- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Virology Journal
(Discontinued)
ISSN: 1874-3579 ― Volume 15, 2021
Understanding Human Coronavirus HCoV-NL63
Sahar Abdul-Rasool1, §, Burtram C Fielding*, 2, §
Abstract
Even though coronavirus infection of humans is not normally associated with severe diseases, the identification of the coronavirus responsible for the outbreak of severe acute respiratory syndrome showed that highly pathogenic coronaviruses can enter the human population. Shortly thereafter, in Holland in 2004, another novel human coronavirus (HCoV-NL63) was isolated from a seven-month old infant suffering from respiratory symptoms. This virus has subsequently been identified in various countries, indicating a worldwide distribution. HCoV-NL63 has been shown to infect mainly children and the immunocommpromised, who presented with either mild upper respiratory symptoms (cough, fever and rhinorrhoea) or more serious lower respiratory tract involvement such as bronchiolitis and croup, which was observed mainly in younger children. In fact, HCoV-NL63 is the aetiological agent for up to 10% of all respiratory diseases. This review summarizes recent findings of human coronavirus HCoV-NL63 infections, including isolation and identification, phylogeny and taxonomy, genome structure and transcriptional regulation, transmission and pathogenesis, and detection and diagnosis.
Article Information
Identifiers and Pagination:
Year: 2010Volume: 4
First Page: 76
Last Page: 84
Publisher Id: TOVJ-4-76
DOI: 10.2174/1874357901004010076
Article History:
Received Date: 13/11/2009Revision Received Date: 25/1/2010
Acceptance Date: 9/4/2010
Electronic publication date: 25/5/2010
Collection year: 2010
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/), which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Molecular Virology Research Laboratory, Medical Microbiology Cluster, Department of Medical Biosciences, Faculty of Natural Sciences, University of the Western Cape, Private Bag X17, Modderdam Road, Bellville, Western Cape 7535, South Africa; Tel: +27-21-9593620; Fax: +27-21-9593125; E-mail: bfielding@uwc.ac.za§ These authors made equal contribution.
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 13-11-2009 |
Original Manuscript | Understanding Human Coronavirus HCoV-NL63 | |
1.. INTRODUCTION
Regardless of geographic location, respiratory tract infections rank among the top three killers of children under five years of age [1Murray CJL, Lopez AD, Mathers CD, Stein C. The global burden of disease 2000 project: aims, methods and data sources Global programme on evidence for health policy Geneva: World Health Organization 2001.]. A significant proportion of these respiratory tract infections have no known cause. Recently, however, a number of novel coronaviruses have been identified as the causative agents for some of these infections [2van der Hoek L, Pyrc K, Jebbink MF, et al. Identification of a new human coronavirus Nat Med 2004; 10: 368-73., 3Woo PC, Lau SK, Chu CM, et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia J Virol 2005; 79: 884-95.].
Coronaviruses (CoVs) belong to the family Coronaviridae in the order nidovirales. Members of the Coronavirus family are positive-strand RNA viruses with large genomes ranging in size from 27–33 kb. The coronavirus genome encodes for a 5′ replicase polyprotein (ORF1a and ORF1b) that, in turn, encodes for all the enzymes required for viral RNA replication. The genome also encodes for the 3′ structural proteins, including spike (S), envelope (E), membrane (M) and nucleocapsid (N), which are common to all coronaviruses. The structural proteins are involved in various viral processes, including virus particle formation [4Pyrc K, Jebbink MF, Berkhout B, van der Hoek L. Genome structure and transcriptional regulation of human coronavirus NL63 Virol J 2004; 1:7]. Additional subgroup-specific accessory genes are found interspersed among the structural genes, which vary in number and location. Recent studies have shown that the proteins encoded by these genes could be modulators of pathogenicity in the natural host [5Casais R, Davies M, Cavanagh D, Britton P. Gene 5 of the avian coronavirus infectious bronchitis virus is not essential for replication J Virol 2005; 79: 8065-78.-7Haijema BJ, Volders H, Rottier PJ. Live, attenuated coronavirus vaccines through the directed deletion of group-specific genes provide protection against feline infectious peritonitis J Virol 2004; 78: 3863-71.].
Five human coronaviruses have been identified to date, four of which are known to continuously circulate in the human population, especially in young children [8van der Hoek L, Pyrc K, Berkhout B. Human coronavirus NL63, a new respiratory virus FEMS Microbiol Rev 2006; 30: 760-3., 9Chen Z, Wang Y, Ratia K, Mesecar AD, Wilkinson KD, Bake SC. Proteolytic processing and deubiquitinating activity of papain-like proteases of human coronavirus NL63 J Virol 2007; 81: 6007-.]. HCoV-OC43 and HCoV-229E, first identified in the mid-1960s [10Tyrrell DA, Bynoe ML. Cultivation of a novel type of common-cold virus in organ cultures Br Med J 1965; 1: 1467-70., 11Hamre D, Procknow JJ. A new virus isolated from the human respiratory tract Proc Soc Exp Biol Med 1966; 121: 190-3.], were shown to cause the common cold [12Bradburne AF, Bynoe ML, Tyrrell DA. Effects of a "new" human respiratory virus in volunteers Br Med J 1967; 3: 767-9.], but rarely infections of the lower respiratory tract [3Woo PC, Lau SK, Chu CM, et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia J Virol 2005; 79: 884-95.]. A third human coronavirus, which causes severe acute respiratory syndrome, SARS-CoV, was identified in 2003 [13Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome N Engl J Med 2003; 348: 1967-76., 14Rota PA, Oberste MS, Monroe SS, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome Science 2003; 300: 1394-9.]. This virus had a worldwide spread, causing acute respiratory illness with a mortality rate of ~10% [15Fielding BC, Tan Y-J. The Singapore contribution in the battle against SARS Issues Infect Dis 2007; 4: 1-22.]. The last reported SARS-CoV infections were laboratory acquired in 2004, and the virus has not been detected in the human population since [16Kahn JS. The widening scope of coronaviruses Curr Opin Pediatr 2006; 18: 42-7., 17Yip CW, Hon CC, Shi M, et al. Phylogenetic perspectives on the epidemiology and origins of SARS and SARS-like coronaviruses Infect Genet Evol 2009; 9: 1185-96.]. More recently, two additional human coronaviruses were identified; HCoV-HKU1 was isolated from a 71-year-old man who presented with fever and cough [3Woo PC, Lau SK, Chu CM, et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia J Virol 2005; 79: 884-95.], and HCoV-NL63 isolated from a seven-month-old baby [2van der Hoek L, Pyrc K, Jebbink MF, et al. Identification of a new human coronavirus Nat Med 2004; 10: 368-73.]. The latter is the topic of this review.
Several groups have studied different aspects of HCoV-NL63 infections, including its worldwide distribution, its association with human disease, and the replication characteristics of the causative virus. In this review we summarize recent findings of human coronavirus HCoV-NL63 infections, including virus isolation and identification, phylogeny and taxonomy, genome structure and transcriptional regulation, transmission and pathogenesis, and detection and diagnosis.
