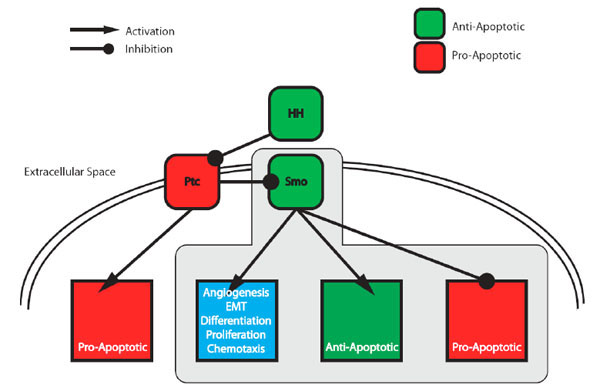 ) and acts through regulating the activity of Smoothened (SMO) [24] - a seven-transmembrane G-protein coupled receptor-like protein. In the absence of HH, Patched (PTC), Hedgehog’s cognate 12-transmembrane receptor protein [25], blocks SMO and as a consequence represses the downstream signalling pathway (Fig. 1
) and acts through regulating the activity of Smoothened (SMO) [24] - a seven-transmembrane G-protein coupled receptor-like protein. In the absence of HH, Patched (PTC), Hedgehog’s cognate 12-transmembrane receptor protein [25], blocks SMO and as a consequence represses the downstream signalling pathway (Fig. 1 ). Upon binding of HH to PTC, SMO is derepressed and activates a signalling cascade of post-translational modifications [15] that promotes activation of a family of Glioma-associated oncogene homolog (GLI) transcription factors [26]. These in turn transcriptionally activate the pathway's target genes that include those involved in cell cycle entry [27], proliferation [22], angiogenesis [28], axonal guidance [29] and epithelial-mesenchymal transition [30].
). Upon binding of HH to PTC, SMO is derepressed and activates a signalling cascade of post-translational modifications [15] that promotes activation of a family of Glioma-associated oncogene homolog (GLI) transcription factors [26]. These in turn transcriptionally activate the pathway's target genes that include those involved in cell cycle entry [27], proliferation [22], angiogenesis [28], axonal guidance [29] and epithelial-mesenchymal transition [30].
Vertebrates encode three family members [31]: GLI-1 (a potent transcriptional activator), -2 (transcriptional activator and repressor functions) and -3 (predominantly a transcriptional repressor). In an unstimulated cell GLI-1 is not expressed and GLI-2 and -3 are protealytically processed and converted into transcriptional repressors. GLI-2 and -3 are maintained in their repressor forms through phosphorylation-mediated ubiquitylation and subsequent cleavage [32]. Upon HH stimulation, both GLI-2 and 3 are no longer cleaved and GLI-1 is now expressed. Together the lack of GLI-2 -3-mediated transcriptional repression, conversion of GLI-2 into an activator and expression of GLI-1 results in transcriptional activation of target genes.
4. Role of Canonical Pathway in Apoptosis
Activation of the canonical Hedgehog pathway can suppress apoptosis through transcriptional upregulation of potent inhibitors of both the extrinsic and intrinsic apoptotic pathways. Hence, aberrant HH signalling in human tumours provides an effective means of evading apoptosis. For example basal cell carcinomas are subjected to apoptotic signals due to expression of the pro-apoptotic TRAIL (Tumour necrosis factor-Related Apoptosis-Inducing Ligand) ligand and its receptors, TRAIL receptor 1/2 (also called DR4 and DR5, respectively) [33]. However, they also harbour mutations in SMO or PTC that result in ligand-independent activation of the canonical HH signalling pathway [34]. Resulting in a reduction of cells’ ability to either promote pro-, or suppress anti-apoptotic pathways. One repressed target of the pathway is the TRAIL receptor 1 and HH-mediated expression of FLIP (FLICE-Like Inhibitory Protein) [33] blocks TRAIL-mediated activation of Caspase-8 and hence the apoptotic caspase cascade. Similarly HH-mediated Bcl2 overexpression [35, 36] also blocks the tumours’ increased apoptotic load generated by oncogene activation and increased metabolic stress [37]. As a potent anti-apoptotic protein frequently overexpressed in numerous cancers, Bcl2 acts to prevent the release of cytochrome c from the mitochondria [38] (and Grant Dewson’s review in this issue). This in turn prevents cyctochrome-c-mediated apoptosome formation, Caspase-9 activation and initiation of the apoptotic caspase cascade. Therefore a combination of potent apoptotic inhibitors, induced by the HH pathway, would render cells impervious to two major routes of apoptotic induction.
