- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Leukemia Journal
(Discontinued)
ISSN: 1876-8164 ― Volume 5, 2013
Malignant T Cells Exhibit CD45 Resistant Stat3 Activation and Proliferation in Cutaneous T-Cell Lymphoma
Thorbjørn Krejsgaard1, 2, Rikke Helvad1, 2, Elisabeth Ralfkiaer3, Karen Astvad3, 4, Kirsten Grønbæk4, Karsten W. Eriksen2, Carsten Geisler2, Katharina Kopp1, 2, Qian Zhang5, Niels Odum1, 2, Anders Woetmann1, 2, *
Abstract
CD45 is a protein tyrosine phosphatase, which is well-known for regulating antigen receptor signalling in T and B cells via its effect on Src kinases. It has recently been shown that CD45 can also dephosphorylate Janus kinases (Jaks) and thereby regulate Signal transducer and activator of transcription (Stat) activation and cytokine-induced proliferation in lymphocytes. Consequently, CD45 dysregulation could be implicated in aberrant Jak/Stat activation and proliferation in lymphoproliferative diseases. Despite high expression of the CD45 ligand, Galectin-1, in skin lesions from cutaneous T-cell lymphoma (CTCL), the malignant T cells exhibit constitutive activation of the Jak3/Stat3 signalling pathway and uncontrolled proliferation. We show that CD45 expression is down-regulated on malignant T cells when compared to non-malignant T cells established from CTCL skin lesions. Moreover, CD45 cross-linking does not suppress the constitutive activation of Stat3 in the malignant T cells and there is no correlation between the level of activated Stat3 and the level of CD45 expression on the malignant T cells. Furthermore, in contrast to non-malignant T cells, the malignant T cells are protected against CD45-mediated inhibition of proliferation. In conclusion, our data suggest that CD45 dysregulation might play a role in the aberrant proliferation and Jak3/Stat3 activation in CTCL.
Article Information
Identifiers and Pagination:
Year: 2010Volume: 3
First Page: 9
Last Page: 15
Publisher Id: TOLEUKEMIAJ-3-9
DOI: 10.2174/1876816401003010009
Article History:
Received Date: 7/7/2009Revision Received Date: 30/10/2009
Acceptance Date: 9/11/2009
Electronic publication date: 13/1/2010
Collection year: 2010
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Department of Biology and Institute of International Health, Immunology and Microbiology, University of Copenhagen, Copenhagen, Denmark; Tel: +45 35 32 79 00; Fax: +45 35 32 78 68; E-mailawoetmann@sund.ku.dk
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 7-7-2009 |
Original Manuscript | Malignant T Cells Exhibit CD45 Resistant Stat3 Activation and Proliferation in Cutaneous T-Cell Lymphoma | |
INTRODUCTION
Cutaneous T-cell lymphomas (CTCLs) are the most frequent primary lymphomas of the skin [1Bradford PT, Devesa SS, Anderson WF. Cutaneous lymphoma incidence patterns in the United States a population-based study of 3884 cases Blood 2009; 113: 5064-73.]. They comprise a wide spectrum of heterogeneous lymphoproliferative disorders characterised by clonal accumulation of neoplastic T lymphocytes in the epidermis. Mycosis fungoides (MF) is the most common representative of CTCL [1Bradford PT, Devesa SS, Anderson WF. Cutaneous lymphoma incidence patterns in the United States a population-based study of 3884 cases Blood 2009; 113: 5064-73.,2Kim EJ, Hess S, Richardson SK. Immunopathogenesis and therapy of cutaneous T cell lymphoma J Clin Invest 2005; 115: 798-812.]. The etiology is unknown but it has been shown that the Janus kinase 3 (Jak3)/Signal transducer and activator of transcription 3 (Stat3) [3Levy DE, Darnell JE Jr. Stats transcriptional control and biological impact Nat Rev Mol Cell Biol 2002; 3: 651-2.] pathway is constitutively active in tumour cell lines obtained from independent skin biopsies and peripheral blood of patients suffering from CTCL [4Nielsen M, Kaltoft K, Nordahl M. Constitutive activation of a slowly migrating isoform of Stat3 in mycosis fungoides tyrphostin AG490 inhibits Stat3 activation and growth of mycosis fungoides tumor cell lines Proc Natl Acad Sci USA 1997; 94: 6764-9.