- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Leukemia Journal
(Discontinued)
ISSN: 1876-8164 ― Volume 5, 2013
Flow Cytometry Evaluation of Minimal Residual Disease in Acute Lymphoblastic Leukaemia Type B
N. Braham Jmili*, 1, M.C. Jacob2, S. Yacoub1, Y. Ben Youssef3, M.A. Laatiri3, Y. Braham4, M. Kottas1
Abstract
Immunophenotyping has become essential to the diagnosis and the treatment management of acute lymphoblastic leukaemia (ALL). We prospectively studied minimal residual disease (MDR) in patients with B lineage ALL who achieved mCR remission. The initial series of patients consisted on 90 cases with B ALL. Sixty-Six patients had bone marrow samples adequate for MDR studies collected on day 35 of remission induction chemotherapy. Strategy of monitoring MRD is based on flow cytometry using quadruple staining according the leukaemia associated immunophenotype found at diagnosis. Data analysis was done using an EPI XL cytometer (Coulter), acquiring 500 000 events. Of the 66 patients 40 (60, 6%) had MRD ≥0, 01%. B lymphoblasts of ALL may morphologically resemble to hematogones (B benign lymphocyte precursors) and their immunophentypes have similarities. Different combinations of antibodies are tested to determine which combinations are more suitable to detect B residual leukaemics cells. The results of this present study indicate that: CD10/CD38/CD19/CD45 and CD10/CD34CD19/CD45 are the more specifics and should be used to distinguish B lymphoblasts of lymphoblastic acute leukaemia from normal hematogones
Article Information
Identifiers and Pagination:
Year: 2010Volume: 3
First Page: 47
Last Page: 54
Publisher Id: TOLEUKEMIAJ-3-47
DOI: 10.2174/1876816401003010047
Article History:
Received Date: 2/2/2010Revision Received Date: 30/4/2010
Acceptance Date: 12/5/2010
Electronic publication date: 16/07/2010
Collection year: 2010
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Laboratory of haematology. Hospital Farhat Hached, 4000 Sousse, Tunisia; Tel: 00 216 73 22 33 11; Fax: 00 216 73 22 67 02; E-mails: jmilinejia@yahoo.fr, brahamyoussef@yahoo.fr
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 2-2-2010 |
Original Manuscript | Flow Cytometry Evaluation of Minimal Residual Disease in Acute Lymphoblastic Leukaemia Type B | |
INTRODUCTION
Acute lymphoblastic acute leukaemia (ALL) is a most common leukaemia in childhood with a peak incidence at 2-5 years of age, and another peak in old age (after 50 years). The overall cure rate in children is 85%.Measurement of minimal residual disease (MRD) during clinical remission after chemotherapy has proven to be a valuable tool for predicting relapses before clinical and haematological manifestations and establishing different risk categories in patients with ALL [1Cavé H, Vander Weeff J, Bosch T. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukaemia N Engl J Med 1998; 339: 591-8.]. The detection of residual leukemic cells is usually based on either molecular or immunophenotypical markers present in leukemic but not in normal cells, allowing for their specific discrimination [2Ciudad J, San Miguel JF, Lopez-Berges MC. Prognosic value of immunophenotypic detection of minimal residual disease in acute lymphoblastic leukemia J Clin Oncol 1998; 16: 3774-8.]. Studies based on semiquantitative polymerase chain reaction (PCR) or immunophenotyping have demonstrated the clinical relevance of MRD investigation; both methods provide similar results [1Cavé H, Vander Weeff J, Bosch T. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukaemia N Engl J Med 1998; 339: 591-8., 3San Miguel JF, Vidriales MB, Lopez-Berges C. Early immunophenotypical evaluation of Minimal Residual Disease in acute myeloid leukemia identifies different patient risk groups and may contribute to podt induction treatment stratification Blood 2001; 98(6): 1746-51.]. Evidence supporting the value of MRD investigation in acute leukaemias by immunophenotyping and/or molecular techniques has been increasingly reported over the last few years [1Cavé H, Vander Weeff J, Bosch T. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukaemia N Engl J Med 1998; 339: 591-8., 2Ciudad J, San Miguel JF, Lopez-Berges MC. Prognosic value of immunophenotypic detection of minimal residual disease in acute lymphoblastic leukemia J Clin Oncol 1998; 16: 3774-8.].
Flow cytometry is nowadays the first line method for immunophenotypic identification of blast cells but is not so usual in limited resources countries [4Feki S, Omri H, Lâatiri MA. Contribution of flow cytometry to acute leukaemia classification in Tunisia Dis Markers 2000; 16: 131-3., 5Braham JN, Ben Abdelaziz A, Nagara M. Profil Epidémiologique et cytologique des leucémies aiguës : A propos de 193 cas colligés au centre Tunisien Revue Fran aise des Laboratoires 2005; 399: 23-8.]. In Tunisia, flow cytometry (FC) is used in diagnosis of acute leukaemia and we think that it is the more suitable method to measure MRD than molecular methods which are not done systematically and are more expensive.
