- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Ornithology Journal
(Discontinued)
ISSN: 1874-4532 ― Volume 13, 2020
Recruitment Rates, Natal and Breeding Dispersal of Montagu’s Harriers (Circus Pygargus) by Means of Microsatellite Analysis
Susann Janowski1, *, Claudia Pürckhauer2, Ralf Krüger3, Dieter Thomas Tietze4, Michael Wink1
Abstract
Introduction:
Adult philopatry as well as juvenile dispersal and recruitment rates are key factors for population development. We investigated these questions for the first time in an increasing German population of Montagu’s harrier in Frankonia using microsatellite markers.
Methods:
By means of 16 loci, we genotyped 2265 samples from juvenile and adult female Montagu’s harriers. Parentage and identity tests were used to reconstruct life histories of birds for a 10 year period. Most of the birds were breeding in one or two years. The longest life history was eight years.
Results:
Adult philopatry was quite high and differed significantly between sexes. We found 73.5% of females to breed < 5 km around the previous nest site (80.4% < 10 km, median nesting distance 2.1 km). All investigated males (n=18) were breeding in a distance of < 5 km (median nesting distance 1.3 km) to the previous nest. Juveniles showed a low recruitment rate (females: 2.9%, males: 4.9%, together 4%). Median natal dispersal distance was 19.1 km for females and 12.3 km for males. We found 29.4% of females and 41.2% of males to be philopatric, as the distance between hatching and first breeding site was < 10 km. Philopatry results mostly agree with data from other European countries.
Discussion:
Due to strict marker and data selection we received high quality life histories of Montagu’s harriers, which demonstrate that microsatellite analyses are valuable tools in ornithology.
Conclusion:
Nevertheless, comparison of philopatry and recruitment rates depend directly on the scale used and investigation method and therefore remain a challenge.
Article Information
Identifiers and Pagination:
Year: 2018Volume: 11
First Page: 39
Last Page: 55
Publisher Id: TOOENIJ-11-39
DOI: 10.2174/1874453201811010039
Article History:
Received Date: 17/5/2018Revision Received Date: 21/9/2018
Acceptance Date: 9/10/2018
Electronic publication date: 22/11/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Department of Biology, Institute of Pharmacy and Molecular Biotechnology, Heidelberg University, Im Neuenheimer Feld 364, D-69120 Heidelberg, Germany, Tel: 0049-6221-54-4847; E-mails: Susann.Janowski@outlook.com; wink@uni-heidelberg.de
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 17-5-2018 |
Original Manuscript | Recruitment Rates, Natal and Breeding Dispersal of Montagu’s Harriers (Circus Pygargus) by Means of Microsatellite Analysis | |
1. INTRODUCTION
Montagu’s harrier (Circus pygargus, Linnaeus, 1758) is one of the most flexible and adaptive migrating raptors in our world and hence in focus of scientific interest. It breeds in Europe and western Asia, but winters six to eight months in semi-arid open habitats of West, East and South Africa, south of the Saharan desert as well as on the Indian sub-continent [1Clarke R. Montagu’s harrier 1996., 2Trierweiler C, Koks B. Montagu’s harrier Circus pygargus. In: Zwarts L, Bijlsma R.G., van der Kamp J. and Wymenga E., Eds. Living on the Edge: Wetlands and Birds in a Changing Sahel. Zeist, KNNV Publishing 2009; pp. 312-27.]. The species is a traditional wetland breeder and used to build its nest in steppe-like grasslands [3Glutz Von Blotzheim U, Bauer KM, Bezzel E. Handbuch der Vögel Mitteleuropas. Akademische Verlagsgesellschaft Frankfurt/Main 1971; Band 4 Falconiformes.], salt meadows [4Sanchez-Zapata JA, Carrete M, Gravilov A, et al. Land use changes and raptor conservation in steppe habitats of Eastern Kazakhstan. Biol Conserv 2003; 111: 71-7.
[http://dx.doi.org/10.1016/S0006-3207(02)00251-3] ] and heathlands [1Clarke R. Montagu’s harrier 1996.]. Since the beginning of the 20th century, breeding in grain fields was observed [5Frionnet C. Les oiseaux de la Haute-Marne. Bulletin de la Société Sciences Naturelles: Haute-Marne (Chaumont) 1925.], which has become the most preferred vegetation type in Europe today [6Arroyo B. Breeding ecology and nest dispersion of Montagu's harrier Circus pygargus in Central Spain. PhD thesis 1995.]. In Europe, the species breeds patchily distributed and is regionally altogether absent [3Glutz Von Blotzheim U, Bauer KM, Bezzel E. Handbuch der Vögel Mitteleuropas. Akademische Verlagsgesellschaft Frankfurt/Main 1971; Band 4 Falconiformes.]. BirdLife International [7 Montagu's Harrier Circus pygargus. Bird Life International; Species factsheet 2014; Downloaded from: http://www.birdlife.org.] classifies Montagu’s harrier population as “least concern”. Nevertheless, many European populations suffer from extensive agriculture, which includes nest destruction, loss of prey, over-use of pesticides as well as improved locust control in wintering areas [8Ferguson-Lees J, Christie DA. Raptors of the World (Helm Identification Guides) 1st ed.2001.]. In Europe, the survival of the ground nesting Montagu’s harrier strongly depends on human conservation management, especially nest protection.
Conservation of a migrating species is a challenge, since research in breeding, wintering and migration areas need to be combined. Montagu’s harrier has been studied by several research teams over the last decades e.g. [6Arroyo B. Breeding ecology and nest dispersion of Montagu's harrier Circus pygargus in Central Spain. PhD thesis 1995., 9Arroyo B, García JT, Bretagnolle V. Conservation of the montagu’s harrier (Circus pygargus) in agricultural areas. Anim Conserv 2002; 5: 283-90.
[http://dx.doi.org/10.1017/S1367943002004031] -22Wiącek J. Benefits and costs of semi-colonial breeding in the Montagu’s harrier Circus pygargus. Belg J Zool 2008; 138: 36-40.]. Research mainly focused on ecology and breeding biology. Especially migration routes have been extensively investigated [23Arroyo B, García JT. Migratory movements of Western European Montagu’s harrier Circus pygargus: A review. Bird Study 1998; 45: 188-94.
[http://dx.doi.org/10.1080/00063659809461090] -28Trierweiler C, Koks BJ, Drent RH, et al. Satellite tracking of two montagu’s harriers (Circus pygargus): Dual pathways during autumn migration. J Ornithol 2007; 148: 513-6.
[http://dx.doi.org/10.1007/s10336-007-0178-5] ]. To study population dynamics, which is important for effective conservation management, many different aspects like spatial distribution, genetic structure and connectivity between breeding areas need to be considered. Key ecological questions include natal and breeding dispersal and philopatry as well as juvenile recruitment rates. The recruitment rate of a population describes the proportion of juvenile birds that return to their breeding area for breeding. Natal dispersal is defined as the movement of juveniles from the place of birth to the place of their first reproduction attempt. Breeding dispersal (or adult philopatry) concerns the movement of adults that had reproduced in one year to a breeding site in the following years [29Greenwood PJ, Harvey PH. The natal and breeding dispersal of birds. Annu Rev Ecol Syst 1982; 13: 1-21.
[http://dx.doi.org/10.1146/annurev.es.13.110182.000245] , 30Greenwood PJ. Mating systems, philopatry and dispersal in birds and mammals. Anim Behav 1980; 28: 1140-62.
[http://dx.doi.org/10.1016/S0003-3472(80)80103-5] ]. Juvenile recruitment, as well as natal and breeding dispersal can influence the genetic structure of populations substantially, since a more or less intensive geographic exchange of individuals lead to connection or isolation of neighbouring groups. Dispersal prevents inbreeding by gene flow, plays a key role in range expansion of metapopulations and influences source-sink dynamics in patchily distributed populations [30Greenwood PJ. Mating systems, philopatry and dispersal in birds and mammals. Anim Behav 1980; 28: 1140-62.
[http://dx.doi.org/10.1016/S0003-3472(80)80103-5] , 31Paradis E, Baillie SR, Sutherland WJ, Gregory RD. Patterns of natal and breeding dispersal in birds. J Anim Ecol 1998; 67: 518-36.
[http://dx.doi.org/10.1046/j.1365-2656.1998.00215.x] ]. Both, natal and breeding dispersal differs between species in its specification, because it is influenced by habitat requirements, social system, geographical range and migratory status of the discussed species [31Paradis E, Baillie SR, Sutherland WJ, Gregory RD. Patterns of natal and breeding dispersal in birds. J Anim Ecol 1998; 67: 518-36.
[http://dx.doi.org/10.1046/j.1365-2656.1998.00215.x] ]. Consequently, knowledge about dispersal and philopatry behaviour is of paramount importance to evaluate conservation status and management strategies of declining or threatened species.
Adult philopatry and natal dispersal data are still missing for many species, including the Montagu’s harrier. Long-term and large-scale observations are needed, which traditionally require mark and recapture methods (ringing, wing-tagging, radio and satellite telemetry). In recent times, the analysis of genetic markers has been established as powerful tools for scientific research including conservation issues [32Davis LA, Roalson EH, Cornell KL, McClanahan KD, Webster MS. Genetic divergence and migration patterns in a north american passerine bird: Implications for evolution and conservation. Mol Ecol 2006; 15(8): 2141-52.
[http://dx.doi.org/10.1111/j.1365-294X.2006.02914.x] [PMID: 16780431] -36Keller LF, Jeffery KJ, Arcese P, et al. Immigration and the ephemerality of a natural population bottleneck: Evidence from molecular markers. Proc Biol Sci 2001; 268(1474): 1387-94.
[http://dx.doi.org/10.1098/rspb.2001.1607] [PMID: 11429139] ].
In this study, we provide evidence from microsatellite analysis for dispersal, philopatry and recruitment in an expanding population of Montagu’s harriers in Mainfranken, Germany. Genetic analyses were conducted by means of 16 recently isolated highly informative microsatellite loci (STRs - short-tandem-repeats) (Janowski et al., 2014). Paternity and identity analyses were used to reveal life histories for individual birds.