-6Krejsgaard T, Vetter-Kauczok CS, Woetmann A. Jak3- and JNK-dependent vascular endothelial growth factor expression in cutaneous T-cell lymphoma Leukemia 2006; 20: 1759-66.]. Importantly, evidence that the Jak3/Stat3 pathway is constitutively active in vivo has also been provided [7Sommer VH, Clemmensen OJ, Nielsen O. In vivo activation of STAT3 in cutaneous T-cell lymphoma.Evidence for an antiapoptotic function of STAT3 Leukemia 2004; 18: 1288-95.,8Zhang Q, Raghunath PN, Xue L. Multilevel dysregulation of STAT3 activation in anaplastic lymphoma kinase-positive T/null-cell lymphoma J Immunol 2002; 168: 466-74.]. Inhibitors of Jak3 block the constitutive activation of Stat3 and inhibit the proliferation of the tumour cells [4Nielsen M, Kaltoft K, Nordahl M. Constitutive activation of a slowly migrating isoform of Stat3 in mycosis fungoides tyrphostin AG490 inhibits Stat3 activation and growth of mycosis fungoides tumor cell lines Proc Natl Acad Sci USA 1997; 94: 6764-9.,5Eriksen KW, Kaltoft K, Mikkelsen G. Constitutive stat3-activation in Sezary syndrome tyrphostin AG490 inhibits stat3-activation interleukin-2 receptor expression and growth of leukemic Sezary cells Leukemia 2001; 15: 787-93.,9Krejsgaard T, Vetter-Kauczok CS, Woetmann A. Ectopic expression of B-lymphoid kinase in cutaneous T-cell lymphoma Blood 2009; 113: 5896-904.]. Furthermore, a dominant negative form of Stat3 blocks spontaneous cytokine production and triggers apoptosis in the malignant T cells [7Sommer VH, Clemmensen OJ, Nielsen O. In vivo activation of STAT3 in cutaneous T-cell lymphoma.Evidence for an antiapoptotic function of STAT3 Leukemia 2004; 18: 1288-95.,10Nielsen M, Nissen MH, Gerwien J. Spontaneous interleukin-5 production in cutaneous T-cell lymphoma lines is mediated by constitutively activated Stat3 Blood 2002; 99: 973-7.]. Collectively, these findings suggest that Jak3/Stat3 activation plays a critical role in the tumorigenesis of CTCL. However, the molecular mechanisms underlying the aberrant Jak3/Stat3 activation remain unknown.
In non-malignant T cells, Stat activation is only transient and strictly controlled by positive and negative regulators. An important class of negative regulators of Stat activation are protein tyrosine phosphatases (PTPs). Some PTPs dephosphorylate and inactivate Jaks, whereas others dephosphorylate specific tyrosine residues in cytokine /growth factor receptors, while yet others inactivate Stats through direct dephosphorylation of critical tyrosine residues [11Chen W, Daines MO, Khurana Hershey GK. Turning off signal transducer and activator of transcription (STAT) the negative regulation of STAT signaling J Allergy Clin Immunol 2004; 114: 476-89.]. Hence, abnormal expression of PTPs is likely to be involved in the constitutive Stat activation. Accordingly, it has been shown that malignant T cells from CTCL patients often have a deficient expression of the PTP SHP-1 due to hypermethylation of the SHP-1 promoter [12Zhang Q, Raghunath PN, Vonderheid E. Lack of phosphotyrosine phosphatase SHP-1 expression in malignant T-cell lymphoma cells results from methylation of the SHP-1 promoter Am J Pathol 2000; 157: 1137-46.,13Zhang Q, Wang HY, Marzec M. STAT3- and DNA methyltransferase 1-mediated epigenetic silencing of SHP-1 tyrosine phosphatase tumor suppressor gene in malignant T lymphocytes Proc Natl Acad Sci USA 2005; 102: 6948-53.]. Interestingly, treatment of the malignant T cells with methylation inhibitors restored the SHP-1 expression and decreased the constitutive phosphorylation of Jak3 [12Zhang Q, Raghunath PN, Vonderheid E. Lack of phosphotyrosine phosphatase SHP-1 expression in malignant T-cell lymphoma cells results from methylation of the SHP-1 promoter Am J Pathol 2000; 157: 1137-46.]. However, not all CTCL cells/patients have deficient expression of SHP-1 suggesting a deficient function of other negative regulators of Jak3/Stat3 signalling [14Witkiewicz A, Raghunath P, Wasik A. Loss of SHP-1 tyrosine phosphatase expression correlates with the advanced stages of cutaneous T-cell lymphoma Hum Pathol 2007; 38: 462-7.].
