- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Leukemia Journal
(Discontinued)
ISSN: 1876-8164 ― Volume 5, 2013
The Etiology of Chronic Lymphocytic Leukemia: Another Look at the Relationship Between B1 cells and CLL
Tara M Nordgren , Shantaram S Joshi*
Abstract
Chronic lymphocytic leukemia (CLL) is a heterogeneous disease of unknown etiological origin. CLL is the only B cell malignancy where a characteristic chromosomal translocation is not involved in cancer initiation. Therefore, the cause of tumorigenesis in CLL patients and the type of cell that is transformed are two questions that have intrigued researchers for decades. However, evidence suggests the CLL cell may be derived from B-1 cells. These B-1 cells, thought to develop during neonatal maturation as a link between innate and adaptive immunity, share multiple phenotypic and genetic patterns with CLL cases. These include signaling molecules sensitivity and expression patterns, B-cell receptor (BCR) specificity, and a unique immune-modulatory phenotype. Through understanding the biological relevance of B-1 cells in immune development and regulation, we may further understand the molecular mechanisms underlying the complexity of CLL. With this understanding, we can provide more optimal care to patients based on their unique diagnosis and pathologic disease course. In this review, based on our current understanding of CLL cells and B1 cells we hypothesize that CLL cells are originated from B1 cells. Following are some of our rationale in deriving this hypothesis
Article Information
Identifiers and Pagination:
Year: 2010Volume: 3
First Page: 69
Last Page: 73
Publisher Id: TOLEUKEMIAJ-3-69
DOI: 10.2174/1876816401003010069
Article History:
Received Date: 8/7/2010Revision Received Date: 31/8/2010
Acceptance Date: 6/9/2010
Electronic publication date: 29/10/2010
Collection year: 2010
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.5/), which permits unrestrictive use, distribution, and reproduction in any medium, provided the original work is properly cited.
* Address correspondence to this author at the University of Nebraska Medical Center Genetics, Cell Biology, and Anatomy 68198-6395, USA; Tel: 402-559-4165; Fax: 402-559-3400; E-mail: ssjoshi@unmc.edu
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 8-7-2010 |
Original Manuscript | The Etiology of Chronic Lymphocytic Leukemia: Another Look at the Relationship Between B1 cells and CLL | |
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is an extremely heterogeneous disease with a highly variable clinical course. Although many patients live and die with the disease, other patients experience an aggressive disease course and ultimately die from the cancer [1Hamblin TJ, Oscier DG. Chronic lymphocytic leukaemia: The nature of the leukaemic cell Blood Rev 1997; 11(3 ): 119-28.]. However, the mystery of CLL remains in the origin of the disease. What causes CLL tumorigenesis, and what cell type is transformed to become CLL? These two questions continue to plague researchers trying to understand this intriguing ailment. By understanding the origination and instigating factors leading to the development of CLL, we hope to elucidate the molecular mechanisms underlying the complexities of this heterogeneous disorder. In so doing, we may provide more optimal care for patients with CLL based on their unique diagnosis and pathologic disease course.
To begin unraveling the mystery of CLL’s origin, the phenotype of CLL cells must be considered. CLL cells are characterized as monoclonal B cells expressing various B cell-associated markers, including the surface CD19 and CD23 markers [2Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia N Engl J Med 2005; 352(8 ): 804-15.]. While the B cell lineage of CLL cells is rarely disputed, the presence of many T cell-associated markers in CLL cells has raised questions as to the exact derivation of CLL cells. For example, the T cell-associated CD4, CD7, and CD8 markers have all been found in certain clinical cases of CLL [3Parisi-Duchene E, Mazurier I, Moskovtchenko P. Aberrant CD8 expression in B-chronic lymphocytic leukemia: Report of five cases Acta Haematol 2006; 115(1-2 ): 74-., 4Jani P, Qi XY, Chang H. Aberrant expression of T-cell-associated markers CD4 and CD7 on B-cell chronic lymphocytic leukemia Am J Hematol 2007; 82(1 ): 73-6.]. Additionally, T cell activation-associated molecule zeta-chain-associated protein kinase 70 (ZAP-70) is expressed in a subset of CLL patients with unmutated immunoglobulin variable (IgVH) region genes, and its expression corresponds to increased B-cell receptor (BCR) signaling and shorter time to treatment in patients [5Wiestner A, Rosenwald A, Barry TS, et al. ZAP-70 expression identifies a chronic lymphocytic leukemia subtype with unmutated immunoglobulin genes, inferior clinical outcome, and distinct gene expression profile Blood 2003; 101(12 ): 4944-51., 6Chen L, Widhopf G, Huynh L, et al. Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia Blood 2002; 100(13 ): 4609-14.]. In fact, one of the primary markers used to separate and identify CLL cells out of samples of peripheral blood mononuclear cells is CD5, which is considered by many to be a pan-T cell marker [7Dalloul A. CD5: A safeguard against autoimmunity and a shield for cancer cells Autoimmun Rev 2009; 8(4 ): 349-53.].