In addition to the anti-apoptotic transcriptional targets, the canonical HH signalling pathway may also use post-translational modifications to regulate the levels and activity of the central apoptotic regulator p53. HH-mediated activation of SMO may promote PI3K-mediated activation of the anti-apoptotic AKT pathway [39] and promote MDM2-mediated p53 degradation [40]. Removal of p53, a key integrator of diverse apoptotic signals, again would cause cells to resist many types of apoptotic insults.
The HH pathway’s ability to protect cells from both intrinsic, through Bcl2, loss of p53 and activation of cell survival pathways, and extrinsic mechanisms, through FLIP, highlights its role as a central player in apoptotic regulation. Therefore, in combination with its more established role in promoting cell growth and division, aberrant activation of the canonical HH pathway can provide both the drive towards, and inhibition of the pro-apoptotic brake(s) against, tumourigenesis. Nevertheless, the HH pathway has additional pro- and anti-apoptotic regulatory roles that will be discussed in the following sections.
5. Hedgehog's Anti-Apoptotic Role in Development
A number of elegant developmental studies with mice and chicken have revealed a major anti-apoptotic role for HH in a range of cells within different tissues. Midline cells (notochord and floorplate) in developing chick [41-43] and mouse [44-46] embryos provide SHH-derived survival and proliferative signals to the surrounding tissue. Surgical procedures that prevent regression of Hensen's node (the avian organiser) prevent the formation of the midline cells, but allow the formation the neural tube [41]. In the absence of midline cells - the source of SHH - all the tissue surrounding the midline-depleted region, including the neural tube, undergo apoptosis. This massive cell death could be rescued by grafting of SHH expressing cells in the midline-depleted animal [41]. Similar observations were also made for midline-derived SHH in promoting the survival of myogenic and chondrogenic cell lineages [43].
In the mouse, ectopic expression of SHH in the dorsal neural tube inhibits differentiation and increased cell numbers [47]. Mice deficient in Shh exhibit gross defects in patterning and tissue organisation and only survive up to or just after birth [45]. Loss of SHH expression in a Shh null animal led to extensive somite and neural crest cell death [45, 48]. In contrast, loss of Ptc function leads to overgrowth phenotypes in both homozygous mouse embryos and heterozygous adults [49]. These observations are consistent with a role for PTC in both promoting cell death and suppressing proliferation. Furthermore, injection of SHH blocking antibodies in the cephalic region of chick embryos led to massive apoptosis in the neural tube and neural crests regions [50, 51].
6. SHH as a Survival Factor in Tumour Cells
Therapeutic intervention to block the HH pathway and its ability to suppress apoptosis, differentiation and promote proliferation has proven extremely effective in the treatment of a number of human tumours [52]. Hedgehog’s mode of action may be cell autonomous or cell non-autonomous, acting in either an autocrine or paracrine manner, respectively [53]. In an autocrine model tumour cells both produce and respond to HH, while in the paracrine model HH produced by the tumour signals to the surrounding stroma. These stromal cells can then indirectly benefit the tumour cells by, for example, mediating angiogenesis [54]. A reverse paracrine model may also function where tumour cells drive tumourigenesis by actively signalling to surrounding stromal cells to produce HH. Furthermore, HH signalling to the stroma may in turn promote production of alternative extracellular signalling molecules such as Insulin-like growth factor (IGF) [55] and directly promote tumour growth and/or survival.
Basal cell carcinoma provided direct evidence for a role for HH in human cancer. Patients harbour mutations in either PTC or SMO that render pathway activation ligand independent [34]. This HH-independent method of activating the pathway seems to be rare in other tumour types, with the majority of tumours being dependent on either autocrine or paracrine modes of actions [56]. Numerous studies have identified HH-signalling as being essential to the survival of tumour cells derived from a wide variety of tissues: small cell lung cancer [57]; oesophageal, stomach, biliary tract and pancreatic cancers [58, 59], multiple myeloma [60, 61], leukemias [62] and gliomas and other brain tumours [63]. Furthermore, in Helicobacter pylori induced gastric cancer, gastric epithelial cells reactivate expression of SHH and, through an autocrine loop, confers resistance to apoptosis [64].