Flow cytometry is a practical tool for monitoring MRD in patients with acute lymphoblastic acute leukaemia (ALL). This approach is based on the identification of immunophenotypes expressed by leukemic cells but not by normal lympho-haematopoietic cells in bone marrow and peripheral blood [6Campana D, Coustan-Smith E. Advances in the immunological monitoring of childhood acute lymphoblastic leukaemia Best Pract Res Clin Haematol 2002; 15(1): 1-19.]. Flow cytometry measurement of MDR in B ALL present some particularities and difficulties to distinguish in the bone marrow neoplastic lymphoblasts of ALL from benign B-lymphocyte precursors known as hematogones [7McKenna RW, Asplund SL, Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) and neoplastic lymphoblasts by 4- color flow cytometry Leuk Lymphoma 2004; 45(2): 277-85., 8Rimsza LM, Larson RS, Winter SS. Benign hematogone-rich lymphoid proliferations can be distinguished from B lineage acute lymphoblastic leukaemia by integration of morphology, immunophenotype, adhesion molecule expression and architectural features Am J Clin Pathol 2000; 114(1): 66-75.].
The aim of this study was analytical: to describe flow cytometric conditions for immunophenotypic analysis at diagnosis of acute leukaemia and to establish a protocol for the measurement of minimal residual disease MRD assayed by four flow cytometry at the end of chemotherapy induction in patients with B ALL in complete morphologic remission using the optimal combination of antibodies. An important question was to determine whether panel of antibodies provided informations of residual cells.
PATIENTS, MATERIALS AND METHODS
Patients
Criteria for entering the study were: Unequivocal diagnosis of B ALL based on morphologic, cytochemical and immunophenotypical criteria [9Imbert M, Joulaut H, Tulliez M. Cytologie des leucémies aigues Rev Prat 996 ; 46: 23-9.-1Cavé H, Vander Weeff J, Bosch T. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukaemia N Engl J Med 1998; 339: 591-8.] phenotypically aberrant blast cells at diagnosis and morphological complete remission (mCR) with induction therapy.
mCR defined by the criteria proposed by BM VIRDIALES et al. [12Vidriales MB, Pérez JJ, López-Berges MC. Minimal residual disease in adolescent (older than 14 years) and adult acute lymphoblastic leukemias: early immunophenotypic evaluation has high clinical value Blood 2003; 101: 4695-700.]:
- Less than 5% blast cells in regenerated bone marrow (BM).
- Absence of extramedullary leukaemia.
- Peripheral blood (PB) Neutrophil count greater than 1.5 109/l and platelet count greater than 100 109/l.
75 consecutive patients with ALL fulfilled these prerequisites and were included in the present study. The initial series of patients consisted of 201 consecutive patients with de novo non acute myeloid leukaemia (non AML). B ALL is diagnosed in 90 cases. Of these patients 85 displayed aberrant immunophenotypes at diagnosis.
Treatment Protocol
Among 85 patients, 75 achieved mCR remission after induction therapy and are the focus of the present study. Distribution according to French- American- British (FAB) classification was/ as follows: L1 (49 patients), L2 (18 patients), L3 (8 patients). All patients were uniformly treated according to the EORTC (European organization for research and treatment of cancer).
The following variables collected at diagnosis were included in the database: age, white blood cell (WBC) count, platelets count, haemoglobin (Hb) level, percentage of blast in BM and absolute number in PB, type of treatment, morphologic FAB classification and karyotype.
BM at Treatment time point: of day 35 are obtained for the prospective investigation of MRD.
Cytological Investigation
Peripheral blood (PB) and Aspiration of bone marrow (sternum puncture in adults and iliac puncture in children) are sent to our laboratory in ethylenediamine tetra-acetic acid tube. After automatic staining of blood and bone marrow smears by using the HEMA TEK slide stainer (AMES Company) and a HEMATEK bloc colorant stain pack (Bayer Diagnostica), cytomorphologic examinations of the blood and the bone marrow slides separately by 2 morphologists are done. Diagnosis of ALL is based on WHO criteria’s. Cytochemical analysis is based on pyronine stain showing myeloperoxidase. Cytological exams of PB and BM are done in diagnosis and in post chemotherapy (day 35).
Flow Cytometry Methods
Cell Isolation: Preparation
Cell count specimens were first done on the Coulter LH 750 blood cell counter. The cells were incubatedfor 15 minutes in the dark with each of the conjugated monoclonal antibodies. Erythrocytes were lysed using lysing solution (optilyse A 11895 - Beckman Coulter) according to the manufacturer’s instruction. Following the lysis step, the samples were washed two times with Phosphate buffered saline (PBS). After centrifugation for 5 minutes at 200x g, the supernatant was removed by aspiration. The cell pellet was conserved at + 4 °C. The cells were resuspended in PBS for acquisition.
