- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Conference Proceedings Journal
(Biological Sciences, Chemical Sciences, Physical Sciences, Medicine, Engineering & Technology)
(Discontinued)
ISSN: 2210-2892 ― Volume 10, 2020
Preliminary Assessment of 99mTc-Salmeterol Xinafoate as a Potential Bone Imaging Agent
W.A. Zaghary1, *, M.A. Motaleb2, M.M. Anwar3
Abstract
Sameterol xinafoate is a selective β2-adrenoceptor agonist used in the treatment of some lung diseases and play a vital role in the regulation of bone mass and bone turnover. 99mTc-salmeterol was formulated for the development of a novel potential diagnostic bone imaging agent with excellent biological properties. Factors influencing the labeling yield such as salmeterol xinafoate amount, pH of the reaction medium, reducing agent amount and the reaction time were studied in details. 99mTc-salmeterol was obtained with a high radiochemical yield of 94.6±0.5% and in vitro stability of 6 h when 0.5 mg of salmeterol was mixed with 10 mg NaBH4 at pH 9 and a reaction time 30 min. The biological distribution showed that 99mTc-salmeterol was highly concentrated in the bone (36.5 ± 2%ID/organ) at 30 min post injection. The bone uptake of 99mTc-salmeterol was remained high (29.3 ± 2ID/organ) for a time up to 2h post injection. The results revealed that 99mTc-salmeterol could solve the 99mTc-phosphonate drawbacks and could be used as a bone imaging agent.
Article Information
Identifiers and Pagination:
Year: 2016Volume: 7
First Page: 80
Last Page: 87
Publisher Id: TOPROCJ-7-1-80
DOI: 10.2174/2210289201607010080
Article History:
Received Date: 28/9/2015Revision Received Date: 23/2/2016
Acceptance Date: 24/2/2016
Electronic publication date: 26/04/2016
Collection year: 2016
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution-Non-Commercial 4.0 International Public License (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/legalcode), which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Correspondence: *Address correspondence to this author at the Department of Pharmaceutical Chemistry, College of Pharmacy, Helwan University, Cairo 11795, Egypt; Tel: 9039926286; Emails: wafaa.zaghary@pharm.helwan.edu.eg, wzaghary@yahoo.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 28-9-2015 |
Original Manuscript | Preliminary Assessment of 99mTc-Salmeterol Xinafoate as a Potential Bone Imaging Agent | |
INTRODUCTION
Radionuclide bone imaging is one of the most useful clinical methods for assessing different bone diseases in the whole body and characterized by its high sensitivity and relatively low specificity [1Thrall, J.H. Technetium-99m labeled agents for skeletal imaging. CRC Crit. Rev. Clin. Radiol. Nucl. Med., 1976, 8(1), 1-31.
[PMID: 789010] -3Marì, C.; Catafau, A.; Carriò, I. Bone scintigraphy and metabolic disorders. Q. J. Nucl. Med., 1999, 43(3), 259-267.
[PMID: 10568141] ]. In the last decades, the use of compounds labeled with radioactive isotopes for the skeletal imaging in the medical field has great interest. This nuclear medicine technique can detect cancerous cells, evaluate fractures in the bones and monitor other bone conditions such as infections, arthritis. Also it can detect cancer that has metastasized to the bone from different primary sites, such as the breast, prostate or lungs. It may also be used to evaluate the bone health before treatment [4Yoneda, T.; Sasaki, A.; Mundy, G.R. Osteolytic bone metastasis in breast cancer. Breast Cancer Res. Treat., 1994, 32(1), 73-84.
[http://dx.doi.org/10.1007/BF00666208] [PMID: 7819589] -6Fogelman, I.; Clarke, S.; Cook, G.; Gnanasegaran, G. An Atlas of Clinical Nuclear Medicine, 3rd ed; CRC Press: USA, 2014. https://books.google.com.eg/books?isbn=1841847526.].
Several 99mTc-labeled bone-imaging agents, 18F-labeled NaF and 68Ga-labeled compounds were widely used for skeletal scintigraphy [7Davis, M.A.; Jones, A.L. Comparison of 99mTc-labeled phosphate and phosphonate agents for skeletal imaging. Semin. Nucl. Med., 1976, 6(1), 19-31.
