- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Virology Journal
(Discontinued)
ISSN: 1874-3579 ― Volume 15, 2021
The Biology of Papillomavirus Latency
Gareth Adam Maglennon1, John Doorbar*, 2
Abstract
The presence of viral DNA in the absence of disease has suggested that papillomaviruses, like many other viruses, can exist as latent infections in the skin or other epithelial sites. In animal models, where detailed investigation has been carried out, papillomavirus DNA can be found at sites of previous infection following immune regression, with the site of latent infection being the epithelial basal layer. Such studies suggest that immune surveillance can restrict viral gene expression in the basal and parabasal layers without efficiently suppressing viral genome replication, most probably through the action of memory T-cells in the skin or dermis. Although gradual papillomavirus genome loss appears to occur over time at latent sites, immunosuppression can arrest this, and can lead to an elevation in viral genome copy number in experimental systems. In addition to immune-mediated latency, it appears that a similar situation can be achieved following infection at low virus titres and/or infection at epithelial sites where the virus life cycle is not properly supported. Such silent of asymptomatic infections do not necessarily involve the host immune system and may be controlled by different mechanisms. It appears that virus reactivation can be triggered by mechanical irritation, wounding or by UV irradiation which changes the local environment. Although the duration of papillomavirus latency in humans is not yet known, it is likely that some of the basic principles will resemble those elucidated in these model systems, and that persistence in the absence of disease may be the default outcome for at least some period of time following regression.
Article Information
Identifiers and Pagination:
Year: 2012Volume: 6
Issue: Suppl 2
First Page: 190
Last Page: 197
Publisher Id: TOVJ-6-190
DOI: 10.2174/1874357901206010190
Article History:
Received Date: 26/4/2012Revision Received Date: 21/9/2012
Acceptance Date: 24/9/2012
Electronic publication date: 28/12/2012
Collection year: 2012
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Division of Virology, National Institute for Medical Research, Mill Hill, London, NW7 1AA, UK; Tel +44 20 8816 2623; Fax +44 20 8906 4477; E-mail: jdoorba@nimr.mrc.ac.uk
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 26-4-2012 |
Original Manuscript | The Biology of Papillomavirus Latency | |
INTRODUCTION
Infection of a host with a virus can have several outcomes. An acute infection may develop that is followed by recovery from the virus (or in some cases, death) and complete elimination of the virus from the host. Alternatively, a chronic or persistent infection may result, with long-term carriage of the virus with or without further bouts of acute disease. Instead of viral clearance following infection, a state of viral latency may develop, during which no clinical signs of disease are apparent and new virus particles are not produced and released. It is possible that such a latent infection may undergo subsequent reactivation leading to new virion synthesis, with or without the re-emergence of clinical disease. Many viruses have a latent stage to their life cycle, and it has long been considered that papillomaviruses are no different. The classic model of viral latency is perhaps the alphaherpesvirus, Herpes Simplex Virus-1 (HSV-1), the cause of labial cold sores and with which at least 90% of the general population are infected [1 Perng GC, Jones C. Towards an understanding of the herpes simplex virus type 1 latency-reactivation cycle Interdiscip Perspect Infect Dis 2010; 2010: 1-18.]. Following an acute and productive infection of epithelial cells usually early in life, HSV-1 migrates by retrograde axonal transport along sensory neurones and enters a latent phase in nuclei in the sensory ganglia [2 Efstathiou S, Preston CM. Towards an understanding of the molecular basis of herpes simplex virus latency Virus Res 2005; 111(2): 108-9.]. This serves as a reservoir of infection from which anterograde transport can lead to recurrent disease later in life, often in response to factors such as immune suppression [3 Decman V, Freeman ML, Kinchington PR, Hendricks RL. Immune control of HSV-1 latency Viral Immunol 2005; 18(3): 466-73.]. HSV-1 latency is very well characterized, but for papillomaviruses, this level of detail does not yet exist. Much of the evidence for latent papillomavirus infections in humans is based on clinical observations and is often anecdotal because of ethical concerns associated with the collection of biopsy samples from humans that may have latent virus, but no signs of clinical disease. Such clinical observations include the higher incidence of cervical papillomavirus infection in human immunodeficiency virus (HIV) seropositive versus seronegative patients, which may result from the reactivation of latent papillomavirus infection following immunosuppression [4 Jamieson DJ, Duerr A, Burk R, et al. Characterization of genital human papillomavirus infection in women who have or who are at risk of having HIV infection Am J Obstet Gynecol 2002; 186(1): 21-7.-6 Palefsky JM, Minkoff H, Kalish LA, et al. Cervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV)-positive and high-risk HIV-negative women J Natl Cancer Inst 1999; 91(3): 226-36.]. Similarly, organ transplant recipients undergoing iatrogenic immunosuppression have a higher incidence of high-risk papillomavirus type infections that are associated with cervical neoplasia [7 Ozsaran AA, Ates T, Dikmen Y, et al. Evaluation of the risk of cervical intraepithelial neoplasia and human papilloma virus infection in renal transplant patients receiving immunosuppressive therapy Eur J Gynaecol Oncol 1999; 20(2 ): 127-30.-9 Veroux M, Corona D, Scalia G, et al. Surveillance of human papilloma virus infection and cervical cancer in kidney transplant recipients: preliminary data Transplant Proc 2009; 41(4 ): 1191-4.] and it has been considered that in many of these cases, reactivation of a dormant or latent infection may be important [10 Savani BN, Stratton P, Shenoy A, Kozanas E, Goodman S, Barrett AJ. Increased risk of cervical dysplasia in long-term survivors of allogeneic stem cell transplantation--implications for screening and HPV vaccination Biol Blood Marrow Transplant 2008; 14(9): 1072-5., 11 Savani BN, Goodman S, Barrett AJ. Can routine posttransplant HPV vaccination prevent commonly occurring epithelial cancers after allogeneic stem cell transplantation? Clin Cancer Res 2009; 15(7): 2219-1.]