Interestingly, although CD5 is considered a T-cell marker, certain subsets of B cells are known to express CD5. The primary subgroup of CD5+ B cells is the B-1 cell type; these cells are thought to develop primarily during neonatal maturation (and possibly within the adult bone marrow) and demonstrate some poly-specificity [8Martin F, Kearney JF. B1 cells: Similarities and differences with other B cell subsets Curr Opin Immunol 2001; 13(2 ): 195-201.]. These B-1 cells serve as a link between the innate and adaptive immune system, developing via a mechanism independent of the B2/traditional developmental program of B cells. Additionally, these cells bear resemblance in function and characteristics to the marginal zone (MZ) subset of B cells (distinct from the follicular, or FO B cells) that may be involved in rapid T-independent or memory immune responses [8Martin F, Kearney JF. B1 cells: Similarities and differences with other B cell subsets Curr Opin Immunol 2001; 13(2 ): 195-201., 9Weill JC, Weller S, Reynaud CA. Human marginal zone B cells Annu Rev Immunol 2009; 27: 267-85.]. Both B1 and MZ subsets of B cells express CD9 and the CD27 memory B-cell marker. In fact, CLL cells have also been characterized as expressing CD27, suggesting their progenitor has an antigen-experienced memory phenotype in at least a majority of CLL cases [10Damle RN, Ghiotto F, Valetto A, et al. B-cell chronic lymphocytic leukemia cells express a surface membrane phenotype of activated, antigen-experienced B lymphocytes Blood 2002; 99(11 ): 4087-93.].
Undeniably, there are a plethora of possible progenitor cells for CLL. Many have suggested that CLL cells could, indeed, be derived from the B-1 cell lineage [11Freedman AS, Boyd AW, Bieber FR, et al. Normal cellular counterparts of B cell chronic lymphocytic leukemia Blood 1987; 70(2 ): 418-27.-15Wong SC, Chew WK, Tan JE, et al. Peritoneal CD5+ B-1 cells have signaling properties similar to tolerant B cells J Biol Chem 2002; 277(34 ): 30707-15.]. On the other hand, others believe that CLL may have an etiology derived from traditional B-2 cells or a heterogeneous etiology varying from patient to patient [1Hamblin TJ, Oscier DG. Chronic lymphocytic leukaemia: The nature of the leukaemic cell Blood Rev 1997; 11(3 ): 119-28., 16Catera R, Silverman GJ, Hatzi K, et al. Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and oxidation Mol Med 2008; 14(11-12 ): 665-74., 17Darzentas N, Hadzidimitriou A, Murray F, et al. A different ontogenesis for chronic lymphocytic leukemia cases carrying stereotyped antigen receptors: Molecular and computational evidence Leukemia 2010; 24(1 ): 125-32.]. While we cannot yet conclude the originating cell type of CLL or how the CLL lineage may play a role in the heterogeneity of the CLL disease course, evidence suggests a similarity between CLL cells and the B-1 cell phenotype. Data in support of this hypothesis will be summarized in the remainder of this short review.