[http://dx.doi.org/10.1016/S0001-2998(76)80033-5] [PMID: 1108208] -10Ogawa, K.; Ishizaki, A.; Takai, K.; Kitamura, Y.; Kiwada, T.; Shiba, K.; Odani, A. Development of novel radiogallium-labeled bone imaging agents using oligo-aspartic acid peptides as carriers. Plos One., 2013, 8(12) e84335.].
Methylene diphosphonate (MDP) and hydroxy-methylene diphosphonate (HMDP) were labeled easily with technetium-99m to be widely used as radiopharmaceuticals for bone imaging [11Subramanian, G.; McAfee, J.G.; Blair, R.J.; Kallfelz, F.A.; Thomas, F.D. Technetium-99m-methylene diphosphonate--a superior agent for skeletal imaging: comparison with other technetium complexes. J. Nucl. Med., 1975, 16(8), 744-755.
[PMID: 170385] -13Shalaby-Rana, E.; Majd, M. (99m)Tc-MDP scintigraphic findings in children with leukemia: value of early and delayed whole-body imaging. J. Nucl. Med., 2001, 42(6), 878-883.
[PMID: 11390551] ]. The most drawbacks of these commercially available skeletal imaging agents were an interval of 2– 6 h is needed between the injection and bone imaging [14Qiu, L.; Cheng, W.; Lin, J.; Luo, S.; Xue, L.; Pan, J. Synthesis and biological evaluation of novel 99mTc-labelled bisphosphonates as superior bone imaging agents. Molecules, 2011, 16(8), 6165-6178.
[http://dx.doi.org/10.3390/molecules16086165] [PMID: 21788926] ]. Decreasing the interval time of patient examination would decrease the burden on both the patients and the medical staff, the total length of the examination and the dose of radiation absorbed.
For a good imaging at an earlier time post injection, a radiopharmaceutical with a higher bone uptake is required [15Ogawa, K.; Mukai, T.; Inoue, Y.; Ono, M.; Saji, H. Development of a novel 99mTc-chelate-conjugated bisphosphonate with high affinity for bone as a bone scintigraphic agent. J. Nucl. Med., 2006, 47(12), 2042-2047.
[PMID: 17138748] ]. Consequently the nature of the organic compounds (pharmaceuticals) is an important factor that determines the advantages of the radiopharmaceuticals for the skeletal imaging [14Qiu, L.; Cheng, W.; Lin, J.; Luo, S.; Xue, L.; Pan, J. Synthesis and biological evaluation of novel 99mTc-labelled bisphosphonates as superior bone imaging agents. Molecules, 2011, 16(8), 6165-6178.
[http://dx.doi.org/10.3390/molecules16086165] [PMID: 21788926] , 16Motaleb, M.A.; Sakr, T.M. Synthesis and preclinical pharmacological evaluation of 99mTc-TEDP as a novel bone imaging agent. J. Labelled Comp. Radiopharm., 2011, 54, 597-601.
[http://dx.doi.org/10.1002/jlcr.1896] ]. Thus, the choice of the most adequate
pharmaceutical for bone targeting must depend on not only the complex formation properties (high labeling yield and stability), but also its biological properties (high bone uptake, in vivo stability and short time interval required for imaging). On the other hand, the pharmacological modulators of β-adrenoceptors play a vital role in the bone mineral density and fracture risk [17Aitken, S.J.; Landao-Bassonga, E.; Ralston, S.H.; Idris, A.I. β2-adrenoreceptor ligands regulate osteoclast differentiation in vitro by direct and indirect mechanisms. Arch. Biochem. Biophys., 2009, 482(1-2), 96-103.
[http://dx.doi.org/10.1016/j.abb.2008.11.012] [PMID: 19059194] ]. Some studies reported that the β2-adrenoceptor agonists influence bone resorption by regulating the expression of RANK-L (receptor activator of nuclear factor kβ ligand) on osteoclast [17Aitken, S.J.; Landao-Bassonga, E.; Ralston, S.H.; Idris, A.I. β2-adrenoreceptor ligands regulate osteoclast differentiation in vitro by direct and indirect mechanisms. Arch. Biochem. Biophys., 2009, 482(1-2), 96-103.
[http://dx.doi.org/10.1016/j.abb.2008.11.012] [PMID: 19059194] -19Bonnet, N.; Benhamou, C.L.; Brunet-Imbault, B.; Arlettaz, A.; Horcajada, M.N.; Richard, O.; Vico, L.; Collomp, K.; Courteix, D. Severe bone alterations under beta2 agonist treatments: bone mass, microarchitecture and strength analyses in female rats. Bone, 2005, 37(5), 622-633.