2. MATERIALS AND METHODS
2.1. The Breeding Population in Mainfranken, Germany
Montagu’s harrier is a regionally threatened bird species in Germany and therefore included in the Red List of Threatened Species to category 2 [37Grüneberg C, Bauer H-G, Haupt H, et al. Rote Liste der Brutvögel Deutschlands. 5. Fassung. Berichte zum Vogelschutz 2016; 52: 19-67.]. In a long-term trend, the German breeding population is apparently growing, primarily due to effective nest protection regimes [38Südbeck P, Bauer H-G, Boschert M, Boye P, Knief W. Rote Liste der Brutvögel Deutschlands. 4. Fassung. Berichte zum Vogelschutz 2007; 44: 23-81., 39Stiefel D. Zur Situation der Wiesenweihe Circus pygargus in Deutschland. Tagung zum Wiesenweihenschutz in der Agrarlandschaft 2010; 46: 18-27.]. Illner 2017 [40Illner H. Brutbestände der Wiesenweihe Circus pygargus und Nestschutz-Maßnahmen in Deutschland 2003 bis 2014. Vogelwelt 2017; 137: 1-13.] summarises that the number of breeding females increased by 15% from an average of 400 in 2004-2007 to an average of 485 in 2011-2014 (estimates of undiscovered broods are included).
Among German populations of Montagu’s harrier, the breeding population in Mainfranken (Bavarian administrative district Unterfranken) is the largest and most successful one with 143 breeding pairs and 254 fledged chicks in 2016 [41Pürckhauer C. Artenhilfsprogramm Wiesenweihe (Circus pygargus) in Bayern. Landesbund für Vogelschutz in Bayern e.V. (LBV). Jahresbericht 2016.]. The breeding population is patchily distributed in a radius of about 40 km around the village Volkach 49°52′N, 10°14′E between the centres Würzburg 49°46′N, 09°56′E in the south and Schweinfurt 50°03′N, 10°14′E in the north. The landscape is characterized by intensively used agricultural habitats, with open and large grain fields. The exact locations of individual breeding sites vary in consecutive years. Since 1999, a conservation program for Montagu’s harriers has been organized by the Bavarian Environment Agency, the Bavarian Association for Bird Protection, the Bavarian State Ministry for Environment and Health and many volunteering bird conservationists. This conservation program is apparently successful as breeding pair numbers are increasing. Montagu’s harrier is now listed in the Red List of Threatened Species of Bavaria to category “extreme rare species and species with geographical restriction” [42Rudolph B-U, Schwandner J, Fünfstück H-J. Red list and list of breeding birds of Bavaria. Bavarian State Office for the Environment (LfU) 2016.].
Montagu’s harriers mainly breed on the ground in fields used to grow cereals. Similar to the situation in other European countries, nest protection can be ensured by leaving an undisturbed area of 50 by 50 m around a nest (i.e. no mowing activity) [43Belting C, Krüger RM. Populationsentwicklung und Schutzstrategien für die Wiesenweihe Circus pygargus in Bayern. Ornithologischer Anzeiger 2002; 41: 87-92.-47Gahrau C, Schmüser H. Artenschutzprojekt Wiesenweihe (Circus pygargus) des Landes Schleswig-Holstein - Brutperiode 2007. Im Rahmen des Monitoringprojekts: Wildtierkataster Schleswig-Holstein 2007.]. Additionally to conservation, a research program was initiated in 2000. Since then, all nests were searched, chicks ringed and marked with wing-tags for identification in future years. Furthermore, blood samples from chicks were collected during the ringing process for genetic analyses and GPS data were recorded of each nest.
2.2. Sample Collection
When visiting harrier nests for ringing between 2000 and 2012, we also took blood samples from chicks by puncturing their brachial vein on one of the two wings. Samples were stored in EDTA buffer {10% EDTA, 0.5% NaF, 0.5% thymol, 1%, Tris-HCL, pH = 7.5 [48Nader W, Werner D, Wink M. Genetic diversity of scarlet macaws Ara macao in reintroduction studies for threatened populations in Costa Rica. Biol Conserv 1999; 87: 269-72.
[http://dx.doi.org/10.1016/S0006-3207(98)00043-3] ]} at 4°C until DNA extraction. Overall, 2068 blood samples were obtained.
For paternity analyses, we furthermore collected blood samples from incubating adult females between May and end of July in the years 2009 to 2012. To minimize disturbance of incubating birds, we did not catch them but obtained blood samples via the “bug method”: Dummy eggs were picked with triatomine bugs to collect blood from an incubating female. Larvae of Dipetalogaster maxima Uhler, 1894 (Heteroptera, Reduviidae) were obtained commercially from G. Schaub (Ruhr University Bochum, Germany). A single third instar larva was placed in an artificial harrier egg (made by R. Nagel, Institute of Avian Research (Vogelwarte Helgoland) Wilhelmshaven, Germany) and put for at least 4 h in a harrier clutch Incubating females quickly return for incubation. As eggs come into close contact with the skin, the bugs can target blood vessels of the female through small holes in the egg. This non-invasive method is widely used to sample blood of sensitive, wild or captured animals [49Arnold JM, Oswald SA, Voigt CC, et al. Taking the stress out of blood collection: Comparison of field blood-sampling techniques for analysis of baseline corticosterone. J Avian Biol 2008; 39: 588-92.
[http://dx.doi.org/10.1111/j.0908-8857.2008.04265.x] -55Stadler A, Meiser CK, Schaub GA. “Living syringes”: Use of hematophagous bugs as blood samplers from small and wild animals. Parasitology Research 2011; 243-71.]. For more information about this method see description in Janowski et al. 2014 [56Janowski S, Grohme MA, Frohme M, Wink M. Development of New Microsatellite (STR) Markers for Montagu’s harrier (Circus pygargus) via 454 Shot-Gun Pyrosequencing. Open Ornithol J 2014; 7: 11-8.
[http://dx.doi.org/10.2174/1874453201407010011] ]. Blood was removed from the bug with a small syringe and stored in an EDTA buffer and cooled to 4°C for later use. DNA remained intact and was not contaminated by insect DNA in this procedure. Altogether 197 blood samples from adult females were collected in that way.
2.3. Sample Processing and Genotyping
DNA was isolated from blood following a standard protocol with proteinase K digestion (Merck, Darmstadt) and phenol-chloroform extraction [57Sambrook J, Fritsch EF, Maniatis T. Moleculer clooning: A labaratuary manual 1989.] for genotyping.
Altogether, 2265 samples from chicks and adult females were genotyped with 19 STR primer pairs that were recently isolated for Montagu’s harrier using next-generation sequencing [56Janowski S, Grohme MA, Frohme M, Wink M. Development of New Microsatellite (STR) Markers for Montagu’s harrier (Circus pygargus) via 454 Shot-Gun Pyrosequencing. Open Ornithol J 2014; 7: 11-8.
[http://dx.doi.org/10.2174/1874453201407010011] ]. These 19 microsatellite loci were amplified with three multiplex PCR sets for high throughput genotyping by capillary array electrophoresis using the MegaBACE 1000 system of Amersham Biosciences (detailed description in Janowski et al., 2014) [56Janowski S, Grohme MA, Frohme M, Wink M. Development of New Microsatellite (STR) Markers for Montagu’s harrier (Circus pygargus) via 454 Shot-Gun Pyrosequencing. Open Ornithol J 2014; 7: 11-8.
[http://dx.doi.org/10.2174/1874453201407010011] ]. In the process of primer development and multiplex arrangement, 10 samples of the same juveniles were genotyped several times (see primer development in Janowski et al., 2014 ) [56Janowski S, Grohme MA, Frohme M, Wink M. Development of New Microsatellite (STR) Markers for Montagu’s harrier (Circus pygargus) via 454 Shot-Gun Pyrosequencing. Open Ornithol J 2014; 7: 11-8.
[http://dx.doi.org/10.2174/1874453201407010011] ]. Furthermore, 192 samples from adult females were genotyped two times. This repetition was carried out as a quality check for amplification consistency in multiplex PCR and reproducibility. Rounding of decimal values (manual binning of peaks to allele-units) was performed as described in Janowski et al., 2014 [56Janowski S, Grohme MA, Frohme M, Wink M. Development of New Microsatellite (STR) Markers for Montagu’s harrier (Circus pygargus) via 454 Shot-Gun Pyrosequencing. Open Ornithol J 2014; 7: 11-8.
[http://dx.doi.org/10.2174/1874453201407010011] ].
All juvenile Montagu’s harriers were genetically sexed using the primers 1237L (sequence 5‘-3‘: GAGAAACTGTGCAAAACAG) and 1272H (sequence 5‘-3‘: TCCAGAATATCTTCTGCTCC) to amplify an intron region in the CHD-gene [58Kahn NW, St.John J, Quinn TW. Chromosome specific intron size difference in the avian CHD gene provide an efficient method for sex identification in birds. Auk 1998; 115: 1074-8.
[http://dx.doi.org/10.2307/4089527] ]. Males show one and females two sex-specific alleles. PCR was conducted with radioactively labelled nucleotides (33P-α-dATP) under the following conditions: A 25 µL reaction volume contained 60 ng of isolated DNA, 10 pmol/µL of each forward and reverse primer, 2.5 µL of a 10x PCR buffer (Bioron), a nucleotide mix containing 0.1 mM of dGTP, dCTP and dTTP, as well as 45 µM of dATP, 0.15 units of Top-Taq DNA polymerase (Bioron, Ludwigshafen), 1 µCi [α-33P]-dATP (Perkin Elmer) and a variable amount of mono distilled water to reach the end volume of 25 µL. Thermocycling was performed in a Tgradient ThermoCycler (Biometra, Göttingen) under the following conditions: Initial denaturing step for 2 min at 94°C, 38 cycles of 30 sec at 94°C, 60 sec at 56°C and 2 min at 72°C, followed by a final extension step for 10 min at 72°C and a pause step at 16°C for storage. After denaturation of PCR products at 95 °C for 5 min, they were separated by vertical high-resolution Polyacrylamide Gel Electrophoresis (PAGE) (containing 5% urea) at 65 W for 1.5 h (run length approximately 40 cm). The gel was dried and analysed by autoradiography using an X-ray film (Fujifilm Super RX).
2.4. Data Selection, Identity Analyses and Parentage Assessments
In order to evaluate the reliability of paternity and identity tests, all 19 loci had to be characterized using the software Cervus 3.0 [59Kalinowski ST, Taper ML, Marshall TC. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 2007; 16(5): 1099-106.
[http://dx.doi.org/10.1111/j.1365-294X.2007.03089.x] [PMID: 17305863] ]. We randomly chose 444 samples from juvenile Montagu’s harriers for characterization tests. Only loci with best assessed values were selected for paternity and identity analyses. Furthermore, a most conservative approach of strict data selection was applied to allow for accurate identification of individuals: All identity and parentage analyses were performed only with fully genotyped samples. Moreover, only families consisting of at least three nest mates (number of eggs per nest in Mainfranken is between two and seven, mostly four to six) were included in parentage tests. These criteria should ascertain correct assignment results. Altogether 166 adult females and 1290 juvenile Montagu’s harriers remained for paternity and identity tests after samples were removed which did not fulfil our quality standards.