7. Pro-Apoptotic Role of SHH
While generally considered to be a survival signal, SHH also seems to play a pro-apoptotic role in some developmental death processes. Cells eliminated at the fusion of the neural folds require SHH-mediated apoptosis, while certain cells in the spinal cord exhibit apoptotic responses to SHH signalling [65]. Furthermore, in the chick developing limb bud, SHH expressing cells in the zone of polarising activity undergo SHH-mediated homeostatic apoptosis. This action is key to maintain the correct spatial positioning and prevent the overexpansion of the zone of polarising activity [66]. Programmed cell death occurring in the developing vertebrate limb is one of the clearest examples of apoptosis as a morphogenetic process [67]. Similarly, the morphogenic role of HH is extremely well characterised [68]. Its exclusive expression in a distinct “polarising region” establishes a morphogen gradient essential for the correct growth and patterning of the vertebrate limb. Within the developing limb four clear areas undergo massive apoptosis that control digit formation and separation - namely the opaque patch, anterior-, and the posterior-necrotic zones and interdigital areas [69].
In the posterior-necrotic zone, exogenous SHH promotes apoptosis but suppresses death in three other regions [66]. Such radical differences in cellular fate most likely reflect differences in an individual cell’s position within a HH concentration gradient and/or the integrated actions of other extracellular signalling pathways. Although in the minority, similar pro-apoptotic effects of exogenous SHH occur in the avian spinal cord that result in a loss of neuronal precursor cells and floor plate cells [65].
Recent work with conditional Ptc knockout mice revealed the importance of paracrine HH signalling in the control of haematopoiesis [70]. Deleting Ptc in haematopoietic cells did not lead to activation of the HH pathway or generate a phenotype. Conversely, loss of Ptc in the surrounding non-haematopoietic cells led to autonomous HH pathway activation and correlated with apoptosis of haematopoietic early T- and B-cell precursors.
8. Patched, the Hedgehog Receptor as a Pro-Apoptotic Dependence Receptor
With the exceptions of the few examples given above, the majority of evidence points to a role for the canonical pathway, via SMO, in suppressing apoptosis. However, more recent work however has highlighted a role for PTC in pro-apoptotic signalling, independent of the SMO-associated canonical pathway [41, 71]. These findings demonstrate a bifurcation of the Hedgehog signalling pathway downstream of the PTC receptor into canonical and non-canonical branches (see Fig. 1 ). Furthermore they suggest that HH-mediated regulation of apoptosis simultaneously relies on regulation of separate intracellular signalling pathways governing (A) PTC-mediated pro-apoptotic signalling and (B) SMO-mediated anti-apoptotic signalling.
). Furthermore they suggest that HH-mediated regulation of apoptosis simultaneously relies on regulation of separate intracellular signalling pathways governing (A) PTC-mediated pro-apoptotic signalling and (B) SMO-mediated anti-apoptotic signalling.
PTC’s pro-apoptotic function in the absence of HH places PTC as a member of a family of "dependence receptors" [72]. The family includes: DCC (deleted in colorectal cancer), TrkC (tyrosine kinase receptor C), ALK (anaplastic lymphoma kinase), RET (rearranged during transfection), αvβ3 integrin, p75NTR (p75 neurotrophin receptor), neogenin, and androgen receptor. These proteins along with PTC are grouped by their shared properties/ abilities to: (1) induce cell death in the absence of their cognate ligand; (2) induce caspase-dependent cell death; (3) undergo a pro-apoptotic modification/alteration of a cytoplasmic domain; (4) play a role in both developmental processes, most commonly neurogenesis, and cancers; and (5) potentially contain a dependence receptor associated transmembrane motif (DART) [73]. PTC matches all five of the above criteria and may explain some of the apoptotic response of cells deprived of HH.
PTC overexpression, in the absence of SHH, activates caspases, promotes DNA fragmentation and is suppressed with the pan-caspase inhibitor z-VAD [41]. PTC deletion and mutation analyses identified a region in the seventh intracellular loop of PTC essential for its apoptotic signalling. Furthermore this region promotes the formation of a pro-apoptotic multiprotein complex named the "dependosome" [71]. Within this region of murine PTC resides a caspase cleavage site (PETD13927#x21D3;H) [41] and cleavage at this site by the effector Caspase-3 and -7, or initiator Caspase-8 was required for PTC-mediated apoptosis. Expression of the amino-terminal cleaved portion (PTC1-1392) was sufficient to induce apoptosis and was refractory to SHH-mediated protection. Moreover a non-caspase-cleavable mutant, PTC PETN1392H, was unable to promote cell death.