Antibodies
Antibodies to the following antigens were used in diagnosis and to specially profile B cell precursors in the measurement of MRD in ALL BCoulter- Immunotech):
IgG1 Fluorescein Isothiocyanate (FITC), HLA-DR (FITC), CD34 (FITC), CD7 (FITC), CD3 (FITC), CD10 (FITC), CD22 (FITC), CD15 (FITC), CD65 (FITC), CD3 (FITC), CD29 (FITC), CD3IntraCytoplasmique (IC) (FITC), CD16 (FITC), CD19 (FITC), CD4 (FITC), CD56 (FITC), CD29 (FITC), Lambda (FITC), Kappa (FITC), IgG1 R-Phycoerythrin (PE), CD34 (PE), CD2 (PE), TCR (PE), CD5 (PE), CD20 (PE), CD22 (PE), CD19 (PE), CD38 (PE), CD79a (PE), CD33 (PE), CD13 (PE), CD117 (PE), MPO (PE), CD14 (PE), CD1a (PE), CD8 (PE), CD58 (PE), CD 19 Allophycocyanin (APC), CD45 R- Phycoerythrincyanin 5.1 (PC 5).
IntraPrep Permeabilization Reagent (Beckman coulter) was used for immunological detection of intracellular leukocyte antigens (CD79a, CD3, MPO, Kappa, Lambda) after a formaldehyde/saponin-based permeabilization. The procedure consists of first, fixing cells with Reagent 1 (formaldehyde), and second, permeabilizing the white cells and lysing red cells using Reagent 2 (saponin).
For compensation of the cytometer we used FLow check. (Réf: 6605359) and Cyto-Comp. (Réf: 6607023).
Protocols
First step: Flow cytometry using triple staining for diagnosis:
Erythrocyte-lysed whole PB and BM samples obtained at diagnosis were analysed, with a large panel of 3 colour combinations of monoclonal antibodies according Table 1, for the identification of phenotypic aberrancies that could be used later in the study of MDR For each experiment, 10 000 cells were measured.
Second step: Immunophentypical investigation of MRD:
Erythrocyte-lysed whole BM samples obtained at 35 day of treatment were analysed by multiparametric flow cytometry using quadruple staining according the leukaemia- associated immunophenotype found at diagnosis. For each experiment, 500 000 cells were measured.
Flow Cytometry Interpretation
PB and BM Samples were acquired with a three and four colour flow cytometer for diagnosis, identification of phenotypic aberrancies and the measurement of MDR. Data analysis was done using an EPICS XL Flow cytometer with Software system II version 3 (Beckman Coulter).
Distinct cell populations (clusters) were identified based on any combination of forward (FSC) and orthogonal light scatter properties (SSC) and fluorescence intensity with various antibody combinations. Each specimen’s event clusters were considered positive or negative compared with the degree of the same specimen stained with the isotypic control antibody.
Analysis following gating :
- Selection of mononuclear cells (A) on the histogram Side scatter (SSC) / Forward Scatter (FSC).
- Detection of lymphoblast at diagnosis (B): Gating on histogram SSC/CD45 (SSC low, CD45low).
- Detection of B cell in MDR analysis: Gating (CD19+ CD45 low).
Immunophenotype of the AL was defined following EGIL (European Group for the Immunological Characterization of Leukaemia) recommendations. B lineage ALL is defined by the expression of at least two of the following three early B cell markers: CD19, CD79a and/or CD22 [10Bene MC, Castoldi G, Knapp W. Proposals for the immunological classification of Acute leukemias Leukemia 1995; 9: 1783-6.].
We prospectively measures the percentage of residual leukaemic cells among bone marrow mononuclear cells collected after remission reinduction (day 35) in the 75 patients with a leukaemia - associated immunophenotype. Statistical analysis was done on logiciel (EPI INFO Version 6).
RESULTS
As mentioned, this study is based on 75 consecutive patients with B lineage ALL diagnosed in cytology as: L1/ L2: 49/18 cases, Burkitt: 8 cases (Table 2) in whom blast cells displayed antigenic phenotypic aberrancies at diagnosis and who achieved mCR after induction therapy.
Antigenic expression on blast cells at diagnosis was systematically analyzed by multiparametric flow cytometry using triple staining. Following phenotype 69.3% of cases were classified as Pre B (Table 3).