. Genital infections with low-risk HPVs such as HPV-6 and HPV-11 are also considered to cause latent infections [12 Lacey CJ. Therapy for genital human papillomavirus-related disease J Clin Virol 2005; 32(Suppl 1): S82-90.], which may account for the high recurrence rates of genital papillomas following treatment [12 Lacey CJ. Therapy for genital human papillomavirus-related disease J Clin Virol 2005; 32(Suppl 1): S82-90., 13 von Krogh G, Lacey CJ, Gross G, Barrasso R, Schneider A. European course on HPV associated pathology: guidelines for primary care physicians for the diagnosis and management of anogenital warts Sex Transm Infect 2000; 76(3): 162-8.]. Similarly, latent HPV-6 and HPV-11 infection of the mucosal lining of the upper airways of individuals with recurrent respiratory papillomatosis (RRP) may be one of the factors responsible for the frequent and multiple recurrences of papillomas [14 Steinberg BM, Topp WC, Schneider PS, Abramson AL. Laryngeal papillomavirus infection during clinical remission N Engl J Med 1983; 308(21): 1261-4.-18 Maran A, Amella CA, Di Lorenzo TP, Auborn KJ, Taichman LB, Steinberg BM. Human papillomavirus type 11 transcripts are present at low abundance in latently infected respiratory tissues Virology 1995; 212(2): 285-94.]. These observations and the limitations of studying latency in humans, has driven investigators to make extensive use of animal models of infection. In most cases, a better understanding of HPV latency has been the primary objective, although it appears that some economically important animal diseases may also be associated with latent infection. These include BPV4-associated urinary bladder cancers in cattle [19 Campo MS. Bovine papillomavirus and cancer Vet J 1997; 154(3): 175-88.-22 Campo MS, Jarrett WF, O'Neil W, Barron RJ. Latent papillomavirus infection in cattle Res Vet Sci 1994; 56(2): 151-7.], and equine sarcoids in horses, which are associated with BPV1 and BPV2 [23 Valentine BA. Survey of equine cutaneous neoplasia in the Pacific Northwest J Vet Diagn Invest 2006; 18(1): 123-6.-27 Bogaert L, Martens A, Van Poucke M, et al. High prevalence of bovine papillomaviral DNA in the normal skin of equine sarcoid-affected and healthy horses Vet Microbiol 2008; 129(1-2): 58-68.]) and which constitute the most common equine skin tumour [23 Valentine BA. Survey of equine cutaneous neoplasia in the Pacific Northwest J Vet Diagn Invest 2006; 18(1): 123-6., 24 Chambers G, Ellsmore VA, O'Brien PM, et al. Association of bovine papillomavirus with the equine sarcoid J Gen Virol 2003; 84(Pt 5): 1055-62.]. BPV 1 and BPV 2 DNA can be detected in the normal skin of horses affected by equine sarcoids, and the occurrence of sarcoids at sites of skin wounding and/or following physical trauma suggests a possible reactivation from latency [25 Carr EA, Theon AP, Madewell BR, Griffey SM, Hitchcock ME. Bovine papillomavirus DNA in neoplastic and nonneoplastic tissues obtained from horses with and without sarcoids in the western United States Am J Vet Res 2001; 62(5): 741-4.-27 Bogaert L, Martens A, Van Poucke M, et al. High prevalence of bovine papillomaviral DNA in the normal skin of equine sarcoid-affected and healthy horses Vet Microbiol 2008; 129(1-2): 58-68.]. Similarly, the frequent detection of BPV-4 DNA in normal bovine bladder mucosa has suggested its presence as a latent infection that can undergo reactivation when cattle graze on pastures rich in bracken fern [21 Borzacchiello G, Iovane G, Marcante ML, et al. Presence of bovine papillomavirus type 2 DNA and expression of the viral oncoprotein E5 in naturally occurring urinary bladder tumours in cows J Gen Virol 2003; 84(Pt 11): 2921-6.]. BPV4 is thus considered as a model of the synergistic actions of a chemical and a biological agent in carcincogenesis as well as a model of papillomavirus latency.
ANIMAL MODELS FOR THE STUDY OF PAPILLOMAVIRUS INFECTION AND LATENCY
Understanding the natural history of papillomavirus infection and latency, has until recently been hampered by the lack of convenient laboratory animal models. Papillomaviruses have been isolated from a diverse range of domestic and wild species, but for laboratory studies, rats and mice are generally preferred because of their small size and because of the availability of validated biological reagents and transgenic mutant mouse strains. Papillomaviruses have been found to cause cutaneous papillomas and sebaceous carcinomas in the European Harvest Mouse (MmPV which infects micromys minutus) [28 O'Banion MK, Reichmann ME, Sundberg JP. Cloning and characterization of a papillomavirus associated with papillomas and carcinomas in the European harvest mouse (Micromys minutus) J Virol 1988; 62(1): 226-33.], as well as the oral cavity of hamsters (mesocricetus auratus) [29 Iwasaki T, Maeda H, Kameyama Y, Moriyama M, Kanai S, Kurata T. Presence of a novel hamster oral papillomavirus in dysplastic lesions of hamster lingual mucosa induced by application of dimethylbenzanthracene and excisional wounding: molecular cloning and complete nucleotide sequence J Gen Virol 1997; 78 (Pt 5): 1087-93.], but transmission to naïve animals and/or laboratory species has not been reported. More recently, papillomaviruses have been detected in clinically normal oral tissues in wild rats (Rattus norvegicus) [30 Schulz E, Gottschling M, Wibbelt G, Stockfleth E, Nindl I. Isolation and genomic characterization of the first Norway rat (Rattus norvegicus) papillomavirus and its phylogenetic position within Pipapillomavirus, primarily infecting rodents J Gen Virol 2009; 90(Pt 11): 2609-14.], and perhaps more importantly, in papillomas from immunocompromised laboratory mice (Mus musculus) [31 Ingle A, Ghim S, Joh J, Chepkoech I, Bennett Jenson A, Sundberg JP. Novel laboratory mouse papillomavirus (MusPV) infection Vet Pathol [Research Suport Non-US Gov't] 2011; 48(2): 500-.]. These rodent papillomaviruses are contained within the Pi Genus [32 Joh J, Jenson AB, Proctor M, et al. Molecular diagnosis of a laboratory mouse papillomavirus (MusPV) Experimental and molecular pathology 2012. in press], and are related (albeit distantly) to the Gamma and Beta types that cause asymptomatic infection in humans. While musPV is not a perfect model of infection and disease caused by the high-risk Alpha types, it will allow us to elucidate the basic mechanisms of PV latency and the role of the immune system in the years to come, and it is likely that much of this work will be relevant to human disease. Other small animal models used to study papillomavirus latency include the African natal multimammate rat (Mastomys natalensis), which is associated with both inapparent disease and cancers in its natural host [33 Nafz J, Kohler A, Ohnesorge M, Nindl I, Stockfleth E, Rosl F. Persistence of Mastomys natalensis papillomavirus in multiple organs identifies novel targets for infection J Gen Virol 2007; 88(Pt 10): 2670-8.-35 Amtmann E, Volm M, Wayss K. Tumour induction in the rodent Mastomys natalensis by activation of endogenous papilloma virus genomes Nature 1984; 308(5956): 291-.]. The multimammate rat is an unconventional laboratory animal however, and as a result of this, its widespread use has been restricted.