CD45, also known as leukocyte-common antigen (LCA), is a transmembrane PTP expressed on all nucleated cells in the haematopoietic system and it is one of the most abundant glycoproteins on the surface of lymphoid cells [15Trowbridge IS, Thomas ML. CD45 an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development Annu Rev Immunol 1994; 12: 85-116.]. It has long been recognised that CD45 has an important positive regulatory effect on T and B cell antigen receptor mediated signalling through its ability to dephosphorylate negative regulatory tyrosine residues on Src kinases [16Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells Annu Rev Immunol 2003; 21: 107-37.]. Recently, an unexpected function of CD45 was identified: CD45 is a Jak phosphatase that negatively regulates cytokine receptor signalling [17Irie-Sasaki J, Sasaki T, Matsumoto W. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling Nature 2001; 409: 349-54.]. Consequently, dysregulation of CD45 could be implicated in Jak/Stat hyper-activity and abnormal regulation of cytokine-induced proliferation in lymphoproliferative diseases. Galectin-1, a member of the (-galactoside binding protein family, is a natural ligand of CD45 and is normally expressed in thymus, spleen, lymph nodes and bone marrow [18Perillo NL, Marcus ME, Baum LG. Galectins versatile modulators of cell adhesion cell proliferation and cell death J Mol Med 1998; 76: 402-12.-20Liu FT. Galectins a new family of regulators of inflammation Clin Immunol 2000; 97: 79-88.]. It has previously been shown that Galectin-1 can inhibit the proliferation and induce apoptosis in human T lymphocytes [21Allione A, Wells V, Forni G. Beta-galactoside-binding protein (beta GBP) alters the cell cycle, up-regulates expression of the alpha- and beta-chains of the IFN-gamma receptor and triggers IFN-gamma-mediated apoptosis of activated human T lymphocytes J Immunol 1998; 161: 2114-9.,22Perillo NL, Pace KE, Seilhamer JJ. Apoptosis of T cells mediated by galectin-1 Nature 1995; 378: 736-9.]. However, despite abundant expression of Galectin-1 in CTCL skin lesions [23Wollina U, Graefe T, Feldrappe S. Galectin fingerprinting by immuno- and lectin histochemistry in cutaneous lymphoma J Cancer Res Clin Oncol 2002; 128: 103-.,24Roberts AA, Amano M, Felten C. Galectin-1-mediated apoptosis in mycosis fungoides the roles of CD7 and cell surface glycosylation Mod Pathol 2003; 16: 543-1.], the malignant T cells exhibit constitutive activation of the Jak3/Stat3 signalling pathway and uncontrolled growth. Therefore, we decided to investigate the possible role of CD45 with respect to the aberrant Stat3 activation and proliferation of the malignant T cells in CTCL.
MATERIALS AND METHODOLOGY
Cell Lines
The malignant T-cell line MyLa2000 and the non-malignant T-cell line MyLa1928 were established from a plaque biopsy of a patient diagnosed with MF [25Kaltoft K, Bisballe S, Dyrberg T. Establishment of two continuous T-cell strains from a single plaque of a patient with mycosis fungoides In Vitro Cell Dev Biol 1992; 28A: 161-7.]. The malignant T-cell lines PB-1, 2A and 2B were established from a patient with progressive CTCL [26Wasik MA, Seldin DC, Butmarc JR. Analysis of IL-2.IL-4 and their receptors in clonally-related cell lines derived from a patient with a progressive cutaneous T-cell lymphoproliferative disorder Leuk Lymphoma 1996; 23: 125-36.]. The Jurkat T-cell line, J-Tag, has been described elsewhere [27Geisler C, Scholler J, Wahi MA. Association of the human CD3-zeta chain with the alpha beta-T cell receptor/CD3 complex.Clues from a T cell variant with a mutated T cell receptor-alpha chain J Immunol 1990; 145: 1761-7.]. The CD4+ human antigen (Ag) specific T-cell lines and peripheral blood lymphocytes (PBLs) derived from healthy donors have been described and characterized previously [28Hofmann B, Odum N, Platz P. Immunological studies in acquired immunodeficiency syndrome.Functional studies of lymphocyte subpopulations Scand J Immunol 1985; 21: 235-43.-31Nielsen M, Odum N, Bendtzen K. MHC class II molecules regulate growth in human T cells Exp Clin Immunogenet 1994; 11: 23-32.].
