- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Environmental Sciences
(Discontinued)
ISSN: 1876-3251 ― Volume 10, 2018
Influence of Paraquat on Four Rhizobacteria Strains: Pantoea agglomerans, Rhizobium nepotum, Rhizobium radiobacter and Rhizobium tibeticum
Mohamed Maldani, Btissam Ben Messaoud, Laila Nassiri, Jamal Ibijbijen*
Abstract
Background:
Soil microorganisms are exposed to herbicides after treatment, which leads to their interaction. The result of this interaction may be the degradation of the herbicides by the microorganisms and by the way, they use the degradation products as an energy source for their own physiological processes, or herbicides have a toxic effect on these microorganisms. Herbicide toxicity becomes severe instantly after application when its concentration in soil is the highest. Paraquat is one of the most widely used herbicides in agriculture; inappropriate use of this herbicide represents an immense pollution problem for soil, therefore on microorganisms. However, the knowledge about the effect of paraquat on soil microorganisms has been limited.
Objectives:
The purpose of the current study was to determine the effect of paraquat application on four nitrogen-fixing bacteria: Pantoea agglomerans, Rhizobium nepotum, Rhizobium tibeticum and Rhizobium radiobacter.
Methods:
Paraquat was applied as the sole source of carbon at a rate (0 g/L, 0.5 g/L, 1 g/L, 3 g/L, 6 g/L and 12 g/L). The effect of paraquat treatments was determined by agar diffusion method and the rate of the growth of bacterial colonies in each treatment.
Results:
In the agar diffusion method, the bacterial strains were inhibited by paraquat, in which the inhibition zone was wider with the increase of paraquat concentration; also, analysis of the Colony Forming Units (CFUs) mostly showed a declining in bacterial growth. In comparison with the control, the growth of the four strains was decreased by increasing the paraquat concentration. Comparing strains with each other, Pantoea agglomerans is the most resistant strain to paraquat.
Conclusion:
Our study has shown the impact of the irrational use of pesticide upon the beneficial bacteria in question. For that, the results of this research have a positive impact on the natural environment, which will have tangible social and economic impacts.
Article Information
Identifiers and Pagination:
Year: 2018Volume: 10
First Page: 48
Last Page: 55
Publisher Id: TOENVIRJ-10-48
DOI: 10.2174/1876325101810010048
Article History:
Received Date: 27/08/2018Revision Received Date: 26/10/2018
Acceptance Date: 29/10/2018
Electronic publication date: 30/11/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Director of Laboratory, Environment & Soil Microbiology Unit, Faculty of Sciences, Moulay Ismail University, B.P. 11201 Zitoune, Meknes, Morocco; Tel:+212670135002, E-mails: j.ibijbijen@fs.umi.ac.ma; jamal_ibijbijen@yahoo.fr.
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 27-08-2018 |
Original Manuscript | Influence of Paraquat on Four Rhizobacteria Strains: Pantoea agglomerans, Rhizobium nepotum, Rhizobium radiobacter and Rhizobium tibeticum | |
1. INTRODUCTION
The soil is one of the most vulnerable ecosystem components, it is easily contaminated by pesticides, however, its remediation may take long periods to restore its initial state. Herbicides are commonly used in agriculture, by the way, its application has many consequences essentially on changing the microbial populations leading to edit the microbial ecological balance and therefore affecting the soil fertility [1Shahid M, Ahmed B, Saghir-Khan M. Evaluation of microbiological management strategy of herbicide toxicity to green gram plants. Biocatal Agric Biotechnol 2018; 14: 96-108.
[http://dx.doi.org/10.1016/j.bcab.2018.02.009] -3Johnen BG, Drew EA. Ecological effects of pesticides on soil microorganisms. Soil Sci 1977; 123: 319-24.
[http://dx.doi.org/10.1097/00010694-197705000-00007] ]. The fate of herbicides applied in agricultural ecosystems is governed by their interaction with soil microorganisms, their transfer and their degradation processes. The increasing reliance of agriculture on herbicides has led to a concern about their eco-toxicological effects on soil microorganisms, this toxicity of herbicides is intensified immediately after their application [4Kumar S, Kaushik G, Dar MA, Nimesh S. Microbial degradation of organophosphate pesticides: A review. Pedosphere 2018; 28: 190-208.
[http://dx.doi.org/10.1016/S1002-0160(18)60017-7] , 5Zaller G, König N, Tiefenbacher A, Muraoka Y. Pesticide seed dressings can affect the activity of various soil organisms and reduce decomposition of plant material. BMC Ecol 2016; 16: 3.
[http://dx.doi.org/10.1186/s12898-016-0092-x] ]. Soil microbial communities play a crucial role in the ecosystem services thanks to the microorganisms engaged in a degradation process that provides them with their original economic value [6Allegrini M, Zabaloy MC, Gómez EV. Ecotoxicological assessment of soil microbial community tolerance to glyphosate. Sci Total Environ 2015; 533: 60-8.
