- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Variety of Crystal Structures of Chiral Schiff Base Lu(III)-Ni(II)/ Cu(II)/Zn(II) and the Related Complexes
Shingo Orita, Takashiro Akitsu*
Abstract
New mononuclear [Cu2(L1)2(H2O)]2H2O・CH3OH] (cyCu)2, multinuclear [Cu2(L1)2K2][Ni(CN)4] (cyCuKNi), and dinuclear Ni(L1)Lu(NO3)3 (cyLuNi), Cu(L1)Lu(NO3)3 (cyLuCu) and Zn(L1)Lu[(NO3)2CH3COO]CH3OH (cyLuZn) complexes incorporating chiral Schiff base ligands were prepared and characterized by various techniques such as solid-state CD spectra, diffuse reflectance electronic spectra, IR spectra, and single crystal X-ray diffraction analysis. Crystal structures were also compared with previous ones, namely [Cu(L2)](H2O) (diCu), Cu(L1)Gd(NO3)3 (cyGdCu). Interestingly, determined crystal structures exhibited drastically different structural characteristics as regards coordination numbers, crystalline solvents, features and strain condition, nevertheless with little modification in 3d metal substitution and/or modified organic ligands. In this paper, comparison of common components of structures will be discussed systematically.
Article Information
Identifiers and Pagination:
Year: 2014Volume: 1
First Page: 1
Last Page: 14
Publisher Id: CHEM-1-1
DOI: 10.2174/1874842201401010001
Article History:
Received Date: 16/06/2014Revision Received Date: 04/08/2014
Acceptance Date: 11/08/2014
Electronic publication date: 03/10/2014
Collection year: 2014
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Department of Chemistry, Faculty of Science, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan; Tel: +81 3 5228 8271; Fax: +81 3 5261 4361; E-mail: akitsu@rs.kagu.tus.ac.jp
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 16-06-2014 |
Original Manuscript | Variety of Crystal Structures of Chiral Schiff Base Lu(III)-Ni(II)/ Cu(II)/Zn(II) and the Related Complexes | |
INTRODUCTION
Chiral Schiff bases transition metal complexes, so-called salen-type ligands, are one of the most studied chiral catalysts in asymmetric synthesis because of their ability to act as chiral catalysts or as co-catalysts [1Bryliakov, KP; Talsi, EP Cr(III)(salen)Cl catalyzed asymmetric epoxidations: insight into the catalytic cycle Inorg. Chem, 2003, 42, 7258-7265.
[http://dx.doi.org/10.1021/ic034578w] -7Nielsen, LPC; Zuend, SJ; Ford, DD; Jacobsen, EN Mechanistic basis for high reactivity of (salen)Co–OTs in the hydrolytic kinetic resolution of terminal epoxides Org. Chem, 2012, 77, 2486-2495.
[http://dx.doi.org/10.1021/jo300181f] ]. Moreover, their binuclear complexes containing transition metals and rare earth metals have gained importance from the viewpoint of special interest in photophysical and magnetic properties arising from interactions between metal ions as well as sole metal ions having suitable electronic states or unpaired electrons [8Xu, L; Zhang, Q; Hou, G; Chen, P; Li, G; Pajerowski, DM; Dennis, CL Syntheses, structures, and magnetic properties of salen type Cu–Gd dimer and hexamer complexes with strong ferromagnetic interactions Polyhedron, 2013, 52, 91-95.-19Lo, WK; Wong, WK; Wong, WY; Guo, J; Yeung, KT; Cheng, YK; Yang, X; Jones, RA Heterobimetallic Zn(II)-Ln(III) phenylene-bridged schiff base complexes, computational studies, and evidence for singlet energy transfer as the main pathway in the sensitization of near-infrared Nd3+ luminescence Inorg. Chem, 2006, 45, 9315-9325.].
We have been interested in chiral salen-type 3d-4f binuclear complexes and have investigated structure-property correlation and their variety resulting from metal substitution and their combination [20Hayashi, T; Shibata, H; Orita, S; Akitsu, T Variety of structures of binuclear chiral schiff base Ce(III)/Pr(III)/Lu(III)-Ni(II)/Cu(II)/ Zn(II) complexes Eur. Chem. Bull, 2013, 2, 49-57.-23Akitsu, T New attempt of empirical approach for magnetic interactions: several examples of 3d-4f [Ln(DMA)2(H2O)4M(CN)6·5H2O]n bimetallic assemblies (DMA = N,N-dimethylacetamide) Asian.
Chem. Lett, 2010, 14, 53-62.]. In this work, in order to investigate structural features due to metal ions, we systematically investigate nine Schiff base complexes (abbreviated as cyCu, cyCuKNi, cyLuNi, cyLuCu, cyLuZn, diLuNi [20Hayashi, T; Shibata, H; Orita, S; Akitsu, T Variety of structures of binuclear chiral schiff base Ce(III)/Pr(III)/Lu(III)-Ni(II)/Cu(II)/ Zn(II) complexes Eur. Chem. Bull, 2013, 2, 49-57.], diLuCu [20Hayashi, T; Shibata, H; Orita, S; Akitsu, T Variety of structures of binuclear chiral schiff base Ce(III)/Pr(III)/Lu(III)-Ni(II)/Cu(II)/ Zn(II) complexes Eur. Chem. Bull, 2013, 2, 49-57.], and diLuZn [20Hayashi, T; Shibata, H; Orita, S; Akitsu, T Variety of structures of binuclear chiral schiff base Ce(III)/Pr(III)/Lu(III)-Ni(II)/Cu(II)/ Zn(II) complexes Eur. Chem. Bull, 2013, 2, 49-57.]) and characterized them with diffuse reflectance electronic spectra, solid-state CD spectra, and X-ray crystal structure analysis. Additionally, these crystal structures were also compared with previously prepared two compounds, namely diCu [21Akitsu, T; Hirarsuka, T; Shibata, H Chiroptical properties of 3d-4f chiral Schiff base magnetic complexes. Magnets:Types, Uses
and Safety; Nova Science Publishers, 2012, pp. 69-84.] and cyGdCu [22Okamoto, Y; Nidaira, K; Akitsu, T Environmental dependence of artifact CD peaks of chiral schiff base 3d-4f complexes in soft mater PMMA matrix Int. J. Mol. Sci, 2011, 12, 6966-6979.
[http://dx.doi.org/10.3390/ijms12106966] ]. Based on the basic structure of mononuclear cyCu having a common component, in which both four-coordinated and five-coordinated Cu(II) complexes are contained in a crystal, the variety and the reason of coordination geometries and structural features caused by systematic modifying ligands and substituting metal ions will be discussed (Fig. 1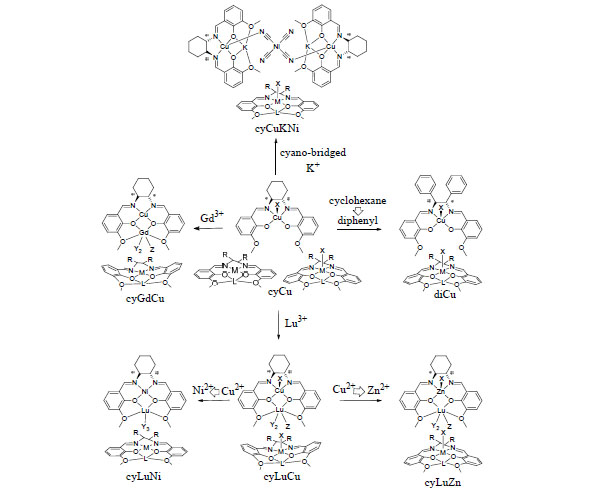 ).