Table 4 resumes the frequencies of aberrant phenotypes found by flow cytometry. 27 leukaemia had one aberrant marker (39%), 11 (14.7%) leukaemia had more than one aberrant marker (9 had 2 markers, 2 had 3 markers). CD 33 (myeloid marker) is aberrantly coexpressed in 14.5% of cases (Fig. 1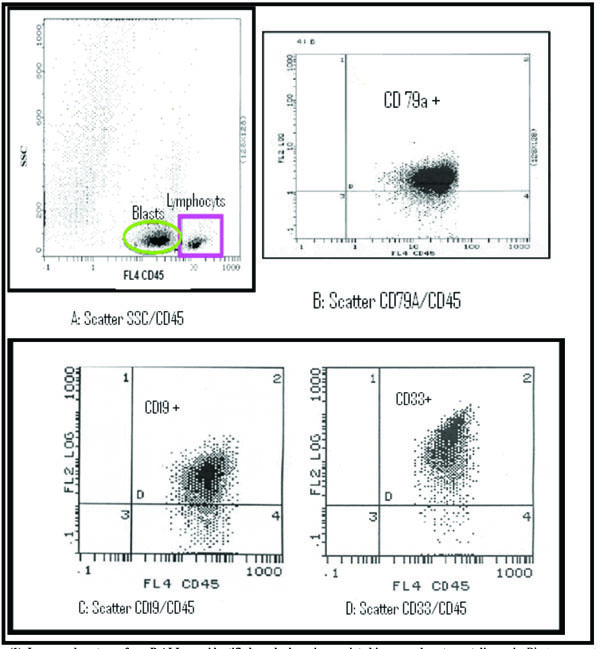 ). Table 5 summarizes the leukaemia associated characteristics used to monitor MRD in the B ALL.
). Table 5 summarizes the leukaemia associated characteristics used to monitor MRD in the B ALL.
Immunophenotypic analysis of the BM in mCR demonstrated that based on the level of residual phenotypically aberrant cells displaying a leukaemia -associated phenotype (LAP+): MRD is detected at less than 10-4 level (10/10 000cells). The level of MRD is defined by the number of lymphoblasts of ALL /10 000 of all acquired cells (Fig. 2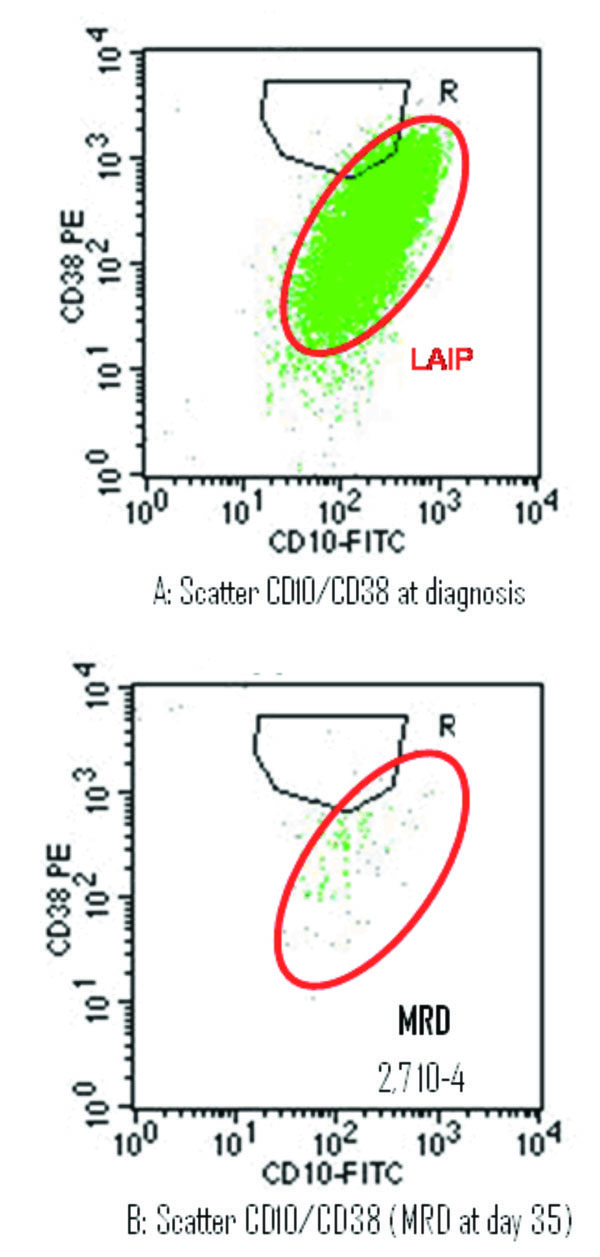 ), 9 of the 75 could not be assessed for MDR measurement for a variety of technical reasons, including lack of adequate numbers of viable cells in the sample or blood dilution (> 50% of neutrophils PNN in BM). Of the 66 patients who achieve mCR and had sufficient cells for flow cytometry studies of MRD, 40 (60.6%) had leukemic cells identifiable by flow cytometry in the BM. Among these patients, the levels of leukaemia were 0.01-<0.1% in 8 patients (12.1%), 0.1-<1% in 20 (30.3%) and ( 1 in 12 (18.1%) (Table 6). Table 7 resumes the level of MRD according clinical and cellular characteristics. The presence or absence of residual leukaemia by flow cytometry at day 35 was not significantly related to age, gender or cellular features.