The limited availability of well-characterized rodent models, (as described above), has meant that most previous work on papillomavirus latency has focused on larger domesticated animals, and in particular on rabbits, dogs and cattle. Canine oral papillomavirus (COPV) is a naturally occurring mucosal papillomavirus that does not usually cause problematic clinical disease, except in rare instances where the virus can cause severe non-regressing papillomatosis [36 Nicholls PK, Klaunberg BA, Moore RA, et al. Naturally occurring, nonregressing canine oral papillomavirus infection: host immunity, virus characterization, and experimental infection Virology 1999; 265(2): 365-74.]. Experimental infection of the oral mucosa leads to the formation of large papillomas within four to eight weeks, followed by spontaneous immune-mediated regression [37 Nicholls PK, Moore PF, Anderson DM, et al. Regression of canine oral papillomas is associated with infiltration of CD4+ and CD8+lymphocytes Virology 2001; 283(1): 31-9.]. As a mucosal virus, COPV has been proposed as an animal model of HPV 6 and 11-associated RRP in humans [38 Jahan-Parwar B, Chhetri DK, Hart S, Bhuta S, Berke GS. Development of a canine model for recurrent respiratory papillomatosis Ann Otol Rhinol Laryngol 2003; 112(12): 1011-3.], and has also been used in vaccine development [39 Johnston KB, Monteiro JM, Schultz LD, et al. Protection of beagle dogs from mucosal challenge with canine oral papillomavirus by immunization with recombinant adenoviruses expressing codon-optimized early genes Virology 2005; 336(2 ): 208-18.-42 Stanley MA, Moore RA, Nicholls PK, et al. Intra-epithelial vaccination with COPV L1 DNA by particle-mediated DNA delivery protects against mucosal challenge with infectious COPV in beagle dogs Vaccine 2001; 19(20-22): 2783-92.], and to investigate the immune response to infection [37 Nicholls PK, Moore PF, Anderson DM, et al. Regression of canine oral papillomas is associated with infiltration of CD4+ and CD8+lymphocytes Virology 2001; 283(1): 31-9., 43 Jain S, Moore RA, Anderson DM, Gough GW, Stanley MA. Cell-mediated immune responses to COPV early proteins Virology 2006; 356(1-2): 23-34.]. Despite the apparent similarities in tissue tropism, COPV and HPVs have a number of organizational differences, which emphasize the general need for caution when using animal models. COPV contains a 1.5kbp regulatory region between E2 and L2 that is not present in HPVs [44 Delius H, Van Ranst MA, Jenson AB, zur Hausen H, Sundberg JP. Canine oral papillomavirus genomic sequence: a unique 1.5-kb intervening sequence between the E2 and L2 open reading frames Virology 1994; 204(1): 447-52.], and in the lesions that COPV causes, viral genome amplification begins in a subset of infected basal cells rather than being restricted to only the suprabasal cell layers [45 Nicholls PK, Doorbar J, Moore RA, Peh W, Anderson DM, Stanley MA. Detection of viral DNA and E4 protein in basal keratinocytes of experimental canine oral papillomavirus lesions Virology 2001; 284(1): 82-98., 46 Peh WL, Middleton K, Christensen N, et al. Life cycle heterogeneity in animal models of human papillomavirus-associated disease J Virol 2002; 76(20): 10401-6.]. Rabbit oral papillomavirus (ROPV) has a similar tropism to COPV, and induces spontaneously regressing and benign papillomas in domestic rabbits [28 O'Banion MK, Reichmann ME, Sundberg JP. Cloning and characterization of a papillomavirus associated with papillomas and carcinomas in the European harvest mouse (Micromys minutus) J Virol 1988; 62(1): 226-33., 47 Parsons RJ, Kidd JG. Oral papillomatosis of rabbits: a virus disease Journal of Experimental Medicine 1942; 77: 233-50.]. Experimental infection gives rise to papillomas that form over a period of four weeks or so, followed by immune-mediated regression [48 Christensen ND, Cladel NM, Reed CA, Han R. Rabbit oral papillomavirus complete genome sequence and immunity following genital infection Virology 2000; 269(2): 451-61., 49 Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression Virology 2011; 414(2): 153-63.]. On the basis of life-cycle organization and genome similarities, ROPV appears to more closely mimic the low-risk mucosal HPV types such as HPV-6 and HPV-11 [46 Peh WL, Middleton K, Christensen N, et al. Life cycle heterogeneity in animal models of human papillomavirus-associated disease J Virol 2002; 76(20): 10401-6.], and infects genital tissue in both male and female rabbits [48 Christensen ND, Cladel NM, Reed CA, Han R. Rabbit oral papillomavirus complete genome sequence and immunity following genital infection Virology 2000; 269(2): 451-61.]. Domestic rabbit (Oryctolagus cuniculus) can also be infected with an entirely different papillomavirus, the Cottontail Rabbit Papillomavirus (CRPV), whose natural host is the cottontail rabbit (Sylvilagus floridanus) native to the Americas. While ROPV has a tropism for oral epithelium, CRPV has a tropism for cutaneous epithelium, with papillomas appearing around four weeks after experimental infection of domestic rabbits [50 Shope RE, Hurst EW. Infectious Papillomatosis of Rabbits : With a Note on the Histopathology J Exp Med 1933; 58(5): 607-24.]. Approximately 10% of papillomas undergo spontaneous regression in domestic rabbits, with 60-75% being persistent and progressing to squamous cell carcinoma [51 Reuter JD, Gomez D, Brandsma JL, Rose JK, Roberts A. Optimization of cottontail rabbit papilloma virus challenge technique J Virol Methods 2001; 98(2): 127-34., 52 Syverton JT. The pathogenesis of the rabbit papilloma-to-carcinoma sequence Ann N Y Acad Sci 1952; 54(6): 1126-40.], and because of this, CRPV has been used as a model of high-risk HPV disease. In addition to the papillomaviruses found in rabbits and dogs, more than 10 different bovine papillomaviruses (BPV) have so far been described. The size of the bovine host, and the expense and difficulties surrounding animal management has limited the use of cattle as an in vivo model system. Finally, a model system of cervical papillomavirus infection does exists in the rhesus macaque, with this model being used to advance our understanding of the biology of HPV cervical infection [53 Roberts JN, Kines RC, Katki HA, Lowy DR, Schiller JT. Effect of Pap smear collection and carrageenan on cervicovaginal human papillomavirus-16 infection in a rhesus macaque model J Natl Cancer Inst 2011; 103(9): 737-43.], although as yet, not our understanding of latent infections.