More recent molecular analysis of PTC receptor complexes identified a number of apoptotic and caspase-associated proteins [71] (Fig. 2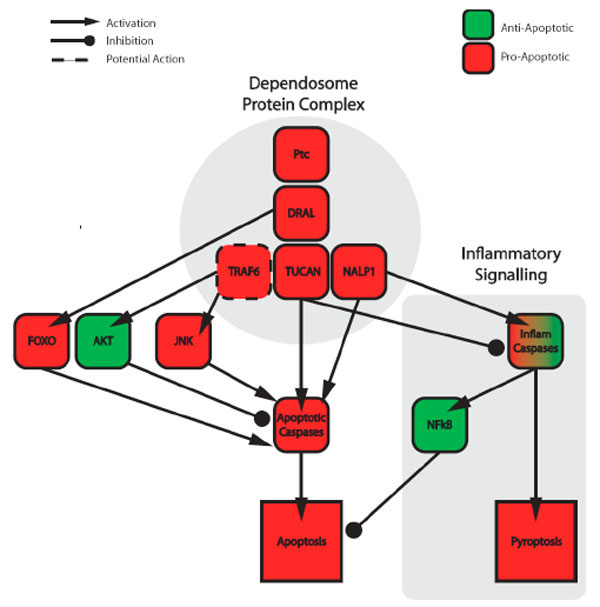 ). Within this complex reside the initiator Caspase-9, the LIM-domain-containing DRAL (Down-regulated in Rhadomyosarcoma) and the CARD-containing proteins TUCAN (tumour-up-regulated CARD-containing antagonist of Caspase nine) and NALP1 (NLR family, pyrin domain containing 1). DRAL bridges PTC to TUCAN, which in turn recruits Caspase-9. The presence of this initiator caspases within the complex provides an obvious link to the intrinsic apoptotic pathway. In the absence of SHH, the multiprotein complex including Caspase-9 assembles and leads to activation of the downstream caspase-cascade. While in the presence of SHH caspases are not activated, the multiprotein complex dissembles and cells do not die.
). Within this complex reside the initiator Caspase-9, the LIM-domain-containing DRAL (Down-regulated in Rhadomyosarcoma) and the CARD-containing proteins TUCAN (tumour-up-regulated CARD-containing antagonist of Caspase nine) and NALP1 (NLR family, pyrin domain containing 1). DRAL bridges PTC to TUCAN, which in turn recruits Caspase-9. The presence of this initiator caspases within the complex provides an obvious link to the intrinsic apoptotic pathway. In the absence of SHH, the multiprotein complex including Caspase-9 assembles and leads to activation of the downstream caspase-cascade. While in the presence of SHH caspases are not activated, the multiprotein complex dissembles and cells do not die.
While Caspase-9 provides the most obvious link to apoptosis [5], the other multi-protein complex components also have links to apoptotic regulation (see following section for more details). Intriguingly, DRAL, TUCAN and NALP1 have known roles in the regulation the NFκB pathway [74]. This pathway has a complex association with apoptosis as it both promotes and suppresses cell death [75]. Nevertheless this link between PTC and the NfκB pathway provides a potential molecular mechanism for PTC-mediated apoptosis. As a number of the proteins identified in the 'dependosome' have been linked to alternative apoptotic and caspase-activational pathways, it is possible that the dependosome may act as a central hub for activating multiple, independent routes for apoptotic initiation.
8.1. Apoptosis-Associated Roles of PTC Dependosome Subunits
8.1.1. DRAL/FHL2
DRAL/FHL2 is a four and a half LIM domain containing protein that primarily acts as a transcriptional co-activator or –repressor for a number of target genes [76, 77]. Caspase-mediated cleavage of PTC is essential for its pro-apoptotic activity and its interaction with the LIM2-containing region of DRAL [71]. Upon overexpression, DRAL promotes apoptosis in a number of cell lines and fits with its role as a p53 target gene [78]. In addition to its above-mentioned role in recruiting Caspase-9, mechanistically, FHL2 may be affecting apoptosis through recruitment of TRAF6 and its associated apoptosis-regulatory complexes [79]. Both pro-apoptotic TGFβ- and RANK-receptors recruit TRAF6, which in turn activates TAK1 and leads to increased JNK activity [80, 81]. As with the HH pathway, under certain circumstances the RANK- and TGFβ-receptors can provide anti-apoptotic signals. Both TGFβ [82] and RANKL [83] use TRAF6 and SRC to mediate anti-apoptotic signalling through the PI3K/AKT survival pathway [84].
