- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Virology Journal
(Discontinued)
ISSN: 1874-3579 ― Volume 15, 2021
Silencing of ORFs C2 and C4 of Tomato Yellow Leaf Curl Virus Engenders Resistant or Tolerant Plants
Yuval Peretz, Assaf Eybishtz , Ilan Sela*
Abstract
The IL-60 system is a transient universal vector system for expression and silencing in plants [1 Peretz Y, Mozes-Koch R, Akad F, Tanne E, Czosnek H, Sela I. A universal expression/silencing vector in plants Plant Physiol 2007; 145: 1251-63.]. This vector has been derived from Tomato yellow leaf curl virus (TYLCV). The viral intergenic region (IR) is a non-coding short (314 b) sequence separating the viral sense-oriented genes from the complementary-oriented genes. IR carries the viral origin of replication as well as a promoter at each end. Placing a gene segment between two IRs at opposite orientations followed by trans-activation of the construct by the plasmid IL-60-BS, caused silencing of the pertinent gene as indicated by the silencing of the endogenous gene PDS.. The viral genes C2 and C4 are implicated as having a role in viral-directed silencing suppression. The silencing of C2 and C4 intervened with the virus ability to counter-react to viral silencing by the host plant, thus engendering resistance or tolerance.
Article Information
Identifiers and Pagination:
Year: 2011Volume: 5
First Page: 141
Last Page: 147
Publisher Id: TOVJ-5-141
DOI: 10.2174/1874357901105010141
Article History:
Received Date: 5/7/2011Revision Received Date: 5/9/2011
Acceptance Date: 21/9/2011
Electronic publication date: 13/12/2011
Collection year: 2011
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Hebrew University of Jerusalem, Robert H. Smith Faculty of Agriculture, Food and Environment, Institute for Plant Sciences and Genetics, Rehovot 76100, Israel; Tel: +972-8-9489377; Fax: +972-8-9473402; E-mail: sela@agri.huji.ac.il
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 5-7-2011 |
Original Manuscript | Silencing of ORFs C2 and C4 of Tomato Yellow Leaf Curl Virus Engenders Resistant or Tolerant Plants | |
INTRODUCTION
The Tomato yellow leaf curl virus (TYLCV) genome is made up of a single-stranded (ss) DNA of approximately 3000 bases (depending on the strain; the Israeli strain which was disarmed and developed into IL-60 is 2787 bases long). The ssDNA is encapsidated, and the virus is transmissible by whiteflies (Bemisia tabaci). The coat protein (CP) directs the viral DNA to the nucleus, where the ssDNA is converted to double-stranded (ds) DNA and viral RNAs are transcribed. The genome carries six overlapping open reading frames (ORFs). Two ORFs, V1 (CP) and V2, are transcribed to RNA in sense orientation, and four ORFs, C1 to C4, are transcribed to RNA in the complementary orientation. Rolling circle replication is initiated by the viral replicase-associated protein (REP, the product of ORF C1), producing progeny ssDNAs. The sense-oriented and complementary-oriented ORFs are separated by a short (314-bases long) intergenic region (IR). The IR carries the viral origin of replication and REP-binding motifs. Strong promoters are situated at both ends of the IR, directing transcription of the sense- and complementary-oriented viral RNAs. TYLCV DNA assembles into minichromosomes, but is accessable to plant factors at the origin of replication and the promoter regions [2Jeske H. Geminiviruses Curr top Microbiol Immunol 2009; 331: 185-226.]. Structure-function relationships in geminiviruses have been described [3Davis JW, Stanley J. Geminivirus genes and vectors Trends Genet 1989; 5: 77-81.-6Gafni Y. Tomato yellow leaf curl virus, the intracellular dynamics of a plant DNA virus Mol Plant Pathol 2003; 4: 9-15.].
