- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Ornithology Journal
(Discontinued)
ISSN: 1874-4532 ― Volume 13, 2020
Brood Parasitism Defense Behaviors Along an Altitudinal Gradient in the American Robin (Turdus Migratorius)
Lisa C. Carmody1, Alexander Cruz1, Jameson F. Chace*, 2
Abstract
Some host species accept eggs from brood parasites over parts of their range and reject them in other areas representing an “evolutionary lag” in the development of rejection behavior or the loss of an adapative behavior when the selection pressure of brood parasitism is removed. Hosts may deter brood parasitism through egg rejection and aggressive nest defense behavior specifically targetting female brood parasites during the egg incubation period. In areas where parasitism frequencies are spatially and temporally variable, anti-parasite behaviors may decline as costs outweigh the benefits. Along the Colorado Front Range, American robins (Turdus migratorius) breed from low elevations where the brood parasitic Brown-headed Cowbird (Molothrus ater) is abundant to near timberline (3700 m) where cowbirds are uncommon. We tested the hypothesis that egg rejection and nest defense behaviors decline with reduced probability of parasitism. We found that robins accepted 100% of immaculate (robin-like) experimental eggs at both low and high elevations, but were more likely to reject spotted (cowbird-like) experimental eggs at low elevations than high elevations. Response to egg size was more variable than to egg color. When presented with a mount of a cowbird and Song Sparrow (Melospiza melodia) near the nest, robins responded more aggressively to cowbird models than to sparrows (control), and nest defense behavior towards cowbirds was longer and more aggressive at the lower elevation sites where cowbirds are common. These results suggest that egg rejection and nest-site aggression are costly adaptations to cowbird parasitism, and these behaviors decline when the threat of parasitism is reduced.
Article Information
Identifiers and Pagination:
Year: 2016Volume: 9
First Page: 39
Last Page: 49
Publisher Id: TOOENIJ-9-39
DOI: 10.2174/1874453201609010039
Article History:
Received Date: 31/05/2016Revision Received Date: 01/09/2016
Acceptance Date: 13/10/2016
Electronic publication date: 21/11/2016
Collection year: 2016
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution-Non-Commercial 4.0 International Public License (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/legalcode), which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Department of Biology and Biomedical Sciences, Salve Regina University, Newport, RI 02840, USA; Tel: 401-341-3204; Fax: 401-341-2993; E-mail: Jameson.chace@salve.edu
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 31-05-2016 |
Original Manuscript | Brood Parasitism Defense Behaviors Along an Altitudinal Gradient in the American Robin (Turdus Migratorius) | |
INTRODUCTION
The Brown-headed Cowbird (Molothrus ater) is an obligate brood parasite [1Friedmann H. Host relations of the parasitic cowbirds. US Nat Museum Bull 1963; 233: 1-276.
[http://dx.doi.org/10.5479/si.03629236.233] -3Ortega CP. Cowbirds and other brood parasites. Tucson, AZ: University of Arizona Press 1998.]. Females do not build their own nests, but lay their eggs in the nests of host species, who often raise young cowbirds to the detriment of their own young [4Payne RB. The ecology of brood parasitism in birds. Annu Rev Ecol Syst 1977; 8: 1-28.
[http://dx.doi.org/10.1146/annurev.es.08.110177.000245] -7Davies NB. Cuckoos, cowbirds and other cheats. London: T. A. D. Poyser 2000.]. Selection should favor anti-parasite defenses that reduce the negative effects of parasitism on the host [8Sealy SG, Neudorf DL, Hobson KA, Gill SA. Nest defense by potential hosts of the brown-headed cowbird: methodological approaches, benefits of defense, and coevolution. In: Rothstein SI, Robinson SK, Eds. Parasitic birds and their hosts. Oxford: Oxford University Press 1998; pp. 194-211.], such as aggression towards the parasite [9Robertson RJ, Norman RF. The function and evolution of aggressive host behavior towards the brown-headed cowbird (Molothrus ater). Can J Zool 1977; 55: 508-18.
[http://dx.doi.org/10.1139/z77-066] -11Gloag R, Fiorini VD, Reboreda JC, Kacelnik A. The wages of violence: mobbing by mockingbirds as a frontline defence against brood-parasitic cowbirds. Anim Behav 2013; 86: 1023-9.
[http://dx.doi.org/10.1016/j.anbehav.2013.09.007] ], rejection of parasitic eggs [2Rothstein SI. An experimental and teleonomic investigation of avian brood parasitism. Condor 1975; 77: 250-71.
[http://dx.doi.org/10.2307/1366221] , 12Peer BD, Robinson SK, Herkert JR. Egg rejection by cowbird hosts in grasslands. Auk 2000; 117: 892-901.
[http://dx.doi.org/10.1642/0004-8038(2000)117[0892:ERBCHI]2.0.CO;2] -15Igic B, Nunez V, Voss HU, et al. Using 3D printed eggs to examine the egg-rejection behaviour of wild birds. Peer J 2015; 3: e965.], and nest desertion [16Hosoi SA, Rothstein SI. Nest desertion and cowbird parasitism: evidence for evolved responses and evolutionary lag. Anim Behav 2000; 59(4): 823-40.
[http://dx.doi.org/10.1006/anbe.1999.1370] [PMID: 10792938] , 17Servedio MR, Hauber ME. To eject or to abandon? Life history traits of hosts and parasites interact to influence the fitness payoffs of alternative anti-parasite strategies. J Evol Biol 2006; 19(5): 1585-94.
[http://dx.doi.org/10.1111/j.1420-9101.2006.01124.x] [PMID: 16910987] ].
The least costly anti-parasite behavior is aggressive nest defense. Agression towards cowbirds can vary across a population, where sympatric hosts may be more aggressive towards cowbirds than allopatric species [18Briskie JV, Sealy SG, Hobson KA. Behavioral defenses against avian brood parasitism in sympatric and allopatric host populations. Evolution 1992; 46: 334-40.
[http://dx.doi.org/10.2307/2409854] ], but see [8Sealy SG, Neudorf DL, Hobson KA, Gill SA. Nest defense by potential hosts of the brown-headed cowbird: methodological approaches, benefits of defense, and coevolution. In: Rothstein SI, Robinson SK, Eds. Parasitic birds and their hosts. Oxford: Oxford University Press 1998; pp. 194-211.]. Even at low levels of parasitism, aggression around the nest should be favored by selection when it reduces the costs of nest predation and parasitism [8Sealy SG, Neudorf DL, Hobson KA, Gill SA. Nest defense by potential hosts of the brown-headed cowbird: methodological approaches, benefits of defense, and coevolution. In: Rothstein SI, Robinson SK, Eds. Parasitic birds and their hosts. Oxford: Oxford University Press 1998; pp. 194-211., 11Gloag R, Fiorini VD, Reboreda JC, Kacelnik A. The wages of violence: mobbing by mockingbirds as a frontline defence against brood-parasitic cowbirds. Anim Behav 2013; 86: 1023-9.
[http://dx.doi.org/10.1016/j.anbehav.2013.09.007] , 19Rothstein SI. Mechanisms of avian egg recognition: which egg parameters elicit responses by rejecter species? Behav Ecol Sociobiol 1982; 11: 229-39.
[http://dx.doi.org/10.1007/BF00299299] ].
“Rejecters” are potential host species that almost always (< 75%) reject the eggs of brood parasites [2Rothstein SI. An experimental and teleonomic investigation of avian brood parasitism. Condor 1975; 77: 250-71.
[http://dx.doi.org/10.2307/1366221] , 20Cruz A, Wiley WJ. The decline of an adaptation in the absence of a presumed selection pressure. Evolution 1989; 43: 55-62.
[http://dx.doi.org/10.2307/2409163] ]. Hosts may accept cowbird eggs because cost of egg rejection, e.g., a host accidentally rejecting or damaging their own eggs is greater than the cost of acceptance, e.g., reduced reproductive output [21Rohwer S, Spaw CD. Evolutionary lag versus bill-size constraints: a comparative study of the acceptance of cowbird eggs by old hosts. Evol Ecol 1988; 2: 27-36.