2.. ISOLATION AND IDENTIFICATION OF HCoV-NL63
HCoV-NL63 was first isolated in Amsterdam in 2004 from the nasopharyngeal aspirate of a seven-month old child; the patient presented with symptoms suggesting respiratory tract infection (coryza, conjunctivitis, and fever), while his chest X-ray showed typical features of bronchiolitis. The aspirate tested negative for all known respiratory viruses. A group of Dutch scientists found that the virus initiated a cytopathic effect when inoculated onto tertiary monkey kidney cells. The group used a new technique, VIDISCA, to clone and amplify the viral genome. VIDISCA is a novel approach that provides a fast and effective tool for amplification of unknown genomes based on cDNA-amplified fragment length polymorphism [2van der Hoek L, Pyrc K, Jebbink MF, et al. Identification of a new human coronavirus Nat Med 2004; 10: 368-73., 18Pyrc K, Jebbink MF, Berkhout B, van der Hoek L. Detection of new viruses by VIDISCA. Virus discovery based on cDNA-amplified fragment length polymorphism Methods Mol Biol 2008; 454: 73-89.]. The virus was identified as a member of the Coronaviridae family. It was shown to be a novel member of Group I coronaviruses because of the similarity of its genome sequence to HCoV-229E. Another group in the Netherlands [19Fouchier RA, Hartwig NG, Bestebroer TM, et al. A previously undescribed coronavirus associated with respiratory disease in humans Proc Natl Acad Sci USA 2004; 101: 6212-.] reported the independent isolation and identification of, essentially, the same virus at about the same time.
3.. PHYLOGENY AND TAXONOMY OF HCoV-NL63
Based on antigenicity, genome organization and sequence homology, coronaviruses are divided into three distinct groups [20Zheng Q, Deng Y, Liu J, van der Hoek L, Berkhout B, Lu M. Core structure of S2 from the human coronavirus NL63 spike glycoprotein Biochemistry 2006; 45: 15205-.]. Group 1 contains transmissible gastroenteritis virus (TGEV), porcine epidemic diarrhea virus (PDEV), feline infectious peritonitis virus (FIPV), canine coronavirus and HCoV-229E, among others. Group 2 contains mouse hepatitis virus (MHV), bovine coronavirus, haemagglutinating encephalomyelitis virus, HCoV-HKU1 and HCoV-OC43, to name a few, with Bat SARS-CoV and SARS-CoV considered distantly related Group 2b coronaviruses. Group 3 contains the avian coronaviruses [21Cheng VC, Lau SK, Woo PC, Yuen KY. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection Clin Microbiol Rev 2007; 20: 660-94.-23Ren W, Li W, Yu M, et al. Full-length genome sequences of two SARS-like coronaviruses in horseshoe bats and genetic variation analysis J Gen Virol 2006; 87: 3355-9.]. Based on phylogenetic analysis, HCoV-NL63 belongs to the Group I coronaviruses [2van der Hoek L, Pyrc K, Jebbink MF, et al. Identification of a new human coronavirus Nat Med 2004; 10: 368-73., 24Pyrc K, Dijkman R, Deng L, et al. Mosaic structure of human coronavirus NL63, one thousand years of evolution J Mol Biol 2006; 364: 964-73.]. Interestingly, evidence of recombination during the evolution of HCoV-NL63 has been reported, and viral isolates have, in fact, a mosaic genome structure. The authors speculate that HCoV-NL63 diverged from a HCoV-229E ancestor in the past, followed by a separation into two distinct HCoV-NL63 lineages. These two lineages recombined during co-infection, giving rise to the two currently observed genotypic subgroups [24Pyrc K, Dijkman R, Deng L, et al. Mosaic structure of human coronavirus NL63, one thousand years of evolution J Mol Biol 2006; 364: 964-73.-27Pyrc K Berkhout, B. van der Hoek L. The novel human coronaviruses NL63 and HKU1 J Virol 2007; 81: 3051-7.]. In fact, recombination between different HCoV-NL63 isolates has been suggested, resulting in a mixture of clinical virus variants circulating in the human population [8van der Hoek L, Pyrc K, Berkhout B. Human coronavirus NL63, a new respiratory virus FEMS Microbiol Rev 2006; 30: 760-3., 27Pyrc K Berkhout, B. van der Hoek L. The novel human coronaviruses NL63 and HKU1 J Virol 2007; 81: 3051-7.-30Minosse C, Selleri M, Zaniratti MS, et al. Phylogenetic analysis of human coronavirus NL63 circulating in Italy J Clin Virol 2008; 43: 114-9.].
3.1.. Genome Structure and Transcriptional Regulation
HCoV-NL63 has a single-stranded RNA genome that is capped and polyadenylated [27Pyrc K Berkhout, B. van der Hoek L. The novel human coronaviruses NL63 and HKU1 J Virol 2007; 81: 3051-7.]. The genome is 27553 bases in size, with the genome order 5′-ORF1a-ORF1b-S-ORF3-E-M-N-polyT-3′ (Fig. 1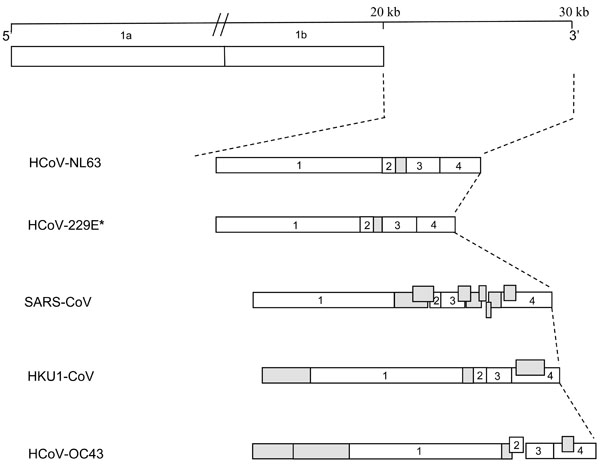 ). Seven distinct ORFs are produced from six distinct mRNAs, which include the full-length genomic RNA and a nested set of five subgenomic (sg) mRNAs [4Pyrc K, Jebbink MF, Berkhout B, van der Hoek L. Genome structure and transcriptional regulation of human coronavirus NL63 Virol J 2004; 1:7]. Coronavirus mRNAs are generated in the membrane-associated replication centers [31Brockway SM, Clay CT, Lu XT, Denison MR. Characterization of the expression, intracellular localization, and replication complex association of the putative mouse hepatitis virus RNA-dependent RNA polymerase J Virol 2003; 77: 10515-27.]. The five sg mRNAs encode for the viral structural and accessory proteins S, ORF3, E, M and N. With the exception of ORF E, a common transcription regulatory sequence (TRS), with core sequence AACUAAA, is located upstream of all the ORFs; this TRS is crucial for sg mRNA formation [27Pyrc K Berkhout, B. van der Hoek L. The novel human coronaviruses NL63 and HKU1 J Virol 2007; 81: 3051-7., 32Pyrc K, Berkhout B, van der Hoek L. Antiviral strategies against human coronaviruses Infect Disord Drug Targets 2007; 7: 59-66.]. HCoV-NL63 uses a discontinuous replication strategy to generate sg mRNAs during the minus strand synthesis [4Pyrc K, Jebbink MF, Berkhout B, van der Hoek L. Genome structure and transcriptional regulation of human coronavirus NL63 Virol J 2004; 1:7, 27Pyrc K Berkhout, B. van der Hoek L. The novel human coronaviruses NL63 and HKU1 J Virol 2007; 81: 3051-7., 32Pyrc K, Berkhout B, van der Hoek L. Antiviral strategies against human coronaviruses Infect Disord Drug Targets 2007; 7: 59-66.], which are then copied into plus strand mRNAs. All plus strand mRNAs share a common ~70 nucleotide leader sequence at their 5’ ends that is identical to the sequence at the 5’ end of the genomic RNA [27Pyrc K Berkhout, B. van der Hoek L. The novel human coronaviruses NL63 and HKU1 J Virol 2007; 81: 3051-7.].