SIGNALING MOLECULES: SENSITIVITY AND EXPRESSION
The B-1 subset of lymphocytes represents a unique B-cell profile. This profile includes an autocrine signaling pathway through interleukin 10 (IL-10), increased surface expression of CXCR5 as compared to B-2 cells, and potential survival and proliferation through the soluble protein APRIL (a proliferation-inducing ligand) [18YenChong S, Lin YC, Czarneski J, et al. Cell cycle effects of IL-10 on malignant B-1 cells Genes Immun 2001; 2(5
): 239-47.-20Planelles L, Carvalho-Pinto CE, Hardenberg G, et al. APRIL promotes B-1 cell-associated neoplasm Cancer Cell 2004; 6(4
): 399-408.]. Interestingly, CLL cells have been shown to utilize similar signaling mechanisms for their own growth and survival (Fig. 1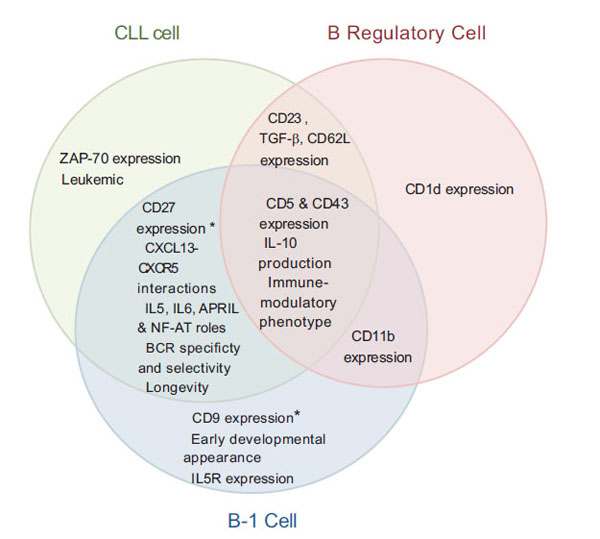 ). For example, serum levels of IL-10 are significantly higher in CLL patients than control subjects, and elevated levels correlated with poor overall survival [21Fayad L, Keating MJ, Reuben JM, et al. Interleukin-6 and interleukin-10 levels in chronic lymphocytic leukemia: Correlation with phenotypic characteristics and outcome Blood 2001; 97(1
): 256-63.]. A murine model of CLL with malignant B-1 cells displayed higher IL-10 mRNA levels than normal B-2 or B-1 cells [12Peng B, Mehta NH, Fernandes H, et al. Growth inhibition of malignant CD5+B (B-1) cells by antisense IL-10 oligonucleotide Leuk Res 1995; 19(3
): 159-67.]. Additionally, recent in vitro data suggest that CLL cells cultured ex vivo show a marked decrease in IL-10 levels, which also corresponds with the induction of spontaneous apoptosis in the cells [22Djurdjevic P, Zelen I, Ristic P, et al. Role of decreased production of interleukin-10 and interferon-gamma in spontaneous apoptosis of B-chronic lymphocytic leukemia lymphocytes in vitro Arch Med Res 2009; 40(5
): 357-63.]. Under physiologic conditions, IL-10 is primarily produced by B-1 cells as compared to other B cell types. This cytokine, which is a known regulator of humoral response activation, is also thought to be an important growth factor for B-1 cells, allowing for their unique longevity and auto-renewing properties [18YenChong S, Lin YC, Czarneski J, et al. Cell cycle effects of IL-10 on malignant B-1 cells Genes Immun 2001; 2(5
): 239-47., 23O'Garra A, Howard M. IL-10 production by CD5 B cells Ann N Y Acad Sci 1992; 651: 182-99.]. Interestingly, the CD5 surface marker that is found on B-1 and CLL cells may be facilitating the upregulation of IL-10 that leads to autocrine pro-survival signaling in these cells [24Gary-Gouy H, Harriague J, Bismuth G, et al. Human CD5 promotes B-cell survival through stimulation of autocrine IL-10 production Blood 2002; 100(13
): 4537-3.] thus highlighting the similar functions IL-10 appears to perform in both the B-1 and CLL cells.
). For example, serum levels of IL-10 are significantly higher in CLL patients than control subjects, and elevated levels correlated with poor overall survival [21Fayad L, Keating MJ, Reuben JM, et al. Interleukin-6 and interleukin-10 levels in chronic lymphocytic leukemia: Correlation with phenotypic characteristics and outcome Blood 2001; 97(1
): 256-63.]. A murine model of CLL with malignant B-1 cells displayed higher IL-10 mRNA levels than normal B-2 or B-1 cells [12Peng B, Mehta NH, Fernandes H, et al. Growth inhibition of malignant CD5+B (B-1) cells by antisense IL-10 oligonucleotide Leuk Res 1995; 19(3
): 159-67.]. Additionally, recent in vitro data suggest that CLL cells cultured ex vivo show a marked decrease in IL-10 levels, which also corresponds with the induction of spontaneous apoptosis in the cells [22Djurdjevic P, Zelen I, Ristic P, et al. Role of decreased production of interleukin-10 and interferon-gamma in spontaneous apoptosis of B-chronic lymphocytic leukemia lymphocytes in vitro Arch Med Res 2009; 40(5
): 357-63.]. Under physiologic conditions, IL-10 is primarily produced by B-1 cells as compared to other B cell types. This cytokine, which is a known regulator of humoral response activation, is also thought to be an important growth factor for B-1 cells, allowing for their unique longevity and auto-renewing properties [18YenChong S, Lin YC, Czarneski J, et al. Cell cycle effects of IL-10 on malignant B-1 cells Genes Immun 2001; 2(5
): 239-47., 23O'Garra A, Howard M. IL-10 production by CD5 B cells Ann N Y Acad Sci 1992; 651: 182-99.]. Interestingly, the CD5 surface marker that is found on B-1 and CLL cells may be facilitating the upregulation of IL-10 that leads to autocrine pro-survival signaling in these cells [24Gary-Gouy H, Harriague J, Bismuth G, et al. Human CD5 promotes B-cell survival through stimulation of autocrine IL-10 production Blood 2002; 100(13
): 4537-3.] thus highlighting the similar functions IL-10 appears to perform in both the B-1 and CLL cells.