Flow Cytometry
For CD45 staining, 1x106 cells were harvested and washed in FACS buffer (PBS, 5% FBS, 0.1% sodium azide). Then, they were stained with phycoerythrin (PE) conjugated anti-CD45 (clone T29/33 which reacts with all CD45 isoforms) or PE conjugated isotype control (mouse IgG2b) antibodies (Abs) from Leinco (St. Louis, MO, USA) for 30 minutes at 4(C in the dark. After final washing, the cells were resuspended in FACS buffer and analysed on FACScan (Becton Dickinson, Franklin Lakes, NJ, USA). For intracellular phospho-Stat3 staining, 1x106 cells were harvested and washed in PBS. Next, the cells were fixed for 10 minutes in 2% paraformaldehyde at 37(C and permabilized in 90% ice-cold methanol for 30 minutes. Finally, the cells were washed in FACS buffer and incubated with Alexa flour 488 conjugated anti-phospho-Stat3(Y705) (4/P-STAT3 from Becton Dickinson) or Alexa flour 488 conjugated isotype control (mouse IgG2a from Leinco) Abs for 1 hour at room temperature in the dark. After thorough washing, the cells were resuspendend in FACS buffer and analysed on FACScan.
CD45 Cross-Linking and Western Blotting
Twelve-well culture plates were pre-coated with 400 μL media or rabbit anti-mouse Abs (40 μg/mL) for 16 hours at 4(C. subsequently, the plates were washed with PBS and incubated with 400μL of media, 15 μg/mL mouse anti-CD45 (clone HI30 which reacts with all CD45 isoforms) (Becton Dickinson) or 15 μg/mL isotype control (Leinco) Abs for 2 hours. After washing, 1x106 cells were added per well and incubated for 90 minutes at 37 degrees in a final volume of 1.2 mL. Then, the cells were lysed and the total cell lysates analyzed by western blotting as previously described [32Krejsgaard T, Gjerdrum LM, Ralfkiaer E. Malignant Tregs express low molecular splice forms of FOXP3 in Sezary syndrome Leukemia 2008; 22: 2230-9.]. Abs used for western blotting were anti-Erk1/2, anti-Actin, anti-CD45 (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-phospho-Stat3(Y705) (Nanotools Denzlingen, Germany).
Proliferation Assays
Assays were performed in 96-well round bottom tissue culture plates essentially as described previously [33Odum N, Martin PJ, Schieven GL. Signal transduction by HLA-DR is mediated by tyrosine kinase(s) and regulated by CD45 in activated T cells Hum Immunol 1991; 32: 85-94.]. In brief, the culture plates were pre-coated overnight with 50 μL rabbit anti-mouse Abs (40 μg/mL) at 4(C and washed extensively in PBS. subsequently, 50 μL of media, 10 μg/mL anti-CD28 mAbs (9.3) [31Nielsen M, Odum N, Bendtzen K. MHC class II molecules regulate growth in human T cells Exp Clin Immunogenet 1994; 11: 23-32.] or 10 μg/mL anti-CD45 mAbs (Leinco) were added to the wells as given and incubated for 2 hours at 37(C. Next, MyLa2000 (2.5x103), Myla1928 (2.5x104) and Ag T cells (2.5x104) were added to the wells. Following incubation for 3 hours at 37(C, media with or without IL-2 (103 U/mL) was added to a final volume of 200 μL. Finally, the cells were incubated for 120 hours and [3H]thymidine (Amersham) (1 μCi/well) was added 24 hours before harvest. The [3H]thymidine incorporation was measured in a scintillation counter and the results were expressed as mean counts per minute (Cpm) from triplicate cultures. Likewise, Jurkat or MyLa2000 T cells were cultured in 96-well round bottom tissue culture plates with or without 3.5 ìM Galectin-1 before determination of thymidine incorporation.