The IL-60 system [1 Peretz Y, Mozes-Koch R, Akad F, Tanne E, Czosnek H, Sela I. A universal expression/silencing vector in plants Plant Physiol 2007; 145: 1251-63.] comprises a series of plasmids derived from a modified disarmed form of TYLCV DNA. Briefly, a deletion of 60 bp in the coat protein ORF of a clone of the Israeli isolate of TYLCV rendered the construct non-symptomatic. In addition, The C1 ORF was interrupted by insertion of a bacterial plasmid (usually BlueScript, ca. 3 Kbp in length). Since no functional C1 can be expressed, the only mode of DNA replication is dsDNA to dsDNA in a REP-independent manner, presumably recombination-depended replication [7Gutierrez C. Geminivirus DNA replication Cell Mol Life Sci 1999; 56: 313-29.]. Various combinations of this system have allowed for replication, movement and expression (when applicable) of foreign sequences in every plant tested to date. The vectors and the genes they carry do not integrate into the plant's genome and are not heritable. Therefore, although active throughout the plant's life span, expression or silencing derived from IL-60 components are not transgenic. When a construct carrying the same sequence in opposite orientations was transcribed, RNA silencing of the pertinent sequence was obtained due to presumed backfolding of the transcript. Thus the IL-60 system provides a universal expression/silencing system in plants. Any gene fused to the IR (IR-X) is stabilized in plants. Challenge infection of these plants with a native TYLCV initiates mobilization, replication and expression of IR-X. Hence, IR-X is a TYLCV-dependent satellite. The disarmed form of TYLCV dsDNA (IL-60-BS) can replace TYLCV as the driving force for movement, replication and expression of IR-X without causing disease [1 Peretz Y, Mozes-Koch R, Akad F, Tanne E, Czosnek H, Sela I. A universal expression/silencing vector in plants Plant Physiol 2007; 145: 1251-63.].
Even though TYLCV is a DNA virus, the host plant reacts to infection by promoting a virus-specific RNA-silencing mechanism, probably induced by a segment of overlapping sense- and complementary-oriented RNAs forming a segment of dsRNA [7Gutierrez C. Geminivirus DNA replication Cell Mol Life Sci 1999; 56: 313-29.-9Vanitharani R, Chellappan P, Fauquet CM. Geminivirus and RNA silencing Trends Plant Sci 2005; 10: 144-51.]. The virus counter-reacts by producing silencing-suppressor proteins. The product of ORF C2 (and its analogs in other geminiviruses) is a transcription activator [10Bisaro DM. Silencing suppression by geminivirus proteins Virology 2006; 344: 158-68., 11Sharma P, Ikegami M. RNA-silencing suppressors of geminiviruses J Gen Plant Pathol 2008; 74: 189-202.] and has also been implicated in silencing suppression [12Sunter G, Bisaro DM. Transactivation in a geminivirus: AL2 gene product is needed for coat protein expression Virology 1991; 180: 416-9.-16Thrinks D, Rajeswaran R, Shivaprasad PV, et al. Suppression of RNA silencing by a geminivirus nuclear protein AC2, correlates with transactivation of host genes J Virol 2005; 79: 2517-7.]. Silencing suppression by C2 appears to be a nuclear process [15Dong XL, van Wezel R, Stanley J, Hong YG. Functional characterization of the nuclear localization signal for a suppressor of posttranslational gene silencing J Virol 2003; 77: 7026-33.]. Since C2 is a transcription activator, it is not surprising that it contributes to silencing suppression indirectly, by upregulating gene expression of the host, leading, among other things, to the intervention of native plant proteins (such as WEL1) in silencing pathways [10Bisaro DM. Silencing suppression by geminivirus proteins Virology 2006; 344: 158-68.]