- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Virology Journal
(Discontinued)
ISSN: 1874-3579 ― Volume 15, 2021
Conformational Differences Unfold a Wide Range of Enterotoxigenic Abilities Exhibited by rNSP4 Peptides from Different Rotavirus Strains
Narayan P Sastri 1, Kiranmayee Pamidimukkala 1, Jagannath R Marathahalli 1, Suguna Kaza 2, C. Durga Rao*, 1
Abstract
NSP4 has been recognized as the rotavirus-encoded enterotoxin. However, a few studies failed to support its diarrheagenic activity. As recombinant NSP4 (rNSP4) peptides of different lengths were used in the limited number of studies, a comparison of relative diarrheagenic potential of NSP4 from different strains could not be possible. To better understand the diarrheagenic potential of NSP4 from different strains, in this report we have evaluated the enterotoxigenic activity of the deletion mutant ΔN72 that lacks the N-terminal 72 residues and the biologically relevant ΔN112 peptide which when derived from SA11 rotavirus strain were previously shown to be highly diarrheagenic in newborn mice. Detailed comparative analysis of biochemical and biophysical properties and diarrheagenic activity of the recombinant ΔN72 peptides from seventeen different strains under identical conditions revealed wide differences among themselves in their resistance to trypsin cleavage, thioflavin T (ThT) binding, multimerization and conformation without any correlation with their diarrhea inducing abilities. These results support our previously proposed concept for the requirement of a unique conformation for optimal biological functions conferred by cooperation between the N- and C-terminal regions of the cytoplasmic tail.
Article Information
Identifiers and Pagination:
Year: 2011Volume: 5
First Page: 124
Last Page: 135
Publisher Id: TOVJ-5-124
DOI: 10.2174/1874357901105010124
Article History:
Received Date: 20/6/2011Revision Received Date: 18/8/2011
Acceptance Date: 6/9/2011
Electronic publication date: 10/11/2011
Collection year: 2011
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http: //creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Department of Microbiology & Cell Biology, Indian Institute of Science, Bangalore 560012, India; Fax: 91-80-23602697; E-mail: cdr@mcbl.iisc.ernet.in
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 20-6-2011 |
Original Manuscript | Conformational Differences Unfold a Wide Range of Enterotoxigenic Abilities Exhibited by rNSP4 Peptides from Different Rotavirus Strains | |
INTRODUCTION
The rotavirus nonstructural protein NSP4, encoded by genome segment 10, is 175 amino acids (aa) in length [1Estes MK. Fields Virology. 4th. Philadelphia: Lippincott Williams and Wilkins 2001; 2: pp. 1747-85.] and has been identified as the viral enterotoxin based on the ability of SA11-NSP4 to induce age-dependent diarrhea in suckling mice [2Ball JM, Tian P, Zeng CQ-Y, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotavirus nonstructural glycoprotein Science 1996; 272 : 101-4.]. Several studies have revealed that the protein is structurally complex and functionally pleiotropic (Fig. 1A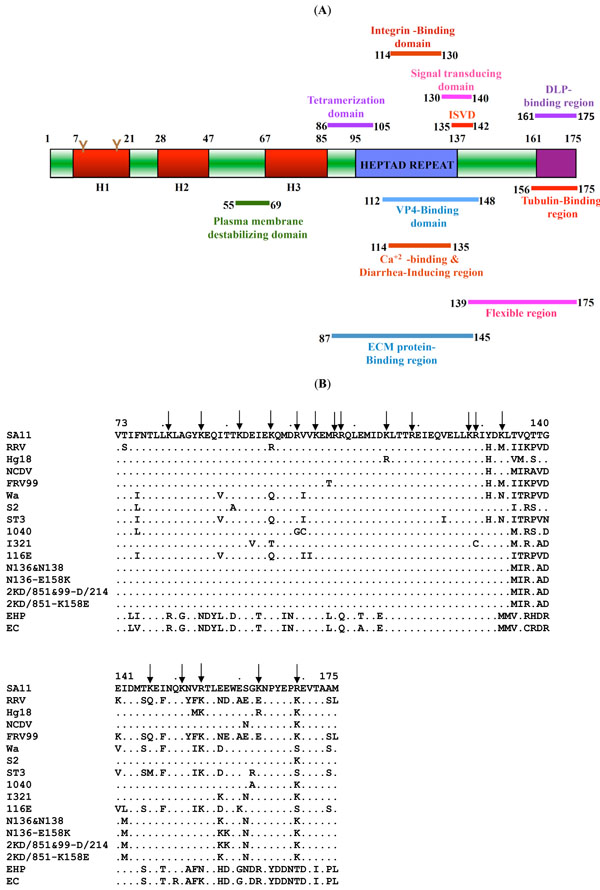 ) [1Estes MK. Fields Virology. 4th. Philadelphia: Lippincott Williams and Wilkins 2001; 2: pp. 1747-85., 3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25.-6Deepa R, Sastri NP, Rao CD, et al. The flexible C-terminus of the rotavirus nonstructural protein NSP4 is an important determinant of its biological properties J Gen Virol 2008; 89: 1485-96.]. NSP4 is critical for rotavirus replication, morphogenesis and pathogenesis [7Lopez T, Camacho M, Zayas M, et al. Silencing the morphogenesis of rotavirus J Virol 2005; 79: 184-92., 8Silvestri LS, Tortorici MA, Vasquez-Del Carpio R, Patton JT. Rotavirus glycoprotein NSP4 is a modulator of viral transcription in the infected cell J Virol 2005; 79: 15165-74.]. It has been reported to exist in multiple forms in the infected cells- as oligomers, higher molecular weight (HMW) complexes [9Maass DR, Atkinson PH. Rotavirus proteins VP7, NS28, and VP4 form oligomeric structures J Virol 1990; 64: 2632-41., 10Taylor JA, Meyer JC, Legge MA, et al. Transient expression and mutational analysis of the rotavirus intracellular receptor: the C-terminal methionine residue is essential for ligand binding J Virol 1992; 66: 3566-72.] and endoplasmic reticulum (ER)- and cytoplasmic membrane-anchored forms [11Bergman CC, Mass D, Poruchynsky M, Atkinson PH, Bellamy AR. Topology of the nonstructural rotavirus receptor glycoptrotein NS28 in the rough endoplasmic reticulum EMBO J 1989; 8: 1695-703.-14Gibbons TF, Storey SM, Williams CV, et al. Rotavirus NSP4: Cell type-dependent transport kinetics to the exofacial plasma membrane and release from intact infected cells Virol J 2011; 8: 278.]. Proteolytically-cleaved and secreted forms were also reported [15Zhang M, Zeng CQ, Morris AP, Estes MK. A functional NSP4 enterotoxin peptide secreted from rotavirus-infected cells J Virol 2000; 74: 11663-70., 16Bugarcic A, Taylor JA. Rotavirus nonstructural glycoprotein NSP4 is secreted from the apical surfaces of polarized epithelial cells J Virol 2006; 80: 12343-9.]. The ER-resident form is anchored through the N-terminal hydrophobic domains and the cytoplasmic tail (CT) of about 131 residues from the C-terminus exhibits all the known important properties associated with the protein including double-layered particle (DLP) binding [11Bergman CC, Mass D, Poruchynsky M, Atkinson PH, Bellamy AR. Topology of the nonstructural rotavirus receptor glycoptrotein NS28 in the rough endoplasmic reticulum EMBO J 1989; 8: 1695-703., 12Chan WK, Au KS, Estes MK. Topology of the simian rotavirus nonstructural glycoproteins (NS28) in the endoplasmic reticulum membrane Virology 1988; 164: 435-2., 17Au K-S, Mattion NM, Estes MK. A subviral particle binding domain on the rotavirus nonstructural glycoprotein NS28 Virology 1993; 194: 665- 73.-19Taylor JA, O’Brien JA, Lord VJ, Meyer JC, Bellamy AR. The RER- localized rotavirus intracellular receptor: A truncated purified soluble form is multivalent and binds virus particles Virology 1993; 194: 807-14.] and diarrhea induction [2Ball JM, Tian P, Zeng CQ-Y, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotavirus nonstructural glycoprotein Science 1996; 272 : 101-4., 3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25.]. Recent studies have also shown that a pentalysine domain (PD) and amphipathic helical domain (AD) located between residue 55-90 together function as a viroporin domain (VD) [20Hyser JM, Collinson-Pautz MR, Utama B, Estes MK. Rotavirus disrupts calcium homeostasis by NSP4 viroporin activity mBio 2010; 1: e00265-10.] (Fig. 1A
