- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Isolation of Phthalates and Terephthalates from Plant Material – Natural Products or Contaminants?
Thies Thiemann1, *
Abstract
Dialkyl phthalates have been used as plasticizers in polymers for decades. As mobile, small weight molecules, phthalates have entered the environment, where they have become ubiquitous. On the other hand, phthalates continue to be isolated from natural sources, plants, bacteria and fungi as bona fide natural products. Here, doubt remains as to whether the phthalates represent actual natural products or whether they should all be seen as contaminants of anthropogenic origin. The following article will review the material as presented in the literature.
Article Information
Identifiers and Pagination:
Year: 2021Volume: 8
First Page: 1
Last Page: 36
Publisher Id: CHEM-8-1
DOI: 10.2174/1874842202108010001
Article History:
Received Date: 28/07/2020Revision Received Date: 13/10/2020
Acceptance Date: 03/11/2020
Electronic publication date: 02/03/2021
Collection year: 2021
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at Department of Chemistry, College of Science, United Arab Emirates University, PO Box 15551, Al Ain, United Arab Emirates; E-mails: thies@uaeu.ac.ae; thiesthiemann@yahoo.de
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 28-07-2020 |
Original Manuscript | Isolation of Phthalates and Terephthalates from Plant Material – Natural Products or Contaminants? | |
1. INTRODUCTION
Phthalates are ubiquitous compounds that have been used as plasticizers in polymers for many decades, starting in the late 1920s – early 1930s, where a number of patents showed the rising interest in these products at the time [1Denune, Y.H. (Schaack Bros. Chem. Works), Esters of hexanol. US Pat. 1702188A, 1928., 2De Witt, G.G.; Eastby, L.W. (EI Du Pont de Nemours Co.), Esters and process for producing them. US Pat. 1993736A, 1931.] and where phthalates started to replace camphor-based plasticizers. Phthalates have been especially associated with the emergence of plastics such as polyvinyl chloride [3Lorz, P.M.; Towae, F.K.; Enke, W.; Jäckh, R.; Bhargava, N.; Hillesheim, W. Phthalic Acid and Derivatives, 2007,
[http://dx.doi.org/10.1002/14356007.a20_181.pub2] ]. At the same time, phthalates, many of which are environmentally quite mobile, have become pervasive pollutants of our biosphere, entering both water and soil. Although especially the low-MW phthalates can be readily degraded hydrolytically [4Huang, J.; Nkrumah, P.N.; Li, Y.; Appiah-Sefah, G. Chemical behavior of phthalates under abiotic conditions in landfills. Rev. Environ. Contam. Toxicol., 2013, 224, 39-52.
[http://dx.doi.org/10.1007/978-1-4614-5882-1_2] [PMID: 23232918] ], photochemically [5Gmurek, M.; Olak-Kucharczyk, M.; Ledakowicz, S. Photochemical decomposition of endocrine disrupting compounds - A review. Chem. Eng. J., 2017, 310, 437-456.
[http://dx.doi.org/10.1016/j.cej.2016.05.014] ] and microbially [6Boll, M.; Geiger, R.; Junghare, M.; Schink, B. Microbial degradation of phthalates: biochemistry and environmental implications. Environ. Microbiol. Rep., 2020, 12(1), 3-15.
[http://dx.doi.org/10.1111/1758-2229.12787] [PMID: 31364812] ], detectable amounts of phthalates can be found almost everywhere, including in our diet [7Wang, Y.; Zhu, H.; Kannan, K. A review of biomonitoring of phthalate exposures. Toxics, 2019, 7(2), 21.
[http://dx.doi.org/10.3390/toxics7020021] [PMID: 30959800] ]. Low-MW phthalates are dermally absorbed relatively easily. This leads to the identification of phthalates and phthalate derivatives in humans, easily detectable in urine [8Philips, E.M.; Santos, S.; Steegers, E.A.P.; Asimakopoulos, A.G.; Kannan, K.; Trasande, L.; Jaddoe, V.W. V. Maternal bisphenol and phthalate urine concentrations and weight gain during pregnancy Environ. Intern., 2020, 105342..], breast milk [9Fan, J.C.; Ren, R.; Jin, Q.; He, H.L.; Wang, S.T. Detection of 20 phthalate esters in breast milk by GC-MS/MS using QuEChERS extraction method. Food Addit. Contam., A, 2019, 36, 1551-1558.], and blood [10Albro, P.W.; Corbett, J.T. Distribution of di- and mono-(2-ethylhexyl) phthalate in human plasma. Transfusion, 1978, 18(6), 750-755.
[http://dx.doi.org/10.1046/j.1537-2995.1978.18679077962.x] [PMID: 83042] ]. It has been found that the concentrations of phthalates in the air are often higher in urban than in rural areas [11Rudel, R.A.; Dodson, R.E.; Perovich, L.J.; Morello-Frosch, R.; Camann, D.E.; Zuniga, M.M.; Yau, A.Y.; Just, A.C.; Brody, J.G. Semivolatile endocrine-disrupting compounds in paired indoor and outdoor air in two northern California communities. Environ. Sci. Technol., 2010, 44(17), 6583-6590.
[http://dx.doi.org/10.1021/es100159c] [PMID: 20681565] , 12He, M.J.; Lu, J.F.; Ma, J.Y.; Wang, H.; Du, X.F. Organophosphate esters and phthalate esters in human hair from rural and urban areas, Chongqing, China: Concentrations, composition profiles and sources in comparison to street dust. Environ. Pollut., 2018, 237, 143-153.
[http://dx.doi.org/10.1016/j.envpol.2018.02.040] [PMID: 29482020] ]. Nevertheless, phthalates have been identified in soil, for instance, as leachates from plastic mulching/plastic film greenhouses [13Shi, M.; Sun, Y.; Wang, Z.; He, G.; Quan, H.; He, H. Plastic film mulching increased the accumulation and human health risks of phthalate esters in wheat grains. Environ. Pollut., 2019, 250, 1-7.
[http://dx.doi.org/10.1016/j.envpol.2019.03.064] [PMID: 30981178] , 14Wang, J.; Chen, G.; Christie, P.; Zhang, M.; Luo, Y.; Teng, Y. Occurrence and risk assessment of phthalate esters (PAEs) in vegetables and soils of suburban plastic film greenhouses. Sci. Total Environ., 2015, 523, 129-137.
[http://dx.doi.org/10.1016/j.scitotenv.2015.02.101] [PMID: 25863503] ], and also because they are used as agricultural adjuvants in pesticides [15Iida, T.; Yanagisawa, K. (Sumitomo Chem. Co., Ltd., Japan) Agrochemical solid formulation, method for preparation and use in pest control, FR 3047639, 2017.] (Fig. 1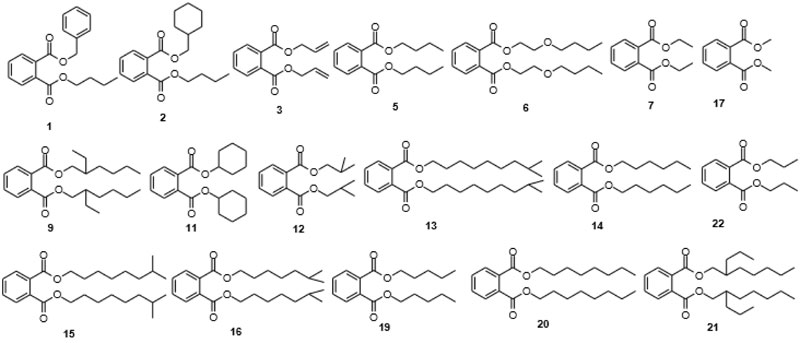 ).
).
Phthalates have been isolated from many plants, from algae, bacteria and fungi. In the natural product isolations, oftentimes, the phthalates were looked upon as plant secondary metabolites. As often is the case, after isolation of the products from the plant, the bioactivity of the compounds was studied. In regard to the phthalates, multiple researchers have remarked on various biological activities of the molecules. Intriguing is the comparison of these studies and the investigations of different governmental organizations on the health effects of phthalates as components of consumer products. Sometimes, there can be a disconnect when scientists in closely related but highly specialized, clearly demarcated research areas do not cross-disseminate their fields with information flow. In the case of the study of phthalates, academically, there seem to be rather different areas of research and development that little overlap and often show little data transfer: the development of new product formulations with phthalates, the analytical detection and quantification of phthalates in our daily products and in our environment, often coupled with the assessment of health implications, the study of the degradation of phthalates by bacteria and other microorganisms and lastly the isolation of phthalates as possible natural products from plants and other organisms. In view of the ubiquity of phthalates in our environment, few research papers on the isolation of phthalates from plants have asked whether these products might not be of anthropogenic origin. Nevertheless, two prior review articles have looked at the possibility that at least some of the isolated phthalates could indeed be natural products [16Zhang, H.; Hua, Y.; Chen, J.; Li, X.; Bai, X.; Wang, H. Organism-derived phthalate derivatives as bioactive natural products J. Environ. Sci., C, 2018, 36125, 17Ortiz, A.; Sansinenea, E. Di-2-ethylhexylphthalate may be a natural product, rather than a pollutant. J. Chem. (Hindawi), 2018.
[http://dx.doi.org/10.1155/2018/6040814] ], with a further essay asking the question of whether medicinal plants are polluted with phthalates [18Saeidnia, S.; Abdollahi, M. Are medicinal plants polluted with phthalates? Daru, 2013, 21(1), 43-45.
[http://dx.doi.org/10.1186/2008-2231-21-43] [PMID: 23718122] ]. In the current contribution, the author reassesses with the aid of published research articles how far phthalates isolated from organisms are indeed natural products or whether they can be seen mostly as contaminants. (Fig. 2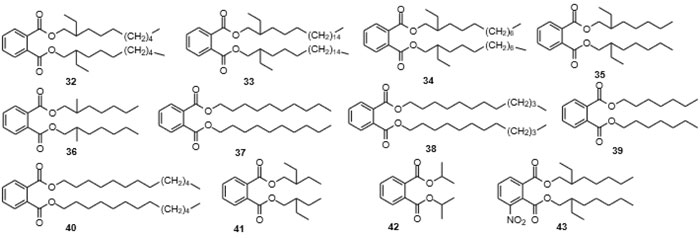 )
)
1.1. Phthalates – Trends in Production and Usage; Health Concerns
Of the roughly 60 different commercially produced phthalates, there are 26 phthalates that are relatively commonly used. The usage of the most common phthalates is shown in Table 1. In 2001, the breakdown of use of different types of phthalates in Europe was reported as follows: di(ethylhexyl) phthalate (DEHP, 9), 51%; diisodecyl phthalate (DIDP), 21%; diisononyl phthalate (DINP, 15), 11%; dibutyl phthalate (5), 2% and others, 17% [19Murphy, J. Additives for plastics – Handbook., (2nd ed. ), 2001,
]. At that time, about 8.4 million tons of plasticizer were produced every year. Of DEHP (9), 3.0 million tons were produced in 2006 alone. In the late 2010s, many of the C3-C6 phthalates were replaced with higher MW C9-C13 phthalates [20Phthalates | Assessing and Managing Chemicals Under TSCA. www.epa.gov2012. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/phthalates Retrieved on July 1stsup>, 2020], with DEHP (9) still abundantly being produced, but in different parts of the world, more and more being replaced by DINP (15). DINP (15) as a “high” phthalate has a longer residency time in plastics, which gives the plastics better durability. It must be noted that while oftentimes the higher branched phthalates are produced as a mixture of isomers, only one isomer for each phthalate is shown in the drawings and tables in the article. Overall, from 2010 onwards, non-phthalate plasticizers are increasingly invading the market. These include terephthalates (Fig. 3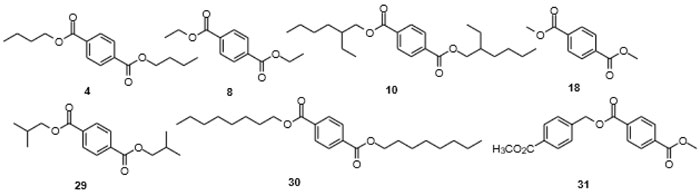 ), epoxy, trimellitates, and some aliphatics/cycloaliphatics (mainly hydrogenated phtha- lates such as diisononyl cyclohexane-1,2-dicarboxylate [DINCH] which is a mixture of isomers which includes 44), alkane α,ω-dicarboxylates such as di(2-ethylhexyl) adipate (DEHA, 49), and alkane tricarboxylates such as acetyl tri-n-butyl citrate (ATBC, 50) and biomass-derived triglycerides [21Jia, P.; Xia, H.; Tang, K.; Zhou, Y. Plasticizers derived from biomass resources: a short review. Polymers (Basel), 2018, 10(12), 1303-1330.
), epoxy, trimellitates, and some aliphatics/cycloaliphatics (mainly hydrogenated phtha- lates such as diisononyl cyclohexane-1,2-dicarboxylate [DINCH] which is a mixture of isomers which includes 44), alkane α,ω-dicarboxylates such as di(2-ethylhexyl) adipate (DEHA, 49), and alkane tricarboxylates such as acetyl tri-n-butyl citrate (ATBC, 50) and biomass-derived triglycerides [21Jia, P.; Xia, H.; Tang, K.; Zhou, Y. Plasticizers derived from biomass resources: a short review. Polymers (Basel), 2018, 10(12), 1303-1330.
[http://dx.doi.org/10.3390/polym10121303] [PMID: 30961228] ]). The market shares of these are forecasted to grow strongly as they continue to replace phthalates [22Plasticizers - Chemical Economics Handbook (CEH). IHS Markit, 2018.]. It is estimated that in 2005, 88% of the plasticizers produced were phthalates. The share of admittedly, a growing market declined to 65% in 2017, and it is predicted to decline even further to about 60% in 2022 [22Plasticizers - Chemical Economics Handbook (CEH). IHS Markit, 2018.].
 |
Fig. (1) Structures of some of the industrially most important phthalates. |
 |
Fig. (2) Phthalates commonly isolated from natural sources. |
 |
Fig. (3) Terephthalates isolated from natural sources. |
This is because of a growing disquiet that phthalates can have harmful effects. DEHP (9) has been found to be an endocrine disruptor [23Barakat, R.; Lin, P.C.; Park, C.J.; Zeineldin, M.; Zhou, S.; Rattan, S.; Brehm, E.; Flaws, J.A.; Ko, C.J. Germline-dependent transmission of male reproductive traits induced by an endocrine disruptor, di-2-ethylhexyl phthalate, in future generations. Sci. Rep., 2020, 10(1), 5705.
[http://dx.doi.org/10.1038/s41598-020-62584-w] [PMID: 32235866] ] and possible carcinogen [24Kim, J.H. Di(2-ethylhexyl) phthalate promotes lung cancer cell line A549 progression via Wnt/β-catenin signaling. J. Toxicol. Sci., 2019, 44(4), 237-244.
[http://dx.doi.org/10.2131/jts.44.237] [PMID: 30944277] ], and DINP (15) has also been put on the list of possible carcinogens [25Tas, I.; Zou, R.; Park, S.Y.; Yang, Y.; Gamage, C.D.B.; Son, Y.J.; Paik, M.J.; Kim, H. Inflammatory and tumorigenic effects of environmental pollutants found in particulate matter on lung epithelial cells. Toxicol. In Vivo, 2019, 59, 300-311.] by the California Office of Environmental Health Hazard Assessment (OEHHA) in 2013. In fact, butyl benzyl phthalate (BBzP, 1), dibutyl phthalate (DnBP, 5), diethyl phthalate (DEP, 7), diisobutyl phthalate (DiBP, 12), diisononyl phthalate (DINP, 15), di-n-octyl phthalate (DnOP, 20), dipentyl phthalate (DNPP, 9), di-isohexyl phthalate, dicyclohexyl phthalate (DcHP, 11), and di-isoheptyl phthalate have all been associated with illnesses and disorders as diverse as attention-deficit hyperactivity disorder [26Hu, D.; Wang, Y.X.; Chen, W.J.; Zhang, Y.; Li, H.H.; Xiong, L.; Zhu, H.P.; Chen, H.Y.; Peng, S.X.; Wan, Z.H.; Zhang, Y.; Du, Y.K. Associations of phthalates exposure with attention deficits hyperactivity disorder: A case-control study among Chinese children. Environ. Pollut., 2017, 229, 375-385.
[http://dx.doi.org/10.1016/j.envpol.2017.05.089] [PMID: 28614761] ], breast cancer [27Zuccarello, P.; Oliveri Conti, G.; Cavallaro, F.; Copat, C.; Cristaldi, A.; Fiore, M.; Ferrante, M. Implication of dietary phthalates in breast cancer. A systematic review. Food Chem. Toxicol., 2018, 118, 667-674.
[http://dx.doi.org/10.1016/j.fct.2018.06.011] [PMID: 29886235] ], obesity [28Zhang, Y.; Dong, T.; Hu, W.; Wang, X.; Xu, B.; Lin, Z.; Hofer, T.; Stefanoff, P.; Chen, Y.; Wang, X.; Xia, Y. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environ. Int., 2019, 123, 325-336.
[http://dx.doi.org/10.1016/j.envint.2018.11.076] [PMID: 30557812] ] and type II diabetes [29Duan, Y.; Wang, L.; Han, L.; Wang, B.; Sun, H.; Chen, L.; Zhu, L.; Luo, Y. Exposure to phthalates in patients with diabetes and its association with oxidative stress, adiponectin, and inflammatory cytokines. Environ. Int., 2017, 109, 53-63.
[http://dx.doi.org/10.1016/j.envint.2017.09.002] [PMID: 28938100] ], neurodevelopmental issues [30Lopez-Carrillo, L.; Cebrian, M.E. Cognitive function.Effects of Persistent and Bioactive Organic Pollutants on Human Health., 2013, 400-420.
[http://dx.doi.org/10.1002/9781118679654.ch15] ], behavioral issues, autism spectrum disorders [31Shin, H.M.; Schmidt, R.J.; Tancredi, D.; Barkoski, J.; Ozonoff, S.; Bennett, D.H.; Hertz-Picciotto, I. Prenatal exposure to phthalates and autism spectrum disorder in the MARBLES study. Environ. Health, 2018, 17(1), 85.
[http://dx.doi.org/10.1186/s12940-018-0428-4] [PMID: 30518373] ], altered reproductive development [32Singh, A.; Kumar, R.; Singh, J.K. Singh; Tanuja, K.S. Di (2-ethylhexyl) phthalate induced toxicological effects on reproductive system of female mice mus-musculus. J. Ecophysiol. Occup. Health, 2019, 19, 71-75.] and male fertility issues [33Herr, C.; zur Nieden, A.; Koch, H.M.; Schuppe, H.C.; Fieber, C.; Angerer, J.; Eikmann, T.; Stilianakis, N.I. Urinary di(2-ethylhexyl)phthalate (DEHP)--metabolites and male human markers of reproductive function. Int. J. Hyg. Environ. Health, 2009, 212(6), 648-653.
[http://dx.doi.org/10.1016/j.ijheh.2009.08.001] [PMID: 19733116] ]. It must be said, however, that in many instances, insufficient data is available to make irrefutable statements on the health impacts of phthalates. Some of the compounds replacing phthalates fare a little better. These include the increasingly used terephthalates such as di(2-ethylhexyl) terephthalate. In addition, dimethyl terephthalate (DMT, 18) which is a starting material in the production of terephthalate based plasticizers as well as in the production of polyalkyl terephthalates [34Gu, J.D. Microbial colonization of polymeric materials for space applications and mechanisms of biodeterioration: a review. Int. Biodeterior. Biodegradation, 2007, 59, 170-179.
[http://dx.doi.org/10.1016/j.ibiod.2006.08.010] , 35Li, J.X.; Gu, J.D.; Pan, L. Transformation of dimethyl phthalate, dimethyl isophthalate and dimethyl terephthalate by Rhodococcus rubber Sa and modeling the processes using the modified Gompertz model. Int. Biodeterior. Biodegradation, 2005, 55, 223-232.
[http://dx.doi.org/10.1016/j.ibiod.2004.12.003] ], especially in the synthesis of polyethylene terephthalate (PET), polytrimethylene terephthalate (PTT), and polybutylene terephthalate (PBT), is looked at as a potential carcinogen and an irritant to skin, eyes and the respiratory tract. As is the case with many monomers and small-molecule starting materials for polymers, also DMT can be found in small concentrations in the respective polymers (Figs. 4 and 5
and 5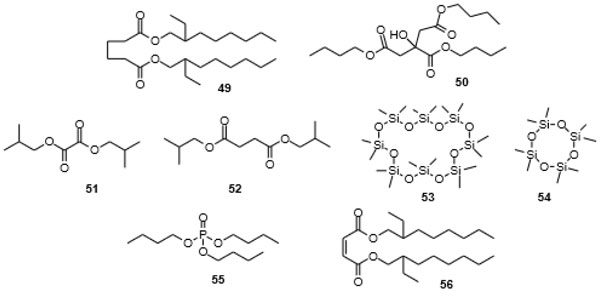 ).
).
1.2. Phthalates in Agricultural Use – Phthalate Content in Soil and Agricultural Produce
There is extensive literature on phthalate content in agricultural produce, whether it be tomatoes grown after biosolids application [36Sablayrolles, C.; Silvestre, J.; Lhoutellier, C.; Montrejaud-Vignoles, M. Phthalates uptake by tomatoes after biosolids application: worst case and operational practice in greenhouse conditions. Fresenius Environ. Bull., 2013, 22, 1061-1069.] or radishes grown with sewage sludge and compost application [37Cai, Q.Y.; Mo, C.H.; Wu, Q.T.; Zeng, Q.Y. Polycyclic aromatic hydrocarbons and phthalic acid esters in the soil-radish (Raphanus sativus) system with sewage sludge and compost application. Bioresour. Technol., 2008, 99(6), 1830-1836.
[http://dx.doi.org/10.1016/j.biortech.2007.03.035] [PMID: 17502135] ]. Extensive developments in analytical chemistry have led to reliable measurement methods for phthalate contents in different types of produce [38Cao, X.L.; Zhao, W.; Dabeka, R. Di-(2-ethylhexyl) adipate and 20 phthalates in composite food samples from the 2013 Canadian Total Diet Study. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess., 2015, 32(11), 1893-1901.
[http://dx.doi.org/10.1080/19440049.2015.1079742] [PMID: 26359692] ] and in processed and packaged foods [39Sannino, A. Development of a gas chromatographic/mass spectrometric method for determination of phthalates in oily foods. J. AOAC Int., 2010, 93(1), 315-322.
[http://dx.doi.org/10.1093/jaoac/93.1.315] [PMID: 20334193] , 40Cocchieri, R.A. Occurrence of phthalate esters in Italian packaged foods. J. Food Prot., 1986, 49(4), 265-266.
[http://dx.doi.org/10.4315/0362-028X-49.4.265] [PMID: 30959656] ]. The occurrence of phthalates in agricultural soils around the world [41Gibson, R.; Wang, M.J.; Padgett, E.; Beck, A.J. Analysis of 4-nonylphenols, phthalates, and polychlorinated biphenyls in soils and biosolids. Chemosphere, 2005, 61(9), 1336-1344.
[http://dx.doi.org/10.1016/j.chemosphere.2005.03.072] [PMID: 15979687] , 42Vikelsøe, J.; Thomsen, M.; Carlsen, L. Phthalates and nonylphenols in profiles of differently dressed soils. Sci. Total Environ., 2002, 296(1-3), 105-116.
[http://dx.doi.org/10.1016/S0048-9697(02)00063-3] [PMID: 12398330] ], with many studies originating in China [43Kong, S.; Ji, Y.; Liu, L.; Chen, L.; Zhao, X.; Wang, J.; Bai, Z.; Sun, Z. Diversities of phthalate esters in suburban agricultural soils and wasteland soil appeared with urbanization in China. Environ. Pollut., 2012, 170, 161-168.
[http://dx.doi.org/10.1016/j.envpol.2012.06.017] [PMID: 22813629] -45Li, K.; Ma, D.; Wu, J.; Chai, C.; Shi, Y. Distribution of phthalate esters in agricultural soil with plastic film mulching in Shandong Peninsula, East China. Chemosphere, 2016, 164, 314-321.
[http://dx.doi.org/10.1016/j.chemosphere.2016.08.068] [PMID: 27596820] ], has been sufficiently established, where DEHP (9) and DnBP (5) are the most abundant phthalates found. The use of plastic mulching in agriculture is still a widespread technique to suppress weed growth and to contain water needs. While biodegradable polymeric films have been advertised, the by far most often used material is both high and low-density polyethene (LDPE and HDPE). Phthalates are mulch PE additives, where it has been shown that these phthalates are released partially into the soil [46Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ., 2016, 550, 690-705.