. Adenosine kinase has been implicated in the methylation cycle of silencing [17Wang H, Hao L, Shung C-Y, Sunter G, Bisaro DM. Adenosine kinase is inactivated by geminivirus AL2 and L2 proteins Plant Cell 2003; 15: 3020-2., 18Wang H, Buckley KJ, Yang X, Buchmann RC, Bisaro DM. Adenosine kinase inhibition and suppression of RNA silencing by geminivirus AL2 and L2 proteins J Virol 2005; 79: 7410-8.], and inhibition of its expression by C2 and its analogs in other geminiviruses brings about silencing suppression, although this might be a case of transcriptional gene silencing rather than a post-transcriptional event [18Wang H, Buckley KJ, Yang X, Buchmann RC, Bisaro DM. Adenosine kinase inhibition and suppression of RNA silencing by geminivirus AL2 and L2 proteins J Virol 2005; 79: 7410-8.]. C4 and its analogs in other geminiviruses have also been pinpointed as silencing suppressors. C4 inhibits accumulation of siRNA, and therefore interferes with the guiding of RISC to its target, resulting in elevated RNA accumulation [19Voinnet O, Pinto YM, Baulcomb DC. Suppression of gene silencing: a general strategy used by diverse DNA and RNA viruses of plants Proc Natl Acad Sci USA 1999; 96: 14147-52., 20Chellappan P, Vanitharani R, Fauquet CM. Short interfering RNA accumulation correlates with host recovery in DNA virus-infected hosts, and gene silencing targets specific for viral sequences J Virol 2004; 78: 7465-7.]. Transgenic plants expressing C4 exhibit virus-like symptoms interpreted as possible intervention in normal developmental processes, in which siRNAs have been demonstrated to participate in several organisms [19Voinnet O, Pinto YM, Baulcomb DC. Suppression of gene silencing: a general strategy used by diverse DNA and RNA viruses of plants Proc Natl Acad Sci USA 1999; 96: 14147-52.-25Neilson JR, Sharp PA. Small RNA regulation of gene expression Cell 2008; 134: 899-902.]. More recently, after the presently reported work was already completed, the product of ORF V2 was also convincingly demonstrated to be a silencing suppressor [26Zrachy A, Glick E, Levy Y, Arazi T, Citovsky V, Gafni Y. Suppresor of RNA silencing encoded by Tomato yellow leaf curl virus-Israel Virology 2007; 358: 159-65., 27Glick E, Zrachya A, Levy Y, et al. Interaction with host SGS3 is required for suppression of DNA silencing by tomato yellow leaf curl virus V2 protein Proc Natl Acad Sci USA 2008; 105: 157-61.]. Here we report that placing a gene segment between two opposing IRs brings about silencing of the inserted gene, and that silencing of C2 and C4 leads to the production of TYLCV-resistant and/or tolerant plants.
MATERIALS AND METHODS
Clones and Constructs
A PCR-amplified segment of TYLCV containing the IR and 159 bp of ORF V2 (bases 61-473, GenBank accession no. X15656) was cloned into the T/A cloning vector pDRIVE. This construct carries the ribosomal binding site of V2. The above IR segment was amplified with primers carrying restriction sites at their 5' ends. A forward IR was cloned into pDRIVE with KpnI and PstI, and the IR in opposite orientation was cloned into the same plasmid with SalI and HindIII. The opposing IRs flanked the T/A cloning site of pDRIVE, enabling the insertion of any PCR product between the two opposing IR promoters. A segment of tomato PDS (bases 943 to 1135, GenBank accession no. M88683) and the entire ORFs of TYLCV C2 and TYLCV C4 (bases 1398 to 1809 and 2343 to 2647, respectively, GenBank accession no. X15656) were inserted between the opposing IRs. The resultant constructs (IR-PDS-RI, IR-C2-RI and IR-C4-RI, respectively) are illustrated in Fig. (1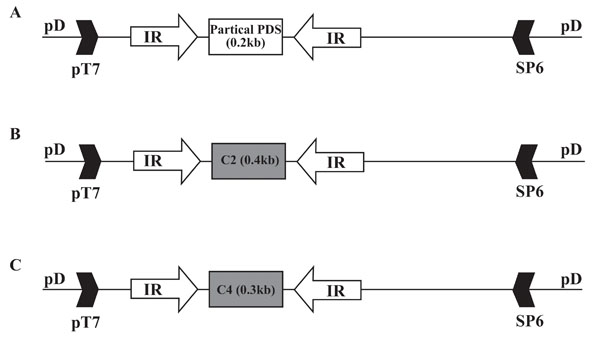 ). IRs in all constructs (including constructs carrying opposing IRs) were positioned so that the transcripts would be regulated by the promoter controlling C1 to C4 transcription. The only viral component in the aforementioned constructs was the IR, and they were therefore considered viral satellites. Constructs were injected into plants along with IL-60-BS [4Argüello-Astorga GR, Guevara-Gonzales RG, Herrera-Estrella LR, Rivera-Bustamante RF. Geminivirus replication origins have a group-specific organization of iterative elements: a model for replication Virology 1994; 203: 90-100.] which promotes replication, movement and transcription of the satellites.