).
 |
Fig. (1) Structures of complexes and summary of structural features determined. |
EXPERIMENTAL SECTION
Materials
Chemicals of the highest commercial grade available (solvents are from Kanto Chemical, organic compounds are from Tokyo Chemical Industry and metal sources from Wako and Aldrich) were used as received without further purification.
Preparation of cyGdCu
Single crystals suitable for X-ray analysis of cyGdCu were prepared according to the procedure given in the literature [22Okamoto, Y; Nidaira, K; Akitsu, T Environmental dependence of artifact CD peaks of chiral schiff base 3d-4f complexes in soft mater PMMA matrix Int. J. Mol. Sci, 2011, 12, 6966-6979.
[http://dx.doi.org/10.3390/ijms12106966] ].
Preparation of cyCu
To a solution of cyGdCu (0.0060 g, 0.0075 mmol) dissolved in methanol (1.5 mL), a solution of potassium tetracyano nickel(II) hydrate (0.0019 g, 0.0075 mmol) dissolved in water (1.5 mL) was added and stirred for 24 h at 298 K. The aqueous solution of potassium tetracyano nickel(II) hydrate played a role in promoting crystallization of good single crystals of cyCu. Purple needle crystals suitable for X-ray crystallography were obtained from the resulting solution (yield 0.0011 g, 32.03 %). Yield 0.0011 g (32.03 %). IR (KBr (cm-1): 426 (w), 463 (w), 506 (w), 560 (m), 600 (m), 668 (w), 732 (s), 781 (w), 881 (w), 921 (w), 977 (m), 1025 (m), 1108 (w), 1163 (w), 1224 (s), 1242 (s), 1321 (s), 1354 (m), 1390 (w), 1442 (s), 1473 (s), 1542 (m), 1600 (s), 1626 (s) (C=N), 2928 (s), 3504 (s).
Preparation of cyCuKNi
Under N2 atmosphere at 298 K, treatment of tetraethylammonium perchlorate (0.640 g, 2.00 mmol) and potassium tetracyano nickel(II) hydrate (0.259 g, 1.00 mmol) in methanol (50 mL) for 72 h gave rise to tetraethylammonium tetracyanonickel(II) hydrate (a precursor). To a 5 mmol/L methanol solution of potassium tetracyanonickel(II) hydrate (1.5 mL), a precursor cyGdCu (0.0060 g, 0.0075 mmol) in methanol (1.5 mL) was added and stirred for 24 h at 298 K. A few grains of brown prismatic crystals suitable for X-ray crystallography were obtained from the resulting solution. Yield 0.0013 g (15.41 %). IR (KBr (cm-1): 562 (s), 668 (w), 748 (s), 853 (w), 970 (m), 1027 (w), 1082 (m), 1212 (s), 1242 (s), 1318 (m), 1440 (s), 1473 (s), 1542 (m), 1629 (s) (C=N), 2118 (m) (C≡N), 2839 (w), 2933 (w), 3423 (s).
Preparation of cyLuNi
To a solution of o-vanillin (0.305 g, 2.00 mmol) dissolved in methanol (60 mL), (1R,2R)-(-)-1,2-cyclohexane-diamine (0.114 g, 1.00 mmol) was added and stirred at 313 K for 2 h to give yellow solution of ligand(L1). Nickel(II)acetate tetrahydrate (0.2545 g, 1.00 mmol) was added to the resulting solution to yield muddy brown solution of the complex. After stirring at 313 K for 3 h, lutetium(III)nitrate hexahydrate (0.3642 g 1.00 mmol) was added to the resulting solution and the mixed solution was refluxed for 4 h at 373 K. After cooling the solution, the resulting orange compound was filtered and recrystallized from methanol/diethyl ether to obtain single crystals. Yield 0.5732 g (71.64 %). Anal. Found: C, 33.22 ; H, 2.91 ; N, 8.61 %. Calc. for C22H24LuN5NiO13: C, 33.02 ; H, 3.02 ; N, 8.75 %. IR (KBr (cm-1):566 (w), 598 (w), 681 (w),740 (m), 786 (w), 810 (w), 869 (w), 964 (m), 1036 (m), 1077 (m), 1168 (w), 1232 (s), 1321 (s), 1383 (m), 1478 (s), 1520 (s), 1619 (s) (C=N), 1700 (s), 2328 (w), 2944 (w), 3401 (s).
Preparation of cyLuCu
To a solution of o-vanillin (0.305 g, 2.00 mmol) dissolved in methanol (60 mL), (1R,2R)-(-)-1,2-cyclohexane-diamine (0.114 g, 1.00 mmol) was added and stirred at 313 K for 2 h to give yellow solution of ligand (L1). Copper(II)acetate hydrate (0.2027 g, 1.00 mmol) was added to the resulting solution to yield muddy purple solution of the complex. After stirring at 313 K for 3 h, lutetium(III)nitrate hexahydrate (0.3610 g, 1.00 mmol) was added to the resulting solution and the reaction was refluxed for 4 h at 373 K. The solution was evaporated under reduced pressure, and deep brown compound was yielded. This compound was filtered and recrystallized from methanol/diethyl ether to give crystals. Yield 0.5962 g (74.06 %). Anal. Found: C, 33.14; H, 3.06; N, 6.80 %. Calc. for C22H24LuN5CuO13: C, 32.83; H, 3.01; N, 8.70 %. IR (KBr (cm-1): 533 (w), 650 (w), 740 (m), 855 (w), 949 (m), 1019 (m), 1070 (m), 1221 (s), 1303 (s), 1383 (m), 1468 (s), 1521 (m), 1630 (s) (C=N), 1652 (s), 2328 (w), 2943 (m), 3399 (s).
Preparation of cyLuZn
To a solution of o-vanillin (0.305 g, 2.00 mmol) dissolved in methanol (60 mL), (1R,2R)-(-)-1,2-cyclohexanediamine (0.114 g, 1.00 mmol) was added and stirred at 313 K for 2 h to give a ligand (L1). Zinc(II)acetate tetrahydrate (0.2243 g, 1.00 mmol) was added to the resulting solution to yield light-yellow solution of the complex. After stirring at 313 K for 3 h, lutetium(III) nitrate hexahydrate (0.3526 g, 1.00 mmol) was added to the resulting solution and the reaction was refluxed for 4 h at 373 K. The solution was evaporated under reduced pressure, and yellow compound was obtained. This compound was filtered and recrystallized from methanol/diethyl ether to obtain single crystals containing (nonstoichometric) CH4O solvent suitable for X-ray analysis. Yield 0.5732 g (68.57 %). Anal. Found: C, 35.86; H, 3.60; N, 6.62 %. Calc. for C25H31LuN4ZnO13: C, 35.92; H, 3.74; N, 6.70 %. IR (KBr (cm-1): 444 (w), 537 (w), 566 (w), 624 (w), 665 (m), 739 (s), 781 (w), 812 (w), 857 (m), 888 (w), 949 (s), 1015 (s), 1073 (m), 1165 (w), 1221 (s), 1268 (s), 1165 (m), 1380 (w), 1384 (m), 1476 (s), 1534 (s), 1556 (m), 1630 (m) (C=N), 1656 (m), 2942 (m), 2424 (s), 3403 (s).