An alternative link to apoptosis includes FHL2’s ability to bind the pro-apoptotic Forkhead transcription factor FOXO1 and blocks its transcriptional activity [85]. While FHL2 also acts on other transcriptional regulators including SMADs 2, 3 and 4 [86] and β-catenin [77] to regulate key cell cycle regulators. FHL2 could also act to suppress survival signalling through binding integrins and blocking integrin-mediated ERK signalling [87].
8.1.2. TUCAN/CARD8
TUCAN is a CARD-containing protein that is expressed as 48kDa and 54kDa isoforms. Through a homophilic CARD-CARD interaction, the smaller form binds Caspase-9 and blocks a variety of intrinsic-pathway activating insults [88, 89]. In contrast the 54kDa, which contains a unique amino-terminus absent in 48kDa form, binds FADD and blocks FasL- and extrinsic Caspase-8-mediated apoptosis [89]. Nevertheless, contradictory findings suggest that TUCAN can also assume pro-apoptotic roles. Upon overexpression TUCAN blocked Caspase-1 processing, the production of IL1-β, suppressed the NFκB pathway and ultimately sensitised cells to PMA-induced apoptosis [90].
8.1.3. NALP1
NALP1 is a member of the NOD-like receptor family involved the recognition of viruses and microbes and subsequent activation of the innate immune system [91]. At the molecular level NALPs promote the formation of large multi-protein signalling complexes called inflammasomes that activate inflammatory Caspases-1, -4 and -5 [92]. While inflammatory caspases are associated with apoptosis of infected macrophage apoptosis - called pyroptosis [93] – these caspases are predominantly involved in processing inflammatory cytokines such as Interleukin-1β and -33 and promoting the inflammatory response [94]. Upon processing, interleukins - as extracellular signalling molecules - bind their cognate receptors and, in an autocrine or paracrine manner, activate the NFκB pathway [95].
In addition to NALP1’s role in the inflammasome, it also binds to the anti-apoptotic Bcl2 and Bcl-XL proteins [96]. These interactions repress NALP1-mediated Caspase-1 activation, however the affect on Bcl2 and Bcl-XL's anti-apoptotic activity remains unknown. Nevertheless, a clear role for NALP1 in promoting apoptosis comes from its ability to interact with the pro-apoptotic apoptosome component Apaf-1 and promote cell death [97].
9. Hedgehog-Mediated Apoptosis via a BMP-Autocrine Loop
In addition to the role of PTC in dependosome-signalling, a second canonical-pathway-associated apoptotic pathway can also promote Caspase-9 activation. In the absence of HH, the cleaved GLI-3R repressor form mediates some of the ectopic apoptosis seen in the neural tube and developing limbs [46, 98, 99]. Aberrant neural tube cell and developing limb apoptosis seen in Shh null mice is rescued in a Shh-/-;Gli-3-/- null animal [46]. Therefore, with respect to cell survival, the canonical SHH pathway primarily acts to prevent the processing of full-length GLI-3 into its cleaved GLI-3R form. A number of observations correlate increased levels of apoptosis with increased expression of GLI-3R and the morphogen BMP4 [98, 99]. Apoptosis associated with BMP signalling occurs in a number of tissues throughout development and includes the developing limb buds [67]. One mechanism acts to activate Caspase-9 through stabilisation of p53 and subsequent expression of the pro-apoptotic BH3-only proteins PUMA [100] or BIM [101].