[http://dx.doi.org/10.1007/BF02071586] -24Rasmussen JL, Underwood TJ, Sealy SG. Functional morphology as a barrier to the evolution of grasp-ejection in hosts of the Brown-headed Cowbird (Molothrus ater). Can J Zool 2010; 88: 1210-7.
[http://dx.doi.org/10.1139/Z10-088] ]. Smaller hosts with bill-size contraint may not be able to effectively remove a parasitic egg without puncture ejection, a process more likely to damage host eggs [25Rothstein SI. Experiments on defenses cedar waxwings use against cowbird parasitism. Auk 1976; 93: 675-91.-27Sealy SG. Evolution of host defenses against brood parasitism: implications of puncture-ejection by a small passerine. Auk 1996; 113: 346-55.
[http://dx.doi.org/10.2307/4088901] ]. Assuming that rejection is almost always advantageous, some acceptor species have not had enough time to develop the ability to recognize a foreign egg [19Rothstein SI. Mechanisms of avian egg recognition: which egg parameters elicit responses by rejecter species? Behav Ecol Sociobiol 1982; 11: 229-39.
[http://dx.doi.org/10.1007/BF00299299] ] and remove it from the nest [28Davies NB, Brooke M. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. I. Host egg discrimination. J Anim Ecol 1989; 58: 207-24.
[http://dx.doi.org/10.2307/4995] -31Rothstein SI, Robinson SK. Parasitic Birds and Their Hosts Studies in Coevolution. New York, NY: Oxford University Press 1998.].
Anti-parasite responses by potential hosts can vary in sympatry and allopatry with brood parasites [18Briskie JV, Sealy SG, Hobson KA. Behavioral defenses against avian brood parasitism in sympatric and allopatric host populations. Evolution 1992; 46: 334-40.
[http://dx.doi.org/10.2307/2409854] , 28Davies NB, Brooke M. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. I. Host egg discrimination. J Anim Ecol 1989; 58: 207-24.
[http://dx.doi.org/10.2307/4995] , 29Davies NB, Brooke M. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. II. Host egg markings, chick discrimination and general discussion. J Anim Ecol 1989; 58: 225-36.
[http://dx.doi.org/10.2307/4996] , 32Lindholm AK. Tests of phenotypic plasticity in reed warbler defences against cuckoo parasitism. Behaviour 2000; 137: 43-60.
[http://dx.doi.org/10.1163/156853900501863] -34Cruz A, Prather JW, Wiley JW, Weaver P. Increase in Egg Rejection in a Population Exposed to Parasitism: Village Weavers on Hispaniola. Behav Ecol 2008; 19: 398-403.
[http://dx.doi.org/10.1093/beheco/arm147] ], and in some cases defensive behaviors may decline under reduced parasitism pressure [20Cruz A, Wiley WJ. The decline of an adaptation in the absence of a presumed selection pressure. Evolution 1989; 43: 55-62.
[http://dx.doi.org/10.2307/2409163] ], but subsequent work by Lahti [35Lahti DC. Persistence of egg recognition in the absence of cuckoo brood parasitism: pattern and mechanism. Evolution 2006; 60(1): 157-68.
[http://dx.doi.org/10.1111/j.0014-3820.2006.tb01090.x] [PMID: 16568640] ] and Cruz et al. [34Cruz A, Prather JW, Wiley JW, Weaver P. Increase in Egg Rejection in a Population Exposed to Parasitism: Village Weavers on Hispaniola. Behav Ecol 2008; 19: 398-403.
[http://dx.doi.org/10.1093/beheco/arm147] ] found high levels of rejection of nonmimetic eggs. Even when parasitism is rare or absent, anti-parasite egg rejection behavior persists in a population when rejection costs are small [29Davies NB, Brooke M. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. II. Host egg markings, chick discrimination and general discussion. J Anim Ecol 1989; 58: 225-36.
[http://dx.doi.org/10.2307/4996] , 33Rothstein SI. Relic behaviours, coevolution and the retention versus loss of host defences after episodes of avian brood parasitism. Anim Behav 2001; 61(1): 95-107.
[http://dx.doi.org/10.1006/anbe.2000.1570] [PMID: 11170700] , 36Lotem A, Nakamura H, Zahavi A. Constraints on egg discrimination and cuckoo-host co-evolution. Anim Behav 1995; 49: 1185-209.
[http://dx.doi.org/10.1006/anbe.1995.0152] -38Kuehn ML, Peer BD, Rothstein SI. Variation in host response to brood parasitism reflects evolutionary differences and not phenotypic plasticity. Anim Behav 2014; 88: 21-8.
[http://dx.doi.org/10.1016/j.anbehav.2013.11.007] ].
In Colorado, cowbirds have historically been associated with bison (Bison bison) from the Great Plains west to elevations around 3800 m and into the mountain parks of central Colorado [39Chace JF, Cruz A. Range of the brown-headed cowbird in Colorado: past and present. Great Basin Nat 1998; 58: 245-9.]. However, when bison declined in Colorado in the 1800’s the range of the cowbird presumably contracted and was limited to lower elevations, only to reexpand following the establishment of domestic livestock at higher elevations [39Chace JF, Cruz A. Range of the brown-headed cowbird in Colorado: past and present. Great Basin Nat 1998; 58: 245-9.]. Thus, potential cowbird hosts breeding at higher elevations may be experiencing an increase in parasitism rates after a period of little or no parasitism.
The American Robin (Turdus migratorius) is a known cowbird egg rejector species [2Rothstein SI. An experimental and teleonomic investigation of avian brood parasitism. Condor 1975; 77: 250-71.
[http://dx.doi.org/10.2307/1366221] , 15Igic B, Nunez V, Voss HU, et al. Using 3D printed eggs to examine the egg-rejection behaviour of wild birds. Peer J 2015; 3: e965., 18Briskie JV, Sealy SG, Hobson KA. Behavioral defenses against avian brood parasitism in sympatric and allopatric host populations. Evolution 1992; 46: 334-40.
[http://dx.doi.org/10.2307/2409854] , 19Rothstein SI. Mechanisms of avian egg recognition: which egg parameters elicit responses by rejecter species? Behav Ecol Sociobiol 1982; 11: 229-39.
[http://dx.doi.org/10.1007/BF00299299] , 25Rothstein SI. Experiments on defenses cedar waxwings use against cowbird parasitism. Auk 1976; 93: 675-91.]. The geographic distribution of cowbirds and robins in Colorado provided an opportunity to perform an experiment to test how defenses are modified in the absence of parasitism. The breeding range of robins in Colorado occurs from the riparian forests in the grasslands to timberline [40Pantle D. American Robin. In: Kingery HE, Ed. Colorado Breeding Bird Atlas. Denver, CO: Colorado Bird Atlas Partnership 1998; pp. 396-7.]. Cowbirds breed across a wide elevational range as well, but because of the distribution of cattle cowbirds probably do not parasitize frequently above 2400 m [39Chace JF, Cruz A. Range of the brown-headed cowbird in Colorado: past and present. Great Basin Nat 1998; 58: 245-9.]. Here, we compare anti-brood parasite behaviors in robins, breeding sympatrically with cowbirds as lower elevations to that of a population that experiences little exposure to cowbirds at higher elevations.