[http://dx.doi.org/10.1016/j.scitotenv.2015.06.096] ]. Moreover, microbial catabolism is a major pathway for the dissipation of herbicides in the environment, some rhizobacteria have beneficial effects on the plant growth and the tolerance to herbicides. For this reason, it is important to study the effect of pesticides on the microbial communities living in the agricultural soils.
Paraquat, the bipyridylium compound, 1, 1’-dimethyl-4, 4’- bipyridylium, has been widely used as an herbicide for many decades [7Camacho-Morales RL, Gerardo-Gerardo JL, Navarro KG, Sánchez JE. Producción de enzimas ligninolíticas durante la degradación del herbicida paraquat por hongos de la pudrición blanca. Rev Argent Microbiol 2017; 49: 189-96.
[http://dx.doi.org/10.1016/j.ram.2016.11.004] -9Roberts TR, Dyson JS, Lane MC. Deactivation of the biological activity of paraquat in the soil environment: A review of long-term environmental fate. J Agric Food Chem 2002; 50: 3623-31.
[http://dx.doi.org/10.1021/jf011323x] ]. Gramoxone is the commercial formulation name for paraquat. It has a persistent half-life of more than 100 days. This compound has a high groundwater contamination potential [10Pizzutti IR, Vela GME, Kok A, Scholten JM. Determination of paraquat and diquat: LC-MS method optimization and validation. Food Chem 2015; 209: 248-55.
[http://dx.doi.org/10.1016/j.foodchem.2016.04.069] , 11Kopytko M, Chalela G, Zauscher F. Biodegradation of two commercial herbicides (Gramoxone and Matancha) by the bacteria Pseudomonas putida. Electron J Biotechnol 2002; 5: 182-95.
[http://dx.doi.org/10.2225/vol5-issue2-fulltext-1] ]. The paraquat is ranked second in Meknes region (Morocco), this ranking also holds internationally after the glyphosate [12Silva R, Palmeira A, Carmo H, Barbosa DJ. P-glycoprotein induction in Caco-2 cells by newly synthetized thioxanthones prevents paraquat cytotoxicity. Arch Toxicol 2015; 89: 1783-800.
[http://dx.doi.org/10.1007/s00204-014-1333-4] ]. Nationally, paraquat is the second most imported herbicide with an increasing yearly-reported [13Maldani M, Dekaki EM, Nassiri L, Ibijbijen J. State of art on the use of pesticides in Meknes region, Morocco. Amer J Agric Sci 2017; 6: 138-48.]. This herbicide affects the photosynthesis by diverting electrons at the exit of photosystem II acids [14Calvet R, Barriuso E, Benoit P, Bedos C. Les pesticides dans le sol: Conséquences agronomiques et environnementales 2005.]. It is used to treat more than 50 crop varieties in more than 120 countries and it has been marketed as an herbicide for over sixty years [12Silva R, Palmeira A, Carmo H, Barbosa DJ. P-glycoprotein induction in Caco-2 cells by newly synthetized thioxanthones prevents paraquat cytotoxicity. Arch Toxicol 2015; 89: 1783-800.
[http://dx.doi.org/10.1007/s00204-014-1333-4] , 15Bromilow RH. Paraquat and sustainable agriculture. Pest Manag Sci 2004; 60: 340-9.
[http://dx.doi.org/10.1002/ps.823] ]. Paraquat persists in soil due to its adsorption on organic matter and clay [16Gondar D, López R, Antelo J, Fiol S. Adsorption of paraquat on soil organic matter: Effect of exchangeable cations and dissolved organic carbon. J Hazard Mater 2012; 235-236: 218-23.
[http://dx.doi.org/10.1016/j.jhazmat.2012.07.044] , 8Amondham W, Parkpian P, Polprasert C, Delaune RD. Paraquat adsorption, degradation, and remobilization in tropical soils of Thailand. J Enviro Sci H. Part B 2006; 41: 485-507.]. Even there are microorganisms, which are able to resist paraquat [17Martani E, Sunarminto BH, Margino S, Handayani S. Herbisida paraquat di lahan gambut: Pengaruhnya terhadap tanah dan pertumbuhan jagung (Paraquat in peat soil its effect on soil and the growth of corn). Manusia Dan Lingkungan 2001; 7: 22-31., 18Carr RJG, Bilton RF, Atkinson T. Mechanisms of biodegradation of paraquat by Lipomyces starkeyi. Appl Environ Microbiol 1985; 49: 1290-794.], several studies have shown that paraquat influenced the growth and activities of soil microorganisms [19Mazhari M, Ferguson J. Bacterial responses to environmental herbicide pollutants (glyphosate and paraquat). Caspian J Environ Sci 2018; 16: 37-5.-21Katayama A, Kuwatsuka S. Microflora with long-term application of paraquat. J Pestic Sci 1992; 17: 137-9.