). IRs in all constructs (including constructs carrying opposing IRs) were positioned so that the transcripts would be regulated by the promoter controlling C1 to C4 transcription. The only viral component in the aforementioned constructs was the IR, and they were therefore considered viral satellites. Constructs were injected into plants along with IL-60-BS [4Argüello-Astorga GR, Guevara-Gonzales RG, Herrera-Estrella LR, Rivera-Bustamante RF. Geminivirus replication origins have a group-specific organization of iterative elements: a model for replication Virology 1994; 203: 90-100.] which promotes replication, movement and transcription of the satellites.
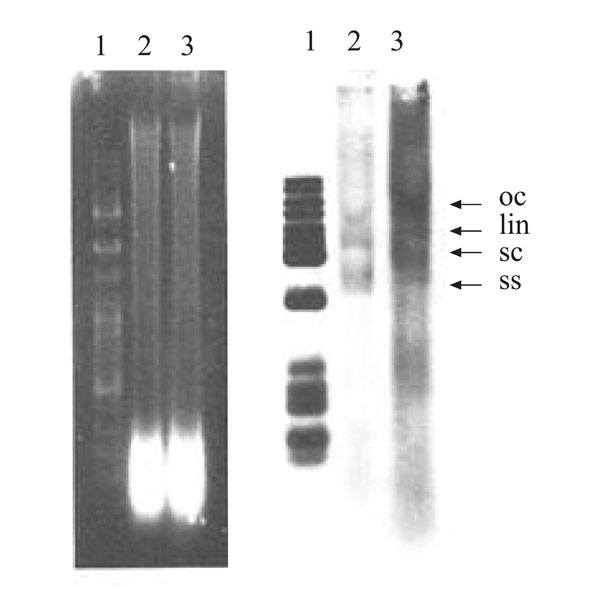 |
Fig. (4) A Southern blot for TYLCV. DNA was extracted from the upper leaves of the plant shown in Fig. (3D |
Propagation of Satellites and their Administration into Plants
Escherichia coli cells were transformed with the pertinent satellite construct, propagated under ampicillin selection, and the construct was extracted by standard procedures [28Sambrook J, Russel DW. Molecular cloning. 3rd ed. New York: Cold Spring Harbor Laboratory Press 2001.]. A stem or leaf petiole of a recipient plant was punctured with a hypodermic needle. A capillary tube was inserted into the resultant hole, and approximately 2 µg of DNA (in 100 µl H2O) was pipetted into the capillary tube until fully absorbed by the plant. Only about 50% of the plants injected with the various satellite forms demonstrated successful administration, as indicated by PCR. Therefore, each experiment was carried out with at least 20 plants, and only plants found by PCR to carry the inserted construct were further analyzed. Each reported experiment was repeated at least twice (more in most cases).
Molecular Analyses
Southern, PCR and semi-quantitative PCR analyses were carried out according to standard procedures [28Sambrook J, Russel DW. Molecular cloning. 3rd ed. New York: Cold Spring Harbor Laboratory Press 2001.]. Unless otherwise stated, 40 cycles were run for each PCR assay. Probes for Southern analyses were labeled by the PCR-DIG procedure (Roche Molecular Biochemicals). Probes were prepared according to the manufacturers' protocols.
Semi-quantitative PCR was carried out by removing aliquots from an ongoing PCR of a target gene (or cDNA) at different cycles and determining the threshold of band appearance. A similar assay, with the same templates, was carried out with primers for a constitutive gene, and the threshold of its band appearance was determined and served as a standardizing parameter for possible quantitative deviations.
Unlike the case of real-time PCR, the exact threshold in a semi-quantitative analysis is only an estimate. Nevertheless we used the following calculation (developed for real-time PCR) to compare quantities between samples. Each treatment threshold was given an arbitrary quantitative value according to the formula ∆ct = 2-(ct target gene - ct constitutive gene), ct being the cycle threshold. The relative quantitative increase/decrease in templates between control and treated plants was estimated from the ratio of their respective ∆cts.