) [1Estes MK. Fields Virology. 4th. Philadelphia: Lippincott Williams and Wilkins 2001; 2: pp. 1747-85., 3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25.-6Deepa R, Sastri NP, Rao CD, et al. The flexible C-terminus of the rotavirus nonstructural protein NSP4 is an important determinant of its biological properties J Gen Virol 2008; 89: 1485-96.]. NSP4 is critical for rotavirus replication, morphogenesis and pathogenesis [7Lopez T, Camacho M, Zayas M, et al. Silencing the morphogenesis of rotavirus J Virol 2005; 79: 184-92., 8Silvestri LS, Tortorici MA, Vasquez-Del Carpio R, Patton JT. Rotavirus glycoprotein NSP4 is a modulator of viral transcription in the infected cell J Virol 2005; 79: 15165-74.]. It has been reported to exist in multiple forms in the infected cells- as oligomers, higher molecular weight (HMW) complexes [9Maass DR, Atkinson PH. Rotavirus proteins VP7, NS28, and VP4 form oligomeric structures J Virol 1990; 64: 2632-41., 10Taylor JA, Meyer JC, Legge MA, et al. Transient expression and mutational analysis of the rotavirus intracellular receptor: the C-terminal methionine residue is essential for ligand binding J Virol 1992; 66: 3566-72.] and endoplasmic reticulum (ER)- and cytoplasmic membrane-anchored forms [11Bergman CC, Mass D, Poruchynsky M, Atkinson PH, Bellamy AR. Topology of the nonstructural rotavirus receptor glycoptrotein NS28 in the rough endoplasmic reticulum EMBO J 1989; 8: 1695-703.-14Gibbons TF, Storey SM, Williams CV, et al. Rotavirus NSP4: Cell type-dependent transport kinetics to the exofacial plasma membrane and release from intact infected cells Virol J 2011; 8: 278.]. Proteolytically-cleaved and secreted forms were also reported [15Zhang M, Zeng CQ, Morris AP, Estes MK. A functional NSP4 enterotoxin peptide secreted from rotavirus-infected cells J Virol 2000; 74: 11663-70., 16Bugarcic A, Taylor JA. Rotavirus nonstructural glycoprotein NSP4 is secreted from the apical surfaces of polarized epithelial cells J Virol 2006; 80: 12343-9.]. The ER-resident form is anchored through the N-terminal hydrophobic domains and the cytoplasmic tail (CT) of about 131 residues from the C-terminus exhibits all the known important properties associated with the protein including double-layered particle (DLP) binding [11Bergman CC, Mass D, Poruchynsky M, Atkinson PH, Bellamy AR. Topology of the nonstructural rotavirus receptor glycoptrotein NS28 in the rough endoplasmic reticulum EMBO J 1989; 8: 1695-703., 12Chan WK, Au KS, Estes MK. Topology of the simian rotavirus nonstructural glycoproteins (NS28) in the endoplasmic reticulum membrane Virology 1988; 164: 435-2., 17Au K-S, Mattion NM, Estes MK. A subviral particle binding domain on the rotavirus nonstructural glycoprotein NS28 Virology 1993; 194: 665- 73.-19Taylor JA, O’Brien JA, Lord VJ, Meyer JC, Bellamy AR. The RER- localized rotavirus intracellular receptor: A truncated purified soluble form is multivalent and binds virus particles Virology 1993; 194: 807-14.] and diarrhea induction [2Ball JM, Tian P, Zeng CQ-Y, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotavirus nonstructural glycoprotein Science 1996; 272 : 101-4., 3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25.]. Recent studies have also shown that a pentalysine domain (PD) and amphipathic helical domain (AD) located between residue 55-90 together function as a viroporin domain (VD) [20Hyser JM, Collinson-Pautz MR, Utama B, Estes MK. Rotavirus disrupts calcium homeostasis by NSP4 viroporin activity mBio 2010; 1: e00265-10.] (Fig. 1A ).
).
A peptide spanning residue 114-135 was reported to be about 800-fold less efficient in diarrhea induction compared to the full-length protein [2Ball JM, Tian P, Zeng CQ-Y, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotavirus nonstructural glycoprotein Science 1996; 272 : 101-4.]. However, the sequence from aa 114-135 is highly conserved among different symptomatic and asymptomatic strains and it alone unlikely determines the optimal diarrhea inducing potential of the protein. Further, a peptide from residue112-175, secreted from rotavirus infected cells, was reported to induce dose-dependent diarrhea in suckling mice similar to the full-length protein [15Zhang M, Zeng CQ, Morris AP, Estes MK. A functional NSP4 enterotoxin peptide secreted from rotavirus-infected cells J Virol 2000; 74: 11663-70.]. Recently, we reported that the ΔN72 deletion mutants from simian SA11 and bovine Hg18 strains were about 20-fold more efficient in diarrhea induction in newborn mice than that reported for the full-length protein, and exhibited efficient DLP-binding activity [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25.].
Rotaviruses exhibit a wide range of DD50 values in newborn mice [21Burns JW, Krishnaney AA, Vo PT, Rouse RV, Anderson LJ, Greenberg HB. Analyses of homologous rotavirus infection in the mouse model Virology 1995; 207: 143-53.-24Ramig RF. The effects of host age, virus dose, and virus strain on heterologous rotavirus infection of suckling mice Microb Pathog 1988; 4: 189-202.]. Rotaviruses can be either symptomatic or asymptomatic, and in some cases NSP4 and a few other viral proteins could cooperatively determine virus virulence [25Bridger JC, Burke B, Beards GM, Desselberger U. The pathogenecity of two porcine rotaviruses differing in their in vitro growth characteristics and gene 4 J Gen Virol 1992; 73: 3011-5.-32Ward RL, Mason BB, Bernstein DI, et al. Attenuation of a human rotavirus vaccine candidate did not correlate with mutations in the NSP4 gene J Virol 1997; 71: 6267-70.]. Analysis of NSP4 sequences from more than 175 strains failed to identify any residue or motif that could be associated with the virulence phenotype [33Lin SL, Tian P. Detailed computational analysis of a comprehensive set of group A rotavirus NSP4 proteins Virus Genes 2003; 26: 271-82.]. Mutations at positions 131, 135 and 138 which were reported to result in loss of a porcine rotavirus virulence and diarrhea-inducing ability of the protein [34Zhang M, Zeng CQ, Dong Y, et al. Mutations in rotavirus nons tructural glycoprotein NSP4 are associated with altered virus virulence J Virol 1998; 72: 3666-72.], did not correlate with the attenuated phenotype of a vaccine strain [32Ward RL, Mason BB, Bernstein DI, et al. Attenuation of a human rotavirus vaccine candidate did not correlate with mutations in the NSP4 gene J Virol 1997; 71: 6267-70.] or the asymptomatic human or feline strains [35Chang KO, Kim YJ, Saif LJ. Comparisons of nucleotide and deduced amino acid sequences of NSP4 genes of virulent and attenuated pairs of group A and C rotaviruses Virus Genes 1999; 18: 229-33.-37Oka T, Nakagomi T, Nakagomi O. A lack of consistent amino acid substitutions in NSP4 between rotaviruses derived from diarrheal and asymptomatically infected kittens Microbiol Immunol 2001; 45: 173-7.]. Further, a limited number of studies reported a lack of correlation between virulence of the rotavirus strain and the DD50 of the cognate NSP4 [22Horie Y, Nakagomi O, Koshimura Y, et al. Diarrhea induction by rotavirus NSP4 in the homologous mouse model system Virology 1999; 262: 398-407., 38Angel J, Tang B, Feng N, Greenberg HB, Bass D. Studies of the role for NSP4 in the pathogenesis of homologous murine rotavirus diarrhea J Infect Dis 1998; 177: 455-8., 39Mori Y, Borgan MA, Ito N, Sugiyama M, Minamoto N. Diarrhea-inducing activity of avian rotavirus glycoproteins, which differ greatly from mammalian rotavirus NSP4 glycoproteins in deduced amino acid sequence in suckling mice J Virol 2002; 76: 5829-34.], some of which could be due to the use of rNSP4 mutants that varied widely in their length. Based on the analysis of a large number of mutants of the highly diarreagenic NSP4 from SA11 and Hg18 strains, we recently reported that the biological functions of rNSP4 proteins are dependent on a unique and complex conformation of the cytoplasmic tail [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25., 6Deepa R, Sastri NP, Rao CD, et al. The flexible C-terminus of the rotavirus nonstructural protein NSP4 is an important determinant of its biological properties J Gen Virol 2008; 89: 1485-96.]. The reported wide variation in diarrhea inducing abilities of a few recombinant proteins could be attributed to improper conformation of the recombinant peptides [22Horie Y, Nakagomi O, Koshimura Y, et al. Diarrhea induction by rotavirus NSP4 in the homologous mouse model system Virology 1999; 262: 398-407., 38Angel J, Tang B, Feng N, Greenberg HB, Bass D. Studies of the role for NSP4 in the pathogenesis of homologous murine rotavirus diarrhea J Infect Dis 1998; 177: 455-8., 39Mori Y, Borgan MA, Ito N, Sugiyama M, Minamoto N. Diarrhea-inducing activity of avian rotavirus glycoproteins, which differ greatly from mammalian rotavirus NSP4 glycoproteins in deduced amino acid sequence in suckling mice J Virol 2002; 76: 5829-34.].