[http://dx.doi.org/10.1584/jpestics.17.2_137] ]. It has been reported by Margino in 2000 [2Margino S, Martani E, Sunarminto BH. Paraquat in peat land: I. Its effect on the dynamics of microbial population. J Perlindungan Tanaman Indonesia 2000; 6: 91-100.] that 20 ppm of paraquat in soil changed the population dynamics of peat soil bacteria and fungi.
Unfortunately, the data about the influence of paraquat on growth and activities of nitrogen-fixing bacteria are limited in Morocco. The lack of information is due to the fact that this type of bacteria have not been sufficiently studied; the amount of paraquat used in Morocco is not under control [13Maldani M, Dekaki EM, Nassiri L, Ibijbijen J. State of art on the use of pesticides in Meknes region, Morocco. Amer J Agric Sci 2017; 6: 138-48.], and therefore, this practice would be causing serious damage to soil bacteria which puts the future of agriculture in that area of the world at risk, for this reason, we carried out this study. To assess the effects of herbicide on soil microorganisms, we investigated four nitrogen-fixing bacteria to explore the relationship between the concentration of paraquat herbicide and its growth response, using paraquat as a source of carbon, and evaluated the growth response of these strains by the method of enumeration in solid medium at the surface.
2. MATERIALS AND METHODS
2.1. Chemicals Used
The paraquat used was a commercial Gramoxone® (containing 200 g active ingredient/L of paraquat, SYNGENTA), purchased from a local dealer’s store in Oujda, Morocco. All other chemicals were of the highest purity commercially available.
To evaluate the tolerance or resistance of bacterial strains by the use of paraquat as a sole source of carbon, a mineral salt medium without a carbon source (MSMC) is used. The composition of the medium (pH 7.0-7.2) was as follows: KH2PO4 (1.5 g/L), Na2HPO4 12H2O (1.5 g/L), NH4SO4 (2 g/L), MgSO4 7H2O (0.2 g/L), CaCl2 (0.01 g/L) and FeSO4 7H2O (0.001 g/L). The media was supplemented with paraquat sterilized by filtration (0.2 µm filter) and were used to test the tolerance or resistance of the four strains to paraquat. Mineral Salt Medium (MSM) with glucose as carbon source was used as a control. The composition of the medium MSM (pH 7.0-7.2) is as follows: C6H12O6 (10 g/L), KH2PO4 (1.5 g/L), Na2HPO4 12H2O (1.5 g/L), NH4SO4 (2 g/L), MgSO4 7H2O (0.2 g/L), CaCl2 (0.01 g/L) and FeSO4 7H2O (0.001 g/L).
2.2. Bacteria Strains
Four bacterial strains were selected to test their tolerance to paraquat. These strains were originally isolated from root nodules of legume “Bituminaria bituminosa” cultivated in the experimental station of the faculty of sciences, Moulay Ismail University-Meknes, Morocco. The selection of strains was based on their ability to fix nitrogen. The selected strains are Pantoea agglomerans, Rhizobium nepotum, Rhizobium radiobacter and Rhizobium tibeticum.
2.3. Preparation of Inoculum
Inoculums were prepared for the four strains by culturing the strains in 50 ml of nutrient medium for three days at 30°C under stirring conditions (150 rpm) until the growth reached the exponential phase. Cells were harvested by centrifugation at 4, 600 g for 5 min, washed with 0.9% sterile saline and were re-suspended to a 0.5 McFarland nephelometer standard (Optical density of 0.108 at 625 nm), this suspension is used as inoculum.
3. TEST OF PARAQUAT EFFECTS
3.1. Agar Diffusion Method
Paraquat effect on the bacterial strains was evaluated by agar diffusion test using filter paper discs (5 mm in diameter). Each strain was grown by surface plating method in Plate Count Agar (PCA) medium, and sterilized paper discs containing a series of paraquat concentrations (0, 0.5, 1, 3, 6 and 12 g/L) were put aseptically on the medium surface. Then they were incubated at 30°C for 72 hours. The assessment of the inhibition effect was based on measuring the diameter of the inhibition zone around the paper disc.
3.2. Quantitative test
Tolerance experiments were designed to examine the influence of five different concentrations on paraquat on bacterial growth. The experiments were performed in flasks (250 ml) containing 100 ml of sterile MSMC with, 0.5 g/L, 1 g/L, 3 g/L, 6 g/L and 12 g/L of paraquat separately. 2 ml of each inoculum was inoculated and triplicate cultures were incubated on a rotary shaker at 150 rpm for 7 days at 30°C.