 |
Fig. (1) Representative Venn diagram highlighting numerous similarities and differences in expression and/or phenotypic patterns between CLL, B-1, and B Regulatory cells, as suggested by current literature. * Denotes notable similarities also shared with MZ B cells [16Catera R, Silverman GJ, Hatzi K, et al. Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and oxidation Mol Med 2008; 14(11-12 ): 665-74., 19Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity Immunity 2002; 16(1 ): 67-76., 20Planelles L, Carvalho-Pinto CE, Hardenberg G, et al. APRIL promotes B-1 cell-associated neoplasm Cancer Cell 2004; 6(4 ): 399-408., 26Lopes JD, Mariano M. B-1 cell: The precursor of a novel mononuclear phagocyte with immuno-regulatory properties An Acad Bras Cienc 2009; 81(3 ): 489-96., 29Burkle A, Niedermeier M, Schmitt-Graff A, et al. Overexpression of the CXCR5 chemokine receptor, and its ligand CXCL13 in B-cell chronic lymphocytic leukemia Blood 2007; 110(9 ): 3316-25., 30Endo T, Nishio M, Enzler T, et al. BAFF and APRIL support chronic lymphocytic leukemia B-cell survival through activation of the canonical NF-kappaB pathway Blood 2007; 109(2 ): 703-10., 34Hayakawa K, Hardy RR, Honda M, et al. Ly-1 B cells: Functionally distinct lymphocytes that secrete IgM autoantibodies Proc Natl Acad Sci USA 1984; 81(8 ): 2494-8., 50Zucchetto A, Sonego P, Degan M, et al. Surface-antigen expression profiling (SEP) in B-cell chronic lymphocytic leukemia (B-CLL): Identification of markers with prognostic relevance J Immunol Methods 2005; 305(1 ): 20-32.-53Mauri C, Ehrenstein MR. The 'short' history of regulatory B cells Trends Immunol 2008; 29(1 ): 34-40.]. |
In fact, IL-10 is not the only cytokine with similar production and effects in both B-1 and CLL cells. Similar to IL-10 serum levels in CLL patients, levels of the pyrogenic cytokine interleukin-6 (IL-6) in CLL serum also appear to be elevated and correspond to an aggressive disease state [21Fayad L, Keating MJ, Reuben JM, et al. Interleukin-6 and interleukin-10 levels in chronic lymphocytic leukemia: Correlation with phenotypic characteristics and outcome Blood 2001; 97(1 ): 256-63.]. Not only have B-1 cells been characterized as producers of IL-6, but Cong et al. (1991) reported that a combination of IL-6 and anti-IgM in culture can induce CD5 expression in previously CD5-negative murine B cells and confer a B-1 phenotype [25Cong YZ, Rabin E, Wortis HH. Treatment of murine CD5- B cells with anti-ig, but not LPS induces surface CD5: Two B-cell activation pathways Int Immunol 1991; 3(5 ): 467-76., 26Lopes JD, Mariano M. B-1 cell: The precursor of a novel mononuclear phagocyte with immuno-regulatory properties An Acad Bras Cienc 2009; 81(3 ): 489-96.]. Therefore, IL-6 may be involved in the promotion and/or maintenance of CLL and B-1 cells.