GLI-mediated transcriptional activation of BMP4 and BMP7 promoters [102] provides a direct molecular link between the HH and BMP morphogen pathways. Therefore, when depleted of ligand, the HH pathway is unable to prevent the formation of GLI-3R, which in turn leads to production of BMPs. Additionally, in the presence of ligand, HH signalling upregulates expression of the BMP antagonist Gremlin [98]. During limb morphogenesis, this BMP-binding protein's expression correlates with a lack of apoptosis and, in its absence, with cell death. In the developing duck embryo, non-apoptotic interdigital tissue expresses Gremlin, while the apoptotic inter-digital tissue of a chick lacks it [103]. Therefore in the absence of SHH, a combination of events promotes BMP-mediated apoptosis: (1) by increased production of BMP4 ligand and (2) decreasing levels of its inhibitory binding-protein Gremlin (Fig. 3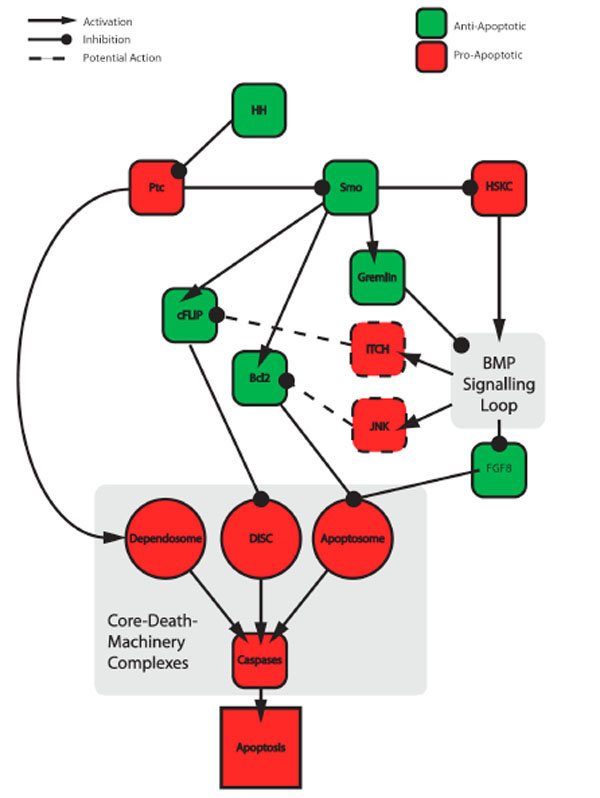 ).
).
The above example demonstrates that when active one morphogen pathway (HH) directly suppresses ligand production of another (BMPs). In turn, when active the BMP pathway represses the expression a third, anti-apoptotic associated morphogen, Fibroblast Growth Factor 8 (FGF8). Therefore, through regulating BMP expression, the HH pathway indirectly promotes FGF8 expression [104]. In the Shh-/- null mouse, apoptotic cells in or around apoptotic tissues not only show elevated levels of BMP 4/7, but also lack FGF8 expression [105]. In the interdigital regions of the developing limbs, FGF8 acts as a cell survival factor [106]. Inactivation of FGF8 results in ectopic cell death in the development of the early limb bud [107, 108], kidney [109, 110] and brain [111, 112]. Activation of both RAS-MAPK and PI3K-AKT cell survival pathways are associated with the FGF receptors engagement [113] and therefore provides a powerful means to control apoptosis.
At the molecular level, BMP-signalling through ligand engagement of the BMP receptor (BmpR1A) mediates loss of FGF8 expression [105]. While this provides a molecular link between the two morphogen-signalling pathways, it is unlikely to be the sole apoptotic consequence of elevated BMP signalling. As with the SHH receptor PTC, ligand engagement of BMP-family Transforming Growth Factor β receptors (TGF-βR) affects two distinct downstream signalling pathways. One relies on a transcriptional output mediated by a group of transcriptional co-regulators called SMADs [114]. Upon binding of the TGFβ ligand, TGFβRI- and TGFβRII-receptors activate SMADs and sensitise cells to apoptosis by transcriptionally downregulating anti-apoptotic BCL-2 [115] and upregulating pro-apoptotic BIM [116].