EVIDENCE FOR THE PERSISTENCE OF PAPILLOMAVIRUSES AS LATENT INFECTIONS
The persistence of papillomaviruses in a latent state necessitates the maintenance of the viral genome in the infected cell, either as an episome or integrated into the host cell DNA. Persistence in the absence of clinical disease does not necessarily exclude viral activities required for genome-maintenance, such as low-level viral genome replication and production of viral transcripts and proteins (described in more detail below). CRPV was one of the first papillomaviruses used to investigate papillomavirus latency, and in this system, the inoculation of rabbit skin with high concentrations of virus rapidly leads to the formation of large papillomas. By contrast, inoculation with low concentrations of virus does not lead to papilloma formation, although viral DNA can be detected at the site of inoculation for as long as 18 weeks after infection [54 Amella CA, Lofgren LA, Ronn AM, Nouri M, Shikowitz MJ, Steinberg BM. Latent infection induced with cottontail rabbit papillomavirus. A model for human papillomavirus latency Am J Pathol 1994; 144(6): 1167-71.] (Fig. 1 ). Such ‘asymptomatic’ or ‘silent’ infections were accompanied by low-level expression of CRPV E1 transcripts, suggesting a possible requirement of the viral E1 helicase [55 Zhang P, Nouri M, Brandsma JL, Iftner T, Steinberg BM. Induction of E6/E7 expression in cottontail rabbit papillomavirus latency following UV activation Virology 1999; 263(2): 388-94.]. While these studies support the general concept of persistence in the absence of overt disease, they have not established whether persistence can also occur following immune-mediated disease-regression (Fig. 1
). Such ‘asymptomatic’ or ‘silent’ infections were accompanied by low-level expression of CRPV E1 transcripts, suggesting a possible requirement of the viral E1 helicase [55 Zhang P, Nouri M, Brandsma JL, Iftner T, Steinberg BM. Induction of E6/E7 expression in cottontail rabbit papillomavirus latency following UV activation Virology 1999; 263(2): 388-94.]. While these studies support the general concept of persistence in the absence of overt disease, they have not established whether persistence can also occur following immune-mediated disease-regression (Fig. 1 ). This has however been examined in separate studies using CRPV and COPV, and more recently, and in more detail, using ROPV [49 Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression Virology 2011; 414(2): 153-63.]. In none of these systems could the site of latent infection be established using DNA in situ hybridization methods, suggesting that the genome copy number per infected cell is very low [45 Nicholls PK, Doorbar J, Moore RA, Peh W, Anderson DM, Stanley MA. Detection of viral DNA and E4 protein in basal keratinocytes of experimental canine oral papillomavirus lesions Virology 2001; 284(1): 82-98., 49 Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression Virology 2011; 414(2): 153-63., 56 Selvakumar R, Schmitt A, Iftner T, Ahmed R, Wettstein FO. Regression of papillomas induced by cottontail rabbit papillomavirus is associated with infiltration of CD8+ cells and
persistence of viral DNA after regression J Virol 1997; 71(7): 5540-8.]. Such approaches have only limited sensitivity however, and do not generally detect papillomavirus genomes even in the basal layer of productive papillomas. Using more sensitive PCR approaches, low levels of papillomavirus DNA could clearly be detected at sites of previous infection in all three systems, with persistence for at least a year post-infection in the absence of clinical signs of disease [45 Nicholls PK, Doorbar J, Moore RA, Peh W, Anderson DM, Stanley MA. Detection of viral DNA and E4 protein in basal keratinocytes of experimental canine oral papillomavirus lesions Virology 2001; 284(1): 82-98., 49 Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression Virology 2011; 414(2): 153-63., 56 Selvakumar R, Schmitt A, Iftner T, Ahmed R, Wettstein FO. Regression of papillomas induced by cottontail rabbit papillomavirus is associated with infiltration of CD8+ cells and
persistence of viral DNA after regression J Virol 1997; 71(7): 5540-8.]. Although the heterogeneous nature of papillomas complicates copy-number estimation in the basal and suprabasal cell layers, the papillomavirus burden was reported to drop from around 7.5 genome copies per cell in warts, to as little as one genome copy per 40-1000 cells following lesion clearance [56 Selvakumar R, Schmitt A, Iftner T, Ahmed R, Wettstein FO. Regression of papillomas induced by cottontail rabbit papillomavirus is associated with infiltration of CD8+ cells and
persistence of viral DNA after regression J Virol 1997; 71(7): 5540-8.]. Our more detailed studies of ROPV-infected rabbits suggest a similar scenario. Following immune-regression, ROPV genomic DNA was present at levels that were up to 6 logs lower than in mature papillomas. Such results are consistent with the presence of genomes in only a small fraction of cells, and with a lack of significant genome amplification in the upper layers of the epithelium [49 Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression Virology 2011; 414(2): 153-63.]. It appears that following experimental infection at least, viral latency is a common sequela to papilloma regression, with latent genomes being detected in the majority of tissue samples obtained from the majority of rabbits [49 Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression Virology 2011; 414(2): 153-63., 56 Selvakumar R, Schmitt A, Iftner T, Ahmed R, Wettstein FO. Regression of papillomas induced by cottontail rabbit papillomavirus is associated with infiltration of CD8+ cells and
persistence of viral DNA after regression J Virol 1997; 71(7): 5540-8.]. For these papillomavirus types, and we suspect for other types too, viral latency may be a typical outcome of disease resolution by the immune system, as happens for example with HSV-1.