Cervus 3.0 [59Kalinowski ST, Taper ML, Marshall TC. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 2007; 16(5): 1099-106.
[http://dx.doi.org/10.1111/j.1365-294X.2007.03089.x] [PMID: 17305863] ] was used to identify identical adult females that could have been sampled repeatedly 2009-2012 by chance (via the bug method). We found 126 adult females for which we had matching genotypes sampled in different years. Furthermore, genotypes of adult females were compared with all female chicks to find individuals which had been sampled previously as chicks. Consequently, discovered hatching years could be used for life history assessment and philopatry analyses. Only 100% identical genotypes were treated as identical.
Parentage analyses were conducted by software Colony 2.0 [60Jones OR, Wang J. COLONY: A program for parentage and sibship inference from multilocus genotype data. Mol Ecol Resour 2010; 10(3): 551-5.
[http://dx.doi.org/10.1111/j.1755-0998.2009.02787.x] [PMID: 21565056] ]. The remaining 1290 samples from chicks comprised 585 females, 691 males and 14 individuals without successful genetic sexing. Our 126 adult females and all juvenile females represented potential mothers in parentage assignments. Since lack of samples from adult males, juvenile males were treated as potential fathers. The following adjustments were considered for a Colony run: We assumed a polygamous mating system without inbreeding, because samples had been collected successively over years. Hence, parents could have produced chicks in different breeding seasons. We chose a medium run length, a full-likelihood method and no sibship prior. We neither gave information about known paternal or maternal sibs, nor excluded paternity, maternity and paternal or maternal sibs, respectively. Consequently, parentage was assigned only by genotyping data. Results for parentage assignment were taken from the “Best Configuration” output-file. An important advantage of Colony programme is the possibility of assigning parentage to sibs, without a corresponding parental genotype. If Colony determines that two or more siblings share the same parent, but a corresponding genotype is missing in the parent data file, the reconstructed genotype of mother and/or father appears with synonym. An allocated mother is presented with a ‘#’ followed by a serial number and a father with a ‘*’ and a serial number, respectively. Thus, it is possible to identify full and half sibs. To check parentage results for assignment mistakes, we compared them with expected full sibs, according to nest number and hatching year.
After paternity tests, we continued with strict data selection. Only clutches, where calculated relationships matched expected ones (checking for ring numbers) and where at least one parent was unambiguously identified were used for further analyses. Furthermore, families were controlled for allele-mismatches between chicks and assigned parents. Allele-mismatches which were not related to interpretation mistakes of peaks or transcription errors led to exclusion of the corresponding sample. Comparison of known mother-chick relationships (where adult females were sampled via the bug-method) revealed no assignment mistakes. This very conservative way of data selection reduced the number of samples to 1276 chicks and 123 adult females.
2.5. Analyses and Statistics
GPS data from nest locations and life histories of individual birds were used to assess philopatry and dispersal. Distances between nests were directly calculated by GPS data in Excel. Life histories were reconstructed for single birds by a ’01-matrix’, where parentage proofs appeared with ‘1’ (else ‘0’). Of course, life histories include times with frequent breeding observations and times where one or more years were without breeding evidence. Since we lack information about ‘missing years’ (brood damage, intermission, breeding outside sampling area or detection failure of broods), adult philopatry was only calculated for birds breeding in consecutive years. An F-test was calculated to test for unequal variance in nesting distances within the sample group, due to unequal sample size of both sexes. Comparison of adult philopatry (nesting distances) between both sexes was hence calculated with a two sided t-test with unequal variances.
Mean age of first breeding was compared between males and females by first testing for unequal variances (F-test) followed by a two sided t-test with unequal variances. We only took broods of the Mainfranken population into account. Birds that performed their first brood outside the investigation area or lost their first brood before sample collection, may distort the values. Therefore, we always refer to the ‘first recognized breeding attempt’ instead of the true first one.
Concerning juvenile dispersal, we calculated distances between the sampled or assigned hatching site and the sampled or assigned nest site of first recognized breeding. Natal dispersal was compared between sexes by first testing for unequal variances (F-test) followed by a two sided t-test with unequal variances.
By referring to Liminana et al., 2012 [61Liminana R, Garcia JT, Gonzalez JM, et al. Philopatry and natal dispersal of Montagu’s harriers (Circus pygargus) breeding in Spain: A review of existing data. Eur J Wildl Res 2012; 58: 549-55.
[http://dx.doi.org/10.1007/s10344-011-0602-2] ], we called juvenile birds to be philopatric, when they were found as breeders < 10 km away from their hatching site. In order to investigate sex specific philopatry rates of returners, percentages of birds, returned to the 10 km area, were compared via χ2-homogenity-test. We also performed a χ2-homogenity-test to compare philopatry in the 10 km area for all analysed 1276 chicks, no matter if they were seen again in Mainfranken or not. Juvenile recruitment was calculated as the number of chicks that were sampled in Mainfranken and returned for breeding.
3. RESULTS
3.1. STR Locus Characteristics
From the initial 19 loci used for genotyping, three had to be excluded from STR analyses: Decimal alleles at locus MS_Cpyg19 could not be rounded to full allele units, MS_Cpyg39 showed indication of null alleles and locus IEAAAG15 [62Busch JD, Katzner TE, Bragin E, Keims P. Tetranucleotide microsatellites for aquila and haliaeetus eagles. Mol Ecol Notes 2005; 5: 29-41.
[http://dx.doi.org/10.1111/j.1471-8286.2004.00823.x] ] showed amplification problems during the genotyping process. Consequently, parentage and identity tests were based on 16 loci. Table 1 summarizes parentage and identity characteristics for the whole marker set, while Table 2 gives detailed characterization results for each individual locus.

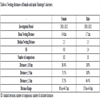
Parentage and identity statistics across 16 loci used for STR analyses of Montagu’s harriers.
NAall: mean number of alleles across all loci; PICall: polymorphism information content across all loci; NE-1P and NE-2P: combined non-exclusion probability for two possible parents when the genotype of the correct parent is unknown (1P) or known (2P). NE-PP: combined non-exclusion probability for parent pairs. NE-I and NE-SI: probability of mistaken identity between two randomly-chosen individuals (I) or full-sibs (SI).
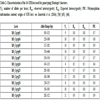
Characterization of the 16 STR loci used for genotyping Montagu’s harriers.
NA: number of alleles per locus; Hobs: observed heterozygosity; Hexp: Expected heterozygosity; PIC: Polymorphism information content; origin of STR loci: see Janowski et al. (2014); [56Janowski S, Grohme MA, Frohme M, Wink M. Development of New Microsatellite (STR) Markers for Montagu’s harrier (Circus pygargus) via 454 Shot-Gun Pyrosequencing. Open Ornithol J 2014; 7: 11-8.
[http://dx.doi.org/10.2174/1874453201407010011] ] [63Topinka JR, May B. Development of polymorphic microsatellite loci in the Northern Goshawk (Accipiter gentilis) and cross-amplification in other raptor species. Conserv Genet 2004; 5: 861-4.
[http://dx.doi.org/10.1007/s10592-004-1973-7] ]; [64Tingay RE, Dawson D, Pandhal J, et al. Isolation of 22 new haliaeetus microsatellite loci and their characterization in the critically endangered Madagascar fish-eagle (Haliaeetus vociferoides) and three other Haliaeetus eagle species. Mol Ecol Notes 2007; 7: 711-5.
[http://dx.doi.org/10.1111/j.1471-8286.2007.01690.x] ].
According to the results, our marker set is proven as highly informative. High information content is revealed by PIC values > 0.5 for 14 out of 16 loci and a PIC value of 0.7 for combined loci. Deviation of Hardy-Weinberg equilibrium could not be detected for any locus. There was no evidence for apparent null alleles in any of these loci, since frequency was always less than 0.05 [59Kalinowski ST, Taper ML, Marshall TC. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 2007; 16(5): 1099-106.
[http://dx.doi.org/10.1111/j.1365-294X.2007.03089.x] [PMID: 17305863] ].
3.2. Life Histories
Reconstruction of individual life histories provides the basis for dispersal and philopatry estimations. Life histories for 123 adult females (sampled via the bug method), and 40 for males (parentage assessment) were reconstructed. Most of the females were identified in a single year only (Table 3), and only a few females could be identified in up to eight years. Likewise, most of the males were detected in a single year only (altogether ranging between one and five years).
3.3. Adult Philopatry
For 102 female and 18 male harriers we could determine distances between their nest sites in two consecutive years. Of them, 80.4% of females could be considered as philopatric, since nesting sites were located in a distance of < 10 km to the one of the previous year (73.5% < 5 km, 37.3% < 1 km) (Fig. 1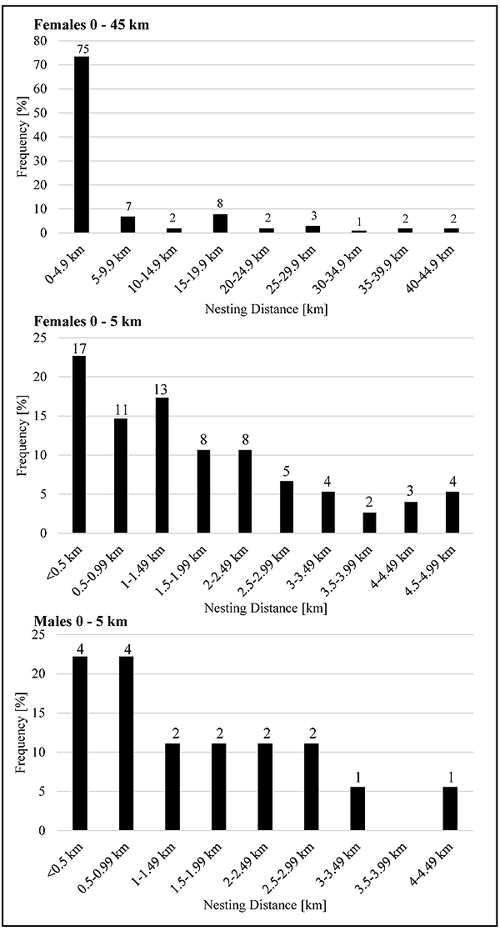 ). The smallest distance was 80 m and the longest 44.7 km within the investigation area (Table 4). All analysed males were apparently breeding in a radius of < 5 km to the nest site of the previous year (44.4% < 1 km) (Fig. 1
). The smallest distance was 80 m and the longest 44.7 km within the investigation area (Table 4). All analysed males were apparently breeding in a radius of < 5 km to the nest site of the previous year (44.4% < 1 km) (Fig. 1 ). Hence, all of males could be classified as philopatric. Distances ranged between 170 m and 4.4 km (Table 4). Nesting distances differed significantly between sexes (F-test: p < 0.01, t-test: p < 0.01). Adult males are thus more philopatric than adult females.