METHODS
Study Area
Experiments were conducted during June and July, 2000-2002 in Boulder County, Colorado, at two distinct elevational locations of five subsites (Fig. 1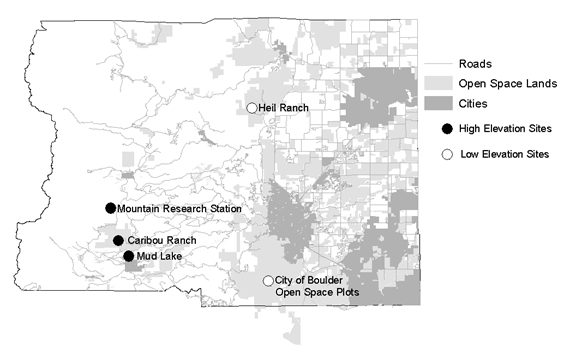 ). Robin nests were located in two low elevation forests, between 1760 m and 1950 m, of foothill ponderosa pine (Pinus ponderosa) primarily on Heil Ranch on Boulder County Open Space but also with City of Boulder Open Space, and in the three high elevation forest sites, between 2600 m and 3350 m, of montane lodgepole pine (Pinus contorta) and Englemann spruce (Picea engelmannii) forests primarily at the University of Colorado’s Mountain Research Station, and also on Boulder County Open Space properties Caribou Ranch and Mud Lake. These three latter sites approach the upper elevational limit of the breeding range of the American Robin in Colorado [41Andrews RA, Righter R. Colorado Birds. Denver, CO: Denver Museum of Natural History 1992.]. Cowbirds were seen and heard daily at the lower elevation sites while at the high elevation sites only one male cowbird was seen at the Mountain Research Station, and male and female cowbirds were seen infrequently at Caribou Ranch and Mud Lake.
). Robin nests were located in two low elevation forests, between 1760 m and 1950 m, of foothill ponderosa pine (Pinus ponderosa) primarily on Heil Ranch on Boulder County Open Space but also with City of Boulder Open Space, and in the three high elevation forest sites, between 2600 m and 3350 m, of montane lodgepole pine (Pinus contorta) and Englemann spruce (Picea engelmannii) forests primarily at the University of Colorado’s Mountain Research Station, and also on Boulder County Open Space properties Caribou Ranch and Mud Lake. These three latter sites approach the upper elevational limit of the breeding range of the American Robin in Colorado [41Andrews RA, Righter R. Colorado Birds. Denver, CO: Denver Museum of Natural History 1992.]. Cowbirds were seen and heard daily at the lower elevation sites while at the high elevation sites only one male cowbird was seen at the Mountain Research Station, and male and female cowbirds were seen infrequently at Caribou Ranch and Mud Lake.
 |
Fig. (1) Study sites in Boulder County, Colorado. Map produced using data Copyright 2001, County of Boulder, Colorado. |
Experimental Parasitism
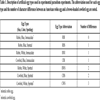
Experimental parasitism was carried out by placing artificial eggs in active robin nests. Robin and cowbird eggs differ in size, ground color, and spotting pattern. Robin eggs are larger than cowbird eggs, blue in ground color as opposed to off-white, and immaculate rather than heavily spotted. By manipulating these characteristics, we tested artificial egg types across a gradient from a robin egg to a cowbird egg (Table 1, Fig. 2 ). Robin-sized eggs (R), were blue immaculate (RBI), blue spotted (RBS), white immaculate (RWI) or white spotted (RWS), while cowbird-sized eggs (C): were blue immaculate (CBI), blue spotted (CBS), or white spotted (CWS). Small, immaculate, white eggs (CWI) were not tested because of their resemblance to fecal sacs, which may elicit rejection for other reasons.
). Robin-sized eggs (R), were blue immaculate (RBI), blue spotted (RBS), white immaculate (RWI) or white spotted (RWS), while cowbird-sized eggs (C): were blue immaculate (CBI), blue spotted (CBS), or white spotted (CWS). Small, immaculate, white eggs (CWI) were not tested because of their resemblance to fecal sacs, which may elicit rejection for other reasons.
Experimental robin egg mimics were tested to ensure that eggs were not rejected based on the artificial qualities of the eggs. Experimental eggs were constructed of self-set Sculpey™ modeling clay, which allowed puncture marks to be detected [36Lotem A, Nakamura H, Zahavi A. Constraints on egg discrimination and cuckoo-host co-evolution. Anim Behav 1995; 49: 1185-209.
[http://dx.doi.org/10.1006/anbe.1995.0152] ]. Eggs were shaped from either white or “robin’s-egg” blue clay. “Turquoise” and “mint” colored clays were mixed to create a blue that matched a robin’s egg. Spotting was created by using acrylic paints in burnt umber and medium gray in a pattern that resembled natural cowbird eggs. Eggs were then placed in a 93ºC oven for 10 min to harden slightly, yet they remained soft enough so that puncture marks could be detected. Finally, the eggs were coated with a waterproof varnish that protected the paint and gave the eggs a slight sheen.
Artificial eggs where within the natural range of egg-size variaion. Robin-sized eggs averaged 28.0 mm in length and 20.5 mm in width, and weighed 6.9 g (n=16). Natural robin eggs average 28.1 mm in length and 20.0 mm in width [42Harrison HH. A field guide to birds’ nests. Boston, MA: Houghton Mifflin Company 1975., 43Baicich PJ, Harrison CJ. A Guide to the Nests, Eggs, and Nestlings of North American Birds. 2nd ed. San Diego, CA: Academic Press 1997.], and weigh 6.3 g [44Sallabanks R, James FC. American robin (Turdus migratorius). In: Pool A, Gill F, Eds. Philadelphia, Pennsylvania: The Birds of North America 1999. No. 462]. Artificial small, cowbird-sized eggs averaged 21.2 mm in length and 16.8 mm in width, and weighed 2.8 g (n=14). Natural cowbird eggs average 21.4 mm in length and 16.4 mm in width [42Harrison HH. A field guide to birds’ nests. Boston, MA: Houghton Mifflin Company 1975., 43Baicich PJ, Harrison CJ. A Guide to the Nests, Eggs, and Nestlings of North American Birds. 2nd ed. San Diego, CA: Academic Press 1997.] and weigh 2.4 g [45Lowther PE. Brown-headed Cowbird (Molothrusater). In: Pool A, Gill F, Eds. Philadelphia, Pennsylvania: The Birds of North America 1993. No. 47]
As part of a larger study, robin nests when found were experimentally parasitized with one of the seven possible egg types (Fig. 2 ) between June 19 and July 18, 2000 (n = 5), May 20 and July 9, 2001 (n = 9), and May 14 and July 1, 2002 (n = 16). Artificial eggs were added to 15 nests at the low elevation sites, all in Heil Ranch, and 15 nests at the high elevation sites but predominately on the Mountain Research Station property (n= 11). In total, two of each artifical egg types (RBI, RBS, RWI, RWS, CBI, CBS) and three of CWS egg type (Fig. 2
) between June 19 and July 18, 2000 (n = 5), May 20 and July 9, 2001 (n = 9), and May 14 and July 1, 2002 (n = 16). Artificial eggs were added to 15 nests at the low elevation sites, all in Heil Ranch, and 15 nests at the high elevation sites but predominately on the Mountain Research Station property (n= 11). In total, two of each artifical egg types (RBI, RBS, RWI, RWS, CBI, CBS) and three of CWS egg type (Fig. 2 ) were added to robin nests at each elevation. All nests were experimentally parasitized during the final egg-laying phase (when clutch was complete) or early incubation stages of nesting. During experimental parasitism one of the artificial eggs (Fig. 2
) were added to robin nests at each elevation. All nests were experimentally parasitized during the final egg-laying phase (when clutch was complete) or early incubation stages of nesting. During experimental parasitism one of the artificial eggs (Fig. 2 ) was added per nest before 1200 h. No robin eggs were removed from the nests when the artificial eggs were added, although that is common during natural parasitism [45Lowther PE. Brown-headed Cowbird (Molothrusater). In: Pool A, Gill F, Eds. Philadelphia, Pennsylvania: The Birds of North America 1993. No. 47]. Several studies have found that rates of egg rejection do not differ when host eggs are removed during experimental parasitism and when they are not [26Davies NB, Brooke M. Cuckoos versus reed warblers: adaptations and counteradaptations. Anim Behav 1988; 36: 262-84.
) was added per nest before 1200 h. No robin eggs were removed from the nests when the artificial eggs were added, although that is common during natural parasitism [45Lowther PE. Brown-headed Cowbird (Molothrusater). In: Pool A, Gill F, Eds. Philadelphia, Pennsylvania: The Birds of North America 1993. No. 47]. Several studies have found that rates of egg rejection do not differ when host eggs are removed during experimental parasitism and when they are not [26Davies NB, Brooke M. Cuckoos versus reed warblers: adaptations and counteradaptations. Anim Behav 1988; 36: 262-84.