), 9 of the 75 could not be assessed for MDR measurement for a variety of technical reasons, including lack of adequate numbers of viable cells in the sample or blood dilution (> 50% of neutrophils PNN in BM). Of the 66 patients who achieve mCR and had sufficient cells for flow cytometry studies of MRD, 40 (60.6%) had leukemic cells identifiable by flow cytometry in the BM. Among these patients, the levels of leukaemia were 0.01-<0.1% in 8 patients (12.1%), 0.1-<1% in 20 (30.3%) and ( 1 in 12 (18.1%) (Table 6). Table 7 resumes the level of MRD according clinical and cellular characteristics. The presence or absence of residual leukaemia by flow cytometry at day 35 was not significantly related to age, gender or cellular features.
 |
Fig. (2) The detection of minimal residual disease (MRD) on day 35 of morphologic remission induction therapy. |
DISCUSSION
The WHO classification of the acute leukaemias incorporates morphologic, immunophenotypic, genetic and clinical features in an attempt to define entities that are biologically homogenous and that have clinical relevance. The acute leukaemias are classified as lymphoid or myeloid based on the lineage of the blast cells. The acute lymphoid leukaemia (ALL) are subdivided into precursor B cell ALL and precursor T ALL [13Valensi F. Classification des Leucémies Aiguës: Nouvelles Approches de l’OMS (Organisation Mondiale de la Santé) Revue Fran aise des Laboratoires 2002; 344: 19-24.]. A systematic immunopheno-typing of acute leukaemia is also interesting for the detection of leukaemia associated phenotypes which are the basis of the minimal residual disease analysis by flow cytometry [14Jouault H. Place de la cytométrie en flux pour le diagnostic et le suivi des leucémies aigues Revue Fran aise des Laboratoires 2002; 6(344): 25-30.]. Eligibility criteria for entering the study were unequivocal diagnosis of de novo B ALL based on morphologic, cytochemical and immunophenotypically aberrant blast cells at diagnosis [3San Miguel JF, Vidriales MB, Lopez-Berges C. Early immunophenotypical evaluation of Minimal Residual Disease in acute myeloid leukemia identifies different patient risk groups and may contribute to podt induction treatment stratification Blood 2001; 98(6): 1746-51.,10Bene MC, Castoldi G, Knapp W. Proposals for the immunological classification of Acute leukemias Leukemia 1995; 9: 1783-6.,13Valensi F. Classification des Leucémies Aiguës: Nouvelles Approches de l’OMS (Organisation Mondiale de la Santé) Revue Fran aise des Laboratoires 2002; 344: 19-24.] mCR with induction therapy and corresponding mCR bone marrows samples for immuno-phenotypical investigation of MRD.
The clinical features of the 75 patients include disorders related to anaemia, neutropenia and thrombocytopenia (Table 2). PB and BM Samples were acquired with a three and four colour flow cytometer for diagnosis, identification of leukaemia associated phenotypes (LAIP) [15Coustan SE, Gajjar A, Hijiya N. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukaemia after relapse Leukemia 2004; 18: 499-504.] Patterns of cell markers expressed on leukaemic cells but not on normal bone marrow cells, with a large panel and the measurement of MDR. Bradsyock et al. [16Bradstock KF, Janossy G, Tidman N. Immunological monitoring of residual disease in treated thymic acute lymphoblastic leukemia Leuk Res 1981; 5(4-5): 301-9.] were the first to report the use of LAIP to investigate several suspicious cells. Similar results are in succession published [17Campana D, Coustan SE, Janossy G. The immunologic detection of minimal residual disease in acute leukaemia Blood 1990; 76(1): 163-71.-20Baruchel A. Maladie résiduelle H matologie 1996; 2(2): 153-68.].
Flow Cytometry Analysis At Diagnosis
In our series, cases were diagnosed by peripheral blood counts, bone marrow cytology, cytochemistry and immunophenotyping. For flow cytometry analysis we have examined the expression of several lineage and maturation linked antigens used in routine immunophenotyping of patients with AL using a 3 colour step panel. Following phenotype 69.3% of cases were classified as Pre B (Table 3). Regarding all subgroups, we did not find differences with literature [10Bene MC, Castoldi G, Knapp W. Proposals for the immunological classification of Acute leukemias Leukemia 1995; 9: 1783-6., 21Zandecki M. Leucémies aiguës lymphoblastiques (LAL) Hematologie Clinique In: Sebahoun G, Ed. Hématologie clinique, Arnette. 2007; pp. 1-7.]. B lineage ALL is defined by the expression of at least two of the following three early B cell markers: CD19, CD79a or CD22. Four categories of B lineage ALL, designated from BI to BIV were established according to the degree of B lymphoid differentiation of the blast cells [10Bene MC, Castoldi G, Knapp W. Proposals for the immunological classification of Acute leukemias Leukemia 1995; 9: 1783-6.].