). Hence, all of males could be classified as philopatric. Distances ranged between 170 m and 4.4 km (Table 4). Nesting distances differed significantly between sexes (F-test: p < 0.01, t-test: p < 0.01). Adult males are thus more philopatric than adult females.
 |
Fig. (1) Adult philopatry of female and male Montagu’s harriers. |
Nesting distances between two consecutive years are given in intervals of 5 km for both sexes (each interval counted without the upper value). Additionally, the interval 0-5 km is detailed for both sexes. Numbers on top of each bar represent numbers of individuals.
There is a slight but not significant trend visible, concerning detection frequency and mean nesting distances (Fig. 2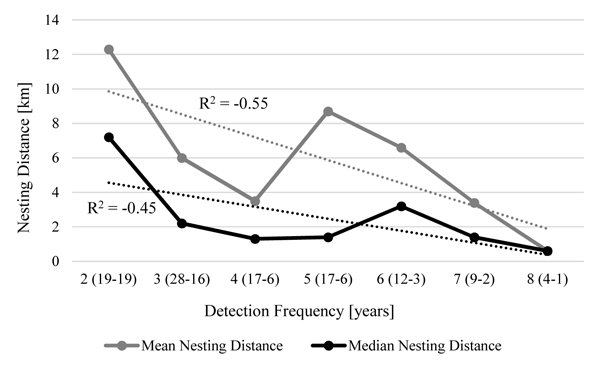 ). Mean nesting distances were lowest for a single female that was recorded in eight breeding seasons. Females that were detected in seven years (2 individuals) and four years (6 individuals) also settled close to the previous nesting site. Females that could only be detected in two years (19 individuals) were breeding further away from the previous site.
). Mean nesting distances were lowest for a single female that was recorded in eight breeding seasons. Females that were detected in seven years (2 individuals) and four years (6 individuals) also settled close to the previous nesting site. Females that could only be detected in two years (19 individuals) were breeding further away from the previous site.
 |
Fig. (2) Nesting Distances of females in two consecutive years depending on detection frequency. |
Mean and median nesting distances are given for females which were observed breeding two to eight times. X-axis show detection number with number of distances counted and number of individuals in brackets. R2 for trend curve (dotted lines) is shown.
Fig. (3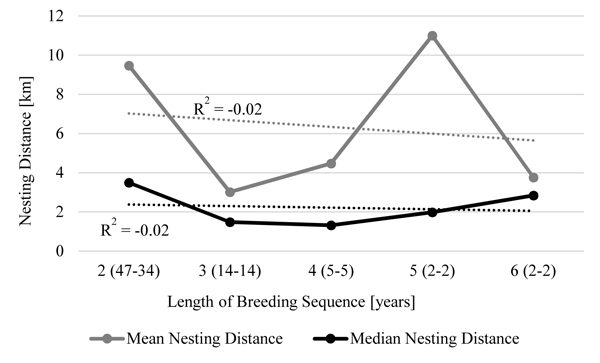 ) shows mean and median nesting distances for females depending on how often they bred consecutively. It need to be considered, that some individuals are counted more than once in the calculation, since their life histories showed for example times where a single individual bred two years in a row (one distance) followed by a pause year and a three year breeding sequence (another two distances) afterwards. Distances spanned over pause years are not included. There is no trend visible that show a relationship between breeding sequence length and nesting distance.
) shows mean and median nesting distances for females depending on how often they bred consecutively. It need to be considered, that some individuals are counted more than once in the calculation, since their life histories showed for example times where a single individual bred two years in a row (one distance) followed by a pause year and a three year breeding sequence (another two distances) afterwards. Distances spanned over pause years are not included. There is no trend visible that show a relationship between breeding sequence length and nesting distance.
 |
Fig. (3) 3: Nesting Distances of females depending on breeding sequence length. |
Mean and median nesting distances are given for females which were observed breeding in two to six years consecutively. X-axis show length of breeding sequence with number of distances counted and number of individuals included in brackets. R2 for trend curve (dotted lines) is shown.
3.4. Age of First Breeding, Natal Dispersal and Recruitment Rate of Juveniles
Reconstructed life histories revealed hatching year and several breeding attempts for 17 females (11 have been sampled as adults via the bug method) and 34 males.
Time between hatching and first recognized breeding attempt ranged between one year and five years for females and between one year and seven years for males. Especially the very long time spans might be incorrect due to incomplete sampling or absence of the respective birds in those years. However, we found a mean age of first breeding of about two years for females and three years for males (differences not significant; F-test: p = 0.44, t-test: p = 0.10). Moreover, we identified three females and one male which started breeding as one year old birds. (Fig. 4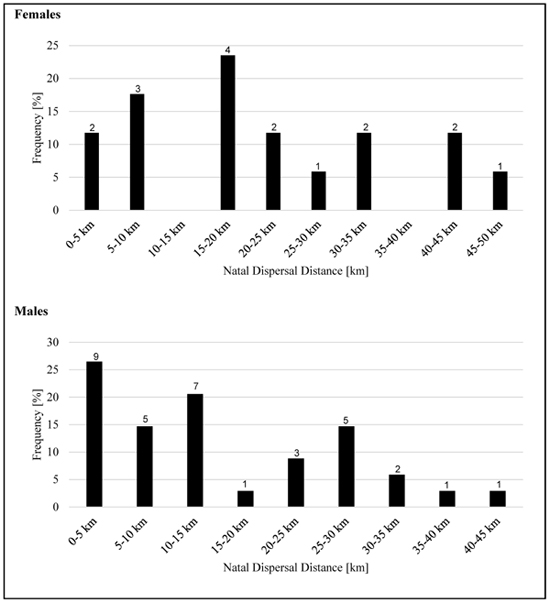 ) illustrates the natal dispersal distances for females and males. Table 5 compares age and distances between hatching and first recognized breeding for both sexes.
) illustrates the natal dispersal distances for females and males. Table 5 compares age and distances between hatching and first recognized breeding for both sexes.
 |
Fig. (4) Natal dispersal distances for female and male Montagu’s harriers. |
Distances between hatching site and place of first recorded breeding attempt is given in intervals of 5 km (each interval counted without the upper value). Numbers on top of each bar represent absolute numbers for each distance.
Distances between hatching site and place of first recorded breeding attempt is given in intervals of 5 km (each interval counted without the upper value). Numbers on top of each bar represent absolute numbers for each distance.
Distances between hatching site and first breeding attempt did not differ between sexes (F-test: p = 0.46, t-test: p = 0.09) (Table 5). We found 29.4% of returned females (5 individuals) and 41.2% of returned males (14 individuals) to be philopatric in our definition (< 10 km). There was no significant difference between the amount of males and females that bred in the 10 km area: χ2=3.03, df = 1, p = 0.08. Concerning all analysed 1276 chicks, philopatry rates (percentage of chicks that were detected breeding < 10 km apart from their hatching site) between females (0.8%) and males (0.6%) were even smaller and not significantly different: χ2 = 0.03, df = 1, p = 0.86. Furthermore, calculated minimal recruitment rate of juveniles amounted 2.9% (17 individuals) for females and 4.9% for males (34 individuals). Hence, minimal overall recruitment rate of chicks that had hatched in Mainfranken and had been analysed (1276 individuals) was 4%.
4. DISCUSSION AND CONCLUSION
4.1. Reconstruction of Life Histories with Microsatellite Markers
Traditionally, the reconstruction of life histories of birds relies on recoveries from ringed or sightings from otherwise marked birds. These methods can work quite well in species which are easy to find and trap. For many other birds it might be difficult to obtain a sufficiently large sample size which allows the calculation of life history variables.
Genetic studies in ornithology are still quite new compared to traditional methods [99Kraus RHS, Wink M. Avian genomics: Fledging into the wild! J Ornithol 2015; 156: 851-65.
[http://dx.doi.org/10.1007/s10336-015-1253-y] ]. In order to be useful, we need methods of high resolution which allow the identification of individuals. Starting with multilocus DNA fingerprinting with labelled probes in the 1980s, the development of microsatellite markers is presently the method of choice for the identification of individuals and for parentage studies [35Jan C, Fumagalli L. Polymorphic DNA microsatellite markers for forensic individual identification and parentage analyses of seven threatened species of parrots (family Psittacidae). Peer J 2016; 4: e2416.
[http://dx.doi.org/10.7717/peerj.2416] [PMID: 27688959] , 65Babb PL, McIntosh AM, Fernandez-Duque E, Di Fiore A, Schurr TG. An optimized microsatellite genotyping strategy for assessing genetic identity and kinship in azara’s owl monkeys (Aotus azarai). Folia Primatol (Basel) 2011; 82(2): 107-17.
[http://dx.doi.org/10.1159/000330564] [PMID: 21912137] -68Wagner AP, Creel S, Kalinowski ST. Estimating relatedness and relationships using microsatellite loci with null alleles. Heredity (Edinb) 2006; 97(5): 336-45.
[http://dx.doi.org/10.1038/sj.hdy.6800865] [PMID: 16868566] ]. Also SNP markers can be useful in this context [99Kraus RHS, Wink M. Avian genomics: Fledging into the wild! J Ornithol 2015; 156: 851-65.
[http://dx.doi.org/10.1007/s10336-015-1253-y] ]. Although microsatellite analyses are widely used today, they are not without inherent problems and much care is needed to correctly establish the genotype of an individual. Therefore, we decided to carry out a most conservative approach of strict data selection and controlling steps. This included characterization and selection of the most informative STR markers, exclusion of non-fully genotyped samples or of families which were too small for parentage analyses.
According to the achieved characterization results, our marker set is of high quality and thus suitable for parentage and identity tests. Neither deviation of Hardy-Weinberg equilibrium, nor evidence for apparent null alleles could be detected for any of the used loci. Accurate identification of individuals and correct assignment results were our highest aim. This led to quite a high reduction of the original sample size. However, our life history of Montagu’s harriers are of high quality and demonstrate that microsatellite analyses are valuable tools in ornithology. As we were able to obtain blood samples from incubating female harriers via the bug method, the STR analysis was much easier and more straightforward for females than for males, whose genotypes could only be reconstructed indirectly via parentage assignment.