[http://dx.doi.org/10.1016/S0003-3472(88)80269-0] , 28Davies NB, Brooke M. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. I. Host egg discrimination. J Anim Ecol 1989; 58: 207-24.
[http://dx.doi.org/10.2307/4995] , 33Rothstein SI. Relic behaviours, coevolution and the retention versus loss of host defences after episodes of avian brood parasitism. Anim Behav 2001; 61(1): 95-107.
[http://dx.doi.org/10.1006/anbe.2000.1570] [PMID: 11170700] , 46Rothstein SI. Evolutionary rates and host defenses against avian brood parasitism. Am Nat 1975; 109: 161-76.
[http://dx.doi.org/10.1086/282984] -48Jackson WM. Egg discrimination and egg-color variability in the northern masked weaver: the importance of conspecific versus interspecific parasitism. In: Rothstein SI, Robinson SK, Eds. Parasitic birds and their hosts. Oxford: Oxford University Press 1998; pp. 407-16.]. Nests were monitored the following day for evidence of acceptance or rejection, and then on the fifth day after the experimental parasitism.
Criteria for Acceptance/Rejection
Two criteria for acceptance were used. Nests were checked approximately 24 hours after experimental parasitism, which was considered the “first-day criterion” (FDC) for rejection [19Rothstein SI. Mechanisms of avian egg recognition: which egg parameters elicit responses by rejecter species? Behav Ecol Sociobiol 1982; 11: 229-39.
[http://dx.doi.org/10.1007/BF00299299] ]. If the eggs remained after one day, the nests were re-checked at five days for the “full-acceptance criterion” (FAC) [19Rothstein SI. Mechanisms of avian egg recognition: which egg parameters elicit responses by rejecter species? Behav Ecol Sociobiol 1982; 11: 229-39.
[http://dx.doi.org/10.1007/BF00299299] ]. Eggs were considered accepted if they remained undamaged in a nest at the time of inspection [19Rothstein SI. Mechanisms of avian egg recognition: which egg parameters elicit responses by rejecter species? Behav Ecol Sociobiol 1982; 11: 229-39.
[http://dx.doi.org/10.1007/BF00299299] , 20Cruz A, Wiley WJ. The decline of an adaptation in the absence of a presumed selection pressure. Evolution 1989; 43: 55-62.
[http://dx.doi.org/10.2307/2409163] , 49Lawes MJ, Kirkman S. Egg recognition and interspecific brood parasitism rates in red bishops (Aves: Ploceidae). Anim Behav 1996; 52: 553-63.
[http://dx.doi.org/10.1006/anbe.1996.0197] ]. Eggs were considered rejected if they were ejected from the nest, damaged, or if the nest was abandoned at the time of inspection.
Analysis of Parasitism Experiments
Rates of egg rejection within and between populations were compared using Fisher’s exact probability tests. For these comparisons, eggs were grouped by size (cowbird vs. robin sized) and coloration (immaculate or spotted).
Nest Defense
Response of Hosts to Parasite Model
A taxidermic mount of a female cowbird was used to assess the aggressive responses of robins to a potential brood parasite. In addition, a Song Sparrow (Melospiza melodia) model was used as a control. The Song Sparrow is similar in shape and size to a cowbird, it is a common breeder in all robin study sites, and poses no threat of predation or parasitism. The mounts were attached to the end of a telescopic pole, which allowed them to be positioned next to nests.
The protocol for model presentations followed those outlined by Sealy et al. [8Sealy SG, Neudorf DL, Hobson KA, Gill SA. Nest defense by potential hosts of the brown-headed cowbird: methodological approaches, benefits of defense, and coevolution. In: Rothstein SI, Robinson SK, Eds. Parasitic birds and their hosts. Oxford: Oxford University Press 1998; pp. 194-211.] and Prather et al. [10Prather JW, Ortega CP, Cruz A. Aggressive responses of red-winged blackbirds (Agelaius phoeniceus) toward brown-headed cowbirds (Molothrus ater) in areas of recent and long-term sympatry. Bird Behav 1999; 13: 1-7.]. Observations were made for 5-minute periods. Behaviors recorded included: no detectable response to the model; distant (greater than 5 m from the model) silent observation; close (less than 5 m from the model) silent observation; vocalization; alarm calling; sitting in the nest; physically attacking the model [9Robertson RJ, Norman RF. The function and evolution of aggressive host behavior towards the brown-headed cowbird (Molothrus ater). Can J Zool 1977; 55: 508-18.
[http://dx.doi.org/10.1139/z77-066] ]. High-pitched calls or screams directed towards the model were considered alarm calls [8Sealy SG, Neudorf DL, Hobson KA, Gill SA. Nest defense by potential hosts of the brown-headed cowbird: methodological approaches, benefits of defense, and coevolution. In: Rothstein SI, Robinson SK, Eds. Parasitic birds and their hosts. Oxford: Oxford University Press 1998; pp. 194-211.]. Other calls or chips were considered vocalizations. The duration of each behavior was recorded in seconds.
Hosts sitting (“cupping”) in their nests in response to a parasite has been described as an anti-parasitic defensive behavior [8Sealy SG, Neudorf DL, Hobson KA, Gill SA. Nest defense by potential hosts of the brown-headed cowbird: methodological approaches, benefits of defense, and coevolution. In: Rothstein SI, Robinson SK, Eds. Parasitic birds and their hosts. Oxford: Oxford University Press 1998; pp. 194-211., 18Briskie JV, Sealy SG, Hobson KA. Behavioral defenses against avian brood parasitism in sympatric and allopatric host populations. Evolution 1992; 46: 334-40.
[http://dx.doi.org/10.2307/2409854] , 50Hobson KA, Sealy SG. Responses of yellow warblers to the threat of cowbird parasitism. Anim Behav 1989; 38: 510-9.
[http://dx.doi.org/10.1016/S0003-3472(89)80044-2] , 51Gill SA, Sealy SG. Nest defence by yellow warblers: recognition of a brood parasite and an avian nest predator. Behaviour 1996; 133: 263-82.
[http://dx.doi.org/10.1163/156853996X00143] ]. Instances of “nest-protection behavior” was scored as “sitting in nest” because the intent could not be determined.
Models were presented at 14 nests at the low elevation sites (Heil Ranch n =13, Boulder Open Space = 1) and 11 nests at the high elevation site (Mountain Research Station n = 10, Caribou Ranch n = 1), between June 20 and July 19, 2000 (n = 13), May 20 and July 9, 2001 (n = 7), and June 18 and July 2, 2002 (n = 5). All observations were carried out once per nest at unparasitized nests during incubation. Both models were each presented once to each nest in random order, sequentially with at least 20 minutes between observations on the same day. Models were presented during the incubation phase of the robin nesting cycle. Because some nests were located after incubation had begun, it was not possible to control for the time since initiation of incubation. However, Knight and Temple [52Knight RL, Temple SA. Why does intensity of avian nest defense increase during the nesting cycle? Auk 1986; 103: 318-27.] reported that aggressive behaviors of robins did not change significantly over the course of the incubation stage of nesting. The models were placed approximately one m from the nest level with the nest edge. Models were set in position while the focal pair was away from the nest to decrease the effects of aggression towards the observer. At least 20 minutes was allowed between presentations to minimize the effects of carry over aggression and habituation to the models [10Prather JW, Ortega CP, Cruz A. Aggressive responses of red-winged blackbirds (Agelaius phoeniceus) toward brown-headed cowbirds (Molothrus ater) in areas of recent and long-term sympatry. Bird Behav 1999; 13: 1-7., 53Robertson RJ, Norman RF. Behavioral defenses to brood parasitism by potential hosts of the brown-headed cowbird. Condor 1976; 78: 166-73.
[http://dx.doi.org/10.2307/1366851] ]. Presentations were video taped and later reviewed.
Analysis of Model Presentation Experiments
Because the behavior of male and female robins was noticeably different during the incubation stage of nesting, only the behaviors of females were analyzed. Females were more likely to be present during the model presentation, and in many cases the male was not seen during the entire observation.