3.3. Enumeration of Bacterial Strains
For each concentration of paraquat (0.5, 1, 3, 6, 12 g/L) including the control, we calculate the bacterial concentration after 7 days of incubation, for this, 1ml of samples, were used to provide a series of dilutions (10-1, 10-2, 10-3, 10-4 and 10-5). For bacterial count 0.1 mL of each dilution were added to the plates, which contained the Plate Count Agar (PCA) medium. The plates were incubated at 28 ± 2°C, for 72h.
3.4. Statistical Analysis
Differences between the mean of treatments were calculated by two-way analysis of variance (ANOVA), using Tukey’s multiple comparison test to carry out a post-hoc pairwise comparison of means. Differences between treatments were considered statistically significant at p < 0.05. IBM SPSS statistics 20 was used for all above statistical analysis.
4. RESULTS AND DISCUSSION
The effect of paraquat treatments on bacterial strains was determined by two test: agar diffusion method and the rate of the growth of bacterial colonies in each treatment. In the agar diffusion method, the bacterial strains were inhibited by paraquat, in which the inhibition zone was wider with the increase of paraquat concentration (Fig. 1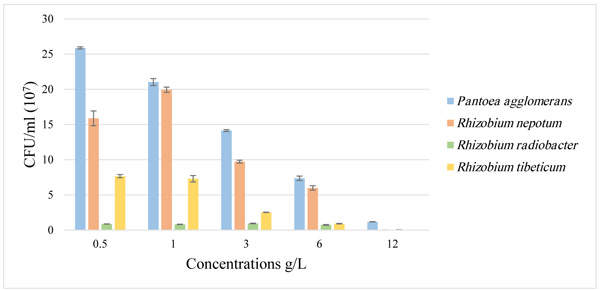 ; Table 1). Furthermore, analysis of the Colony Forming Units (CFUs) mostly showed a declining in bacterial growth, in comparison with the control, the growth of the four strains: Pantoea agglomerans, Rhizobium nepotum, Rhizobium radiobacter and Rhizobium tibeticum decreased with the increase of the paraquat concentration (Fig. 2
; Table 1). Furthermore, analysis of the Colony Forming Units (CFUs) mostly showed a declining in bacterial growth, in comparison with the control, the growth of the four strains: Pantoea agglomerans, Rhizobium nepotum, Rhizobium radiobacter and Rhizobium tibeticum decreased with the increase of the paraquat concentration (Fig. 2 ). Statistically, the strains showed different degrees of sensitivity to the paraquat, a significant difference was observed between the four strains (p < 0.05). The Pantoea agglomerans strain showed a tolerance to paraquat, in both tests there was no significant difference between the control and 0.5 g/L treatment (p > 0.05). We observed a low decrease in bacterial growth in the 1 g/L treatment, this decrease intensified with the increase of the concentration (3, 6, and 12 g/L) (Fig. 3
). Statistically, the strains showed different degrees of sensitivity to the paraquat, a significant difference was observed between the four strains (p < 0.05). The Pantoea agglomerans strain showed a tolerance to paraquat, in both tests there was no significant difference between the control and 0.5 g/L treatment (p > 0.05). We observed a low decrease in bacterial growth in the 1 g/L treatment, this decrease intensified with the increase of the concentration (3, 6, and 12 g/L) (Fig. 3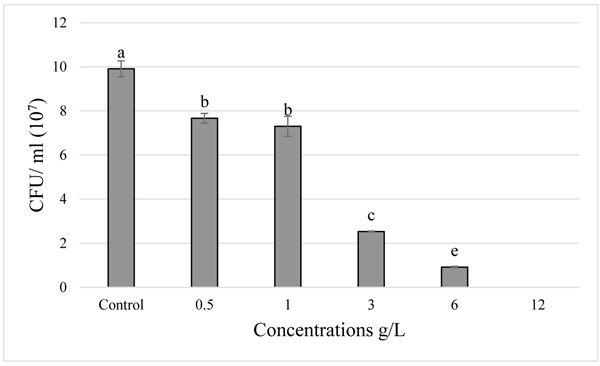 ). While in the Rhizobium tibeticum strain, compared to the control, the treatment with 0.5 g/L and 1 g/L showed a slight decrease in the bacterial growth, whereas with high concentrations (3 and 6 g/L) the bacterial growth was minimal and there was no growth at 12 g/L concentration (Fig. 4
). While in the Rhizobium tibeticum strain, compared to the control, the treatment with 0.5 g/L and 1 g/L showed a slight decrease in the bacterial growth, whereas with high concentrations (3 and 6 g/L) the bacterial growth was minimal and there was no growth at 12 g/L concentration (Fig. 4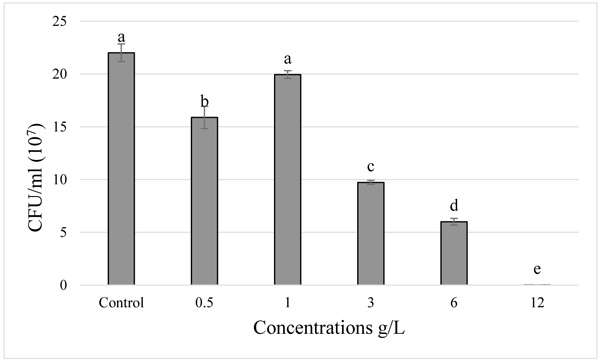 ). For the Rhizobium nepotum strain relative to the control, there was no significant difference in the treatment with 1 g/L, whereas a very slight decrease was observed in 0.5 g/L treatment, for the treatments 3 and 6 g/L the bacterial growth decreased, respectively Fig. (5