[http://dx.doi.org/10.1016/j.scitotenv.2016.01.153] [PMID: 26849333] ]. The delivery of phthalates from the plastic film covers of the agricultural plants themselves has been studied in some detail [47Wang, K.; Song, N.; Cui, M.; Shi, Y. Phthalate esters migration from two kinds of plastic films and the enrichment in peanut plant. Fresenius Environ. Bull., 2017, 26, 4409-4415.]. In addition, sewage sludge [42Vikelsøe, J.; Thomsen, M.; Carlsen, L. Phthalates and nonylphenols in profiles of differently dressed soils. Sci. Total Environ., 2002, 296(1-3), 105-116.
[http://dx.doi.org/10.1016/S0048-9697(02)00063-3] [PMID: 12398330] ], agrochemicals [48Fernández, M.A.; Gómara, B.; González, M.J. The Handbook of Environmental Chemistry, 2012, , 337-374.], wastewater irrigation [49Zhang, Y.; Liang, Q.; Gao, R.; Hou, H.; Tan, W.; He, X.; Zhang, H.; Yu, M.; Ma, L.; Xi, B.; Wang, X.; Ma, L.; Xi, B.; Wang, X. Contamination of phthalate esters (PAEs) in typical wastewater-irrigated agricultural soils in Hebei, North China. PLoS One, 2015, 10(9)e0137998
[http://dx.doi.org/10.1371/journal.pone.0137998] [PMID: 26360905] ] and the atmosphere [50Ligocki, M.P.; Leuenberger, C.; Pankow, J.F. Trace organic compounds in rain—II. Gas scavenging of neutral organic compounds. Atmos. Environ., 1985, 19, 1609-1617.
[http://dx.doi.org/10.1016/0004-6981(85)90213-6] , 51Ligocki, M.P.; Leuenberger, C.; Pankow, J.F. Trace organic compounds in rain—III. Particle scavenging of neutral organic compounds. Atmos. Environ., 1985, 19, 1619-1626.
[http://dx.doi.org/10.1016/0004-6981(85)90214-8] ] itself deliver phthalates to the soil [52Hongjun, Y.; Wenjun, X.; Qing, L.; Jingtao, L.; Hongwen, Y.; Zhaohua, L. Distribution of phthalate esters in topsoil: a case study in the Yellow River Delta. China. Environ. Monit. Assess., 2013, 185, 8489-8500.
[http://dx.doi.org/10.1007/s10661-013-3190-7] [PMID: 23609921] -54He, L.; Gielen, G.; Bolan, N.S.; Zhang, X.; Qin, H.; Huang, H.; Wang, H. Contamination and remediation of phthalic acid esters in agricultural soils in China: a review. Agron. Sustain. Dev., 2014, 35, 519-534.
[http://dx.doi.org/10.1007/s13593-014-0270-1] ]. Especially, the phthalate uptake of plants from sludge has been a worry [55Gonzalez-Villa, F.J.; Saiz-Jimenez, C.; Martin, F. Identification of free organic chemicals found in composted municipal refuse. J. Environ. Qual., 1982, 11, 251-254.
[http://dx.doi.org/10.2134/jeq1982.00472425001100020021x] ], where Shea et al. [56Shea, P.J.; Weber, J.B.; Overcash, M.R. Uptake and phytotoxicity of di-n-butyl phthalate in corn (Zea mays). Bull. Environ. Contam. Toxicol., 1982, 29(2), 153-158.
[http://dx.doi.org/10.1007/BF01606143] [PMID: 7126902] ] reported a di-n-butyl phthalate (DnBP, 5) uptake in corn at 0.32 ppm from soil contaminated with 100 ppm DnBP (5). The removal of phthalates from agricultural crop derived extracts designated for food or pharmaceutical use is sometimes seen as a necessity and different processes have been developed to that regard [57Yi, N.; Zhao, Y.; Li, J.; Wei, Z.; Zhao, Y. (Hunan Zhongmao Biotechnology Co., Ltd.) Method for removing plasticizer from plant-derived flavonoid extract CN 109320561, 2019.].
Phthalates can be cleaved to monophthalates and to phthalic acid by UV light [58Hankett, J.M.; Collin, W.R.; Chen, Z. Molecular structural changes of plasticized PVC after UV light exposure. J. Phys. Chem. B, 2013, 117(50), 16336-16344.
[http://dx.doi.org/10.1021/jp409254y] [PMID: 24283894] ], but this is a slow process, at least in water with photochemical half-lives of over 100 days (for butyl benzyl phthalate, 1), 3 years (for dimethyl phthalate, 17) and 1000 years [for bis(ethylhexyl)phthalate, 9] [59Gledhill, W.E.; Kaley, R.G.; Adams, W.J.; Hicks, O.; Michael, P.R.; Saeger, V.W.; Leblanc, G.A. An environmental safety assessment of butyl benzyl phthalate. Environ. Sci. Technol., 1980, 14(3), 301-305.
[http://dx.doi.org/10.1021/es60163a001] [PMID: 22276719] , 60Staples, C.A.; Peterson, D.R.; Parkerton, T.F.; Adams, W.J. The environmental fate of phthalates esters: a literature review. Chemosphere, 1997, 35, 667-749.
[http://dx.doi.org/10.1016/S0045-6535(97)00195-1] ]. However, as the phthalates penetrate the soil, the degradation pathway open to them is the aerobic or anaerobic ester hydrolysis with subsequent cleavage of the aromatic ring system [61Cartwright, C.D.; Thompson, I.P.; Burns, R.G. Degradation and impact of phthalate plasticizers on soil microbial communities. Environ. Toxicol. Chem., 2000, 19, 1253-1261.
[http://dx.doi.org/10.1002/etc.5620190506] , 62Nallii, S.; Cooper, D.G.; Nicell, J.A. Biodegradation of plasticizers by Rhodococcus rhodochrous. Biodegradation, 2002, 13(5), 343-352.
[http://dx.doi.org/10.1023/A:1022313810852] [PMID: 12688586] ]. Short-chain phthalates such as diethyl phthalate (DEP, 7) are degraded more easily by microorganisms than longer chain phthalates such as bis(2-ethylhexyl) phthalate (DEHP, 9), which can only be co-metabolically degraded in the presence of an additional carbon source [61Cartwright, C.D.; Thompson, I.P.; Burns, R.G. Degradation and impact of phthalate plasticizers on soil microbial communities. Environ. Toxicol. Chem., 2000, 19, 1253-1261.
[http://dx.doi.org/10.1002/etc.5620190506] , 62Nallii, S.; Cooper, D.G.; Nicell, J.A. Biodegradation of plasticizers by Rhodococcus rhodochrous. Biodegradation, 2002, 13(5), 343-352.
[http://dx.doi.org/10.1023/A:1022313810852] [PMID: 12688586] ]. Different microbial strains have been isolated and used to remove phthalates from different matrices, such as natural water (sea water [63Paluselli, A.; Fauvelle, V.; Galgani, F.; Sempéré, R. Phthalate release from plastic fragments and degradation in seawater. Environ. Sci. Technol., 2019, 53(1), 166-175.
[http://dx.doi.org/10.1021/acs.est.8b05083] [PMID: 30479129] ]:), soils [64Carrara, S.M.; Morita, D.M.; Boscov, M.E. Biodegradation of di(2-ethylhexyl)phthalate in a typical tropical soil. J. Hazard. Mater., 2011, 197, 40-48.
[http://dx.doi.org/10.1016/j.jhazmat.2011.09.058] [PMID: 22014440] ], sediments [65Chang, B.V.; Liao, C.S.; Yuan, S.Y. Anaerobic degradation of diethyl phthalate, di-n-butyl phthalate, and di-(2-ethylhexyl) phthalate from river sediment in Taiwan. Chemosphere, 2005, 58(11), 1601-1607.
[http://dx.doi.org/10.1016/j.chemosphere.2004.11.031] [PMID: 15694480] ], wastewater [66Camacho-Munoz, G.A.; Llanos, C.H.; Berger, P.A.; Miosso, C.J.; da Rocha, A.F. Occurrence of pharmaceutical compounds in wastewater and sludge from wastewater treatment plants: removal and ecotoxicological impact of wastewater discharges and sludge disposal. Conf. Proc. IEEE Eng. Med. Biol. Soc., 2012, •••, 6508-6513.], and landfills [67Boonyaroj, V.; Chiemchaisri, C.; Chiemchaisri, W.; Yamamoto, K. Removal of organic micro-pollutants from solid waste landfill leachate in membrane bioreactor operated without excess sludge discharge. Water Sci. Technol., 2012, 66(8), 1774-1780.
[http://dx.doi.org/10.2166/wst.2012.324] [PMID: 22907464] ]. A recent review of the microbial degradation of phthalates is available [6Boll, M.; Geiger, R.; Junghare, M.; Schink, B. Microbial degradation of phthalates: biochemistry and environmental implications. Environ. Microbiol. Rep., 2020, 12(1), 3-15.
[http://dx.doi.org/10.1111/1758-2229.12787] [PMID: 31364812] ].
1.3. Phthalates in Aquatic Environments and Isolated from Marine Produce
Monitoring phthalate concentrations in aquatic environments and in marine products has been going on for a long time. A study from Japan, compares the phthalate level of fish caught in the Uji river in 1973 [68Kamata, I.; Tsutsui, G.; Takana, J.; Shirai, T. Phthalic acid esters in fish in the Uji river, Kyoto-fu. Eisei Kogai Kenkyusho Nenpo, 1977, 22, 114-116.] with fish caught there in 1946! A report from 1986 tells us that in the upper Pickwick reservoirs in north Alabama, USA, phthalates have been found to an appreciable degree in turtles, but not in fish or clams [69Dycus, D.L. Technical report series: North Alabama water quality assessment Contaminants in biota, 1986, 7(TVA/ONRED/AWR-86/33) Order No. DE87900603.].
 |
Fig. (4) Isophthalates 46-48, dinonyl cyclohexane-1,2-dicarboxylate isomer (44), and trimellitate 45 as substi-tutes for phthalates as plasticizers. |
 |
Fig. (5) Dialkyl alkanedioates and alkenedioates 49, 51, 52, and 56, trialkyl phosphates 55 and cyclosiloxanes 53 and 54, compounds that often accompany phthalates from natural sources. |
A study from Portland, Maine, of 1983 tells us the concentration of dibutyl phthalate (5) and di(2-ethylhexyl)phthalate (9) in clams were less than those measured in the sediment [70Ray, L.E.; Murray, H.E.; Giam, C.S. Organic pollutants in marine samples from Portland, Maine. Chemosphere, 1983, 12, 1031-1038.
[http://dx.doi.org/10.1016/0045-6535(83)90255-2] ]. A Chinese study from 2003 looked at the concentration of dibutyl phthalate (5), diethyl phthalate (7) and di(2-ethylhexyl) phthalate (9) in water, soil, sediments, and aquatic organisms, including shrimps, fish and clams from Shanghai, Hangzhou Bay, the Grand Canal and surrounding areas [71Zhang, Y.H.; Chen, B.H.; Zheng, L.X. Determination of phthalates in environmental samples. Huanjing Yu Jiankang Zazhi, 2003, 20, 283-286.]. The presence of phthalates in sponges has been explained to have potentially originated from bacteria on the sponges [72Wahidullah, S.; Naik, B.G.; Al-Fadhli, A.A. Chemotaxonomic study of the demosponge Cinachyrella cavernosa (Lamarck). Biochem. Syst. Ecol., 2015, 58, 91-96.
[http://dx.doi.org/10.1016/j.bse.2014.11.001] ].
Meanwhile, there is also beginning to be a good overview of phthalate concentrations in the aquatic environment in different parts of the world, whether it is in the German Bight [73Xie, Z.; Ebinghaus, R.; Temme, C.; Lohmann, R.; Caba, A.; Ruck, W. Atmospheric concentrations and air–sea exchanges of phthalates in the North Sea (German Bight). Atmos. Environ., 2005, 39, 3209-3219.
[http://dx.doi.org/10.1016/j.atmosenv.2005.02.021] ], the Bay of Marseille [74Paluselli, A.; Fauvelle, V.; Schmidt, N.; Galgani, F.; Net, S.; Sempéré, R. Distribution of phthalates in Marseille Bay (NW Mediterranean Sea). Sci. Total Environ., 2018, 621, 578-587.
[http://dx.doi.org/10.1016/j.scitotenv.2017.11.306] [PMID: 29195205] ], or the Asan lake in S.-Korea [75Lee, Y.M.; Lee, J.E.; Choe, W.; Kim, T.; Lee, J.Y.; Kho, Y.; Choi, K.; Zoh, K.D. Distribution of phthalate esters in air, water, sediments, and fish in the Asan Lake of Korea. Environ. Int., 2019, 126, 635-643.
[http://dx.doi.org/10.1016/j.envint.2019.02.059] [PMID: 30856451] ]. In addition, the mobility of phthalates and their movement between the atmosphere and water [73Xie, Z.; Ebinghaus, R.; Temme, C.; Lohmann, R.; Caba, A.; Ruck, W. Atmospheric concentrations and air–sea exchanges of phthalates in the North Sea (German Bight). Atmos. Environ., 2005, 39, 3209-3219.
[http://dx.doi.org/10.1016/j.atmosenv.2005.02.021] , 76Xie, Z.; Ebinghaus, R.; Temme, C.; Lohmann, R.; Caba, A.; Ruck, W. Occurrence and air-sea exchange of phthalates in the Arctic. Environ. Sci. Technol., 2007, 41(13), 4555-4560.
[http://dx.doi.org/10.1021/es0630240] [PMID: 17695896] ] or between sediments and water become more understood. Investigations were carried out with deuterated di-n-octylphthalate to better comprehend the uptake of phthalates by mollusks from sediments [77Foster, G.D.; Baksi, S.M.; Means, J.C. Bioaccumulation of trace organic contaminants from sediment by Baltic clams (Macoma balthica) and soft-shell clams (Mya arenaria). Environ. Toxicol. Chem., 1987, 6, 969-976.
[http://dx.doi.org/10.1002/etc.5620061209] ].
Phthalates in marine produce are watched carefully. Thus, phthalate concentrations have been assessed and monitored in commercial marine pelagic fish species such as Atlantic bluefin tuna from Sardinia [9-14.62 ng/g DEHP (9); 15.-6.3 ng/g MEHP] [78Guerranti, C.; Cau, A.; Renzi, M.; Badini, S.; Grazioli, E.; Perra, G.; Focardi, S.E. Phthalates and perfluorinated alkylated substances in Atlantic bluefin tuna (Thunnus thynnus) specimens from Mediterranean Sea (Sardinia, Italy): Levels and risks for human consumption. J. Environ. Sci. Health B, 2016, 51(10), 661-667.
[http://dx.doi.org/10.1080/03601234.2016.1191886] [PMID: 27323803] ], Atlantic herring, Atlantic mackerel [17-27 μg/g DIHP (14)] [79Musial, C.J.; Uthe, J.; Sirota, G.R.; Burns, B.G.; Gilgan, M.W.; Zitko, V.; Matheson, R.A. Di-n-hexyl phthalate (DHP), a newly identified contaminant in Atlantic herring (Clupea harengus harengus) and Atlantic mackerel (Scomber scombrus). Can. J. Fish. Aquat. Sci., 1981, •••, 856-859.
[http://dx.doi.org/10.1139/f81-113] ], Baltic herring [5.6 μg/g DMP (17)] and codfish [1.9 μg/g DMP (17)] [80Ostrovsky, I.; Čabala, R.; Kubinec, R.; Górová, R.; Blaško, J.; Kubincová, J.; Řimnáčová, L.; Lorenz, W. Determination of phthalate sum in fatty food by gas chromatography. Food Chem., 2011, 124, 392-395.
[http://dx.doi.org/10.1016/j.foodchem.2010.06.045] ]. Phthalate concentrations have also been measured in farmed fish such as in common carp [0.26 mg/kg – 0.72 mg/kg DEHP (9), 0.31 mg/kg - 0.56 mg/kg DnBP, (5)] [81Jarašová, A.; Puškárová, L.; Di Stancová, V. -2-ethylhexyl phthalate and di-n-butyl phthalate in tissues of common carp (Cyprinus carpio L.) after harvest and after storage in fish storage tank. J. Microbiol. Biotechnol. Food Sci., 2012, 1, 277-286.]. How all this translates to exposure of humans to phthalates is an issue of major concern. Already in 1973, D. Williams noted levels of dibutyl phthalate [5, 0-78 ppm] and di(2-ethylhexyl) phthalate [0-160 ppb] in 21 samples of fish available to the Canadian consumer [82Williams, D.T. Dibutyl- and di-(2-ethylhexyl)phthalate in fish. J. Agric. Food Chem., 1973, 21(6), 1128-1129.
[http://dx.doi.org/10.1021/jf60190a028] [PMID: 4755838] ]. Since then, a number of studies have looked into the risk of human contact to phthalates through consumption of different types of produce [83Servaes, K.; Van Holderbeke, M.; Geerts, L.; Sioen, I.; Fierens, T.; Voorspels, S.; Vanermen, G. Phthalate contamination in food: occurrence on the Belgian market and possible contamination pathways. Organohalogen Compd., 2011, 73, 291-294.-86Cheng, Z.; Nie, X.P.; Wang, H.S.; Wong, M.H. Risk assessments of human exposure to bioaccessible phthalate esters through market fish consumption. Environ. Int., 2013, 57-58, 75-80.
[http://dx.doi.org/10.1016/j.envint.2013.04.005] [PMID: 23688402] ], be it in Belgian, UK, Tunisian or Chinese markets. Overviews of phthalates in food have been published [87Cao, X.L. Phthalate esters in foods: sources, occurrence, and analytical methods. Compr. Rev. Food Sci. Food Saf., 2010, 9(1), 21-43.
[http://dx.doi.org/10.1111/j.1541-4337.2009.00093.x] [PMID: 33467808] ].
1.4. Phthalates Isolated from Organisms as Natural Products – Isolation of Phthalate Replacement Products from Organisms
Against this background, there is a large body of literature concerning the isolation of phthalates from organisms as natural products, i.e., not as or not expressly as contaminants found within the organisms (Table 2). In a number of papers listed here, though, the connection is made between phthalates and plasticizers [88Jeevitha, T.; Deepa, K.; Michael, A. In vitro study on the anti-microbial efficacy of Aloe vera against Candida albicans. Afr. J. Microbiol. Res., 2018, 12, 930-937.
[http://dx.doi.org/10.5897/AJMR2015.7631] ], although it is not precisely noted that the phthalates isolated from the organisms are true of anthropogenic nature. In certain cases, it was not relevant for the authors whether the isolated phthalates were actual plant metabolites or anthropogenic contaminants, such as in the case of identifying the volatile components that make up the bouquet of pineapples of different degrees of ripeness [89Umano, K.; Hagi, Y.; Nakahara, K.; Shoji, A.; Shibamoto, T. Volatile constituents of green and ripened pineapple (Ananas comosus [L.] Merr.). J. Agric. Food Chem., 1992, 40, 599-603.
[http://dx.doi.org/10.1021/jf00016a014] ], where both dibutyl phthalate (5) and diisobutyl phthalate (12) were found; or in the case of identifying the flavor components of clams and mussels such as from the Hongdao clam [90Lan, X.; Xue, Y.; Chen, X.; Yang, Y. Aromatic components of Hongdao clam by HS-SPME and GC-MS. Adv. Mat. Res. (Durnten-Zurich), 2013, 709, 49-52.
[http://dx.doi.org/10.4028/www.scientific.net/AMR.709.49] ], where dibutyl phthalate (5) and diethyl phthalate were isolated, or from the muscles of four other Chinese sea clams, where dibutyl phthalate (5) and butyl benzyl phthalate (1) were isolated [91Liu, J.; Lu, B.T.; Xiao, S.Y. Analysis and evaluation of flavor substances in four sea clam muscles. Huanjing Yu Jiankang Zazhi, 2008, 25, 633-634.]. In other cases, the authors were very much aware of the possibility of the isolated phthalate being a contaminant. Thus, Silva at al. [92Silva, F.A.; Liotti, R.G.; Boleti, A.P.A.; Reis, É.M.; Passos, M.B.S.; Dos Santos, E.L.; Sampaio, O.M.; Januário, A.H.; Branco, C.L.B.; Silva, G.F.D.; Mendonça, E.A.F.; Soares, M.A. Diversity of cultivable fungal endophytes in Paullinia cupana (Mart.) Ducke and bioactivity of their secondary metabolites. PLoS One, 2018, 13(4)e0195874
[http://dx.doi.org/10.1371/journal.pone.0195874] [PMID: 29649297] ]. were clearly aware that DEHP (9) could be a contaminant from laboratory equipment and state that DEHP (9) was only isolated from Diaporthe phaseolorum and not from other organisms that they worked with under identical conditions. S. E. McKenzie et al. isolated bis (2-ethylhexyl) phthalate from the marine fungus Corollospora lacera, but showed that the compound was indeed an artifact stemming from the culturing and extraction process [93MacKenzie, S.E.; Gurusamy, G.S.; Piórko, A.; Strongman, D.B.; Hu, T.; Wright, J.L.C. Isolation of sterols from the marine fungus Corollospora lacera. Can. J. Microbiol., 2004, 50(12), 1069-1072.
[http://dx.doi.org/10.1139/w04-103] [PMID: 15714238] ]. Laboratory contamination with phthalates can happen facilely as the author has also experienced in his laboratory, once both in Japan and in the United Arab Emirates. Typical sources of contamination are solvent bottles made of plastic and cling films. Sources, incidents and remedies of such contaminations have been reviewed by Nguyen et al. [94Nguyen, D.H.; Nguyen, D.T.M.; Kim, E.K. Effects of di-(2-ethylhexyl) phthalate (DEHP) released from laboratory equipments. Korean J. Chem. Eng., 2008, 25, 1136-1139.
[http://dx.doi.org/10.1007/s11814-008-0186-z] ] and Reid et al. [95Reid, A.M.; Brougham, C.A.; Fogarty, A.M.; Roche, J.J. An investigation into possible sources of phthalate contamination in the environmental analytical laboratory. Int. J. Environ. Anal. Chem., 2007, 87, 125-133.
[http://dx.doi.org/10.1080/03067310601071183] ]. Nevertheless, the overwhelming number of reports on the isolation of phthalates from natural organisms originate from the exposure of these organisms to anthropogenic environmental pollution rather than from contamination of the samples in the laboratory. Oftentimes, phthalates are isolated as a bouquet of different phthalates from the organisms [96Yang, T.; Zhang, C.X.; Cai, E.B.; Bao, J.C.; Zheng, Y.L. Analysis of chemical composition of volatile oil from underground part of Astilbe chinensis (Maxim.) Franch.et Sav using GC – MS. Ziyuan Kaifa Yu Shichang, 2011, 27, 106-107.-98Qin, L.Q.; Liu, X.X.; Luo, S.Y.; Yang, D. GC-MS comparative analysis of low-polarity compounds of Amaranthus caudatus L. Anhui Nongye Kexue, 2015, 43, 81-83.], often hand-in-hand with alkanedioates such as diisobutyl oxalate (51) and diisobutyl succinate (52) in addition to siloxanes such as hexadecamethylcyclooctasiloxane (53) and octamethylcyclotetrasiloxane (54) that all are known additives in polymers [96Yang, T.; Zhang, C.X.; Cai, E.B.; Bao, J.C.; Zheng, Y.L. Analysis of chemical composition of volatile oil from underground part of Astilbe chinensis (Maxim.) Franch.et Sav using GC – MS. Ziyuan Kaifa Yu Shichang, 2011, 27, 106-107.]. From the fruits of Acanthopanax sessiliflorus (Araliaceae), 13 different phthalates were isolated [99Asilbekova, D.T.; Gusakova, S.D.; Glushenkova, A.I. Lipids in fruits of Acanthopanax sessiliflorus., 1985, , 760-766.]. In 2020, N. Kumari et al. published the isolation of dibutyl phthalate (5) as secondary metabolites of an actinomycetes strain grown on actinomycete isolation agar. However, in the same study tert-butylcalix [4Huang, J.; Nkrumah, P.N.; Li, Y.; Appiah-Sefah, G. Chemical behavior of phthalates under abiotic conditions in landfills. Rev. Environ. Contam. Toxicol., 2013, 224, 39-52.
[http://dx.doi.org/10.1007/978-1-4614-5882-1_2] [PMID: 23232918] ] arene, clearly, a synthetic product, was also found as a purported secondary metabolite of the actinomycetes strain [100Kumari, N.; Menghani, E.; Mithal, R. GCMS analysis & assessment of antimicrobial potential of rhizospheric Actinomycetes of AIA3 isolate. Indian J. Tradit. Knowl., 2020, 19, 111-119.].