Preparation of diCu
Single crystals suitable for X-ray analysis of diCu were prepared according to the procedure given in the literature [21Akitsu, T; Hirarsuka, T; Shibata, H Chiroptical properties of 3d-4f chiral Schiff base magnetic complexes. Magnets:Types, Uses and Safety; Nova Science Publishers, 2012, pp. 69-84.].
Physical Measurements
Elemental analyses (C, H, N) were carried out with a Perkin-Elmer 2400II CHNS/O analyzer at Tokyo University of Science. Infrared spectra were recorded using KBr pellets on a JASCO FT-IR 4200 plus spectrophotometer in the range of 4000-400 cm-1 at 298 K. Diffuse reflectance spectra were measured on a JASCO V-570 UV/VIS/NIR spectrophotometer equipped with an integrating sphere in the range of 800-200 nm at 298 K. Circular dichroism (CD) spectra were measured as KBr pellets on a JASCO J-820 spectropolarimeter in a range of 800-300 nm at 298 K. Fluorescence spectra in the solid state were recorded on a JASCO FP-6200 spectrophotometer at 298 K.
X-Ray Crystallography
Prismatic single crystals of purple cyCu, brown cyGdCu, brown cyCuKNi, orange cyLuNi, purple cyLuCu, pale yellow cyLuZn, and green diCu were glued on top of a glass fiber and coated with a thin layer of epoxy resin. Intensity data were collected on a Bruker APEX2 CCD diffractometer with graphite monochromated Mo Kα radiation (λ = 0.71073 A). Data analysis was carried out with a SAINT program package. The structures were solved by direct methods with a SHELXS-97 [24Sheldrick, GM A short history of SHELX Acta. Crystallogr. A, 2008, 64, 112-22.
[http://dx.doi.org/10.1107/S0108767307043930] ] and expanded by Fourier techniques and refined by full-matrix least-squares methods based on F2 using the program SHELXL-97 [24Sheldrick, GM A short history of SHELX Acta. Crystallogr. A, 2008, 64, 112-22.
[http://dx.doi.org/10.1107/S0108767307043930] ]. A multi-scan absorption correction was applied by a program SADABS. All non-hydrogen atoms were readily located and refined by anisotropic thermal parameters. All hydrogen atoms were located at geometrically calculated positions and refined using riding models.
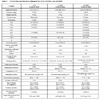
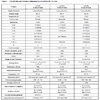
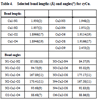
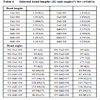
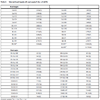
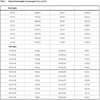
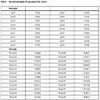
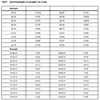
The crystallographic data for “cyCu, cyGdCu, and CyCuKNi”, “cyLuM (M=Ni, Cu, and Zn)”, and “diCu”are summarized in Tables 1, 2, and 3, respectively.
RESULTS AND DISCUSSION
Structure Description of cyCu
Complex cyCu crystallizes in monoclinic, space group P21 with Z = 2. As shown in Figs. (2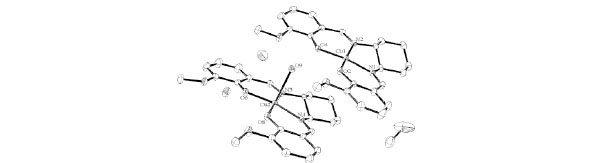 ) and
S1, the asymmetric unit of cyCu contains crystallographically two independent molecules of four-coordinated and five coordinated mononuclear Cu(II) complexes. One of the Cu(II) complex affords a four-coordinated square planar [CuN2O2] geometry with the four donor atoms of the tetradentete Schiff base forming the equatorial plane with Cu1–N1, Cu1-N2, Cu1-O2, and Cu1-O4 distances ranging from 1.8946(19) to 1.937(2) Å (Table 4).
) and
S1, the asymmetric unit of cyCu contains crystallographically two independent molecules of four-coordinated and five coordinated mononuclear Cu(II) complexes. One of the Cu(II) complex affords a four-coordinated square planar [CuN2O2] geometry with the four donor atoms of the tetradentete Schiff base forming the equatorial plane with Cu1–N1, Cu1-N2, Cu1-O2, and Cu1-O4 distances ranging from 1.8946(19) to 1.937(2) Å (Table 4).
 |
Fig. (2) Molecular structures of cyCu showing selected atom labeling scheme. Hydrogen atoms are omitted for clarity. |
The other Cu(II) complex affords a five-coordinated square pyramidal [CuN2O3] geometry with additional aqua ligand occupying the axial sites with Cu2–N3, Cu2-N4, Cu2-O6, and Cu2-O8 distances ranging from 1.9114(19) to 1.951(2) Å and axial O atom from coordinate aqua ligand with Cu2–O9 distance of 2.453(2) Å (Table 4). The displacement of Cu1 and Cu2 from the A-plane and A’-plane (Fig. 3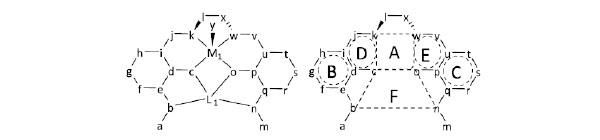 ) is 0.0198(13) Å and 0.1407(13) Å, respectively. The chiral (1R,2R)-(-)-1,2-cyclohexanediamine moieties adopt a λ configuration with torsion angles N1-C9-C20-N2 = -47.2(3)° and N3-C31-C42-N4 = -45.8(3)°, respectively.
) is 0.0198(13) Å and 0.1407(13) Å, respectively. The chiral (1R,2R)-(-)-1,2-cyclohexanediamine moieties adopt a λ configuration with torsion angles N1-C9-C20-N2 = -47.2(3)° and N3-C31-C42-N4 = -45.8(3)°, respectively.
 |
Fig. (3) Definition of mean planes for comparison of common moieties. |
The dihedral angles between the B-plane and C-plane and between the B’-plane and C’-plane are 13.86(6) and 5.45(5)°, respectively. The angles between the A-plane and B-plane and between the A’-plane and B’-plane are 9.65(4) and 8.77(4)°, respectively. And the angles between the A-plane and C-plane and between the A’-plane and C’-plane are 4.21(6) and 3.42(3)°, respectively. Moreover, the angles between the A-plane and D-plane, between the A-plane and E-plane, and between the D-plane and E-plane are 8.24(4), 6.82(6), and 14.65(6)°, and between the A’-plane and D’-plane, between the A’-plane and E’-planes, and between the D’-plane and E’-plane are 7.84, 4.81, and 6.53°, respectively.
The displacement of O9 from the F-plane is 0.6961(32) Å for the four-coordinated molecule taking planar structure (see Fig. 4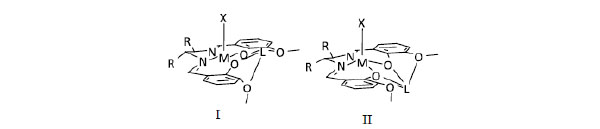 ). Whereas the displacement of O10 from the F’-plane is 0.9007(33) Å for the five-coordinated molecule (see Fig. 4
). Whereas the displacement of O10 from the F’-plane is 0.9007(33) Å for the five-coordinated molecule (see Fig. 4 ). Since axial water ligands and crystalline water are in the same side, the overall molecule takes slightly distorted umbrella structure towards the opposite side of the axial ligand (see Fig. 5
). Since axial water ligands and crystalline water are in the same side, the overall molecule takes slightly distorted umbrella structure towards the opposite side of the axial ligand (see Fig. 5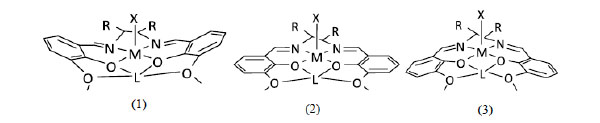 ).