In addition to a SMAD-mediated transcriptional response, post-translational modifications can also drive TGFβ-mediated apoptotic responses. Upon TGFβ-receptor ligation, TGβR-mediated phosphorylation activates the E3 ubiquitin-protein ligase TRAF6 and promotes its non-degradative K63-linked autoubiquitylation [80]. TGF-β-activated kinase 1 (TAK1) is then activated upon binding to ubiquitylated TRAF6. Once active TAK1 initiates a kinase cascade to activate the stress-activated kinases JNK and p38 [81] (see Fig. 3 ). These kinases are capable of promoting both developmental and stress-induced apoptosis and are activated in response to a range of cellular insults [117]. As discussed earlier PTC may also recruit TRAF6 via FHL2 to activate JNK/p38 and promote apoptosis. Regardless of the route of JNK/p38 activation, these kinases effect apoptosis [117, 118] via (1) nuclear translocation of c-JUN and subsequent expression of pro-apoptotic genes; (2) direct phosphorylation and activation of p53; (3) degradation of FLIP, via phosphorylation and activation of the ubiquitin-protein ligase ITCH; and (4) activation and suppression of pro-apoptotic BH3-only protein and anti-apoptotic Bcl2 family members, respectively.
). These kinases are capable of promoting both developmental and stress-induced apoptosis and are activated in response to a range of cellular insults [117]. As discussed earlier PTC may also recruit TRAF6 via FHL2 to activate JNK/p38 and promote apoptosis. Regardless of the route of JNK/p38 activation, these kinases effect apoptosis [117, 118] via (1) nuclear translocation of c-JUN and subsequent expression of pro-apoptotic genes; (2) direct phosphorylation and activation of p53; (3) degradation of FLIP, via phosphorylation and activation of the ubiquitin-protein ligase ITCH; and (4) activation and suppression of pro-apoptotic BH3-only protein and anti-apoptotic Bcl2 family members, respectively.
CLOSING REMARKS
In summary, components of the HH-pathway can both directly promote and suppress apoptosis (Fig. 3 ). Unfortunately, a clear link between the presence, or absence, of ligand and promotion or suppression of apoptosis cannot currently be made. In the presence of ligand the HH-pathway can actively suppress apoptosis through both blocking pro- and activating anti-apoptotic proteins. However, under certain developmental circumstances, the presence of HH and activation of the pathway promotes apoptosis (see Section 8). Nevertheless, in the absence of ligand, apoptosis does seem to predominate, with two separate signalling pathways converging on caspases to promote cell death. The first stems from PTC-mediated formation of the "dependosome", which should prompt the apoptotic field to rethink the exclusive association between extracellular (extrinsic) pathways and Caspase-8 activation. And the second stems from SMO-associated processing of GLI-3 into its GLI-3R form. These two pathways significantly differ, with the former directly activating Caspase-9 and the latter acting indirectly through autocrine action of second messengers (BMPs).
). Unfortunately, a clear link between the presence, or absence, of ligand and promotion or suppression of apoptosis cannot currently be made. In the presence of ligand the HH-pathway can actively suppress apoptosis through both blocking pro- and activating anti-apoptotic proteins. However, under certain developmental circumstances, the presence of HH and activation of the pathway promotes apoptosis (see Section 8). Nevertheless, in the absence of ligand, apoptosis does seem to predominate, with two separate signalling pathways converging on caspases to promote cell death. The first stems from PTC-mediated formation of the "dependosome", which should prompt the apoptotic field to rethink the exclusive association between extracellular (extrinsic) pathways and Caspase-8 activation. And the second stems from SMO-associated processing of GLI-3 into its GLI-3R form. These two pathways significantly differ, with the former directly activating Caspase-9 and the latter acting indirectly through autocrine action of second messengers (BMPs).
With the majority of our knowledge being derived from in vivo animal studies, such results reflect the complex environment an individual cell experiences in a particular tissue and timeframe. Within these settings individual cells perceive temporally and spatially restricted instructions from a wide array of extracellular signalling molecules (see Review by Paul Ekert in this issue) - that include morphogens. In the case of morphogens, cellular responses are not only dependent on the presence or absence of the ligand, but also slope of the ligand gradient. In combination with the other extracellular signalling molecules, cells must translate and integrate this information through intracellular pathways. These in turn initiate molecular programmes that effect cellular outcomes. Due to a significant degree of cross-talk and cross-regulation between different pathways [19] the molecular and cellular outcomes of morphogen-stimulation can be altered. Such contextual determinants may help explain the apparent pro- and anti-apoptotic duality of HH-signalling. Only more detailed in vivo studies will allow us to identify the molecular circuits that ultimately determine morphogen-associated outputs.
ACKNOWLEDGEMENTS
I would like to thank Dr. Val Brunton for her input in improving the manuscript. Mark Ditzel is funded by the University of Edinburgh as a Senior Research Fellowship and as a BBSRC New Investigator Award.