). This has however been examined in separate studies using CRPV and COPV, and more recently, and in more detail, using ROPV [49 Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression Virology 2011; 414(2): 153-63.]. In none of these systems could the site of latent infection be established using DNA in situ hybridization methods, suggesting that the genome copy number per infected cell is very low [45 Nicholls PK, Doorbar J, Moore RA, Peh W, Anderson DM, Stanley MA. Detection of viral DNA and E4 protein in basal keratinocytes of experimental canine oral papillomavirus lesions Virology 2001; 284(1): 82-98., 49 Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression Virology 2011; 414(2): 153-63., 56 Selvakumar R, Schmitt A, Iftner T, Ahmed R, Wettstein FO. Regression of papillomas induced by cottontail rabbit papillomavirus is associated with infiltration of CD8+ cells and
persistence of viral DNA after regression J Virol 1997; 71(7): 5540-8.]. Such approaches have only limited sensitivity however, and do not generally detect papillomavirus genomes even in the basal layer of productive papillomas. Using more sensitive PCR approaches, low levels of papillomavirus DNA could clearly be detected at sites of previous infection in all three systems, with persistence for at least a year post-infection in the absence of clinical signs of disease [45 Nicholls PK, Doorbar J, Moore RA, Peh W, Anderson DM, Stanley MA. Detection of viral DNA and E4 protein in basal keratinocytes of experimental canine oral papillomavirus lesions Virology 2001; 284(1): 82-98., 49 Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression Virology 2011; 414(2): 153-63., 56 Selvakumar R, Schmitt A, Iftner T, Ahmed R, Wettstein FO. Regression of papillomas induced by cottontail rabbit papillomavirus is associated with infiltration of CD8+ cells and
persistence of viral DNA after regression J Virol 1997; 71(7): 5540-8.]. Although the heterogeneous nature of papillomas complicates copy-number estimation in the basal and suprabasal cell layers, the papillomavirus burden was reported to drop from around 7.5 genome copies per cell in warts, to as little as one genome copy per 40-1000 cells following lesion clearance [56 Selvakumar R, Schmitt A, Iftner T, Ahmed R, Wettstein FO. Regression of papillomas induced by cottontail rabbit papillomavirus is associated with infiltration of CD8+ cells and
persistence of viral DNA after regression J Virol 1997; 71(7): 5540-8.]. Our more detailed studies of ROPV-infected rabbits suggest a similar scenario. Following immune-regression, ROPV genomic DNA was present at levels that were up to 6 logs lower than in mature papillomas. Such results are consistent with the presence of genomes in only a small fraction of cells, and with a lack of significant genome amplification in the upper layers of the epithelium [49 Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression Virology 2011; 414(2): 153-63.]. It appears that following experimental infection at least, viral latency is a common sequela to papilloma regression, with latent genomes being detected in the majority of tissue samples obtained from the majority of rabbits [49 Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression Virology 2011; 414(2): 153-63., 56 Selvakumar R, Schmitt A, Iftner T, Ahmed R, Wettstein FO. Regression of papillomas induced by cottontail rabbit papillomavirus is associated with infiltration of CD8+ cells and
persistence of viral DNA after regression J Virol 1997; 71(7): 5540-8.]. For these papillomavirus types, and we suspect for other types too, viral latency may be a typical outcome of disease resolution by the immune system, as happens for example with HSV-1.
For low-risk papillomavirus types such as ROPV or HPV11, it has been suggested that papilloma formation requires virus entry and genome maintenance in an epithelial stem cell [57 Schmitt A, Rochat A, Zeltner R, et al. The primary target cells of the high-risk cottontail rabbit papillomavirus colocalize with hair follicle stem cells J Virol 1996; 70(3 ): 1912-22.]. Lesion-persistence may depend on the longevity of this cell, with viral genome-containing daughter cells (i.e. the infected transiently amplifying cells) populating the epithelial basal layer around the infected stem cell as it undergoes normal cell-division. Following immune-regression, it is thought that such infected stem-cells may harbour latent papillomavirus genomes, with reactivation occurring following changes in host immune-status, or following changes in the level of hormones, cytokines and/or growth factors [45 Nicholls PK, Doorbar J, Moore RA, Peh W, Anderson DM, Stanley MA. Detection of viral DNA and E4 protein in basal keratinocytes of experimental canine oral papillomavirus lesions Virology 2001; 284(1): 82-98., 55 Zhang P, Nouri M, Brandsma JL, Iftner T, Steinberg BM. Induction of E6/E7 expression in cottontail rabbit papillomavirus latency following UV activation Virology 1999; 263(2): 388-94.]. In our ROPV study, we were able to specifically localize latent viral genomes using laser capture microscopy to the basal cells of the epithelium [49 Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression Virology 2011; 414(2): 153-63.]. In the population of basal cells examined using this approach, viral genomes were often detected at less than one copy per cell, suggesting that only a subset of basal cells, and possibly the basal stem cells were infected [58 Koster MI. Making an epidermis Ann N Y Acad Sci 2009; 1170: 7-10., 59 Watt FM, Lo Celso C, Silva-Vargas V. Epidermal stem cells: an update Curr Opin Genet Dev 2006; 16(5 ): 518-24.]. Despite observing viral DNA in the basal layer, we only rarely found evidence of viral genome amplification in the cell layers above, suggesting that the virus may be maintained in a quiescent or inactive state in cells with a capacity for self-renewal. As seen in asymptomatic CRPV infections [55 Zhang P, Nouri M, Brandsma JL, Iftner T, Steinberg BM. Induction of E6/E7 expression in cottontail rabbit papillomavirus latency following UV activation Virology 1999; 263(2): 388-94.], viral transcripts were reproducibly present in latently infected ROPV tissues [49 Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression Virology 2011; 414(2): 153-63.], and in addition to E1 transcripts, other early mRNA species (E2, E6), including spliced forms, were detected. It is possible that the control of viral gene expression following immune regression may be different from that seen following low-titre infection where the low-level expression of E1 mRNAs were observed. Despite extensive analysis of tissue sections, we have not yet been able to detect late viral proteins at sites of previous infection (E1^E4 and L1), supporting the idea that genome amplification and virus synthesis does not occur or is rare. Thus, for ROPV infection at least, the post-regression genomes appear truly latent.
ACTIVATION AND REACTIVATION OF LATENT PAPILLOMAVIRUS