).
 |
Fig. (4) Distortion of L (I and II distort toward the same side and the opposite side of the axial ligand). |
 |
Fig. (5) Distortion of overall molecular structures. |
Structure Description of cyGdCu
Complex cyGdCu crystallizes in monoclinic, space group P21 with Z = 4. As shown in Fig. (6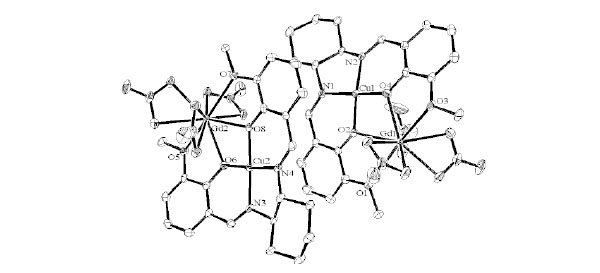 ) and
S2, the Gd(III) ion affords a ten-coordinated environment with distorted square pyramidal towards bridging nitrate ligands. While Cu(II) ion affords a four-coordinated square planar [CuN2O2] geometry with Cu1–N1, Cu1-N2, Cu1-O2, and Cu1-O4 distances ranging from 1.898(5) to 1.916(5) Å (Table 5), the displacement of Cu1 from the A-plane is 0.0357(25) Å (see Fig. 3
) and
S2, the Gd(III) ion affords a ten-coordinated environment with distorted square pyramidal towards bridging nitrate ligands. While Cu(II) ion affords a four-coordinated square planar [CuN2O2] geometry with Cu1–N1, Cu1-N2, Cu1-O2, and Cu1-O4 distances ranging from 1.898(5) to 1.916(5) Å (Table 5), the displacement of Cu1 from the A-plane is 0.0357(25) Å (see Fig. 3 ). The chiral (1R,2R)-(-)-1,2-cyclohexanediamine moiety adopts a λ configuration, with torsion angle N1-C9-C20-N2 = -49.1(6)°.
). The chiral (1R,2R)-(-)-1,2-cyclohexanediamine moiety adopts a λ configuration, with torsion angle N1-C9-C20-N2 = -49.1(6)°.
 |
Fig. (6) Molecular structures of cyGdCu showing selected atom labeling scheme. Hydrogen atoms are omitted for clarity. |
The overall molecular structure of planar is an umbrella shape. The dihedral angle between the B-plane and C-plane is 24.82°. The angles between the A-plane and B-plane and between the A-plane and C-plane are 19.18 and 6.45°, respectively. Moreover, the angles between the A-plane and D-plane, between the A-plane and E-planes, and between the D-plane and E-plane are 13.70, 5.94 and, 19.39°, respectively. The displacement of Lu1 from the F-plane is 0.4675(29) Å.
Structure Description of cyCuKNi
Complex cyCuKNi crystallizes in triclinic, space group P1 with Z = 1. As shown in Figs. (7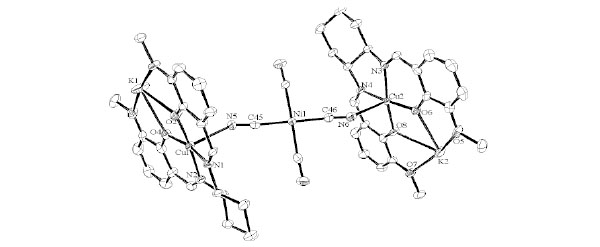 and
S3), there are four-coordinated and five-coordinated K(I) ions in the same molecule. Moreover, square planar [Ni(CN)4]2- units are bridged to form a one-dimensional chain as shown in Fig. (8
and
S3), there are four-coordinated and five-coordinated K(I) ions in the same molecule. Moreover, square planar [Ni(CN)4]2- units are bridged to form a one-dimensional chain as shown in Fig. (8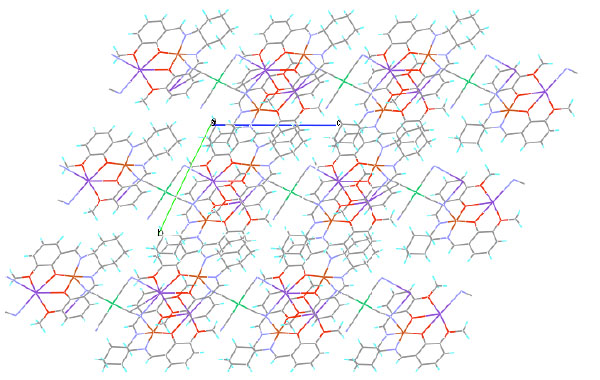 ). The Cu(II) ion affords a five-coordinated square pyramidal [CuN3O2] geometry with Cu1–N1, Cu1-N2, Cu1-O2, and Cu1-O4 distances ranging from 1.885(7) to 1.989(8) Å and the axial Cu1-N5 (cyanide) bond distance being 2.435(8) Å (Table 6). The displacement of Cu1 from the A-plane (see Fig. 3
). The Cu(II) ion affords a five-coordinated square pyramidal [CuN3O2] geometry with Cu1–N1, Cu1-N2, Cu1-O2, and Cu1-O4 distances ranging from 1.885(7) to 1.989(8) Å and the axial Cu1-N5 (cyanide) bond distance being 2.435(8) Å (Table 6). The displacement of Cu1 from the A-plane (see Fig. 3 ) is 0.1640(32) Å. The chiral (1R,2R)-(-)-1,2-cyclohexanediamine moiety adopts a λ configuration, with torsion angle N1-C9-C20-N2 =43.1(8)°.
) is 0.1640(32) Å. The chiral (1R,2R)-(-)-1,2-cyclohexanediamine moiety adopts a λ configuration, with torsion angle N1-C9-C20-N2 =43.1(8)°.
 |
Fig. (7) Molecular structures of cyCuKNi showing selected atom labeling scheme. Hydrogen atoms are omitted for clarity. |
 |
Fig. (8) Crystal packing of cyCuKNi viewed down from the a axis. |
The dihedral angle between the B-plane and C-plane is 6.06°. The angles between the A-plane and B-plane and between the A-plane and C-plane are 9.68, and 3.63°, respectively. Moreover, the angles between the A-plane and D-plane, between the A-plane and E-planes, and between the D-plane and E-plane are 9.49, 4.89, and 6.51°, respectively.
The displacement of K1 from the F-plane is 0.0307(50) Å, and there is K ion almost in the F-plane. Detailed structural description for the other molecules with Cu2 is omitted because of similarity.