4.2. Adult Philopatry
Dispersal depends on age and sex. In most species, juveniles disperse more than adults which have established a breeding territory. Both, female biased natal and breeding dispersal has been reported for many bird species [30Greenwood PJ. Mating systems, philopatry and dispersal in birds and mammals. Anim Behav 1980; 28: 1140-62.
[http://dx.doi.org/10.1016/S0003-3472(80)80103-5] ]. Factors influencing such a sex-biased dispersal could be resource competition (between age classes and sexes), intrasexual competition for mates and inbreeding avoidance [30Greenwood PJ. Mating systems, philopatry and dispersal in birds and mammals. Anim Behav 1980; 28: 1140-62.
[http://dx.doi.org/10.1016/S0003-3472(80)80103-5] , 69Dobson FS, Jones WT. Multiple causes of dispersal. Am Nat 1985; 126: 855-8.
[http://dx.doi.org/10.1086/284457] , 70Johnson ML, Gaines MS. Evolution of dispersal - theoretical-models and empirical tests using birds and mammals. Annu Rev Ecol Syst 1990; 21: 449-80.
[http://dx.doi.org/10.1146/annurev.es.21.110190.002313] ]. Moreover, female-biased dispersal is seen as a result of a monogamous mating system. Most of avian raptors are monogamous {only one breeding partner per season or even the same partner for many years [71Newton I. Population ecology of raptors. 1st ed, T & AD Poyser 1979; pp.399.-73Wittenberger JF, Tilson RL. The evolution of monogamy: Hypotheses and evidence. Annu Rev Ecol Syst 1980; 11: 197-232.
[http://dx.doi.org/10.1146/annurev.es.11.110180.001213] ]}, which should favour philopatric males. These males gain advantages when staying in or returning to their natal area to acquire or defend a territory and resources to attract females. Philopatric males could benefit from familiarity with competitors and predators, territorial circumstances (breeding and hunting habitat) and resource availability [30Greenwood PJ. Mating systems, philopatry and dispersal in birds and mammals. Anim Behav 1980; 28: 1140-62.
[http://dx.doi.org/10.1016/S0003-3472(80)80103-5] , 74Newton I, Marquiss M. Fidelity to breeding area and mate in sparrowhawks Accipiter nisus. J Anim Ecol 1982; 51: 327-41.
[http://dx.doi.org/10.2307/4327] ]. Also in raptors, females disperse more than males [75Dennhardt AJ, Wakamiya SM. Effective dispersal of Peregrine falcons (Falco peregrinus) in the midwest, USA. J Raptor Res 2013; 47: 262-70.
[http://dx.doi.org/10.3356/JRR-12-30.1] -77Whitfield DP, Duffy K, Mcleod DRA, et al. Juvenile dispersal of white-tailed eagles in Western Scotland. J Raptor Res 2009; 43: 110-20.
[http://dx.doi.org/10.3356/JRR-08-54.1] ], but exceptions towards males or sex-consistent dispersal behaviour also exist [78Booms TL, Talbot SL, Sage GK, et al. Nest-site fidelity and dispersal of gyrfalcons estimated by noninvasive genetic sampling. Condor 2011; 113: 768-78.
[http://dx.doi.org/10.1525/cond.2011.100178] ]. Most raptors return to their breeding sites of the previous year and hence are quite philopatric.
Since male Montagu’s harriers provide food for their females during breeding and for their offspring during rearing and post-fledging period [6Arroyo B. Breeding ecology and nest dispersion of Montagu's harrier Circus pygargus in Central Spain. PhD thesis 1995., 13Kitowski I. Behaviour of montagu’s harrier Circus pygargus juveniles during the post-fledging dependency period in SE Poland. Berkut 2002; 12: 201-7.], being philopatric might also improve their ability of gaining the required resources. Moreover, the species is a semi-colonial breeder. Colonies can be maintained over years and may provide advantages regarding predator defence [9Arroyo B, García JT, Bretagnolle V. Conservation of the montagu’s harrier (Circus pygargus) in agricultural areas. Anim Conserv 2002; 5: 283-90.
[http://dx.doi.org/10.1017/S1367943002004031] , 79Arroyo BE, Mougeot F, Bretagnolle V. Colonial breeding and nest defence in Montagu’s harrier (Circus pygargus). Behav Ecol Sociobiol 2001; 50: 109-15.
[http://dx.doi.org/10.1007/s002650100342] ].
In our study, adult males were more philopatric than adult females and nesting site distances differed significantly between both sexes. This result might be somehow biased by the large difference of sample sizes between males and females.
There was a slight but not significant trend visible, concerning detection frequency and mean nesting distances for females. Females that breed more than two years in Mainfranken tend to breed closer to the nest in the previous year. Although we don’t have knowledge about the age for most of the birds, the group of two-year breeders might be characterized by young and inexperienced females, which are still searching for a (new) breeding territory. Moreover, it is likely that young birds cannot compete with older ones for good breeding territories due to their deficiency in habitat familiarity. Older birds are more experienced in resource acquisition and predator defence, since they already bred for several years in the area. On the other hand, nesting distance between two consecutive years does not depend on length of breeding sequence. Females that bred in three consecutive years were not found to breed closer to their previous nest than those ones that bred in five or six years successively. This finding reflects the species flexibility and mobility to react on ecological and environmental changes. Montagu’s harriers depend on vole abundance in the breeding area. Voles are their main prey during breeding season in Germany [80Götz S. Untersuchungen zur Brut- und Ernährungsbiologie der Wiesenweihe (Circus pygnrgas L) auf den Mainfränkischen Platten. Universität Regensburg 2002., 81Hölker M, Wagner T. Nahrungsökologie der Wiesenweihe Circus pygargus in der ackerbaulich intensiv genutzten Feldlandschaft der Hellwegbörde, Nordrhein-Westfalen. Vogelwelt 2006; 127: 37-50.], as in other European regions [82Arroyo BE. Diet of Montagu’s harrier Circus pygargus in Central Spain: Analysis of temporal and geographic variation. Ibis 1997; 139: 664-72.
[http://dx.doi.org/10.1111/j.1474-919X.1997.tb04689.x] -87Terraube J, Guixe D, Arroyo B. Diet composition and foraging success in generalist predators: Are specialist individuals better foragers? Basic Appl Ecol 2014; 15: 616-24.
[http://dx.doi.org/10.1016/j.baae.2014.08.008] ]. Vole abundance fluctuates from year to year and can vary between different localities. Even during breeding season change of vole abundance is possible, so that birds can change their breeding site in reaction to that (after nest predation). Agricultural environment also influences adult philopatry. Rotating crop cultivation directly navigate adequate breeding sites. Nevertheless, our results agree with the few data in the literature: Breeding adults seem to be relatively philopatric to their previous breeding site M. Salamolard, A. Butet, A. Leroux, V. Bretagnolle unpublished data in [20Salamolard M, Butet A, Leroux A, Bretagnolle V. Responses of an avian predator to variations in prey density at a temperate latitude. Ecology 2000; 81: 2428-41.
[http://dx.doi.org/10.1890/0012-9658(2000)081[2428:ROAAPT]2.0.CO;2] ]. For hen harriers Circus cyaneus similar data have been published [88Picozzi N. Dispersion, breeding and prey of Hen harrier Circus cyaneus in glen dye, kincardineshire. Ibis 1978; 120: 498-509.
[http://dx.doi.org/10.1111/j.1474-919X.1978.tb06814.x] ].
4.3. Philopatry and Recruitment Rate of Juveniles
4.3.1. Age of First Breeding
For the first time, we could provide evidence for the age of mean first breeding of Montagu’s harriers in Mainfranken. Age of recognized first breeding is two years for females and three years for males. This finding is in agreement with data from Spain (female: two years, male: three years) and France (female: Three years, male: four years) [89Arroyo B, García JT, Bretagnolle V. Circus pygargus Montagu's harrier. Oxford: Oxford University Press 2004.]. Moreover, first-year breeders (three females and one male in Mainfranken) are also reported in Spain for males [90Arroyo B. Successful breeding by a first-year male Montagu’s harrier. Bird Study 1996; 43: 383-4.
[http://dx.doi.org/10.1080/00063659609461033] ]) and probably also for females [20Salamolard M, Butet A, Leroux A, Bretagnolle V. Responses of an avian predator to variations in prey density at a temperate latitude. Ecology 2000; 81: 2428-41.
[http://dx.doi.org/10.1890/0012-9658(2000)081[2428:ROAAPT]2.0.CO;2] ].
4.3.2. Recruitment Rate
In agreement with published data, the overall recruitment rates of juvenile Montagu’s harriers in Mainfranken were quite low: Only 2.9% of females and 4.9% of males, which had been sampled as chicks, were found to breed in Mainfranken in later years (all together only 4% of 1,276 sampled individuals). This value must be regarded as a minimum estimate, as we could not assess harriers which had bred outside the investigation area. Similar findings are known from France and Spain: In France, a mean recruitment rate of juveniles of about 8% (2.7-20.6% in different years) was estimated [91Leroux ABA. Montagu's harrier Circus pygargus in Western France: Philopatry and demographic parameters. RRF/HOT European Meeting 1993.] using ringing and wing-tag data. In Spain, 6.6% of 217 tagged juvenile birds were observed near the natal area in the next years, but only 4.2% started their first breeding attempt [6Arroyo B. Breeding ecology and nest dispersion of Montagu's harrier Circus pygargus in Central Spain. PhD thesis 1995.]. Moreover, Arroyo and Bretagnolle [92Arroyo BE, Bretagnolle V. Evaluating the long-term effectiveness of conservation practices in Montagu's harrier Circus pygargus. In: Raptors at Risk, Bodmin 2000; 403-8.] reported that 15% of returned birds were breeding in a distance of more than 50 km away from the natal nest. Juvenile recruitment, of course, is affected directly by juvenile survival and dispersal. The juvenile survival rate is assumed between 31% and 69% [61Liminana R, Garcia JT, Gonzalez JM, et al. Philopatry and natal dispersal of Montagu’s harriers (Circus pygargus) breeding in Spain: A review of existing data. Eur J Wildl Res 2012; 58: 549-55.
[http://dx.doi.org/10.1007/s10344-011-0602-2] ] and 67-75% after the second winter [93Millon A, Bretagnolle V. Predator population dynamics under a cyclic prey regime: Numerical responses, demographic parameters and growth rates. Oikos 2008; 117: 1500-10.