). For the Rhizobium nepotum strain relative to the control, there was no significant difference in the treatment with 1 g/L, whereas a very slight decrease was observed in 0.5 g/L treatment, for the treatments 3 and 6 g/L the bacterial growth decreased, respectively Fig. (5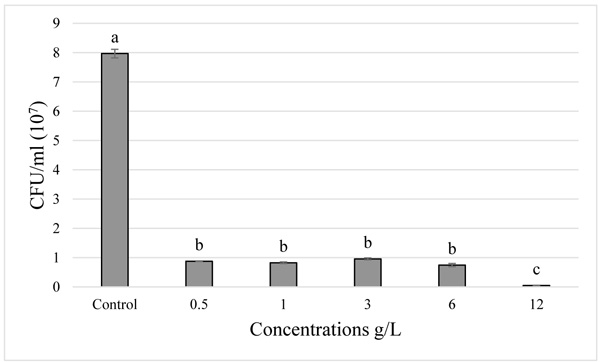 ). For Rhizobium radiobacter, which is the most sensitive strain to paraquat, a high rate of decrease in bacterial growth was observed for the treatments 0.5, 1, 3, and 6 g/L. Moreover, the treatment 12 g/L represented a lethal dose. In the agar diffusion test, higher concentrations induced a longer diameter of inhibition zone. Generally, the increase of paraquat concentration affected the growth of all the tested strains.
). For Rhizobium radiobacter, which is the most sensitive strain to paraquat, a high rate of decrease in bacterial growth was observed for the treatments 0.5, 1, 3, and 6 g/L. Moreover, the treatment 12 g/L represented a lethal dose. In the agar diffusion test, higher concentrations induced a longer diameter of inhibition zone. Generally, the increase of paraquat concentration affected the growth of all the tested strains.
 |
Fig. (1) Comparison of paraquat effect on four strains. |
 |
Fig. (2) Effect of increasing paraquat concentration on the growth of Pantoea agglomerans. |
 |
Fig. (3) Effect of increasing paraquat concentration on the growth of Rhizobium tibeticum. |
 |
Fig. (4) Effect of increasing paraquat concentration on the growth of Rhizobium nepotum. |
 |
Fig. (5) Effect of increasing paraquat concentration on the growth of Rhizobium radiobacter. |
In our study, the four strains had different behaviours towards the paraquat. Pantoea agglomerans, Rhizobium nepotum and Rhizobium tibeticum have shown, successively, a resistance to paraquat at 1, 3 and 6 g/L. This resistance was characterized by a decrease in the bacterial density that disappeared at 12 g/L. Similar results were reported by Katayama in 1992 [21Katayama A, Kuwatsuka S. Microflora with long-term application of paraquat. J Pestic Sci 1992; 17: 137-9.
[http://dx.doi.org/10.1584/jpestics.17.2_137] ], which have shown that some rhizobacteria resist to paraquat up to 1g/L. This may be due to the adaptation of the strains to this herbicide via the synthesis of enzymes able to degrade the paraquat and by the way the detoxification of the soil [17Martani E, Sunarminto BH, Margino S, Handayani S. Herbisida paraquat di lahan gambut: Pengaruhnya terhadap tanah dan pertumbuhan jagung (Paraquat in peat soil its effect on soil and the growth of corn). Manusia Dan Lingkungan 2001; 7: 22-31., 18Carr RJG, Bilton RF, Atkinson T. Mechanisms of biodegradation of paraquat by Lipomyces starkeyi. Appl Environ Microbiol 1985; 49: 1290-794.]. This adaptation towards the paraquat can be explained by the fact that the strains use the herbicides metabolites as a source of nutrients or energy [22Baboo M, Pasayat M, Samal A, Kujur Maharana JK. Effect of four herbicides on soil organic carbon, microbial biomass, enzyme activity and microbial populations in agricultural soil. Int J Res Enviro Sci Technol 2013; 3: 100-12., 23Sebiomo A, Ogundero VW, Bankole SA. Effect of four herbicides on microbial population, soil organic matter and dehydrogenase activity. Afr J Biotechnol 2011; 10: 770-8.]. Deng in 2015 and Bera in 2013 [24Deng S, Chen Y, Wang D, Shi T. Rapid biodegradation of organophosphorus pesticides by Stenotrophomonas sp. G1. J Hazard Mater 2015; 297: 17-24.