Most of the time, the structures of the phthalates were determined by GC-MS, relying on the retention time of the compounds on the specific column material used and the mass spectrometric data as analyzed by a computer-accessible database. While mostly the data analysis is expected to be correct, such an analysis is not without its danger. Furthermore, isomeric structures, especially of long-chain esters, which in the case of the phthalates are known to be produced industrially oftentimes as isomeric mixtures, are not easily distinguished and need human aided analysis, with multiple mass spectrometric analyses carried out under different conditions. In a number of cases, there is a mismatch between reported and accepted physical data of the phthalates. Thus, DEHP (9) has been isolated from Diaporthe phaseolorum as a dark yellow solid but resembles a colorless oil [92Silva, F.A.; Liotti, R.G.; Boleti, A.P.A.; Reis, É.M.; Passos, M.B.S.; Dos Santos, E.L.; Sampaio, O.M.; Januário, A.H.; Branco, C.L.B.; Silva, G.F.D.; Mendonça, E.A.F.; Soares, M.A. Diversity of cultivable fungal endophytes in Paullinia cupana (Mart.) Ducke and bioactivity of their secondary metabolites. PLoS One, 2018, 13(4)e0195874
[http://dx.doi.org/10.1371/journal.pone.0195874] [PMID: 29649297] ]. Similarly, di-n-butyl phthalate (5), isolated as a secondary metabolite of an endophytic fungal strain of Rumex madaio, has been reported as a solid, although again, the compound is a colorless oil at room temperature [101Bai, X.L.; Yu, R.L.; Li, M.Z.; Zhang, H.W. Antimicrobial assay of endophytic fungi from Rumex madaio and chemical study of strain R1. Bangladesh J. Pharmacol., 2019, 14, 129-135.
[http://dx.doi.org/10.3329/bjp.v14i3.41598] ]. In many cases, however, extensive NMR spectroscopic analyses bear out the structures of the compounds perfectly.
Dialkyl phthalates are initially metabolized to monoalkyl phthalates by a number of microorganisms [104Eaton, R.W.; Ribbons, D.W. Metabolism of dibutylphthalate and phthalate by Micrococcus sp. strain 12B. J. Bacteriol., 1982, 151(1), 48-57.
[http://dx.doi.org/10.1128/JB.151.1.48-57.1982] [PMID: 7085570] ], and it has been realized that in the digestive lumen and liver of fish, monoalkyl phthalates (MPA) may also be produced from dialkyl phthalates [105Fourgous, C.; Chevreuil, M.; Alliot, F.; Amilhat, E.; Faliex, E.; Paris-Palacios, S.; Teil, M.J.; Goutte, A. Phthalate metabolites in the European eel (Anguilla anguilla) from Mediterranean coastal lagoons. Sci. Total Environ., 2016, 569-570, 1053-1059.
[http://dx.doi.org/10.1016/j.scitotenv.2016.06.159] [PMID: 27412480] ]. This leads to 913 ± 885 ng/g MPA in European eel (Anguilla anguilla) muscles, collected in two French lagoons in the Mediterranean Sea [105Fourgous, C.; Chevreuil, M.; Alliot, F.; Amilhat, E.; Faliex, E.; Paris-Palacios, S.; Teil, M.J.; Goutte, A. Phthalate metabolites in the European eel (Anguilla anguilla) from Mediterranean coastal lagoons. Sci. Total Environ., 2016, 569-570, 1053-1059.
[http://dx.doi.org/10.1016/j.scitotenv.2016.06.159] [PMID: 27412480] ], to 0.54 ng/g for benzyl phthalate (MBzP, 24) to 82 ng/g for n-butyl phthalate (MnBP, 25) in the muscles of juvenile Shiner Perch (Cymatogaster aggregata) [106McConnell, M.L. Distribution of phthalate monoesters in an aquatic food web. Master Report No. 426. School of Resource and Environmental Management, Simon Frazer University, 2007.] and to 0.24–1.1 ng/g for ethylhexyl phthalate (MEHP, 26, 6.63–60.9 ng/g for MnBP (25) in the white-spotted greenling (Hexagrammos stelleri) [107Blair, J.D.; Ikonomou, M.G.; Kelly, B.C.; Surridge, B.; Gobas, F.A. Ultra-trace determination of phthalate ester metabolites in seawater, sediments, and biota from an urbanized marine inlet by LC/ESI-MS/MS. Environ. Sci. Technol., 2009, 43(16), 6262-6268.
[http://dx.doi.org/10.1021/es9013135] [PMID: 19746723] ]. In these cases again, the substrate phthalates will be of anthropogenic origin. Monoalkyl phthalates such as MEHP (26) and MnBP (25) have been isolated from a number of bacteria, algae and fungi, but also from terrestrial plants (Table 2 ). Monoalkyl phthalates are being used as biomarkers for the original presence of dialkyl phthalates in organisms. The question remains in how far certain occurrences of phthalates in natural organisms indicate that they are natural products of these organisms. Table 2 shows a selection of reports of phthalates found in various organisms, especially in plants, bacteria, and fungi that do not specifically mention a possible anthropogenic origin of the phthalates. Of the 26 industrially most produced phthalates, only of diisoundecyl-, diisotridecyl- and of diallyl phthalate (3), no reports could be found regarding their isolation as products from plants. Interestingly, larger phthalates such as diisotridecyl phthalate, which is used in heat-resistant cables, have not been reported from plant isolates, either, yet. On the other hand, it would have been interesting to find the isolation of dialkyl phthalates that are known not to have been synthesized industrially. This data is hard to come by. Thus, it has been mentioned that one sign that bis(2-methylheptyl) phthalate were produced by Hypericum hyssopifolium (Guttiferae) itself, was that the compound was not used in the chemical industry [102Cakir, A.; Mavi, A.; Yildirim, A.; Duru, M.E.; Harmandar, M.; Kazaz, C. Isolation and characterization of antioxidant phenolic compounds from the aerial parts of Hypericum hyssopifolium L. by activity-guided fractionation. J. Ethnopharmacol., 2003, 87(1), 73-83.
). Monoalkyl phthalates are being used as biomarkers for the original presence of dialkyl phthalates in organisms. The question remains in how far certain occurrences of phthalates in natural organisms indicate that they are natural products of these organisms. Table 2 shows a selection of reports of phthalates found in various organisms, especially in plants, bacteria, and fungi that do not specifically mention a possible anthropogenic origin of the phthalates. Of the 26 industrially most produced phthalates, only of diisoundecyl-, diisotridecyl- and of diallyl phthalate (3), no reports could be found regarding their isolation as products from plants. Interestingly, larger phthalates such as diisotridecyl phthalate, which is used in heat-resistant cables, have not been reported from plant isolates, either, yet. On the other hand, it would have been interesting to find the isolation of dialkyl phthalates that are known not to have been synthesized industrially. This data is hard to come by. Thus, it has been mentioned that one sign that bis(2-methylheptyl) phthalate were produced by Hypericum hyssopifolium (Guttiferae) itself, was that the compound was not used in the chemical industry [102Cakir, A.; Mavi, A.; Yildirim, A.; Duru, M.E.; Harmandar, M.; Kazaz, C. Isolation and characterization of antioxidant phenolic compounds from the aerial parts of Hypericum hyssopifolium L. by activity-guided fractionation. J. Ethnopharmacol., 2003, 87(1), 73-83.
[http://dx.doi.org/10.1016/S0378-8741(03)00112-0] [PMID: 12787957] ]. It must be noted, however, that two patents existed for the production and use of the compounds at that time, one by BASF and one by Casio Computer Co [103JP 57063379 (Casio Computer Co.) Guest-host effect liquid crystal display devices, 1982.].
It is interesting to screen the frequency of articles reporting on the isolation of phthalates with one short and one long alkyl chain, which is not that frequently found as additives in consumer products. These would include methyl propyl phthalate (57), n-butyl methyl phthalate (58), methyl n-pentyl phthalate (59), 2-ethylhexyl methyl phthalate (60) and the corresponding alkyl ethyl phthalates (Fig. 6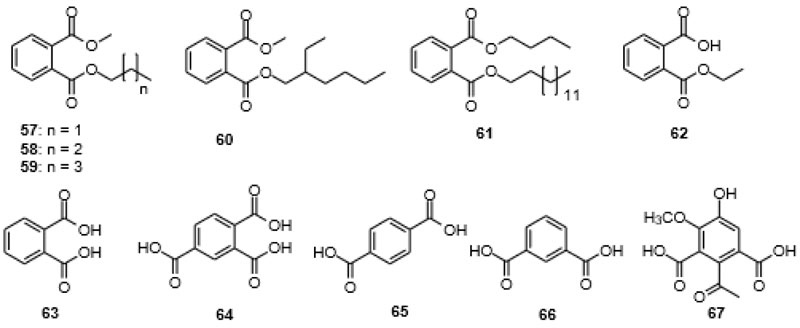 ). The interesting finding is that quite a few reports of isolation of these “mis-matched”, non-symmetric, less produced phthalates from different organisms exist [108Lucas, E.M.F.; Abreu, L.M.; Marriel, I.E.; Pfenning, L.H.; Takahashi, J.A. Phthalates production from Curvularia senegalensis (Speg.) Subram, a fungal species associated to crops of commercial value. Microbiol. Res., 2008, 163(5), 495-502.
). The interesting finding is that quite a few reports of isolation of these “mis-matched”, non-symmetric, less produced phthalates from different organisms exist [108Lucas, E.M.F.; Abreu, L.M.; Marriel, I.E.; Pfenning, L.H.; Takahashi, J.A. Phthalates production from Curvularia senegalensis (Speg.) Subram, a fungal species associated to crops of commercial value. Microbiol. Res., 2008, 163(5), 495-502.
[http://dx.doi.org/10.1016/j.micres.2007.02.003] [PMID: 17462873] ]. n-Butyl n-tetradecyl phthalate (61) was isolated from the leaves of Urtica dioica L [109Dar, S.A.; Yousuf, A.R.; Ganai, F.A.; Sharma, P.; Kumar, N.; Singh, R. Bioassay guided isolation and identification of anti-inflammatory and antimicrobial compounds from Urtica dioica L. (Urticaceae) leaves. Afr. J. Biotechnol., 2012, 11, 12910-12920.]. together with a number of other compounds of anthropogenic origin such as di-n-butylphthalate (DnBP, 5), di-2-ethylhexylphthalate (DEHP, 9), tributyl phosphate (55), and bis(2-ethylhexyl)maleate (56), all used as plasticizers, sealants or hydraulic fluids. Ethyl methyl phthalate (EMP, 23) was found in the stems of thorn apple [Datura stramonium L.] [110Durak, H.; Aysu, T. Structural analysis of bio-oils from subcritical and supercritical hydrothermal liquefaction of Datura stramonium L. J. Supercrit. Fluids, 2016, 108, 123-135.
[http://dx.doi.org/10.1016/j.supflu.2015.10.016] ], in Italian thistle [Carduus pycnocephalus L.] [111Al-Shammari, L.A.; Hassan, W.H.B.; Al-Youssef, H.M. Chemical composition and antimicrobial activity of the essential oil and lipid content of Carduus pycnocephalus L. growing in Saudi Arabia. J. Chem. Pharm. Res., 2012, 4, 1281-1287.], and dyer’s woad [Isatis indigotica] [112Wu, J.; Sun, D.; Li, X.; Chen, J.; He, L.; Dong, W. GC-MS analysis of liposoluble constituents in Isatis indigotica. Zhongguo Yaofang, 2008, 19, 2354-2356.], among other plants. Research has shown that primary biodegradation of DEP mostly follows two paths, namely firstly the hydrolysis to monoethyl phthalate (MEP, 62) and then to phthalic acid (PA, 63) and secondly the de-methylation and trans-esterification to form ethyl methyl phthalate (EMP, 23). These pathways have been shown to operate in Pseudomonas sp. DNE-S1 [113Tao, Y.; Li, H.; Gu, J.; Shi, H.; Han, S.; Jiao, Y.; Zhong, G.; Zhang, Q.; Akindolie, M.S.; Lin, Y.; Chen, Z.; Zhang, Y. Metabolism of diethyl phthalate (DEP) and identification of degradation intermediates by Pseudomonas sp. DNE-S1. Ecotoxicol. Environ. Saf., 2019, 173, 411-418.
[http://dx.doi.org/10.1016/j.ecoenv.2019.02.055] [PMID: 30798184] ] and in Sphingobium yanoikuyae SHJ [114Wang, Y.; Liu, H.; Peng, Y.E.; Tong, L.; Feng, L.; Ma, K. New pathways for the biodegradation of diethyl phthalate by Sphingobium yanoikuyae SHJ, Proc. Biochem., 2018, 71, 152-158.]. Enzymatic trans-esterification in natural organisms of industrial phthalates to mixed phthalates should be considered but has not been studied, to the best of the author’s knowledge. Finally, phthalic acid has been found in a number of plant extracts, such as in the ethyl acetate extract of Bridelia ovata [115Poofery, J.; Khaw-on, P.; Subhawa, S.; Sripanidkulchai, B.; Tantraworasin, A.; Saeteng, S.; Siwachat, S. Lertprasertsuke, N.; Banjerdpongchai, R. Potential of Thai herbal extracts on lung cancer treatment by inducing apoptosis and synergizing chemotherapy. Molecules, 2020, 25, 231-261.
[http://dx.doi.org/10.3390/molecules25010231] ] and ethanolic extracts of licorice (Glycyrrhiza glabra) leaves [116Vijayalakshmi, U.; Shourie, A. Comparative GC-MS analysis of secondary metabolites from leaf, stem and callus of Glycyrrhiza glabra. World J. Pharm. Res., 2019, 8, 1915-1923.], sometimes in concert with phthalates [117Mohan, S.C.; Anand, T. Comparative study of identification of bioactive compounds from Barringtonia acutangula leaves and bark extracts and its biological activity. J. Appl. Sci. (Faisalabad), 2019, 19, 528-536.
[http://dx.doi.org/10.3923/jas.2019.528.536] ]. It must be noted, however, that phthalic acid (63) also can derive from the oxidation of naphthalenes as VOCs in the atmosphere. On the other hand, terephthalic acid (65) (see below) can derive from the burning of plastic, while 1,2,4-benzenetricarboxylic acid (64) can originate from the oxidation of polycyclic aromatic hydrocarbons (PAHs). Thus, phthalic acid, as well as terephthalic acid (65) and 1,2,4-benzenetricarboxylic acid (64), have been found residing on PM2.5 in the atmosphere (eg., at a max. of 73.2 ng/m3 collected air space over Nanhai, China; 178.5 ng/m3; and 43.4 ng/m3, respectively) [118He, X.; Huang, X.H.H.; Chow, K.S.; Wang, Q.; Zhang, T.; Wu, D.; Yu, J.Z. Abundance and sources of phthalic acids, benzene-tricarboxylic acids, and phenolic acids in PM2.5 at urban and suburban sites in Southern China. ACS Earth Space Chem., 2018, 2, 147-158.
[http://dx.doi.org/10.1021/acsearthspacechem.7b00131] ].
As mentioned above, terephthalates, trimellitates and ring-hydrogenated analogs of phthalates, i.e., cyclohexane-1,2-dicarboxylates, have partially replaced phthalates as plasticizers in recent times, and they have started to appear in the environment in different concentrations. Thus, humans are equally exposed to terephthalates as has been shown in a recent German study on phthalate content in urine samples from 1999 to 2017, which indicated that the human exposure to para-phthalates (terephthalates) continues to grow [119Lessmann, F.; Kolossa-Gehring, M.; Apel, P.; Rüther, M.; Pälmke, C.; Harth, V.; Brüning, T.; Koch, H.M. German Environmental Specimen Bank: 24-hour urine samples from 1999 to 2017 reveal rapid increase in exposure to the para-phthalate plasticizer di(2-ethylhexyl) terephthalate (DEHTP). Environ. Int., 2019, 132105102
[http://dx.doi.org/10.1016/j.envint.2019.105102] [PMID: 31491609] ] as these are replacing the phthalates as less regulated plasticizers. Here, the author tried to find whether this is also reflected in their isolation from natural sources. Indeed, reports on the isolation of terephthalates, especially from plant sources, could be found (Table 3), as could be on the isolation of isophthalates (dialkyl 1,3-phthalates, Table 4). Isophthalates in the form of ethylene terephthalate-isophthalate copolymers have been used in food packaging films, but have also been formulated as diluents in polymers such as polyethylene terephthalates [120Zekriardehani, S.; Joshi, A.S.; Jabarin, S.A.; Gidley, D.W.; Coleman, M.R. Effect of dimethyl terephthalate and dimethyl isophthalate on the free volume and barrier properties of poly(ethylene terephthalate) (PET): amorphous PET. Macromolecules, 2018, 51, 456-467.
[http://dx.doi.org/10.1021/acs.macromol.7b02230] ]. Benzene-1,3-dicarboxylic acid (isophthalic acid, 66) has been isolated from a number of plants. Typical isolations have been reported from the essential oil of Dendrobium nobile [121Huang, X.; Yi, Y.; Zhang, X.; Yang, L.; Xu, X. Constituents in essential oil of Dendrobium nobile with GC-MS. Shizhen Guoyi Guoyao, 2010, 21, 889-891.], and stems of cultivated Dendrobium officinale, and Dendrobium huoshanense [122Jin, Q.; Jiao, C.; Sun, S.; Cheng, C.; Yongping, L.; Fan, H.; Zhu, Y. Metabolic analysis of medicinal Dendrobium officinale and Dendrobium huoshanense during different growth years. PLoS One, 2016, 11, e0146607/1-e0146607/17.] (Orchidaceae), from the air-dried parts of the whole plant Swertia angustifolia (Gentianaceae) [123He, K.; Cao, T.W.; Wang, H.L.; Geng, C.A.; Zhang, X.M.; Chen, J.J. [Chemical constituents of Swertia angustifolia.]. Zhongguo Zhongyao Zazhi, 2015, 40(18), 3603-3607.
[PMID: 26983208] ], from the leaves of Cerbera manghas (sea mango, Apocynaceae) [124Zhang, X.; Pei, Y.; Liu, M.; Kang, S.; Zhang, J. Organic acids from leaves of Cerbera manghas. Zhongcaoyao, 2010, 41, 1763-1765.], and from the culture filtrate of the yeast Candida tropicalis [125Abbasi, A.; Zaidi, Z.H. Isolation of isophthalic acid from Candida tropicalis. J. Chem. Soc. Pak., 1980, 2, 49-49.]. In addition, ring-substituted isophthalic acids have been found, such as 2-acetyl-5-hydroxy-4-methoxyisophthalic acid (67, Fig. 6 ) in the fungus Talaromyces flavus (Trichocomaceae) [126He, J.; Yang, L.; Wang, Y.; Mu, Z.; Zou, Z.; Wang, X.; Zhong, G. A new isophthalic acid from Talaromyces flavus. Chem. Nat. Compd., 2017, 53, 409-411.
) in the fungus Talaromyces flavus (Trichocomaceae) [126He, J.; Yang, L.; Wang, Y.; Mu, Z.; Zou, Z.; Wang, X.; Zhong, G. A new isophthalic acid from Talaromyces flavus. Chem. Nat. Compd., 2017, 53, 409-411.
[http://dx.doi.org/10.1007/s10600-017-2010-7] ]. Few examples of the isolation of trimellitic acid esters as natural products could be located (Table 4), even though these also had been forwarded as additives to agrochemical powder preparations [127Hokko Chem. Ind., Ltd. Trimellitic acid esters for quality improvement of agrochemical powder preparation JP 57159702, 1982.] such as to fertilizers [128Chen, Y. (P.R.C.) Long-acting slow release fertilizer dedicated for apple CN 104892245, 2015.]. Moreover, to date, no report could be found of the isolation of diisononyl cyclohexane-1,2-dicarboxylate from a plant or other organism as a natural product.
 |
Fig. (6) Non-symmetric phthalates 57-61, ethyl phthalate 62, benzenedicarboxylic and tricarboxylic acids 63-67. |
1.5. Uncommon Phthalates Isolated from Organisms as an Indication that These are Natural Products and not Products of Anthropogenic Origin
2-Methyl-, 2-ethyl, and 2-propylalkyl phthalates such as compounds 9, 21 36, and 43 exhibit stereocenter(s), where it must be noted that industrial phthalates are produced as stereoisomeric mixtures from the racemic alcohols. A number of papers have reported on the isolation of enantiopure or at least enantio-enriched phthalates [129Singh, N.; Mahmood, U.; Kaul, V.K.; Jirovetz, L. A new phthalic acid ester from Ajuga bracteosa. Nat. Prod. Res., 2006, 20(6), 593-597.
[http://dx.doi.org/10.1080/14786410500185550] [PMID: 16835093] , 130Afza, N.; Yasmeen, S.; Ferheen, S.; Malik, A.; Ali, M.I.; Kalhoro, M.A.; Ifzal, R. New aromatic esters from Galinsoga parviflora. J. Asian Nat. Prod. Res., 2012, 14(5), 424-428.
[http://dx.doi.org/10.1080/10286020.2012.657181] [PMID: 22348678] ], indicating the natural origin of these phthalates. In the isolation of bis(2S-methylheptyl) phthalate (S-36) from the evergreen perennial plant Ajuga bracteosa, the authors did not forward any analytical result that indicated that the isolated substance was enantiopure or indeed chiral. The structure presented in the paper is that of the meso form of the compound, (R/S)-bis(2-methylheptyl) phthalate (36) [129Singh, N.; Mahmood, U.; Kaul, V.K.; Jirovetz, L. A new phthalic acid ester from Ajuga bracteosa. Nat. Prod. Res., 2006, 20(6), 593-597.
[http://dx.doi.org/10.1080/14786410500185550] [PMID: 16835093] ], but the isolation of the compound could potentially be that of a mixture of stereoisomers. Different is the case of the isolation of bis(2S-methylheptyl) phthalate from Galinsoga parviflora, a herbaceous plant of the Asteraceae family, where the isolated compound shows a specific optical rotation [α]D23 of 193.5° (c = 0.075M, MeOH). Here, the question remains as to whether selective enzymatic hydrolysis of a mixture of bis(2-methylheptyl) phthalate stereoisomers has led to (R/S)-bis(2-methylheptyl) phthalate (36) as the one remaining dialkyl phthalate or whether the phthalate as a whole has been biosynthetically created (Fig. 7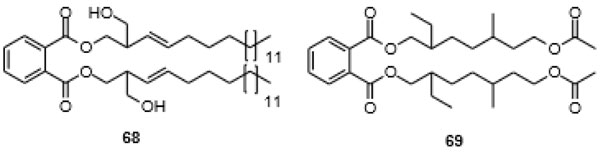 ).
).
Undoubtedly, there are phthalates that have been isolated from organisms that thus far have had no place in the industry. One such is kurraminate [bis(2-hydroxymethylnonadec-3E-enyl) phthalate] (68) isolated from flowering plant Nepeta kurramensis at Khyber Pakhtunkhwa, Pakistan [131Ur Rehman, N.; Ahmad, N.; Hussain, J.; Liaqit, A.; Hussain, H.; Bakht, N.; Al-Harrasi, A.; Shinwari, Z.K. One new phthalate derivative from Nepeta kurramensis. Chem. Nat. Compd., 2017, 53, 426-428.
[http://dx.doi.org/10.1007/s10600-017-2014-3] ], along with known bis(2-ethyleicosyl) phthalate (33), which was also isolated from Phyllanthus muellerianus in West Africa [132Saleem, M.; Nazir, M.; Akhtar, N.; Onocha, P.A.; Riaz, N.; Jabbar, A.; Shaiq Ali, M.; Sultana, N. New phthalates from Phyllanthus muellerianus (Euphorbiaceae). J. Asian Nat. Prod. Res., 2009, 11(11), 974-977.
[http://dx.doi.org/10.1080/10286020903341388] [PMID: 20183263] ]. Also, 33 is not produced industrially. Bis(7-acetoxy-2-ethyl-5-methylheptyl) phthalate (69) has been isolated from translucent honeysuckle (Lonicera quinquelocularis). This terminally hydroxylated phthalate, which possesses four stereocenters, not discussed by the authors, again is apparently not of anthropogenic origin [133Khan, D.; Zhao, W.; Ahmad, S.; Khan, S. New antioxidant and cholinesterase inhibitory constituents from Lonicera quinquelocularis. J. Med. Plants Res., 2014, 8, 313-317.
[http://dx.doi.org/10.5897/JMPR2013.5245] , 134Khan, D.; Khan, H.U.; Khan, F.; Khan, S.; Badshah, S.; Khan, A.S.; Samad, A.; Ali, F.; Khan, I.; Muhammad, N. New cholinesterase inhibitory constituents from Lonicera quinquelocularis. PLoS One, 2014, 9(4)e94952
[http://dx.doi.org/10.1371/journal.pone.0094952] [PMID: 24733024] ]. The phthalate has been isolated together with the common anthropogenic phthalates DEHP (9) and di-n-octyl phthalate (20). 69 shows an acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activity with IC50 of 1.65 and 5.98 μM.