RESULTS
The Artificial Satellites
Construction of the IR-carrying artificial satellites is described in detail in Materials and Methods. In general, the TYLCV IR was cloned into a bacterial plasmid. In satellites designed for silencing, a segment of the gene to be silenced was inserted between two opposing IRs (IR-X-RI), generating transcripts in both orientations and providing dsRNA which, in turn, brought about silencing. The stronger IR promoter, transcribing the viral antisense genes, was oriented towards the inserts. In these constructs, we placed a segment of the endogenous phytoene desaturase gene (PDS, as a positive control for silencing) or the complete C2 and C4 ORFs of TYLCV. The bacterial plasmid component for all of the IR-carrying constructs was pDRIVE (Qiagen), but other plasmids, such as pBluescript (Stratagene), have also been used successfully. Replication, expression and movement of the satellites were promoted by IL-60-BS [4Argüello-Astorga GR, Guevara-Gonzales RG, Herrera-Estrella LR, Rivera-Bustamante RF. Geminivirus replication origins have a group-specific organization of iterative elements: a model for replication Virology 1994; 203: 90-100.]. The various types of IR-carrying satellites are illustrated in Fig. (1 ). Note that the only TYLCV-derived sequence in these satellites is the IR.
). Note that the only TYLCV-derived sequence in these satellites is the IR.
Satellite-Mediated Silencing of an Endogenous Gene
The first step was to determine whether a sequence placed between two opposing IRs would bring about silencing of the pertinent gene following TYLCV infection. The gene for PDS was selected for this assay. PDS is a key enzyme in the plant's carotenoid-biosynthesis pathway, leading to the production of zeaxanthin, which protects chlorophyll from excessive light bleaching [29Demmig-Adams B, Adams WW III. The role of xanthophylls cycle carotenoids in the protection of photosynthesis Trends Plant Sci 1996; 1: 21-6.-31DellaPenna D, Pogson BJ. Vitamin synthesis in plants: tocopherols and carotenoids Ann Rev Plant Biol 2006; 57: 711-38.]. PDS-silencing results in chlorophyll bleaching under strong light and provides a distinctive, easily recognizable phenotype.
A segment of the tomato PDS gene was inserted into a satellite between two opposing IRs as described in Materials and Methods and illustrated in Fig. (1A ). We expected that transcription would result in PDS-related RNA in both orientations and that the resultant dsRNA would engender RNA silencing of PDS. The construct (IR-PDS-RI) was injected into tomato plants and the plants were whitefly-inoculated with TYLCV 7 days post-injection. Bleaching along the veins appeared about a month after TYLCV inoculation and then progressed rapidly to all parts of the plant (Fig. 2A
). We expected that transcription would result in PDS-related RNA in both orientations and that the resultant dsRNA would engender RNA silencing of PDS. The construct (IR-PDS-RI) was injected into tomato plants and the plants were whitefly-inoculated with TYLCV 7 days post-injection. Bleaching along the veins appeared about a month after TYLCV inoculation and then progressed rapidly to all parts of the plant (Fig. 2A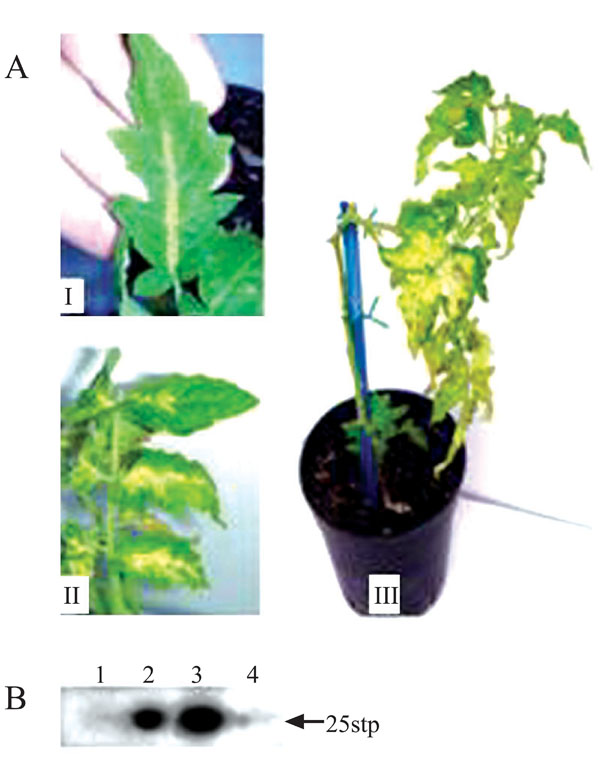 ). Bleaching symptoms are distinguishable from TYLCV-induced symptoms, and to the naked eye, PDS-silenced plants either did not exhibit TYLCV-derived symptoms or developed only very mild ones. Silencing of PDS was corroborated by the appearance in treated plants of siRNAs corresponding to the PDS sequence (Fig. 2B