). Seven distinct ORFs are produced from six distinct mRNAs, which include the full-length genomic RNA and a nested set of five subgenomic (sg) mRNAs [4Pyrc K, Jebbink MF, Berkhout B, van der Hoek L. Genome structure and transcriptional regulation of human coronavirus NL63 Virol J 2004; 1:7]. Coronavirus mRNAs are generated in the membrane-associated replication centers [31Brockway SM, Clay CT, Lu XT, Denison MR. Characterization of the expression, intracellular localization, and replication complex association of the putative mouse hepatitis virus RNA-dependent RNA polymerase J Virol 2003; 77: 10515-27.]. The five sg mRNAs encode for the viral structural and accessory proteins S, ORF3, E, M and N. With the exception of ORF E, a common transcription regulatory sequence (TRS), with core sequence AACUAAA, is located upstream of all the ORFs; this TRS is crucial for sg mRNA formation [27Pyrc K Berkhout, B. van der Hoek L. The novel human coronaviruses NL63 and HKU1 J Virol 2007; 81: 3051-7., 32Pyrc K, Berkhout B, van der Hoek L. Antiviral strategies against human coronaviruses Infect Disord Drug Targets 2007; 7: 59-66.]. HCoV-NL63 uses a discontinuous replication strategy to generate sg mRNAs during the minus strand synthesis [4Pyrc K, Jebbink MF, Berkhout B, van der Hoek L. Genome structure and transcriptional regulation of human coronavirus NL63 Virol J 2004; 1:7, 27Pyrc K Berkhout, B. van der Hoek L. The novel human coronaviruses NL63 and HKU1 J Virol 2007; 81: 3051-7., 32Pyrc K, Berkhout B, van der Hoek L. Antiviral strategies against human coronaviruses Infect Disord Drug Targets 2007; 7: 59-66.], which are then copied into plus strand mRNAs. All plus strand mRNAs share a common ~70 nucleotide leader sequence at their 5’ ends that is identical to the sequence at the 5’ end of the genomic RNA [27Pyrc K Berkhout, B. van der Hoek L. The novel human coronaviruses NL63 and HKU1 J Virol 2007; 81: 3051-7.].
 |
Fig. (1) Schematic comparison of the genome organization of coronaviruses infecting humans. Genomic maps shown are based on the complete genome sequences (NCBI accession numbers are shown in brackets): HCoV-NL63: Human coronavirus HCOV-NL63 (NC_005831); HCoV-229E: Human coronavirus 229E (NC_002645); SARS-CoV: Severe acute respiratory syndrome coronavirus (NC_004718); HKU1-CoV: Human coronavirus HKU1 (NC_006577); HCoV-OC43: Human coronavirus OC43 (NC_005147). ORFs S (1), E (2), M (3) and N (4) are shown and open reading frames encoding for accessory genes are shaded in grey. *ORF4 of HCoV-229E is shown as a single open reading frame [34Dijkman R, Jebbink MF, Wilbrink B, et al. Human coronavirus 229E encodes a single ORF4 protein between the spike and the envelope genes Virol J 2006; 3: 106.]. |
3.1.1. . Protein 1a/1b
HCoV-NL63 ORF1a/1b contains a putative elaborated pseudoknot structure that triggers a -1 ribosomal frameshift to translate the complete 1ab polyprotein; for a mini-review of the putative ORF1a/1b products and functions see Pryc et al. (2007) [27Pyrc K Berkhout, B. van der Hoek L. The novel human coronaviruses NL63 and HKU1 J Virol 2007; 81: 3051-7.] and Van der Hoek et al. (2006) [8van der Hoek L, Pyrc K, Berkhout B. Human coronavirus NL63, a new respiratory virus FEMS Microbiol Rev 2006; 30: 760-3.]. Chen and colleagues identified processed products nsp3 and nsp4 of the HCoV-NL63 replicase polyprotein, which could be detected at 24 hours post-infection. These products localize in the peri-nuclear sites of virus infected cells. Also, the group identified and characterized two viral papain-like proteases, PLP1 and PLP2, which process the viral replicase polyprotein. Interestingly, the PLP2 protease has deubiquitinating activity [9Chen Z, Wang Y, Ratia K, Mesecar AD, Wilkinson KD, Bake SC. Proteolytic processing and deubiquitinating activity of papain-like proteases of human coronavirus NL63 J Virol 2007; 81: 6007-.], although the function of this is not clear in viral replication.
3.1.2. . Spike Protein
The species tropism and virulence of a particular coronavirus are largely determined by the spike (S) glycoprotein. Coronavirus S proteins mediate attachment to the cellular receptors and subsequent fusion of the virus and cell membrane [20Zheng Q, Deng Y, Liu J, van der Hoek L, Berkhout B, Lu M. Core structure of S2 from the human coronavirus NL63 spike glycoprotein Biochemistry 2006; 45: 15205-.]. The N-terminal portion of HCoV-NL63 S contains a unique 179 amino acid domain not present in other coronaviruses. This region represents the most variable region of the HCoV-NL63 genome and a role in immune evasion for this region has been proposed [8van der Hoek L, Pyrc K, Berkhout B. Human coronavirus NL63, a new respiratory virus FEMS Microbiol Rev 2006; 30: 760-3., 27Pyrc K Berkhout, B. van der Hoek L. The novel human coronaviruses NL63 and HKU1 J Virol 2007; 81: 3051-7.]. HCoV-NL63 S is a single-chain glycoprotein and consists of an N-terminal receptor-binding domain (S1) and a C-terminal transmembrane fusion domain (S2). S2 consists of two highly conserved heptad-repeat (HR) sequences that are larger than the corresponding regions for the group II and group III coronaviruses [20Zheng Q, Deng Y, Liu J, van der Hoek L, Berkhout B, Lu M. Core structure of S2 from the human coronavirus NL63 spike glycoprotein Biochemistry 2006; 45: 15205-., 27Pyrc K Berkhout, B. van der Hoek L. The novel human coronaviruses NL63 and HKU1 J Virol 2007; 81: 3051-7., 33Bosch BJ, Martina BE, Van Der Zee R, et al. Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides Proc Natl Acad Sci USA 2004; 101: 8455-60.]. Initial proteolytic studies of the S2 fusion core identified an α-helical domain consisting of a trimer of the HR segments N57 and C42. The resolved crystal structure of this trimeric complex shows distinctive high-affinity conformations of interacting cross-sectional layers of six helices. It has been suggested that the larger HR regions of the group I coronaviruses may be required to prime the S proteins for their fusion-activating conformational changes during entry of the virus [20Zheng Q, Deng Y, Liu J, van der Hoek L, Berkhout B, Lu M. Core structure of S2 from the human coronavirus NL63 spike glycoprotein Biochemistry 2006; 45: 15205-.].