Behaviors were grouped into three categories: non-aggressive, mildly aggressive, or strongly aggressive [10Prather JW, Ortega CP, Cruz A. Aggressive responses of red-winged blackbirds (Agelaius phoeniceus) toward brown-headed cowbirds (Molothrus ater) in areas of recent and long-term sympatry. Bird Behav 1999; 13: 1-7.]. No response and distant silent observation were categorized as non-aggressive; close silent observation,vocalization were classified as mildly aggressive; and alarm calling and physically attacking the model were classified as strongly aggressive. Because behavioral data were not normally distributed, nonparametric tests of variance were performed using JMP11.0 (SAS Institute) to determine if there were significant within and between site differences in time females spent responding non-aggressively, mildly aggressively, and strongly aggressively towards the parasite and control models. One-way Wilcoxon two-sample tests with Bonferoni corrections were used to statistically compare differences in robin responses to sparrow and cowbird models as well as differences to cowbird models between low and high elevation sites.
RESULTS
Experimental Parasitism
There were no cases of nest desertion in response to artificial parasitism. No eggs that remained in the nest, either at one day or five days, appeared to have been damaged. No experimentally parasitized nests were preyed upon while artificial eggs were in the nest.
Differences Between Populations in Rejection Rates
Cowbird-sized eggs were more likely to be rejected at both elevations than robin-sized eggs; however there were no significant differences in rejection rates based on egg size (Table 1). Cowbird-sized eggs were equally likely to be rejected after five days at high and low elevation sites (one-tailed Fisher’s Exact test, P < 0.05; Table 2).
All immaculate, mimetic robin, eggs, were accepted for five days at both the high and low elevation sites (Table 2). Spotted eggs were rejected more often than immaculate eggs at low elevation sites (one-tailed Fisher’s Exact Test, P < 0.05), while there was no significant difference in rejection rates at high elevation sites (Table 2). Rejection rates of spotted eggs was higher at low elevation sites than high elevation sites (one-tailed Fisher’s Exact Test, P < 0.05; Table 2).
Conspecific Brood Parasitism
No evidence was found of conspecific brood parasitism at either the high or low elevation sites. Of 52 nests monitored and/or experimented, no instances of gaps in the egg laying cycle, multiple eggs appearing on one day, or abnormally high clutch sizes were observed.
Nest Defense
Within Sites Comparisons
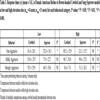
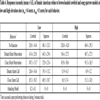
At the low elevation sites, female robins (n = 14) spent nearly significantly (adjusted alpha = 0.008) more time responding non-aggressively to the sparrow model than towards the cowbird model (Z = 3.8385, df = 1, P = 0.05). However, there was no significant difference between mildly aggressive or strongly aggressive behaviors directed towards the cowbird or sparrow models (Tables 3 and 4).
At the high elevation sites, female robins (n = 11) spent statistically equal time in all three aggressive behavioral categories towards sparrow and cowbird models. There was no significant difference between female robins’ behaviors directed towards either the cowbird or sparrow models (Tables 3 and 4).
Between Sites Comparisons
Female robins spent significantly more time responding non-aggressively to the cowbird model at the high elevation sites than at the low elevation sites (Tables 3 and 4). There was a nearly significant difference (adjusted alpha = 0.02) in time female robins spent performing strongly aggressive behaviors towards the cowbird model at the high and low elevation sites (Z= 4.0721, df = 1, P = 0.044; Table 3).
DISCUSSION
Egg Rejection
The results of experimental parasitism suggest that differences exist between the high and low elevation sites in regards to egg recognition and rejection. While mimetic robin eggs and eggs that deviated from robin eggs by only one character were accepted at both the high and low elevation sites, cowbird eggs were rejected less frequently at the high elevation sites than at the low elevation sites.
Similar to Rothstein’s [19Rothstein SI. Mechanisms of avian egg recognition: which egg parameters elicit responses by rejecter species? Behav Ecol Sociobiol 1982; 11: 229-39.
[http://dx.doi.org/10.1007/BF00299299] ] findings that that size is relatively more important than color or spotting pattern in eliciting an early rejection response, our study found that robins were likely to reject smaller eggs at the low elevation sites (Table 2). Smaller eggs may be rejected more quickly because the size difference can be detected by the host through tactile perception [19Rothstein SI. Mechanisms of avian egg recognition: which egg parameters elicit responses by rejecter species? Behav Ecol Sociobiol 1982; 11: 229-39.
[http://dx.doi.org/10.1007/BF00299299] ]. We also detected a possible “threshold effect” for egg rejection in robins at the low elevation sites, similar to the results of Rothstein [19Rothstein SI. Mechanisms of avian egg recognition: which egg parameters elicit responses by rejecter species? Behav Ecol Sociobiol 1982; 11: 229-39.
[http://dx.doi.org/10.1007/BF00299299] ], where only eggs that differed by two or more parameters were rejected. “Runt eggs” (substantially smaller) have been reported to occur frequently in robins, and white or spotted eggs are reported to occur rarely [44Sallabanks R, James FC. American robin (Turdus migratorius). In: Pool A, Gill F, Eds. Philadelphia, Pennsylvania: The Birds of North America 1999. No. 462]. However, the probability of two of these abnormalities occurring simultaneously in any egg is very low, and so a threshold of two differences for rejection minimizes rejection errors.
The results of the parasitism experiments suggest that while some differences exist in rejection rates of cowbird eggs between sites where cowbirds are present and sites where cowbirds are absent or rare, the majority of cowbird eggs were rejected at both sites. This might be explained in a few ways.
First, robins may have retained egg recognition abilities over the period of time in which cowbirds have been absent. If this were the case, it would support the evolutionary lag hypothesis for acceptance versus rejection that suggests that once egg rejection appears in a population, it will remain unchanged. Retention of egg rejection would indicate that the costs of egg rejection behavior are sufficiently small such that the behavior can be retained at essentially no cost to the host [33Rothstein SI. Relic behaviours, coevolution and the retention versus loss of host defences after episodes of avian brood parasitism. Anim Behav 2001; 61(1): 95-107.
[http://dx.doi.org/10.1006/anbe.2000.1570] [PMID: 11170700] , 38Kuehn ML, Peer BD, Rothstein SI. Variation in host response to brood parasitism reflects evolutionary differences and not phenotypic plasticity. Anim Behav 2014; 88: 21-8.
[http://dx.doi.org/10.1016/j.anbehav.2013.11.007] ].
A second explanation is that brood parasitism is more common at the high elevation site than originally thought. This explanation seems unlikely, even though it is known that cowbirds parasitize hosts in other areas at 3000 m and above [39Chace JF, Cruz A. Range of the brown-headed cowbird in Colorado: past and present. Great Basin Nat 1998; 58: 245-9.]. Although the nests of other host species were not monitored to estimate overall rates of cowbird parasitism at the high elevation sites, cowbird sightings at the high elevation sites were infrequent, as compared to sightings being common at the low elevation sites (pers. obs.). It is therefore unlikely that parasitism occurred commonly in the absence of observed adult parasites.
Third, there may be sufficiently high rates of gene flow between the populations of robins, which would result in the maintenance of “rejecter genes” in the high elevation population. Briskie et al. [18Briskie JV, Sealy SG, Hobson KA. Behavioral defenses against avian brood parasitism in sympatric and allopatric host populations. Evolution 1992; 46: 334-40.
[http://dx.doi.org/10.2307/2409854] ] hypothesized that egg rejection in an unparasitized population of robins in Canada may have been due to gene flow between that population and others farther to the south where brood parasites are found. The study sites of Briskie et al. [18Briskie JV, Sealy SG, Hobson KA. Behavioral defenses against avian brood parasitism in sympatric and allopatric host populations. Evolution 1992; 46: 334-40.
[http://dx.doi.org/10.2307/2409854] ] were separated by around 500 km, whereas the sites in this study are no more than 25 km apart along an altitudinal gradient. Because the sites are in such close proximity to one another, gene flow between populations is likely.
Even though gene flow may occur between populations, differences may still exist. A parasitized populations reed warblers (Acrocephalus scirpaceus) in England often reject parasitic Common Cuckoo (Cuculus canorus) eggs, while other unparasitized populations accept parasitic eggs [54Lindholm AK, Thomas RJ. Differences between populations of reed warblers in defences against brood parasitism. Behaviour 2000; 137: 25-42.