RESULTS
CD45 Expression is Down-Regulated on Malignant T Cells when Compared to Non-Malignant T Cells Derived from CTCL
Initially, we measured the expression of CD45 on malignant and non-malignant skin T cells derived from a CTCL plaque stage biopsy of the same patient. Interestingly, we found that the CD45 expression was strongly down-regulated on the malignant T cells when compared with the non-malignant T cells (Fig. 1A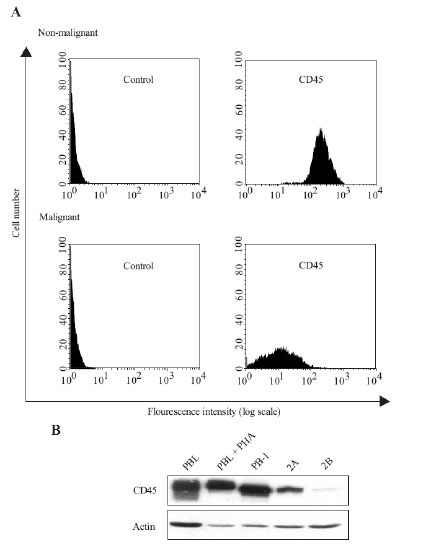 ). We also examined the expression of CD45 in the three malignant T cells (PB-1, 2A, 2B) established from another patient with CTCL. The PB-1 T-cell line is from a relatively early, clinically indolent disease stage, whereas the 2A and 2B T-cell lines are established from later and more aggressive disease stages [26Wasik MA, Seldin DC, Butmarc JR. Analysis of IL-2.IL-4 and their receptors in clonally-related cell lines derived from a patient with a progressive cutaneous T-cell lymphoproliferative disorder Leuk Lymphoma 1996; 23: 125-36.]. As seen in Fig. (1B
). We also examined the expression of CD45 in the three malignant T cells (PB-1, 2A, 2B) established from another patient with CTCL. The PB-1 T-cell line is from a relatively early, clinically indolent disease stage, whereas the 2A and 2B T-cell lines are established from later and more aggressive disease stages [26Wasik MA, Seldin DC, Butmarc JR. Analysis of IL-2.IL-4 and their receptors in clonally-related cell lines derived from a patient with a progressive cutaneous T-cell lymphoproliferative disorder Leuk Lymphoma 1996; 23: 125-36.]. As seen in Fig. (1B ), the expression of CD45 in PB-1 cells was similar to that in peripheral blood lymphocytes (PBL) and PBLs treated with phytohaemagglutinin (PHA). In contrast, the expression of CD45 was severely down-regulated in 2A and 2B cells when compared to that of PBLs and PB-1 cells (Fig. 1B
), the expression of CD45 in PB-1 cells was similar to that in peripheral blood lymphocytes (PBL) and PBLs treated with phytohaemagglutinin (PHA). In contrast, the expression of CD45 was severely down-regulated in 2A and 2B cells when compared to that of PBLs and PB-1 cells (Fig. 1B ) suggesting that CD45 expression is down-regulated during progression from indolent to aggressive disease. Because deficient expression of the PTP SHP-1 in CTCL cell lines is caused by hypermethylation of the SHP-1 promoter [12Zhang Q, Raghunath PN, Vonderheid E. Lack of phosphotyrosine phosphatase SHP-1 expression in malignant T-cell lymphoma cells results from methylation of the SHP-1 promoter Am J Pathol 2000; 157: 1137-46.], we treated the malignant T cells with the DNA methyltransferase inhibitor 5-aza-2’-deoxycytidine. However, this did not effect the CD45 expression (data not shown) indicating that down-regulation of CD45 expression is not caused by hypermethylation of the CD45 promoter.
) suggesting that CD45 expression is down-regulated during progression from indolent to aggressive disease. Because deficient expression of the PTP SHP-1 in CTCL cell lines is caused by hypermethylation of the SHP-1 promoter [12Zhang Q, Raghunath PN, Vonderheid E. Lack of phosphotyrosine phosphatase SHP-1 expression in malignant T-cell lymphoma cells results from methylation of the SHP-1 promoter Am J Pathol 2000; 157: 1137-46.], we treated the malignant T cells with the DNA methyltransferase inhibitor 5-aza-2’-deoxycytidine. However, this did not effect the CD45 expression (data not shown) indicating that down-regulation of CD45 expression is not caused by hypermethylation of the CD45 promoter.