[http://dx.doi.org/10.1111/j.0030-1299.2008.16458.x] ], respectively. Furthermore, return rates are also influenced by the migration behaviour of a species. Wintering ranges of Montagu’s harriers originating from different breeding populations partly overlap. Birds migrate between different home ranges, tracking seasonal changes in food availability [94Trierweiler C, Mullié WC, Drent RH, et al. A Palaearctic migratory raptor species tracks shifting prey availability within its wintering range in the Sahel. J Anim Ecol 2013; 82(1): 107-20.
[http://dx.doi.org/10.1111/j.1365-2656.2012.02036.x] [PMID: 23137184] ]. During foraging migration, they get in contact with individuals from other populations and hence, potential breeding partners [21Trierweiler C, Klaassen RHG, Drent RH, et al. Migratory connectivity and population-specific migration routes in a long-distance migratory bird. Proc Biol Sci 2014; 281(1778): 20132897.
[http://dx.doi.org/10.1098/rspb.2013.2897] [PMID: 24430850] ], which might also influence dispersal and recruitment.
4.3.3. Natal Dispersal
In general, a very low recruitment rate and natal philopatry have been assumed [61Liminana R, Garcia JT, Gonzalez JM, et al. Philopatry and natal dispersal of Montagu’s harriers (Circus pygargus) breeding in Spain: A review of existing data. Eur J Wildl Res 2012; 58: 549-55.
[http://dx.doi.org/10.1007/s10344-011-0602-2] , 95Arroyo B, García JT, Bretagnolle V. Circus pygargus Montagu's harrier. Oxford: Oxford University Press 2004.]. Our present study confirms the investigations using wing-tags and PVC-rings. Natal dispersal distances in Mainfranken did not differ between sexes, which is in agreement with findings in different Spanish breeding areas [61Liminana R, Garcia JT, Gonzalez JM, et al. Philopatry and natal dispersal of Montagu’s harriers (Circus pygargus) breeding in Spain: A review of existing data. Eur J Wildl Res 2012; 58: 549-55.
[http://dx.doi.org/10.1007/s10344-011-0602-2] ]. In Spain, only 7% of 1,662 tagged juvenile birds could be identified as breeders in later years. Only 4.2% of tagged females and 3.2% of males were detected breeding within 10 km of their natal nest and hence were considered to be philopatric. However, results varied clearly between regions, monitoring intensity and marking technique, respectively, leading to an overall recruitment rate between 0-25%.
In Mainfranken, the philopatry rate of 1276 juvenile birds was comparatively low, with 0.8% for females and 0.6% for males. Reports of juvenile birds, that had been wing-tagged in Mainfranken and observed as breeders e.g. in Tattendorf, Austria; Račiněves, Czech Republic and Éguilly-sous-Bois, France (unpublished data), also indicate a pronounced dispersal and low philopatry. Discussing and comparing philopatry rates is always difficult, since results depend directly on the scale used. We have restricted our genetic sampling to the Mainfranken breeding population, although it is clear that birds easily migrate between Mainfranken and close neighbouring areas, e.g. Nördlinger Ries (administrative districts Donau-Ries, Bavaria and Ostalb, Baden-Württemberg).
Differences in regional and sex specific philopatry rates may be due to different carrying capacities of the environment, lower survival rates of adult birds or differences in sex-specific survival of juveniles (recruits) [61Liminana R, Garcia JT, Gonzalez JM, et al. Philopatry and natal dispersal of Montagu’s harriers (Circus pygargus) breeding in Spain: A review of existing data. Eur J Wildl Res 2012; 58: 549-55.
[http://dx.doi.org/10.1007/s10344-011-0602-2] , 96Hernandez-Matias A, Real J, Pradel R. Determinants of territorial recruitment in bonelli’s eagle (Aquila fasciata) populations. Auk 2010; 127: 173-84.
[http://dx.doi.org/10.1525/auk.2009.09143] , 97Soutullo A, Limiñana R, Urios V, Surroca M, A Gill J. Density-dependent regulation of population size in colonial breeders: Allee and buffer effects in the migratory Montagu’s harrier. Oecologia 2006; 149(3): 543-52.
[http://dx.doi.org/10.1007/s00442-006-0465-5] [PMID: 16794831] ]. The small sex specific difference (though not significant) in philopatry rates in Spanish and French studies by Leroux and Bretagnolle [16Leroux A, Bretagnolle V. Sex ratio variations in broods of montagu’s harriers Circus pygargus. J Avian Biol 1996; 27: 63-9.
[http://dx.doi.org/10.2307/3676962] ] and Arroyo [6Arroyo B. Breeding ecology and nest dispersion of Montagu's harrier Circus pygargus in Central Spain. PhD thesis 1995.] are interpreted in the context with self-regulation of breeding colonies. The authors suggested that especially small breeding colonies favour the production of the more philopatric sex that would return to the colony and hence, would contribute to colony preservation: In France, males dominate juvenile sex ratios with 55.2% and are considered to be more philopatric [16Leroux A, Bretagnolle V. Sex ratio variations in broods of montagu’s harriers Circus pygargus. J Avian Biol 1996; 27: 63-9.
[http://dx.doi.org/10.2307/3676962] ], while in Spain this is found for females (54% quota in sex ratios) [98Arroyo BE. Fledgling sex ratio variation and future reproduction probability in montagu’s harrier Circus pygargus. Behav Ecol Sociobiol 2002; 52: 109-16.
[http://dx.doi.org/10.1007/s00265-002-0496-9] ]. Our data from Mainfranken appear to agree with this hypothesis: more returned males (41.2%) bred in a distance of less than 10 km to the hatching site than returned females (29.4%). Additionally, males were the predominant sex of chicks between 2000-2012 (53.1% males vs. 46.9% females) (unpublished data).
For the first time microsatellite analysis offered important basic information concerning natal and breeding dispersal in German Montagu’s harriers. Nevertheless, more studies are needed to understand the biology of the species and to develop strategies of its conservation [99Kraus RHS, Wink M. Avian genomics: Fledging into the wild! J Ornithol 2015; 156: 851-65.
[http://dx.doi.org/10.1007/s10336-015-1253-y] ].
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was analyzed and approved by: Regierung von Unterfranken, D-97064 Würzburg, no.: 55.2-2531.01 -46/11.
HUMAN AND ANIMAL RIGHTS
No Laboratory Animals were used for the studies that on the bases of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest. Financial support was provided by the Bayerisches Landesamt für Umwelt. S. Janowski thanks the Landesgraduiertenförderung Baden-Württemberg and the Gerhard und Ellen Zeidler-Stiftung for scholarships.
ACKNOWLEDGEMENTS
We are thankful to the Landesbund für Vogelschutz in Bayern e.V. and several volunteers for their aid and support during sample collection in Mainfranken. We thank Hedwig Sauer-Gürth and her trainees for help with DNA analyses. Heidi Staudter helped with literature research. G. Schaub (Ruhr Universität Bochum, Germany) produced the Dipetalogaster maxima for us and R. Nagel (Institut für Vogelforschung (Vogelwarte Helgoland), Wilhelmshaven, Germany) built the artificial harrier eggs. We thank them for their support.
REFERENCES
| [1] | Clarke R. Montagu’s harrier 1996. |
| [2] | Trierweiler C, Koks B. Montagu’s harrier Circus pygargus. In: Zwarts L, Bijlsma R.G., van der Kamp J. and Wymenga E., Eds. Living on the Edge: Wetlands and Birds in a Changing Sahel. Zeist, KNNV Publishing 2009; pp. 312-27. |
| [3] | Glutz Von Blotzheim U, Bauer KM, Bezzel E. Handbuch der Vögel Mitteleuropas. Akademische Verlagsgesellschaft Frankfurt/Main 1971; Band 4 Falconiformes. |
| [4] | Sanchez-Zapata JA, Carrete M, Gravilov A, et al. Land use changes and raptor conservation in steppe habitats of Eastern Kazakhstan. Biol Conserv 2003; 111: 71-7. [http://dx.doi.org/10.1016/S0006-3207(02)00251-3] |
| [5] | Frionnet C. Les oiseaux de la Haute-Marne. Bulletin de la Société Sciences Naturelles: Haute-Marne (Chaumont) 1925. |
| [6] | Arroyo B. Breeding ecology and nest dispersion of Montagu's harrier Circus pygargus in Central Spain. PhD thesis 1995. |
| [7] | Montagu's Harrier Circus pygargus. Bird Life International; Species factsheet 2014; Downloaded from: http://www.birdlife.org. |
| [8] | Ferguson-Lees J, Christie DA. Raptors of the World (Helm Identification Guides) 1st ed.2001. |
| [9] | Arroyo B, García JT, Bretagnolle V. Conservation of the montagu’s harrier (Circus pygargus) in agricultural areas. Anim Conserv 2002; 5: 283-90. [http://dx.doi.org/10.1017/S1367943002004031] |
| [10] | Arroyo BE, Bretagnolle V, Leroux A. Interactive effects of food and age on breeding in the Montagu’s harrier Circus pygargus. Ibis 2007; 149: 806-13. [http://dx.doi.org/10.1111/j.1474-919X.2007.00716.x] |
| [11] | Clarke R. British Montagu’s harriers- what governs their numbers? Ornithologischer Anzeiger 2002; 41: 143-58. |
| [12] | Garcia JT, Arroyo BE. Effect of abiotic factors on reproduction in the centre and periphery of breeding ranges: A comparative analysis in sympatric harriers. Ecography 2001; 24: 393-402. [http://dx.doi.org/10.1034/j.1600-0587.2001.d01-195.x] |
