- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Cancer Immunology Journal
(Discontinued)
ISSN: 1876-4010 ― Volume 8, 2020
Transforming Growth Factor-Beta1 and Myeloid-Derived Suppressor Cells Interplay in Cancer
Juan F. Santibanez1, 2, *, Suncica Bjelica1
Abstract
Background:
Transforming growth factor-beta1 (TGF-β1) is a pleiotropic cytokine with a double role in cancer through its capacity to inhibit early stages of tumors while enhancing tumor progression at late stages of tumor progression. Moreover, TGF-β1 is a potent immunosuppressive cytokine within the tumor microenvironment that allows cancer cells to escape from immune surveillance, which largely contributes to the tumor progression.
Method:
It has been established that the cancer progression is commonly associated with increased number of Myeloid-derived suppressor cells (MDSC) that are a hallmark of cancer and a key mechanism of immune evasion.
Result:
MDSC represent a population of heterogeneous myeloid cells comprised of macrophages, granulocytes and dendritic cells at immature stages of development. MDSC promote tumor progression by regulating immune responses as well as tumor angiogenesis and cancer metastasis.
Conclusion:
In this review, we present an overview of the main key functions of both TGF-β1 and MDSC in cancer and in the immune system. Furthermore, the mutual contribution between TGF-β1 and MDSC in the regulation of immune system and cancer development will be analyzed.
Article Information
Identifiers and Pagination:
Year: 2017Volume: 06
First Page: 1
Last Page: 14
Publisher Id: TOCIJ-6-1
DOI: 10.2174/1876401001706010001
Article History:
Received Date: 31/05/2017Revision Received Date: 08/08/2017
Acceptance Date: 18/08/2017
Electronic publication date: 12/10/2017
Collection year: 2017
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Molecular oncology group, Institute for Medical Research, University of Belgrade, Dr Subotica 4, POB 102, 11129 Belgrade, Serbia; Tel: 381112685788; Fax: 381112643691; E-mail: jfsantibanez@imi.bg.ac.rs
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 31-05-2017 |
Original Manuscript | Transforming Growth Factor-Beta1 and Myeloid-Derived Suppressor Cells Interplay in Cancer | |
1. INTRODUCTION
TGF-β1 is a pleiotropic cytokine implicated in almost all aspects of cell biology including cell proliferation, survival and migration, and cell differentiation. TGF-β1, depending on the cell context, can modulate either negatively or positively the transcription of target genes [1Trikha P, Carson WE 3rd. Signaling pathways involved in MDSC regulation. Biochim Biophys Acta 2014; 1846: 55-65.]. In cancer, TGF-β can act either as a tumor suppressor in the early stages of tumorigenesis or as a tumor promoter in the late stages of tumor progression. TGF-β1 plays a key role in promoting cancer progression at multiple stages of the metastatic process, including epithelial to mesenchymal transition (EMT) [2Santibanez JF, Krstic J, Quintanilla M, Bernabeu C. TGF–β Signalling and Its Role in Cancer Progression and Metastasis. eLS John Wiley and Sons Ltd 2016; 1-9.
[http://dx.doi.org/10.1002/9780470015902.a0025045] , 3Colak S, Ten Dijke P. Targeting TGF-β Signaling in Cancer. Trends Cancer 2017; 3(1): 56-71.
[http://dx.doi.org/10.1016/j.trecan.2016.11.008] [PMID: 28718426] ]. TGF-β1 expression is increased within the tumor compared with the normal surrounding tissue and elevated expression of TGF-β1 is a poor prognosis marker. Actually, TGF-β1 is implicated in the increased malignancy features of cancer cells due to its capacities to induce cell motility, extracellular matrix degradation and angiogenesis [4Morikawa M, Derynck R, Miyazono K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb Perspect Biol 2016; 8(5): 27141051., 5Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011; 20(5): 576-90.
[http://dx.doi.org/10.1016/j.ccr.2011.09.009] [PMID: 22094253] ].
Within the tumor microenvironment (TM), TGF-β1 is considered to be one of the main factors regulating the inflammatory response by modulating the activity of the innate and adaptive immune systems. Furthermore, TGF-β1 is produced and it acts on the different types of immune cells such as T and B cells, natural killer (NK) cells and macrophages among others [6Yang L, Pang Y, Moses HL. TGF-β and immune cells: An important regulatory axis in the tumor microenvironment and progression. Trends Immunol 2010; 31(6): 220-7.
[http://dx.doi.org/10.1016/j.it.2010.04.002] [PMID: 20538542] ].
In tumors, Myeloid-derived suppressor cells (MDSC) are considered as one of the main orchestrators of cancer-related inflammation. Within TM, MDSC can express different polarized features that contribute to the inflammatory milieu and cancer cell out-growth promotion. Moreover, MDSC in both human cancer and cancer mouse models are implicated in the subversion of the immune surveillance by downregulating T cell immunity. Furthermore, MDSC can
be recruited and expanded by cancer cells-expressed factors, while the pathological increase of MDSC frequency into TM establishes an immune permissive microenvironment that contributes to the tumor progression [7Sica A, Porta C, Amadori A, Pastò A. Tumor-associated myeloid cells as guiding forces of cancer cell stemness. Cancer Immunol Immunother 2017; 66(8): 1025-36.