This study was undertaken to attempt to resolve the reported inconsistency in the diarrheagenic activity reported for a few rNSP4 peptides, that differed in length, by the analysis of the enterotoxigenic activity of a single polypeptide (ΔN72) of uniform size from a large number of strains. We have evaluated ThT binding, resistance to trypsin, multimerization/oligomerization and conformational properties of the proteins to understand if there is any correlation between any of these properties and their diarrheagenic potential.
MATERiALs AND METHODOLOGY
Viruses and Cells
The rotavirus strains, their G and P serotype/genotype associations and the host from which they were isolated are listed in Table 1. The Vellore neonatal strains were kindly provided by Dr. G. Kang, Christian Medical College, Vellore, India. Except for IS2, 1040, EHP, EC and Vellore strains, viruses were grown in MA104 cells.
Cloning, Expression and Purification of Different NSP4ΔN72 and Δ112 Peptides
The genomic RNA from EC, EHP, IS2, 1040, I321 and Vellore strains was extracted from the fecal samples and that from others was isolated from infected cell culture supernatants as described previously [36Jagannath MR, Vethanayagam RR, Reddy BSY, Raman S, Rao CD. Characterization of human symptomatic rotavirus isolates MP409 and MP480 having ‘long’ RNA electropherotype and subgroup I specificity, highly related to the P6[1], G8 type bovine rotavirus A5, from Mysore, India Arch Virol 2000; 145: 1339-57., 43Das M, Dunn SJ, Woode GN, Greenberg HB, Rao CD. Both surface proteins (VP4 and VP7) of an asymptomatic neonatal rotavirus strain (I321) have high levels of sequence identity with the homologous proteins of a serotype 10 bovine rotavirus Virology 1993; 194: 374-9.]. Cloning of the NSP4 gene, its ΔN72 region spanning aa 73 to 175 and generation of the pET22-NH vector for expression of proteins in fusion with an N-terminal His-tag have been described [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25.]. The ΔN112 region was cloned using strain- and position-specific primers. The nucleotide sequence of ΔN72 and ΔN112 in pBluescript KS+ (pBS) or pET22-NH was determined using T7, M13 forward and/or reverse primers (Macrogen, Korea). All NSP4ΔN72 and ΔN112 peptides expressed in E. coli BL21 (DE3) were highly soluble and were purified by Ni2+-NTA-agarose (QIAGEN) chromatography after binding in presence 0.5% NP-40 and washing extensively in its absence [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25.]. Purity of the proteins was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and mass spectrometry. Molecular masses of the peptides were determined by size exclusion chromatography (SEC) using Sephacryl S-200 column (GE Healthcare) on a Bio-Rad FPLC chromatography system as well as by mass spectrometry using Ultraflex time of flight mass spectrometer (Bruker Daltonics) [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25., 46Karas M, Hillenkamp F. Laser desorption ionization of proteins with molecular masses exceeding 10000 Daltons Anal Chem 1988; 60: 2299-301.].
Thioflavin T Fluorescence Assay
ΔN72 peptides dialyzed against a buffer containing 10 mM sodium phosphate pH 7.6 and 100 mM NaCl at 100 μM each of the protein and the dye were used. ThT-binding assays were performed by mixing 50 µl of 60 µM protein solution with 450 µl of 10 µM ThT. Readings were recorded in a Shimadzu RF-5301 PC spectrofluorometer at 25°C. The excitation wave length was 450 nm, and the emission was monitored between 450 and 600 nm [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25., 47Naiki H, Higuchi K, Hosokawa M, Takeda T. Fluorometric determination of amyloid fibrils in vivo using the fluorescent dye, thioflavin T Anal Biochem 1989; 177: 244-9.].
Determination of Diarrhoeal Dose 50 (DD50) of NSP4ΔN72 and Δ112 Peptides
Prior to animal experiments, the ThT binding ability of the ΔN72 peptides was evaluated [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25.]. Peptides exhibiting high ThT fluorescence were tested between 1 and 100 pmol and those showing highly reduced or lack of ThT binding were evaluated between 50 pmol and 10 nmol in 5-7 day-old BALB/c mouse pups. The recombinant ΔN72 and ΔN112 peptides in 50 μl of sterile PBS were administered intraperitoneally. DD50 and mean diarrheal scores, on a scale of 1-4, were determined as described [2Ball JM, Tian P, Zeng CQ-Y, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotavirus nonstructural glycoprotein Science 1996; 272 : 101-4., 3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25.]. At each dose, 8 mouse pups were used and the experiment was repeated three to four times. The fold efficiency of diarrhea induction of different NSP4ΔN72 peptides was calculated with reference to the DD50 of SA11ΔN72 which exhibited the lowest value.
Circular Dichroism (CD) Spectroscopy
Secondary structural differences among different ΔN72 and ΔN112 peptides were examined employing Far UV-CD spectroscopy. The percent α-helical, β-sheet and random conformation contents were determined using the k2d program [48Andrade MA, Chacon P, Merelo JJ, Moran F. Evaluation of secondary structure of proteins from UV circular dichroism using an unsupervised learning neural network Protein Eng 1993; 6: 383-90.]. CD spectra of the proteins, in 5 mM sodium phosphate buffer pH 7.4 containing 5 mM NaCl, were recorded on a JASCO J-715 spectropolarimeter at a protein concentration of 10 μM and the molar residue ellipticity was calculated as described [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25.].
Trypsin Resistance Analysis of Different NSP4 Proteins
Purified ΔN72 peptides were digested with sequencing grade trypsin (Promega) at 37°C, the trypsin-cleaved products were analyzed by Tricine-SDS-PAGE [49Schagger H, von Jagaw G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range of 1 to 100 kDa Anal Biochem 1987; 166: 368-79.] followed by Coomassie Blue staining and the molecular masses of the cleaved products were determined by mass spectrometry as previously described [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25.]. Relative trypsin resistance on a scale of 0 to 100% was determined by densitometric measurement of intensities of bands corresponding to all the protected fragments of the helical region post 2 hr incubation with respect to control peptide with 75 to 100% being highly resistant and 0 to 25% corresponding to undetectable level of protected fragments.
RESULTS
Thioflavin T Binding Ability of Different NSP4ΔN72 Peptides
Recently, we have shown that rNSP4ΔN72 peptides from SA11 and Hg18 were highly diarrheagenic, formed highly ordered higher molecular weight (HMW) complexes, exhibited high α-helical content, thioflavin T binding and resistance to trypsin of the region from residue 73-146 in contrast to a large number of their N- and C-terminal deletion mutants or amino acid (aa) substitution mutants [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25., 6Deepa R, Sastri NP, Rao CD, et al. The flexible C-terminus of the rotavirus nonstructural protein NSP4 is an important determinant of its biological properties J Gen Virol 2008; 89: 1485-96.]. Mutations in ΔN72 affected the diarrhea-inducing and ThT-binding activities to different extents with DD50 increases ranging between 20-2080-fold (DD50 0.05 to >10 nmol) [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25., 6Deepa R, Sastri NP, Rao CD, et al. The flexible C-terminus of the rotavirus nonstructural protein NSP4 is an important determinant of its biological properties J Gen Virol 2008; 89: 1485-96.]. These studies suggested a correlation between optimal ThT binding and efficient diarrhea induction and that efficient ThT binding is dependent on a unique conformation that is significantly affected by aa substitutions throughout the length of the peptide. Further, a specific conformation only in the ordered multimeric forms of SA11- and Hg18-NSP4ΔN72, but not other HMW complexes of mutant NSP4ΔN72 peptides, is recognized by ThT. Since NSP4s from different strains exhibit significant aa variations in the flexible C-terminal region of the cytoplasmic tail (CT) (Fig. 1B ), it is likely that different NSP4 peptides would differ significantly from each other in their ThT binding property.