Structure Description of cyLuNi, cyLuCu, and cyLuZn
Complexes cyLuNi, cyLuCu, and cyLuZn crystallize in triclinic, space group P1 with Z = 2, P21 with Z = 4, and P1 with Z = 2, respectively. As shown in Figs. (9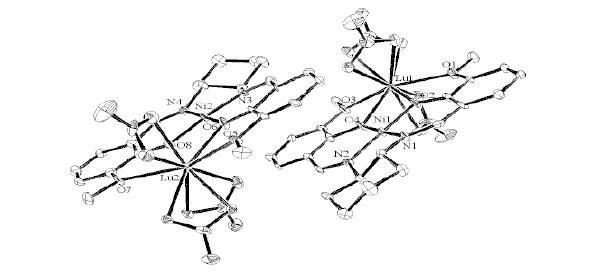 -11
-11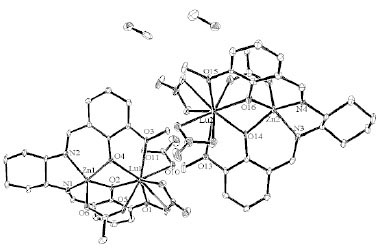 and
S4-
S6), Lu(III) ion affords a ten-, nine-, and nine-coordinated environment with distorted square pyramidal from the plane made by two nitrate ligands. Ni(II) ion affords a four-coordinated square planar [NiN2O2] geometry with Ni1–N1, Ni1-N2, Ni1-O2, and Ni1-O4 distances ranging from 1.814(14) to 1.888(14) Å (Table 7). Cu(II) ion affords a five-coordinated square pyramidal [CuN2O3] geometry with Cu1-N1, Cu1-N2, Cu1-O2, and Cu1-O4 distances ranging from 1.893(5) to 1.920(4) Å and axial O atom from bridging nitrate ligand with Cu1–O9 distance of 2.399(5) Å (Table 8). Zn(II) ion affords a five-coordinated square pyramidal [ZnN2O3] geometry with Zn1–N1, Zn1-N2, Zn1-O2, and Zn1-O4 distances ranging from 2.015(9) to 2.054(9) Å and axial O atom from bridging acetate ligand with Zn1–O9 distance of 1.996(9) Å (Table 9). The displacement of Ni1, Cu1, and Zn1 atoms from the A-plane (see Fig. 3
and
S4-
S6), Lu(III) ion affords a ten-, nine-, and nine-coordinated environment with distorted square pyramidal from the plane made by two nitrate ligands. Ni(II) ion affords a four-coordinated square planar [NiN2O2] geometry with Ni1–N1, Ni1-N2, Ni1-O2, and Ni1-O4 distances ranging from 1.814(14) to 1.888(14) Å (Table 7). Cu(II) ion affords a five-coordinated square pyramidal [CuN2O3] geometry with Cu1-N1, Cu1-N2, Cu1-O2, and Cu1-O4 distances ranging from 1.893(5) to 1.920(4) Å and axial O atom from bridging nitrate ligand with Cu1–O9 distance of 2.399(5) Å (Table 8). Zn(II) ion affords a five-coordinated square pyramidal [ZnN2O3] geometry with Zn1–N1, Zn1-N2, Zn1-O2, and Zn1-O4 distances ranging from 2.015(9) to 2.054(9) Å and axial O atom from bridging acetate ligand with Zn1–O9 distance of 1.996(9) Å (Table 9). The displacement of Ni1, Cu1, and Zn1 atoms from the A-plane (see Fig. 3 ) is 0.0071(85) Å, 0.0619(20) Å, and 0.6150(45) Å, respectively. The chiral (1R,2R)-(-)-1,2-cyclohexanediamine group adopts a λ configuration, with torsion angle N1-C9-C20-N2 = -43.3(13)°, -42.0(5)°, and -43.4(9)°, respectively.
) is 0.0071(85) Å, 0.0619(20) Å, and 0.6150(45) Å, respectively. The chiral (1R,2R)-(-)-1,2-cyclohexanediamine group adopts a λ configuration, with torsion angle N1-C9-C20-N2 = -43.3(13)°, -42.0(5)°, and -43.4(9)°, respectively.
 |
Fig. (9) Molecular structures of cyLuNi showing selected atom labeling scheme. Hydrogen atoms are omitted for clarity. |
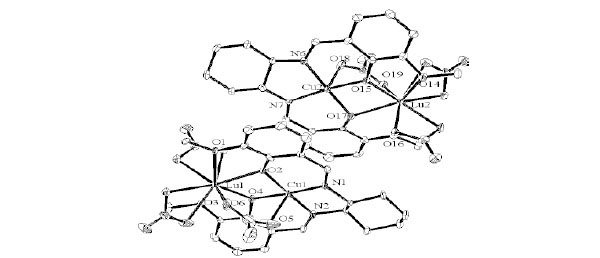 |
Fig. (10) Molecular structures of cyLuCu showing selected atom labeling scheme. Hydrogen atoms are omitted for clarity. |
 |
Fig. (11) Molecular structures of cyLuZn showing selected atom labeling scheme. Hydrogen atoms are omitted for clarity. |
The dihedral angles between the B-plane and C-plane are 3.08°, 16.91°, and 32.59°, respectively. The angles between the A-plane and B-plane and between the A-plane and C-plane are 8.98, and 6.00°, 10.08, and 10.99°, and 11.36 and 21.32°, respectively. Moreover, the angles between the A-plane and D-plane, between the A-plane and E-planes, and between the D-plane and E-plane are 11.34, 7.41, and 5.59°, 1.44, 9.78, and 11.02°, and 14.07, 23.01, and 37.07°, respectively.
Structure Description of diCu
Complex diCu crystallizes in monoclinic, space group P21 with Z = 4. As shown in Figs. (12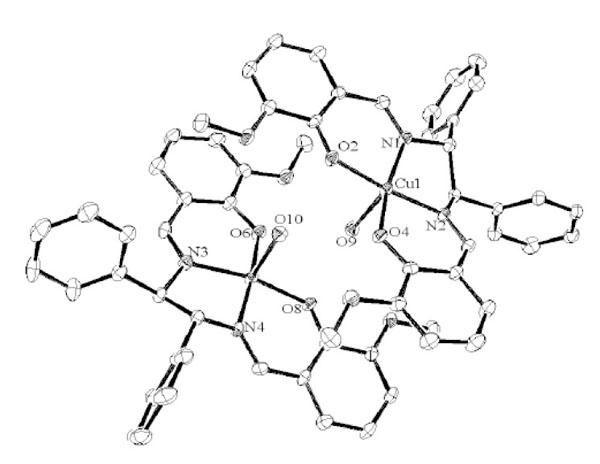 and
S7), Cu(II) ion affords a five-coordinated square pyramidal [CuN2O3] geometry with Cu1–N1, Cu1-N2, Cu1-O2, Cu1-O4 distances ranging from 1.921(2) to 1.964(3) Å and axial O atom from coordinate aqua ligand with Cu1–O9 distance of 2.340(3) Å (Table 10). The displacement of Cu1 atom from the A-plane (see Fig. 3
and
S7), Cu(II) ion affords a five-coordinated square pyramidal [CuN2O3] geometry with Cu1–N1, Cu1-N2, Cu1-O2, Cu1-O4 distances ranging from 1.921(2) to 1.964(3) Å and axial O atom from coordinate aqua ligand with Cu1–O9 distance of 2.340(3) Å (Table 10). The displacement of Cu1 atom from the A-plane (see Fig. 3 ) is 0.1634(13) Å. The chiral (1R,2R)-(+)-1,2-diphenylethylenediamine group adopts a λ configuration, with torsion angle N1-C9-C24-N2 and C10-C9-C24-C25 = -47.6(3)° and 58.0(3)°, respectively.