3.1.3.. Accessory Protein ORF3
Coronavirus genomes contain accessory genes found interspersed among the structural genes, which vary in number and location between the different coronavirus groups (Fig. 1 ). Both HCoV-NL63 and HCoV-229E are group I coronaviruses and encode for only one accessory gene product between the S and E genes [34Dijkman R, Jebbink MF, Wilbrink B, et al. Human coronavirus 229E encodes a single ORF4 protein between the spike and the envelope genes Virol J 2006; 3: 106.]. Accessory genes are poorly characterized and the functions of the gene products are not well understood. Initial research into the functions of these genes has shown that they are non-essential and dispensable for virus growth in cell culture [5Casais R, Davies M, Cavanagh D, Britton P. Gene 5 of the avian coronavirus infectious bronchitis virus is not essential for replication J Virol 2005; 79: 8065-78., 6de Haan CA, Masters PS, Shen X, Weiss S, Rottier PJ. The group-specific murine coronavirus genes are not essential, but their deletion, by reverse genetics, is attenuating in the natural host Virology 2002; 296: 177-89., 35Hodgson T, Britton P, Cavanagh D. Neither the RNA nor the proteins of open reading frames 3a and 3b of the coronavirus infectious bronchitis virus are essential for replication J Virol 2006; 80: 296-305.]. More recent studies have shown that the accessory genes are required for in vivo infection and pathogenecity in the natural host [6de Haan CA, Masters PS, Shen X, Weiss S, Rottier PJ. The group-specific murine coronavirus genes are not essential, but their deletion, by reverse genetics, is attenuating in the natural host Virology 2002; 296: 177-89., 7Haijema BJ, Volders H, Rottier PJ. Live, attenuated coronavirus vaccines through the directed deletion of group-specific genes provide protection against feline infectious peritonitis J Virol 2004; 78: 3863-71., 36Cavanagh D, Casais R, Armesto M, et al. Manipulation of the infectious bronchitis coronavirus genome for vaccine development and analysis of the accessory proteins Vaccine 2007; 25: 5558-62.-38Pewe L, Zhou H, Netland J, et al. A SARS-CoV-specific protein enhances virulence of an attenuated strain of mouse hepatitis virus Adv Exp Med Biol 2006; 581: 493-8.].
). Both HCoV-NL63 and HCoV-229E are group I coronaviruses and encode for only one accessory gene product between the S and E genes [34Dijkman R, Jebbink MF, Wilbrink B, et al. Human coronavirus 229E encodes a single ORF4 protein between the spike and the envelope genes Virol J 2006; 3: 106.]. Accessory genes are poorly characterized and the functions of the gene products are not well understood. Initial research into the functions of these genes has shown that they are non-essential and dispensable for virus growth in cell culture [5Casais R, Davies M, Cavanagh D, Britton P. Gene 5 of the avian coronavirus infectious bronchitis virus is not essential for replication J Virol 2005; 79: 8065-78., 6de Haan CA, Masters PS, Shen X, Weiss S, Rottier PJ. The group-specific murine coronavirus genes are not essential, but their deletion, by reverse genetics, is attenuating in the natural host Virology 2002; 296: 177-89., 35Hodgson T, Britton P, Cavanagh D. Neither the RNA nor the proteins of open reading frames 3a and 3b of the coronavirus infectious bronchitis virus are essential for replication J Virol 2006; 80: 296-305.]. More recent studies have shown that the accessory genes are required for in vivo infection and pathogenecity in the natural host [6de Haan CA, Masters PS, Shen X, Weiss S, Rottier PJ. The group-specific murine coronavirus genes are not essential, but their deletion, by reverse genetics, is attenuating in the natural host Virology 2002; 296: 177-89., 7Haijema BJ, Volders H, Rottier PJ. Live, attenuated coronavirus vaccines through the directed deletion of group-specific genes provide protection against feline infectious peritonitis J Virol 2004; 78: 3863-71., 36Cavanagh D, Casais R, Armesto M, et al. Manipulation of the infectious bronchitis coronavirus genome for vaccine development and analysis of the accessory proteins Vaccine 2007; 25: 5558-62.-38Pewe L, Zhou H, Netland J, et al. A SARS-CoV-specific protein enhances virulence of an attenuated strain of mouse hepatitis virus Adv Exp Med Biol 2006; 581: 493-8.].
Unlike the SARS-CoV genome, the HCoV-NL63 genome encodes for only one accessory protein, ORF3 (Fig. 1 ). ORF3 is expressed from distinct subgenomic (sg) mRNA 3, which is one of at least six distinct mRNAs [4Pyrc K, Jebbink MF, Berkhout B, van der Hoek L. Genome structure and transcriptional regulation of human coronavirus NL63 Virol J 2004; 1:7]. The ORF3 gene encodes for a putative 225 amino acid protein, about 25.6 kDa in size. Pyrc et al. (2004) reports that the HCoV-NL63 ORF3 gene has a unique nucleotide composition and appears as a U-rich and A-poor region within the genome, indicating a recent gene transfer event from another viral or cellular origin [4Pyrc K, Jebbink MF, Berkhout B, van der Hoek L. Genome structure and transcriptional regulation of human coronavirus NL63 Virol J 2004; 1:7]. The ORF3 protein of HCoV-NL63 is homologous to proteins of the other Group 1 coronaviruses, with an amino acid sequence most similar (43% identity and 62% similarity) to HCoV-229E ORF4. Based on comparative in silico analysis of HCoV-NL63 ORF3 and other human coronavirus ORF3-like homologues, Fielding and Suliman speculate that the protein could contribute to pathogenesis in the natural host [39Fielding BC, Suliman T. Comparative analysis of human coronavirus-NL63 ORF3 protein homologues Afr J Biotechnol 2009; 8: 3175-8.]. More recently, studies have shown that HCoV-NL63 localizes along the secretory pathway (ERGIC, Golgi, plasma membrane) and co-localizes with the structural proteins M and E in the ERGIC. Also, studies have shown that this N-glycosylated protein is incorporated into virions during assembly. This further suggests an important function for HCoV-NL63 ORF3, particularly in virus assembly and/or budding from the infected cell [40Muller MA, van der Hoek L, Voss D, et al. Human coronavirus NL63 open reading frame 3 encodes a virion-incorporated N-glycosylated membrane protein Virol J 2010; 7: 6.].
). ORF3 is expressed from distinct subgenomic (sg) mRNA 3, which is one of at least six distinct mRNAs [4Pyrc K, Jebbink MF, Berkhout B, van der Hoek L. Genome structure and transcriptional regulation of human coronavirus NL63 Virol J 2004; 1:7]. The ORF3 gene encodes for a putative 225 amino acid protein, about 25.6 kDa in size. Pyrc et al. (2004) reports that the HCoV-NL63 ORF3 gene has a unique nucleotide composition and appears as a U-rich and A-poor region within the genome, indicating a recent gene transfer event from another viral or cellular origin [4Pyrc K, Jebbink MF, Berkhout B, van der Hoek L. Genome structure and transcriptional regulation of human coronavirus NL63 Virol J 2004; 1:7]. The ORF3 protein of HCoV-NL63 is homologous to proteins of the other Group 1 coronaviruses, with an amino acid sequence most similar (43% identity and 62% similarity) to HCoV-229E ORF4. Based on comparative in silico analysis of HCoV-NL63 ORF3 and other human coronavirus ORF3-like homologues, Fielding and Suliman speculate that the protein could contribute to pathogenesis in the natural host [39Fielding BC, Suliman T. Comparative analysis of human coronavirus-NL63 ORF3 protein homologues Afr J Biotechnol 2009; 8: 3175-8.]. More recently, studies have shown that HCoV-NL63 localizes along the secretory pathway (ERGIC, Golgi, plasma membrane) and co-localizes with the structural proteins M and E in the ERGIC. Also, studies have shown that this N-glycosylated protein is incorporated into virions during assembly. This further suggests an important function for HCoV-NL63 ORF3, particularly in virus assembly and/or budding from the infected cell [40Muller MA, van der Hoek L, Voss D, et al. Human coronavirus NL63 open reading frame 3 encodes a virion-incorporated N-glycosylated membrane protein Virol J 2010; 7: 6.].