MRD was studied in the 75 patients with B lineage ALL using CD10FITC/CD38PE/CD19APC/CD45PC5. Leukaemia associated immunophenotype(LAIP) was identified at diagnosis (scatter A) and it was out of the gating prevue for the normal B cell progenitor (gating R). Residual leukaemia was present at the percentage indicated (scatter B).
We used several standardized antibody combinations with a large panel of antibodies to screen ALL samples at diagnosis for leukaemia – associated aberrations. Aberrant characteristics were judged relevant only if they were sufficiently strong or homogenous on a majority of blasts of a leukaemia sample. 4 main types of aberrant phenotypes: Cross-lineage antigen expression (Myeloid/lymphoid), asynchronous antigen expression, antigen overexpression and abnormal light scatter pattern. These phenotypes can identify one leukemic cell among 10 000 normal cells and currently applicable to at least 90% of patients with ALL [3San Miguel JF, Vidriales MB, Lopez-Berges C. Early immunophenotypical evaluation of Minimal Residual Disease in acute myeloid leukemia identifies different patient risk groups and may contribute to podt induction treatment stratification Blood 2001; 98(6): 1746-51.].
Flow cytometry of bone marrow aspirations detected aberrant expression of myeloid or lymphoid T antigens in B ALL. These mixed lineages of leukaemic cells represent the capacity of leukaemia for trilineal expression of leucocytes. For each case, one or more marker combinations that allowed the identification of leukemic cell were selected at diagnosis and applied to study MRD at the end of remission induction. MDR by FC is possible in patients with AL who have aberrant phenotypic features.
Minimal Residual Disease Measurement
BM obtained at the post induction remission were analyzed for detection of MRD by multiparametric flow cytometry using four colour antibodies according to B cell maturation. In order to explore MRD a multivariate analysis must be conducted considering LAIP detected at diagnosis. Detectable MRD was defined as 0.01% or more leukemic cells among mononuclear cells in sample [15Coustan SE, Gajjar A, Hijiya N. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukaemia after relapse Leukemia 2004; 18: 499-504.].
Our aim was to define within leukemic cells those phenotypes that are frequent, sensitive and specific to detecting B cell lymphoblasts by using 6 dimensional space formed by the 2 light scatter parameters; forward scatter (FSC) and side scatter (SSC) and the 4 fluorescence-associated characteristics. Several analytical protocols for the study of MRD by FC were described in literature [3San Miguel JF, Vidriales MB, Lopez-Berges C. Early immunophenotypical evaluation of Minimal Residual Disease in acute myeloid leukemia identifies different patient risk groups and may contribute to podt induction treatment stratification Blood 2001; 98(6): 1746-51.,6Campana D, Coustan-Smith E. Advances in the immunological monitoring of childhood acute lymphoblastic leukaemia Best Pract Res Clin Haematol 2002; 15(1): 1-19.,21Zandecki M. Leucémies aiguës lymphoblastiques (LAL) Hematologie Clinique In: Sebahoun G, Ed. Hématologie clinique, Arnette. 2007; pp. 1-7.]. A very important limitation for these methods was the identification of patients for MDR study. This is probably because of the technical difficulties related to the immunologic characterisation of acute leukaemias and the detection of LAIP.
According Dario and co-workers, only 35% to 40% are suitable for MRD investigations [17Campana D, Coustan SE, Janossy G. The immunologic detection of minimal residual disease in acute leukaemia Blood 1990; 76(1): 163-71.]. Recently, identifying irregularities in the intensity of antigen expression leukaemia by FC define additional LAIP combinations exploitable for MRD. Measurement of MRD by FC is more difficult when the population of interest is rare. We were able to circumvent this problem by acquiring a large number of viable cells (500 000events) and using a gating strategy incorporating different parameters: cell size (FSC), granularity (SSC), expression of CD45 and CD19.