From the work described thus far in animal models, it has been shown that papillomavirus genomes can persist in the absence of clinical or microscopic signs of disease. However, of greater importance is to attribute some significance to this and to define the importance and potential consequences of such persistence. Anecdotal tales suggest that latent human papillomavirus infections can sometimes undergo reactivation leading to disease recrudescence. The high recurrence rate of low-risk genital HPV-6 and 11 infections following treatment is often attributed to the reactivation of latent papillomavirus infections [12 Lacey CJ. Therapy for genital human papillomavirus-related disease J Clin Virol 2005; 32(Suppl 1): S82-90.]. Immune suppression, either iatrogenic and drug-induced following organ transplantation, or secondary to HIV infection, appears to facilitate papillomavirus reactivation, and has been well documented in the case of Beta HPV disease [10 Savani BN, Stratton P, Shenoy A, Kozanas E, Goodman S, Barrett AJ. Increased risk of cervical dysplasia in long-term survivors of allogeneic stem cell transplantation--implications for screening and HPV vaccination Biol Blood Marrow Transplant 2008; 14(9): 1072-5., 11 Savani BN, Goodman S, Barrett AJ. Can routine posttransplant HPV vaccination prevent commonly occurring epithelial cancers after allogeneic stem cell transplantation? Clin Cancer Res 2009; 15(7): 2219-1., 60 Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women J Natl Cancer Inst 2005; 97(8 ): 577-86.-63 Harwood CA, Proby CM. Human papillomavirus and immunosuppression In: Sterling J, Tyring SK, Eds. Human papillomaviruses. London: Arnold 2001; pp. 102-7.]. Finally, in individuals suffering from recurrent respiratory papillomatosis (RRP), the high recurrence rates at sites of previous infection, and the demonstration of viral DNA and RNA transcripts in the absence of clinical lesions has led to the suggestion that latency may underlie recurrence in susceptible patients [15 Abramson AL, Nouri M, Mullooly V, Fisch G, Steinberg BM. Latent Human Papillomavirus infection is comparable in the larynx and trachea J Med Virol 2004; 72(3): 473-7.-18 Maran A, Amella CA, Di Lorenzo TP, Auborn KJ, Taichman LB, Steinberg BM. Human papillomavirus type 11 transcripts are present at low abundance in latently infected respiratory tissues Virology 1995; 212(2): 285-94., 64 Gissmann L, Diehl V, Schultz-Coulon HJ, zur Hausen H. Molecular cloning and characterization of human papilloma virus DNA derived from a laryngeal papilloma J Virol 1982; 44(1 ): 393-400., 65 Mounts P, Shah KV, Kashima H. Viral etiology of juvenile- and adult-onset squamous papilloma of the larynx Proc Natl Acad Sci USA 1982; 79(17): 5425-9.]. The study of animal models has provided an opportunity to define the factors involved in reactivation more precisely. Mastomys Natalensis Papillomavirus (MnPV) infection of the multimammate rat appears to represent an ‘endogenous infection’, with extrachromosomal viral genomes being present in a variety of organs in laboratory colonies [35 Amtmann E, Volm M, Wayss K. Tumour induction in the rodent Mastomys natalensis by activation of endogenous papilloma virus genomes Nature 1984; 308(5956): 291-.]. Although an unusual model system, MnPV has given us some intriguing insights into viral latency. Typically in the young animal, MnPV DNA is present at low copy number in the skin in the absence of clinical signs of disease. Following repeated chronic mechanical irritation of the skin with fine glasspaper over a period of eight weeks, a 58-fold increase in viral DNA copy number was detected compared to non-irritated skin [34 Siegsmund M, Wayss K, Amtmann E. Activation of latent papillomavirus genomes by chronic mechanical irritation J Gen Virol 1991; 72 (Pt 11): 2787-9.]. With the same treatment regime applied over a period of 67 weeks, there was a significant increase in the formation of cutaneous tumours when compared to the skin of non-irritated mice. A similar effect was described when a tumour promoter, tetradecanoyl-phorbol-13-acetate (TPA) was applied to the skin [35 Amtmann E, Volm M, Wayss K. Tumour induction in the rodent Mastomys natalensis by activation of endogenous papilloma virus genomes Nature 1984; 308(5956): 291-.]. These findings would suggest that stimulation of cellular proliferation and induction of epithelial hyperplasia, either by chronic mechanical irritation or by application of compounds, is sufficient to promote the activation of apparently latent papillomavirus genomes. The importance of mechanical irritation has also been demonstrated in the establishment of CRPV papillomas. Mechanical irritation of rabbit skin prior to infection with CRPV virions or genomes enhanced infectivity, but it is not clear whether or not such treatment has an effect on latent CRPV infection [66 Cladel NM, Hu J, Balogh K, Mejia A, Christensen ND. Wounding prior to challenge substantially improves infectivity of cottontail rabbit papillomavirus and allows for standardization of infection J Virol Methods 2008; 148(1-2 ): 34-9.]. In our own studies on rabbits infected with ROPV, we were not able to reactivate latent infection by mechanically wounding previously-infected tongue tissue, although this may be largely due to accessibility difficulties and the sensitive nature of the tissue which precluded multiple treatments being applied over time. It would appear from the MnPV model that chronic mechanical irritation is necessary. The CRPV model has however provided additional useful insights. After inoculation of Cottontail Rabbits with a low dose of virus, Zhang et al. investigated the response of latent viral genomes to exposure to ultraviolet light [55 Zhang P, Nouri M, Brandsma JL, Iftner T, Steinberg BM. Induction of E6/E7 expression in cottontail rabbit papillomavirus latency following UV activation Virology 1999; 263(2): 388-94.]. During the asymptomatic phase of infection, when viral genomes were present in the absence of lesions, analysis of tissue biopsies by reverse-transcription PCR and Southern blotting revealed the presence of E1 transcripts which may be necessary for the maintenance of the viral genomes in infected cells [67 Wilson VG, West M, Woytek K, Rangasamy D. Papillomavirus E1 proteins: form, function, and features Virus Genes 2002; 24(3 ): 275-90.], but not E6 and E7 transcripts associated with stimulation of cellular proliferation. As little as one week after irradiation with ultraviolet light, the authors were able to detect E6 and E7 mRNA at the sites of infection, with papillomas developing subsequently at some locations. It appears in this system, that expression of E1 may play a role in maintenance of the virus as a latent infection, but that E6 and E7 expression were needed for activation to form clinical lesions. The universal requirement for E1 has recently been questioned however, and it has been speculated that some PV types may be able to persist in the basal layer in the absence of this PV-specific replication protein [68 Egawa N, Nakahara T, Ohno S, et al. The E1 protein of human papillomavirus type 16 is dispensable for maintenance replication of the viral genome J Virol 2012; 86(6 ): 3276-83.]. In our work on rabbits infected with ROPV, we detected E6 and E7 transcripts as well as E1 and E2 transcripts at low levels during latency, but at vastly reduced copy number compared to productive infection [49 Maglennon GA, McIntosh P, Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression Virology 2011; 414(2): 153-63.]. The detection of transcripts cannot be equated to expression of protein, but it seems possible that low levels of early viral proteins may be necessary during papillomavirus latency, but that the precise requirements may differ depending on papillomavirus type and possibly also on whether latency is controlled by the immune system or has resulted from low titre infection. In both situations however, the expression of viral proteins is likely to be low in order to avoid immune stimulation.