One of the most compelling examples that phthalates can indeed be of natural origin is the isolation of a row of diethylene glycol phthalate ester oligomers from the marine-derived fungus Cochliobolus lunatus [135Chen, M.; Zhang, W.; Shao, C.L.; Chi, Z.M.; Wang, C.Y. DNA methyltransferase inhibitor induced fungal biosynthetic products: Diethylene glycol phthalate ester oligomers from the marine-derived fungus Cochliobolus lunatus. Mar. Biotechnol. (NY), 2016, 18(3), 409-417.
[http://dx.doi.org/10.1007/s10126-016-9703-y] [PMID: 27245469] ], which was subjected to epigenetic manipulation with the DNA transferase inhibitor 5-azacytidine (70). This led to the isolation of the seven diethylene glycol phthalate esters 72, 75-79, and 82 (Fig. 9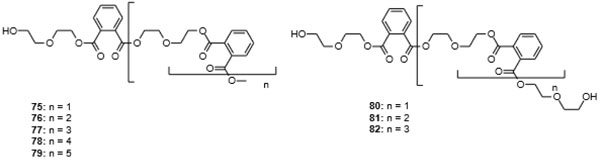 ), in addition to the known compounds 71, 74, 80, and 81 (Figs. 8
), in addition to the known compounds 71, 74, 80, and 81 (Figs. 8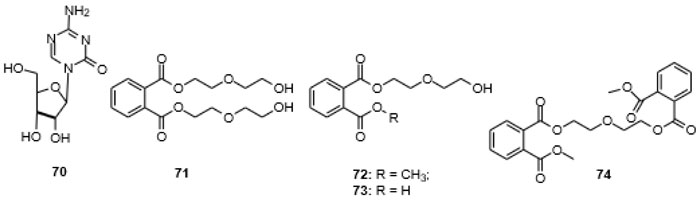 and 9
and 9 ). The compounds have been analyzed by NMR spectroscopic methods, and there is no question as to their identity. The fungus itself was obtained from a piece of fresh tissue from the inner part of the sea anemone Palythoa haddoni, collected from the Weizhou coral reef in the South China Sea [135Chen, M.; Zhang, W.; Shao, C.L.; Chi, Z.M.; Wang, C.Y. DNA methyltransferase inhibitor induced fungal biosynthetic products: Diethylene glycol phthalate ester oligomers from the marine-derived fungus Cochliobolus lunatus. Mar. Biotechnol. (NY), 2016, 18(3), 409-417.
). The compounds have been analyzed by NMR spectroscopic methods, and there is no question as to their identity. The fungus itself was obtained from a piece of fresh tissue from the inner part of the sea anemone Palythoa haddoni, collected from the Weizhou coral reef in the South China Sea [135Chen, M.; Zhang, W.; Shao, C.L.; Chi, Z.M.; Wang, C.Y. DNA methyltransferase inhibitor induced fungal biosynthetic products: Diethylene glycol phthalate ester oligomers from the marine-derived fungus Cochliobolus lunatus. Mar. Biotechnol. (NY), 2016, 18(3), 409-417.
[http://dx.doi.org/10.1007/s10126-016-9703-y] [PMID: 27245469] ]. The linear polyether motif is not common in nature. The central building block, bis[2-(2-hydroxyethoxy)ethyl] phthalate (71), as an additive in polyurethanes, however, has been subject to a large number of patents [136Cai, R.; Tu, S. (Suzhou Liansheng Chemistry Co., Ltd.) Nontoxic and odorless dye carrier with good promoting efficacy for polyester fiber and manufacture method CN 107541968, 2016., 137Ikejiri, Y.; Numakura, T.; Tsuchimochi, Y.; Kawakami, K. (Kawasaki Kasei Chemicals, Ltd.) Fluidity improvers, thermoplastic resin compositions, moldings comprising them with good mechanical strength, and their manufacture JP 2007191685 A, 2006.], appearing in patents as early as 1957 [138Patton, T.C.; Hall, F.M. (Baker Castor Oil Co.) Plastigels containing hydroxy fatty acid soaps US Pat. 2794791, 1953.], and of directed synthesis [139Chen, S.; Wang, Q.; Chen, X. Synthesis of phthalic anhydride diglycol ester. Gongye Cuihua, 2008, 16, 45-48.]. [2-(2-Hydroxyethoxy)ethyl] methyl phthalate (72) had not been reported previously, but [2-(2-hydroxyethoxy)ethyl] phthalate (73) has been covered in a number of patents and also phthalate 74 has appeared in a patent [140Fujii, K.; Ohigashi, T.; Yokoyama, N.; Komehana, N. (Daicel Chemical Industries, Ltd., Japan; Daihachi Chemical Industry Co., Ltd.) Phthalic ester dimers as plasticizers and cellulose fatty acid ester resin compositions JP 11349537 A, 1998.].
A further strong indication that certain phthalates can be of natural origin comes from studies of C.Y. Chen et al., who showed with a 14C inclusion experiment that the red algae Bangia atropurpurea can de novo synthesize DEHP (9) and DnBP (5) [141Chen, C.Y. Biosynthesis of di-(2-ethylhexyl) phthalate (DEHP) and di-n-butyl phthalate (DBP) from red alga--Bangia atropurpurea. Water Res., 2004, 38(4), 1014-1018.
[http://dx.doi.org/10.1016/j.watres.2003.11.029] [PMID: 14769421] ]. B. atropurpurea filaments were cultured in a medium containing NaH14CO3. After two weeks, the radioactivity of DEHP (9) and DnBP (5) fractionated by HPLC from cultured filaments was analyzed, where single peak fractions of DEHP (160.00 cpm) and DnBP (4786.67 cpm) were found to have significantly higher radioactivities than the background (28.00 cpm) [141Chen, C.Y. Biosynthesis of di-(2-ethylhexyl) phthalate (DEHP) and di-n-butyl phthalate (DBP) from red alga--Bangia atropurpurea. Water Res., 2004, 38(4), 1014-1018.
[http://dx.doi.org/10.1016/j.watres.2003.11.029] [PMID: 14769421] ]. It is not clear, though, whether carbon-14 isotope was built into the structures indiscriminately or whether, for instance, it was only built into the alkyl chain of the esters.
 |
Fig. (7) Phthalates 68 and 69, two phthalates that are not produced industrially. |
 |
Fig. (8) 5-Azacytidine (70), bis[2-(2-hydroxyethoxy)ethyl] phthalate (71), [2-(2-hydroxyethoxy)ethyl] methyl phthalate (72), [2-(2-hydroxyethoxy)ethyl] phthalate (73) and bis-phthalate 74 [135Chen, M.; Zhang, W.; Shao, C.L.; Chi, Z.M.; Wang, C.Y. DNA methyltransferase inhibitor induced fungal biosynthetic products: Diethylene glycol phthalate ester oligomers from the marine-derived fungus Cochliobolus lunatus. Mar. Biotechnol. (NY), 2016, 18(3), 409-417. [http://dx.doi.org/10.1007/s10126-016-9703-y] [PMID: 27245469] ]. |
 |
Fig. (9) Diethylene glycol phthalate ester oligomers isolated from the marine-derived fungus Cochliobolus lunatus [135Chen, M.; Zhang, W.; Shao, C.L.; Chi, Z.M.; Wang, C.Y. DNA methyltransferase inhibitor induced fungal biosynthetic products: Diethylene glycol phthalate ester oligomers from the marine-derived fungus Cochliobolus lunatus. Mar. Biotechnol. (NY), 2016, 18(3), 409-417. [http://dx.doi.org/10.1007/s10126-016-9703-y] [PMID: 27245469] ]. |
Looking at many of the known biosynthetic pathways involving aromatic structures such as the phenylpropanoid pathway, it is evident that these rarely give rise to aromatic substances with more than one carboxylic acid group substituting the arene unit. In fact, it is very uncommon to find an aromatic natural compound with two electron-withdrawing groups that is not an intermediate. More often than not, electron-donating hydroxyl, alkoxyl or amino groups are in evidence in naturally occurring aromatic compounds such as in the aromatic building blocks of plant lignins in the form of ferulates 83, hydroxycinnamates 84, with coniferyl alcohol (85) as a building block, in aromatic alkaloids such as bufotenin (86), flavonoids such as 2-phenylchromen-4-one (87), chalcones such as xanthohumol (88) and amino acids such tyrosine (89) and tryptophane (90) (Fig. 10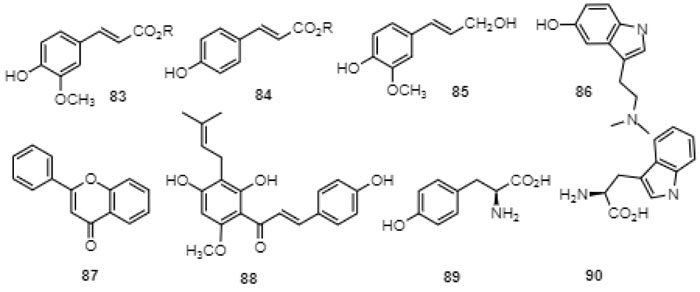 ).
).
C. Tian et al. showed that DnBP (5) is produced by naturally occurring filamentous fungi Penicillium lanosum PTN121, Trichoderma asperellum PTN7 and Aspergillus niger PTN42, cultured in an artificial medium [142Tian, C.; Ni, J.; Chang, F.; Liu, S.; Xu, N.; Sun, W.; Xie, Y.; Guo, Y.; Ma, Y.; Yang, Z.; Dang, C.; Huang, Y.; Tian, Z.; Wang, Y. Bio-Source of di-n-butyl phthalate production by filamentous fungi. Sci. Rep., 2016, 6, 19791.
[http://dx.doi.org/10.1038/srep19791] [PMID: 26857605] ]. Using an enzyme excreted by the fungi, the authors were able to enzymatically produce DnBP (5) under cell-free conditions from D-glucose (91) alone, from D-glucose and 1-butanol, from protocatechuic acid (94) and 1-butanol, and from phthalic acid (63) and 1-butanol (Fig. 11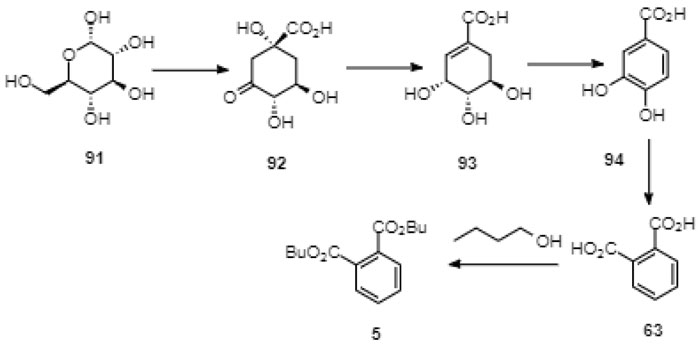 ). This result indicates that DnBP (5) could be produced by the shikimic acid pathway [142Tian, C.; Ni, J.; Chang, F.; Liu, S.; Xu, N.; Sun, W.; Xie, Y.; Guo, Y.; Ma, Y.; Yang, Z.; Dang, C.; Huang, Y.; Tian, Z.; Wang, Y. Bio-Source of di-n-butyl phthalate production by filamentous fungi. Sci. Rep., 2016, 6, 19791.
). This result indicates that DnBP (5) could be produced by the shikimic acid pathway [142Tian, C.; Ni, J.; Chang, F.; Liu, S.; Xu, N.; Sun, W.; Xie, Y.; Guo, Y.; Ma, Y.; Yang, Z.; Dang, C.; Huang, Y.; Tian, Z.; Wang, Y. Bio-Source of di-n-butyl phthalate production by filamentous fungi. Sci. Rep., 2016, 6, 19791.
[http://dx.doi.org/10.1038/srep19791] [PMID: 26857605] ], although the mechanism of the transformation of protocatechuic acid (94) to phthalic acid (63) is not clear, yet (Figs. 11 -13
-13 ).
).
 |
Fig. (10) Typical natural products with an aromatic subunit. |
 |
Fig. (11) Proposed pathway from glucose (91) to dibutyl phthalate (5) [142Tian, C.; Ni, J.; Chang, F.; Liu, S.; Xu, N.; Sun, W.; Xie, Y.; Guo, Y.; Ma, Y.; Yang, Z.; Dang, C.; Huang, Y.; Tian, Z.; Wang, Y. Bio-Source of di-n-butyl phthalate production by filamentous fungi. Sci. Rep., 2016, 6, 19791. [http://dx.doi.org/10.1038/srep19791] [PMID: 26857605] ]. |
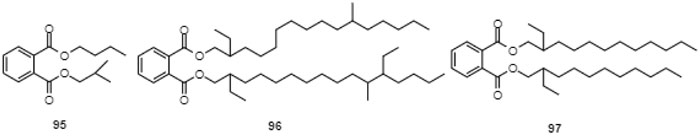 |
Fig. (12) Butyl isobutyl phthalate (95) and 2,12-diethyl-11-methylhexadecyl 2-ethyl-11-methylhexadecyl phthalate (96) and 2-ethyldecyl 2-ethylundecyl phthalate (97), isolated from the seahorse Hippocampus Kuda Bleeler [152Li, Y.; Qian, Z.J.; Kim, S.K. Cathepsin B inhibitory activities of three new phthalate derivatives isolated from seahorse, Hippocampus Kuda Bleeler. Bioorg. Med. Chem. Lett., 2008, 18(23), 6130-6134. [http://dx.doi.org/10.1016/j.bmcl.2008.10.016] [PMID: 18938081] ]. |
 |
Fig. (13) Phthalates introduced in the tables. |
1.6. Biological Activities of Phthalates Isolated from Organisms and Comparison to Activities and Hazard Assessment Associated with Industrial Phthalates
The biological assessment carried out on phthalates isolated from plants as potential natural products is quite different from that carried out on phthalates as industrial plasticizers. In the former, phthalates have been screened for their potentially benevolent effects such as antitumour compounds, antimicrobial products and larvicidal agents. In the latter, potential health and environmental risks associated with the compounds have been assessed, also for regulative purposes, which results, for instance, in the testing of these compounds for their hormonal activity. Both of these biological assessment series are nicely complementary.
In many reports of the isolation of phthalates from natural sources, the authors have tested plant extracts containing, apart from the phthalates, a plethora of other components. In these cases, it is difficult to tie the respective biological activity of the extract to the phthalate ingredient. However, there are also a number of reports of testing the biological activity of isolated phthalates collected from natural organisms. In this regard, bis(2-ethylhexyl)phthalate (9) isolated from the flower of Procera gigantea was found to be active against the gram-positive bacteria Staphylococcus aureus, Bacillus subtilis, Streptococcus equosemens and Sarcina lutea [143Habib, M.R.; Karim, M.R. Antimicrobial and cytotoxic activity of di-(2-ethylhexyl)phthalate and anhydrosophoradiol-3-acetate isolated from Calotropis gigantea (Linn.) flower. Mycobiology, 2009, 37(1), 31-36.
[http://dx.doi.org/10.4489/MYCO.2009.37.1.031] [PMID: 23983504] , 144Di El-Sayed, M.H. -(2-ethylhexyl) phthalate, a major bioactive metabolite with antimicrobial and cytotoxic activity isolated from the culture filtrate of newly isolated soil Streptomyces (Streptomyces mirabilis strain NSQu-25). World Appl. Sci. J., 2012, 20, 1202-1212.] and against the gram-negative bacteria Closteridium perfringens, Escherchia coli, Pseudomonas aeruginosa, Shigella sonnei, Shigella shiga and Shigella dysenteriae [143Habib, M.R.; Karim, M.R. Antimicrobial and cytotoxic activity of di-(2-ethylhexyl)phthalate and anhydrosophoradiol-3-acetate isolated from Calotropis gigantea (Linn.) flower. Mycobiology, 2009, 37(1), 31-36.
[http://dx.doi.org/10.4489/MYCO.2009.37.1.031] [PMID: 23983504] , 144Di El-Sayed, M.H. -(2-ethylhexyl) phthalate, a major bioactive metabolite with antimicrobial and cytotoxic activity isolated from the culture filtrate of newly isolated soil Streptomyces (Streptomyces mirabilis strain NSQu-25). World Appl. Sci. J., 2012, 20, 1202-1212.]. The compound was found to inactive against Bacillus megaterium [143Habib, M.R.; Karim, M.R. Antimicrobial and cytotoxic activity of di-(2-ethylhexyl)phthalate and anhydrosophoradiol-3-acetate isolated from Calotropis gigantea (Linn.) flower. Mycobiology, 2009, 37(1), 31-36.
[http://dx.doi.org/10.4489/MYCO.2009.37.1.031] [PMID: 23983504] ]. Bis(2-ethylhexyl)phthalate (9) showed activity against the fungus Aspergillus flavus as well. Aspergillus fumigatus, Aspergillus niger, and Fusarium sp. were found to be resistant against the compound [143Habib, M.R.; Karim, M.R. Antimicrobial and cytotoxic activity of di-(2-ethylhexyl)phthalate and anhydrosophoradiol-3-acetate isolated from Calotropis gigantea (Linn.) flower. Mycobiology, 2009, 37(1), 31-36.
[http://dx.doi.org/10.4489/MYCO.2009.37.1.031] [PMID: 23983504] ]. Not all biological activity tests have led to unanimous results, nevertheless a more detailed compilation of the antimicrobial test results of isolated and purified phthalates from the literature can be found in Table 5.
Both di-n-hexyl phthalate (14) and bis(2-propylheptyl) phthalate (21), isolated from the rhizopheric soil of the tobacco plant, have been found to have allelochemical properties versus lettuce (Lactuca sativa). Also, they showed autotoxic effects on the flue-cured tobacco plant itself [145Ren, X.; He, X.; Zhang, Z.; Yan, Z.; Jin, H.; Li, X.; Qin, B. Isolation, Identification, and Autotoxicity Effect of Allelochemicals from Rhizosphere Soils of Flue-Cured Tobacco. J. Agric. Food Chem., 2015, 63(41), 8975-8980.
[http://dx.doi.org/10.1021/acs.jafc.5b03086] [PMID: 26416408] ] (Table 6). Allelochemical properties of phthalates are not unusual. Thus, dimethyl phthalate has been found to be a typical allelochemical of the perennial invasive plant Solidago canadensis (Canadian goldenrod) [146Sun, B.Y.; Tan, J.Z.; Wan, Z.G.; Gu, F.G.; Zhu, M.D. Allelopathic effects of extracts from Solidago canadensis L. against seed germination and seedling growth of some plants. J. Environ. Sci. (China), 2006, 18, 304-309.], which leads to delayed seed germination and reduced seedling growth of a number of plants such as wheat and mulberry. Dibutyl phthalate (DnBP, 5) and diisobutyl phthalate (DIBP, 12) were detected in high concentrations in naturally decomposed cotton stalk extracts. These strongly inhibited the cotton seedling growth in a bioassay, indicating autotoxic effects [147Li, Y.B.; Zhang, Q. Effects of naturally and microbially decomposed cotton stalks on cotton seedling growth. Arch. Agron. Soil Sci., 2016, 62, 1264-1270.
[http://dx.doi.org/10.1080/03650340.2015.1135327] ]. However, it must be noted that in all the cases above, it was not ascertained that the phthalates were indeed authentic natural products of the plants; also, it must be observed that such allelopathic effects should be taken into account when applying plastic mulch in plant production.
M. Uyeda et al. showed that DEHP (9) aggregates the gram-negative bacteria Proteus vulgaris and Serratia marcescens as well as HeLa cells [148Uyeda, M.; Suzuki, K.; Shibata, M. 3315-AF2, a cell aggregation factor produced by Streptomyces sp. strain No. A-3315. Agric. Biol., 1990, 54, 251-252.
[http://dx.doi.org/10.1271/bbb1961.54.251] ]. Butyl isobutyl phthalate (95), this time isolated from the brown alga Laminaria japonica (Saccharina japonica), showed non-competitive inhibitory in vitro activity against α-glucosidase [149Bu, T.; Liu, M.; Zheng, L.; Guo, Y.; Lin, X. α-Glucosidase inhibition and the in vivo hypoglycemic effect of butyl-isobutyl-phthalate derived from the Laminaria japonica rhizoid. Phytother. Res., 2010, 24(11), 1588-1591.
[http://dx.doi.org/10.1002/ptr.3139] [PMID: 21031613] ], toted at one time as a possible drug to help treat type II diabetes. Di-n-octyl phthalate (20) found in the plant Pachygone ovata (Poir.) Miers ex Hook. F. & Thomson is most likely of anthropogenic origin. The tests conducted with the isolated compound once again show the acute biological activity of such environmental pollutants. Di-n-octyl phthalate (20) was found to be cytotoxic towards MCF-7 breast cancer cells with an IC50 of 42.5 μg/mL. The compound was found to upregulate CASPASEs 3 and 9 and downregulate BCL2 gene expression, inducing BCL2 regulated apoptosis [150Amalarasi, L.E.; Jothi, G.J. BCL2 mediated apoptotic induction potential of the DNOP isolated from Pachygone ovata (Poir.) Miers ex Hook. F. & Thomson IN MCF-7 (human breast carcinoma). Int. Res. J. Pharm., 2019, 10, 190-194.
[http://dx.doi.org/10.7897/2230-8407.1003103] ]. Dioctyl phthalate, here isolated from Nigella glandulifera Freyn, was also identified as a tyrosinase inhibitor, which leads to an inhibition of melanogenesis [151Nguyen, D.T.M.; Nguyen, D.H.; Lyun, H.L.; Lee, H.B.; Shin, J.H.; Kim, E.K. Inhibition of melanogenesis by dioctyl phthalate isolated from Nigella glandulifera Freyn. J. Microbiol. Biotechnol., 2007, 17(10), 1585-1590.
[PMID: 18156772] ]. Other phthalates were found to be cytotoxic to MCF-7 (see Table 7).
Finally, the four phthalates, bis(2-ethylheptyl) phthalate (35), 2,12-diethyl-11-methylhexadecyl 2-ethyl-11-methylhexa- decyl phthalate (96), 2-ethyldecyl 2-ethylundecyl phthalate (97), and bis(2-ethyldodecyl) phthalate (34) isolated from the seahorse Hippocampus Kuda Bleeler, showed dose-dependent cathepsin B inhibition activities with IC50 values of 0.13 mM (1), 0.21 mM (2), 0.18 mM (3), and 0.29 mM (4), respectively [152Li, Y.; Qian, Z.J.; Kim, S.K. Cathepsin B inhibitory activities of three new phthalate derivatives isolated from seahorse, Hippocampus Kuda Bleeler. Bioorg. Med. Chem. Lett., 2008, 18(23), 6130-6134.
[http://dx.doi.org/10.1016/j.bmcl.2008.10.016] [PMID: 18938081] ]. Cathepsin B is a lysosomal cysteine protease of the papain family, which functions in intracellular protein catabolism.
CONCLUSION
Phthalates have been isolated from a multitude of different natural sources. Oftentimes, the phthalates are isolated as a bouquet of different phthalates, sometimes in conjunction with siloxanes, which definitely are of anthropogenic origin. This tends to signalize that in most of the cases, the phthalates themselves are of anthropogenic origin. While it is known that phthalates can stem from contamination from laboratory equipment, most of the phthalates found in natural sources may originate from fertilizers, other agrochemicals, irrigation water, or by import through the atmosphere. On the other hand, there are a few phthalates found in nature that are not produced industrially. As these differ solely in their O-alkyl groups, it must be considered whether enzymatic esterification/trans-esterification, starting from anthropogenic phthalates, may play a role. Furthermore, few phthalates have been found in nature in which the aromatic core is substituted further. Nevertheless, it has been shown that carbon-14 is built into secreted phthalates by B. atropurpurea filaments when cultured in a medium containing NaH14CO3. While a detailed, clear, and plausible biogenetic route to phthalates, should they be natural products, has not yet been forwarded, it could be shown with a fungal enzyme that dibutyl phthalate could be produced from glucose, indicating that a natural shikimic acid pathway to phthalates may exist. As phthalates upon isolation from various sources were seen as natural products, many of the biological assays typically carried out on newly identified natural compounds were also performed on them, leading to the recognition of their antimicrobial activity. These tests nicely complement the mandatory tests carried out on them as plasticizers produced industrially on a large scale.