) is 0.1634(13) Å. The chiral (1R,2R)-(+)-1,2-diphenylethylenediamine group adopts a λ configuration, with torsion angle N1-C9-C24-N2 and C10-C9-C24-C25 = -47.6(3)° and 58.0(3)°, respectively.
 |
Fig. (12) Molecular structures of diCu showing selected atom labeling scheme. Hydrogen atoms are omitted for clarity. |
The dihedral angle between the B-plane and C-plane is 9.00°. The angles between the A-plane and B-plane and between the A-plane and C-plane are 11.36 and 8.44°, respectively. Moreover, the angles between the A-plane and D-plane, between the A-plane and E-planes, and between the D-plane and E-plane are 14.12, 4.90, and 15.02°, respectively.
Two axial water ligands of two molecules face each other inside the two dimeric molecules (see Fig. 4 ) and the displacement of O10 from the F-plane is 0.7791(35) Å. The overall molecular structure is slightly distorted umbrella shape to the opposite direction of the axial ligand (see Fig. 5
) and the displacement of O10 from the F-plane is 0.7791(35) Å. The overall molecular structure is slightly distorted umbrella shape to the opposite direction of the axial ligand (see Fig. 5 ).
).
As shown in Figs. (3 -5
-5 ), the least square mean planes (A-plane , B-plane, C-plane, D-plane, E-plane, and F-plane) are defined using the atom groups (c/k/w/o, d/e/f/g/h/i , p/q/r/s/t/u , c/d/i/j/k ,o/p/u/v/w, and b/c/o/n) for the molecule with M1 atom. Similarly, the least square mean planes (A’-plane , B’-plane, C’-plane, D’-plane, E’-plane, and F’-plane) are defined for the molecule with M atom.
), the least square mean planes (A-plane , B-plane, C-plane, D-plane, E-plane, and F-plane) are defined using the atom groups (c/k/w/o, d/e/f/g/h/i , p/q/r/s/t/u , c/d/i/j/k ,o/p/u/v/w, and b/c/o/n) for the molecule with M1 atom. Similarly, the least square mean planes (A’-plane , B’-plane, C’-plane, D’-plane, E’-plane, and F’-plane) are defined for the molecule with M atom.
Similar to previous binuclear 3d-4f complexes [10Koner, R; Lee, GH; Wang, Y; Wei, HH; Mohanta, S Two new diphenoxo-bridged discrete dinuclear Cu(II)Gd(III) compounds with cyclic diimino moieties: syntheses, structures, and magnetic properties Eur. J. Inorg. Chem, 2005, 8, 1500-1505.
[http://dx.doi.org/10.1002/ejic.200400858] -13Jana, A; Majumder, S; Carrella, L; Nayak, M; Weyhermueller, T; Dutta, S; Schollmeyer, D; Rentschler, E; Koner, R; Mohanta, S Syntheses, structures, and magnetic properties of diphenoxo-bridged Cu(II)Ln(III) and NiII(low-spin)Ln(III) compounds derived from a compartmental ligand (Ln = Ce-Yb) Inorg. Chem, 2010, 49, 9012-9025.
[http://dx.doi.org/10.1021/ic101445n] , 20Hayashi, T; Shibata, H; Orita, S; Akitsu, T Variety of structures of binuclear chiral schiff base Ce(III)/Pr(III)/Lu(III)-Ni(II)/Cu(II)/ Zn(II) complexes Eur. Chem. Bull, 2013, 2, 49-57.-22Okamoto, Y; Nidaira, K; Akitsu, T Environmental dependence of artifact CD peaks of chiral schiff base 3d-4f complexes in soft mater PMMA matrix Int. J. Mol. Sci, 2011, 12, 6966-6979.
[http://dx.doi.org/10.3390/ijms12106966] ], Lu(III) ions of most of the complexes described in this paper adopt ten-coordinated environment. However, nine-coordinated Lu(III) ions are also found for diLuZn [20Hayashi, T; Shibata, H; Orita, S; Akitsu, T Variety of structures of binuclear chiral schiff base Ce(III)/Pr(III)/Lu(III)-Ni(II)/Cu(II)/ Zn(II) complexes Eur. Chem. Bull, 2013, 2, 49-57.], cyLuCu, and cyLuZn, and both nine- and ten-coordinated Lu(III) ions are found for diLuCu [20Hayashi, T; Shibata, H; Orita, S; Akitsu, T Variety of structures of binuclear chiral schiff base Ce(III)/Pr(III)/Lu(III)-Ni(II)/Cu(II)/ Zn(II) complexes Eur. Chem. Bull, 2013, 2, 49-57.]. Formation of nine-coordinated Lu(III) environment is attributed to the smallest ionic radii due to lanthanide contraction as well as significantly steric hindrance of nitrate ions to eliminate [10Koner, R; Lee, GH; Wang, Y; Wei, HH; Mohanta, S Two new diphenoxo-bridged discrete dinuclear Cu(II)Gd(III) compounds with cyclic diimino moieties: syntheses, structures, and magnetic properties Eur. J. Inorg. Chem, 2005, 8, 1500-1505.
[http://dx.doi.org/10.1002/ejic.200400858] -13Jana, A; Majumder, S; Carrella, L; Nayak, M; Weyhermueller, T; Dutta, S; Schollmeyer, D; Rentschler, E; Koner, R; Mohanta, S Syntheses, structures, and magnetic properties of diphenoxo-bridged Cu(II)Ln(III) and NiII(low-spin)Ln(III) compounds derived from a compartmental ligand (Ln = Ce-Yb) Inorg. Chem, 2010, 49, 9012-9025.
[http://dx.doi.org/10.1021/ic101445n] , 20Hayashi, T; Shibata, H; Orita, S; Akitsu, T Variety of structures of binuclear chiral schiff base Ce(III)/Pr(III)/Lu(III)-Ni(II)/Cu(II)/ Zn(II) complexes Eur. Chem. Bull, 2013, 2, 49-57.-22Okamoto, Y; Nidaira, K; Akitsu, T Environmental dependence of artifact CD peaks of chiral schiff base 3d-4f complexes in soft mater PMMA matrix Int. J. Mol. Sci, 2011, 12, 6966-6979.
[http://dx.doi.org/10.3390/ijms12106966] ].
Bridged structures for cyLuCu, cyLuZn, and diLuZn [20Hayashi, T; Shibata, H; Orita, S; Akitsu, T Variety of structures of binuclear chiral schiff base Ce(III)/Pr(III)/Lu(III)-Ni(II)/Cu(II)/ Zn(II) complexes Eur. Chem. Bull, 2013, 2, 49-57.] may be ascribed to axial ligands of Cu(II) and Zn(II) complexes. Distortion of Lu(III) coordination environment toward the axial ligand side may be due to stabilization of bond lengths (Fig. 4 ). Overall molecular shape also depends on flexibility of 3d ions, namely flexible Cu(II) and Ni(II) for flexible while rigid Zn(II) for bent ones (Fig. 5
). Overall molecular shape also depends on flexibility of 3d ions, namely flexible Cu(II) and Ni(II) for flexible while rigid Zn(II) for bent ones (Fig. 5 ).
).