4.. TRANSMISSION AND PATHOGENESIS
4.1.. Mode of Entry into the Cell
It is well documented that SARS-CoV and HCoV-NL63 use the same receptor, angiotensin converting enzyme (ACE)-2, for entry into the host cell [41Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury Nat Med 2005; 11: 875-9., 42Hofmann H, Pyrc K, van der Hoek L, Geier M, Berkhout B, Pohlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry Proc Natl Acad Sci USA 2005; 102: 7988-93.]. However, the consequences following the entry are very different; SARS-CoV causes severe respiratory distress, while HCoV-NL63 might lead to a mild respiratory infection. This was attributed to the ability of each virus receptor-binding protein, spike or S protein, to bind to ACE-2 on the cell surface. It was found that the HCoV-NL63 S protein has a weaker interaction with ACE-2 than the SARS-CoV S protein. This lower-affinity interaction with ACE-2 might partly explain the different pathological consequences of infection by SARS-CoV and HCoV-NL63 [43Mathewson AC, Bishop A, Yao Y. Interaction of severe acute respiratory syndrome-coronavirus and NL63 coronavirus spike proteins with angiotensin converting enzyme-2 J Gen Virol 2008; 89: 2741-5.].
Nevertheless, high pathogenicity is expected to evolve with coronaviruses. As for HCoV-NL63, the possibility of the development of a recombinant virus variant is high because of its high prevalence and the possibility of a recombination event through co-infection [24Pyrc K, Dijkman R, Deng L, et al. Mosaic structure of human coronavirus NL63, one thousand years of evolution J Mol Biol 2006; 364: 964-73.]. Moreover, the virus is able to survive for up to seven days in an aqueous solution and respiratory secretions and remains infective at room temperature. In heavily populated regions, direct person-to-person transmission is considered the major route of HCoV-NL63 spread [42Hofmann H, Pyrc K, van der Hoek L, Geier M, Berkhout B, Pohlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry Proc Natl Acad Sci USA 2005; 102: 7988-93., 44Muller A, Tillmann RL, Muller A, Simon A, Schildgen O. Stability of human metapneumovirus and human coronavirus NL63 on medical instruments and in the patient environment J Hosp Infect 2008; 69: 406-8.].
4.2.. Seasonal Incidence
The virus has been shown to have a worldwide distribution and was observed primarily in the winter season in temperate climates. On the other hand, countries with extreme weather, like Canada, have also shown virus activity around January to March, although milder symptoms were reported [45Bastien N, Anderson K, Hart L, et al. Human coronavirus NL63 infection in Canada J Infect Dis 2005; 191: 503-6.]. Interestingly, seasonal variations have been reported in China where infection with HCoV-NL63 appeared mainly in spring and summer [26Chiu SS, Chan KH, Chu KW, et al. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China Clin Infect Dis 2005; 40: 1721-9.]. Also, a recent study of coronaviruses in Thailand did not show any seasonal predilection [46Dare RK, Fry AM, Chittaganpitch M, et al. Human coronavirus infections in rural Thailand: a comprehensive study using real-time reverse-transcription polymerase chain reaction assays J Infect Dis 2007; 196: 1321-8.], while Wu et al. (2007) reported that the virus is detected during the autumn season in Taiwan [47Wu PS, Chang LY, Berkhout B, et al. Clinical manifestations of human coronavirus NL63 infection in children in Taiwan Eur J Pediatr 2008; 167: 75-80.]. It is evident that the virus has no predilection to a particular season and is not affected by temperature variations as infections can occur throughout the year (Table 1).
4.3.. Prevalence
The human coronaviruses account for a significant number of hospitalization for children under 18 years of age, the elderly and immunocompromised individuals. In fact, a one-year study of children hospitalized in Hong Kong (China) has shown that respiratory tract infection with coronaviruses accounts for 4.4% of all admissions for acute respiratory infections. Of these, HCoV-NL63 was the most common coronavirus identified with an incidence of 2.6% [48Chan KC, Tang NL, Hui DS, et al. Absence of association between angiotensin converting enzyme polymorphism and development of adult respiratory distress syndrome in patients with severe acute respiratory syndrome: a case control study BMC Infect Dis 2005; 5: 26.]. Moreover, a study in Japan has shown that out of 419 specimens that tested negative for common respiratory viruses, five (1.2%) were positive for human coronavirus HCoV-NL63 [49Suzuki A, Okamoto M, Ohmi A, Watanabe O, Miyabayashi S, Nishimura H. Detection of human coronavirus-NL63 in children in Japan Pediatr Infect Dis J 2005; 24: 645-.]. Another Japanese report has indicated that out of 118 nasopharyngeal swab samples obtained from hospitalized children younger than two years of age, three (2.5%) were positive for HCoV-NL63 [50Ebihara T, Endo R, Ma X, Ishiguro N, Kikuta H. Detection of human coronavirus NL63 in young children with bronchiolitis J Med Virol 2005; 75: 463-5.].
In Europe, high prevalence was also noted. In Italy, a study conducted on 322 infants suffering from acute respiratory disease has shown that 8.7% of the cases examined were caused by coronaviruses, with HCoV-NL63 accounting for 21.4% of the latter [51Canducci F, Debiaggi M, Sampaolo M, et al. Two-year prospective study of single infections and co-infections by respiratory syncytial virus and viruses identified recently in infants with acute respiratory disease J Med Virol 2008; 80: 716-23.]. In France, 300 respiratory specimens were checked for HCoV-NL63 presence; of the 300 samples, 28 (9.3%) were positive [29Vabret A, Mourez T, Dina J, et al. Human coronavirus NL63, France Emerg Infect Dis 2005; 11: 1225-9.]. Likewise, seven cases were reported in a one-year study in Belgium [28Moes E, Vijgen L, Keyaerts E, et al. A novel pancoronavirus RT-PCR assay: frequent detection of human coronavirus NL63 in children hospitalized with respiratory tract infections in Belgium BMC Infect Dis 2005; 5: 6.]. Interestingly, the Dutch group that first described the virus has obtained 949 samples from a German group who conducted a population-based study on lower respiratory tract infection in children under three years of age [52Forster J, Ihorst G, Rieger CH, et al. Prospective population-based study of viral lower respiratory tract infections in children under 3 years of age (the PRI.DE study) Eur J Pediatr 2004; 163: 709-16.]; the group re-analyzed the samples and detected a 5.2% incidence of HCoV-NL63 [53van der Hoek L, Sure K, Ihorst G, et al. Croup is associated with the novel coronavirus NL63 PLoS Med 2005; 2 e240]. In Australia, the virus was detected in Melbourne, where a study conducted on 543 patients with respiratory symptoms has shown 18 cases (3.3%) of human coronavirus HCoV-NL63 infection [54Lambert SB, Allen KM, Druce JD, et al. Community epidemiology of human metapneumovirus, human coronavirus NL63, and other respiratory viruses in healthy preschool-aged children using parent-collected specimens Pediatrics 2007; 120: 929-37.]. Moreover, in the USA and Canada, the virus was reported in 79 (8.8%) out of 895 and 19 (3.6%) out of 525 patients, respectively.