LIST OF ABBREVIATIONS
| BBzP | = n-Butyl benzyl phthalate (1) |
| BCP | = n-Butyl cyclohexyl phthalate(2) |
| DAP | = Diallyl phthalate(3) |
| DnBT | = Di-n-butyl terephthalate(4) |
| DnBP | = Di-n-butyl phthalate(5) |
| DBEP | = Dibutoxy ethyl phthalate(6) |
| DEP | = Diethyl phthalate (7) |
| DET | = Diethyl terephthalate (8) |
| DEHP | = Bis(2-ethylhexyl) phthalate (9) |
| DEHT | = Di(2-ethylhexyl) terephthalate (10) |
| DcHP | = Dicyclohexyl phthalate (11) |
| DiBP | = Diisobutyl phthalate (12) |
| DIDP | = Diisodecyl phthalate (13) |
| DIHP | = Di-n-hexyl phthalate (14) |
| DINP | = Diisononyl phthalate (15) |
| DIOP | = Diisooctyl phthalate (16) |
| DMP | = Dimethyl phthalate (17) |
| DMT | = Dimethyl terephthalate (18) |
| DNPP | = Dipentyl phthalate (diamyl phthalate)(19) |
| DnOP | = Di-n-octyl phthalate (20) |
| DPHP | = Bis(2-propylheptyl) phthalate (21) |
| DPP | = Di-n-propyl phthalate (22) |
| EMP | = Ethyl methyl phthalate (23) |
| HDPE | = High density polyethene |
| LDPE | = Low density polyethene |
| MBzP | = Benzyl phthalate (24) |
| MnBP | = n-Butyl phthalate (25) |
| MEHP | = Ethylhexyl phthalate (26) |
| MnHP | = n-Hexyl phthalate (27) |
| MnOP | = n-Octyl phthalate (28) |
| MPA | = Monoalkyl phthalate |
| MW | = molecular weight |
| PVA | = Polyvinyl acetate |
| PVC | = Polyvinyl chloride |
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
PRISMA guidelines were followed in this study.
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
| [1] | Denune, Y.H. (Schaack Bros. Chem. Works), Esters of hexanol. US Pat. 1702188A, 1928. |
| [2] | De Witt, G.G.; Eastby, L.W. (EI Du Pont de Nemours Co.), Esters and process for producing them. US Pat. 1993736A, 1931. |
| [3] | Lorz, P.M.; Towae, F.K.; Enke, W.; Jäckh, R.; Bhargava, N.; Hillesheim, W. Phthalic Acid and Derivatives, 2007, [http://dx.doi.org/10.1002/14356007.a20_181.pub2] |
| [4] | Huang, J.; Nkrumah, P.N.; Li, Y.; Appiah-Sefah, G. Chemical behavior of phthalates under abiotic conditions in landfills. Rev. Environ. Contam. Toxicol., 2013, 224, 39-52. [http://dx.doi.org/10.1007/978-1-4614-5882-1_2] [PMID: 23232918] |
| [5] | Gmurek, M.; Olak-Kucharczyk, M.; Ledakowicz, S. Photochemical decomposition of endocrine disrupting compounds - A review. Chem. Eng. J., 2017, 310, 437-456. [http://dx.doi.org/10.1016/j.cej.2016.05.014] |
| [6] | Boll, M.; Geiger, R.; Junghare, M.; Schink, B. Microbial degradation of phthalates: biochemistry and environmental implications. Environ. Microbiol. Rep., 2020, 12(1), 3-15. [http://dx.doi.org/10.1111/1758-2229.12787] [PMID: 31364812] |
| [7] | Wang, Y.; Zhu, H.; Kannan, K. A review of biomonitoring of phthalate exposures. Toxics, 2019, 7(2), 21. [http://dx.doi.org/10.3390/toxics7020021] [PMID: 30959800] |
| [8] | Philips, E.M.; Santos, S.; Steegers, E.A.P.; Asimakopoulos, A.G.; Kannan, K.; Trasande, L.; Jaddoe, V.W. V. Maternal bisphenol and phthalate urine concentrations and weight gain during pregnancy Environ. Intern., 2020, 105342.. |
| [9] | Fan, J.C.; Ren, R.; Jin, Q.; He, H.L.; Wang, S.T. Detection of 20 phthalate esters in breast milk by GC-MS/MS using QuEChERS extraction method. Food Addit. Contam., A, 2019, 36, 1551-1558. |
| [10] | Albro, P.W.; Corbett, J.T. Distribution of di- and mono-(2-ethylhexyl) phthalate in human plasma. Transfusion, 1978, 18(6), 750-755. [http://dx.doi.org/10.1046/j.1537-2995.1978.18679077962.x] [PMID: 83042] |
| [11] | Rudel, R.A.; Dodson, R.E.; Perovich, L.J.; Morello-Frosch, R.; Camann, D.E.; Zuniga, M.M.; Yau, A.Y.; Just, A.C.; Brody, J.G. Semivolatile endocrine-disrupting compounds in paired indoor and outdoor air in two northern California communities. Environ. Sci. Technol., 2010, 44(17), 6583-6590. [http://dx.doi.org/10.1021/es100159c] [PMID: 20681565] |
| [12] | He, M.J.; Lu, J.F.; Ma, J.Y.; Wang, H.; Du, X.F. Organophosphate esters and phthalate esters in human hair from rural and urban areas, Chongqing, China: Concentrations, composition profiles and sources in comparison to street dust. Environ. Pollut., 2018, 237, 143-153. [http://dx.doi.org/10.1016/j.envpol.2018.02.040] [PMID: 29482020] |
| [13] | Shi, M.; Sun, Y.; Wang, Z.; He, G.; Quan, H.; He, H. Plastic film mulching increased the accumulation and human health risks of phthalate esters in wheat grains. Environ. Pollut., 2019, 250, 1-7. [http://dx.doi.org/10.1016/j.envpol.2019.03.064] [PMID: 30981178] |
| [14] | Wang, J.; Chen, G.; Christie, P.; Zhang, M.; Luo, Y.; Teng, Y. Occurrence and risk assessment of phthalate esters (PAEs) in vegetables and soils of suburban plastic film greenhouses. Sci. Total Environ., 2015, 523, 129-137. [http://dx.doi.org/10.1016/j.scitotenv.2015.02.101] [PMID: 25863503] |
| [15] | Iida, T.; Yanagisawa, K. (Sumitomo Chem. Co., Ltd., Japan) Agrochemical solid formulation, method for preparation and use in pest control, FR 3047639, 2017. |
| [16] | Zhang, H.; Hua, Y.; Chen, J.; Li, X.; Bai, X.; Wang, H. Organism-derived phthalate derivatives as bioactive natural products J. Environ. Sci., C, 2018, 36125 |
| [17] | Ortiz, A.; Sansinenea, E. Di-2-ethylhexylphthalate may be a natural product, rather than a pollutant. J. Chem. (Hindawi), 2018. [http://dx.doi.org/10.1155/2018/6040814] |
| [18] | Saeidnia, S.; Abdollahi, M. Are medicinal plants polluted with phthalates? Daru, 2013, 21(1), 43-45. [http://dx.doi.org/10.1186/2008-2231-21-43] [PMID: 23718122] |
| [19] | Murphy, J. Additives for plastics – Handbook., (2nd ed. ), 2001, |
| [20] | Phthalates | Assessing and Managing Chemicals Under TSCA. www.epa.gov2012. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/phthalates Retrieved on July 1stsup>, 2020 |
| [21] | Jia, P.; Xia, H.; Tang, K.; Zhou, Y. Plasticizers derived from biomass resources: a short review. Polymers (Basel), 2018, 10(12), 1303-1330. [http://dx.doi.org/10.3390/polym10121303] [PMID: 30961228] |
| [22] | Plasticizers - Chemical Economics Handbook (CEH). IHS Markit, 2018. |
| [23] | Barakat, R.; Lin, P.C.; Park, C.J.; Zeineldin, M.; Zhou, S.; Rattan, S.; Brehm, E.; Flaws, J.A.; Ko, C.J. Germline-dependent transmission of male reproductive traits induced by an endocrine disruptor, di-2-ethylhexyl phthalate, in future generations. Sci. Rep., 2020, 10(1), 5705. [http://dx.doi.org/10.1038/s41598-020-62584-w] [PMID: 32235866] |
| [24] | Kim, J.H. Di(2-ethylhexyl) phthalate promotes lung cancer cell line A549 progression via Wnt/β-catenin signaling. J. Toxicol. Sci., 2019, 44(4), 237-244. [http://dx.doi.org/10.2131/jts.44.237] [PMID: 30944277] |
| [25] | Tas, I.; Zou, R.; Park, S.Y.; Yang, Y.; Gamage, C.D.B.; Son, Y.J.; Paik, M.J.; Kim, H. Inflammatory and tumorigenic effects of environmental pollutants found in particulate matter on lung epithelial cells. Toxicol. In Vivo, 2019, 59, 300-311. |
| [26] | Hu, D.; Wang, Y.X.; Chen, W.J.; Zhang, Y.; Li, H.H.; Xiong, L.; Zhu, H.P.; Chen, H.Y.; Peng, S.X.; Wan, Z.H.; Zhang, Y.; Du, Y.K. Associations of phthalates exposure with attention deficits hyperactivity disorder: A case-control study among Chinese children. Environ. Pollut., 2017, 229, 375-385. [http://dx.doi.org/10.1016/j.envpol.2017.05.089] [PMID: 28614761] |
| [27] | Zuccarello, P.; Oliveri Conti, G.; Cavallaro, F.; Copat, C.; Cristaldi, A.; Fiore, M.; Ferrante, M. Implication of dietary phthalates in breast cancer. A systematic review. Food Chem. Toxicol., 2018, 118, 667-674. [http://dx.doi.org/10.1016/j.fct.2018.06.011] [PMID: 29886235] |
| [28] | Zhang, Y.; Dong, T.; Hu, W.; Wang, X.; Xu, B.; Lin, Z.; Hofer, T.; Stefanoff, P.; Chen, Y.; Wang, X.; Xia, Y. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environ. Int., 2019, 123, 325-336. [http://dx.doi.org/10.1016/j.envint.2018.11.076] [PMID: 30557812] |
| [29] | Duan, Y.; Wang, L.; Han, L.; Wang, B.; Sun, H.; Chen, L.; Zhu, L.; Luo, Y. Exposure to phthalates in patients with diabetes and its association with oxidative stress, adiponectin, and inflammatory cytokines. Environ. Int., 2017, 109, 53-63. [http://dx.doi.org/10.1016/j.envint.2017.09.002] [PMID: 28938100] |
| [30] | Lopez-Carrillo, L.; Cebrian, M.E. Cognitive function.Effects of Persistent and Bioactive Organic Pollutants on Human Health., 2013, 400-420. [http://dx.doi.org/10.1002/9781118679654.ch15] |
| [31] | Shin, H.M.; Schmidt, R.J.; Tancredi, D.; Barkoski, J.; Ozonoff, S.; Bennett, D.H.; Hertz-Picciotto, I. Prenatal exposure to phthalates and autism spectrum disorder in the MARBLES study. Environ. Health, 2018, 17(1), 85. [http://dx.doi.org/10.1186/s12940-018-0428-4] [PMID: 30518373] |
| [32] | Singh, A.; Kumar, R.; Singh, J.K. Singh; Tanuja, K.S. Di (2-ethylhexyl) phthalate induced toxicological effects on reproductive system of female mice mus-musculus. J. Ecophysiol. Occup. Health, 2019, 19, 71-75. |
| [33] | Herr, C.; zur Nieden, A.; Koch, H.M.; Schuppe, H.C.; Fieber, C.; Angerer, J.; Eikmann, T.; Stilianakis, N.I. Urinary di(2-ethylhexyl)phthalate (DEHP)--metabolites and male human markers of reproductive function. Int. J. Hyg. Environ. Health, 2009, 212(6), 648-653. [http://dx.doi.org/10.1016/j.ijheh.2009.08.001] [PMID: 19733116] |
| [34] | Gu, J.D. Microbial colonization of polymeric materials for space applications and mechanisms of biodeterioration: a review. Int. Biodeterior. Biodegradation, 2007, 59, 170-179. [http://dx.doi.org/10.1016/j.ibiod.2006.08.010] |
| [35] | Li, J.X.; Gu, J.D.; Pan, L. Transformation of dimethyl phthalate, dimethyl isophthalate and dimethyl terephthalate by Rhodococcus rubber Sa and modeling the processes using the modified Gompertz model. Int. Biodeterior. Biodegradation, 2005, 55, 223-232. [http://dx.doi.org/10.1016/j.ibiod.2004.12.003] |
| [36] | Sablayrolles, C.; Silvestre, J.; Lhoutellier, C.; Montrejaud-Vignoles, M. Phthalates uptake by tomatoes after biosolids application: worst case and operational practice in greenhouse conditions. Fresenius Environ. Bull., 2013, 22, 1061-1069. |
| [37] | Cai, Q.Y.; Mo, C.H.; Wu, Q.T.; Zeng, Q.Y. Polycyclic aromatic hydrocarbons and phthalic acid esters in the soil-radish (Raphanus sativus) system with sewage sludge and compost application. Bioresour. Technol., 2008, 99(6), 1830-1836. [http://dx.doi.org/10.1016/j.biortech.2007.03.035] [PMID: 17502135] |
| [38] | Cao, X.L.; Zhao, W.; Dabeka, R. Di-(2-ethylhexyl) adipate and 20 phthalates in composite food samples from the 2013 Canadian Total Diet Study. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess., 2015, 32(11), 1893-1901. [http://dx.doi.org/10.1080/19440049.2015.1079742] [PMID: 26359692] |
| [39] | Sannino, A. Development of a gas chromatographic/mass spectrometric method for determination of phthalates in oily foods. J. AOAC Int., 2010, 93(1), 315-322. [http://dx.doi.org/10.1093/jaoac/93.1.315] [PMID: 20334193] |
| [40] | Cocchieri, R.A. Occurrence of phthalate esters in Italian packaged foods. J. Food Prot., 1986, 49(4), 265-266. [http://dx.doi.org/10.4315/0362-028X-49.4.265] [PMID: 30959656] |
| [41] | Gibson, R.; Wang, M.J.; Padgett, E.; Beck, A.J. Analysis of 4-nonylphenols, phthalates, and polychlorinated biphenyls in soils and biosolids. Chemosphere, 2005, 61(9), 1336-1344. [http://dx.doi.org/10.1016/j.chemosphere.2005.03.072] [PMID: 15979687] |
| [42] | Vikelsøe, J.; Thomsen, M.; Carlsen, L. Phthalates and nonylphenols in profiles of differently dressed soils. Sci. Total Environ., 2002, 296(1-3), 105-116. [http://dx.doi.org/10.1016/S0048-9697(02)00063-3] [PMID: 12398330] |
| [43] | Kong, S.; Ji, Y.; Liu, L.; Chen, L.; Zhao, X.; Wang, J.; Bai, Z.; Sun, Z. Diversities of phthalate esters in suburban agricultural soils and wasteland soil appeared with urbanization in China. Environ. Pollut., 2012, 170, 161-168. [http://dx.doi.org/10.1016/j.envpol.2012.06.017] [PMID: 22813629] |
| [44] | Niu, L.; Xu, Y.; Xu, C.; Yun, L.; Liu, W. Status of phthalate esters contamination in agricultural soils across China and associated health risks. Environ. Pollut., 2014, 195, 16-23. [http://dx.doi.org/10.1016/j.envpol.2014.08.014] [PMID: 25194267] |
| [45] | Li, K.; Ma, D.; Wu, J.; Chai, C.; Shi, Y. Distribution of phthalate esters in agricultural soil with plastic film mulching in Shandong Peninsula, East China. Chemosphere, 2016, 164, 314-321. [http://dx.doi.org/10.1016/j.chemosphere.2016.08.068] [PMID: 27596820] |
| [46] | Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ., 2016, 550, 690-705. [http://dx.doi.org/10.1016/j.scitotenv.2016.01.153] [PMID: 26849333] |
| [47] | Wang, K.; Song, N.; Cui, M.; Shi, Y. Phthalate esters migration from two kinds of plastic films and the enrichment in peanut plant. Fresenius Environ. Bull., 2017, 26, 4409-4415. |
| [48] | Fernández, M.A.; Gómara, B.; González, M.J. The Handbook of Environmental Chemistry, 2012, , 337-374. |
| [49] | Zhang, Y.; Liang, Q.; Gao, R.; Hou, H.; Tan, W.; He, X.; Zhang, H.; Yu, M.; Ma, L.; Xi, B.; Wang, X.; Ma, L.; Xi, B.; Wang, X. Contamination of phthalate esters (PAEs) in typical wastewater-irrigated agricultural soils in Hebei, North China. PLoS One, 2015, 10(9)e0137998 [http://dx.doi.org/10.1371/journal.pone.0137998] [PMID: 26360905] |
| [50] | Ligocki, M.P.; Leuenberger, C.; Pankow, J.F. Trace organic compounds in rain—II. Gas scavenging of neutral organic compounds. Atmos. Environ., 1985, 19, 1609-1617. [http://dx.doi.org/10.1016/0004-6981(85)90213-6] |
| [51] | Ligocki, M.P.; Leuenberger, C.; Pankow, J.F. Trace organic compounds in rain—III. Particle scavenging of neutral organic compounds. Atmos. Environ., 1985, 19, 1619-1626. [http://dx.doi.org/10.1016/0004-6981(85)90214-8] |
| [52] | Hongjun, Y.; Wenjun, X.; Qing, L.; Jingtao, L.; Hongwen, Y.; Zhaohua, L. Distribution of phthalate esters in topsoil: a case study in the Yellow River Delta. China. Environ. Monit. Assess., 2013, 185, 8489-8500. [http://dx.doi.org/10.1007/s10661-013-3190-7] [PMID: 23609921] |
| [53] | Wang, J.; Luo, Y.; Teng, Y.; Ma, W.; Christie, P.; Li, Z. Soil contamination by phthalate esters in Chinese intensive vegetable production systems with different modes of use of plastic film. Environ. Pollut., 2013, 180, 265-273. [http://dx.doi.org/10.1016/j.envpol.2013.05.036] [PMID: 23792387] |
| [54] | He, L.; Gielen, G.; Bolan, N.S.; Zhang, X.; Qin, H.; Huang, H.; Wang, H. Contamination and remediation of phthalic acid esters in agricultural soils in China: a review. Agron. Sustain. Dev., 2014, 35, 519-534. [http://dx.doi.org/10.1007/s13593-014-0270-1] |
| [55] | Gonzalez-Villa, F.J.; Saiz-Jimenez, C.; Martin, F. Identification of free organic chemicals found in composted municipal refuse. J. Environ. Qual., 1982, 11, 251-254. [http://dx.doi.org/10.2134/jeq1982.00472425001100020021x] |
| [56] | Shea, P.J.; Weber, J.B.; Overcash, M.R. Uptake and phytotoxicity of di-n-butyl phthalate in corn (Zea mays). Bull. Environ. Contam. Toxicol., 1982, 29(2), 153-158. [http://dx.doi.org/10.1007/BF01606143] [PMID: 7126902] |
| [57] | Yi, N.; Zhao, Y.; Li, J.; Wei, Z.; Zhao, Y. (Hunan Zhongmao Biotechnology Co., Ltd.) Method for removing plasticizer from plant-derived flavonoid extract CN 109320561, 2019. |
| [58] | Hankett, J.M.; Collin, W.R.; Chen, Z. Molecular structural changes of plasticized PVC after UV light exposure. J. Phys. Chem. B, 2013, 117(50), 16336-16344. [http://dx.doi.org/10.1021/jp409254y] [PMID: 24283894] |
| [59] | Gledhill, W.E.; Kaley, R.G.; Adams, W.J.; Hicks, O.; Michael, P.R.; Saeger, V.W.; Leblanc, G.A. An environmental safety assessment of butyl benzyl phthalate. Environ. Sci. Technol., 1980, 14(3), 301-305. [http://dx.doi.org/10.1021/es60163a001] [PMID: 22276719] |
| [60] | Staples, C.A.; Peterson, D.R.; Parkerton, T.F.; Adams, W.J. The environmental fate of phthalates esters: a literature review. Chemosphere, 1997, 35, 667-749. [http://dx.doi.org/10.1016/S0045-6535(97)00195-1] |
| [61] | Cartwright, C.D.; Thompson, I.P.; Burns, R.G. Degradation and impact of phthalate plasticizers on soil microbial communities. Environ. Toxicol. Chem., 2000, 19, 1253-1261. [http://dx.doi.org/10.1002/etc.5620190506] |
| [62] | Nallii, S.; Cooper, D.G.; Nicell, J.A. Biodegradation of plasticizers by Rhodococcus rhodochrous. Biodegradation, 2002, 13(5), 343-352. [http://dx.doi.org/10.1023/A:1022313810852] [PMID: 12688586] |
| [63] | Paluselli, A.; Fauvelle, V.; Galgani, F.; Sempéré, R. Phthalate release from plastic fragments and degradation in seawater. Environ. Sci. Technol., 2019, 53(1), 166-175. [http://dx.doi.org/10.1021/acs.est.8b05083] [PMID: 30479129] |
| [64] | Carrara, S.M.; Morita, D.M.; Boscov, M.E. Biodegradation of di(2-ethylhexyl)phthalate in a typical tropical soil. J. Hazard. Mater., 2011, 197, 40-48. [http://dx.doi.org/10.1016/j.jhazmat.2011.09.058] [PMID: 22014440] |
| [65] | Chang, B.V.; Liao, C.S.; Yuan, S.Y. Anaerobic degradation of diethyl phthalate, di-n-butyl phthalate, and di-(2-ethylhexyl) phthalate from river sediment in Taiwan. Chemosphere, 2005, 58(11), 1601-1607. [http://dx.doi.org/10.1016/j.chemosphere.2004.11.031] [PMID: 15694480] |
| [66] | Camacho-Munoz, G.A.; Llanos, C.H.; Berger, P.A.; Miosso, C.J.; da Rocha, A.F. Occurrence of pharmaceutical compounds in wastewater and sludge from wastewater treatment plants: removal and ecotoxicological impact of wastewater discharges and sludge disposal. Conf. Proc. IEEE Eng. Med. Biol. Soc., 2012, •••, 6508-6513. |
| [67] | Boonyaroj, V.; Chiemchaisri, C.; Chiemchaisri, W.; Yamamoto, K. Removal of organic micro-pollutants from solid waste landfill leachate in membrane bioreactor operated without excess sludge discharge. Water Sci. Technol., 2012, 66(8), 1774-1780. [http://dx.doi.org/10.2166/wst.2012.324] [PMID: 22907464] |
| [68] | Kamata, I.; Tsutsui, G.; Takana, J.; Shirai, T. Phthalic acid esters in fish in the Uji river, Kyoto-fu. Eisei Kogai Kenkyusho Nenpo, 1977, 22, 114-116. |
| [69] | Dycus, D.L. Technical report series: North Alabama water quality assessment Contaminants in biota, 1986, 7(TVA/ONRED/AWR-86/33) Order No. DE87900603. |
| [70] | Ray, L.E.; Murray, H.E.; Giam, C.S. Organic pollutants in marine samples from Portland, Maine. Chemosphere, 1983, 12, 1031-1038. [http://dx.doi.org/10.1016/0045-6535(83)90255-2] |
| [71] | Zhang, Y.H.; Chen, B.H.; Zheng, L.X. Determination of phthalates in environmental samples. Huanjing Yu Jiankang Zazhi, 2003, 20, 283-286. |
| [72] | Wahidullah, S.; Naik, B.G.; Al-Fadhli, A.A. Chemotaxonomic study of the demosponge Cinachyrella cavernosa (Lamarck). Biochem. Syst. Ecol., 2015, 58, 91-96. [http://dx.doi.org/10.1016/j.bse.2014.11.001] |
| [73] | Xie, Z.; Ebinghaus, R.; Temme, C.; Lohmann, R.; Caba, A.; Ruck, W. Atmospheric concentrations and air–sea exchanges of phthalates in the North Sea (German Bight). Atmos. Environ., 2005, 39, 3209-3219. [http://dx.doi.org/10.1016/j.atmosenv.2005.02.021] |