), it is likely that different NSP4 peptides would differ significantly from each other in their ThT binding property.
The observation that high ThT fluorescence exhibited by SA11- and Hg-18ΔN72 peptides correlated with their efficient diarrhea-inducing ability suggested that this property can be used to significantly reduce the number of mouse pups required to determine the DD50 of the large number of NSP4 peptides used in this study. Thus a protein showing high ThT binding need not be tested at high concentration and those that exhibit weak binding can be tested only at high concentration.
The ΔN72 peptides from 17 different human (sympto-matic and asymptomatic) and animal strains (Table 1) were expressed, purified (Fig. 2A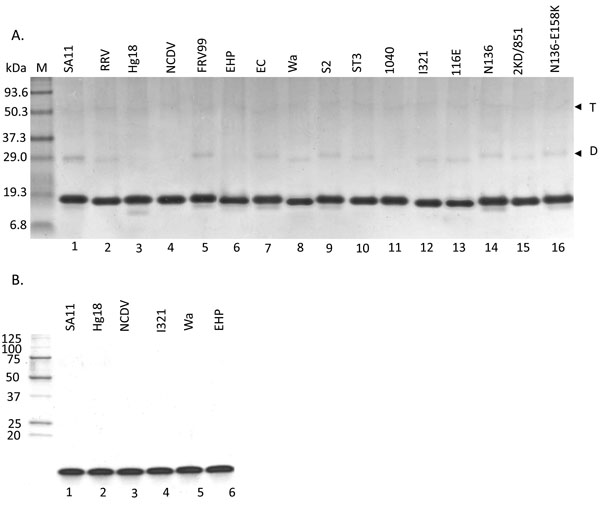 ) and their ThT binding property was evaluated. As shown in Fig. (3
) and their ThT binding property was evaluated. As shown in Fig. (3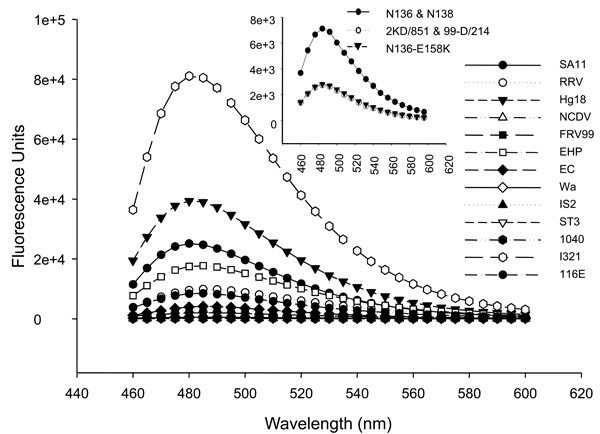 ), only I321-, SA11-, Hg18- and EHP- NSP4ΔN72 peptides exhibited significant ThT fluorescence and those from all other strains showed either highly reduced or total lack of ThT binding. Of note, whilst NSP4 from the human asymptomatic strain I321 showed about 2.0-3.3-fold more ThT fluorescence than that of Hg18 and SA11, that from other human asymptomatic strains116E and ST3 exhibited highly reduced or negligible ThT fluorescence. The peptides from the two murine strains EHP and EC also varied significantly in their ThT binding ability. Thus in contrast to the previous observation with SA11- and Hg18- NSP4ΔN72 peptides which exhibited high level of ThT binding, the present analysis using similar peptides from a large number of strains revealed that the ThT binding ability varied widely among different peptides.
), only I321-, SA11-, Hg18- and EHP- NSP4ΔN72 peptides exhibited significant ThT fluorescence and those from all other strains showed either highly reduced or total lack of ThT binding. Of note, whilst NSP4 from the human asymptomatic strain I321 showed about 2.0-3.3-fold more ThT fluorescence than that of Hg18 and SA11, that from other human asymptomatic strains116E and ST3 exhibited highly reduced or negligible ThT fluorescence. The peptides from the two murine strains EHP and EC also varied significantly in their ThT binding ability. Thus in contrast to the previous observation with SA11- and Hg18- NSP4ΔN72 peptides which exhibited high level of ThT binding, the present analysis using similar peptides from a large number of strains revealed that the ThT binding ability varied widely among different peptides.
NSP4ΔN72 and ΔN112 Peptides Exhibit Similar Pattern in their Diarrheagenic Abilities But with Different DD50 Values
Evaluation of the diarrhea inducing ability of ΔN72 peptides from 17 different strains suggested that different NSP4ΔN72 peptides used in this study can be identified as either efficient diarrhea inducers represented by SA11, Hg18 and EHP which exhibited a low DD50 of 0.005-0.05 nmol, or inefficient/poor diarrhea inducers represented by other NSP4s exhibiting DD50 > 0.05 nmol (Table 2). Although the efficient diarrhea-inducing activity of the former group correlated, in general, with high ThT fluorescence and spontaneous diarrhea within 30 minutes of administration, the inefficient diarrhea inducers differed widely among themselves without any correspondence between ThT fluorescence and DD50 (Table 2). Further, NSP4s from symptomatic and asymptomatic strains could not be distinguished by their diarrhea inducing abilities. While the NSP4 peptides from human asymptomatic strains ST3, I321, and 116E exhibited DD50 values between 0.07 and 0.5 nmol, those from animal strains and human symptomatic strains (RRV, NCDV, FRV18, EC, and Wa, IS2 and 1040) showed DD50 in the range of 0.75 to >10 nmol. Also, the ΔN72 peptides from the Vellore symptomatic strains (N136 and N138) were about 33-fold less efficient in their ability to induce diarrhea in newborn mouse pups than those from the asymptomatic strains 2KD/851 and 99-D/214. Of note, I321 NSP4 which bound ThT better than SA11 and Hg18 NSP4ΔN72 peptides, was about 1000-fold less efficient in diarrhea induction (Table 2). Whilst ΔN72 from SA11, Hg18 and EHP induced spontaneous diarrhea within 30-40 min of protein administration at the DD50 value with a mean diarrheal score of 3.2, the inefficient diarrheagenic peptides induced diarrheal stools at or below DD50 values between 1 and 2 hr post administration only after gentle palpitation of the abdomen with mean diarrhoeal score of 2.0. Among the latter group, NSP4 from ST3 and NCDV exhibited comparatively lower DD50 (0.07 and 0.075 nmol, respectively). While the peptides from RRV, Wa, equine strain FRV99 and the G2 strain 1040 showed 100-200-fold higher DD50, that from another G2 strain IS2 exhibited 1000-fold higher DD50 than SA11-NSP4ΔN72 (Table 2). Of significance, the murine EC-NSP4ΔN72 failed to induce diarrhea even with 10 nmol of the protein (precise DD50 not determined) compared to that of another murine strain EHP (0.05 nmol). Though IS2-NSP4ΔN72 peptide was very inefficient in diarrheagenic activity (5.0 nmol) among the diarrheagenic ΔN72 peptides, it appears to be significantly better than the full-length proteins from avian strains (41-138 nmol) (Table 2) [23Mori Y, Sugiyama M, Takayama M, Atoji Y, Masegi T, Minamoto N. Avian-to-mammal transmission of an avian rotavirus: Analysis of its pathogenicity in a heterologous mouse model Virology 2001; 288: 63-70., 39Mori Y, Borgan MA, Ito N, Sugiyama M, Minamoto N. Diarrhea-inducing activity of avian rotavirus glycoproteins, which differ greatly from mammalian rotavirus NSP4 glycoproteins in deduced amino acid sequence in suckling mice J Virol 2002; 76: 5829-34.].