[http://dx.doi.org/10.1016/j.jhazmat.2015.04.052] , 25Bera S, Ghosh RK. Soil microflora and weed management as influenced by Atrazine 50% WP in sugarcane. Univers J Agric Res 2013; 1: 41-7.], reported that many soil microorganisms are able to decompose herbicides such as pasteureanum Clostridium, Achromobacter sp., Aerobacter aerogenes, Pseudomonas fluorescens and Agrobacterium tumefaciens, which degrade paraquat [18Carr RJG, Bilton RF, Atkinson T. Mechanisms of biodegradation of paraquat by Lipomyces starkeyi. Appl Environ Microbiol 1985; 49: 1290-794.]. Other researchers have also shown that some strains of Rhizobium sp. can degrade pesticides [17Martani E, Sunarminto BH, Margino S, Handayani S. Herbisida paraquat di lahan gambut: Pengaruhnya terhadap tanah dan pertumbuhan jagung (Paraquat in peat soil its effect on soil and the growth of corn). Manusia Dan Lingkungan 2001; 7: 22-31.].
On the contrary, several microorganisms of soil are sensitive to herbicides, which may alter the quality, the density and the diversity of the microorganisms of soil. Several studies have reported the effect of herbicides on soil microbial life. According to Greaves in 1976 [26Greaves MP, Davies HA, Marsh JAP. WingField GI. Herbicides and soil microorganisms. Crit Rev Microbiol 1976; 5: 1-38.
[http://dx.doi.org/10.3109/10408417609102308] ], the use of herbicides can reduce global microbial populations in soil. Zain in 2013 [27Zain NMM, Mohamad RB, Sijam K, Morshed MM. Effects of selected herbicides on soil microbial populations in oil palm plantation of Malaysia: A microcosm experiment. Afr J Microbiol Res 2013; 7: 367-74.
[http://dx.doi.org/10.5897/AJMR12.1277] ] confirmed that herbicide treatments significantly inhibit the development of soil microorganisms, and the degree of inhibition is closely related to the rates of their concentrations. These results are similar to our results obtained for Rhizobium radiobacter. This strain was sensitive to paraquat, even on applying low concentrations, this can be explained by the toxicity of paraquat, or the bacteria were unable to degrade paraquat. This result is in agreement with other research, which proved that paraquat inhibits the growth of rhizobacteria [17Martani E, Sunarminto BH, Margino S, Handayani S. Herbisida paraquat di lahan gambut: Pengaruhnya terhadap tanah dan pertumbuhan jagung (Paraquat in peat soil its effect on soil and the growth of corn). Manusia Dan Lingkungan 2001; 7: 22-31., 19Mazhari M, Ferguson J. Bacterial responses to environmental herbicide pollutants (glyphosate and paraquat). Caspian J Environ Sci 2018; 16: 37-5., 27Zain NMM, Mohamad RB, Sijam K, Morshed MM. Effects of selected herbicides on soil microbial populations in oil palm plantation of Malaysia: A microcosm experiment. Afr J Microbiol Res 2013; 7: 367-74.
[http://dx.doi.org/10.5897/AJMR12.1277] -29Mekwatanakarn P, Sivasithamparam K. Effect of certain herbicides on soil microbial populations and their influence on saprophytic growth in soil and pathogenicity of take-all fungus. Biol Fertil Soils 1987; 5: 175.
[http://dx.doi.org/10.1007/BF00257655] ]. These results are similar to the observations made by Adhikary in 2014 [30Adhikary P, Shil S, Patra PS. Effect of herbicides on soil microorganisms in transplanted Chili. Global J Bio Agric Health Sci 2014; 3: 236-8.], which suggest that the herbicide (pendimethalin, oxyfluorfen and propaquizafop) application to soil causes impacts on microorganisms growth.
Scientific research indicates that there is no general pattern of herbicide effect on soil microorganisms [31Trimurtulu N, Ashok S, Latha M, Subramanyeswara RA. Influence of preemergence herbicides on the soil microflora during the crop growth of black gram, Vigna mungo. L. Int J Curr Microbiol Appl Sci 2015; 4: 539-46.-33Radivojević LJ, Gašić S, Santrić LJ, Stanković-kalezić R. The impact of atrazine on several biochemical properties of chernozem soil. J Serb Chem Soc 2008; 73: 951-9.