[http://dx.doi.org/10.1016/j.bone.2005.07.012] [PMID: 16157516] ]. Other studies showed that the β-adrenoceptor antagonists inhibit bone resorption [20de Vries, F.; Pouwels, S.; Bracke, M.; Leufkens, H.G.; Cooper, C.; Lammers, J.W.; van Staa, T.P. Use of beta-2 agonists and risk of hip/femur fracture: a population-based case-control study. Pharmacoepidemiol. Drug Saf., 2007, 16(6), 612-619.
[http://dx.doi.org/10.1002/pds.1318] [PMID: 16998945] , 21Reid, I.R.; Gamble, G.D.; Grey, A.B.; Black, D.M.; Ensrud, K.E.; Browner, W.S. Beta-Blocker use, BMD, and fractures in the study of osteoporotic fractures. J. Bone Miner. Res., 2005, 20(4), 613-618.
[http://dx.doi.org/10.1359/JBMR.041202] [PMID: 15765180] ]. Depending on these findings, it was found the modulators of β-adrenoceptors play an important pharmacological efficacy in the regulation of bone mass and bone turnover. Also, salmeterol xinafoate has been developed as a selective β2-adrenoceptor agonist having the desired pharmacological profile of a long-acting bronchodilator [22Brogden, R.N.; Faulds, D. Salmeterol xinafoate. A review of its pharmacological properties and therapeutic potential in reversible obstructive airways disease. Drugs, 1991, 42(5), 895-912.
[http://dx.doi.org/10.2165/00003495-199142050-00010] [PMID: 1723379] -25Anwar, M.M.; El-Haggar, R.S.; Zaghary, W.A. Salmeterol xinafoate. Profiles Drug Subst. Excip. Relat. Methodol., 2015, 40, 321-369.
[http://dx.doi.org/10.1016/bs.podrm.2015.02.002] [PMID: 26051688] ].
For the purpose of developing new bone imaging agent with excellent biological properties, a new non-phosphonate β2-agonist compound (salmeterol xinafoate) (Fig. 1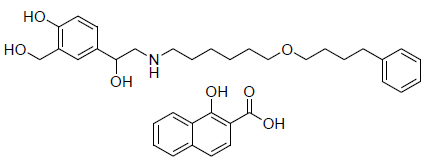 ) was labeled with technetium-99m and its biological properties was investigated.
) was labeled with technetium-99m and its biological properties was investigated.
The aim of this study is assessing efficiently the performance labeling yield of salmeterol xinafoate with technetium-99m efficiently and studying the factors affecting the labeling yield in details. Moreover, this study revealed the biodistribution of 99mTc-salmeterol xinfoate and evaluated it as a useful radiotracer used for in-vivo bone imaging.
 |
Fig. (1) Salmeterol xinfoate. |
EXPERIMENTAL
All of the chemical reagents were of analytical reagent grade and bidistilled water was used for solution preparation. Salmeterol xinafoate was obtained as a gift from King Saud University. Albino Swiss mice, each of 20–25 g were used for the biological distribution study. A NaI(Tl) γ-ray scintillation counter (Scaler Ratemeter SR7, Nuclear Enterprises, Edinburgh, UK) was used for the measurement of γ-ray radioactivity.
Preparation of 99mTc-Salmeterol Xinafoate Complex
Labeling Procedure
Exactly 0.5 mg of salmeterol xinafoate dissolved in ethanol (1mL) was separately transferred to penicillin vials. Exactly 10 mg of NaBH4 was added to each vial along with different amounts of NaOH (0.1 N) or HCl (0.1 N) to adjust the pH value in a range of 3-12. Then, freshly eluted 99mTcO4− (400 MBq) (1mL) was added to each vial. The reaction mixtures were left at room temperature for 30 min. The same procedure was repeated with varying NaBH4 amounts (2-20 mg), varying salmeterol amounts (0.1-1 mg) at different reaction times (1-360 min).