Whether or not viral proteins are produced during latency, the host immune system is likely to be central to the regulation of the latent state. Studies of experimental animal infections have been valuable in determining the sequence of events that lead to the spontaneous regression of papillomas and in defining the nature of the host immune response to papillomavirus infection. Regression of papillomas is typically associated with a heavy infiltrate of T-cells and macrophages into the epithelium and underlying stromal tissue leading to rapid lesion clearance [37 Nicholls PK, Moore PF, Anderson DM, et al. Regression of canine oral papillomas is associated with infiltration of CD4+ and CD8+lymphocytes Virology 2001; 283(1): 31-9., 56 Selvakumar R, Schmitt A, Iftner T, Ahmed R, Wettstein FO. Regression of papillomas induced by cottontail rabbit papillomavirus is associated with infiltration of CD8+ cells and
persistence of viral DNA after regression J Virol 1997; 71(7): 5540-8., 69 Wilgenburg BJ, Budgeon LR, Lang CM, Griffith JW, Christensen
ND. Characterization of immune responses during regression of
rabbit oral papillomavirus infections Comp Med 2005; 55(5
): 431-9.]. In these systems, the cell-mediated immune response would appear to be directed particularly against the early proteins E6 and E2 and is maximal just prior to regression [43 Jain S, Moore RA, Anderson DM, Gough GW, Stanley MA. Cell-mediated immune responses to COPV early proteins Virology 2006; 356(1-2): 23-34., 70 Selvakumar R, Ahmed R, Wettstein FO. Tumor regression is
associated with a specific immune response to the E2 protein of
cottontail rabbit papillomavirus Virology 1995; 208(1
): 298-302.]. Following regression, antibodies directed against the L1 capsid protein can be detected and may protect against subsequent challenge [36 Nicholls PK, Klaunberg BA, Moore RA, et al. Naturally occurring, nonregressing canine oral papillomavirus infection: host immunity, virus characterization, and experimental infection Virology 1999; 265(2): 365-74.]. We believe that continual immune surveillance is key in maintaining papillomaviruses in a latent state as is the case with other DNA viruses that enter latency, such as HSV-1 [3 Decman V, Freeman ML, Kinchington PR, Hendricks RL. Immune control of HSV-1 latency Viral Immunol 2005; 18(3): 466-73., 71 Amon W, Farrell PJ. Reactivation of Epstein-Barr virus from
latency Rev Med Virol 2005; 15(3
): 149-56.], and the presence of memory T-cells in the epithelium provides us with a mechanism by which this may be achieved [72 Foster CA, Yokozeki H, Rappersberger K, et al. Human epidermal
T cells predominantly belong to the lineage expressing alpha/beta T
cell receptor J Exp Med 1990; 171(4
): 997-1013.] (Fig. 2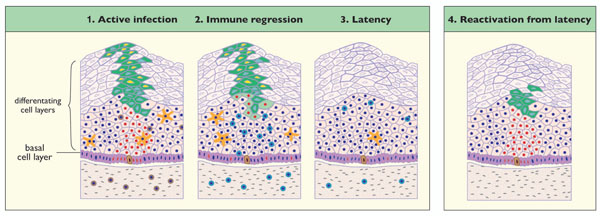 ). Interestingly, recent studies in humans have suggested that latent HPV infections may be associated with particular methylation patterns on the viral episome and presumably also with limited gene expression [73 Vinokurova S, von Knebel Doeberitz M. Differential methylation
of the HPV 16 upstream regulatory region during epithelial
differentiation and neoplastic transformation PLoS One 2011; 6(9
): e24451.]. If our hypothesis that recurrence in HIV-infected individuals and renal transplant recipients is due to the reactivation of latent infections, then a waning immune response would appear to be necessary to facilitate recurrence. In the CRPV model of asymptomatic infection described earlier, a low level virus inoculation was found to be insufficient to allow papilloma formation, and probably also insufficient to stimulate a strong cell-mediated immune response against viral antigens [54 Amella CA, Lofgren LA, Ronn AM, Nouri M, Shikowitz MJ, Steinberg BM. Latent infection induced with cottontail rabbit papillomavirus. A model for human papillomavirus latency Am J Pathol 1994; 144(6): 1167-71., 55 Zhang P, Nouri M, Brandsma JL, Iftner T, Steinberg BM. Induction of E6/E7 expression in cottontail rabbit papillomavirus latency following UV activation Virology 1999; 263(2): 388-94.]. Therefore, subsequent exposure to ultraviolet light and induction of a DNA damage response would be sufficient to allow activation of the virus in the absence of a robust immune response. According to this model, we have examined whether latent ROPV can be reactivated by drug-induced suppression of the host immune system. Our unpublished data suggest that a prolonged period of immune suppression does stimulate early events during reactivation, with evidence of an elevation in viral genome copy number at sites of latent infection. These experiments have however been challenging because of the difficulty of inducing a chronic state of immune suppression in rabbits. Recently, a novel papillomavirus (MusPV) has been isolated from a laboratory mouse [31 Ingle A, Ghim S, Joh J, Chepkoech I, Bennett Jenson A, Sundberg JP. Novel laboratory mouse papillomavirus (MusPV) infection Vet Pathol [Research Suport Non-US Gov't] 2011; 48(2): 500-.]. MusPV has a tropism for cutaneous epithelium, and crude virus preparations and DNA have been successfully used to induce the appearance of discrete benign papillomas in immunocompromised mice. The genome of MusPV has been fully sequenced and has been classified as a member of the Pi genus, with it’s closest relatives being other rodent papillomaviruses [74 Joh J, Jenson AB, King W, et al. Genomic analysis of the first
laboratory-mouse papillomavirus J Gen Virol 2011; 92(Pt 3): 692-8.]. Such a mouse model has obvious value for the study of papillomavirus latency, given the availability of immunodeficient strains of Mus musculus and the ability to suppress and deplete components of the host immune system and it is hoped that the MusPV genome will soon become available to the papillomavirus research community. Furthermore, by causing cutaneous disease on readily accessible areas of the body, opportunities may be afforded to investigate other factors that are important in reactivation, such as chronic wounding and application of substances.
). Interestingly, recent studies in humans have suggested that latent HPV infections may be associated with particular methylation patterns on the viral episome and presumably also with limited gene expression [73 Vinokurova S, von Knebel Doeberitz M. Differential methylation
of the HPV 16 upstream regulatory region during epithelial
differentiation and neoplastic transformation PLoS One 2011; 6(9
): e24451.]. If our hypothesis that recurrence in HIV-infected individuals and renal transplant recipients is due to the reactivation of latent infections, then a waning immune response would appear to be necessary to facilitate recurrence. In the CRPV model of asymptomatic infection described earlier, a low level virus inoculation was found to be insufficient to allow papilloma formation, and probably also insufficient to stimulate a strong cell-mediated immune response against viral antigens [54 Amella CA, Lofgren LA, Ronn AM, Nouri M, Shikowitz MJ, Steinberg BM. Latent infection induced with cottontail rabbit papillomavirus. A model for human papillomavirus latency Am J Pathol 1994; 144(6): 1167-71., 55 Zhang P, Nouri M, Brandsma JL, Iftner T, Steinberg BM. Induction of E6/E7 expression in cottontail rabbit papillomavirus latency following UV activation Virology 1999; 263(2): 388-94.]. Therefore, subsequent exposure to ultraviolet light and induction of a DNA damage response would be sufficient to allow activation of the virus in the absence of a robust immune response. According to this model, we have examined whether latent ROPV can be reactivated by drug-induced suppression of the host immune system. Our unpublished data suggest that a prolonged period of immune suppression does stimulate early events during reactivation, with evidence of an elevation in viral genome copy number at sites of latent infection. These experiments have however been challenging because of the difficulty of inducing a chronic state of immune suppression in rabbits. Recently, a novel papillomavirus (MusPV) has been isolated from a laboratory mouse [31 Ingle A, Ghim S, Joh J, Chepkoech I, Bennett Jenson A, Sundberg JP. Novel laboratory mouse papillomavirus (MusPV) infection Vet Pathol [Research Suport Non-US Gov't] 2011; 48(2): 500-.]. MusPV has a tropism for cutaneous epithelium, and crude virus preparations and DNA have been successfully used to induce the appearance of discrete benign papillomas in immunocompromised mice. The genome of MusPV has been fully sequenced and has been classified as a member of the Pi genus, with it’s closest relatives being other rodent papillomaviruses [74 Joh J, Jenson AB, King W, et al. Genomic analysis of the first
laboratory-mouse papillomavirus J Gen Virol 2011; 92(Pt 3): 692-8.]. Such a mouse model has obvious value for the study of papillomavirus latency, given the availability of immunodeficient strains of Mus musculus and the ability to suppress and deplete components of the host immune system and it is hoped that the MusPV genome will soon become available to the papillomavirus research community. Furthermore, by causing cutaneous disease on readily accessible areas of the body, opportunities may be afforded to investigate other factors that are important in reactivation, such as chronic wounding and application of substances.