| [13] | Kitowski I. Behaviour of montagu’s harrier Circus pygargus juveniles during the post-fledging dependency period in SE Poland. Berkut 2002; 12: 201-7. |
| [14] | Koks BJ, Trierweiler C, Visser EG, Dijkstra C, Komdeur J. Conservation of montagu’s harrier Circus pygargus: Do voles make agricultural habitat attractive? IBIS 2007; 149: 575-86. [http://dx.doi.org/10.1111/j.1474-919X.2007.00683.x] |
| [15] | Laminana R, Soutullo A, Urios V, Surroca M. Vegetation hight selection in Montagu’s harriers Circus pygargus breeding in a natural habitat. Ardea 2006; 94: 1-5. |
| [16] | Leroux A, Bretagnolle V. Sex ratio variations in broods of montagu’s harriers Circus pygargus. J Avian Biol 1996; 27: 63-9. [http://dx.doi.org/10.2307/3676962] |
| [17] | Millon A, Bourrioux JL, Riols C, Bretagnolle V. Comparative breeding biology of Hen harrier and Montagu's harrier: An 8-year study in North-eastern France. Ibis 2002; 144: 94-105. [http://dx.doi.org/10.1046/j.0019-1019.2001.00009.x] |
| [18] | Mougeot F, Arroyo BE, Bretagnolle V. Paternity assurance responses to first-year and adult male territorial intrusions in a courtship-feeding raptor. Anim Behav 2006; 71: 101-8. [http://dx.doi.org/10.1016/j.anbehav.2005.03.036] |
| [19] | Rutkowski R, Krupinski D, Kitowski I, et al. Genetic structure and diversity of breeding Montagu’s harrier (Circus pygargus) in Europe. Eur J Wildl Res 2015; 61: 691-701. [http://dx.doi.org/10.1007/s10344-015-0943-3] |
| [20] | Salamolard M, Butet A, Leroux A, Bretagnolle V. Responses of an avian predator to variations in prey density at a temperate latitude. Ecology 2000; 81: 2428-41. [http://dx.doi.org/10.1890/0012-9658(2000)081[2428:ROAAPT]2.0.CO;2] |
| [21] | Trierweiler C, Klaassen RHG, Drent RH, et al. Migratory connectivity and population-specific migration routes in a long-distance migratory bird. Proc Biol Sci 2014; 281(1778): 20132897. [http://dx.doi.org/10.1098/rspb.2013.2897] [PMID: 24430850] |
| [22] | Wiącek J. Benefits and costs of semi-colonial breeding in the Montagu’s harrier Circus pygargus. Belg J Zool 2008; 138: 36-40. |
| [23] | Arroyo B, García JT. Migratory movements of Western European Montagu’s harrier Circus pygargus: A review. Bird Study 1998; 45: 188-94. [http://dx.doi.org/10.1080/00063659809461090] |
| [24] | Ganesh T, Kanniah P. Roost counts of harriers Circus spanning seven winters in Andhra Pradesh, India. Forktail 2000; 16: 1-3. |
| [25] | Klaassen RHG, Hake M, Strandberg R, et al. When and where does mortality occur in migratory birds? Direct evidence from long-term satellite tracking of raptors. J Anim Ecol 2014; 83(1): 176-84. [http://dx.doi.org/10.1111/1365-2656.12135] [PMID: 24102110] |
| [26] | Liminana R, Soutullo A, Urios V. Autumn migration of Montagu’s harriers Circus pygargus tracked by satellite telemetry. J Ornithol 2007; 148: 517-23. [http://dx.doi.org/10.1007/s10336-007-0182-9] |
| [27] | Spaar R, Bruderer B. Migration by flapping or soaring: Flight strategies of marsh, montagu’s and pallid harriers in Southern Israel. Condor 1997; 99: 458-69. [http://dx.doi.org/10.2307/1369952] |
| [28] | Trierweiler C, Koks BJ, Drent RH, et al. Satellite tracking of two montagu’s harriers (Circus pygargus): Dual pathways during autumn migration. J Ornithol 2007; 148: 513-6. [http://dx.doi.org/10.1007/s10336-007-0178-5] |
| [29] | Greenwood PJ, Harvey PH. The natal and breeding dispersal of birds. Annu Rev Ecol Syst 1982; 13: 1-21. [http://dx.doi.org/10.1146/annurev.es.13.110182.000245] |
| [30] | Greenwood PJ. Mating systems, philopatry and dispersal in birds and mammals. Anim Behav 1980; 28: 1140-62. [http://dx.doi.org/10.1016/S0003-3472(80)80103-5] |
| [31] | Paradis E, Baillie SR, Sutherland WJ, Gregory RD. Patterns of natal and breeding dispersal in birds. J Anim Ecol 1998; 67: 518-36. [http://dx.doi.org/10.1046/j.1365-2656.1998.00215.x] |
| [32] | Davis LA, Roalson EH, Cornell KL, McClanahan KD, Webster MS. Genetic divergence and migration patterns in a north american passerine bird: Implications for evolution and conservation. Mol Ecol 2006; 15(8): 2141-52. [http://dx.doi.org/10.1111/j.1365-294X.2006.02914.x] [PMID: 16780431] |
| [33] | Delaney KS, Riley SPD, Fisher RN. A rapid, strong, and convergent genetic response to urban habitat fragmentation in four divergent and widespread vertebrates. PLoS One 2010; 5(9): 5. [http://dx.doi.org/10.1371/journal.pone.0012767] [PMID: 20862274] |
| [34] | Evans SR, Sheldon BC. Interspecific patterns of genetic diversity in birds: Correlations with extinction risk. Conserv Biol 2008; 22(4): 1016-25. [http://dx.doi.org/10.1111/j.1523-1739.2008.00972.x] [PMID: 18616741] |
| [35] | Jan C, Fumagalli L. Polymorphic DNA microsatellite markers for forensic individual identification and parentage analyses of seven threatened species of parrots (family Psittacidae). Peer J 2016; 4: e2416. [http://dx.doi.org/10.7717/peerj.2416] [PMID: 27688959] |
| [36] | Keller LF, Jeffery KJ, Arcese P, et al. Immigration and the ephemerality of a natural population bottleneck: Evidence from molecular markers. Proc Biol Sci 2001; 268(1474): 1387-94. [http://dx.doi.org/10.1098/rspb.2001.1607] [PMID: 11429139] |
| [37] | Grüneberg C, Bauer H-G, Haupt H, et al. Rote Liste der Brutvögel Deutschlands. 5. Fassung. Berichte zum Vogelschutz 2016; 52: 19-67. |
| [38] | Südbeck P, Bauer H-G, Boschert M, Boye P, Knief W. Rote Liste der Brutvögel Deutschlands. 4. Fassung. Berichte zum Vogelschutz 2007; 44: 23-81. |
| [39] | Stiefel D. Zur Situation der Wiesenweihe Circus pygargus in Deutschland. Tagung zum Wiesenweihenschutz in der Agrarlandschaft 2010; 46: 18-27. |
| [40] | Illner H. Brutbestände der Wiesenweihe Circus pygargus und Nestschutz-Maßnahmen in Deutschland 2003 bis 2014. Vogelwelt 2017; 137: 1-13. |
| [41] | Pürckhauer C. Artenhilfsprogramm Wiesenweihe (Circus pygargus) in Bayern. Landesbund für Vogelschutz in Bayern e.V. (LBV). Jahresbericht 2016. |
| [42] | Rudolph B-U, Schwandner J, Fünfstück H-J. Red list and list of breeding birds of Bavaria. Bavarian State Office for the Environment (LfU) 2016. |
| [43] | Belting C, Krüger RM. Populationsentwicklung und Schutzstrategien für die Wiesenweihe Circus pygargus in Bayern. Ornithologischer Anzeiger 2002; 41: 87-92. |
| [44] | Pürckhauer C. Artenhilfsprogramm Wiesenweihe (Circus pygargus) in Bayern - Landesbund für Vogelschutz in Bayern e.V. (LBV), Jahresbericht 2008. |
| [45] | Illner H. Schutzprogramm für Wiesenweihen und Rohrweihen in Mittelwestfalen -Arbeitsgemeinschaft Biologischer Umweltschutz im Kreis Soest eV 2007. |
| [46] | Koks BJ, Visser EG. Montagu’s harriers Circus pygargus in the Netherlands: Does nest protection prevent extinction? Ornithologischer Anzeiger 2002; 41: 159-66. |
| [47] | Gahrau C, Schmüser H. Artenschutzprojekt Wiesenweihe (Circus pygargus) des Landes Schleswig-Holstein - Brutperiode 2007. Im Rahmen des Monitoringprojekts: Wildtierkataster Schleswig-Holstein 2007. |
| [48] | Nader W, Werner D, Wink M. Genetic diversity of scarlet macaws Ara macao in reintroduction studies for threatened populations in Costa Rica. Biol Conserv 1999; 87: 269-72. [http://dx.doi.org/10.1016/S0006-3207(98)00043-3] |
| [49] | Arnold JM, Oswald SA, Voigt CC, et al. Taking the stress out of blood collection: Comparison of field blood-sampling techniques for analysis of baseline corticosterone. J Avian Biol 2008; 39: 588-92. [http://dx.doi.org/10.1111/j.0908-8857.2008.04265.x] |
| [50] | Becker PH, Voigt CC, Arnold JM, Nagel R. A non-invasive technique to bleed incubating birds without trapping: A blood-sucking bug in a hollow egg. J Ornithol 2006; 147: 115-8. [http://dx.doi.org/10.1007/s10336-005-0027-3] |
| [51] | Thomsen R, Voigt CC. Non-invasive blood sampling from primates using laboratory-bred blood-sucking bugs (Dipetalogaster maximus; reduviidae, heteroptera). Primates 2006; 47(4): 397-400. [http://dx.doi.org/10.1007/s10329-006-0194-8] [PMID: 16741605] |
| [52] | Voigt CC, Fassbender M, Dehnhard M, et al. Validation of a minimally invasive blood-sampling technique for the analysis of hormones in domestic rabbits, Oryctolagus cuniculus (lagomorpha). Gen Comp Endocrinol 2004; 135(1): 100-7. [http://dx.doi.org/10.1016/j.ygcen.2003.08.005] [PMID: 14644649] |
| [53] | Voigt CC, Peschel U, Wibbelt G, Frölich K. An alternative, less invasive blood sample collection technique for serologic studies utilizing triatomine bugs (heteroptera; insecta). J Wildl Dis 2006; 42(2): 466-9. [http://dx.doi.org/10.7589/0090-3558-42.2.466] [PMID: 16870877] |
| [54] | Von Helversen O, Volleth M, Núnez J. A new method for obtaining blood from a small mammal without injuring the animal: Use of triatomid bugs. Experientia 1986; 42: 809-10. [http://dx.doi.org/10.1007/BF01941531] |