). Bleaching symptoms are distinguishable from TYLCV-induced symptoms, and to the naked eye, PDS-silenced plants either did not exhibit TYLCV-derived symptoms or developed only very mild ones. Silencing of PDS was corroborated by the appearance in treated plants of siRNAs corresponding to the PDS sequence (Fig. 2B ).
).
Engendering of TYLCV Resistance/Tolerance/Immunity by Satellite-Mediated Silencing of Putative Viral-Silencing Suppressor
The IL-60 system has also been developed as an inductive system comprising of two elements: The expressing/silencing element (the target element) carries a sequence to be expressed/silenced under the control of IR. The driver element provides all necessary factors for replication, expression and movement. TYLCV itself is a driver element, and in order to avoide disease IL-60-BS has been developed as a driver [4Argüello-Astorga GR, Guevara-Gonzales RG, Herrera-Estrella LR, Rivera-Bustamante RF. Geminivirus replication origins have a group-specific organization of iterative elements: a model for replication Virology 1994; 203: 90-100.]. We have demonstrated the ability of the IL-60 system to silence the aforementioned endogenous gene (PDS) by the opposing promoters approach driven by IL-60-BS, and a stem-loop folding structure for the silencing of a viral gene (p10 of GV; 4) we attempted to find out whether TYLCV itself can activate a IR-X-RI construct, where X is a viral gene, and thus promote silencing of its own transcripts.
Post-transcriptional gene silencing has been reported to play a role in the plant's reaction to infection. In the case of TYLCV, the products of ORFs C2 and C4 have been reported to be involved in the suppression of this viral silencing (summarized in 6). We therefore attempted to engender non-transgenic resistance by silencing C2 or C4, thereby arresting the virus's ability to exercise counter-silencing measures.
The C4 ORF was placed between two opposing IR promoters (IR-C4-RI), as described in Materials and Methods and illustrated in Fig. (1C ). The construct was injected into tomato plants. As already stated, this construct cannot replicate in the plant, but by virtue of its inherent IR, is stable and can be induced by the invading virus to replicate, move and be expressed. Transcription from IR-C4-RI was expected to provide RNAs in both orientations, with the resultant dsRNA initiating silencing of C4-carrying transcripts. In the absence (or presence of reduced levels) of C4, the virus can no longer effectively suppress its own silencing by the host plant, and resistance (or tolerance) may thus be engendered. In contrast to other cases (such as the P10 in 4) the resistance machinery was not pre-introduced, and the virus does not find a protection mechanism "waiting" for it. Here, the virus itself activates resistance. In effect, the system directs the invading virus to commit suicide.
). The construct was injected into tomato plants. As already stated, this construct cannot replicate in the plant, but by virtue of its inherent IR, is stable and can be induced by the invading virus to replicate, move and be expressed. Transcription from IR-C4-RI was expected to provide RNAs in both orientations, with the resultant dsRNA initiating silencing of C4-carrying transcripts. In the absence (or presence of reduced levels) of C4, the virus can no longer effectively suppress its own silencing by the host plant, and resistance (or tolerance) may thus be engendered. In contrast to other cases (such as the P10 in 4) the resistance machinery was not pre-introduced, and the virus does not find a protection mechanism "waiting" for it. Here, the virus itself activates resistance. In effect, the system directs the invading virus to commit suicide.