| [74] | Paluselli, A.; Fauvelle, V.; Schmidt, N.; Galgani, F.; Net, S.; Sempéré, R. Distribution of phthalates in Marseille Bay (NW Mediterranean Sea). Sci. Total Environ., 2018, 621, 578-587. [http://dx.doi.org/10.1016/j.scitotenv.2017.11.306] [PMID: 29195205] |
| [75] | Lee, Y.M.; Lee, J.E.; Choe, W.; Kim, T.; Lee, J.Y.; Kho, Y.; Choi, K.; Zoh, K.D. Distribution of phthalate esters in air, water, sediments, and fish in the Asan Lake of Korea. Environ. Int., 2019, 126, 635-643. [http://dx.doi.org/10.1016/j.envint.2019.02.059] [PMID: 30856451] |
| [76] | Xie, Z.; Ebinghaus, R.; Temme, C.; Lohmann, R.; Caba, A.; Ruck, W. Occurrence and air-sea exchange of phthalates in the Arctic. Environ. Sci. Technol., 2007, 41(13), 4555-4560. [http://dx.doi.org/10.1021/es0630240] [PMID: 17695896] |
| [77] | Foster, G.D.; Baksi, S.M.; Means, J.C. Bioaccumulation of trace organic contaminants from sediment by Baltic clams (Macoma balthica) and soft-shell clams (Mya arenaria). Environ. Toxicol. Chem., 1987, 6, 969-976. [http://dx.doi.org/10.1002/etc.5620061209] |
| [78] | Guerranti, C.; Cau, A.; Renzi, M.; Badini, S.; Grazioli, E.; Perra, G.; Focardi, S.E. Phthalates and perfluorinated alkylated substances in Atlantic bluefin tuna (Thunnus thynnus) specimens from Mediterranean Sea (Sardinia, Italy): Levels and risks for human consumption. J. Environ. Sci. Health B, 2016, 51(10), 661-667. [http://dx.doi.org/10.1080/03601234.2016.1191886] [PMID: 27323803] |
| [79] | Musial, C.J.; Uthe, J.; Sirota, G.R.; Burns, B.G.; Gilgan, M.W.; Zitko, V.; Matheson, R.A. Di-n-hexyl phthalate (DHP), a newly identified contaminant in Atlantic herring (Clupea harengus harengus) and Atlantic mackerel (Scomber scombrus). Can. J. Fish. Aquat. Sci., 1981, •••, 856-859. [http://dx.doi.org/10.1139/f81-113] |
| [80] | Ostrovsky, I.; Čabala, R.; Kubinec, R.; Górová, R.; Blaško, J.; Kubincová, J.; Řimnáčová, L.; Lorenz, W. Determination of phthalate sum in fatty food by gas chromatography. Food Chem., 2011, 124, 392-395. [http://dx.doi.org/10.1016/j.foodchem.2010.06.045] |
| [81] | Jarašová, A.; Puškárová, L.; Di Stancová, V. -2-ethylhexyl phthalate and di-n-butyl phthalate in tissues of common carp (Cyprinus carpio L.) after harvest and after storage in fish storage tank. J. Microbiol. Biotechnol. Food Sci., 2012, 1, 277-286. |
| [82] | Williams, D.T. Dibutyl- and di-(2-ethylhexyl)phthalate in fish. J. Agric. Food Chem., 1973, 21(6), 1128-1129. [http://dx.doi.org/10.1021/jf60190a028] [PMID: 4755838] |
| [83] | Servaes, K.; Van Holderbeke, M.; Geerts, L.; Sioen, I.; Fierens, T.; Voorspels, S.; Vanermen, G. Phthalate contamination in food: occurrence on the Belgian market and possible contamination pathways. Organohalogen Compd., 2011, 73, 291-294. |
| [84] | Bradley, E.L.; Burden, R.A.; Bentayeb, K.; Driffield, M.; Harmer, N.; Mortimer, D.N.; Speck, D.R.; Ticha, J.; Castle, L. Exposure to phthalic acid, phthalate diesters and phthalate monoesters from foodstuffs: UK total diet study results. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess., 2013, 30(4), 735-742. [http://dx.doi.org/10.1080/19440049.2013.781684] [PMID: 23641808] |
| [85] | Beltifa, A.; Feriani, A.; Machreki, M.; Ghorbel, A.; Ghazouani, L.; Di Bella, G.; Van Loco, J.; Reyns, T.; Mansour, H.B. Plasticizers and bisphenol A, in packaged foods sold in the Tunisian markets: study of their acute in vivo toxicity and their environmental fate. Environ. Sci. Pollut. Res. Int., 2017, 24(28), 22382-22392. [http://dx.doi.org/10.1007/s11356-017-9861-0] [PMID: 28801775] |
| [86] | Cheng, Z.; Nie, X.P.; Wang, H.S.; Wong, M.H. Risk assessments of human exposure to bioaccessible phthalate esters through market fish consumption. Environ. Int., 2013, 57-58, 75-80. [http://dx.doi.org/10.1016/j.envint.2013.04.005] [PMID: 23688402] |
| [87] | Cao, X.L. Phthalate esters in foods: sources, occurrence, and analytical methods. Compr. Rev. Food Sci. Food Saf., 2010, 9(1), 21-43. [http://dx.doi.org/10.1111/j.1541-4337.2009.00093.x] [PMID: 33467808] |
| [88] | Jeevitha, T.; Deepa, K.; Michael, A. In vitro study on the anti-microbial efficacy of Aloe vera against Candida albicans. Afr. J. Microbiol. Res., 2018, 12, 930-937. [http://dx.doi.org/10.5897/AJMR2015.7631] |
| [89] | Umano, K.; Hagi, Y.; Nakahara, K.; Shoji, A.; Shibamoto, T. Volatile constituents of green and ripened pineapple (Ananas comosus [L.] Merr.). J. Agric. Food Chem., 1992, 40, 599-603. [http://dx.doi.org/10.1021/jf00016a014] |
| [90] | Lan, X.; Xue, Y.; Chen, X.; Yang, Y. Aromatic components of Hongdao clam by HS-SPME and GC-MS. Adv. Mat. Res. (Durnten-Zurich), 2013, 709, 49-52. [http://dx.doi.org/10.4028/www.scientific.net/AMR.709.49] |
| [91] | Liu, J.; Lu, B.T.; Xiao, S.Y. Analysis and evaluation of flavor substances in four sea clam muscles. Huanjing Yu Jiankang Zazhi, 2008, 25, 633-634. |
| [92] | Silva, F.A.; Liotti, R.G.; Boleti, A.P.A.; Reis, É.M.; Passos, M.B.S.; Dos Santos, E.L.; Sampaio, O.M.; Januário, A.H.; Branco, C.L.B.; Silva, G.F.D.; Mendonça, E.A.F.; Soares, M.A. Diversity of cultivable fungal endophytes in Paullinia cupana (Mart.) Ducke and bioactivity of their secondary metabolites. PLoS One, 2018, 13(4)e0195874 [http://dx.doi.org/10.1371/journal.pone.0195874] [PMID: 29649297] |
| [93] | MacKenzie, S.E.; Gurusamy, G.S.; Piórko, A.; Strongman, D.B.; Hu, T.; Wright, J.L.C. Isolation of sterols from the marine fungus Corollospora lacera. Can. J. Microbiol., 2004, 50(12), 1069-1072. [http://dx.doi.org/10.1139/w04-103] [PMID: 15714238] |
| [94] | Nguyen, D.H.; Nguyen, D.T.M.; Kim, E.K. Effects of di-(2-ethylhexyl) phthalate (DEHP) released from laboratory equipments. Korean J. Chem. Eng., 2008, 25, 1136-1139. [http://dx.doi.org/10.1007/s11814-008-0186-z] |
| [95] | Reid, A.M.; Brougham, C.A.; Fogarty, A.M.; Roche, J.J. An investigation into possible sources of phthalate contamination in the environmental analytical laboratory. Int. J. Environ. Anal. Chem., 2007, 87, 125-133. [http://dx.doi.org/10.1080/03067310601071183] |
| [96] | Yang, T.; Zhang, C.X.; Cai, E.B.; Bao, J.C.; Zheng, Y.L. Analysis of chemical composition of volatile oil from underground part of Astilbe chinensis (Maxim.) Franch.et Sav using GC – MS. Ziyuan Kaifa Yu Shichang, 2011, 27, 106-107. |
| [97] | Kavitha, A.; Prabhakar, P.; Vijayalakshmi, M.; Venkateswarlu, Y. Production of bioactive metabolites by Nocardia levis MK-VL_113. Lett. Appl. Microbiol., 2009, 49(4), 484-490. [http://dx.doi.org/10.1111/j.1472-765X.2009.02697.x] [PMID: 19708882] |
| [98] | Qin, L.Q.; Liu, X.X.; Luo, S.Y.; Yang, D. GC-MS comparative analysis of low-polarity compounds of Amaranthus caudatus L. Anhui Nongye Kexue, 2015, 43, 81-83. |
| [99] | Asilbekova, D.T.; Gusakova, S.D.; Glushenkova, A.I. Lipids in fruits of Acanthopanax sessiliflorus., 1985, , 760-766. |
| [100] | Kumari, N.; Menghani, E.; Mithal, R. GCMS analysis & assessment of antimicrobial potential of rhizospheric Actinomycetes of AIA3 isolate. Indian J. Tradit. Knowl., 2020, 19, 111-119. |
| [101] | Bai, X.L.; Yu, R.L.; Li, M.Z.; Zhang, H.W. Antimicrobial assay of endophytic fungi from Rumex madaio and chemical study of strain R1. Bangladesh J. Pharmacol., 2019, 14, 129-135. [http://dx.doi.org/10.3329/bjp.v14i3.41598] |
| [102] | Cakir, A.; Mavi, A.; Yildirim, A.; Duru, M.E.; Harmandar, M.; Kazaz, C. Isolation and characterization of antioxidant phenolic compounds from the aerial parts of Hypericum hyssopifolium L. by activity-guided fractionation. J. Ethnopharmacol., 2003, 87(1), 73-83. [http://dx.doi.org/10.1016/S0378-8741(03)00112-0] [PMID: 12787957] |
| [103] | JP 57063379 (Casio Computer Co.) Guest-host effect liquid crystal display devices, 1982. |
| [104] | Eaton, R.W.; Ribbons, D.W. Metabolism of dibutylphthalate and phthalate by Micrococcus sp. strain 12B. J. Bacteriol., 1982, 151(1), 48-57. [http://dx.doi.org/10.1128/JB.151.1.48-57.1982] [PMID: 7085570] |
| [105] | Fourgous, C.; Chevreuil, M.; Alliot, F.; Amilhat, E.; Faliex, E.; Paris-Palacios, S.; Teil, M.J.; Goutte, A. Phthalate metabolites in the European eel (Anguilla anguilla) from Mediterranean coastal lagoons. Sci. Total Environ., 2016, 569-570, 1053-1059. [http://dx.doi.org/10.1016/j.scitotenv.2016.06.159] [PMID: 27412480] |
| [106] | McConnell, M.L. Distribution of phthalate monoesters in an aquatic food web. Master Report No. 426. School of Resource and Environmental Management, Simon Frazer University, 2007. |
| [107] | Blair, J.D.; Ikonomou, M.G.; Kelly, B.C.; Surridge, B.; Gobas, F.A. Ultra-trace determination of phthalate ester metabolites in seawater, sediments, and biota from an urbanized marine inlet by LC/ESI-MS/MS. Environ. Sci. Technol., 2009, 43(16), 6262-6268. [http://dx.doi.org/10.1021/es9013135] [PMID: 19746723] |
| [108] | Lucas, E.M.F.; Abreu, L.M.; Marriel, I.E.; Pfenning, L.H.; Takahashi, J.A. Phthalates production from Curvularia senegalensis (Speg.) Subram, a fungal species associated to crops of commercial value. Microbiol. Res., 2008, 163(5), 495-502. [http://dx.doi.org/10.1016/j.micres.2007.02.003] [PMID: 17462873] |
| [109] | Dar, S.A.; Yousuf, A.R.; Ganai, F.A.; Sharma, P.; Kumar, N.; Singh, R. Bioassay guided isolation and identification of anti-inflammatory and antimicrobial compounds from Urtica dioica L. (Urticaceae) leaves. Afr. J. Biotechnol., 2012, 11, 12910-12920. |
| [110] | Durak, H.; Aysu, T. Structural analysis of bio-oils from subcritical and supercritical hydrothermal liquefaction of Datura stramonium L. J. Supercrit. Fluids, 2016, 108, 123-135. [http://dx.doi.org/10.1016/j.supflu.2015.10.016] |
| [111] | Al-Shammari, L.A.; Hassan, W.H.B.; Al-Youssef, H.M. Chemical composition and antimicrobial activity of the essential oil and lipid content of Carduus pycnocephalus L. growing in Saudi Arabia. J. Chem. Pharm. Res., 2012, 4, 1281-1287. |
| [112] | Wu, J.; Sun, D.; Li, X.; Chen, J.; He, L.; Dong, W. GC-MS analysis of liposoluble constituents in Isatis indigotica. Zhongguo Yaofang, 2008, 19, 2354-2356. |
| [113] | Tao, Y.; Li, H.; Gu, J.; Shi, H.; Han, S.; Jiao, Y.; Zhong, G.; Zhang, Q.; Akindolie, M.S.; Lin, Y.; Chen, Z.; Zhang, Y. Metabolism of diethyl phthalate (DEP) and identification of degradation intermediates by Pseudomonas sp. DNE-S1. Ecotoxicol. Environ. Saf., 2019, 173, 411-418. [http://dx.doi.org/10.1016/j.ecoenv.2019.02.055] [PMID: 30798184] |
| [114] | Wang, Y.; Liu, H.; Peng, Y.E.; Tong, L.; Feng, L.; Ma, K. New pathways for the biodegradation of diethyl phthalate by Sphingobium yanoikuyae SHJ, Proc. Biochem., 2018, 71, 152-158. |
| [115] | Poofery, J.; Khaw-on, P.; Subhawa, S.; Sripanidkulchai, B.; Tantraworasin, A.; Saeteng, S.; Siwachat, S. Lertprasertsuke, N.; Banjerdpongchai, R. Potential of Thai herbal extracts on lung cancer treatment by inducing apoptosis and synergizing chemotherapy. Molecules, 2020, 25, 231-261. [http://dx.doi.org/10.3390/molecules25010231] |
| [116] | Vijayalakshmi, U.; Shourie, A. Comparative GC-MS analysis of secondary metabolites from leaf, stem and callus of Glycyrrhiza glabra. World J. Pharm. Res., 2019, 8, 1915-1923. |
| [117] | Mohan, S.C.; Anand, T. Comparative study of identification of bioactive compounds from Barringtonia acutangula leaves and bark extracts and its biological activity. J. Appl. Sci. (Faisalabad), 2019, 19, 528-536. [http://dx.doi.org/10.3923/jas.2019.528.536] |
| [118] | He, X.; Huang, X.H.H.; Chow, K.S.; Wang, Q.; Zhang, T.; Wu, D.; Yu, J.Z. Abundance and sources of phthalic acids, benzene-tricarboxylic acids, and phenolic acids in PM2.5 at urban and suburban sites in Southern China. ACS Earth Space Chem., 2018, 2, 147-158. [http://dx.doi.org/10.1021/acsearthspacechem.7b00131] |
| [119] | Lessmann, F.; Kolossa-Gehring, M.; Apel, P.; Rüther, M.; Pälmke, C.; Harth, V.; Brüning, T.; Koch, H.M. German Environmental Specimen Bank: 24-hour urine samples from 1999 to 2017 reveal rapid increase in exposure to the para-phthalate plasticizer di(2-ethylhexyl) terephthalate (DEHTP). Environ. Int., 2019, 132105102 [http://dx.doi.org/10.1016/j.envint.2019.105102] [PMID: 31491609] |
| [120] | Zekriardehani, S.; Joshi, A.S.; Jabarin, S.A.; Gidley, D.W.; Coleman, M.R. Effect of dimethyl terephthalate and dimethyl isophthalate on the free volume and barrier properties of poly(ethylene terephthalate) (PET): amorphous PET. Macromolecules, 2018, 51, 456-467. [http://dx.doi.org/10.1021/acs.macromol.7b02230] |
| [121] | Huang, X.; Yi, Y.; Zhang, X.; Yang, L.; Xu, X. Constituents in essential oil of Dendrobium nobile with GC-MS. Shizhen Guoyi Guoyao, 2010, 21, 889-891. |
| [122] | Jin, Q.; Jiao, C.; Sun, S.; Cheng, C.; Yongping, L.; Fan, H.; Zhu, Y. Metabolic analysis of medicinal Dendrobium officinale and Dendrobium huoshanense during different growth years. PLoS One, 2016, 11, e0146607/1-e0146607/17. |
| [123] | He, K.; Cao, T.W.; Wang, H.L.; Geng, C.A.; Zhang, X.M.; Chen, J.J. [Chemical constituents of Swertia angustifolia.]. Zhongguo Zhongyao Zazhi, 2015, 40(18), 3603-3607. [PMID: 26983208] |
| [124] | Zhang, X.; Pei, Y.; Liu, M.; Kang, S.; Zhang, J. Organic acids from leaves of Cerbera manghas. Zhongcaoyao, 2010, 41, 1763-1765. |
| [125] | Abbasi, A.; Zaidi, Z.H. Isolation of isophthalic acid from Candida tropicalis. J. Chem. Soc. Pak., 1980, 2, 49-49. |
| [126] | He, J.; Yang, L.; Wang, Y.; Mu, Z.; Zou, Z.; Wang, X.; Zhong, G. A new isophthalic acid from Talaromyces flavus. Chem. Nat. Compd., 2017, 53, 409-411. [http://dx.doi.org/10.1007/s10600-017-2010-7] |
| [127] | Hokko Chem. Ind., Ltd. Trimellitic acid esters for quality improvement of agrochemical powder preparation JP 57159702, 1982. |
| [128] | Chen, Y. (P.R.C.) Long-acting slow release fertilizer dedicated for apple CN 104892245, 2015. |
| [129] | Singh, N.; Mahmood, U.; Kaul, V.K.; Jirovetz, L. A new phthalic acid ester from Ajuga bracteosa. Nat. Prod. Res., 2006, 20(6), 593-597. [http://dx.doi.org/10.1080/14786410500185550] [PMID: 16835093] |
| [130] | Afza, N.; Yasmeen, S.; Ferheen, S.; Malik, A.; Ali, M.I.; Kalhoro, M.A.; Ifzal, R. New aromatic esters from Galinsoga parviflora. J. Asian Nat. Prod. Res., 2012, 14(5), 424-428. [http://dx.doi.org/10.1080/10286020.2012.657181] [PMID: 22348678] |
| [131] | Ur Rehman, N.; Ahmad, N.; Hussain, J.; Liaqit, A.; Hussain, H.; Bakht, N.; Al-Harrasi, A.; Shinwari, Z.K. One new phthalate derivative from Nepeta kurramensis. Chem. Nat. Compd., 2017, 53, 426-428. [http://dx.doi.org/10.1007/s10600-017-2014-3] |
| [132] | Saleem, M.; Nazir, M.; Akhtar, N.; Onocha, P.A.; Riaz, N.; Jabbar, A.; Shaiq Ali, M.; Sultana, N. New phthalates from Phyllanthus muellerianus (Euphorbiaceae). J. Asian Nat. Prod. Res., 2009, 11(11), 974-977. [http://dx.doi.org/10.1080/10286020903341388] [PMID: 20183263] |
| [133] | Khan, D.; Zhao, W.; Ahmad, S.; Khan, S. New antioxidant and cholinesterase inhibitory constituents from Lonicera quinquelocularis. J. Med. Plants Res., 2014, 8, 313-317. [http://dx.doi.org/10.5897/JMPR2013.5245] |
| [134] | Khan, D.; Khan, H.U.; Khan, F.; Khan, S.; Badshah, S.; Khan, A.S.; Samad, A.; Ali, F.; Khan, I.; Muhammad, N. New cholinesterase inhibitory constituents from Lonicera quinquelocularis. PLoS One, 2014, 9(4)e94952 [http://dx.doi.org/10.1371/journal.pone.0094952] [PMID: 24733024] |
| [135] | Chen, M.; Zhang, W.; Shao, C.L.; Chi, Z.M.; Wang, C.Y. DNA methyltransferase inhibitor induced fungal biosynthetic products: Diethylene glycol phthalate ester oligomers from the marine-derived fungus Cochliobolus lunatus. Mar. Biotechnol. (NY), 2016, 18(3), 409-417. [http://dx.doi.org/10.1007/s10126-016-9703-y] [PMID: 27245469] |
| [136] | Cai, R.; Tu, S. (Suzhou Liansheng Chemistry Co., Ltd.) Nontoxic and odorless dye carrier with good promoting efficacy for polyester fiber and manufacture method CN 107541968, 2016. |
| [137] | Ikejiri, Y.; Numakura, T.; Tsuchimochi, Y.; Kawakami, K. (Kawasaki Kasei Chemicals, Ltd.) Fluidity improvers, thermoplastic resin compositions, moldings comprising them with good mechanical strength, and their manufacture JP 2007191685 A, 2006. |
| [138] | Patton, T.C.; Hall, F.M. (Baker Castor Oil Co.) Plastigels containing hydroxy fatty acid soaps US Pat. 2794791, 1953. |
| [139] | Chen, S.; Wang, Q.; Chen, X. Synthesis of phthalic anhydride diglycol ester. Gongye Cuihua, 2008, 16, 45-48. |
| [140] | Fujii, K.; Ohigashi, T.; Yokoyama, N.; Komehana, N. (Daicel Chemical Industries, Ltd., Japan; Daihachi Chemical Industry Co., Ltd.) Phthalic ester dimers as plasticizers and cellulose fatty acid ester resin compositions JP 11349537 A, 1998. |
| [141] | Chen, C.Y. Biosynthesis of di-(2-ethylhexyl) phthalate (DEHP) and di-n-butyl phthalate (DBP) from red alga--Bangia atropurpurea. Water Res., 2004, 38(4), 1014-1018. [http://dx.doi.org/10.1016/j.watres.2003.11.029] [PMID: 14769421] |
| [142] | Tian, C.; Ni, J.; Chang, F.; Liu, S.; Xu, N.; Sun, W.; Xie, Y.; Guo, Y.; Ma, Y.; Yang, Z.; Dang, C.; Huang, Y.; Tian, Z.; Wang, Y. Bio-Source of di-n-butyl phthalate production by filamentous fungi. Sci. Rep., 2016, 6, 19791. [http://dx.doi.org/10.1038/srep19791] [PMID: 26857605] |
| [143] | Habib, M.R.; Karim, M.R. Antimicrobial and cytotoxic activity of di-(2-ethylhexyl)phthalate and anhydrosophoradiol-3-acetate isolated from Calotropis gigantea (Linn.) flower. Mycobiology, 2009, 37(1), 31-36. [http://dx.doi.org/10.4489/MYCO.2009.37.1.031] [PMID: 23983504] |
| [144] | Di El-Sayed, M.H. -(2-ethylhexyl) phthalate, a major bioactive metabolite with antimicrobial and cytotoxic activity isolated from the culture filtrate of newly isolated soil Streptomyces (Streptomyces mirabilis strain NSQu-25). World Appl. Sci. J., 2012, 20, 1202-1212. |
| [145] | Ren, X.; He, X.; Zhang, Z.; Yan, Z.; Jin, H.; Li, X.; Qin, B. Isolation, Identification, and Autotoxicity Effect of Allelochemicals from Rhizosphere Soils of Flue-Cured Tobacco. J. Agric. Food Chem., 2015, 63(41), 8975-8980. [http://dx.doi.org/10.1021/acs.jafc.5b03086] [PMID: 26416408] |
| [146] | Sun, B.Y.; Tan, J.Z.; Wan, Z.G.; Gu, F.G.; Zhu, M.D. Allelopathic effects of extracts from Solidago canadensis L. against seed germination and seedling growth of some plants. J. Environ. Sci. (China), 2006, 18, 304-309. |
| [147] | Li, Y.B.; Zhang, Q. Effects of naturally and microbially decomposed cotton stalks on cotton seedling growth. Arch. Agron. Soil Sci., 2016, 62, 1264-1270. [http://dx.doi.org/10.1080/03650340.2015.1135327] |
| [148] | Uyeda, M.; Suzuki, K.; Shibata, M. 3315-AF2, a cell aggregation factor produced by Streptomyces sp. strain No. A-3315. Agric. Biol., 1990, 54, 251-252. [http://dx.doi.org/10.1271/bbb1961.54.251] |
| [149] | Bu, T.; Liu, M.; Zheng, L.; Guo, Y.; Lin, X. α-Glucosidase inhibition and the in vivo hypoglycemic effect of butyl-isobutyl-phthalate derived from the Laminaria japonica rhizoid. Phytother. Res., 2010, 24(11), 1588-1591. [http://dx.doi.org/10.1002/ptr.3139] [PMID: 21031613] |