[http://dx.doi.org/10.1007/s00262-017-1997-8] [PMID: 28401258] , 8Barnie PA, Zhang P, Lv H, et al. Myeloid-derived suppressor cells and myeloid regulatory cells in cancer and autoimmune disorders. Exp Ther Med 2017; 13(2): 378-88.
[http://dx.doi.org/10.3892/etm.2016.4018] [PMID: 28352304] ]. In this review, we attempt to describe the main aspects in the interplay of TGF-β1 and MDSC in cancer, and the positive pernicious loop between TGF-β1 and MDSC that contributes to the escape of cancer cells from immune surveillance, which finally increases tumor malignancy.
2. TRANSFORMING GROWTH FACTOR BETA-1, MICROENVIRONMENT AND IMMUNE SYSTEM IN CANCER
2.1. Transforming Growth Factor Beta-1
Transforming growth factors (TGF-βs) were described according to their capacity to “transform” fibroblast rat cells in vitro [9Anzano MA, Roberts AB, Smith JM, Sporn MB, De Larco JE. Sarcoma growth factor from conditioned medium of virally transformed cells is composed of both type alpha and type beta transforming growth factors. Proc Natl Acad Sci USA 1983; 80(20): 6264-8.
[http://dx.doi.org/10.1073/pnas.80.20.6264] [PMID: 6604914] ]. Three isoforms [TGF-β1, -β2, -β3] were found to be expressed in mammals sharing a degree of homology from 64 to 82% [10Govinden R, Bhoola KD. Genealogy, expression, and cellular function of transforming growth factor-beta. Pharmacol Ther 2003; 98(2): 257-65.
[http://dx.doi.org/10.1016/S0163-7258(03)00035-4] [PMID: 12725873] ]. To date, more than 40 secreted ligands have been described that comprise the TGF-β superfamily. These include several subfamilies, such as TGF-βs per se, BMPs (bone morphogenetic proteins), GDFs (growth and differentiation factors), MIF (Müllerian inhibitory factor), activins and inhibins. Regardless of their structural similarities, TGF-β superfamily factors function as regulators of a variety of processes during embryogenesis and later on in adult tissue homeostasis [11Santibañez JF, Quintanilla M, Bernabeu C. TGF-β/TGF-β receptor system and its role in physiological and pathological conditions. Clin Sci 2011; 121(6): 233-51.
[http://dx.doi.org/10.1042/CS20110086] [PMID: 21615335] , 12Gordon KJ, Blobe GC. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta 2008; 197-228.].
Namely, TGF-β1 has been involved in a plethora of distinct biological process, which includes cell growth, differentiation and development, angiogenesis, suppression of immune response and promotion of tumorigenesis [11Santibañez JF, Quintanilla M, Bernabeu C. TGF-β/TGF-β receptor system and its role in physiological and pathological conditions. Clin Sci 2011; 121(6): 233-51.
[http://dx.doi.org/10.1042/CS20110086] [PMID: 21615335] , 13Sun N, Taguchi A, Hanash S. Switching Roles of TGF-β in Cancer Development: Implications for Therapeutic Target and Biomarker Studies. J Clin Med 2016; 5(12): 109.
[http://dx.doi.org/10.3390/jcm5120109] [PMID: 27916872] ]. TGF-β1 is synthesized as precursor of 75kDa that comprises two main domains: The latency associated peptide (LAP) and TGF-β1. Later on, this precursor is subjected to cleavage by the furin-type convertase, this produces an inactive small latent complex (SLC) via a non covalent bond between mature TGF-β1 and LAP [14Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci 2003; 116(2): 217-24.
[http://dx.doi.org/10.1242/jcs.00229] [PMID: 12482908] ]. Moreover, a large latent complex (LCC) can be produced by the binding of SLC with the latent TGF-β1 binding protein (LTBP). This complex is secreted and remains covalently associated to the extracellular matrix (ECM) for further activation. Several TGF-β1 activation mechanisms have been described, which include acidic microenvironments, proteolytic cleavage by plasmin and metalloproteinases and oxidative stress [14Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci 2003; 116(2): 217-24.
[http://dx.doi.org/10.1242/jcs.00229] [PMID: 12482908] , 15Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 2000; 14(2): 163-76.