Radiochemical Yield of 99mTc-Salmeterol Xinafoate Complex
The labeling yield percent of the labeled 99mTc-salmeterol xinafoate complex was determined using the ascending paper chromatographic technique. Strips of Whatman No.1 paper chromatography (Whatman International Ltd, Maidstone, Kent, UK) of 13 cm long and 0.5 cm wide were marked at a distance of 2 cm from the lower end and lined into sections, 1 cm each up to 10 cm. A spot from 99mTc-salmeterol xinafoate complex solution was applied using hypodermic syringe and then the strip was developed in an ascending manner in a closed jar. The used developing solvent was acetone. After complete development, the strip was dried and cut into fragments, 1 cm each. Then the sections were assayed in a NaI(Tl) γ-ray scintillation counter.
In Vitro Stability of 99mTc-Salmeterol Complex
The In vitro stability of 99mTc-salmeterol xinafoate complex was investigated as a function of time up to 6 h after labeling.
Biodistribution Studies in Animals
Experiments were performed using the procedure that was approved by the animal ethics committee and was in accordance with the guidelines set out by the Egyptian Atomic Energy Authority. Male Albino Swiss mice weighing 20-25 g were used. The biodistribution of the 99mTc-salmeterol xinafoate complex was evaluated in male Albino Swiss mice (body mass 25-30 g). For the quantitative determination of organ distribution, five mice were used for each experiment and 0.1 mL of about 18 MBq of 99mTc-salmeterol solution was injected into the tail vein of mice. Then the mice were anesthetized and blood was obtained by cardiac puncture. Samples of fresh blood, bone and muscle were collected in pre-weighed vials and counted. The different organs were removed, counted and compared with a standard solution of the labeled salmeterol. The average percent values of the administrated dose/organ were calculated. Blood, bone and muscles were assumed to be 7, 10 and 40%, respectively, of the total body weight [26Rhodes, B.A. Considerations in the radiolabeling of albumin. Semin. Nucl. Med., 1974, 4(3), 281-293.
[http://dx.doi.org/10.1016/S0001-2998(74)80015-2] [PMID: 4601680] ]. Corrections were made for the background radiation and the physical decay during the experiment [27Motaleb, M.A. Preparation, quality control and stability of 99mTc-sparafloxacin complex, a novel agent for detecting sites of infection. J. Label Comp. Radiopharm., 2009, 52, 415-418.
Available from: http://onlinelibrary.wiley.com/doi/10.1002/jlcr.1619/abstract.
[http://dx.doi.org/10.1002/jlcr.1619] ].
RESULTS AND DISCUSSION
Radiolabeling of 99mTc-Salmeterol Xinafoate Complex
Using the ascending paper chromatographic technique, the radiochemical purity of the formed 99mTc-salmeterol xinafoate complex was checked by using acetone as a developing solvent where free 99m TcO4− was moved with the solvent front (Rf = 1), while99mTc-salmeterol xinafoate complex remained at the point of spotting. The radiochemical yield is the mean value of three experiments.
Effect of Ligand Concentration
As shown in Fig. (2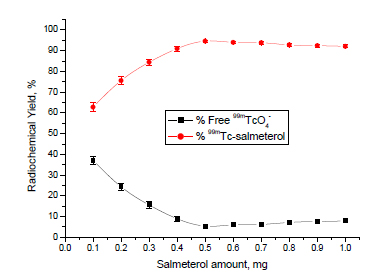 ), the labeling yield of 99mTc-Salmeterol xinafoate complex was low (62.8%) at 0.1 mg salmeterol because the amount of salmeterol is not enough to form complex with all of the reduced technetium-99m [28Bekheet1, Safaa; Tawoosy, M. El ; Massoud, A. A.; Borei, I. H.; Ghanem, H. M.; Motaleb, M. A. 99mTc-Labeled ceftazidime and biological evaluation in experimental animals for detection of bacterial infection. Am. J. Biochem., 2014, 4(2), 15-24.
Available from: http://article.sapub.org/10.5923.j.ajb.20140402.01.html.
] and the yield was increased with increasing the amount of salmeterol till reaching the maximum value of 94.6% at 0.5 mg. The amount of salmeterol at 0.5 mg was sufficient enough to form 99mTc-salmeterol xinafoate complex with a high labeling yield. The labeling efficiency remained stable even with increasing the amount of salmeterol xinafoate above 0.5 mg.
), the labeling yield of 99mTc-Salmeterol xinafoate complex was low (62.8%) at 0.1 mg salmeterol because the amount of salmeterol is not enough to form complex with all of the reduced technetium-99m [28Bekheet1, Safaa; Tawoosy, M. El ; Massoud, A. A.; Borei, I. H.; Ghanem, H. M.; Motaleb, M. A. 99mTc-Labeled ceftazidime and biological evaluation in experimental animals for detection of bacterial infection. Am. J. Biochem., 2014, 4(2), 15-24.