Another cytokine that appears to function in both B-1 and CLL cells is interleukin-5 (IL-5). It has been shown that the IL-5 receptor is expressed by CD5+ B-1 cells, which secrete IgM upon stimulation with IL-5 [27Hitoshi Y, Yamaguchi N, Mita S, et al. Distribution of IL-5 receptor-positive B cells, expression of IL-5 receptor on ly-1(CD5)+ B cells J Immunol 1990; 144(11 ): 4218-25.]. As it is known that many B-1 antibodies are auto-reactive, an over-production of IL-5 leading to expansion of B-1 cells should increase the susceptibility to auto-immune disease. However, a study performed in an IL-5 transgenic mouse susceptible to systemic lupus erythematosus (SLE) showed that the increase in B-1 cells was protective against SLE while causing a CLL phenotype in the mice [28Wen X, Zhang D, Kikuchi Y, et al. Transgene-mediated hyper-expression of IL-5 inhibits autoimmune disease but increases the risk of B cell chronic lymphocytic leukemia in a model of murine lupus Eur J Immunol 2004; 34(10 ): 2740-9.]. This study therefore highlights the importance of IL-5 in the functioning of B-1 cells and suggests a potential link between B-1 and CLL cells, at least in this murine model.
The chemokine receptor-ligand interaction between CXCR5 and CXCL13 has recently been studied for its role in the homing, proliferation, and survival of CLL cells. Data suggest that overexpression of CXCR5 by CLL cells allows them to home to lymphoid tissues where they can interact with CD68+ stromal cells that provide the CLL cells with important pro-survival signals. Additionally, CLL patients exhibit significantly higher levels of CXCL13 in serum than control subjects, suggesting a role for this CXCR5-CXCL13 interaction in the disease state [29Burkle A, Niedermeier M, Schmitt-Graff A, et al. Overexpression of the CXCR5 chemokine receptor, and its ligand CXCL13 in B-cell chronic lymphocytic leukemia Blood 2007; 110(9 ): 3316-25.]. Similar to CLL cells, B-1 cells have been shown to express higher levels of CXCR5 than their B-2 cell counterparts, and this expression is necessary for the B-1 cells to home to the peritoneal cavities where they function in immune response via antibody production [19Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity Immunity 2002; 16(1 ): 67-76.].
Yet another molecule that may play a role in the proliferation/survival of both CLL and B-1 cells is APRIL. This soluble protein has been shown to activate the NFκB pathway in CLL cells, leading to resistance to apoptosis and prolonged survival. APRIL has also been found to be elevated in CLL patients as compared to control subjects [20Planelles L, Carvalho-Pinto CE, Hardenberg G, et al. APRIL promotes B-1 cell-associated neoplasm Cancer Cell 2004; 6(4 ): 399-408., 30Endo T, Nishio M, Enzler T, et al. BAFF and APRIL support chronic lymphocytic leukemia B-cell survival through activation of the canonical NF-kappaB pathway Blood 2007; 109(2 ): 703-10.]. Interestingly, overexpression of APRIL in transgenic mice confers an abnormal expansion of B-1 cells in the peritoneal cavities that leads to lymphoid tumor development in this murine model [20Planelles L, Carvalho-Pinto CE, Hardenberg G, et al. APRIL promotes B-1 cell-associated neoplasm Cancer Cell 2004; 6(4 ): 399-408.]. Thus, these studies suggest a tumorigenic role for APRIL in B-1 cells and CLL.
NF-AT (nuclear factor of activated T cells) is another signaling molecule that has been implicated in the activation of both CLL and B-1 cells. In B-2 cells, NF-AT appears to be activated upon BCR crosslinking, whereas its expression is constitutive in B-1 cells [15Wong SC, Chew WK, Tan JE, et al. Peritoneal CD5+ B-1 cells have signaling properties similar to tolerant B cells J Biol Chem 2002; 277(34 ): 30707-15.]. A similar phenomenon is seen in CLL cells, where a patient’s normal B cells can be distinguished from transformed CLL cells by the constitutive nuclear localization of NF-AT in the CLL cells [31Schuh K, Avots A, Tony HP, et al. Nuclear NF-ATp is a hallmark of unstimulated B cells from B-CLL patients Leuk Lymphoma 1996; 23(5-6 ): 583-92.]. The increased expression of NF-AT in CLL samples has also been directly correlated with increased CD38 expression, which is considered an indicator of poor clinical prognosis [32Joshi AD, Hegde GV, Dickinson JD, et al. ATM CTLA4 MNDA and HEM1 in high versus low CD38 expressing B-cell chronic lymphocytic leukemia Clin Cancer Res 2007; 13(18 Pt 1 ): 5295-304.]. In addition, the activation of NF-AT may be of particular importance to B-1 and CLL cells, as studies have shown that NF-AT has multiple binding sites for the CD5 gene and may therefore regulate the characteristic expression of CD5 in these cell types [15Wong SC, Chew WK, Tan JE, et al. Peritoneal CD5+ B-1 cells have signaling properties similar to tolerant B cells J Biol Chem 2002; 277(34 ): 30707-15., 33Berland R, Wortis HH. An NFAT-dependent enhancer is necessary for anti-IgM-mediated induction of murine CD5 expression in primary splenic B cells J Immunol 1998; 161(1 ): 277-85.]. Taken together, these data indicate similar signaling patterns may be responsible for the regulation of both B-1 and CLL cells, highlighting the cells’ resemblance.