[http://dx.doi.org/10.2298/JSC0810951R] ]. The herbicide may be toxic to some microorganisms as it may be beneficial for others by using it as a source of carbon, phosphorus, or nitrogen after degradation, as observed by Amondham in 2006 [8Amondham W, Parkpian P, Polprasert C, Delaune RD. Paraquat adsorption, degradation, and remobilization in tropical soils of Thailand. J Enviro Sci H. Part B 2006; 41: 485-507.], who showed that paraquat has no effect on soil microorganisms and their activities.Moreover, Adomako in 2016 [28Adomako MO, Akyeampong S. Effect of some commonly used herbicides on soil microbial population. J Environ Earth Sci 2016; 6: 30-8.] reported that the activity of bacteria increased with the application of paraquat. The herbicides toxicity intensifies when high herbicide concentrations are applied. Several farmers who overdose the use of herbicides consequently destroy the soil flora as a result in long-term.
CONCLUSION
In conclusion, we have obtained evidence that paraquat herbicide affects the viability of four nitrogen-fixing bacteria, especially at high concentrations. In addition, the results showed that microbial response to this herbicide depends on the bacterial species, whose effect is reflected by the different degrees of tolerance to the paraquat.
ABBREVIATIONS
| CFUs | = Colony Forming Units |
| MSM | = Mineral Salt Medium |
| MSMC | = Mineral Salt Medium without Carbon source |
| PCA | = Plate Count Agar |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Our special thanks to Environment & Soil Microbiology Unit, Department of Biology, Faculty of Sciences, Moulay Ismail University, Meknes, Morocco and all those who helped us to accomplish this work.
REFERENCE
| [1] | Shahid M, Ahmed B, Saghir-Khan M. Evaluation of microbiological management strategy of herbicide toxicity to green gram plants. Biocatal Agric Biotechnol 2018; 14: 96-108. [http://dx.doi.org/10.1016/j.bcab.2018.02.009] |
| [2] | Margino S, Martani E, Sunarminto BH. Paraquat in peat land: I. Its effect on the dynamics of microbial population. J Perlindungan Tanaman Indonesia 2000; 6: 91-100. |
| [3] | Johnen BG, Drew EA. Ecological effects of pesticides on soil microorganisms. Soil Sci 1977; 123: 319-24. [http://dx.doi.org/10.1097/00010694-197705000-00007] |
| [4] | Kumar S, Kaushik G, Dar MA, Nimesh S. Microbial degradation of organophosphate pesticides: A review. Pedosphere 2018; 28: 190-208. [http://dx.doi.org/10.1016/S1002-0160(18)60017-7] |
| [5] | Zaller G, König N, Tiefenbacher A, Muraoka Y. Pesticide seed dressings can affect the activity of various soil organisms and reduce decomposition of plant material. BMC Ecol 2016; 16: 3. [http://dx.doi.org/10.1186/s12898-016-0092-x] |
| [6] | Allegrini M, Zabaloy MC, Gómez EV. Ecotoxicological assessment of soil microbial community tolerance to glyphosate. Sci Total Environ 2015; 533: 60-8. [http://dx.doi.org/10.1016/j.scitotenv.2015.06.096] |
| [7] | Camacho-Morales RL, Gerardo-Gerardo JL, Navarro KG, Sánchez JE. Producción de enzimas ligninolíticas durante la degradación del herbicida paraquat por hongos de la pudrición blanca. Rev Argent Microbiol 2017; 49: 189-96. [http://dx.doi.org/10.1016/j.ram.2016.11.004] |
| [8] | Amondham W, Parkpian P, Polprasert C, Delaune RD. Paraquat adsorption, degradation, and remobilization in tropical soils of Thailand. J Enviro Sci H. Part B 2006; 41: 485-507. |
| [9] | Roberts TR, Dyson JS, Lane MC. Deactivation of the biological activity of paraquat in the soil environment: A review of long-term environmental fate. J Agric Food Chem 2002; 50: 3623-31. [http://dx.doi.org/10.1021/jf011323x] |
| [10] | Pizzutti IR, Vela GME, Kok A, Scholten JM. Determination of paraquat and diquat: LC-MS method optimization and validation. Food Chem 2015; 209: 248-55. [http://dx.doi.org/10.1016/j.foodchem.2016.04.069] |
| [11] | Kopytko M, Chalela G, Zauscher F. Biodegradation of two commercial herbicides (Gramoxone and Matancha) by the bacteria Pseudomonas putida. Electron J Biotechnol 2002; 5: 182-95. [http://dx.doi.org/10.2225/vol5-issue2-fulltext-1] |