Currently, the accuracy of the percentage of detection is hampered by two main problems: firstly, the suitability of the samples examined: a recent study has shown that there are differences between respiratory samples collected by nose/throat swabs and by nasopharyngeal aspirates, specifically regarding their potential to detect and identify respiratory pathogens [55Kleines M, Scheithauer S, Rackowitz A, Ritter K, Hausler M. High prevalence of human bocavirus detected in young children with severe acute lower respiratory tract disease by use of a standard PCR protocol and a novel real-time PCR protocol J Clin Microbiol 2007; 45: 1032-4.]. The second problem is that diagnostic tests for HCoVs are not frequently used in the routine testing for viruses, which probably results in the percentage of HCoVs infections being greatly underestimated [56van Elden LJ, van Loon AM, van Alphen F, et al. Frequent detection of human coronaviruses in clinical specimens from patients with respiratory tract infection by use of a novel real-time reverse-transcriptase polymerase chain reaction J Infect Dis 2004; 189: 652-7.]. Moreover, throughout the years several methods of variable sensitivity were used to determine the incidence of the virus [57van de Pol AC, van Loon AM, Wolfs TF, et al. Increased detection of respiratory syncytial virus, influenza viruses, parainfluenza viruses, and adenoviruses with real-time PCR in samples from patients with respiratory symptoms J Clin Microbiol 2007; 45: 2260-.-59Lu RJ, Zhang LL, Tan WJ. Development and comparison of real-time and conventional RT-PCR assay for detection of human coronavirus NL63 and HKU1 Bing Du Xue Bao 2008; 24: 305-11.].
4.4.. Co-Infection
Many groups have reported that the occurrence of co-infections with HCoV-NL63 and other respiratory viruses, including other human coronaviruses, influenza A virus, respiratory syncytial virus (RSV), parainfluenza virus and human metapneumovirus (hMPV), are common [26Chiu SS, Chan KH, Chu KW, et al. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China Clin Infect Dis 2005; 40: 1721-9., 30Minosse C, Selleri M, Zaniratti MS, et al. Phylogenetic analysis of human coronavirus NL63 circulating in Italy J Clin Virol 2008; 43: 114-9., 46Dare RK, Fry AM, Chittaganpitch M, et al. Human coronavirus infections in rural Thailand: a comprehensive study using real-time reverse-transcription polymerase chain reaction assays J Infect Dis 2007; 196: 1321-8., 47Wu PS, Chang LY, Berkhout B, et al. Clinical manifestations of human coronavirus NL63 infection in children in Taiwan Eur J Pediatr 2008; 167: 75-80., 51Canducci F, Debiaggi M, Sampaolo M, et al. Two-year prospective study of single infections and co-infections by respiratory syncytial virus and viruses identified recently in infants with acute respiratory disease J Med Virol 2008; 80: 716-23., 53van der Hoek L, Sure K, Ihorst G, et al. Croup is associated with the novel coronavirus NL63 PLoS Med 2005; 2 e240, 54Lambert SB, Allen KM, Druce JD, et al. Community epidemiology of human metapneumovirus, human coronavirus NL63, and other respiratory viruses in healthy preschool-aged children using parent-collected specimens Pediatrics 2007; 120: 929-37., 60Kaiser L, Regamey N, Roiha H, Deffernez C, Frey U. Human coronavirus NL63 associated with lower respiratory tract symptoms in early life Pediatr Infect Dis J 2005; 24: 1015-7., 61Yoo SJ, Kuak EY, Shin BM. Detection of 12 respiratory viruses with two-set multiplex reverse transcriptase-PCR assay using a dual priming oligonucleotide system Korean J Lab Med 2007; 27: 420-7.]. Also, co-infected patients are more likely to be hospitalized, indicating the severity of this kind of superinfection. In a study from Germany, RSV-A and HCoV-NL63 was the most common co-infection indentified in children less than three years of age. This is probably due to the high incidence of RSV-A in winter and the overlap in seasonality of the viruses [53van der Hoek L, Sure K, Ihorst G, et al. Croup is associated with the novel coronavirus NL63 PLoS Med 2005; 2 e240]. Also, in Italy, HCoV-NL63 circulates as a mixture of variant strains and is often associated with other viral infections [30Minosse C, Selleri M, Zaniratti MS, et al. Phylogenetic analysis of human coronavirus NL63 circulating in Italy J Clin Virol 2008; 43: 114-9.]. In South Africa, co-infection of patients with HCoV-NL63 and bocavirus in hospitalized children is reported. Nasopharyngeal and bronchoalveolar lavage samples from 341 patients were screened for common respiratory viruses, and the co-presence of HCoV-NL63 and bocavirus in at least one sample was reported [62Smuts H, Hardie D. Human bocavirus in hospitalized children, South Africa Emerg Infect Dis 2006; 12: 1457-8.].
Interestingly, the viral load of HCoV-NL63 is lower in co-infected patients than in patients infected with HCOV-NL63 only. There are various possible explanations for this phenomenon [53van der Hoek L, Sure K, Ihorst G, et al. Croup is associated with the novel coronavirus NL63 PLoS Med 2005; 2 e240]:
- HCoV-NL63 might be the initial infection that weakens the immune system enough for a second infection to gain a foothold. By the time this second infection shows symptoms, the HCoV-NL63 infection might have already have been brought under control by the host immune system,
- the two viruses may be in competition for the same receptor or target cell in the respiratory organs,
- the elevated activation of the innate immune response triggered by the second respiratory virus may cause inhibition of HCoV-NL63 or,
- prolonged persistence of HCoV-NL63 at low levels.
The high prevalence of co-infections of HCoV-NL63 and other respiratory viruses increases the chances of genetic recombination with these human or zoonotically transmitted viruses. In fact, Pryc et al. (2006) states HCoV-NL63 resulted from a recombination event between PEDV and an ancestral HCoV-NL63 strain. Theoretically, these types of recombination events could enable highly pathogenic virus variants to arise [24Pyrc K, Dijkman R, Deng L, et al. Mosaic structure of human coronavirus NL63, one thousand years of evolution J Mol Biol 2006; 364: 964-73.].
4.5.. Clinical Features
Recent scientific and clinical evidence has indicated that the virus is found during upper and lower respiratory tract infections, causing symptoms and signs that do not differ greatly from the symptoms described for the 'old' viruses HCoV-229E and HCoV-OC43. Other systems involvement is still controversial [63Vabret A, Dina J, Brison E, Brouard J, Freymuth F. Human coronaviruses Pathol Biol (Paris) 2008; 57: 147-60.].