Though ΔN72 from SA11 and Hg18 was very efficient in diarrhea induction, this mutant form of the protein is not detected in the virus infected cells. It is possible that the wide differences observed in the diarrheagenic properties of ΔN72 peptides from different strains could be a manifestation of the unnatural mutation. Since the full-length protein could not be expressed in E. coli and that expressed in insect cells was difficult to purify to homogeneity, we expressed and purified the recombinant ΔN112 peptide (Fig. 2B ), which is similar to the biologically active peptide secreted from the virus infected or gene-transfected cells [14Gibbons TF, Storey SM, Williams CV, et al. Rotavirus NSP4: Cell type-dependent transport kinetics to the exofacial plasma membrane and release from intact infected cells Virol J 2011; 8: 278.], from six different strains (SA11, Hg18, NCDV, I321, EHP and Wa) and determined their DD50 in newborn mouse pups. As shown in Table 2, though the pattern of diarrhea inducing ability of the ΔN112 peptides from SA11, Hg18, NCDV, Wa and I321 was very similar to that of the corresponding ΔN72 peptides, the former exhibited high DD50 values than the latter. These results suggest that both ΔN72 and ΔN112 peptides from different strains exhibit very similar pattern in their diarrhea inducing properties, but differ in their relative diarrhea inducing efficiencies. The relatively high DD50 values exhibited by the ΔN112 peptides in comparison to those of ΔN72 peptides could be attributed to their lack of the N-terminal amphipathic domain which was shown to potentiate the biological function of the protein [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25., 20Hyser JM, Collinson-Pautz MR, Utama B, Estes MK. Rotavirus disrupts calcium homeostasis by NSP4 viroporin activity mBio 2010; 1: e00265-10.].
), which is similar to the biologically active peptide secreted from the virus infected or gene-transfected cells [14Gibbons TF, Storey SM, Williams CV, et al. Rotavirus NSP4: Cell type-dependent transport kinetics to the exofacial plasma membrane and release from intact infected cells Virol J 2011; 8: 278.], from six different strains (SA11, Hg18, NCDV, I321, EHP and Wa) and determined their DD50 in newborn mouse pups. As shown in Table 2, though the pattern of diarrhea inducing ability of the ΔN112 peptides from SA11, Hg18, NCDV, Wa and I321 was very similar to that of the corresponding ΔN72 peptides, the former exhibited high DD50 values than the latter. These results suggest that both ΔN72 and ΔN112 peptides from different strains exhibit very similar pattern in their diarrhea inducing properties, but differ in their relative diarrhea inducing efficiencies. The relatively high DD50 values exhibited by the ΔN112 peptides in comparison to those of ΔN72 peptides could be attributed to their lack of the N-terminal amphipathic domain which was shown to potentiate the biological function of the protein [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25., 20Hyser JM, Collinson-Pautz MR, Utama B, Estes MK. Rotavirus disrupts calcium homeostasis by NSP4 viroporin activity mBio 2010; 1: e00265-10.].
A Single aa Mutation in the Flexible C-Terminus of NSP4 from the Vellore Neonatal G10P[11Bergman CC, Mass D, Poruchynsky M, Atkinson PH, Bellamy AR. Topology of the nonstructural rotavirus receptor glycoptrotein NS28 in the rough endoplasmic reticulum EMBO J 1989; 8: 1695-703.]-Type Strains Correlates with Virus Virulence without Correspondence with the DD50 of the Cognate rNSP4ΔN72
Recently, G10P [11Bergman CC, Mass D, Poruchynsky M, Atkinson PH, Bellamy AR. Topology of the nonstructural rotavirus receptor glycoptrotein NS28 in the rough endoplasmic reticulum EMBO J 1989; 8: 1695-703.] strains associated with symptomatic or asymptomatic infections in neonates in Vellore, India, were reported. However, sequence analysis of NSP4 from a few isolates failed to differentiate the virulent from the avirulent strains [45Iturriza-Gomara MI, Kang G, Mammen A, et al. Characterization of G10P[11] rotaviruses causing acute gastroenteritis in neonates and infants in Vellore, India J Clin Microbiol 2004; 42: 2541-7.]. Sequence analysis, in our laboratory, of NSP4ΔN72 from two symptomatic Vellore isolates (N136 and N138) for which the sequence was not reported, and two asymptomatic isolates (2KD/851 and 99-D/214) for which the sequence was available, revealed a single aa difference at position 158 (E158K) between the virulent and avirulent pairs. While the symptomatic isolates contained Glu at this position, the asymptomatic strains possessed Lys (Fig. 1B ). Only Glu was reported at position 158 in both symptomatic and asymptomatic strains [45Iturriza-Gomara MI, Kang G, Mammen A, et al. Characterization of G10P[11] rotaviruses causing acute gastroenteritis in neonates and infants in Vellore, India J Clin Microbiol 2004; 42: 2541-7.]. To understand if the virulence phenotype of the Vellore strains correlates with the diarrheagenic activity of cognate NSP4, the DD50 of ΔN72 peptides from symptomatic and asymptomatic isolates in suckling mice was determined. Unexpectedly, NSP4ΔN72 peptide from the symptomatic isolates N136 and N138 exhibited DD50 (2.0 nmol) that was about 33-fold higher than that of the asymptomatic isolates 2KD/851 and 99-D/214 (0.06 nmol). Association of the E158K mutation with altered diarrhea induction was further evident from the DD50 values of the E158K and K158E mutant peptides which were indistinguishable from those of the peptides from asymptomatic and symptomatic strains, respectively (Table 2). Of note, N136-ΔN72 consistently showed >50% higher ThT fluorescence of that of 2KD/851, though significantly less than that showed by SA11 and Hg18, (Fig. 3
). Only Glu was reported at position 158 in both symptomatic and asymptomatic strains [45Iturriza-Gomara MI, Kang G, Mammen A, et al. Characterization of G10P[11] rotaviruses causing acute gastroenteritis in neonates and infants in Vellore, India J Clin Microbiol 2004; 42: 2541-7.]. To understand if the virulence phenotype of the Vellore strains correlates with the diarrheagenic activity of cognate NSP4, the DD50 of ΔN72 peptides from symptomatic and asymptomatic isolates in suckling mice was determined. Unexpectedly, NSP4ΔN72 peptide from the symptomatic isolates N136 and N138 exhibited DD50 (2.0 nmol) that was about 33-fold higher than that of the asymptomatic isolates 2KD/851 and 99-D/214 (0.06 nmol). Association of the E158K mutation with altered diarrhea induction was further evident from the DD50 values of the E158K and K158E mutant peptides which were indistinguishable from those of the peptides from asymptomatic and symptomatic strains, respectively (Table 2). Of note, N136-ΔN72 consistently showed >50% higher ThT fluorescence of that of 2KD/851, though significantly less than that showed by SA11 and Hg18, (Fig. 3 ) but was relatively inefficient in diarrhea induction than 2KD/851.
) but was relatively inefficient in diarrhea induction than 2KD/851.
ΔN72 Peptides from Different Strains Differ Significantly in their Resistance to Cleavage by Trypsin
Though ThT is frequently used to detect β-sheet structures in amyloid fibrils and ordered polymeric proteins [50Blanchard BJ, Chen A, Rozeboom LM, Stafford KA, Weigele P, Ingram VM. Efficient reversal of Alzheimer’s disease fibril formation and elimination neurotoxocity by a small molecule Proc Natl Acad Sci USA 2004; 101: 14326-32., 51Devlin GL, Chow MKM, Howlett GJ, Bottomley SP. Acid denaturation of α1-antitrypsin: characterization of a novel mechanism of serpin polymerization J Mol Biol 2002; 324: 859-70.], studies on acetylcholinesterase [52Ferrari GV, Mallender WD, Inestrosa NC, Rosenberry TL. Thioflavin T is a fluorescent probe of the acetylcholinesterase peripheral site that reveals conformational interactions between the peripheral and acylation sites J Biol Chem 2001; 276: 23282-7.] revealed that ThT binds efficiently to the peripheral ligand binding site which lacks β-sheet structures characteristic of amyloid protein. Though the precise nature of interaction is not understood, these as well as our results [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25., 6Deepa R, Sastri NP, Rao CD, et al. The flexible C-terminus of the rotavirus nonstructural protein NSP4 is an important determinant of its biological properties J Gen Virol 2008; 89: 1485-96.] suggested that ThT could bind to proteins independent of the amyloid β-sheet structures and that different amino acid substitution and deletion mutant NSP4ΔN72 peptides differ significantly in their conformation.