Tomato seedlings were injected with IR-C4-RI. Seven days later, these plants, as well as their control, non-injected counterparts, were whitefly-inoculated with TYLCV (30 viruliferous insects per plant). Sixty days post-inoculation, all of the control plants exhibited severe symptoms, while none of the IR-C4-RI-treated plants showed any. By 90 days post-infection, about 60% of the IR-C4-RI-treated plants had developed very mild symptoms. However, the deleterious effects of the disease were not observed: these plants (symptomless or showing mild symptoms) looked normal in size and set flowers and fruits. The experiment was repeated twice with 30 treated plants in each experiment. An estimation of the virus titer by semi-quantitative PCR indicated an over 10 million-fold reduction in viral DNA in the C4-silenced, non-symptomatic plants. Development of resistance was detected, by semi-quantitative PCR and Southern-blot analysis, as early as 1 week post-inoculation (data not shown). Furthermore, recovery from infection was obtained following injection of IR-C4-RI into plants which were already heavily infected (inoculated in the laboratory or collected in the field). The new growth was asymptomatic, and the plants recovered from stunting and developed flowers and fruits. In this case, semi-quantitative PCR indicated an over 100,000-fold reduction in TYLCV titer in leaves above the site of injection relative to leaves below this site in the same treated plant, the latter retaining disease symptoms. The results of the engendered resistance/tolerance and recovery are shown in Figs. (3 , 4
, 4 ).
).
The viral C2 gene was also placed between two opposing IRs as described in Materials and Methods (Fig. 1B ). IR-C2-RI was injected into plants which were later whitefly-inoculated with TYLCV as described for C4. The experiment consisted of 40 treated plants and was repeated twice. Symptoms appeared following inoculation but all new growth was symptomless. The plants grew to normal size and (except for some symptoms on their lower part) were indistinguishable from healthy plants (Fig. 5A
). IR-C2-RI was injected into plants which were later whitefly-inoculated with TYLCV as described for C4. The experiment consisted of 40 treated plants and was repeated twice. Symptoms appeared following inoculation but all new growth was symptomless. The plants grew to normal size and (except for some symptoms on their lower part) were indistinguishable from healthy plants (Fig. 5A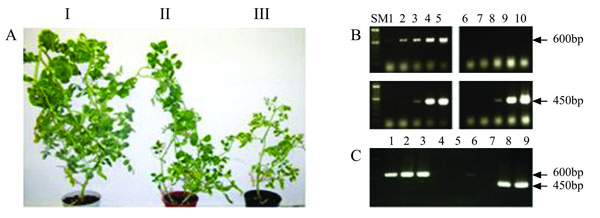 ). Semi-quantitative RT-PCR could not detect any TYLCV at all (Fig. 5B
). Semi-quantitative RT-PCR could not detect any TYLCV at all (Fig. 5B ). Total suppression of infection was also indicated by comparison of TYLCV titers in symptomatic versus asymptomatic leaves of the very same plant (Fig. 5C
). Total suppression of infection was also indicated by comparison of TYLCV titers in symptomatic versus asymptomatic leaves of the very same plant (Fig. 5C ). C2-siRNA was detected in IR-C2-RI-tretaed plants before and after TYLCV inoculation (data not shown). It appears that the new growth had become immune to TYLCV. Silencing-suppressor-derived resistance/tolerance/immunity is discussed further on.
). C2-siRNA was detected in IR-C2-RI-tretaed plants before and after TYLCV inoculation (data not shown). It appears that the new growth had become immune to TYLCV. Silencing-suppressor-derived resistance/tolerance/immunity is discussed further on.
DISCUSSION
This paper describes a rapidly responding platform for silencing in plants. The IR-carrying segment of TYLCV fused to a foreign gene and introduced into plants is stable, possibly due to its inherent IR sequence. A helper geminivirus may be introduced at a later time, contributing trans-activating factors and engendering the spread of, and expression from the IR-carrying segment [4Argüello-Astorga GR, Guevara-Gonzales RG, Herrera-Estrella LR, Rivera-Bustamante RF. Geminivirus replication origins have a group-specific organization of iterative elements: a model for replication Virology 1994; 203: 90-100.]. By expressing constructs leading to the formation of dsRNA, the system can also engender silencing of a target gene.