BCR SPECIFICITY
One of the unique characteristics of B-1 cells is their BCR specificities. The B-1 cells are thought to be a link between innate and adaptive immunity because they have BCRs with natural- and auto-reactivity [34Hayakawa K, Hardy RR, Honda M, et al. Ly-1 B cells: Functionally distinct lymphocytes that secrete IgM autoantibodies Proc Natl Acad Sci USA 1984; 81(8 ): 2494-8.]. These BCRs include a limited repertoire of specificities with heavy- and light-chain selectivity in the B-1-cell subsets that are thought to play a pivotal role in T-independent/innate immune responses [8Martin F, Kearney JF. B1 cells: Similarities and differences with other B cell subsets Curr Opin Immunol 2001; 13(2 ): 195-201., 35Rowley B, Tang L, Shinton S, et al. Autoreactive B-1 B cells: Constraints on natural autoantibody B cell antigen receptors J Autoimmun 2007; 29(4 ): 236-45.]. Interestingly, many CLL cases have been shown to have similar BCR characteristics as B-1 cells. At least 20% of CLL patients have been characterized as having stereotypic heavy chain complementarity-determining region 3 sequences [36Stamatopoulos K, Belessi C, Moreno C, et al. Over 20% of patients with chronic lymphocytic leukemia carry stereotyped receptors: Pathogenetic implications and clinical correlations Blood 2007; 109(1 ): 259-70.]. In particular, the BCRs of CLL patients tend to exhibit outgrowth of the same immunoglobulin clones that utilize limited Ig genes such as VH5, VH4, VKI, and the G6 cross-reactive idiotype [37Kipps TJ, Tomhave E, Pratt LF, et al. Developmentally restricted immunoglobulin heavy chain variable region gene expressed at high frequency in chronic lymphocytic leukemia Proc Natl Acad Sci USA 1989; 86(15 ): 5913-7.-39Hashimoto S, Dono M, Wakai M, et al. Somatic diversification and selection of immunoglobulin heavy and light chain variable region genes in IgG+ CD5+ chronic lymphocytic leukemia B cells J Exp Med 1995; 181(4 ): 1507-7.]. Also, many antibodies produced from CLL cells are able to bind apoptotic cells, recognizing self-antigens associated with apoptosis and/or cellular stress. These antibodies also tend to be unmutated and auto- and poly-reactive — a phenotype similar to the B-1 cells [13Caligaris-Cappio F. B-chronic lymphocytic leukemia: A malignancy of anti-self B cells Blood 1996; 87(7 ): 2615-0., 16Catera R, Silverman GJ, Hatzi K, et al. Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and oxidation Mol Med 2008; 14(11-12 ): 665-74.].
IMMUNO-SUPPRESSIVE PROFILE: B REGULATORY LINK?
Although a role for the T regulatory cell has been substantiated within the immune repertoire for some time, it is only in the past few years that researchers have appreciated a humoral component for immune down-modulation: the CD5+CD1d+CD11b+ B regulatory cell (Breg). While not much is known about this subset of cells, Bregs are thought to cause immune-suppression through complex interactions with other immune effector and stromal cells mediated by the Bregs’ production of IL-10 [40Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation Immunol Rev 2008; 224, 41Lemoine S, Morva A, Youinou P, et al. Regulatory B cells in autoimmune diseases: How do they work? Ann N Y Acad Sci 2009; 1173: 260-7.]. Certain IL-10-secreting Bregs are expanded in cases of endogenous autoimmunity, whereas cases of exogenously-perturbed autoimmune disorders have corresponded with decreased numbers of Breg cells. This suggests a complex paradoxical relationship between immune regulation and auto-immunity [42Yanaba K, Bouaziz JD, Matsushita T, et al. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals J Immunol 2009; 182(12 ): 7459-2.]. Interestingly, this same contradictory relationship has also been seen with B-1 cells, where B-1 cells have been associated with the generation of immune tolerance and with the induction of autoimmunity [26Lopes JD, Mariano M. B-1 cell: The precursor of a novel mononuclear phagocyte with immuno-regulatory properties An Acad Bras Cienc 2009; 81(3 ): 489-96., 35Rowley B, Tang L, Shinton S, et al. Autoreactive B-1 B cells: Constraints on natural autoantibody B cell antigen receptors J Autoimmun 2007; 29(4 ): 236-45.].