| [12] | Silva R, Palmeira A, Carmo H, Barbosa DJ. P-glycoprotein induction in Caco-2 cells by newly synthetized thioxanthones prevents paraquat cytotoxicity. Arch Toxicol 2015; 89: 1783-800. [http://dx.doi.org/10.1007/s00204-014-1333-4] |
| [13] | Maldani M, Dekaki EM, Nassiri L, Ibijbijen J. State of art on the use of pesticides in Meknes region, Morocco. Amer J Agric Sci 2017; 6: 138-48. |
| [14] | Calvet R, Barriuso E, Benoit P, Bedos C. Les pesticides dans le sol: Conséquences agronomiques et environnementales 2005. |
| [15] | Bromilow RH. Paraquat and sustainable agriculture. Pest Manag Sci 2004; 60: 340-9. [http://dx.doi.org/10.1002/ps.823] |
| [16] | Gondar D, López R, Antelo J, Fiol S. Adsorption of paraquat on soil organic matter: Effect of exchangeable cations and dissolved organic carbon. J Hazard Mater 2012; 235-236: 218-23. [http://dx.doi.org/10.1016/j.jhazmat.2012.07.044] |
| [17] | Martani E, Sunarminto BH, Margino S, Handayani S. Herbisida paraquat di lahan gambut: Pengaruhnya terhadap tanah dan pertumbuhan jagung (Paraquat in peat soil its effect on soil and the growth of corn). Manusia Dan Lingkungan 2001; 7: 22-31. |
| [18] | Carr RJG, Bilton RF, Atkinson T. Mechanisms of biodegradation of paraquat by Lipomyces starkeyi. Appl Environ Microbiol 1985; 49: 1290-794. |
| [19] | Mazhari M, Ferguson J. Bacterial responses to environmental herbicide pollutants (glyphosate and paraquat). Caspian J Environ Sci 2018; 16: 37-5. |
| [20] | Yu Q, Cairns A, Powles S. Glyphosate, paraquat and ACCase multiple herbicide resistance evolved in a Lolium rigidum biotype?. Planta 2006; 225: 499-513. [http://dx.doi.org/10.1007/s00425-006-0364-3] |
| [21] | Katayama A, Kuwatsuka S. Microflora with long-term application of paraquat. J Pestic Sci 1992; 17: 137-9. [http://dx.doi.org/10.1584/jpestics.17.2_137] |
| [22] | Baboo M, Pasayat M, Samal A, Kujur Maharana JK. Effect of four herbicides on soil organic carbon, microbial biomass, enzyme activity and microbial populations in agricultural soil. Int J Res Enviro Sci Technol 2013; 3: 100-12. |
| [23] | Sebiomo A, Ogundero VW, Bankole SA. Effect of four herbicides on microbial population, soil organic matter and dehydrogenase activity. Afr J Biotechnol 2011; 10: 770-8. |
| [24] | Deng S, Chen Y, Wang D, Shi T. Rapid biodegradation of organophosphorus pesticides by Stenotrophomonas sp. G1. J Hazard Mater 2015; 297: 17-24. [http://dx.doi.org/10.1016/j.jhazmat.2015.04.052] |
| [25] | Bera S, Ghosh RK. Soil microflora and weed management as influenced by Atrazine 50% WP in sugarcane. Univers J Agric Res 2013; 1: 41-7. |
| [26] | Greaves MP, Davies HA, Marsh JAP. WingField GI. Herbicides and soil microorganisms. Crit Rev Microbiol 1976; 5: 1-38. [http://dx.doi.org/10.3109/10408417609102308] |
| [27] | Zain NMM, Mohamad RB, Sijam K, Morshed MM. Effects of selected herbicides on soil microbial populations in oil palm plantation of Malaysia: A microcosm experiment. Afr J Microbiol Res 2013; 7: 367-74. [http://dx.doi.org/10.5897/AJMR12.1277] |
| [28] | Adomako MO, Akyeampong S. Effect of some commonly used herbicides on soil microbial population. J Environ Earth Sci 2016; 6: 30-8. |
| [29] | Mekwatanakarn P, Sivasithamparam K. Effect of certain herbicides on soil microbial populations and their influence on saprophytic growth in soil and pathogenicity of take-all fungus. Biol Fertil Soils 1987; 5: 175. [http://dx.doi.org/10.1007/BF00257655] |
| [30] | Adhikary P, Shil S, Patra PS. Effect of herbicides on soil microorganisms in transplanted Chili. Global J Bio Agric Health Sci 2014; 3: 236-8. |
| [31] | Trimurtulu N, Ashok S, Latha M, Subramanyeswara RA. Influence of preemergence herbicides on the soil microflora during the crop growth of black gram, Vigna mungo. L. Int J Curr Microbiol Appl Sci 2015; 4: 539-46. |
| [32] | Ahemad M, Khan MS. Effects of pesticides on plant growth promoting traits of mesorhizobium strain MRC4. J Saudi Soc Agric Sci 2012; 11: 63-71. [http://dx.doi.org/10.1016/j.jssas.2011.10.001] |
| [33] | Radivojević LJ, Gašić S, Santrić LJ, Stanković-kalezić R. The impact of atrazine on several biochemical properties of chernozem soil. J Serb Chem Soc 2008; 73: 951-9. [http://dx.doi.org/10.2298/JSC0810951R] |