The Constitutive Activation of Stat3 is CD45 Resistant
It has previously been documented that CD45 cross-linking inhibits common (-chain cytokine-mediated Stat3 phosphorylation [34Blank N, Kriegel M, Hieronymus T. CD45 tyrosine phosphatase controls common gamma-chain cytokine-mediated STAT and extracellular signal-related kinase phosphorylation in activated human lymphoblasts: inhibition of proliferation without induction of apoptosis J Immunol 2001; 166: 6034-40.]. Accordingly, we investigated if CD45 cross-linking could inhibit the constitutive activation of Stat3 in the malignant T cells. However, as seen in Fig. (2A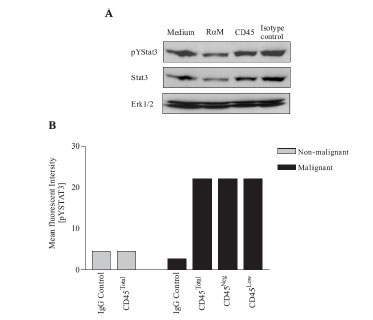 ), cross-linking of CD45 using an anti-CD45 mAb had no inhibitory effect on the level of phosphorylated Stat3 (pYStat3) in the malignant T cells. The expression of CD45 was relatively heterogeneous in the population of malignant T cells (Fig. 1
), cross-linking of CD45 using an anti-CD45 mAb had no inhibitory effect on the level of phosphorylated Stat3 (pYStat3) in the malignant T cells. The expression of CD45 was relatively heterogeneous in the population of malignant T cells (Fig. 1 ). Some cells were essentially CD45 negative (CD45Neg) while others had low expression of CD45 (CD45Low). Therefore, we examined whether CD45 expression was inversely correlated with pYStat3. As seen in Fig. (1A
). Some cells were essentially CD45 negative (CD45Neg) while others had low expression of CD45 (CD45Low). Therefore, we examined whether CD45 expression was inversely correlated with pYStat3. As seen in Fig. (1A ), the level of pYStat3 was similar in CD45Neg and CD45Low cells and, additionally, both resembled pYStat3 levels of the total cell population (CD45Total). Thus, the low levels of CD45 expressed on the malignant T cells were not sufficient to counteract the aberrant activity of Stat3. This prompted us to explore if higher levels of CD45 expression could suppress the constitutive activation of Stat3. Consequently, we transfected the malignant T cells with full length CD45, a truncated form of CD45 or an empty vector and subsequently tried to measure the level of pYStat3 in the transfected cells. However, the level of pYStat3 could not be reliably determined in cells transfected with full length CD45 as the majority (>95%) died shortly after transfection (data not shown). In contrast, transfection with truncated CD45 or an empty control vector did not induce cell death in the malignant T cells.
), the level of pYStat3 was similar in CD45Neg and CD45Low cells and, additionally, both resembled pYStat3 levels of the total cell population (CD45Total). Thus, the low levels of CD45 expressed on the malignant T cells were not sufficient to counteract the aberrant activity of Stat3. This prompted us to explore if higher levels of CD45 expression could suppress the constitutive activation of Stat3. Consequently, we transfected the malignant T cells with full length CD45, a truncated form of CD45 or an empty vector and subsequently tried to measure the level of pYStat3 in the transfected cells. However, the level of pYStat3 could not be reliably determined in cells transfected with full length CD45 as the majority (>95%) died shortly after transfection (data not shown). In contrast, transfection with truncated CD45 or an empty control vector did not induce cell death in the malignant T cells.
Malignant, But Not Non-Malignant, T Cells is Resistant to CD45 Mediated Inhibition of Proliferation
Recently, Blank et al. [34Blank N, Kriegel M, Hieronymus T. CD45 tyrosine phosphatase controls common gamma-chain cytokine-mediated STAT and extracellular signal-related kinase phosphorylation in activated human lymphoblasts: inhibition of proliferation without induction of apoptosis J Immunol 2001; 166: 6034-40.] reported that cross-linking of CD45 by mAbs inhibits common (-chain cytokine mediated proliferation of activated human lymphoblasts. Therefore, we examined if CD45 cross-linking could inhibit the proliferation of the malignant T cells. As shown in Fig. (3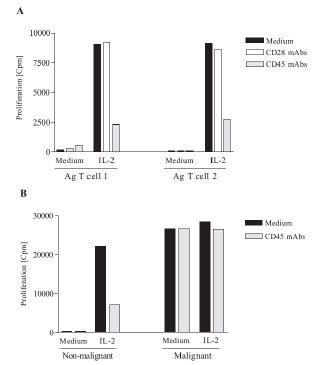 ), CD45 cross-linking by an immobilised anti-CD45 mAb mediated a profound inhibition of IL-2-induced proliferation of antigen-specific (Ag) T cells from healthy donors (Fig. 3A
), CD45 cross-linking by an immobilised anti-CD45 mAb mediated a profound inhibition of IL-2-induced proliferation of antigen-specific (Ag) T cells from healthy donors (Fig. 3A ) and likewise in non-malignant T cells from a CTCL patient (Fig. 3B