In addition to being the primary B cell producers of IL-10, further evidence suggests that B-1 cells may compose at least a proportion of the recently discovered Bregs [26Lopes JD, Mariano M. B-1 cell: The precursor of a novel mononuclear phagocyte with immuno-regulatory properties An Acad Bras Cienc 2009; 81(3
): 489-96., 43De-Gennaro LA, Popi AF, Almeida SR, et al. Mariano M. B-1 cells modulate oral tolerance in mice Immunol Lett 2009; 124(2
): 63-9.]. In support of this hypothesis, B-1 cells have been previously suggested to play an important role in immune tolerance and down-modulation [15Wong SC, Chew WK, Tan JE, et al. Peritoneal CD5+ B-1 cells have signaling properties similar to tolerant B cells J Biol Chem 2002; 277(34
): 30707-15., 26Lopes JD, Mariano M. B-1 cell: The precursor of a novel mononuclear phagocyte with immuno-regulatory properties An Acad Bras Cienc 2009; 81(3
): 489-96., 35Rowley B, Tang L, Shinton S, et al. Autoreactive B-1 B cells: Constraints on natural autoantibody B cell antigen receptors J Autoimmun 2007; 29(4
): 236-45.]. Recent data from our laboratory also suggests an immune-suppressive or tolerogenic signature in many CLL patient cases based on microarray analysis. Our results indicate that microenvironmental interactions allow CLL cells to elicit tolerizing signals to surrounding immune cells [44Mittal A, Gilling C, Iqbal J, et al. Clinical heterogeneity of CLL: Role for immune dysregulation mediated by the lymph node microenvironment Blood (ASH Ann Meet Abst) 2009; 114: 1243.]. In contrast to producing an immunosuppressive environment, autoimmune disorders often co-present with CLL, suggesting that CLL cells may also be involved in complex immune-regulatory processes similar to B-1 and Breg cells. These processes could potentially be mediated through increased IL-10 expression and other immune-modulatory interactions in various microenvironmental niches [21Fayad L, Keating MJ, Reuben JM, et al. Interleukin-6 and interleukin-10 levels in chronic lymphocytic leukemia: Correlation with phenotypic characteristics and outcome Blood 2001; 97(1
): 256-63., 45Ghia P, Scielzo C, Frenquelli M, et al. From normal to clonal B cells: Chronic lymphocytic leukemia (CLL) at the crossroad between neoplasia and autoimmunity Autoimmun Rev 2007; 7(2
): 127-31.]. These evidences suggest that there may be an etiological link between B-1, Breg, and CLL cells (Fig. 1 ), as their phenotypic (such as CD5, CD23, and CD43 expression) and discussed functional aspects bear strong resemblance to each other in multiple contexts.
), as their phenotypic (such as CD5, CD23, and CD43 expression) and discussed functional aspects bear strong resemblance to each other in multiple contexts.