Table 1 shows that patients diagnosed with the virus have presented with mild symptoms, indicating upper respiratory tract infection such as fever, cough and rhinorrhoea. On the other hand, the disease is also known to cause significant more alarming lower respiratory tract infection. One of the most alarming symptoms is bronchiolitis, an inflammation of the membranes lining the bronchioles. This symptom was reported by several research groups [25Arden KE, Nissen MD, Sloots TP, Mackay IM. New human coronavirus, HCoV-NL63, associated with severe lower respiratory tract disease in Australia J Med Virol 2005; 75: 455-62., 50Ebihara T, Endo R, Ma X, Ishiguro N, Kikuta H. Detection of human coronavirus NL63 in young children with bronchiolitis J Med Virol 2005; 75: 463-5., 64Bastien N, Robinson JL, Tse A, Lee BE, Hart L, Li Y. Human coronavirus NL-63 infections in children: a 1-year study J Clin Microbiol 2005; 43: 4567-73.], and although a population-based study in China did not report an association of HCoV-NL63 with bronchiolitis [26Chiu SS, Chan KH, Chu KW, et al. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China Clin Infect Dis 2005; 40: 1721-9.], it is still believed to be one of the presenting symptoms. Several research groups have linked HCoV-NL63 to croup [26Chiu SS, Chan KH, Chu KW, et al. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China Clin Infect Dis 2005; 40: 1721-9., 53van der Hoek L, Sure K, Ihorst G, et al. Croup is associated with the novel coronavirus NL63 PLoS Med 2005; 2 e240]. Croup children present with pharangitis, sore throat and hoarseness of voice, and are considered for hospitalization. Of the rare findings, a group has reported the association between HCoV-NL63 and Kawasaki disease, a form of childhood vasculitis that is presented as fever, polymorphic exanthema, oropharyngeal erythema and bilateral conjuctivitis [65Esper F, Shapiro ED, Weibel C, Ferguson D, Landry ML, Kahn JS. Association between a novel human coronavirus and Kawasaki disease J Infect Dis 2005; 191: 499-502.]. However, others fail to report on this association [66McIntosh K. Coronaviruses in the limelight J Infect Dis 2005; 191: 489-91., 67Shimizu C, Shike H, Baker SC, et al. Human coronavirus NL63 is not detected in the respiratory tracts of children with acute Kawasaki disease J Infect Dis 2005; 192: 1767-71.].
It is noteworthy to say that the report of symptoms in young children, who represent the majority of patients, is based mainly on parental observations, where other possible subjective signs and symptoms fail to be recognized by the parents. Moreover, most of the studies were conducted on patients reporting to hospitals suffering from acute respiratory tract infection. To date, there are only few population-based studies and the question arises whether larger numbers of such studies might reveal the involvement of other body systems.
5.. LABORATORY DIAGNOSIS AND DETECTION
It is difficult to distinguish clinical symptoms caused by HCoV-NL63 from those caused by many of the other human viruses, which could make diagnosis and detection of the virus complex. Treatment of patients without a clear diagnosis could result in implementation of expensive and disruptive public health measures, as well as to an increased spread of the disease [68Fox JD. Respiratory virus surveillance and outbreak investigation J Clin Virol 2007; 40(Suppl 1): S24-30.]. Therefore, improved surveillance and diagnosis of respiratory illness could reduce health-care costs drastically [68Fox JD. Respiratory virus surveillance and outbreak investigation J Clin Virol 2007; 40(Suppl 1): S24-30.-70Halasa NB, Williams JV, Wilson GJ, Walsh WF, Schaffner W, Wright PF. Medical and economic impact of a respiratory syncytial virus outbreak in a neonatal intensive care unit Pediatr Infect Dis J 2005; 24: 1040-4.].
5.1.. Detection of Viral RNA
Due to its high specificity and sensitivity, reverse transcription polymerase chain reaction (RT-PCR) and other nucleic acid tests are the preferred methods for diagnosis of coronavirus infections, such as SARS-CoV [15Fielding BC, Tan Y-J. The Singapore contribution in the battle against SARS Issues Infect Dis 2007; 4: 1-22.]. The majority of nucleic acid amplification tests is designed with the ORF1a/1b, which is genetically stable in coronaviruses, and the nucleocapsid (N) or spike (S) genes (Table 1) [25Arden KE, Nissen MD, Sloots TP, Mackay IM. New human coronavirus, HCoV-NL63, associated with severe lower respiratory tract disease in Australia J Med Virol 2005; 75: 455-62., 26Chiu SS, Chan KH, Chu KW, et al. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China Clin Infect Dis 2005; 40: 1721-9., 28Moes E, Vijgen L, Keyaerts E, et al. A novel pancoronavirus RT-PCR assay: frequent detection of human coronavirus NL63 in children hospitalized with respiratory tract infections in Belgium BMC Infect Dis 2005; 5: 6., 30Minosse C, Selleri M, Zaniratti MS, et al. Phylogenetic analysis of human coronavirus NL63 circulating in Italy J Clin Virol 2008; 43: 114-9., 45Bastien N, Anderson K, Hart L, et al. Human coronavirus NL63 infection in Canada J Infect Dis 2005; 191: 503-6., 46Dare RK, Fry AM, Chittaganpitch M, et al. Human coronavirus infections in rural Thailand: a comprehensive study using real-time reverse-transcription polymerase chain reaction assays J Infect Dis 2007; 196: 1321-8., 64Bastien N, Robinson JL, Tse A, Lee BE, Hart L, Li Y. Human coronavirus NL-63 infections in children: a 1-year study J Clin Microbiol 2005; 43: 4567-73., 67Shimizu C, Shike H, Baker SC, et al. Human coronavirus NL63 is not detected in the respiratory tracts of children with acute Kawasaki disease J Infect Dis 2005; 192: 1767-71., 71Choi EH, Lee HJ, Kim SJ, et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000-2005 Clin Infect Dis 2006; 43: 585-92.-73Kistler A, Avila PC, Rouskin S, et al. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity J Infect Dis 2007; 196: 817-25.]. Based on in vitro coronavirus expression studies, the N gene has the theoretical advantage of being more abundant in infected cells and therefore of higher sensitivity for PCR assays, but this has not been clearly proven in clinical studies [74Cheng VC, Lau SK, Woo PC, Yuen KY. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection Clin Microbiol Rev 2007; 20: 660-94.]. RT-PCR of nasopharyngeal samples, both frozen and fresh, is the most popular choice for detection of HCoV-NL63 (Table 1) and viral culture is frequently used for confirmation of infection [67Shimizu C, Shike H, Baker SC, et al. Human coronavirus NL63 is not detected in the respiratory tracts of children with acute Kawasaki disease J Infect Dis 2005; 192: 1767-71., 73Kistler A, Avila PC, Rouskin S, et al. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity J Infect Dis 2007; 196: 817-25.].