A PROPOSED MODEL OF PAPILLOMAVIRUS LATENCY
Primarily through studies of experimental animal infections, we are beginning to define and understand papillomavirus latency. Drawing together results of experiments performed in animals, supported by clinical observations in humans, we can build a proposed model of how a latent stage of the papillomavirus life cycle may occur. Although some papillomaviruses may have specific ways to access the epithelial basal cell layer (e.g. via the squamo-columnar junction or via hair follicles), in many cases it is thought that papillomaviruses gain access to basal cells of the epithelium via cuts or abrasions, and that the establishment and maintenance of infection requires entry into a long-lived cell such as the epithelial stem cell [57 Schmitt A, Rochat A, Zeltner R, et al. The primary target cells of
the high-risk cottontail rabbit papillomavirus colocalize with hair
follicle stem cells J Virol 1996; 70(3
): 1912-22.]. Following infection, there are two potential outcomes. A clinically apparent lesion may form as is seen for example with ROPV infection of the rabbit tongue with virions. In other cases, a clinically silent infection may develop, that may or may not involve the completion of the full productive virus life cycle (Fig. 1 ). Such an infection has been observed in rabbits infected with a low dose of CRPV [54 Amella CA, Lofgren LA, Ronn AM, Nouri M, Shikowitz MJ, Steinberg BM. Latent infection induced with cottontail rabbit papillomavirus. A model for human papillomavirus latency Am J Pathol 1994; 144(6): 1167-71., 55 Zhang P, Nouri M, Brandsma JL, Iftner T, Steinberg BM. Induction of E6/E7 expression in cottontail rabbit papillomavirus latency following UV activation Virology 1999; 263(2): 388-94.]. Where new virions are not formed, this may represent a form of viral latency, but in this situation, there is unlikely to be immune cell involvement. Following the ‘apparent’ clearance of an acute productive infection, viral latency may also ensue, in which a fraction of basal epithelial cells (possibly the epithelial stem cells or stem-like cells) retain viral genomes, most probably in the form of episomal DNA (Fig. 2
). Such an infection has been observed in rabbits infected with a low dose of CRPV [54 Amella CA, Lofgren LA, Ronn AM, Nouri M, Shikowitz MJ, Steinberg BM. Latent infection induced with cottontail rabbit papillomavirus. A model for human papillomavirus latency Am J Pathol 1994; 144(6): 1167-71., 55 Zhang P, Nouri M, Brandsma JL, Iftner T, Steinberg BM. Induction of E6/E7 expression in cottontail rabbit papillomavirus latency following UV activation Virology 1999; 263(2): 388-94.]. Where new virions are not formed, this may represent a form of viral latency, but in this situation, there is unlikely to be immune cell involvement. Following the ‘apparent’ clearance of an acute productive infection, viral latency may also ensue, in which a fraction of basal epithelial cells (possibly the epithelial stem cells or stem-like cells) retain viral genomes, most probably in the form of episomal DNA (Fig. 2 ). In the absence of a strong immune response, factors such as a DNA damage response, or the stimulation of cellular proliferation by wounding will stimulate proliferation of cells harbouring viral DNA. In turn, lesions may form, as appears to occur in latent CRPV infection, following exposure to UV light [55 Zhang P, Nouri M, Brandsma JL, Iftner T, Steinberg BM. Induction of E6/E7 expression in cottontail rabbit papillomavirus latency following UV activation Virology 1999; 263(2): 388-94.]. Alternatively, where latency has developed following lesion regression driven by a strong cell-mediated immune response, we believe that subsequent virus reactivation may occur upon suppression of the immune system. A weakened immune system is likely to allow virus-infected cells to undergo proliferation with completion of the productive virus life cycle, with or without the re-emergence of lesions (Fig. 2
). In the absence of a strong immune response, factors such as a DNA damage response, or the stimulation of cellular proliferation by wounding will stimulate proliferation of cells harbouring viral DNA. In turn, lesions may form, as appears to occur in latent CRPV infection, following exposure to UV light [55 Zhang P, Nouri M, Brandsma JL, Iftner T, Steinberg BM. Induction of E6/E7 expression in cottontail rabbit papillomavirus latency following UV activation Virology 1999; 263(2): 388-94.]. Alternatively, where latency has developed following lesion regression driven by a strong cell-mediated immune response, we believe that subsequent virus reactivation may occur upon suppression of the immune system. A weakened immune system is likely to allow virus-infected cells to undergo proliferation with completion of the productive virus life cycle, with or without the re-emergence of lesions (Fig. 2 ). For studies of virus reactivation in particular, we expect that animal models will play a key role in the further development of these models.
). For studies of virus reactivation in particular, we expect that animal models will play a key role in the further development of these models.
CONCLUDING REMARKS
Clinical observations of humans and animals infected with papillomaviruses have for a long time led to the assumption that papillomaviruses can form latent infections. Our understanding of papillomavirus latency has been substantiated by a limited number of studies using experimental animal models of infection carried out in relatively few labs. However, despite the important advances that have been made, our overall understanding of papillomavirus latency is still very much in it’s infancy, particularly when compared to the vast wealth of knowledge pertaining to other viral infections. The ubiquity of HPVs and their mounting association with a growing range of diseases (including severe papillomatosis and diverse cancers), is directing more interest towards the understanding of papillomavirus latency. The recent discovery of a laboratory mouse papillomavirus is a significant and long-awaited advancement in the field, and one that will hopefully allow us to address the many unanswered questions that remain.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The authors were funded by a program grant from the United Kingdom Medical Research Council (U117584278). Gareth Maglennon received funding from Sanofi Pasteur MSD (Lyon, France) and from the Wellcome Trust in the form of a Research Training Fellowship (082155/Z/07/Z).