[http://dx.doi.org/10.1163/156853900501854] ]. The unparasitized populations in England were separated from parasitized populations by no more than 80 km [54Lindholm AK, Thomas RJ. Differences between populations of reed warblers in defences against brood parasitism. Behaviour 2000; 137: 25-42.
[http://dx.doi.org/10.1163/156853900501854] ]. It seems likely that egg rejection is a plastic trait in these populations. Because parasitism by cuckoos is variable across these habitats, it benefits individuals to express rejection facultatively based on perceived costs of rejection [34Cruz A, Prather JW, Wiley JW, Weaver P. Increase in Egg Rejection in a Population Exposed to Parasitism: Village Weavers on Hispaniola. Behav Ecol 2008; 19: 398-403.
[http://dx.doi.org/10.1093/beheco/arm147] , 54Lindholm AK, Thomas RJ. Differences between populations of reed warblers in defences against brood parasitism. Behaviour 2000; 137: 25-42.
[http://dx.doi.org/10.1163/156853900501854] , 55Stokke BG, Hafstad I, Rudolfsen G, et al. Predictors of resistance to brood parasitism within and among reed warbler populations. Behav Ecol 2008; 19: 612-20.
[http://dx.doi.org/10.1093/beheco/arn007] ].
Although robins seem to have retained the ability to recognize foreign eggs, in that the high elevation robins rejected two of three cowbird eggs, their rejection response appears to have been relaxed. Relaxation of rejection behaviors in the absence of the selective pressures of parasitism suggests that there is some cost to egg rejection. These costs must be sufficient enough [56Croston R, Hauber ME. A recoverable cost of brood parasitism during the nestling stage of the American Robin (Turdus migratorius): implications for the evolution of egg rejection behaviors in a host of the Brown-headed Cowbird (Molothrus ater). Ethol Ecol Evol 2015; 27: 42-55.
[http://dx.doi.org/10.1080/03949370.2013.872195] ] to favor a reduced rate of egg rejection or increased tolerance of foreign eggs. If egg rejection is lost or relaxed in the absence of parasitism, then the equilibrium hypothesis for the evolution of egg rejection behavior is supported. The opposing hypothesis, evolutionary lag, suggests that once egg rejection appears in a population, it will remain unchanged because the costs of rejection are insignificant [33Rothstein SI. Relic behaviours, coevolution and the retention versus loss of host defences after episodes of avian brood parasitism. Anim Behav 2001; 61(1): 95-107.
[http://dx.doi.org/10.1006/anbe.2000.1570] [PMID: 11170700] ]. If egg recognition errors occur at nests of unparasitized populations, acceptance would be favored because these costs would outweigh the nonexistent benefits of rejection. While some studies have found that rejection errors in unparasitized populations can be costly enough to favor acceptance [23Lotem A, Nakamura N. Evolutionary equilibria in avian brood parasitism: an alternative to the ‘arms race-evolutionary lag’ concept. In: Rothstein SI, Robinson SK, Eds. Parasitic birds and their hosts. Oxford: Oxford University Press 1998; pp. 223-35., 37Davies NB, Brooke M, Kacelnik A. Recognition errors and probability of parasitism determine whether reed warblers should accept of reject mimetic cuckoo eggs. Proc Biol Sci 1996; 263: 925-31.
[http://dx.doi.org/10.1098/rspb.1996.0137] , 57Marchetti K. Costs to host defence and the persistence of parasitic cuckoos. Proc Biol Sci 1992; 248(1321): 41-5.
[http://dx.doi.org/10.1098/rspb.1992.0040] [PMID: 1355910] ], other studies suggest that these costs are negligible [33Rothstein SI. Relic behaviours, coevolution and the retention versus loss of host defences after episodes of avian brood parasitism. Anim Behav 2001; 61(1): 95-107.
[http://dx.doi.org/10.1006/anbe.2000.1570] [PMID: 11170700] ]. Ejection costs for American robins have been estimated from 0.03 to 0.08 host eggs lost per ejection [25Rothstein SI. Experiments on defenses cedar waxwings use against cowbird parasitism. Auk 1976; 93: 675-91., 58Lorenzana JC, Sealy SG. Fitness costs and benefits of cowbird egg ejection by gray catbirds. Behav Ecol 2001; 12: 325-9.
[http://dx.doi.org/10.1093/beheco/12.3.325] ], an average cost of ejection for grasp-ejecters (0.06 host eggs lost per ejection) [58Lorenzana JC, Sealy SG. Fitness costs and benefits of cowbird egg ejection by gray catbirds. Behav Ecol 2001; 12: 325-9.
[http://dx.doi.org/10.1093/beheco/12.3.325] ].
Relaxation of egg discrimination in the absence of parasitism suggests that rejection costs may not be negligible for unparasitized populations and sufficient to cause an increased tolerance towards foreign eggs in areas where parasites are absent. Similar to robins in this study, gray catbirds (Dumetella carolinensis) in Bermuda, where no brown-headed cowbirds are present, have demonstrated some degree of increased tolerance in the absence of parasitism [33Rothstein SI. Relic behaviours, coevolution and the retention versus loss of host defences after episodes of avian brood parasitism. Anim Behav 2001; 61(1): 95-107.
[http://dx.doi.org/10.1006/anbe.2000.1570] [PMID: 11170700] , 38Kuehn ML, Peer BD, Rothstein SI. Variation in host response to brood parasitism reflects evolutionary differences and not phenotypic plasticity. Anim Behav 2014; 88: 21-8.
[http://dx.doi.org/10.1016/j.anbehav.2013.11.007] ].
Egg rejection may function as a plastic behavior in robins. Robins at the high elevation sites rejected the majority of cowbird eggs, suggesting that the majority of robins can recognize parasitic eggs. The evolution of egg rejection behavior may involve the ability to recognize one’s own eggs, as well as the decision to reject odd eggs [29Davies NB, Brooke M. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. II. Host egg markings, chick discrimination and general discussion. J Anim Ecol 1989; 58: 225-36.
[http://dx.doi.org/10.2307/4996] ]. In the absence of parasitism, hosts need not lose their ability to reject eggs altogether. Instead, they may relax their discrimination against foreign eggs [33Rothstein SI. Relic behaviours, coevolution and the retention versus loss of host defences after episodes of avian brood parasitism. Anim Behav 2001; 61(1): 95-107.
[http://dx.doi.org/10.1006/anbe.2000.1570] [PMID: 11170700] , 34Cruz A, Prather JW, Wiley JW, Weaver P. Increase in Egg Rejection in a Population Exposed to Parasitism: Village Weavers on Hispaniola. Behav Ecol 2008; 19: 398-403.
[http://dx.doi.org/10.1093/beheco/arm147] ], as was found in this study. Phenotypic plasticity of egg rejection behavior suggests that parasitized and unparasitized populations are equally able to recognize parasitic eggs, but that differences exist in decisions to reject those eggs [34Cruz A, Prather JW, Wiley JW, Weaver P. Increase in Egg Rejection in a Population Exposed to Parasitism: Village Weavers on Hispaniola. Behav Ecol 2008; 19: 398-403.
[http://dx.doi.org/10.1093/beheco/arm147] , 54Lindholm AK, Thomas RJ. Differences between populations of reed warblers in defences against brood parasitism. Behaviour 2000; 137: 25-42.
[http://dx.doi.org/10.1163/156853900501854] ]. If cowbirds are rare or absent, as at the high elevation site, then selection would favor minimizing the costs of rejection and increasing the tolerance of foreign eggs.
Although rejecton may be acting in a plastic manner, we cannot rule out the possibility that robins may have retained egg recognition abilities as a result of robin populations that were exposed to parasitism historically [38Kuehn ML, Peer BD, Rothstein SI. Variation in host response to brood parasitism reflects evolutionary differences and not phenotypic plasticity. Anim Behav 2014; 88: 21-8.