Available from: http://article.sapub.org/10.5923.j.ajb.20140402.01.html.
] and the yield was increased with increasing the amount of salmeterol till reaching the maximum value of 94.6% at 0.5 mg. The amount of salmeterol at 0.5 mg was sufficient enough to form 99mTc-salmeterol xinafoate complex with a high labeling yield. The labeling efficiency remained stable even with increasing the amount of salmeterol xinafoate above 0.5 mg.
Effect of NaBH4 Concentration
The effect of NaBH4 amount was summarized in Fig. (3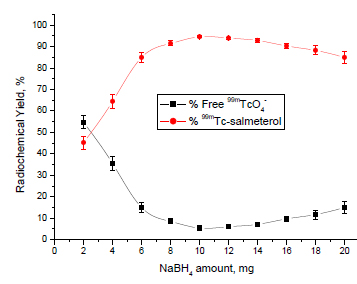 ). The data showed that the radiochemical yield was dependent on the amount of NaBH4 present in the reaction mixture. At NaBH4 of 2 mg, the labeling yield of 99mTc-salmeterol xinafoate was 45.3% because NaBH4 concentration was insufficient to reduce all pertechnetate so the percentage of 99m TcO4- was relatively high (54.7%). The labeling yield was significantly increased by increasing the amount of NaBH4 where the maximum labeling yield of 94.6% was obtained at 10 mg NaBH4. By increasing the amount of NaBH4 above the optimum concentration value, the labeling yield decreased again (85% at 20 mg NaBH4).
). The data showed that the radiochemical yield was dependent on the amount of NaBH4 present in the reaction mixture. At NaBH4 of 2 mg, the labeling yield of 99mTc-salmeterol xinafoate was 45.3% because NaBH4 concentration was insufficient to reduce all pertechnetate so the percentage of 99m TcO4- was relatively high (54.7%). The labeling yield was significantly increased by increasing the amount of NaBH4 where the maximum labeling yield of 94.6% was obtained at 10 mg NaBH4. By increasing the amount of NaBH4 above the optimum concentration value, the labeling yield decreased again (85% at 20 mg NaBH4).
Effect of pH
Radiochemical yield of 99mTc-salmeterol xinafoate complex was affected by changes in the pH values as graphically illustrated in Fig. (4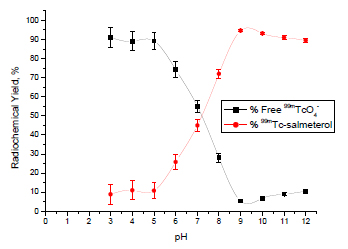 ). The maximum yield was obtained at pH 9. At pH 3, the radiochemical yield was low (8.9%) compared to 94.6% at pH 9. At pH 12, the labeling yield of 99mTc-salmeterol complex was relatively low and equal to 89.5%.
). The maximum yield was obtained at pH 9. At pH 3, the radiochemical yield was low (8.9%) compared to 94.6% at pH 9. At pH 12, the labeling yield of 99mTc-salmeterol complex was relatively low and equal to 89.5%.
Effect of Reaction Time
Fig. (5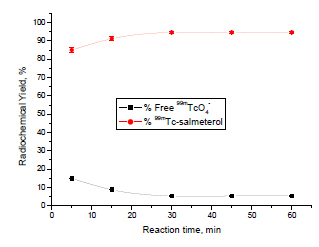 ) describes the labeling yield of 99mTc-salmeterol xinafoate complex at different reaction times. At 5 min reaction time, the percentage of 99mTc-salmeterol complex was relatively small and equal to 85%, this may be because the time required for the reaction between the reduced 99mTc and salmeterol xinafoate was not enough to give the maximum labeling yield. The labeling yield increased with time till reaching its maximum value of 94.6% at 30 min. So, 30 min is the optimum reaction time required to obtain the maximum labeling yield.
) describes the labeling yield of 99mTc-salmeterol xinafoate complex at different reaction times. At 5 min reaction time, the percentage of 99mTc-salmeterol complex was relatively small and equal to 85%, this may be because the time required for the reaction between the reduced 99mTc and salmeterol xinafoate was not enough to give the maximum labeling yield. The labeling yield increased with time till reaching its maximum value of 94.6% at 30 min. So, 30 min is the optimum reaction time required to obtain the maximum labeling yield.