The ΔN72 peptide region from different strains contains 17 trypsin cleavage sites that are conserved among the strains (Fig. 1B ). Previously, we have shown that single amino acid substitutions in the CT of the highly diarrheagenic SA11-NSP4ΔN72 affected its diarrhea inducing ability and resistance to trypsin digestion [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25.]. Since different NSP4s significantly differ in their sequence in the unstructured C-terminal region, it is likely that NSP4s from different strains would vary in their susceptibility to trypsin cleavage. As shown in Fig. (4
). Previously, we have shown that single amino acid substitutions in the CT of the highly diarrheagenic SA11-NSP4ΔN72 affected its diarrhea inducing ability and resistance to trypsin digestion [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25.]. Since different NSP4s significantly differ in their sequence in the unstructured C-terminal region, it is likely that NSP4s from different strains would vary in their susceptibility to trypsin cleavage. As shown in Fig. (4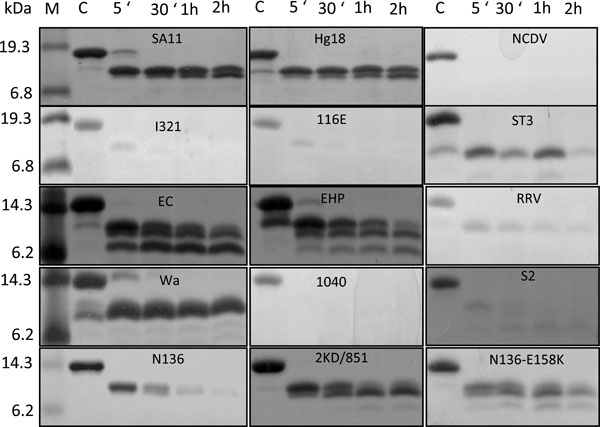 ), the order of trypsin resistance on a scale of 0 to 100 (100 being the highest) was SA11, Hg18, Wa, EHP, EC, 2KD/851 and N136-E158K (75-100%) > ST3, RRV and N136 (50 to 75%) > IS2, I321, 116E (25 to 50%) > NCDV and 1040 (0 to 25%), with the SA11 group being highly resistant and NCDV group being extremely susceptible. While ΔN72 from SA11, Hg18 and WA yielded a 9.95 kDa stable fragment, that from the murine strains yielded additional smaller stable fragments (Fig. 4
), the order of trypsin resistance on a scale of 0 to 100 (100 being the highest) was SA11, Hg18, Wa, EHP, EC, 2KD/851 and N136-E158K (75-100%) > ST3, RRV and N136 (50 to 75%) > IS2, I321, 116E (25 to 50%) > NCDV and 1040 (0 to 25%), with the SA11 group being highly resistant and NCDV group being extremely susceptible. While ΔN72 from SA11, Hg18 and WA yielded a 9.95 kDa stable fragment, that from the murine strains yielded additional smaller stable fragments (Fig. 4 ). Further, the non-diarrheagenic EC-NSP4ΔN72 was as resistant to trypsin as EHP-NSP4ΔN72. It may be noted that NSP4s from the murine strains exhibit high sequence divergence compared to that of other group A rotaviruses and form a distinct genetic group [22Horie Y, Nakagomi O, Koshimura Y, et al. Diarrhea induction by rotavirus NSP4 in the homologous mouse model system Virology 1999; 262: 398-407.] (Fig. 1B
). Further, the non-diarrheagenic EC-NSP4ΔN72 was as resistant to trypsin as EHP-NSP4ΔN72. It may be noted that NSP4s from the murine strains exhibit high sequence divergence compared to that of other group A rotaviruses and form a distinct genetic group [22Horie Y, Nakagomi O, Koshimura Y, et al. Diarrhea induction by rotavirus NSP4 in the homologous mouse model system Virology 1999; 262: 398-407.] (Fig. 1B ). Of significance, ΔN72 from the symptomatic strain N136 was highly susceptible to trypsin and about 33-fold less diarrheagenic compared to that from the asymptomatic strain 2KD/851 or the N136-E158K mutant in spite of the protein from N136 exhibiting relatively higher ThT fluorescence (Figs. 3
). Of significance, ΔN72 from the symptomatic strain N136 was highly susceptible to trypsin and about 33-fold less diarrheagenic compared to that from the asymptomatic strain 2KD/851 or the N136-E158K mutant in spite of the protein from N136 exhibiting relatively higher ThT fluorescence (Figs. 3 , 4
, 4 and Table 2).
and Table 2).
rNSP4ΔN72 Peptides from Different Strains Differ in their Conformation and Multimerization Properties
The wide differences in ThT binding and susceptibility to trypsin digestion suggest conformational differences among the recombinant ΔN72 peptides from different strains. CD spectroscopic studies were employed to confirm this prediction. As shown in Fig. (5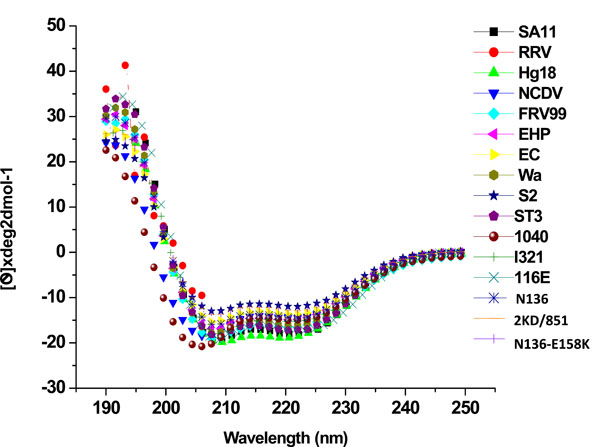 ) and Table 2, all the peptides exhibited highly negative CD spectral values as expected suggesting that they contained the expected coiled coil domain (CCD) and the highly diarrheagenic SA11-, Hg18- and EHP-ΔN72 peptides consistently showed high (-helical content compared to majority of the peptides as observed earlier [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25.]. Thus while high (-helical content correlated well with the low DD50 values of the efficient diarrhea inducers, the inefficient diarrhea inducers differed significantly among themselves in their conformation contents without correlation to their relative DD50 values. For example, while NSP4ΔN72 from Wa, ST3, 116E, N136 and N138 exhibited about 56-58%, that from all other strains showed only 30-48% (-helical content. Generally, proteins exhibiting high (-helicity contained less than 9% (-sheet content and those with low (-helical content showed between 16 and 26%. NCDV-NSP4ΔN72 differed from all others as it failed to bind ThT and exhibited very high (54%) random conformation content in contrast to that of other proteins which ranged between 32 and 44%. The SA11ΔN112 peptide contained very low (-helical content compared to the corresponding ΔN72 peptide. Hence the secondary structural contents of ΔN112 peptides from other strains were not evaluated.
) and Table 2, all the peptides exhibited highly negative CD spectral values as expected suggesting that they contained the expected coiled coil domain (CCD) and the highly diarrheagenic SA11-, Hg18- and EHP-ΔN72 peptides consistently showed high (-helical content compared to majority of the peptides as observed earlier [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25.]. Thus while high (-helical content correlated well with the low DD50 values of the efficient diarrhea inducers, the inefficient diarrhea inducers differed significantly among themselves in their conformation contents without correlation to their relative DD50 values. For example, while NSP4ΔN72 from Wa, ST3, 116E, N136 and N138 exhibited about 56-58%, that from all other strains showed only 30-48% (-helical content. Generally, proteins exhibiting high (-helicity contained less than 9% (-sheet content and those with low (-helical content showed between 16 and 26%. NCDV-NSP4ΔN72 differed from all others as it failed to bind ThT and exhibited very high (54%) random conformation content in contrast to that of other proteins which ranged between 32 and 44%. The SA11ΔN112 peptide contained very low (-helical content compared to the corresponding ΔN72 peptide. Hence the secondary structural contents of ΔN112 peptides from other strains were not evaluated.