5.2.. Antigen and Antibody Detection Assays
Serum samples have been screened for antibodies against HCoV-NL63 using enzyme-linked immunosorbent assays (ELISA) [75Dijkman R, Jebbink MF, El Idrissi NB, et al. Human coronavirus NL63 and 229E seroconversion in children J Clin Microbiol 2008; 46: 2368-73., 76Shao X, Guo X, Esper F, Weibel C, Kahn JS. Seroepidemiology of group I human coronaviruses in children J Clin Virol 2007; 40: 207-13.]. An ELISA assay based on the N protein showed high seropositive results for serum samples from children younger than 20 years old [76Shao X, Guo X, Esper F, Weibel C, Kahn JS. Seroepidemiology of group I human coronaviruses in children J Clin Virol 2007; 40: 207-13.]. Interestingly, the presence of maternally-acquired N-directed antibodies was detected in serum by ELISA [75Dijkman R, Jebbink MF, El Idrissi NB, et al. Human coronavirus NL63 and 229E seroconversion in children J Clin Microbiol 2008; 46: 2368-73., 76Shao X, Guo X, Esper F, Weibel C, Kahn JS. Seroepidemiology of group I human coronaviruses in children J Clin Virol 2007; 40: 207-13.], usually decreasing within the first 4-5 months of life [76Shao X, Guo X, Esper F, Weibel C, Kahn JS. Seroepidemiology of group I human coronaviruses in children J Clin Virol 2007; 40: 207-13.]. Potent neutralizing activity directed against HCoV-NL63 S protein was detected in virtually all serum samples from patients eight years of age or older, suggesting that HCoV-NL63 infection of humans is common and usually acquired during childhood [42Hofmann H, Pyrc K, van der Hoek L, Geier M, Berkhout B, Pohlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry Proc Natl Acad Sci USA 2005; 102: 7988-93.]. Alarmingly, SARS-CoV infections appear to stimulate cross-reactive antibody responses to other human coronaviruses, including HCoV-NL63 [77Chan KH, Cheng VC, Woo PC, et al. Serological responses in patients with severe acute respiratory syndrome coronavirus infection and cross-reactivity with human coronaviruses 229E, OC43, and NL63 Clin Diagn Lab Immunol 2005; 12: 1317-21.]. Such cross reaction between human coronaviruses has been reported previously for immunoflourescence and complement fixation tests [78McIntosh K, Chao RK, Krause HE, Wasil R, Mocega HE, Mufson MA. Coronavirus infection in acute lower respiratory tract disease of infants J Infect Dis 1974; 130: 502-7., 79Monto AS, Rhodes LM. Detection of coronavirus infection of man by immunofluorescence Proc Soc Exp Biol Med 1977; 155: 143-8.]. In fact, false positive results were also reported for SARS-CoV detection using ELISA tests based on the recombinant N antigen [80Woo PC, Lau SK, Wong BH, et al. False-positive results in a recombinant severe acute respiratory syndrome-associated coronavirus (SARS-CoV) nucleocapsid enzyme-linked immunosorbent assay due to HCoV-OC43 and HCoV-229E rectified by Western blotting with recombinant SARS-CoV spike polypeptide J Clin Microbiol 2004; 42: 5885-8.]. This cross-reactivity is most likely due to the presence of cross-reactive antigenic epitopes of the coronaviruses [77Chan KH, Cheng VC, Woo PC, et al. Serological responses in patients with severe acute respiratory syndrome coronavirus infection and cross-reactivity with human coronaviruses 229E, OC43, and NL63 Clin Diagn Lab Immunol 2005; 12: 1317-21.]. An awareness of this cross-reactivity is important when developing antigen- and/or antibody-diagnostic assays and selecting the most suitable antigen or antigen target is important.
6.. CONCLUSION
The discovery of the HCoV-NL63 and other novel human coronaviruses does not necessarily represent a sudden increase in emerging infections by ‘new’ coronaviruses. In fact, in the early 1960’s, viral agents causing upper respiratory tract infections were isolated from patients and were subsequently shown to be coronaviruses by electron microscopy. At least four of these were shown to be serologically distinct from the known coronaviruses at the time [10Tyrrell DA, Bynoe ML. Cultivation of a novel type of common-cold virus in organ cultures Br Med J 1965; 1: 1467-70., 11Hamre D, Procknow JJ. A new virus isolated from the human respiratory tract Proc Soc Exp Biol Med 1966; 121: 190-3., 81Almeida JD, Tyrrell DA. The morphology of three previously uncharacterized human respiratory viruses that grow in organ culture J Gen Virol 1967; 1: 175-8.]. However, with the clinical samples of these no longer available for study, it will never be known whether these ‘old’ viruses and the ‘new’ viruses represent the same strains [82Kahn JS. The widening scope of coronaviruses Curr Opin Pediatr 2006; 18: 42-7.].
Molecular clock analysis is the average rate at which a species' genome accumulates mutations and is used to measure that species’ evolutionary divergence. The reliability of molecular dating is dependent on the validity of the molecular clock hypothesis, which assumes that the substitution rate is roughly constant [83Bromham L, Penny D. The modern molecular clock Nat Rev Genet 2003; 4: 216-4.], with an average substitution rate for coronaviruses estimated to be 10− 4 substitutions per year per site [84Sanchez CM, Gebauer F, Sune C, Mendez A, Dopazo J, Enjuanes L. Genetic evolution and tropism of transmissible gastroenteritis coronaviruses Virology 1992; 190: 92-105., 85Vijgen L, Lemey P, Keyaerts E, Van Ranst M. Genetic variability of human respiratory coronavirus OC43 J Virol 2005; 79: 3223-4. author reply 3224-5]. With the lack of sufficient sequence data available for HCoV-NL63, the substitution rate for HCoV-229E, using partial sequences of the S gene from different known dates, was calculated. Then, assuming a constant evolutionary rate in time and a constant evolutionary rate between the branches for HCoV-NL63 and HCoV229E, the time to their most recent common ancestor was dated to the 11th century [24Pyrc K, Dijkman R, Deng L, et al. Mosaic structure of human coronavirus NL63, one thousand years of evolution J Mol Biol 2006; 364: 964-73.]. This shows that HCoV-NL63 has been present in the human population for centuries. In support of this finding, the virus described by Fouchier et al. was originally isolated from an eight-month old child in 1988 [19Fouchier RA, Hartwig NG, Bestebroer TM, et al. A previously undescribed coronavirus associated with respiratory disease in humans Proc Natl Acad Sci USA 2004; 101: 6212-.]. More recent studies in the Netherlands also supported the notion that only SARS-CoV has recently been introduced to the human population from an animal source, while HCoV-NL63 has been circulating in humans for a while [86van der Hoek L. Human coronaviruses: what do they cause? Antivir Ther 2007; 12: 651-8.]. With the exception of SARS-CoV, the coronaviruses infecting humans are not well studied. This is partly due to the prevailing view that they are only involved in mild respiratory tract infections. The introduction of sensitive molecular and cell biology techniques have aided in identifying three ‘new’ human coronaviruses, which has improved our understanding of the classification of the coronaviruses. These modern tools now need to be developed further to make the sensitive and accurate detection of coronaviruses in clinical samples possible. This would increase our understanding of the extent to which coronaviruses affect human health. Importantly, Donaldson and colleagues has recently reported the assembly of a full-length infectious HCoV-NL63 clone, which will make the study of this virus easier, improving our understanding of the role of each of the proteins encoded by the HCoV-NL63 genome [87Donaldson EF, Yount B, Sims AC, Burkett S, Pickles RJ, Baric RS. Systematic assembly of a full-length infectious clone of human coronavirus NL63 J Virol 2008; 82: 11948-57.]. In the long run, molecular and cell biology tools could help elucidate the link, if any, between human coronaviruses and human diseases of the respiratory tract, the vascular system, the central nervous system and the gastrointestinal tract [82Kahn JS. The widening scope of coronaviruses Curr Opin Pediatr 2006; 18: 42-7.].
ACKNOWLEDGEMENTS
The authors receive funding from the University of the Western Cape. BCF also receives funding from the National Research Foundation, South Africa. We apologize to any author whose work was inadvertently omitted from this review.