In Vitro Stability
The stability of 99mTc-salmeterol xinafoate was studied in order to determine the suitable time for injection to avoid the formation of the undesired products that result from the radiolysis of the labeled compound. These undesired radioactive products may be accumulated in the non-target organs [29Moustapha, M.E.; Motaleb, M. A.; Ibrahim, I. T. Synthesis of 99mTc-oxybutynin for M3-receptor-mediated imaging of urinary bladder. J. Radioanal. Nucl. Chem., 2011, 287, 35-40. http://link.springer.com/article/10.1007%2Fs10967-010-0794-z
[http://dx.doi.org/10.1007/s10967-010-0794-z] ]. The results showed that 99mTc-salmeterol complex is stable at ~94% up to 6 h, as shown in Fig. (6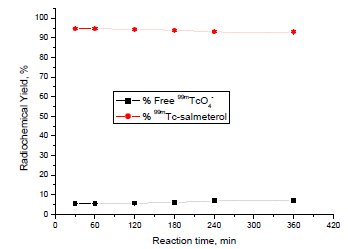 ).
).
Biological Distribution Study
Table 1 shows the biodistribution of 99mTc-salmeterol xinafoate complex in different body organs and fluids in mice at different time intervals after intravenous administration of 99mTc-salmeterol xinafoate complex. The washout of the radioactivity from the body was through both urinary and hepatobiliary pathways where the activity in urine and intestine were about 23 and 14 % at 2 h post injection.
The bone uptake started high and equal to ~ 36.5 % at 30 min then the activity remained relatively high at this level (36.5 - 29 %) up to 2 h post injection. So, excellent skeletal uptake can be obtained faster with 99mTc-salmeterol than with other commercially available radio pharmaceuticals used for skeletal imaging [14Qiu, L.; Cheng, W.; Lin, J.; Luo, S.; Xue, L.; Pan, J. Synthesis and biological evaluation of novel 99mTc-labelled bisphosphonates as superior bone imaging agents. Molecules, 2011, 16(8), 6165-6178.
[http://dx.doi.org/10.3390/molecules16086165] [PMID: 21788926] ]. So, 99mTc-salmeterol could be used to scan the skeletal body bones faster than the other radio pharmaceuticals with low expose of the patients and the operating staff to a high radiation dose.
CONCLUSION
99mTc-salmeterol xinafoate complex is a novel non phosphonate radiopharmaceutical. It was achieved by using 0.5 mg salmeterol, 10 mg NaBH4, at pH 9 and 30 min reaction time. Biological distribution of 99mTc-salmeterol in Albino Swiss mice revealed that 99mTc-salmeterol was rapidly accumulated in the bone with a higher uptake of 36.5% at 30 min post injection than the other commercially available radiopharmaceuticals for bone imaging. The bone uptake was remained high for a time up to 2 h (29%) sufficient for imaging without expose the patient and the medical staff to a high radiation dose. So, 99mTc-salmeterol xinafoate complex could be used for bone imaging and solve the drawbacks of the other commercially available 99mTc-phosphonate derivatives.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared None.