Because the key point of the approach is the identification of phenotypic aberrancies that could be used later for detection of residual blast cells displaying the same phenotypic profile in the BM in mcR obtained after induction therapy: large panels of monoclonal antibodies are needed to cover all the different lymphoid/myeloid lineages and we have designed a gating strategy that can be used to measure residual B cells. However, the neoplastic lymphoblasts of precursor B ALL may morphologically resemble hematogones (benign B lymphocyte precursors), and their immunophenotype also has features in common. Thus, distinction of hematogones and neoplastic lymphoblasts of B cells present in bone marrow may cause diagnostic problems due to their morphologic and immunophenotypic similarities [7McKenna RW, Asplund SL, Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) and neoplastic lymphoblasts by 4- color flow cytometry Leuk Lymphoma 2004; 45(2): 277-85., 22Babusikova O, Zeleznikova T, Mlcakova A. The knowledge on the 3rd type hematogones could contribute to more precise detection of small numbers of precursor B-acute lymphoblastic leukaemia Neoplasma 2005; 52(6): 502-9.-24Chen W, karandikar NJ, McKenna RW, Kroft SH. Stability of leukaemia- associated immunophenotypes in precursor B-lymphoblastic leukaemia a single institution experience Am J Clin Pathol 2007; 127(1): 39-46.] Distinction in the bone marrow of benign B-lymphocyte precursors known as hematogones from neoplastic lymphoblasts of ALL is critical for disease management (in post-chemotherapy and post-bone marrow transplant regenerating marrow) [7McKenna RW, Asplund SL, Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) and neoplastic lymphoblasts by 4- color flow cytometry Leuk Lymphoma 2004; 45(2): 277-85., 25Riley RS, Massey D, Jackson-Cook C. Immunophenotypic analysis of acute lymphocytic leukaemia Hematol Oncol Clin North Am 2002; 16(2): 245-99.]. It has been reported that the number of hematogones in bone marrow is variable; the hematogones are present in higher numbers in children and they are often increased in regene-rating marrow and in some clinical conditions particularly in patients with cytopenias and neoplastic diseases [7McKenna RW, Asplund SL, Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) and neoplastic lymphoblasts by 4- color flow cytometry Leuk Lymphoma 2004; 45(2): 277-85., 26Guiziry DE, Farahat N, Hassab H. Phenotypic analysis of bone marrow lymphocytes from children with acute thrombocytopenic purpura Egypt J Immunol 2005; 12(1): 9-14., 27McKenna RW, Waschington LT, Aquino DB. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) in 662 consecutive bone marrow specimens by 4- color flow cytometry Blood 2001; 98(8): 2498-507.]. Hematogones may be particularly prominent in the regeneration phase following chemotherapy. In some instan-ces they constitute 5 % to more than 50 % of cells [7McKenna RW, Asplund SL, Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) and neoplastic lymphoblasts by 4- color flow cytometry Leuk Lymphoma 2004; 45(2): 277-85., 28Vargas SO, Hasegawa SL, Dorfman DM. Hematogones as an internal control in flow cytometric analysis of suspected acute lymphoblastic leukemaia Pediatr Dev Pathol 2001; 4(5): 505-11.-30Davis BH, Scwartz M. ZAP-70 expression is low in normal precursor B cells or hematogones Cytometry B Clin Cytom 2006; 70B(4): 314-8.].
The presence of benign immature B cells has been noted to interfere with the flow cytometric analysis of cases of suspected acute lymphoblastic leukaemia because their immunophenotype (positive for CD19, CD10, CD34 and terminal deoxynucleotidyl transferase) is similar to that of pre B cells lymphoblasts and they simulate acute lymphoblastic leukaemia or lymphoma [28Vargas SO, Hasegawa SL, Dorfman DM. Hematogones as an internal control in flow cytometric analysis of suspected acute lymphoblastic leukemaia Pediatr Dev Pathol 2001; 4(5): 505-11., 29Davis RE, Longacre TA, Cornbleet PJ. Hematogones in the bone marrow of adults Immunophenotypic features clinical settings and differential diagnosis Am J Clin Pathol 1994; 102(2): 201-11., 31Hurford MT, Altman AJ, DiGiuseppe JA. Unique pattern of nuclear TdT immunofluorescence distinguishes normal precursor B cells (Hematogones) from lymphoblasts of precursor B-lymphoblastic leukaemia Am J Clin Pathol 2008; 129(5): 700-5.]. The presence of hematogones in clinical samples should be recognized so as not to adversely influence prognostic studies [30Davis BH, Scwartz M. ZAP-70 expression is low in normal precursor B cells or hematogones Cytometry B Clin Cytom 2006; 70B(4): 314-8., 31Hurford MT, Altman AJ, DiGiuseppe JA. Unique pattern of nuclear TdT immunofluorescence distinguishes normal precursor B cells (Hematogones) from lymphoblasts of precursor B-lymphoblastic leukaemia Am J Clin Pathol 2008; 129(5): 700-5.]. Flow cytometry is reported to distinguish between these cell populations in nearly all instances. In the medical literature that we consulted, the neoplastic lymphoblasts in precursor B ALL deviate from the normal B-lineage maturation spectrum and exhibit maturation arrest and over-, under-, and asynchronous expression of antigens observed on normal B-cell precursors and they often aberrantly express myeloid-associated antigens [22Babusikova O, Zeleznikova T, Mlcakova A. The knowledge on the 3rd type hematogones could contribute to more precise detection of small numbers of precursor B-acute lymphoblastic leukaemia Neoplasma 2005; 52(6): 502-9.].