| [150] | Amalarasi, L.E.; Jothi, G.J. BCL2 mediated apoptotic induction potential of the DNOP isolated from Pachygone ovata (Poir.) Miers ex Hook. F. & Thomson IN MCF-7 (human breast carcinoma). Int. Res. J. Pharm., 2019, 10, 190-194. [http://dx.doi.org/10.7897/2230-8407.1003103] |
| [151] | Nguyen, D.T.M.; Nguyen, D.H.; Lyun, H.L.; Lee, H.B.; Shin, J.H.; Kim, E.K. Inhibition of melanogenesis by dioctyl phthalate isolated from Nigella glandulifera Freyn. J. Microbiol. Biotechnol., 2007, 17(10), 1585-1590. [PMID: 18156772] |
| [152] | Li, Y.; Qian, Z.J.; Kim, S.K. Cathepsin B inhibitory activities of three new phthalate derivatives isolated from seahorse, Hippocampus Kuda Bleeler. Bioorg. Med. Chem. Lett., 2008, 18(23), 6130-6134. [http://dx.doi.org/10.1016/j.bmcl.2008.10.016] [PMID: 18938081] |
| [153] | Kumar, K.A.; Setty, S.R.; Narsu, M.L. GC-MS analysis of n-hexane extracts of Hibiscus micranthus Linn. Asian J. Chem., 2011, 23, 561-565. |
| [154] | Cheriti, A.; Saad, A.; Belboukhari, N.; Ghezali, S. Chemical composition of the essential oil of Launaea arborescens from Algerian Sahara. Chem. Nat. Compd., 2006, 42, 360-361. [http://dx.doi.org/10.1007/s10600-006-0123-5] |
| [155] | Abou Zeid, A.H.S.; Saleh, M.M.; Sleem, A.A.; Mohamed, R.S.; Hifnawy, M.S. Phytochemical investigation and biological activity of the aerial parts of Calliandra haematocephala Hassk. Bull. Fac. Pharm. (Cairo U.), 2006, 44, 127-147. |
| [156] | Al-Bari, M.A.A.; Bhuiyan, M.S.A.; Flores, M.E.; Petrosyan, P.; García-Varela, M.; Islam, M.A.U. Streptomyces bangladeshensis sp. nov., isolated from soil, which produces bis-(2-ethylhexyl)phthalate. Int. J. Syst. Evol. Microbiol., 2005, 55(Pt 5), 1973-1977. [http://dx.doi.org/10.1099/ijs.0.63516-0] [PMID: 16166697] |
| [157] | Lee, K.H.; Kim, J.H.; Lim, D.S.; Kim, C.H. Anti-leukaemic and anti-mutagenic effects of di(2-ethylhexyl)phthalate isolated from Aloe vera Linne. J. Pharm. Pharmacol., 2000, 52(5), 593-598. [http://dx.doi.org/10.1211/0022357001774246] [PMID: 10864149] |
| [158] | Hayashi, S.; Asakawa, Y.; Ishida, T.; Matsuura, T. Phthalate esters of Cryptotaenia canadensis DC. var. Japonica Makino (Umbelliferae). Tetrahedron Lett., 1967, •••, 5061-5063. [http://dx.doi.org/10.1016/S0040-4039(01)89914-7] |
| [159] | Muharni, M.; Fitrya, F.; Ruliza, M.O.; Susanti, D.A.; Di Elfita, E. -(2-ethylhexyl)phthalate and pyranon derivated from endophytic fungi Penicillium sp the leave of kunyit putih (Curcuma zedoaria). Indonesian J. Chem., 2014, 14, 290-296. [http://dx.doi.org/10.22146/ijc.21241] |
| [160] | Habib, M.R.; Karim, M.R. Antitumour evaluation of di-(2-ethylhexyl) phthalate (DEHP) isolated from Calotropis gigantea L. flower. Acta Pharm., 2012, 62(4), 607-615. [http://dx.doi.org/10.2478/v10007-012-0035-9] [PMID: 23333892] |
| [161] | Mavar-Manga, H.; Haddad, M.; Pieters, L.; Baccelli, C.; Penge, A.; Quetin-Leclercq, J. Anti-inflammatory compounds from leaves and root bark of Alchornea cordifolia (Schumach. & Thonn.) Müll. Arg. J. Ethnopharmacol., 2008, 115(1), 25-29. [http://dx.doi.org/10.1016/j.jep.2007.08.043] [PMID: 17942256] |
| [162] | Abdel-Aziz, M.S. GHareeb, M.A.; Saad, A.M.; Refahy, L.A.; Hamed, A.A. Chromatographic isolation and structural elucidation of secondary metabolites from the soil-inhabiting fungus Aspergillus fumigatus 3T-EGY. Acta Chromatogr., 2018, 30, 243-249. [http://dx.doi.org/10.1556/1326.2017.00329] |
| [163] | Lotfy, M.M.; Hassan, H.M.; Hetta, M.H.; El-Gendy, A.O.; Di Mohammed, R. -(2-ethylhexyl) phthalate, a major bioactive metabolite with antimicrobial and cytotoxic activity isolated from river Nile derived fungus Aspergillus awamori, Beni-Suef Univ. J. Basic Appl. Sci., 2018, 7, 263-269. |
| [164] | Rajamanikyam, M.; Vadlapudi, V.; Parvathaneni, S.P.; Koude, D.; Sripadi, P.; Misra, S.; Amanchy, R.; Upadhyayula, S.M. Isolation and characterization of phthalates from Brevibacterium mcbrellneri that cause cytotoxicity and cell cycle arrest. EXCLI J., 2017, 16, 375-387. [PMID: 28507481] |
| [165] | Kalinovskaya, N.I.; Romanenko, L.A.; Kalinovsky, A.I. Antibacterial low-molecular-weight compounds produced by the marine bacterium Rheinheimera japonica KMM 9513T. Antonie van Leeuwenhoek, 2017, 110(5), 719-726. [http://dx.doi.org/10.1007/s10482-017-0839-1] [PMID: 28176144] |
| [166] | Qi, S-H.; Xu, Y.; Xiong, H-R.; Qian, P-Y.; Zhang, S. Antifouling and antibacterial compounds from a marine fungus Cladosporium sp. F14. World J. Microbiol. Biotechnol., 2009, 25, 399-406. [http://dx.doi.org/10.1007/s11274-008-9904-2] |
| [167] | Driche, E.H.; Belghit, S.; Bijani, C.; Zitouni, A.; Sabaou, N.; Mathieu, F.; Badji, B. A new Streptomyces strain isolated from Saharan soil produces di-(2-ethylhexyl) phthalate, a metabolite active against methicillin-resistant Staphylococcus aureus. Ann. Microbiol., 2015, 65, 1341-1350. [http://dx.doi.org/10.1007/s13213-014-0972-2] |
| [168] | Awad, N.E. Volatile constituents, carbohydrate compositions and topical anti-inflammatory of the two green algae Caulerpa racemosa (O. Dargent) and Codium tomentosum (Stackhouse) Bull. Fac. Pharm. (Cairo U.), 2002, 40, 233-243. |
| [169] | Wahidulla, S.; de Souza, L. Phthalate esters from the brown alga Stoechospermum marginatum (C. agardh) Kuetzing. Bot. Mar., 1995, 38, 333-334. [http://dx.doi.org/10.1515/botm.1995.38.1-6.333] |
| [170] | Goldhaber-Pasillas, D.; Bye, R.; Chávez-Ávila, V.; Mata, R. In vitro morphogenetic responses and comparative analysis of phthalides in the highly valued medicinal plant Ligusticum porteri Coulter & Rose. Plant Growth Regul., 2012, 67, 107-119. [http://dx.doi.org/10.1007/s10725-012-9666-6] |
| [171] | Lazreg-Aref, H.; Mars, M.; Fekih, A.; Aouni, M.; Said, K. Chemical composition and antibacterial activity of a hexane extract of Tunisian capri latex from the unripe fruit of Ficus carica. Pharm. Biol., 2012, 50(4), 407-412. [http://dx.doi.org/10.3109/13880209.2011.608192] [PMID: 22136172] |
| [172] | Karthika, S.; Ravishankar, M.; Mariajancyrani, J.; Chandramohan, G. Study on phytoconstituents from Moringa oleifera leaves. Asian J. Plant Sci. Res., 2013, 3, 63-69. |
| [173] | Qian, Z.J.; Kang, K.H.; Kim, S-K. Isolation and antioxidant activity evaluation of two new phthalate derivatives from seahorse, Hippocampus kuda Bleeler, Biotechnol. Bioproc. E, 2012, 17, 1031-1040. |
| [174] | Moushumi Priya, A.; Jayachandran, S. Induction of apoptosis and cell cycle arrest by Bis (2-ethylhexyl) phthalate produced by marine Bacillus pumilus MB 40. Chem. Biol. Interact., 2012, 195(2), 133-143. [http://dx.doi.org/10.1016/j.cbi.2011.11.005] [PMID: 22155658] |
| [175] | Imam, A.A.; Ezema, M.D.; Muhammed, I.U.; Atiku, M.K.; Alhassan, A.J.; Idi, A.; Abdullahi, H.; Mohammed, A. In vivo antimalarial activity of solvents extracts of Alstonia boonei stem bark and partial characterization of most active extract(s). Annu. Res. Rev. Biol., 2017, 17 [http://dx.doi.org/10.9734/ARRB/2017/36235] |
| [176] | Shah, S.M.; Shah, A.A.; Ullah, F.; Hussain, S.; Khan, S.B.; Asiri, A.M.; Ahmad, S.; Khan, H.; Farooq, U. A new trypsin inhibitory phthalic acid ester from Heliotropium strigosum. Med. Chem. Res., 2014, 23, 2712-2714. [http://dx.doi.org/10.1007/s00044-013-0864-1] |
| [177] | Bagalkotkar, G. Isolation and characterization of compounds from ‘naga buana’ (Phyllanthus pulcher) and ‘similit matinggi’ (Casearia capitellata) and their cytotoxic effects on cancer cell lines Doctor thesis, Universiti Putra Malaysia, Kuala Lumpur, Malaysia, 2007. |
| [178] | Anju, K.M.; Archana, M.M.; Mohandas, C.; Nambisan, B. An antimicrobial phthalate derivative from Bacillus cereus, the symbiotic bacterium associated with a novel entomopathogenic nematode, Rhabditis (Oscheius) sp. Int. J. Pharm. Pharm. Sci., 2015, 7, 238-242. |
| [179] | Hoang, V.L.T.; Li, Y.; Kim, S-K. Cathepsin B inhibitory activities of phthalates isolated from a marine Pseudomonas strain. Bioorg. Med. Chem. Lett., 2008, 18(6), 2083-2088. [http://dx.doi.org/10.1016/j.bmcl.2008.01.097] [PMID: 18289850] |
| [180] | Chua, B.L.; Zunoliza, A.; Kar, Y.P.; Luqman, C.A.; Thomas, S.; Yaw, C.; Umi, K.Y. Isolation, structure elucidation, identification and quantitative analysis of di(2-ethylhexyl) phthalate (DEHP) from the roots of Chlorophytum boriviliuanum (Safed Musli). Res. J. Pharm. Biol. Chem. Sci., 2015, 6, 1090-1095. |
| [181] | Islam, M.T.; Ahn, S.Y.; Cho, S.M.; Yun, H.K. Isolation of antibacterial compounds from hairy vetch (Vicia villosa) against grapevine crown gall pathogen. Hortic. Environ. Biotechnol., 2013, 54, 338-345. [http://dx.doi.org/10.1007/s13580-013-0028-8] |
| [182] | Rameshthangam, P.; Ramasamy, P. Antiviral activity of bis(2-methylheptyl)phthalate isolated from Pongamia pinnata leaves against White Spot Syndrome Virus of Penaeus monodon Fabricius. Virus Res., 2007, 126(1-2), 38-44. [http://dx.doi.org/10.1016/j.virusres.2007.01.014] [PMID: 17328984] |
| [183] | Liu, M.; Ding, Y.; Du, L. Chemical components of traditional Dai medicine Arundina graminifolia (D. Don) Hochr. Zhongcaoyao, 2007, 38, 676-677. |
| [184] | Ren, L.; Zeng, L.; Lu, Y.; Huang, S.; Zhang, M. Deterrence effect of Lantana camara L. essential oil on adult of Liriomyza sativae Blanchard. Guangxi Nongye Shengwu Kexue, 2006, 25, 43-47. |
| [185] | Son, A.R.; Choi, J.Y.; Kim, J.A.; Cho, S.H. Isolation of melanogenesis inhibitors from Ponciri fructus. Saengyak Hakhoechi, 2005, 36, 1-8. |
| [186] | Xu, G.H.; Kim, J.A.; Kim, S.Y.; Ryu, J.C.; Kim, Y.S.; Jung, S.H.; Kim, M.K.; Lee, S.H. Terpenoids and coumarins isolated from the fruits of Poncirus trifoliata. Chem. Pharm. Bull. (Tokyo), 2008, 56(6), 839-842. [http://dx.doi.org/10.1248/cpb.56.839] [PMID: 18520091] |
| [187] | Jung, H.W.; Choi, J.Y.; Lee, J.G.; Choi, E.H.; Oh, J.S.; Kim, D.C.; Kim, J.A.; Park, S.H.; Son, J.K.; Lee, S.H. Isolation of melanogenesis inhibitors from Cinnamomi cortex. Saengyak Hakhoechi, 2007, 38, 382-386. |
| [188] | Lee, J.G.; Choi, J.Y.; Oh, J.S.; Jung, H.W.; Choi, E.H.; Lee, H.S.; Kim, J.A.; Chang, T.S.; Son, J.K.; Lee, S.H. Isolation of melanin biosynthesis inhibitory compounds from the Phellodendri cortex. Saengyak Hakhoechi, 2007, 38, 387-393. |
| [189] | Zhang, C.X.; He, X.X.; Zhang, J.; Guo, Q.; Lei, L-F.; Su, J-Y.; Zeng, L-M. New precursor of tetraterpenoids from the soft coral Sarcophyton glaucum. Nat. Prod. Res., 2013, 27(9), 782-786. [http://dx.doi.org/10.1080/14786419.2012.701211] [PMID: 22889207] |
| [190] | Bhardwaj, N.R.; Kumar, J. Characterization of volatile secondary metabolites from Trichoderma asperellum. J. Appl. Nat. Sci., 2017, 9, 954-959. [http://dx.doi.org/10.31018/jans.v9i2.1303] |
| [191] | Zhang, Y.; Mu, J.; Gu, X.; Zhao, C.; Wang, X.; Xie, Z. A marine sulfate-reducing bacterium producing multiple antibiotics: biological and chemical investigation. Mar. Drugs, 2009, 7(3), 341-354. [http://dx.doi.org/10.3390/md7030341] [PMID: 19841718] |
| [192] | Zhang, B.; Li, S.; Xin, G.; Li, T.; Wu, J. Analysis of volatile component in different part of durian fruit by GC/MS. Shipin Yanjiu Yu Kaifa, 2012, 33, 130-134. |
| [193] | Zhu, F.; Yuan, B.; Huang, T.; Feng, Y.; Jiang, J. GC-MS analysis of puny polar fractions from Fomitiporia punctata. Yaowu Fenxi Zazhi, 2011, 31, 732-734. |
| [194] | Mishra, A.; Dixit, S.; Ratan, V.; Srivastava, M.; Trivedi, S.; Srivastava, Y.K. Identification and in silico screening of biologically active secondary metabolites isolated from Trichoderma harzianum. Ann. Phytomed., 2018, 7, 78-86. [http://dx.doi.org/10.21276/ap.2018.7.1.9] |
| [195] | Kavitha, A.; Lakshmi Narasu, M. Comparative evaluation of antimicrobial activities of root, stem and leaves of Holoptelea integrifolia against pathogenic bacteria. Asian J. Microbiol. Biotechnol. Environ. Sci., 2014, 16, 145-154. |
| [196] | Hossain, M.A.; Siddique, A.B.; Rahman, S.M.M. Chemical composition of the essential oils of Stevia rebaudiana Bertoni leaves. Asian J. Tradit. Med., 2010, 5, 56-61. |
| [197] | Hussain, A.; Rather, M.A.; Dar, M.S.; Aga, M.A.; Ahmad, N.; Manzoor, A.; Qayum, A.; Shah, A.; Mushtaq, S.; Ahmad, Z.; Hassan, Q.P. Novel bioactive molecules from Lentzea violacea strain AS 08 using one strain-many compounds (OSMAC) approach. Bioorg. Med. Chem. Lett., 2017, 27(11), 2579-2582. [http://dx.doi.org/10.1016/j.bmcl.2017.03.075] [PMID: 28400238] |
| [198] | Wang, D.; Luo, X.; Jiang, B. Chemical constituents in twigs and leaves of Melodinus fusiformis. Zhongcaoyao, 2012, 43, 653-657. |
| [199] | Liu, R.H.; Wen, X.C.; Li, Y.Y.; Zhang, P.Z.; Shao, F.; Huang, H.L.; Tang, F.R. Chemical constituents from Dalbergia cochinchinensis. Zhong Yao Cai, 2015, 38(9), 1868-1871. [PMID: 26930980] |
| [200] | Fujita, E.; Saeki, Y.; Ochiai, M.; Inoue, T. Neutral constituents of Lythrum salicaria, Bull. Inst. Chem. Res. Kyoto U., 1972, 50, 327-331. |
| [201] | Sajid, M.; Khan, M.R.; Shah, S.A.; Majid, M.; Ismail, H.; Maryam, S.; Batool, R.; Younis, T. Investigations on anti-inflammatory and analgesic activities of Alnus nitida Spach (Endl). stem bark in Sprague Dawley rats. J. Ethnopharmacol., 2017, 198, 407-416. [http://dx.doi.org/10.1016/j.jep.2017.01.041] [PMID: 28119101] |
| [202] | Kala, S.C.; Ammani, K. GC–MS analysis of biologically active compounds in Canthium parviflorum Lam. leaf and callus extracts. Int. J. Chemtech Res., 2017, 10, 1039-1058. |
| [203] | Sharma, A.; Kumar, V.; Kanwar, M.K.; Thukral, A.K.; Bhardwaj, R. Phytochemical profiling of the leaves of Brassica juncea L. using GC-MS. Int. Food Res. J., 2017, 24, 547-551. |
| [204] | Singariya, P.; Mourya, K.K.; Kumar, P. Gas chromatography-mass spectrometric analysis of acetone extract of Cenchrus ciliaris (Dhaman grass). Int. J. Sci. Nature, 2015, 6, 652-661. |
| [205] | Keire, D.A.; Anton, P.; Faull, K.F.; Ruth, E.; Walsh, J.H.; Chew, P.; Quisimoro, D.; Territo, M.; Reeve, J.R., Jr Diethyl phthalate, a chemotactic factor secreted by Helicobacter pylori. J. Biol. Chem., 2001, 276(52), 48847-48853. [http://dx.doi.org/10.1074/jbc.M109811200] [PMID: 11677249] |
| [206] | Faizi, S.; Sumbul, S.; Versiani, M.A.; Saleem, R.; Sana, A.; Siddiqui, H. GC/GCMS analysis of the petroleum ether and dichloromethane extracts of Moringa oleifera roots. Asian Pac. J. Trop. Biomed., 2014, 4(8), 650-654. [http://dx.doi.org/10.12980/APJTB.4.201414B141] [PMID: 25183335] |
| [207] | Hashem, F.A.; Sleem, A.A. Chemical characterization and anti-inflammatory activity of Tecoma radicans Bull. Nat. Res. Centre (Egypt), 2006, 31, 489-499. |
| [208] | Bhattacharya, E.; Dutta, R.; Chakraborty, S.; Mandal Biswas, S. Phytochemical profiling of Artocarpus lakoocha Roxb. leaf methanol extract and its antioxidant, antimicrobial and antioxidative activities. Asian Pac. J. Trop. Biomed., 2019, 9, 484-492. [http://dx.doi.org/10.4103/2221-1691.270984] |
| [209] | Amade, P.; Mallea, M.; Bouaïcha, N. Isolation, structural identification and biological activity of two metabolites produced by Penicillium olsonii Bainier and Sartory. J. Antibiot. (Tokyo), 1994, 47(2), 201-207. [http://dx.doi.org/10.7164/antibiotics.47.201] [PMID: 8150716] |
| [210] | Acevedo, F.; Torres, P.; Oomah, B.D.; de Alencar, S.M.; Massarioli, A.P.; Martín-Venegas, R.; Albarral-Ávila, V.; Burgos-Díaz, C.; Ferrer, R.; Rubilar, M. Volatile and non-volatile/semi-volatile compounds and in vitro bioactive properties of Chilean Ulmo (Eucryphia cordifolia Cav.) honey. Food Res. Int., 2017, 94, 20-28. [http://dx.doi.org/10.1016/j.foodres.2017.01.021] [PMID: 28290363] |
| [211] | Mehta, B.K.; Nigam, V.; Nigam, R.; Singh, A. Gas chromatography mass spectrometry (GC-MS) analysis of the hexane extract of the Syzygium cumini bark. J. Med. Plants Res., 2012, 6, 4163-4166. |
| [212] | Babu, B.; Wu, J-T. Production of phthalate esters by nuisance freshwater algae and cyanobacteria. Sci. Total Environ., 2010, 408(21), 4969-4975. [http://dx.doi.org/10.1016/j.scitotenv.2010.07.032] [PMID: 20692018] |
| [213] | Ganti, V.S.; Kim, K.H.; Bhattarai, H.D.; Shin, H.W. Isolation and characterisation of some antifouling agents from the brown alga Sargassum confusum. J. Asian Nat. Prod. Res., 2006, 8(4), 309-315. [http://dx.doi.org/10.1080/10286020500034980] [PMID: 16864441] |
| [214] | El-Naggar, M.Y.M. Dibutyl phthalate and the antitumor agent F5A1, two metabolites produced by Streptomyces nasri submutant H35. Biom. Lett., 1997, 55, 125-131. |
| [215] | Namikoshi, M.; Fujiwara, T.; Nishikawa, T.; Ukai, K. Natural abundance 14C content of dibutyl phthalate (DBP) from three marine algae. Mar. Drugs, 2006, 4, 290-297. [http://dx.doi.org/10.3390/md404290] |
| [216] | Lee, D-S. Dibutyl phthalate, an α-glucosidase inhibitor from Streptomyces melanosporofaciens. J. Biosci. Bioeng., 2000, 89(3), 271-273. [http://dx.doi.org/10.1016/S1389-1723(00)88832-5] [PMID: 16232742] |
| [217] | Roy, R.N.; Laskar, S.; Sen, S.K. Dibutyl phthalate, the bioactive compound produced by Streptomyces albidoflavus 321.2. Microbiol. Res., 2006, 161(2), 121-126. [http://dx.doi.org/10.1016/j.micres.2005.06.007] [PMID: 16427514] |
| [218] | Yang, J.; Gu, D.; Ji, Z.; Fang, C.; Xu, F.; Yang, Y. Comprehensive separation of major compositions from Sophora japonica var. violacea by counter-current chromatography using a liquid-liquid extraction strategy. Ind. Crops Prod., 2018, 124, 363-368. [http://dx.doi.org/10.1016/j.indcrop.2018.08.003] |
| [219] | Wang, X.; Zhang, M.; Zhao, Y.; Wang, H.; Liu, T.; Xin, Z. Pentadecyl ferulate, a potent antioxidant and antiproliferative agent from the halophyte Salicornia herbacea. Food Chem., 2013, 141(3), 2066-2074. [http://dx.doi.org/10.1016/j.foodchem.2013.05.043] [PMID: 23870929] |
| [220] | Kavitha, A.; Prabhakar, P.; Narasimhulu, M.; Vijayalakshmi, M.; Venkateswarlu, Y.; Rao, K.V.; Raju, V.B.S. Isolation, characterization and biological evaluation of bioactive metabolites from Nocardia levis MK-VL_113. Microbiol. Res., 2010, 165(3), 199-210. [http://dx.doi.org/10.1016/j.micres.2009.05.002] [PMID: 19577444] |
| [221] | Chen, M.; Zhou, J.R.; Li, C.; Song, Y.Y.; Xie, L.J.; Chen, S.; Zeng, R.S. Isolation, identification and bioactivity of allelochemicals of Streptomyces sp. strain 6803. Allelopathy J., 2009, 23, 411-424. |
| [222] | Khatiwora, E.; Adsul, V.B.; Ruikar, A.D.; Gadkari, T.; Deshpande, N.R.; Kashalkar, R.V. Isolation and characterization of dibutyl phthalate from Ipomoea carnea stem and its quantification by HPTLC. J. Pharm. Res., 2011, 4, 3264-3265. |
| [223] | Adsul, V.B.; Khatiwora, E.; Arbale, V.A.; Deshpande, N.R. Isolation and characterization of dibutyl phthalate from leaves of Ipomoea carnea. Chem. Nat. Compd., 2012, 48, 712-713. [http://dx.doi.org/10.1007/s10600-012-0362-6] |
| [224] | Wang, D.M.; Pu, W.J.; Wang, Y.H.; Zhang, Y.J.; Wang, S.S. A new isorhamnetin glycoside and other phenolic compounds from Callianthemum taipaicum. Molecules, 2012, 17(4), 4595-4603. [http://dx.doi.org/10.3390/molecules17044595] [PMID: 22510608] |