REFERENCES
| [1] | Thrall, J.H. Technetium-99m labeled agents for skeletal imaging. CRC Crit. Rev. Clin. Radiol. Nucl. Med., 1976, 8(1), 1-31. [PMID: 789010] |
| [2] | Love, C.; Din, A.S.; Tomas, M.B.; Kalapparambath, T.P.; Palestro, C.J. Radionuclide bone imaging: an illustrative review. Radiographics, 2003, 23(2), 341-358. [http://dx.doi.org/10.1148/rg.232025103] [PMID: 12640151] |
| [3] | Marì, C.; Catafau, A.; Carriò, I. Bone scintigraphy and metabolic disorders. Q. J. Nucl. Med., 1999, 43(3), 259-267. [PMID: 10568141] |
| [4] | Yoneda, T.; Sasaki, A.; Mundy, G.R. Osteolytic bone metastasis in breast cancer. Breast Cancer Res. Treat., 1994, 32(1), 73-84. [http://dx.doi.org/10.1007/BF00666208] [PMID: 7819589] |
| [5] | Zaman, M.U.; Fatima, N.; Sajjad, Z.; Zaman, U.; Zaman, A.; Tahseen, R. “Pseudo-thyroid lobe”: A diagnostic conundrum caused by ossified anterior longitudinal ligament on bone scan. Indian J. Nucl. Med., 2015, 30(1), 78-79. [http://dx.doi.org/10.4103/0972-3919.147554] [PMID: 25589815] |
| [6] | Fogelman, I.; Clarke, S.; Cook, G.; Gnanasegaran, G. An Atlas of Clinical Nuclear Medicine, 3rd ed; CRC Press: USA, 2014. https://books.google.com.eg/books?isbn=1841847526. |
| [7] | Davis, M.A.; Jones, A.L. Comparison of 99mTc-labeled phosphate and phosphonate agents for skeletal imaging. Semin. Nucl. Med., 1976, 6(1), 19-31. [http://dx.doi.org/10.1016/S0001-2998(76)80033-5] [PMID: 1108208] |
| [8] | Grant, F.D.; Fahey, F.H.; Packard, A.B.; Davis, R.T.; Alavi, A.; Treves, S.T. Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J. Nucl. Med., 2008, 49(1), 68-78. [http://dx.doi.org/10.2967/jnumed.106.037200] [PMID: 18077529] |
| [9] | Fellner, M.; Biesalski, B.; Bausbacher, N.; Kubícek, V.; Hermann, P.; Rösch, F.; Thews, O. (68)Ga-BPAMD: PET-imaging of bone metastases with a generator based positron emitter. Nucl. Med. Biol., 2012, 39(7), 993-999. [http://dx.doi.org/10.1016/j.nucmedbio.2012.04.007] [PMID: 22633217] |
| [10] | Ogawa, K.; Ishizaki, A.; Takai, K.; Kitamura, Y.; Kiwada, T.; Shiba, K.; Odani, A. Development of novel radiogallium-labeled bone imaging agents using oligo-aspartic acid peptides as carriers. Plos One., 2013, 8(12) e84335. |
| [11] | Subramanian, G.; McAfee, J.G.; Blair, R.J.; Kallfelz, F.A.; Thomas, F.D. Technetium-99m-methylene diphosphonate--a superior agent for skeletal imaging: comparison with other technetium complexes. J. Nucl. Med., 1975, 16(8), 744-755. [PMID: 170385] |
| [12] | Cole, T.J.; Balseiro, J.; Lippman, H.R. Technetium-99m-methylene diphosphonate (MDP) uptake in a sympathetic effusion: an index of malignancy and a review of the literature. J. Nucl. Med., 1991, 32(2), 325-327. [PMID: 1992037] |
| [13] | Shalaby-Rana, E.; Majd, M. (99m)Tc-MDP scintigraphic findings in children with leukemia: value of early and delayed whole-body imaging. J. Nucl. Med., 2001, 42(6), 878-883. [PMID: 11390551] |
| [14] | Qiu, L.; Cheng, W.; Lin, J.; Luo, S.; Xue, L.; Pan, J. Synthesis and biological evaluation of novel 99mTc-labelled bisphosphonates as superior bone imaging agents. Molecules, 2011, 16(8), 6165-6178. [http://dx.doi.org/10.3390/molecules16086165] [PMID: 21788926] |
| [15] | Ogawa, K.; Mukai, T.; Inoue, Y.; Ono, M.; Saji, H. Development of a novel 99mTc-chelate-conjugated bisphosphonate with high affinity for bone as a bone scintigraphic agent. J. Nucl. Med., 2006, 47(12), 2042-2047. [PMID: 17138748] |
| [16] | Motaleb, M.A.; Sakr, T.M. Synthesis and preclinical pharmacological evaluation of 99mTc-TEDP as a novel bone imaging agent. J. Labelled Comp. Radiopharm., 2011, 54, 597-601. [http://dx.doi.org/10.1002/jlcr.1896] |