Rimsza, has demonstrated that hematogone-rich lymphoid proliferations exhibit a spectrum of B- lymphoid differentiation antigen expression with predominance of intermediate and mature B lineage cells [8Rimsza LM, Larson RS, Winter SS. Benign hematogone-rich lymphoid proliferations can be distinguished from B lineage acute lymphoblastic leukaemia by integration of morphology, immunophenotype, adhesion molecule expression and architectural features Am J Clin Pathol 2000; 114(1): 66-75.]. Flow cytometry revealed in this study that intermediately differentiated cells (CD10+, CD19+) predominated followed in frequency by CD20+ [8Rimsza LM, Larson RS, Winter SS. Benign hematogone-rich lymphoid proliferations can be distinguished from B lineage acute lymphoblastic leukaemia by integration of morphology, immunophenotype, adhesion molecule expression and architectural features Am J Clin Pathol 2000; 114(1): 66-75.]. Hematogone populations always exhibit a continuous and complete maturation spectrum of antigen expression typical of the normal evolution of B-lineage precursors; they lack aberrant or asynchronous antigen expression [22Babusikova O, Zeleznikova T, Mlcakova A. The knowledge on the 3rd type hematogones could contribute to more precise detection of small numbers of precursor B-acute lymphoblastic leukaemia Neoplasma 2005; 52(6): 502-9.]. Hematogones are precursors which were defined by CD19 positivity and CD45 bright, the expression of antigen of immaturity: HLA DR and CD34, and the coexpression of more mature markers CD19, CD20, CD22. These cells are blended and confused with those of mature B lymphocytes (CD10 negative) on CD45/SSC and could be better recognized on CD10 gating [7McKenna RW, Asplund SL, Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) and neoplastic lymphoblasts by 4- color flow cytometry Leuk Lymphoma 2004; 45(2): 277-85.]. With flow cytometry using optimal antibodies in combination, the distinction can nearly always be made. However, we have to emphasize the difficulties in distinguishing these cells from residual marrow blasts after chemotherapy [23Rimsza LM, Douglas VK, Tighe P. Benign B-cell precursors (hematogones) are the predominant lymphoid population in the bone marrow of preterm infants Biol Neonate 2004; 86(4): 247-53., 24Chen W, karandikar NJ, McKenna RW, Kroft SH. Stability of leukaemia- associated immunophenotypes in precursor B-lymphoblastic leukaemia a single institution experience Am J Clin Pathol 2007; 127(1): 39-46., 32Kallkury BV, Hartmann DP, Cossman J, et al. Posttherapy surveillance of B cell precursor acute lymphoblastic leukaemia. Value of polymerase chain reaction and limitation of flow cytometry Am J Clin Pathol 1999; 111(6): 759-6.]. Identification of normal hematogones B contribute to better clarify the detection of small numbers of blasts B of acute lymphoblastic leukaemia [3San Miguel JF, Vidriales MB, Lopez-Berges C. Early immunophenotypical evaluation of Minimal Residual Disease in acute myeloid leukemia identifies different patient risk groups and may contribute to podt induction treatment stratification Blood 2001; 98(6): 1746-51., 15Coustan SE, Gajjar A, Hijiya N. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukaemia after relapse Leukemia 2004; 18: 499-504.].
An important question is mentioned in all series describing flow cytometrics measurements of residual disease and using different combinations of antibodies with large panels: which panel of monoclonal antibodies provided more informations of residual cells? The result of this present study indicates that two combinations are suitable for the monitoring of MRD by FC in B ALL: CD10/CD38/ CD19/CD45 and CD10/CD34/CD19/CD45, because they allow the screening of LAIP which are frequent and distinguish B lymphoblasts from normal hematogones. As shown in the FC data analysis, our results are entirely satisfactory and in general, are in concordance with literature [12Vidriales MB, Pérez JJ, López-Berges MC. Minimal residual disease in adolescent (older than 14 years) and adult acute lymphoblastic leukemias: early immunophenotypic evaluation has high clinical value Blood 2003; 101: 4695-700., 25Riley RS, Massey D, Jackson-Cook C. Immunophenotypic analysis of acute lymphocytic leukaemia Hematol Oncol Clin North Am 2002; 16(2): 245-99.]. The strategy of monitoring MRD described previously is relatively simple and standardized but remains limited by the quality of FC analysis at diagnosis and screening of aberrant phenotypes.
CONCLUSION
FC is a very powerful approach to study MRD. The result of this prospective study proposes a strategy to monitoring MDR by FC that could be helpful in the treatment stratification and the management of patients with B lineage ALL. A strong correlation between flow cytometric measurements of MRD during clinical remission and treatment outcome has been demonstrated, suggesting that these assays should be incorporated into treatment protocols. For that purpose, new analytical approaches must be performed in order to be used in the investigation of MDR.