| [225] | Johnson, J.A.; Citarasu, T.; Manjusha, W.A. Antimicrobial screening and identification of bioactive compounds present in marine sponge Zygomycale sp. collected from Kanyakumari coast. J. Chem. Biol. Phys. Sci., 2012, 2, 1842-1848. |
| [226] | Cui, J.L.; Wang, C.L.; Guo, S.; Yang, L.; Xiao, P.; Wang, M. Evaluation of fungus-induced agilawood from Aquilaria sinensis in China. Symbiosis, 2013, 60, 37-44. [http://dx.doi.org/10.1007/s13199-013-0237-z] |
| [227] | Blazević, I.; Radonić, A.; Mastelić, J.; Zekić, M.; Skocibusić, M.; Maravić, A. Hedge mustard (Sisymbrium officinale): chemical diversity of volatiles and their antimicrobial activity. Chem. Biodivers., 2010, 7(8), 2023-2034. [http://dx.doi.org/10.1002/cbdv.200900234] [PMID: 20730965] |
| [228] | Chen, Y.; Zheng, W.; Wang, L-M.; Cui, H-L.; Li, G-X. Liu, X.-G.; Han, C.-C.; Zeng, R.-S. Effect of toxins isolated from Exserohilum monoceras (Drechsler) Leonard and Suggs on Echinochloa crusgalli (L.) Beauv. Agric. Sci. China, 2009, 8, 972-978. [http://dx.doi.org/10.1016/S1671-2927(08)60302-8] |
| [229] | Gu, X.J.; Mu, J.; Zhang, Y.; Liang, Y.T.; Li, W. Study of antibacterial substances from a marine anaerobic denitrifying bacterial strain Pseudomonas stutzeri. J. Dalian Jiaotong Univ., 2011, 32, 66-69. |
| [230] | Basaran, P.; Demirbas, R.M. Spectroscopic detection of pharmaceutical compounds from an aflatoxigenic strain of Aspergillus parasiticus. Microbiol. Res., 2010, 165(6), 516-522. [http://dx.doi.org/10.1016/j.micres.2009.09.006] [PMID: 19879117] |
| [231] | Wang, Y-L.; Dong, P-P.; Liang, J-H.; Li, N.; Sun, C-P.; Tian, X-G.; Huo, X-K.; Zhang, B-J.; Ma, X-C.; Lv, C-Z. Phytochemical constituents from Uncaria rhynchophylla in human carboxylesterase 2 inhibition: Kinetics and interaction mechanism merged with docking simulations. Phytomedicine, 2018, 51, 120-127. [http://dx.doi.org/10.1016/j.phymed.2018.10.006] [PMID: 30466609] |
| [232] | Nidhal, N.; Zhou, X.; Chen, G.; Zhang, B.; Han, C.; Song, X. Chemical constituents of Leucas zeylanica and their chemotaxonomic significance. Biochem. Syst. Ecol., 2020, 89104006 [http://dx.doi.org/10.1016/j.bse.2020.104006] |
| [233] | Wei, H.; He, C.; Peng, Y.; Ma, G.; Xiao, P. [Chemical constituents of Dolomiaea souliei]. Zhongguo Zhongyao Zazhi, 2012, 37(9), 1249-1253. [PMID: 22803370] |
| [234] | Al-Amier, H.; El-Hela, A.A.; Al-Khadrawy, F.M.; Craker, L.E. Comparative evaluation of the volatile constituents in some verbena species cultivated in Egypt. J. Herbs Spices Med. Plants, 2005, 11, 25-33. [http://dx.doi.org/10.1300/J044v11n03_03] |
| [235] | Takshak, S.; Agrawal, S.B. The role of supplemental ultraviolet-B radiation in altering the metabolite profile, essential oil content and composition, and free radical scavenging activities of Coleus forskohlii, an indigenous medicinal plant. Environ. Sci. Pollut. Res. Int., 2016, 23(8), 7324-7337. [http://dx.doi.org/10.1007/s11356-015-5965-6] [PMID: 26681329] |
| [236] | Paranjothi, C.C.J.; Murali, S.R. Antibacterial activity and GCMS analysis of the extract of leaves of Rhizophora apiculata (a mangrove plant). World J. Pharm. Res., 2018, 7, 1-8. |
| [237] | Ding, Z.; He, Y.; Ding, J. The chemical constituents of Lysimachia microcarpa, Yunnan Zhiwu Yanjiu. Yunnan Zhi Wu Yan Jiu, 1993, 15, 201-204. |
| [238] | Han, X.; Niu, Y.; Du, G.; Cao, Z. Chemical components identification of Capsicum chinense root exudates by nutrient solution culture. Guizhou Nongye Kexue, 2015, 43, 143-148. |
| [239] | Badawy, M.E.I.; Kherallah, I.E.A.; Mohareb, A.S.O.; Salem, M.Z.M.; Yousef, H.A. Chemical composition and antimicrobial activity of bark and leaf extracts of Cupressus sempervirens and Juniperus phoenicea grown in Al-Jabel Al-Akhdar Region, Libya. Nat. Prod. J., 2019, 9, 268-279. [Bentham]. [http://dx.doi.org/10.2174/2210315508666180223151210] |
| [240] | Nasution, R.; Marianne, C.J.; Mustanir, M. Roswita, Anti-obesity compounds from the leaves of plants Morus alba (MORACEAE). Int. J. Chemtech Res., 2015, 8, 228-234. |
| [241] | Yang, Y.; Zeng, G.; Tan, J.; Li, X.; Feng, X.; Wang, Y.; Zhou, Y. Chemical constituents from Morus alba L. Zhongnan Yaoxue, 2011, 9, 92-95. |
| [242] | Coqueiro, A.; Regasini, L.O.; Leme, G.M.; Polese, L.; Nogueira, C.T.; Del Cistia, M.L.; Graminhab, M.A.S.; Bolzani, V.S. Leishmanicidal activity of Brosimum glaziovii (Moraceae) and chemical composition of the bioactive fractions by using high-resolution gas chromatography and GC-MS. J. Braz. Chem. Soc., 2014, 25, 1839-1847. |
| [243] | Ren, G.; Hu, Z.C.; Xiang, H.Y.; Peng, J.B.; Liu, R.H.; Hunag, H.L.; Shao, F. Chemical constituents from the fruiting branches of Artocarpus nanchuanensis endemic to China. Biochem. Syst. Ecol., 2013, 51, 98-100. [http://dx.doi.org/10.1016/j.bse.2013.08.019] |
| [244] | Darwish, F.M.M. Phytochemical study of Ficus benghalensis L. Bull. Fac. Pharm., 2002, 40, 249-258. |
| [245] | Bhimba, V.; Meenupriya, J.; Joel, E.L.; Naveena, D.E.; Kumar, S.; Thanharaj, M. Antibacterial activity and characterization of secondary metabolites isolated from mangrove plant Avicennia officinalis. Asian Pac. J. Trop. Med., 2010, 54, 544-546. [http://dx.doi.org/10.1016/S1995-7645(10)60131-9] |
| [246] | Mangamuri, U.; Muvva, V.; Poda, S.; Naragani, K.; Munaganti, R.K.; Chitturi, B.; Yenamandra, V. Bioactive metabolites produced by Streptomyces cheonanensis VUK-A from Coringa mangrove sediments: isolation, structure, elucidation and bioactivity 3 Biotech., 2016, 6, 63-70. |
| [247] | Tian, J.; Wang, A.; Wu, L.; Hu, X.; Cheng, Y. Study on chemical constituents from volatile oil of Ficus carica L. Zhongguo Zhongyao Zazhi, 2005, 30, 474-476. |
| [248] | Eseyin, O.A.; Daniel, A.; Paul, T.S.; Attih, E.; Emmanuel, E.; Ekarika, J.; Munavvar Zubaid, A.S.; Ashfaq, A.; Afzal, S.; Ukeme, A. Phytochemical analysis and antioxidant activity of the seed of Telfairia occidentalis Hook (Cucurbitaceae). Nat. Prod. Res., 2018, 32(4), 444-447. [http://dx.doi.org/10.1080/14786419.2017.1308366] [PMID: 28361553] |
| [249] | Elsherbiny, E.A.; Saad, A.; Zaghloul, M.G.; El-Sheshtawi, M. Efficiency assessment of the antifungal metabolites from Sclerotium cepivorum against onion white rot disease. Eur. J. Plant Pathol., 2015, 142, 843-854. [http://dx.doi.org/10.1007/s10658-015-0656-1] |
| [250] | Gabriel, A.F.; Okwute, S.K. Isolation and characterization of lup-20(29)-en-3-one and diisononyl phthalate from antimicrobial Pterocarpus erinaceus (Poir) stem bark. J. Chem. Soc. Nigeria, 2009, 34, 156-161. |
| [251] | Kim, J.S.; Kim, Y.T.; Kim, C. Constituents from the roots of Astragalus membranaceus (I). Saengyak Hakhoechi, 1996, 27, 336-341. |
| [252] | Zhang, J.F.; Chen, Y.P.; Liang, Z.Y.; Ji, S.S.; Lin, Z.H. Research on the chemical constituents in leaf of Sindora glabra Merr. ex De Wit. Anhui Nongye Kexue, 2016, 44, 17-18. |
| [253] | Liang, H.Y.; Rong, X.; Chen, P.T.; Ma, Z.Q.; Zhang, X. Anti-TMV activity and isolation of active ingredients in fruit from Chaenomeles sinensis. Zhongguo Nong Ye Ke Xue, 2013, 46, 3571-3579. |
| [254] | Xie, M.; Yan, Z.; Ren, X.; Li, X.; Qin, B. Codonopilate A, a triterpenyl ester as main autotoxin in cultivated soil of Codonopsis pilosula (Franch.) Nannf. J. Agric. Food Chem., 2017, 65(10), 2032-2038. [http://dx.doi.org/10.1021/acs.jafc.6b04320] [PMID: 28240886] |
| [255] | Gautam, V.; Sharma, A.; Arora, S.; Bhardwaj, R. Bioactive compounds in the different extracts of flowers of Rhododendron arboreum Sm. J. Chem. Pharm. Res., 2016, 8, 439-444. |
| [256] | Liu, X.P.; Zhang, C.; Tan, Z.W.; Liu, Y.X.; Tian, D.T.; Yu, A.N. Study on chemical components of volatile oil from Patrinia villosa Juss. Anhui Nongye Kexue, 2008, 36, 410. |
| [257] | Cai, J.; Lin, P.; Zhu, X.; Su, Q. Comparative analysis of clary sage (S. sclarea L.) oil volatiles by GC-FTIR and GC-MS. Food Chem., 2006, 99, 401-407. [http://dx.doi.org/10.1016/j.foodchem.2005.07.041] |
| [258] | Ramachandran, A.; Thangappan, B.S.; Ponnusamy, P. Evaluation of secondary metabolites of Hirsutella citriformis against Udaspes folus infecting Curcuma longa L. J. Pharm. Res., 2013, 7, 7-14. [http://dx.doi.org/10.1016/j.jopr.2013.01.019] |
| [259] | Xu, F.Q.; Guo, L.; Yan, B.L. GC-MS analysis of essential oil of Polygonum aviculare. Shizhen Guoyi Guoyao, 2012, 23, 1190-1191. |
| [260] | Lin, N.; Chen, J.; Zhang, W.K.; Yi, B. Study on volatile oil components from lignum Aquilariae resinatum produced in Hainan. Hainan Med. J., 2016, 27, 1383-1385. |
| [261] | Jassbi, A.R.; Zare, M.; Jamebozorgi, F.H. Chemical composition and biological activity of the essential oil and solvent extracts of Scaligeria nodosa, The Open Bioact. Compd. J., 2017, 5, 16-22. |
| [262] | Sastry, V.M.V.S.; Rao, G.R.K. Dioctyl phthalate and antibacterial compound from the marine brown alga Sargassum wightii. J. Appl. Phycol., 1995, 7, 185-186. [http://dx.doi.org/10.1007/BF00693066] |
| [263] | Abd-Elnaby, H.; Abo-Elala, G.; Abdel-Raouf, U.; Abd-elwahab, A.; Hamed, M. Antibacterial and anticancer activity of marine Streptomyces parvus: optimization and application. Biotechnol. Biotechnol. Equip., 2016, 30, 180-191. [http://dx.doi.org/10.1080/13102818.2015.1086280] |
| [264] | Shrivastava, R.; Mishra, J. Extraction, phytochemical screening, isolation and identification of bioactive compounds from extract of the plant Euphorbia thymifolia Linn. J. Drug Deliv. Ther., 2019, 9, 107-113. [http://dx.doi.org/10.22270/jddt.v9i3.2608] |
| [265] | Abdel-Kareem, M.M.; Rasmey, A.M.; Zohri, A.A. The action mechanism and biocontrol potentiality of novel isolates of Saccharomyces cerevisiae against the aflatoxigenic Aspergillus flavus. Lett. Appl. Microbiol., 2019, 68(2), 104-111. [PMID: 30554415] |
| [266] | Sharma, I.; Mathur, M.; Singh, G.P. Gas chromatography-mass spectrometry analysis and phytochemical screening of methanolic leaf extract of Plumbago zeylanica Linn. Int. J. Pharm. Sci. Rev. Res., 2015, 33, 315-320. |
| [267] | Dilip, H.; Kadu, S. Morphotaxonomy and GC-MS analysis of memnoniella. Eur. J. Biomed. Pharm. Sci., 2015, 2, 743-751. |
| [268] | Bello, S.S.; Evalde, N.; Samuel, C.; Okai, I.R.; Kelechi, E.C.; Chinyere, I.C. In vitro DNA damage inhibition activity of aqueous and ethyl acetate extract of Vernonia amygdalina leaves. World J. Pharm. Res., 2018, 7, 256-266. |
| [269] | Lu, X.; Chen, Q.; Cui, X.; Abozeid, A.; Liu, Y.; Liu, J.; Tang, Z. Comparative metabolomics of two saline-alkali tolerant plants Suaeda glauca and Puccinellia tenuiflora based on GC-MS platform. Nat. Prod. Res., 2019, ••• [http://dx.doi.org/10.1080/14786419.2019.1633647] [PMID: 31282217] |
| [270] | Dharani, J.; Sripathi, R.; Ravi, S. Chemical composition of Cyanthillium cinereum (L.) H. Rob essential oil and its molecular docking study against bacterial proteins. J. Pharm. Sci. Res., 2018, 10, 2216-2220. |
| [271] | Wei, J.F.; Wang, J.X.; Kang, W.Y. Analysis of changes in physiological indices and essential oil of Lysimachia nummularia aurea under NaCl stress. Zhongguo Yaoxue Zazhishe, 2013, 48, 423-427. |
| [272] | Hussain, A.Z.; Kumaresan, S. GC-MS analysis and antimicrobial activity of Hygrophila auriculata. Arch. Appl. Sci. Res., 2013, 5, 163-168. |
| [273] | Lu, Y.; Fang, X.; Hu, G. Comparative research on volatile oil components in different parts of Magnolia officinalis Rehd, et Wils. Guangpu Shiyanshi, 2011, 28, 3139-3142. |
| [274] | Rosaline, D.; Sakthivelkumar, S.; Rajendran, K.; Janarthanan, S. Detection of antibacterial activity and its characterization from the marine macro-algae Sargassum wightii (Greville ex J. Agardh 1848). Indian J. Geo-Mar. Sci., 2016, 45, 1365-1371. |
| [275] | Yamaguchi, I.; Mega, N.; Sanada, H. Components of the gel of Aloe vera (L.) burm. f. Biosci. Biotechnol. Biochem., 1993, 57(8), 1350-1352. [http://dx.doi.org/10.1271/bbb.57.1350] [PMID: 7764018] |
| [276] | Refaat, J.; Samy, M.N.; Desoukey, S.Y.; Ramadan, M.A.; Sugimoto, S.; Matsunami, K.; Kamel, M.S. Chemical constituents from Chorisia chodatii flowers and their biological activities. Med. Chem. Res., 2015, 24, 2939-2949. [http://dx.doi.org/10.1007/s00044-015-1342-8] |
| [277] | Akwu, N.A.; Naidoo, Y.; Singh, M.; Nundkumar, N.; Lin, J. Phytochemical screening, in vitro evaluation of the antimicrobial, antioxidant and cytotoxicity potentials of Grewia lasiocarpa E. Mey. ex Harv. S. Afr. J. Bot., 2019, 123, 180-192. [http://dx.doi.org/10.1016/j.sajb.2019.03.004] |
| [278] | Xu, Y.; Tian, S.; Zhu, H. A new lactone aldehyde compound isolated from secondary metabolites of marine fungus Penicillium griseofulvum. Tianran Chanwu Yanjiu Yu Kaifa, 2015, 27, 559-561. |
| [279] | Jing, J.; Zhang, J.; Li, H.; Huang, S.; Shan, L.; Zhou, X. Study on chemical constituents of Codonopsis thalictrifolia wall. var. mollis (Chipp) L. T. Shen. Shizhen Guoyi Guoyao, 2013, 24, 2340-2342. |
| [280] | Feng, N.; Xie, G.; Cui, Z.; Chen, F.; Tu, P. Chemical constituents from roots of Semiaquilegia adoxoides. J. Chin. Pharm. Sci., 2006, 15, 251-254. |
| [281] | Zhang, J.; Yang, T.T.; Li, G.Q.; Wang, W.J.; Zhang, X.Q.; Ye, W.C. [Chemical constituents from twigs and leaves of Melodinus hemsleyanus]. Zhongguo Zhongyao Zazhi, 2013, 38(9), 1390-1393. [PMID: 23944075] |
| [282] | Shen, L.; Jiang, S.; Zhu, H. Chemical constituents in herb of Incarvillea younghusbandii Sprague. Tianran Chanwu Yanjiu Yu Kaifa, 2012, 24, 1210-1213. |
| [283] | Peng, G.; Liu, J.; Wang, K.; An, L.; Liu, W. Determination of compounds in essential oil from ordinary powder and micronized powder of Typhonium giganteum Engl. Zhonghua Zhongyiyao Zazhi, 2010, 25, 1119-1121. |
| [284] | Chen, L.; Song, Z.Y.; Wang, J.J.; Song, H.T.; Zhang, G.G.; Wang, J.H. [Studies on the chemical constituents from aerial parts of Gynura divaricata]. Zhong Yao Cai, 2010, 33(3), 373-376. [PMID: 20681301] |
| [285] | Liu, H.; Zhang, D.; Luo, Y. Studies on chemical constituents of Mosla chinensis ‘jiangxiangru’. Zhongguo Shiyan Fangjixue Zazhi, 2010, 16, 56-59. |
| [286] | Tian, W.; Ma, J.; Zhang, H.; Zhang, H. Study on volatile compounds and antibacterial effects of Indigofera bungeana. Lanzhou Daxue Xuebao Yizueban, 2006, 32, 34-37. |
| [287] | Jiang, H.; Yuan, J.; Ma, Q.; Zhao, Y. Phenolic compounds from Mangifera indica. Chem. Nat. Compd., 2019, 55, 147-150. [http://dx.doi.org/10.1007/s10600-019-02639-1] |
| [288] | Feng, X.; He, Y.; Wang, Y.; Zhang, J.; Huang, L. Analysis of dichloromethane extracts of Radix paeoniae rubra with GC-MS and combined chemometric resolution methods. Yaowu Fenxi Zazhi, 2014, 34, 238-242. |
| [289] | Lu, Y.L.; Huang, J.; Xu, S.T.; Gao, Z.R.; Shi, H.L.; Li, Z. GC-MS analysis of volatile compounds of Elsholtzia communis. Zhongguo Yaofang, 2013, 24, 1403-1406. |
| [290] | Nandika, D.; Syamsu, K. Arinana, Kusumawardhani, D.T.; Fitriana, Y. Bioactivities of catechin from Gambir (Uncaria gambir Roxb.) against wood-decaying fungi. BioResources, 2019, 14, 5646-5656. |
| [291] | Shi, J.; Zhao, Q.; Jia, T. Chemical constituents of Myristica argentea. Tianran Chanwu Yanjiu Yu Kaifa, 2010, 22, 987-990. |
| [292] | Moharam, B.A.; Jantan, I.; Jalil, J.; Ahmad, F. Inhibitory effect of compounds from Goniothalamus tapis Miq. and Goniothalamus uvaroides King on platelet-activating factor receptor binding. Phytother. Res., 2012, 26(5), 687-691. [http://dx.doi.org/10.1002/ptr.3620] [PMID: 22002630] |
| [293] | Gallo, M.B.C.; Chagas, F.O.; Almeida, M.O.; Macedo, C.C.; Cavalcanti, B.C.; Barros, F.W.A.; de Moraes, M.O.; Costa-Lotufo, L.V.; Pessoa, C.; Bastos, J.K.; Pupo, M.T. Endophytic fungi found in association with Smallanthus sonchifolius (Asteraceae) as resourceful producers of cytotoxic bioactive natural products. J. Basic Microbiol., 2009, 49(2), 142-151. [http://dx.doi.org/10.1002/jobm.200800093] [PMID: 18798172] |
| [294] | Murniasih, T.; Indriany, E.A.; Putra, M.Y.; Untari, F. The antibacterial capacity of marine bacteria isolated from sponge Acanthella cavernosa collected from Lombok Island. J. Coast. Life Med., 2016, 4, 775-778. [http://dx.doi.org/10.12980/jclm.4.2016J6-189] |
| [295] | Lu, Z.G.; Liu, X.Y.; Su, R.J. GC-MS analysis of Yatay palm fruits essential oil using comparative injection method, Beijing Gongshang Daxue Xuebao. Ziran Kexueban, 2008, 26, 9-12. |
| [296] | Zheng, S.Z.; Ma, X.M.; Sheng, Q.; Hu, H.B.; Shen, X.M. Analysis on chemical components in Elsholtxia fruticosa Rehd by gas chromatography and mass spectrometry. Xibei Shifan Daxue Xuebao. Ziran Kexue Ban, 2004, 40, 48-50. |
| [297] | Najib, A.; Handayani, V.; Ahmad, A.R.; Anisa, R. Chemoprofiling of active n-hexane fraction as alpha-glucosidase inhibitors from Kanunang (Cordia myxa L.) leaves from Enrekang South Sulawesi. J. Glob. Pharma Technol., 2019. |
| [298] | Aynur, R.; Nurmamat, A.; Mehsum, H.; Kudret, H.; Nie, L. GC-MS analysis of volatile oil in Elaeagnus angustifolia flower extracted by CO2 supercritical fluid. Xibei Yaoxue Zazhi, 2015, 30, 9-14. |
| [299] | Ara, K.M.; Karami, M.; Raofie, F. Application of response surface methodology for the optimization of supercritical carbon dioxide extraction and ultrasound-assisted extraction of Capparis spinosa seed oil. J. Supercrit. Fluids, 2014, 85, 173-182. [http://dx.doi.org/10.1016/j.supflu.2013.10.016] |
| [300] | Bai, X.; Wang, H.; Zeng, X.; Tan, A.; Guo, L.; Quan, H.; Chen, Z. GC-MS combined with retention indices for identification of numb-taste components in Liangpin pomelo (Citrus maxima (Burm.) Merr. cv. Liangpin Yu) in comparison with Zanthoxylum schinifolium fruit. Shipin Kexue (Beijing, China), 2015, 36, 103-107. |
| [301] | Liu, J.; Ji, L.; Song, W.; Miao, D.; Li, X.; Ma, X.; An, J. A GC/MS analysis of fatty acid and ether-soluble components in Pinctada martensii flesh. Jiangxi Nongye Daxue Xuebao, 2011, 33, 791-795. |
| [302] | Smaoui, S.; Mellouli, L.; Lebrihi, A.; Coppel, Y.; Fguira, L.F.; Mathieu, F. Purification and structure elucidation of three naturally bioactive molecules from the new terrestrial Streptomyces sp. TN17 strain. Nat. Prod. Res., 2011, 25(8), 806-814. [http://dx.doi.org/10.1080/14786410902986225] [PMID: 21331973] |
| [303] | Al-Bari, M.A.A.; Abu Sayeed, M.; Rahman, M.S.; Mossadik, M.A. Characterization and antimicrobial activities of a phthalic acid derivative produced by Streptomyces bangladeshiensis, a novel species collected in Bangladesh, Res. J. Medicine & Med. Sci., 2006, 1, 77-81. |
| [304] | Lyutskanova, D.; Ivanova, V.; Stoilova-Disheva, M.; Kolarova, M.; Aleksieva, K.; Peltekova, V. Isolation and characterization of a psychrotolerant Streptomyces strain from permafrost soil in Spitsbergen, producing phthalic acid ester. Biotechnol. Biotechnol. Equip., 2009, 23, 1220-1224. [http://dx.doi.org/10.1080/13102818.2009.10817642] |
| [305] | Barakat, K.M.; Beltagy, E.A. Bioactive phthalate from marine Streptomyces ruber EKH2 against virulent fish pathogens. Egypt. J. Aquat. Res., 2015, 41, 49-56. [http://dx.doi.org/10.1016/j.ejar.2015.03.006] |