| [17] | Aitken, S.J.; Landao-Bassonga, E.; Ralston, S.H.; Idris, A.I. β2-adrenoreceptor ligands regulate osteoclast differentiation in vitro by direct and indirect mechanisms. Arch. Biochem. Biophys., 2009, 482(1-2), 96-103. [http://dx.doi.org/10.1016/j.abb.2008.11.012] [PMID: 19059194] |
| [18] | Elefteriou, F.; Ahn, J.D.; Takeda, S.; Starbuck, M.; Yang, X.; Liu, X.; Kondo, H.; Richards, W.G.; Bannon, T.W.; Noda, M.; Clement, K.; Vaisse, C.; Karsenty, G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature, 2005, 434(7032), 514-520. [http://dx.doi.org/10.1038/nature03398] [PMID: 15724149] |
| [19] | Bonnet, N.; Benhamou, C.L.; Brunet-Imbault, B.; Arlettaz, A.; Horcajada, M.N.; Richard, O.; Vico, L.; Collomp, K.; Courteix, D. Severe bone alterations under beta2 agonist treatments: bone mass, microarchitecture and strength analyses in female rats. Bone, 2005, 37(5), 622-633. [http://dx.doi.org/10.1016/j.bone.2005.07.012] [PMID: 16157516] |
| [20] | de Vries, F.; Pouwels, S.; Bracke, M.; Leufkens, H.G.; Cooper, C.; Lammers, J.W.; van Staa, T.P. Use of beta-2 agonists and risk of hip/femur fracture: a population-based case-control study. Pharmacoepidemiol. Drug Saf., 2007, 16(6), 612-619. [http://dx.doi.org/10.1002/pds.1318] [PMID: 16998945] |
| [21] | Reid, I.R.; Gamble, G.D.; Grey, A.B.; Black, D.M.; Ensrud, K.E.; Browner, W.S. Beta-Blocker use, BMD, and fractures in the study of osteoporotic fractures. J. Bone Miner. Res., 2005, 20(4), 613-618. [http://dx.doi.org/10.1359/JBMR.041202] [PMID: 15765180] |
| [22] | Brogden, R.N.; Faulds, D. Salmeterol xinafoate. A review of its pharmacological properties and therapeutic potential in reversible obstructive airways disease. Drugs, 1991, 42(5), 895-912. [http://dx.doi.org/10.2165/00003495-199142050-00010] [PMID: 1723379] |
| [23] | Ball, D.I.; Brittain, R.T.; Coleman, R.A.; Denyer, L.H.; Jack, D.; Johnson, M.; Lunts, L.H.; Nials, A.T.; Sheldrick, K.E.; Skidmore, I.F. Salmeterol, a novel, long-acting beta 2-adrenoceptor agonist: characterization of pharmacological activity in vitro and in vivo. Br. J. Pharmacol., 1991, 104(3), 665-671. [http://dx.doi.org/10.1111/j.1476-5381.1991.tb12486.x] [PMID: 1686740] |
| [24] | Adkins, J.C.; McTavish, D. Salmeterol. A review of its pharmacological properties and clinical efficacy in the management of children with asthma. Drugs, 1997, 54(2), 331-354. [http://dx.doi.org/10.2165/00003495-199754020-00011] [PMID: 9257086] |
| [25] | Anwar, M.M.; El-Haggar, R.S.; Zaghary, W.A. Salmeterol xinafoate. Profiles Drug Subst. Excip. Relat. Methodol., 2015, 40, 321-369. [http://dx.doi.org/10.1016/bs.podrm.2015.02.002] [PMID: 26051688] |
| [26] | Rhodes, B.A. Considerations in the radiolabeling of albumin. Semin. Nucl. Med., 1974, 4(3), 281-293. [http://dx.doi.org/10.1016/S0001-2998(74)80015-2] [PMID: 4601680] |
| [27] | Motaleb, M.A. Preparation, quality control and stability of 99mTc-sparafloxacin complex, a novel agent for detecting sites of infection. J. Label Comp. Radiopharm., 2009, 52, 415-418.
Available from: http://onlinelibrary.wiley.com/doi/10.1002/jlcr.1619/abstract.
[http://dx.doi.org/10.1002/jlcr.1619] |
| [28] | Bekheet1, Safaa; Tawoosy, M. El ; Massoud, A. A.; Borei, I. H.; Ghanem, H. M.; Motaleb, M. A. 99mTc-Labeled ceftazidime and biological evaluation in experimental animals for detection of bacterial infection. Am. J. Biochem., 2014, 4(2), 15-24. Available from: http://article.sapub.org/10.5923.j.ajb.20140402.01.html. |
| [29] | Moustapha, M.E.; Motaleb, M. A.; Ibrahim, I. T. Synthesis of 99mTc-oxybutynin for M3-receptor-mediated imaging of urinary bladder. J. Radioanal. Nucl. Chem., 2011, 287, 35-40. http://link.springer.com/article/10.1007%2Fs10967-010-0794-z [http://dx.doi.org/10.1007/s10967-010-0794-z] |