We demonstrated inducible silencing of an endogenous gene (PDS). We proceeded to construct a system in which virus infection stimulates the silencing of one of its own genes, bringing about resistance/tolerance. The treated plants became resistant/tolerant within a few days of injection, as compared to conventional breeding which, after many years of development, has been only partially successful. After the completion of this work, a new candidate gene for TYLCV silencing suppression (V2) was reported [26Zrachy A, Glick E, Levy Y, Arazi T, Citovsky V, Gafni Y. Suppresor of RNA silencing encoded by Tomato yellow leaf curl virus-Israel Virology 2007; 358: 159-65., 27Glick E, Zrachya A, Levy Y, et al. Interaction with host SGS3 is required for suppression of DNA silencing by tomato yellow leaf curl virus V2 protein Proc Natl Acad Sci USA 2008; 105: 157-61.]. The IR constructs employed in this study carried 159 bp segment of V2 harboring the gene's ribosomal binding site. The V2 segment was also bidirectionally transcribed which conceivably could have resulted in V2 silencing as well. The presence of three genes for silencing suppression in a virus carrying only six genes is quite peculiar and may suggest that TYLCV is particularly prone to silencing. Therefore, the interplay between the plant's silencing of TYLCV infection and the viral counter-activity in suppression of its own silencing is a major factor in infectivity. The mere fact that TYLCV infection may be counteracted by disarming the virus from its silencing-suppression capability indicates that an innate host immune response is a considerable factor in the plant's survival following infection. The IL-60 platform offers some advantages in plant manipulation. It is non-species-specific, it can be inducible, and can be used for both expression as well as silencing. It is stable compared to the PVX system and, in contrast to the TRV system, is applicable to many plant species (including monocots and woody plant) A disadvantage is the need for injection (which is not always successful) in order to introduce the plasmids into the plant.
Silencing of viral genes coupled with the development of resistance or tolerance has been demonstrated. Since X silencing engendered by IR-X-RI develops into a phenotype, it is apparent that the satellite continues to transcribe and is not turned off due to self silencing. This can be explained by the unique features of the IRs at both ends of the insert. The IR carries strong signals for RNA-polymerase II termination [14Platt T. Transcription termination and the regulation of gene expression Ann Rev Biochem 1986; 55: 339-72.]: A C:G rich stem-loop structure starting at position 147, a polyadenylation signal (AATCAAA) at position 234-240 followed by a downstream AGTGTTCA. Therefore, it is conceivable that transcription stops at both ends of X, and the formed dsRNA, leading to silencing, represent only sequences of X (which is silenced) and not of the entire plasmid (which is not affected).
In addition, involvement of transcription gene silencing (TGS) can not be ruled out. Once siRNAs are formed they may affect chromatin remodeling [32Verdel A, Jia S, Gerber S, et al. RNAi-mediated targeting of heterochromatin by the RITS complex Science 2004; 303: 672-.], TYLCV-dsDNAs appears as histone-coated minichromosomes [33Pilartz M, Jeske H. Abutilon mosaic geminivirus double-stranded DNA is packed into minichromosomes Virology 1992; 189: 800-2., 34Pilartz M, Jeske H. Mapping of abutilon mosaic geminivirus minichromosomes J Virol 2003; 77: 10808-8.] with exposed active sites. The IR may be the origin of assembly of minichromosomes. Such remodeling may turn promoter regions inaccessible, shutting down transcription from those sites. siRNA may also cause TGS by engendering DNA methylation.
In conclusion, a plasmid of the type IR-X-RI brings about X silencing by engendering the production of dsRNA which is processed to siRNA and presumably resulting in post-transcriptional gene silencing. However, TGS is not ruled out. The in-trans down regulation of TYLCV may be due to siRNA- depended silencing as well as to competition between an efficient satellite and the virus.
ACKNOWLEDGEMENT
The study was supported by Morflora LLC, Miami, FL, USA