[http://dx.doi.org/10.1016/j.anbehav.2013.11.007] ]. As Kuehn et al. [38Kuehn ML, Peer BD, Rothstein SI. Variation in host response to brood parasitism reflects evolutionary differences and not phenotypic plasticity. Anim Behav 2014; 88: 21-8.
[http://dx.doi.org/10.1016/j.anbehav.2013.11.007] ] noted, in allopatric robins (no cowbirds) in Alaska and sympatric populations in Conneticut and Michigan the “nearly identical responses of sympatric and naïve populations indicates that a high level of exposure to cowbirds is not required for the full expression of rejection behaviour, which suggests that phenotypic plasticity may not explain the reduced responsiveness of allopatric populations.” Similarly, in our study the non-mimetic eggs were rejected at both the high and low elevations sites, but in a more relaxed fashion at the high elevation sites.
Nest Aggression
The results of the model presentation experiments showed that robins at the lower elevation sites alarm called more often when presented with a cowbird model than with a sparrow model, whereas this difference was not found at the high elevation site. Female robins at high elevation sites spent less time responding aggressively to the cowbird model than robins at the low elevation sites while no differences were found between sites in responses to the sparrow model. These results suggest a relaxation of nest defense behaviors of robins at the high elevation sites where cowbirds are infrequent.
It is difficult to determine from these data if the aggressive nest defense behaviors of robins are specific to the threat of brood parasitism or are a generalized response to intruders near the nest. Robins at the high elevation sites were less aggressive towards the cowbird model, yet avian nest predators, e.g., Steller’s Jay (Cyanocitta stelleri), are common at this site. These robins are therefore encountering predators at their nests and would have no reason to have a relaxed generalized response towards any intruder.
CONCLUSION
Studying host defenses against brood parasitism can be useful for understanding the evolution and maintenance of anti-parasitic behaviors. Nest defense may function as a plastic behavior in hosts, that is heightened in regions where the selection pressure is greater and relaxed when the brood parasites are less common. There is a generalized aggressive response to intruders at the nest that would benefit the host because the potential threats of different nest predators are common and suite of potential nest predators varied. The costs of nest defense are minimal compared to the potential costs of recognizing and rejecting a host egg rather than a cowbird egg. While nest defense may be a more general response, egg rejection is very specific to a single species interaction. We found relaxed egg recognition and egg rejection of foreign eggs by robins in high elevations areas with lower cowbird populations, but no difference in the low-cost generalized aggressive response to any intruder at the nest.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
We gratefully acknowledge the field assistance provided by Brian Weaver, Cheryl Todd, and John Prather. Thanks to the City of Boulder Open Space and Mountain Parks, Boulder County Open Space, and the University of Colorado Mountain Research Station for allowing work on their property. Financial support was provided by the Undergraduate Research Opportunities program and the Edna Bailey Sussman Foundation.
REFERENCES
| [1] | Friedmann H. Host relations of the parasitic cowbirds. US Nat Museum Bull 1963; 233: 1-276. [http://dx.doi.org/10.5479/si.03629236.233] |
| [2] | Rothstein SI. An experimental and teleonomic investigation of avian brood parasitism. Condor 1975; 77: 250-71. [http://dx.doi.org/10.2307/1366221] |
| [3] | Ortega CP. Cowbirds and other brood parasites. Tucson, AZ: University of Arizona Press 1998. |
| [4] | Payne RB. The ecology of brood parasitism in birds. Annu Rev Ecol Syst 1977; 8: 1-28. [http://dx.doi.org/10.1146/annurev.es.08.110177.000245] |
| [5] | Marvil RE, Cruz A. Host-parasite interactions between Solitary Vireos (Vireo solitarius) and Brown-headed Cowbirds (Molothrus ater). Auk 1989; 106: 476-80. |
| [6] | Trine CL, Robinson WD, Robinson SK. Consequences of brown-headed cowbird brood parasitism for host population dynamics. In: Rothstein SI, Robinson SK, Eds. Parasitic birds and their hosts. Oxford: Oxford University Press 1998; pp. 194-211. |
| [7] | Davies NB. Cuckoos, cowbirds and other cheats. London: T. A. D. Poyser 2000. |
| [8] | Sealy SG, Neudorf DL, Hobson KA, Gill SA. Nest defense by potential hosts of the brown-headed cowbird: methodological approaches, benefits of defense, and coevolution. In: Rothstein SI, Robinson SK, Eds. Parasitic birds and their hosts. Oxford: Oxford University Press 1998; pp. 194-211. |
| [9] | Robertson RJ, Norman RF. The function and evolution of aggressive host behavior towards the brown-headed cowbird (Molothrus ater). Can J Zool 1977; 55: 508-18. [http://dx.doi.org/10.1139/z77-066] |
| [10] | Prather JW, Ortega CP, Cruz A. Aggressive responses of red-winged blackbirds (Agelaius phoeniceus) toward brown-headed cowbirds (Molothrus ater) in areas of recent and long-term sympatry. Bird Behav 1999; 13: 1-7. |
| [11] | Gloag R, Fiorini VD, Reboreda JC, Kacelnik A. The wages of violence: mobbing by mockingbirds as a frontline defence against brood-parasitic cowbirds. Anim Behav 2013; 86: 1023-9. [http://dx.doi.org/10.1016/j.anbehav.2013.09.007] |
| [12] | Peer BD, Robinson SK, Herkert JR. Egg rejection by cowbird hosts in grasslands. Auk 2000; 117: 892-901. [http://dx.doi.org/10.1642/0004-8038(2000)117[0892:ERBCHI]2.0.CO;2] |
| [13] | Peer BD, Rothstein SI, Kuehn MJ, Fleischer RC. Host defenses against cowbird (Molothrus spp.) parasitism. Implications for cowbird management. Ornithol Monogr 2005; 57: 84-97. [http://dx.doi.org/10.2307/40166816] |
| [14] | Hauber ME, Moskát C, Bán M. Experimental shift in hosts acceptance threshold of inaccurate-mimic brood parasite eggs. Biol Lett 2006; 2(2): 177-80. [http://dx.doi.org/10.1098/rsbl.2005.0438] [PMID: 17148357] |
| [15] | Igic B, Nunez V, Voss HU, et al. Using 3D printed eggs to examine the egg-rejection behaviour of wild birds. Peer J 2015; 3: e965. |
| [16] | Hosoi SA, Rothstein SI. Nest desertion and cowbird parasitism: evidence for evolved responses and evolutionary lag. Anim Behav 2000; 59(4): 823-40. [http://dx.doi.org/10.1006/anbe.1999.1370] [PMID: 10792938] |
| [17] | Servedio MR, Hauber ME. To eject or to abandon? Life history traits of hosts and parasites interact to influence the fitness payoffs of alternative anti-parasite strategies. J Evol Biol 2006; 19(5): 1585-94. [http://dx.doi.org/10.1111/j.1420-9101.2006.01124.x] [PMID: 16910987] |
| [18] | Briskie JV, Sealy SG, Hobson KA. Behavioral defenses against avian brood parasitism in sympatric and allopatric host populations. Evolution 1992; 46: 334-40. [http://dx.doi.org/10.2307/2409854] |
| [19] | Rothstein SI. Mechanisms of avian egg recognition: which egg parameters elicit responses by rejecter species? Behav Ecol Sociobiol 1982; 11: 229-39. [http://dx.doi.org/10.1007/BF00299299] |
| [20] | Cruz A, Wiley WJ. The decline of an adaptation in the absence of a presumed selection pressure. Evolution 1989; 43: 55-62. [http://dx.doi.org/10.2307/2409163] |