) and likewise in non-malignant T cells from a CTCL patient (Fig. 3B , left). The inhibition was specific, as an anti-CD28 mAb of same isotype had no inhibitory effect. Similar inhibition was observed with different CD45 mAbs indicating that this was a general feature of CD45 Abs (data not shown). Importantly, CD45 cross-linking had no effect on the proliferation of the malignant T cells (Fig. 3B
, left). The inhibition was specific, as an anti-CD28 mAb of same isotype had no inhibitory effect. Similar inhibition was observed with different CD45 mAbs indicating that this was a general feature of CD45 Abs (data not shown). Importantly, CD45 cross-linking had no effect on the proliferation of the malignant T cells (Fig. 3B , right). Galectin-1 is a natural CD45 ligand which has been shown to be abundantly expressed in CTCL skin lesions [23Wollina U, Graefe T, Feldrappe S. Galectin fingerprinting by immuno- and lectin histochemistry in cutaneous lymphoma J Cancer Res Clin Oncol 2002; 128: 103-.,24Roberts AA, Amano M, Felten C. Galectin-1-mediated apoptosis in mycosis fungoides the roles of CD7 and cell surface glycosylation Mod Pathol 2003; 16: 543-1.]. It has previously been published that Galectin-1 can inhibit the proliferation of T cells [21Allione A, Wells V, Forni G. Beta-galactoside-binding protein (beta GBP) alters the cell cycle, up-regulates expression of the alpha- and beta-chains of the IFN-gamma receptor and triggers IFN-gamma-mediated apoptosis of activated human T lymphocytes J Immunol 1998; 161: 2114-9.], and as expected, Galectin-1 inhibited the proliferation of Jurkat T cells (Fig. 4
, right). Galectin-1 is a natural CD45 ligand which has been shown to be abundantly expressed in CTCL skin lesions [23Wollina U, Graefe T, Feldrappe S. Galectin fingerprinting by immuno- and lectin histochemistry in cutaneous lymphoma J Cancer Res Clin Oncol 2002; 128: 103-.,24Roberts AA, Amano M, Felten C. Galectin-1-mediated apoptosis in mycosis fungoides the roles of CD7 and cell surface glycosylation Mod Pathol 2003; 16: 543-1.]. It has previously been published that Galectin-1 can inhibit the proliferation of T cells [21Allione A, Wells V, Forni G. Beta-galactoside-binding protein (beta GBP) alters the cell cycle, up-regulates expression of the alpha- and beta-chains of the IFN-gamma receptor and triggers IFN-gamma-mediated apoptosis of activated human T lymphocytes J Immunol 1998; 161: 2114-9.], and as expected, Galectin-1 inhibited the proliferation of Jurkat T cells (Fig. 4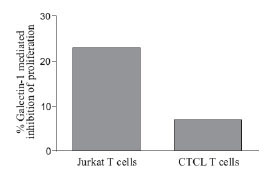 ). However, in line with our previous findings, the malignant T cells from CTCL were less sensitive to Galectin-1 mediated suppression of proliferation (Fig. 4
). However, in line with our previous findings, the malignant T cells from CTCL were less sensitive to Galectin-1 mediated suppression of proliferation (Fig. 4 ). Collectively, these results indicate that CD45 down-regulation can protect the malignant T cells from CD45-mediated inhibition of proliferation.
). Collectively, these results indicate that CD45 down-regulation can protect the malignant T cells from CD45-mediated inhibition of proliferation.
DISCUSSION
In the present study, we show that CD45 expression is down-regulated on malignant T cells when compared to non-malignant T cells established from CTCL skin lesions. A finding which is in agreement with previous observations [35Clavio M, Rossi E, Truini M. Anaplastic large cell lymphoma a clinicopathologic study of 53 patients Leuk Lymphoma 1996; 22: 319-27.-37Ralfkiaer E, Thomsen K, Vejlsgaard GL. Expression of a cell adhesion protein (VLA beta) in normal and diseased skin Br J Dermatol 1991; 124: 527-32.]. Furthermore, our results suggest that the down-regulation of CD45 expression primarily occurs during progression from indolent to more aggressive disease stages. Loss of CD45 expression has also been reported in other haematological malignancies such as acute lymphoblastic leukaemia [38Ratei R, Sperling C, Karawajew L. Immunophenotype and clinical characteristics of CD45-negative and CD45-positive childhood acute lymphoblastic leukemia Ann Hematol 1998; 77: 107-4.] chronic lymphocytic leukemia [39Dang AM, Phillips JA, Lin T. Raveche ES.Altered CD45 expression in malignant B-1 cells Cell Immunol 1996; 169: 196-207.] and Hodgkin’s lymphoma [40Ozdemirli M, Mankin HJ, Aisenberg AC. Hodgkin's disease presenting as a solitary bone tumor.A report of four cases and review of the literature Cancer 1996; 77: 79-88.] indicating that down-regulation of CD45 expression could play a role in the development and pathology of various haematological malignancies.