Solid-state CD and Electronic Spectra
Figs. (13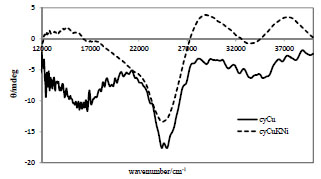 and
S8) show CD spectra for cyCu and cyCuKNi and diffuse reflectance electronic spectra for cyCu and cyCuKNi, respectively. Figs. (
S9 and
S10) show CD spectra for cyLuNi, cyLuCu, and cyLuZn and diffuse reflectance electronic spectra for cyLuNi, cyLuCu, and cyLuZn, respectively.
and
S8) show CD spectra for cyCu and cyCuKNi and diffuse reflectance electronic spectra for cyCu and cyCuKNi, respectively. Figs. (
S9 and
S10) show CD spectra for cyLuNi, cyLuCu, and cyLuZn and diffuse reflectance electronic spectra for cyLuNi, cyLuCu, and cyLuZn, respectively.
 |
Fig. (13) Solid-state CD spectra for cyCu,and cyCuKNi. |
The d-d bands (only for Ni(II) or Cu(II) moieties) and CT bands of CD spectra appeared at about 16000-18000 cm-1 and 24500-26800 cm-1, respectively. The corresponding d-d bands of electronic spectra appeared at about 18400-18600 cm-1, the changes of which are attributed to substitution and/or coordination environment of 3d metal ions [20Hayashi, T; Shibata, H; Orita, S; Akitsu, T Variety of structures of binuclear chiral schiff base Ce(III)/Pr(III)/Lu(III)-Ni(II)/Cu(II)/ Zn(II) complexes Eur. Chem. Bull, 2013, 2, 49-57., 21Akitsu, T; Hirarsuka, T; Shibata, H Chiroptical properties of 3d-4f chiral Schiff base magnetic complexes. Magnets:Types, Uses and Safety; Nova Science Publishers, 2012, pp. 69-84.].
Solid-state Fluorescence Spectra
Fig. (14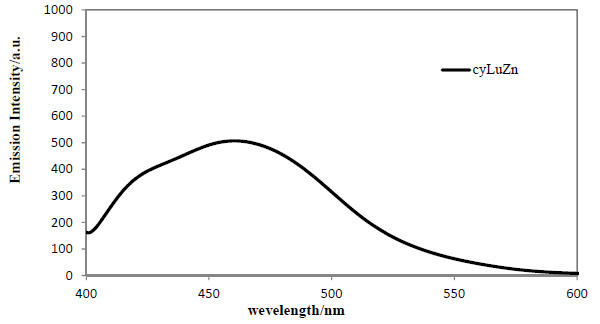 ) depicts fluorescence spectra of cyLuZn at 300 K in the solid states. Under this condition determined based on the electronic spectra, florescence peak for cyLuZn appeared at 460 nm due to the ligand. Unfortunately, the other complexes could not be observed in the fluorescence spectra in the solid state under the same conditions.
) depicts fluorescence spectra of cyLuZn at 300 K in the solid states. Under this condition determined based on the electronic spectra, florescence peak for cyLuZn appeared at 460 nm due to the ligand. Unfortunately, the other complexes could not be observed in the fluorescence spectra in the solid state under the same conditions.
 |
Fig. (14) Solid-state fluorescence spectra (λex = 360 nm) for cyLuZn at 300 K. |
CONCLUSION
Consequently, Fig. (1 ) also summarized structural features for a series of salen-type complexes mentioned. This study systematically reports on Lu(III) complexes (the smallest ion radii among 4f ions) of ten-coordinated for diLuNi [20Hayashi, T; Shibata, H; Orita, S; Akitsu, T Variety of structures of binuclear chiral schiff base Ce(III)/Pr(III)/Lu(III)-Ni(II)/Cu(II)/ Zn(II) complexes Eur. Chem. Bull, 2013, 2, 49-57.], cyLuNi, nine- and ten-coordinated for diLuCu[20Hayashi, T; Shibata, H; Orita, S; Akitsu, T Variety of structures of binuclear chiral schiff base Ce(III)/Pr(III)/Lu(III)-Ni(II)/Cu(II)/ Zn(II) complexes Eur. Chem. Bull, 2013, 2, 49-57.], and nine-coordinated for cyLuCu, cydiZn, cyLuZn. Therefore, desorption of nitrate ions is facilitated by significantly steric hindrance. As for 3d ions, axial ligands and degree of distortion from planar basal plane exhibited variety among them. Distortion of overall molecular structures depends on 3d metal ions mainly. So Ni(II) complex is planar, and Zn(II) complex is an umbrella shape toward the opposite direction to the axial ligand. However, as for flexible Cu(II) complex, distortion of overall molecular structures depends on 4f metal ions. Flexibility of coordination environment of Cu(II) ions brings about the diversification of the structures.
) also summarized structural features for a series of salen-type complexes mentioned. This study systematically reports on Lu(III) complexes (the smallest ion radii among 4f ions) of ten-coordinated for diLuNi [20Hayashi, T; Shibata, H; Orita, S; Akitsu, T Variety of structures of binuclear chiral schiff base Ce(III)/Pr(III)/Lu(III)-Ni(II)/Cu(II)/ Zn(II) complexes Eur. Chem. Bull, 2013, 2, 49-57.], cyLuNi, nine- and ten-coordinated for diLuCu[20Hayashi, T; Shibata, H; Orita, S; Akitsu, T Variety of structures of binuclear chiral schiff base Ce(III)/Pr(III)/Lu(III)-Ni(II)/Cu(II)/ Zn(II) complexes Eur. Chem. Bull, 2013, 2, 49-57.], and nine-coordinated for cyLuCu, cydiZn, cyLuZn. Therefore, desorption of nitrate ions is facilitated by significantly steric hindrance. As for 3d ions, axial ligands and degree of distortion from planar basal plane exhibited variety among them. Distortion of overall molecular structures depends on 3d metal ions mainly. So Ni(II) complex is planar, and Zn(II) complex is an umbrella shape toward the opposite direction to the axial ligand. However, as for flexible Cu(II) complex, distortion of overall molecular structures depends on 4f metal ions. Flexibility of coordination environment of Cu(II) ions brings about the diversification of the structures.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
SUPPLEMENTARY MATERIAL
CCDC 978339-978345 contain the supplementary crystallographic data. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: (+44) 1223-336-033; or E-mail: deposit@ccdc.cam.ac.uk
ACKNOWLEDGEMENTs
This work was supported by Research Foundation for Opto-Science and Technology.
REFERENCES
| [1] | Bryliakov, KP; Talsi, EP Cr(III)(salen)Cl catalyzed asymmetric epoxidations: insight into the catalytic cycle Inorg. Chem, 2003, 42, 7258-7265. [http://dx.doi.org/10.1021/ic034578w] |
| [2] | Jain, S; Zheng, X; Jones, CW; Weck, M; Davis, RJ Importance of counterion reactivity on the deactivation of co-salen catalysts in the hydrolytic kinetic resolution of epichlorohydrin Inorg. Chem, 2007, 46, 8887-8896. [http://dx.doi.org/10.1021/ic700782f] |
| [3] | Adao, P; Pessoa, JC; Henriques, RT; Kuznetsov, ML; Avecilla, F; Maurya, MR; Kumar, U; Correia, I Synthesis, characterization, and application of vanadium-salan complexes in oxygen transfer reactions Inorg. Chem, 2009, 48, 3542-3561. [http://dx.doi.org/10.1021/ic8017985] |
| [4] | Oxford, GAE; Snurr, RQ; Broadbelt, LJ Hybrid quantum mechanics/molecular mechanics investigation of (salen)Mn for use in metal-organic frameworks Ind. Eng. Chem. Res, 2010, 49, 10965-10973. [http://dx.doi.org/10.1021/ie100165j] |
| [5] | Zhao, ZP; Li, MS; Zhang, JY; Li, HN; Zhu, PP; Liu, WF New chiral catalytic membranes created by coupling uvphotografting with covalent immobilization of salen–Co(III) for hydrolytic kinetic resolution of racemic epichlorohydrin Ind. Eng. Chem.