Conformational differences appeared to reflect the ordered multimerization ability of the different recombinant proteins. We recently showed that the highly diarrheagenic NSP4ΔN72 from SA11 and Hg18 existed in HMW complexes in E. coli, and that the purified protein formed highly ordered multimers and mutations in the N- or C-terminal regions significantly perturbed the conformation and equilibrium between the multimeric and oligomeric forms to different extents [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25., 6Deepa R, Sastri NP, Rao CD, et al. The flexible C-terminus of the rotavirus nonstructural protein NSP4 is an important determinant of its biological properties J Gen Virol 2008; 89: 1485-96.]. As shown in Table 1, while the highly diarrheagenic SA11- and Hg18-ΔN72 peptides exhibited efficient multimerization even at low concentration as reported earlier [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25.], all other ΔN72 peptides differed widely in their multimerization property. Some showed concentration-dependent multimerization (EHP and RRV), whereas others either existed predominantly in oligomeric form or failed to multimerize even at high concentration (Table 2). Though the rNSP4ΔN72 from the symptomatic and asymptomatic Vellore strains differing in a single amino acid showed comparable multimerization/oligomerization properties, they differed significantly in their ThT binding, trypsin resistance and conformation contents. NCDV-NSP4ΔN72 though failed to multimerize at low concentration, about 60% existed as HMW form at high concentration. Interestingly, its diarrhea-inducing ability showed a sharp decline below DD50 value, which probably correlates with the extreme instability of the oligomers at low concentration as seen by the broad peak in SEC (data not shown).
DISCUSSION
Our recent studies based on SA11-and Hg18-ΔN72 peptides and their mutants suggested that efficient diarrhea induction is correlated with high ThT fluorescence, trypsin resistance, ∝-helical content and/or ordered multimerization [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25., 6Deepa R, Sastri NP, Rao CD, et al. The flexible C-terminus of the rotavirus nonstructural protein NSP4 is an important determinant of its biological properties J Gen Virol 2008; 89: 1485-96.]. However, the present analysis of different properties of NSP4ΔN72 peptides on a wider scale revealed that efficient diarrhea inducers exhibited high-level of ThT binding, ∝-helical content, trypsin resistance, and multimerization. However, no such correlation between the relative DD50 values of the inefficient diarrheagenic peptides and any of the studied properties was observed. (Table 2, Figs. 3 -5
-5 ). Of interest, ΔN72 peptides from asymptomatic strains differed significantly among themselves in their properties. Whilst ST3-NSP4ΔN72 failed to bind ThT and exhibited DD50 of 0.07 nmol, that of I321, though exhibited high ThT fluorescence, was about 7 fold less efficient in diarrhea induction than the former. 116EΔN72, though showed DD50 similar to that of I321, exhibited negligible ThT binding. Also, the DD50 of ΔN72 peptides from the asymptomatic strains was either very similar to, or significantly lower than that of some of the symptomatic strains. Further, the NSP4 ΔN72 peptide from the symptomatic Vellore strains N136 and N138 was about 33-fold less efficient than that of the asymptomatic strains 2KD/851and 99-D/214 in spite of the former showing higher ∝-helical content and ThT binding. Linear regression analysis of the data from Table 2 using DD50 as dependent variable and ThT fluorescence, ∝-helical content, trypsin resistance or percent multimerization as independent variables conclusively revealed a lack of correspondence between diarrheagenic activity and any of the biochemical and biophysical properties (data not shown).
). Of interest, ΔN72 peptides from asymptomatic strains differed significantly among themselves in their properties. Whilst ST3-NSP4ΔN72 failed to bind ThT and exhibited DD50 of 0.07 nmol, that of I321, though exhibited high ThT fluorescence, was about 7 fold less efficient in diarrhea induction than the former. 116EΔN72, though showed DD50 similar to that of I321, exhibited negligible ThT binding. Also, the DD50 of ΔN72 peptides from the asymptomatic strains was either very similar to, or significantly lower than that of some of the symptomatic strains. Further, the NSP4 ΔN72 peptide from the symptomatic Vellore strains N136 and N138 was about 33-fold less efficient than that of the asymptomatic strains 2KD/851and 99-D/214 in spite of the former showing higher ∝-helical content and ThT binding. Linear regression analysis of the data from Table 2 using DD50 as dependent variable and ThT fluorescence, ∝-helical content, trypsin resistance or percent multimerization as independent variables conclusively revealed a lack of correspondence between diarrheagenic activity and any of the biochemical and biophysical properties (data not shown).
Though it may be argued that ΔN72 peptide does not correspond to the full-length protein or a biologically relevant peptide in the infected cells, it is the longest and highly diarrheagenic peptide from SA11 and Hg18 that could be purified to homogeneity in large quantities from E. coli [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25.]. Present studies using the biologically relevant ΔN112 peptides revealed that both ΔN72 and ΔN112 peptides from different strains exhibit similar pattern of diarrhea inducing properties though differ in the relative range of their DD50 values. The ΔN72 peptide from SA11 and Hg18 is about 20- and 200-fold more efficient in diarrhea induction than that reported for the full-length protein and the secreted ΔN112 peptide [2Ball JM, Tian P, Zeng CQ-Y, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotavirus nonstructural glycoprotein Science 1996; 272 : 101-4., 3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25., 15Zhang M, Zeng CQ, Morris AP, Estes MK. A functional NSP4 enterotoxin peptide secreted from rotavirus-infected cells J Virol 2000; 74: 11663-70.], respectively in newborn mice and exhibits efficient DLP-binding activity suggesting that this region exhibits optimal biological properties associated with full-length protein. The observation that ΔN112 peptide was less efficient than ΔN72 further supports our previous observation that both the N-terminal AD and C-terminal flexible regions are important for optimal biological functions of the protein [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25., 6Deepa R, Sastri NP, Rao CD, et al. The flexible C-terminus of the rotavirus nonstructural protein NSP4 is an important determinant of its biological properties J Gen Virol 2008; 89: 1485-96.]. A recent report of the viroporin activity of the N-terminal region from residue 55-90 further supports this observation [20Hyser JM, Collinson-Pautz MR, Utama B, Estes MK. Rotavirus disrupts calcium homeostasis by NSP4 viroporin activity mBio 2010; 1: e00265-10.]. Since full-length NSP4 could neither be expressed in E. coli due to the presence of hydrophobic domains at the N-terminus nor could that expressed in insect cells be purified to homogeneity [2Ball JM, Tian P, Zeng CQ-Y, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotavirus nonstructural glycoprotein Science 1996; 272 : 101-4.], different studies used deletion mutants that varied widely in their length and/or the presence or absence of a tag at the N-terminus which might have contributed to the wide differences in the DD50 values reported in the limited number of studies [2Ball JM, Tian P, Zeng CQ-Y, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotavirus nonstructural glycoprotein Science 1996; 272 : 101-4., 3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25., 15Zhang M, Zeng CQ, Morris AP, Estes MK. A functional NSP4 enterotoxin peptide secreted from rotavirus-infected cells J Virol 2000; 74: 11663-70., 23Mori Y, Sugiyama M, Takayama M, Atoji Y, Masegi T, Minamoto N. Avian-to-mammal transmission of an avian rotavirus: Analysis of its pathogenicity in a heterologous mouse model Virology 2001; 288: 63-70., 39Mori Y, Borgan MA, Ito N, Sugiyama M, Minamoto N. Diarrhea-inducing activity of avian rotavirus glycoproteins, which differ greatly from mammalian rotavirus NSP4 glycoproteins in deduced amino acid sequence in suckling mice J Virol 2002; 76: 5829-34.]. Results in our laboratory indicated that presence of a His-tag at the N-terminus of SA11- and Hg18-ΔN72 did not affect its biological function.