CD45 can inhibit Jak/Stat activation and cytokine-induced proliferation in lymphocytes [17Irie-Sasaki J, Sasaki T, Matsumoto W. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling Nature 2001; 409: 349-54.,34Blank N, Kriegel M, Hieronymus T. CD45 tyrosine phosphatase controls common gamma-chain cytokine-mediated STAT and extracellular signal-related kinase phosphorylation in activated human lymphoblasts: inhibition of proliferation without induction of apoptosis J Immunol 2001; 166: 6034-40.]. Accordingly, we found that cross-linking of CD45 resulted in a profound inhibition of IL-2-induced proliferation of Ag T cells from healthy donors and non-malignant T cells from a CTCL patient. In contrast, CD45 cross-linking did not inhibit the proliferation of the malignant T cells. Likewise, CD45 cross-linking did not inhibit the constitutive activation of Stat3 in the malignant T cells and we found no correlation between the level of activated Stat3 and CD45 expression on the malignant T cells. Thus, our results suggest that the uncontrolled proliferation and aberrant Stat3 activation observed in the malignant T cells is resistant to CD45 cross-linking. This resistance could either be a consequence of the low CD45 expression, a dysfunction of CD45 or both. In vivo, CD45 cross-linking could be achieved through binding to one of its putative physiologic ligands, such as Galectin-1. Galectin-1 is highly expressed in CTCL skin lesions [23Wollina U, Graefe T, Feldrappe S. Galectin fingerprinting by immuno- and lectin histochemistry in cutaneous lymphoma J Cancer Res Clin Oncol 2002; 128: 103-.,24Roberts AA, Amano M, Felten C. Galectin-1-mediated apoptosis in mycosis fungoides the roles of CD7 and cell surface glycosylation Mod Pathol 2003; 16: 543-1.]
indicating that CTCL cells are exposed to agents capable of cross-linking CD45 in vivo. Thus, CD45 resistance could give the malignant T cells a growth advantage by protecting them from CD45 mediated inhibition of proliferation. In line with this idea, Asosingh et al. [41Asosingh K, De RH, Croucher P. In vivo homing and differentiation characteristics of mature (CD45-) and immature (CD45+) 5T multiple myeloma cells Exp Hematol 2001; 29: 77-84.] found that CD45 negative cells have a higher proliferative rate compared with CD45 positive cells in a murine model of Multiple Myeloma (MM). Furthermore, insulin-like growth factor 1 (IGF-1) has been shown to induce proliferation of human MM cells [42Ge NL, Rudikoff S. Insulin-like growth factor I is a dual effector of multiple myeloma cell growth Blood 2000; 96: 2856-61.]. Induction of CD45 expression in such cells inhibit IGF-1 signalling [43Descamps G, Pellat-Deceunynck C, Szpak Y. The magnitude of Akt/phosphatidylinositol 3'-kinase proliferating signaling is related to CD45 expression in human myeloma cells J Immunol 2004; 173: 4953-9.] indicating that down-regulation of CD45 expression facilitates IGF-1 mediated proliferation of MM cells.
Inhibition of Stat3 activity has been shown to induce apoptosis in malignant T cells from CTCL [7Sommer VH, Clemmensen OJ, Nielsen O. In vivo activation of STAT3 in cutaneous T-cell lymphoma.Evidence for an antiapoptotic function of STAT3 Leukemia 2004; 18: 1288-95.]. Accordingly, we speculated that forced expression of CD45 in the malignant T cells would inhibit the activity of Stat3 and trigger apoptosis. In agreement with this hypothesis, the malignant T cells died shortly after transfection with full length CD45 but not a truncated form of CD45 or an empty vector. However, due to the quick induction of cell death, we were not experimentally able to resolve if the cell death was preceded by an inhibition of Stat3 activity. Therefore, we can’t exclude that the cell death was triggered by events independently of CD45 function or by technicalities.
In conclusion, our results suggest that CD45 dysregulation might play a role in the aberrant proliferation and Jak3/Stat3 activation in CTCL.
ACKNOWLEDGEMENTS
This work was supported by grants from The University of Copenhagen, The Danish Research Councils, The Foundation of 17-12-1981, The Novo Nordic Foundation, The Danish Cancer Society, The Neye Foundation, The Lundbeck Foundation, and The National Cancer Institute (CA89194: MA Wasik). We wish to thank Keld Kaltoft (Århus University and CellCure Århus, Denmark) for the generous gift of the MyLa cell lines. The project part concerning establishment and study of CTCL cell lines by Dr. Keld Kaltoft has been approved by "Den videnskabsetiske Kommite i Århus Amt" (The science-ethical committee in Århus County).