| [55] | Stadler A, Meiser CK, Schaub GA. “Living syringes”: Use of hematophagous bugs as blood samplers from small and wild animals. Parasitology Research 2011; 243-71. |
| [56] | Janowski S, Grohme MA, Frohme M, Wink M. Development of New Microsatellite (STR) Markers for Montagu’s harrier (Circus pygargus) via 454 Shot-Gun Pyrosequencing. Open Ornithol J 2014; 7: 11-8. [http://dx.doi.org/10.2174/1874453201407010011] |
| [57] | Sambrook J, Fritsch EF, Maniatis T. Moleculer clooning: A labaratuary manual 1989. |
| [58] | Kahn NW, St.John J, Quinn TW. Chromosome specific intron size difference in the avian CHD gene provide an efficient method for sex identification in birds. Auk 1998; 115: 1074-8. [http://dx.doi.org/10.2307/4089527] |
| [59] | Kalinowski ST, Taper ML, Marshall TC. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 2007; 16(5): 1099-106. [http://dx.doi.org/10.1111/j.1365-294X.2007.03089.x] [PMID: 17305863] |
| [60] | Jones OR, Wang J. COLONY: A program for parentage and sibship inference from multilocus genotype data. Mol Ecol Resour 2010; 10(3): 551-5. [http://dx.doi.org/10.1111/j.1755-0998.2009.02787.x] [PMID: 21565056] |
| [61] | Liminana R, Garcia JT, Gonzalez JM, et al. Philopatry and natal dispersal of Montagu’s harriers (Circus pygargus) breeding in Spain: A review of existing data. Eur J Wildl Res 2012; 58: 549-55. [http://dx.doi.org/10.1007/s10344-011-0602-2] |
| [62] | Busch JD, Katzner TE, Bragin E, Keims P. Tetranucleotide microsatellites for aquila and haliaeetus eagles. Mol Ecol Notes 2005; 5: 29-41. [http://dx.doi.org/10.1111/j.1471-8286.2004.00823.x] |
| [63] | Topinka JR, May B. Development of polymorphic microsatellite loci in the Northern Goshawk (Accipiter gentilis) and cross-amplification in other raptor species. Conserv Genet 2004; 5: 861-4. [http://dx.doi.org/10.1007/s10592-004-1973-7] |
| [64] | Tingay RE, Dawson D, Pandhal J, et al. Isolation of 22 new haliaeetus microsatellite loci and their characterization in the critically endangered Madagascar fish-eagle (Haliaeetus vociferoides) and three other Haliaeetus eagle species. Mol Ecol Notes 2007; 7: 711-5. [http://dx.doi.org/10.1111/j.1471-8286.2007.01690.x] |
| [65] | Babb PL, McIntosh AM, Fernandez-Duque E, Di Fiore A, Schurr TG. An optimized microsatellite genotyping strategy for assessing genetic identity and kinship in azara’s owl monkeys (Aotus azarai). Folia Primatol (Basel) 2011; 82(2): 107-17. [http://dx.doi.org/10.1159/000330564] [PMID: 21912137] |
| [66] | Gerber S, Mariette S, Streiff R, Bodénès C, Kremer A. Comparison of microsatellites and amplified fragment length polymorphism markers for parentage analysis. Mol Ecol 2000; 9(8): 1037-48. [http://dx.doi.org/10.1046/j.1365-294x.2000.00961.x] [PMID: 10964223] |
| [67] | Selkoe KA, Toonen RJ. Microsatellites for ecologists: A practical guide to using and evaluating microsatellite markers. Ecol Lett 2006; 9(5): 615-29. [http://dx.doi.org/10.1111/j.1461-0248.2006.00889.x] [PMID: 16643306] |
| [68] | Wagner AP, Creel S, Kalinowski ST. Estimating relatedness and relationships using microsatellite loci with null alleles. Heredity (Edinb) 2006; 97(5): 336-45. [http://dx.doi.org/10.1038/sj.hdy.6800865] [PMID: 16868566] |
| [69] | Dobson FS, Jones WT. Multiple causes of dispersal. Am Nat 1985; 126: 855-8. [http://dx.doi.org/10.1086/284457] |
| [70] | Johnson ML, Gaines MS. Evolution of dispersal - theoretical-models and empirical tests using birds and mammals. Annu Rev Ecol Syst 1990; 21: 449-80. [http://dx.doi.org/10.1146/annurev.es.21.110190.002313] |
| [71] | Newton I. Population ecology of raptors. 1st ed, T & AD Poyser 1979; pp.399. |
| [72] | Orians GH. On the evolution of mating systems in birds and mammals. Am Nat 1969; 589-603. [http://dx.doi.org/10.1086/282628] |
| [73] | Wittenberger JF, Tilson RL. The evolution of monogamy: Hypotheses and evidence. Annu Rev Ecol Syst 1980; 11: 197-232. [http://dx.doi.org/10.1146/annurev.es.11.110180.001213] |
| [74] | Newton I, Marquiss M. Fidelity to breeding area and mate in sparrowhawks Accipiter nisus. J Anim Ecol 1982; 51: 327-41. [http://dx.doi.org/10.2307/4327] |
| [75] | Dennhardt AJ, Wakamiya SM. Effective dispersal of Peregrine falcons (Falco peregrinus) in the midwest, USA. J Raptor Res 2013; 47: 262-70. [http://dx.doi.org/10.3356/JRR-12-30.1] |
| [76] | Forero MG, Donazar JA, Hiraldo F. Causes and fitness consequences of natal dispersal in a population of black kites. Ecology 2002; 83: 858-72. [http://dx.doi.org/10.1890/0012-9658(2002)083[0858:CAFCON]2.0.CO;2] |
| [77] | Whitfield DP, Duffy K, Mcleod DRA, et al. Juvenile dispersal of white-tailed eagles in Western Scotland. J Raptor Res 2009; 43: 110-20. [http://dx.doi.org/10.3356/JRR-08-54.1] |
| [78] | Booms TL, Talbot SL, Sage GK, et al. Nest-site fidelity and dispersal of gyrfalcons estimated by noninvasive genetic sampling. Condor 2011; 113: 768-78. [http://dx.doi.org/10.1525/cond.2011.100178] |
| [79] | Arroyo BE, Mougeot F, Bretagnolle V. Colonial breeding and nest defence in Montagu’s harrier (Circus pygargus). Behav Ecol Sociobiol 2001; 50: 109-15. [http://dx.doi.org/10.1007/s002650100342] |
| [80] | Götz S. Untersuchungen zur Brut- und Ernährungsbiologie der Wiesenweihe (Circus pygnrgas L) auf den Mainfränkischen Platten. Universität Regensburg 2002. |
| [81] | Hölker M, Wagner T. Nahrungsökologie der Wiesenweihe Circus pygargus in der ackerbaulich intensiv genutzten Feldlandschaft der Hellwegbörde, Nordrhein-Westfalen. Vogelwelt 2006; 127: 37-50. |
| [82] | Arroyo BE. Diet of Montagu’s harrier Circus pygargus in Central Spain: Analysis of temporal and geographic variation. Ibis 1997; 139: 664-72. [http://dx.doi.org/10.1111/j.1474-919X.1997.tb04689.x] |
| [83] | Garcia JT, Arroyo BE. Food-niche differentiation in sympatric Hen Circus cyaneus and montagu’s harriers Circus pygargus. Ibis 2005; 147: 144-54. [http://dx.doi.org/10.1111/j.1474-919x.2004.00377.x] |
| [84] | Hiraldo F. Diet of the Montagu’s harrier Circus pygargus in southwestern spain. Doñana. Acta Vertebrata 1975; 2: 25-55. |
| [85] | Kitowski I. Age-related differences in foraging behavior of Montagu’s harrier Circus pygargus males in South-east Poland. Acta Ethol 2003; 6: 35-8. [http://dx.doi.org/10.1007/s10211-003-0078-5] |
| [86] | Koks BJ, Trierweiler C, Visser eg, Dijkstra C, Komdeur J. Do voles make agricultural habitat attractive to Montagu’s harrier Circus pygargus? Ibis 2007; 149: 575-86. [http://dx.doi.org/10.1111/j.1474-919X.2007.00683.x] |
| [87] | Terraube J, Guixe D, Arroyo B. Diet composition and foraging success in generalist predators: Are specialist individuals better foragers? Basic Appl Ecol 2014; 15: 616-24. [http://dx.doi.org/10.1016/j.baae.2014.08.008] |
| [88] | Picozzi N. Dispersion, breeding and prey of Hen harrier Circus cyaneus in glen dye, kincardineshire. Ibis 1978; 120: 498-509. [http://dx.doi.org/10.1111/j.1474-919X.1978.tb06814.x] |
| [89] | Arroyo B, García JT, Bretagnolle V. Circus pygargus Montagu's harrier. Oxford: Oxford University Press 2004. |
| [90] | Arroyo B. Successful breeding by a first-year male Montagu’s harrier. Bird Study 1996; 43: 383-4. [http://dx.doi.org/10.1080/00063659609461033] |
| [91] | Leroux ABA. Montagu's harrier Circus pygargus in Western France: Philopatry and demographic parameters. RRF/HOT European Meeting 1993. |
| [92] | Arroyo BE, Bretagnolle V. Evaluating the long-term effectiveness of conservation practices in Montagu's harrier Circus pygargus. In: Raptors at Risk, Bodmin 2000; 403-8. |
| [93] | Millon A, Bretagnolle V. Predator population dynamics under a cyclic prey regime: Numerical responses, demographic parameters and growth rates. Oikos 2008; 117: 1500-10. [http://dx.doi.org/10.1111/j.0030-1299.2008.16458.x] |
| [94] | Trierweiler C, Mullié WC, Drent RH, et al. A Palaearctic migratory raptor species tracks shifting prey availability within its wintering range in the Sahel. J Anim Ecol 2013; 82(1): 107-20. [http://dx.doi.org/10.1111/j.1365-2656.2012.02036.x] [PMID: 23137184] |
| [95] | Arroyo B, García JT, Bretagnolle V. Circus pygargus Montagu's harrier. Oxford: Oxford University Press 2004. |
| [96] | Hernandez-Matias A, Real J, Pradel R. Determinants of territorial recruitment in bonelli’s eagle (Aquila fasciata) populations. Auk 2010; 127: 173-84. [http://dx.doi.org/10.1525/auk.2009.09143] |
| [97] | Soutullo A, Limiñana R, Urios V, Surroca M, A Gill J. Density-dependent regulation of population size in colonial breeders: Allee and buffer effects in the migratory Montagu’s harrier. Oecologia 2006; 149(3): 543-52. [http://dx.doi.org/10.1007/s00442-006-0465-5] [PMID: 16794831] |
| [98] | Arroyo BE. Fledgling sex ratio variation and future reproduction probability in montagu’s harrier Circus pygargus. Behav Ecol Sociobiol 2002; 52: 109-16. [http://dx.doi.org/10.1007/s00265-002-0496-9] |
| [99] | Kraus RHS, Wink M. Avian genomics: Fledging into the wild! J Ornithol 2015; 156: 851-65. [http://dx.doi.org/10.1007/s10336-015-1253-y] |