ONGOING STUDIES
While there are substantial similarities between B-1 and CLL cells, some researchers still question the etiological origin of CLL and suggest that B-1 cells may not necessarily be responsible for CLL leukemogenesis. For example, some argue that the CD5 marker present on both B-1 and CLL cells may not be an indication of common origin, but rather a marker for a specific differentiation or activation state in the cells [1Hamblin TJ, Oscier DG. Chronic lymphocytic leukaemia: The nature of the leukaemic cell Blood Rev 1997; 11(3 ): 119-28., 46Su I, Tarakhovsky A. B-1 cells: Orthodox or conformist? Curr Opin Immunol 2000; 12(2 ): 191-4.]. This idea is supported by evidence that incubation with IL-6 and anti-IgM can induce CD5 expression and confer a B-1 cell phenotype in CD5-negative murine B-1 cells [25Cong YZ, Rabin E, Wortis HH. Treatment of murine CD5- B cells with anti-ig, but not LPS induces surface CD5: Two B-cell activation pathways Int Immunol 1991; 3(5 ): 467-76.]. Others suggest that there may not be one single etiological origin for CLL and that the heterogeneity of clinical disease may actually derive from different CLL cases having diverse pre-cancerous origins. For example, a recent study identified two groups of CLL cases: one group having heterogeneous BCRs and the other having stereotypical BCRs. The authors suggested this result may be attributed to certain CLL cases arising from traditional B-2 cells (those with heterogeneous BCRs), while other CLL cases may arise from B-1 cells (those having stereotypical BCRs) [17Darzentas N, Hadzidimitriou A, Murray F, et al. A different ontogenesis for chronic lymphocytic leukemia cases carrying stereotyped antigen receptors: Molecular and computational evidence Leukemia 2010; 24(1 ): 125-32.]. Therefore, while evidence exists in support of the hypothesis that CLL cells are derived from B-1 cells, this data is not conclusive, leaving the etiological origin of CLL cells an unsolved mystery.
Given this information, future studies are necessary to resolve the predecessor of the CLL cell. Gratuitously, current methodologies and models lend themselves to this task. For example, despite the lack of a characteristic translocation associated with leukemogenesis in CLL, it is possible that other alterations or epigenetic changes may be taking place. The varying roles of methylation, histone modification, and miRNAs in promoting the leukemic phenotype have all been recently reviewed [47Chen J, Odenike O, Rowley JD. Leukaemogenesis: More than mutant genes Nat Rev Cancer 2010; 10(1 ): 23-36.]. Therefore, the epigenetic patterns of B-1, B-2, and CLL cells may not only provide insight as to the etiology of CLL, but may also help determine the confounding factors leading to cancer initiation in CLL patients.
In addition to looking at the epigenetics of CLL, it may also be pertinent to further standardize and utilize murine models that recapitulate the CLL phenotype. In fact, a recent animal model of CLL, the Eì-TCL1 transgenic mouse, has been utilized to investigate epigenetic changes in CLL that are correlative to patient cases, thus highlighting its value in investigating the CLL phenotype [48Chen SS, Raval A, Johnson AJ, et al. Epigenetic changes during disease progression in a murine model of human chronic lymphocytic leukemia Proc Natl Acad Sci USA 2009; 106(32 ): 13433-8., 49Chen SS, Sherman MH, Hertlein E, et al. Epigenetic alterations in a murine model for chronic lymphocytic leukemia Cell Cycle 2009; 8(22 ): 3663-7.]. Also, as CLL cells are known to readily undergo apoptosis in vitro, microenvironmental and immune-modulatory studies are vital to understanding the role of the various niches of CLL and how they promote survival and proliferation; these studies may only be possible through utilization of an animal model.
CONCLUSION
In conclusion, the etiology of CLL is still not entirely understood. The unique phenotype of these cells expressing both B and T cell molecules, various different activation markers, and unique signaling molecules all confound our understanding of their original derivation. However, evidence does suggest based on their signaling molecules expression and sensitivity, BCR specificity, and unique immune-modulatory profile that these cells share a similar phenotype with the B-1 cells of the immune system (Fig. 1 ). Through further investigation of the normal function of B-1 cells as well as their potential actions as Bregs, we may be able to determine the instigating factor—whether it be through a pathogenic insult or a combination of genomic and epigenetic events—leading to leukemogenesis in CLL patients. Additionally, investigating the roles of B-1 and Bregs in their normal physiological niches may facilitate our understanding of the unique mechanisms by which CLL cells exhibit prolonged survival, immune modulation, and other effects that play a role in the tumorigenesis of this heterogeneous disease.
). Through further investigation of the normal function of B-1 cells as well as their potential actions as Bregs, we may be able to determine the instigating factor—whether it be through a pathogenic insult or a combination of genomic and epigenetic events—leading to leukemogenesis in CLL patients. Additionally, investigating the roles of B-1 and Bregs in their normal physiological niches may facilitate our understanding of the unique mechanisms by which CLL cells exhibit prolonged survival, immune modulation, and other effects that play a role in the tumorigenesis of this heterogeneous disease.
CONFLICT OF INTEREST
The authors of this manuscript have no conflicts of interest in publishing this review, and do not have any financial relationship with commercial entities with interest in CLL.