Res, 2012, 51, 9531-9539. [http://dx.doi.org/10.1021/ie3011935] |
| [6] | Kurahashi, T; Hada, M; Fujii, H Critical role of external axial ligands in chirality amplification of trans-cyclohexane-1,2-diamine in salen complexes J. Am. Chem. Soc, 2009, 131, 12394-12405. [http://dx.doi.org/10.1021/ja904635n] |
| [7] | Nielsen, LPC; Zuend, SJ; Ford, DD; Jacobsen, EN Mechanistic basis for high reactivity of (salen)Co–OTs in the hydrolytic kinetic resolution of terminal epoxides Org. Chem, 2012, 77, 2486-2495. [http://dx.doi.org/10.1021/jo300181f] |
| [8] | Xu, L; Zhang, Q; Hou, G; Chen, P; Li, G; Pajerowski, DM; Dennis, CL Syntheses, structures, and magnetic properties of salen type Cu–Gd dimer and hexamer complexes with strong ferromagnetic interactions Polyhedron, 2013, 52, 91-95. |
| [9] | Sui, Y; Liu, DS; Hu, RH; Huang, JG One-dimensional zigzag chain of Cu–Gd coordination polymers derived from chiral hexadentate Schiff base ligands: Synthesis, structure and magnetic properties Inorg. Chem. Acta, 2013, 395, 225-229. [http://dx.doi.org/10.1016/j.ica.2012.10.035] |
| [10] | Koner, R; Lee, GH; Wang, Y; Wei, HH; Mohanta, S Two new diphenoxo-bridged discrete dinuclear Cu(II)Gd(III) compounds with cyclic diimino moieties: syntheses, structures, and magnetic properties Eur. J. Inorg. Chem, 2005, 8, 1500-1505. [http://dx.doi.org/10.1002/ejic.200400858] |
| [11] | Costes, JP; Dahan, F; Dupuis, A Influence of anionic ligands (x) on the nature and magnetic properties of dinuclear LCuGdX3.nH2O complexes (LH2 standing for tetradentate schiff base ligands deriving from 2-hydroxy-3-methoxybenzaldehyde and X being Cl, N3C2, and CF3COO) Inorg. Chem, 2000, 39, 165-168. [http://dx.doi.org/10.1021/ic990865h] |
| [12] | Margeat, O; Acroix, PG; Costes, JP; Donnadieu, B; Lepetit, C Synthesis, structures, and physical properties of copper(II)-gadolinium(III) complexes combining ferromagnetic coupling and quadratic nonlinear optical properties Inorg. Chem, 2004, 43, 4743-4750. [http://dx.doi.org/10.1021/ic049801j] |
| [13] | Jana, A; Majumder, S; Carrella, L; Nayak, M; Weyhermueller, T; Dutta, S; Schollmeyer, D; Rentschler, E; Koner, R; Mohanta, S Syntheses, structures, and magnetic properties of diphenoxo-bridged Cu(II)Ln(III) and NiII(low-spin)Ln(III) compounds derived from a compartmental ligand (Ln = Ce-Yb) Inorg. Chem, 2010, 49, 9012-9025. [http://dx.doi.org/10.1021/ic101445n] |
| [14] | Wong, WK; Yang, X; Jones, RA; Rivers, JH; Lynch, V; Lo, WK; Xiao, D; Oye, MM; Holmes, AL Multinuclear luminescent schiff-base Zn-Nd sandwich complexes Inorg. Chem, 2006, 45, 4340-4345. [http://dx.doi.org/10.1021/ic051866e] |
| [15] | Pasatoiu, TD; Madalan, AM; Kumke, MU; Tiseanu, C; Andruh, M Temperature switch of LMCT role: from quenching to sensitization of europium emission in a Zn(II)-Eu(III) binuclear complex Inorg. Chem, 2010, 49, 2310-2315. [http://dx.doi.org/10.1021/ic902169s] |
| [16] | Pasatoiu, TD; Sutter, JP; Madalan, AM; Zohra, F; Fellah, C; Duhayon, C; Andruh, M Preparation, crystal structures, and magnetic features for a series of dinuclear [Ni(II)Ln(III)] schiff-base complexes: evidence for slow relaxation of the magnetization for the Dy(III) derivative Inorg. Chem, 2011, 50, 5890-5898. |
| [17] | Andruh, M; Costes, JP; Diaz, C; Gao, S 3d-4f combined chemistry: synthetic strategies and magnetic properties Inorg. Chem, 2009, 48, 3342-3359. [http://dx.doi.org/10.1021/ic801027q] |
| [18] | Pasatoiu, TD; Tiseanu, C; Madalan, AM; Jurca, B; Duhayon, C; Sutter, JP; Andruh, M Study of the luminescent and magnetic properties of a series of heterodinuclear [Zn(II)Ln(III)] complexes Inorg. Chem, 2011, 50, 5879-5889. [http://dx.doi.org/10.1021/ic200426w] |
| [19] | Lo, WK; Wong, WK; Wong, WY; Guo, J; Yeung, KT; Cheng, YK; Yang, X; Jones, RA Heterobimetallic Zn(II)-Ln(III) phenylene-bridged schiff base complexes, computational studies, and evidence for singlet energy transfer as the main pathway in the sensitization of near-infrared Nd3+ luminescence Inorg. Chem, 2006, 45, 9315-9325. |
| [20] | Hayashi, T; Shibata, H; Orita, S; Akitsu, T Variety of structures of binuclear chiral schiff base Ce(III)/Pr(III)/Lu(III)-Ni(II)/Cu(II)/ Zn(II) complexes Eur. Chem. Bull, 2013, 2, 49-57. |
| [21] | Akitsu, T; Hirarsuka, T; Shibata, H Chiroptical properties of 3d-4f chiral Schiff base magnetic complexes. Magnets:Types, Uses and Safety; Nova Science Publishers, 2012, pp. 69-84. |
| [22] | Okamoto, Y; Nidaira, K; Akitsu, T Environmental dependence of artifact CD peaks of chiral schiff base 3d-4f complexes in soft mater PMMA matrix Int. J. Mol. Sci, 2011, 12, 6966-6979. [http://dx.doi.org/10.3390/ijms12106966] |
| [23] | Akitsu, T New attempt of empirical approach for magnetic interactions: several examples of 3d-4f [Ln(DMA)2(H2O)4M(CN)6·5H2O]n bimetallic assemblies (DMA = N,N-dimethylacetamide) Asian. Chem. Lett, 2010, 14, 53-62. |
| [24] | Sheldrick, GM A short history of SHELX Acta. Crystallogr. A, 2008, 64, 112-22. [http://dx.doi.org/10.1107/S0108767307043930] |