Recent studies from our laboratory [3Jagannath MR, Kesavulu MM, Deepa R, et al. N- and C-terminal cooperation in rotavirus enterotoxin: novel mechanism of modulation of the properties of a multifunctional protein by a structurally and functionally overlapping conformational domain J Virol 2006; 80: 412-25., 6Deepa R, Sastri NP, Rao CD, et al. The flexible C-terminus of the rotavirus nonstructural protein NSP4 is an important determinant of its biological properties J Gen Virol 2008; 89: 1485-96.] also suggested that sequence variations observed in the flexible C-terminus compared to the other regions of NSP4s in different strains [33Lin SL, Tian P. Detailed computational analysis of a comprehensive set of group A rotavirus NSP4 proteins Virus Genes 2003; 26: 271-82.] (Fig. 1B ) would affect conformation, ThT binding and biological properties of the recombinant protein. The significant differences in ThT binding, resistance to trypsin digestion, conformation and DD50 values exhibited by the recombinant proteins from the symptomatic and asymptomatic G10P [11Bergman CC, Mass D, Poruchynsky M, Atkinson PH, Bellamy AR. Topology of the nonstructural rotavirus receptor glycoptrotein NS28 in the rough endoplasmic reticulum EMBO J 1989; 8: 1695-703.] strains that differed at a single amino acid position 158 in the flexible C-terminal region strongly supports this hypothesis. Further, it may be noted that the EC-NSP4 differs from that of EHP at four positions 137, 150, 154 and 161 in the unstructured C-terminus (Fig. 1B
) would affect conformation, ThT binding and biological properties of the recombinant protein. The significant differences in ThT binding, resistance to trypsin digestion, conformation and DD50 values exhibited by the recombinant proteins from the symptomatic and asymptomatic G10P [11Bergman CC, Mass D, Poruchynsky M, Atkinson PH, Bellamy AR. Topology of the nonstructural rotavirus receptor glycoptrotein NS28 in the rough endoplasmic reticulum EMBO J 1989; 8: 1695-703.] strains that differed at a single amino acid position 158 in the flexible C-terminal region strongly supports this hypothesis. Further, it may be noted that the EC-NSP4 differs from that of EHP at four positions 137, 150, 154 and 161 in the unstructured C-terminus (Fig. 1B ). The observation that rNSP4ΔN72 peptides from the murine EHP and EC strains exhibit contrasting diarrhea inducing properties in spite of both viruses being highly virulent in the homologous host strongly suggests a conformational conundrum to the differences in the biological function of the recombinant polypeptides. In the infected cells, NSP4 interaction with other viral [1Estes MK. Fields Virology. 4th. Philadelphia: Lippincott Williams and Wilkins 2001; 2: pp. 1747-85., 9Maass DR, Atkinson PH. Rotavirus proteins VP7, NS28, and VP4 form oligomeric structures J Virol 1990; 64: 2632-41., 20Hyser JM, Collinson-Pautz MR, Utama B, Estes MK. Rotavirus disrupts calcium homeostasis by NSP4 viroporin activity mBio 2010; 1: e00265-10.] and cellular proteins [53Mirazimi A, Nilsson M, Svensson L. The molecular chaperone calnexin interacts with the NSP4 enterotoxin of rotavirus in vivo and in vitro J Virol 1998; 72: 8705-9.-57Seo N-S, Zeng CQ-Y, Hyser JM, et al. Integrins α1β1 and α2β1 are receptors for the rotavirus enterotoxin Proc Natl Acad Sci USA 2008; 105: 8811-., 64Berkova Z, Crawford SE, Blatt SE, Morris AP, Estes MK. Expression of rotavirus NSP4 alters the actin network organization through the actin remodeling protein cofilin J Virol 2007; 81: 3545-53.] might facilitate conformational maturation and the multitude of its biological functions [2Ball JM, Tian P, Zeng CQ-Y, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotavirus nonstructural glycoprotein Science 1996; 272 : 101-4., 6Deepa R, Sastri NP, Rao CD, et al. The flexible C-terminus of the rotavirus nonstructural protein NSP4 is an important determinant of its biological properties J Gen Virol 2008; 89: 1485-96., 53Mirazimi A, Nilsson M, Svensson L. The molecular chaperone calnexin interacts with the NSP4 enterotoxin of rotavirus in vivo and in vitro J Virol 1998; 72: 8705-9., 54Xu A, Bellamy AR, Taylor JA. Immobilization of the early secretory pathway by a virus glycoprotein that binds to microtubules EMBO J 2000; 19: 6465-74., 57Seo N-S, Zeng CQ-Y, Hyser JM, et al. Integrins α1β1 and α2β1 are receptors for the rotavirus enterotoxin Proc Natl Acad Sci USA 2008; 105: 8811-.-67Ousingsawat J, Mirza M, Tian Y, et al. Rotavirus toxin NSP4 induces diarrhea by activation of TMEM16A and inhibition of Na+ absorption Pflugers Arch-Eur J Physiol 2011; 461: 579-89.]. The present studies also suggest that mutations in different NSP4s that could severely affect their biological functions would vary from strain to strain and depend on the overall sequence context of the complete CT, and that rNSP4s, irrespective of their origin, would exhibit DD50 values with inconsistent correlation to the virulence of their virus strains. In this context, our earlier observation that the biological properties of the highly diarrheagenic SA11-NSP4ΔN72 are dependent on a unique and complex conformation in the cytoplasmic tail, mediated by cooperation between the N-terminal amphipathic domain and the extreme C-terminus, is of relevance. The wide variation in conformation among the different peptides, supported by the wide range of differences in susceptibility to trypsin cleavage, ThT binding and multimerization, strongly suggests that unlike the highly enterotoxigenic rNSP4 peptides from SA11 and Hg18, those from majority of the strains fail to attain the proper conformation required for optimal diarrheagenic function.
). The observation that rNSP4ΔN72 peptides from the murine EHP and EC strains exhibit contrasting diarrhea inducing properties in spite of both viruses being highly virulent in the homologous host strongly suggests a conformational conundrum to the differences in the biological function of the recombinant polypeptides. In the infected cells, NSP4 interaction with other viral [1Estes MK. Fields Virology. 4th. Philadelphia: Lippincott Williams and Wilkins 2001; 2: pp. 1747-85., 9Maass DR, Atkinson PH. Rotavirus proteins VP7, NS28, and VP4 form oligomeric structures J Virol 1990; 64: 2632-41., 20Hyser JM, Collinson-Pautz MR, Utama B, Estes MK. Rotavirus disrupts calcium homeostasis by NSP4 viroporin activity mBio 2010; 1: e00265-10.] and cellular proteins [53Mirazimi A, Nilsson M, Svensson L. The molecular chaperone calnexin interacts with the NSP4 enterotoxin of rotavirus in vivo and in vitro J Virol 1998; 72: 8705-9.-57Seo N-S, Zeng CQ-Y, Hyser JM, et al. Integrins α1β1 and α2β1 are receptors for the rotavirus enterotoxin Proc Natl Acad Sci USA 2008; 105: 8811-., 64Berkova Z, Crawford SE, Blatt SE, Morris AP, Estes MK. Expression of rotavirus NSP4 alters the actin network organization through the actin remodeling protein cofilin J Virol 2007; 81: 3545-53.] might facilitate conformational maturation and the multitude of its biological functions [2Ball JM, Tian P, Zeng CQ-Y, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotavirus nonstructural glycoprotein Science 1996; 272 : 101-4., 6Deepa R, Sastri NP, Rao CD, et al. The flexible C-terminus of the rotavirus nonstructural protein NSP4 is an important determinant of its biological properties J Gen Virol 2008; 89: 1485-96., 53Mirazimi A, Nilsson M, Svensson L. The molecular chaperone calnexin interacts with the NSP4 enterotoxin of rotavirus in vivo and in vitro J Virol 1998; 72: 8705-9., 54Xu A, Bellamy AR, Taylor JA. Immobilization of the early secretory pathway by a virus glycoprotein that binds to microtubules EMBO J 2000; 19: 6465-74., 57Seo N-S, Zeng CQ-Y, Hyser JM, et al. Integrins α1β1 and α2β1 are receptors for the rotavirus enterotoxin Proc Natl Acad Sci USA 2008; 105: 8811-.-67Ousingsawat J, Mirza M, Tian Y, et al. Rotavirus toxin NSP4 induces diarrhea by activation of TMEM16A and inhibition of Na+ absorption Pflugers Arch-Eur J Physiol 2011; 461: 579-89.]. The present studies also suggest that mutations in different NSP4s that could severely affect their biological functions would vary from strain to strain and depend on the overall sequence context of the complete CT, and that rNSP4s, irrespective of their origin, would exhibit DD50 values with inconsistent correlation to the virulence of their virus strains. In this context, our earlier observation that the biological properties of the highly diarrheagenic SA11-NSP4ΔN72 are dependent on a unique and complex conformation in the cytoplasmic tail, mediated by cooperation between the N-terminal amphipathic domain and the extreme C-terminus, is of relevance. The wide variation in conformation among the different peptides, supported by the wide range of differences in susceptibility to trypsin cleavage, ThT binding and multimerization, strongly suggests that unlike the highly enterotoxigenic rNSP4 peptides from SA11 and Hg18, those from majority of the strains fail to attain the proper conformation required for optimal diarrheagenic function.
ACKNOWLEDGEMENTS
Financial support from the Indian Council of Medical Research and the Structural Genomics program under the Genomics Initiative at the Indian Institute of Science funded by the Department of Biotechnology, Indian Council of Medical Research, Government of India is acknowledged. We gratefully acknowledge the use of mass spectrometry and CD facilities in the Molecular Biophysics Unit and the CD facility in the Department of Biochemistry at the Indian Institute of Science. Technical assistance from Mr. Senthil Kumar is acknowledged.