| [21] | Rohwer S, Spaw CD. Evolutionary lag versus bill-size constraints: a comparative study of the acceptance of cowbird eggs by old hosts. Evol Ecol 1988; 2: 27-36. [http://dx.doi.org/10.1007/BF02071586] |
| [22] | Brooker M, Brooker L. Acceptance by the splendid fairy-wren of parasitism by Horsfield’s bronze-cuckoo: further evidence for evolutionary equilibrium in brood parasitism. Behav Ecol 1996; 7: 395-407. [http://dx.doi.org/10.1093/beheco/7.4.395] |
| [23] | Lotem A, Nakamura N. Evolutionary equilibria in avian brood parasitism: an alternative to the ‘arms race-evolutionary lag’ concept. In: Rothstein SI, Robinson SK, Eds. Parasitic birds and their hosts. Oxford: Oxford University Press 1998; pp. 223-35. |
| [24] | Rasmussen JL, Underwood TJ, Sealy SG. Functional morphology as a barrier to the evolution of grasp-ejection in hosts of the Brown-headed Cowbird (Molothrus ater). Can J Zool 2010; 88: 1210-7. [http://dx.doi.org/10.1139/Z10-088] |
| [25] | Rothstein SI. Experiments on defenses cedar waxwings use against cowbird parasitism. Auk 1976; 93: 675-91. |
| [26] | Davies NB, Brooke M. Cuckoos versus reed warblers: adaptations and counteradaptations. Anim Behav 1988; 36: 262-84. [http://dx.doi.org/10.1016/S0003-3472(88)80269-0] |
| [27] | Sealy SG. Evolution of host defenses against brood parasitism: implications of puncture-ejection by a small passerine. Auk 1996; 113: 346-55. [http://dx.doi.org/10.2307/4088901] |
| [28] | Davies NB, Brooke M. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. I. Host egg discrimination. J Anim Ecol 1989; 58: 207-24. [http://dx.doi.org/10.2307/4995] |
| [29] | Davies NB, Brooke M. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. II. Host egg markings, chick discrimination and general discussion. J Anim Ecol 1989; 58: 225-36. [http://dx.doi.org/10.2307/4996] |
| [30] | Ward D, Lindholm AK, Smith JN. Multiple parasitism of the red-winged blackbird: further experimental evidence of evolutionary lag in a common host of the brown-headed cowbird. Auk 1996; 113: 408-13. [http://dx.doi.org/10.2307/4088907] |
| [31] | Rothstein SI, Robinson SK. Parasitic Birds and Their Hosts Studies in Coevolution. New York, NY: Oxford University Press 1998. |
| [32] | Lindholm AK. Tests of phenotypic plasticity in reed warbler defences against cuckoo parasitism. Behaviour 2000; 137: 43-60. [http://dx.doi.org/10.1163/156853900501863] |
| [33] | Rothstein SI. Relic behaviours, coevolution and the retention versus loss of host defences after episodes of avian brood parasitism. Anim Behav 2001; 61(1): 95-107. [http://dx.doi.org/10.1006/anbe.2000.1570] [PMID: 11170700] |
| [34] | Cruz A, Prather JW, Wiley JW, Weaver P. Increase in Egg Rejection in a Population Exposed to Parasitism: Village Weavers on Hispaniola. Behav Ecol 2008; 19: 398-403. [http://dx.doi.org/10.1093/beheco/arm147] |
| [35] | Lahti DC. Persistence of egg recognition in the absence of cuckoo brood parasitism: pattern and mechanism. Evolution 2006; 60(1): 157-68. [http://dx.doi.org/10.1111/j.0014-3820.2006.tb01090.x] [PMID: 16568640] |
| [36] | Lotem A, Nakamura H, Zahavi A. Constraints on egg discrimination and cuckoo-host co-evolution. Anim Behav 1995; 49: 1185-209. [http://dx.doi.org/10.1006/anbe.1995.0152] |
| [37] | Davies NB, Brooke M, Kacelnik A. Recognition errors and probability of parasitism determine whether reed warblers should accept of reject mimetic cuckoo eggs. Proc Biol Sci 1996; 263: 925-31. [http://dx.doi.org/10.1098/rspb.1996.0137] |
| [38] | Kuehn ML, Peer BD, Rothstein SI. Variation in host response to brood parasitism reflects evolutionary differences and not phenotypic plasticity. Anim Behav 2014; 88: 21-8. [http://dx.doi.org/10.1016/j.anbehav.2013.11.007] |
| [39] | Chace JF, Cruz A. Range of the brown-headed cowbird in Colorado: past and present. Great Basin Nat 1998; 58: 245-9. |
| [40] | Pantle D. American Robin. In: Kingery HE, Ed. Colorado Breeding Bird Atlas. Denver, CO: Colorado Bird Atlas Partnership 1998; pp. 396-7. |
| [41] | Andrews RA, Righter R. Colorado Birds. Denver, CO: Denver Museum of Natural History 1992. |
| [42] | Harrison HH. A field guide to birds’ nests. Boston, MA: Houghton Mifflin Company 1975. |
| [43] | Baicich PJ, Harrison CJ. A Guide to the Nests, Eggs, and Nestlings of North American Birds. 2nd ed. San Diego, CA: Academic Press 1997. |
| [44] | Sallabanks R, James FC. American robin (Turdus migratorius). In: Pool A, Gill F, Eds. Philadelphia, Pennsylvania: The Birds of North America 1999. No. 462 |
| [45] | Lowther PE. Brown-headed Cowbird (Molothrusater). In: Pool A, Gill F, Eds. Philadelphia, Pennsylvania: The Birds of North America 1993. No. 47 |
| [46] | Rothstein SI. Evolutionary rates and host defenses against avian brood parasitism. Am Nat 1975; 109: 161-76. [http://dx.doi.org/10.1086/282984] |
| [47] | Ortega CP, Cruz A. Mechanisms of egg acceptance by marsh-dwelling blackbirds. Condor 1988; 90: 349-58. [http://dx.doi.org/10.2307/1368563] |
| [48] | Jackson WM. Egg discrimination and egg-color variability in the northern masked weaver: the importance of conspecific versus interspecific parasitism. In: Rothstein SI, Robinson SK, Eds. Parasitic birds and their hosts. Oxford: Oxford University Press 1998; pp. 407-16. |
| [49] | Lawes MJ, Kirkman S. Egg recognition and interspecific brood parasitism rates in red bishops (Aves: Ploceidae). Anim Behav 1996; 52: 553-63. [http://dx.doi.org/10.1006/anbe.1996.0197] |
| [50] | Hobson KA, Sealy SG. Responses of yellow warblers to the threat of cowbird parasitism. Anim Behav 1989; 38: 510-9. [http://dx.doi.org/10.1016/S0003-3472(89)80044-2] |
| [51] | Gill SA, Sealy SG. Nest defence by yellow warblers: recognition of a brood parasite and an avian nest predator. Behaviour 1996; 133: 263-82. [http://dx.doi.org/10.1163/156853996X00143] |
| [52] | Knight RL, Temple SA. Why does intensity of avian nest defense increase during the nesting cycle? Auk 1986; 103: 318-27. |
| [53] | Robertson RJ, Norman RF. Behavioral defenses to brood parasitism by potential hosts of the brown-headed cowbird. Condor 1976; 78: 166-73. [http://dx.doi.org/10.2307/1366851] |
| [54] | Lindholm AK, Thomas RJ. Differences between populations of reed warblers in defences against brood parasitism. Behaviour 2000; 137: 25-42. [http://dx.doi.org/10.1163/156853900501854] |
| [55] | Stokke BG, Hafstad I, Rudolfsen G, et al. Predictors of resistance to brood parasitism within and among reed warbler populations. Behav Ecol 2008; 19: 612-20. [http://dx.doi.org/10.1093/beheco/arn007] |
| [56] | Croston R, Hauber ME. A recoverable cost of brood parasitism during the nestling stage of the American Robin (Turdus migratorius): implications for the evolution of egg rejection behaviors in a host of the Brown-headed Cowbird (Molothrus ater). Ethol Ecol Evol 2015; 27: 42-55. [http://dx.doi.org/10.1080/03949370.2013.872195] |
| [57] | Marchetti K. Costs to host defence and the persistence of parasitic cuckoos. Proc Biol Sci 1992; 248(1321): 41-5. [http://dx.doi.org/10.1098/rspb.1992.0040] [PMID: 1355910] |
| [58] | Lorenzana JC, Sealy SG. Fitness costs and benefits of cowbird egg ejection by gray catbirds. Behav Ecol 2001; 12: 325-9. [http://dx.doi.org/10.1093/beheco/12.3.325] |