[PMID: 10652271] ]. Bioavailable and active TGF-β1 binds first to cell-surface serine/threonine kinase type II receptors (TβRII) which activate and form heteromeric complex with the TGF-β1 type I receptor (TβRI) Fig. (1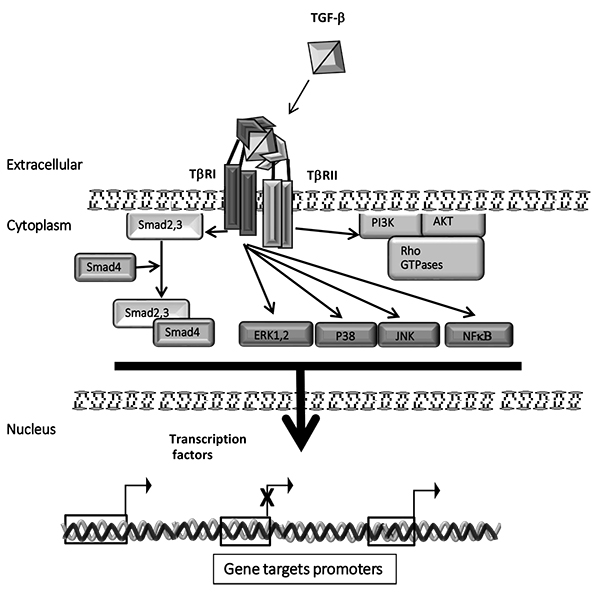 ). Then, TβRI phosphorylates Smad2 and Smad3, which induces their release from the inner face of plasma membrane to form a heteromeric complex with the common Smad4. Next, this complex is translocated into the nucleus to regulate genes target expression [14Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci 2003; 116(2): 217-24.
). Then, TβRI phosphorylates Smad2 and Smad3, which induces their release from the inner face of plasma membrane to form a heteromeric complex with the common Smad4. Next, this complex is translocated into the nucleus to regulate genes target expression [14Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci 2003; 116(2): 217-24.
[http://dx.doi.org/10.1242/jcs.00229] [PMID: 12482908] , 16Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003; 113(6): 685-700.
[http://dx.doi.org/10.1016/S0092-8674(03)00432-X] [PMID: 12809600] , 17Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol 2005; 21: 659-93.
[http://dx.doi.org/10.1146/annurev.cellbio.21.022404.142018] [PMID: 16212511] ]. In turn, TGF-β1 signaling is regulated by the expression of other components of Smads, the inhibitory Smads proteins (Smad6 and Smad7 or I-Smads) [18Itoh S, ten Dijke P. Negative regulation of TGF-beta receptor/Smad signal transduction. Curr Opin Cell Biol 2007; 19(2): 176-84.
[http://dx.doi.org/10.1016/j.ceb.2007.02.015] [PMID: 17317136] ].
Beyond the canonical Smad2,3 pathway, TGF-β1 activates several non-canonical intracellular signal pathways Fig. (1 ), named also non-Smads pathways, which include: mitogen-activated protein kinases (MAPK) ERK1,2, JNK and p38; PI3K (phosphoinositide 3-kinase)/ AKT1,2 and mTOR; NF-κB (nuclear factor κB), Cyclooxygenase-2 and prostaglandins; the small GTPase proteins Ras, Rho family of GTPases, among others [19Mu Y, Gudey SK, Landström M. Non-Smad signaling pathways. Cell Tissue Res 2012; 347(1): 11-20.
), named also non-Smads pathways, which include: mitogen-activated protein kinases (MAPK) ERK1,2, JNK and p38; PI3K (phosphoinositide 3-kinase)/ AKT1,2 and mTOR; NF-κB (nuclear factor κB), Cyclooxygenase-2 and prostaglandins; the small GTPase proteins Ras, Rho family of GTPases, among others [19Mu Y, Gudey SK, Landström M. Non-Smad signaling pathways. Cell Tissue Res 2012; 347(1): 11-20.
[http://dx.doi.org/10.1007/s00441-011-1201-y] [PMID: 21701805] ]. The plethora of the TGF-β1 signal transduction pathways in part explains the capacity of TGF-β1 to regulate many cellular functions at both molecularand biological levels.
2.2. Tumor Microenvironment Overview
The tumor microenvironment or tumor stroma consists mainly of the cellular components, the surrounding extracellular matrix, and interstitial fluid. These factors interact with each other, contributing to the hallmarks of cancer having significant influence on immune responses against the tumor [20Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144(5): 646-74.
[http://dx.doi.org/10.1016/j.cell.2011.02.013] [PMID: 21376230] ]. In this sense, cancer cells avoid recognition by the immune surveillance and simultaneously secrete inflammatory mediators to establish and maintain a constant state of inflammation [21Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986; 315(26): 1650-9.
[http://dx.doi.org/10.1056/NEJM198612253152606] [PMID: 3537791] ]. In turn, the cellular components in the tumor include tumor cells themselves, associated stromal cells such as fibroblasts and mesenchymal stromal cells, endothelial cells, and infiltrating immune cells. In this aspect, it is important to note that TM infiltrating immune cells play essential and paradoxical roles in immune responses against cancer. For instance, particular subsets of immune cells, such as cytotoxic T lymphocytes, NK, mature dendritic cells (DC) or M1 tumor-associated macrophages (TAM), participate in tumor growth and progression restrain. Conversely, other infiltrating immune cells, such as M2 TAM, neutrophils, mast cells, regulatory T cells (Treg), immature DC or MDSC, tumor growth and progression [22Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21(3): 309-22.
[http://dx.doi.org/10.1016/j.ccr.2012.02.022] [PMID: 22439926] -24Law AM, Lim E, Ormandy CJ, Gallego-Ortega D. The innate and adaptive infiltrating immune systems as targets for breast cancer immunotherapy. Endocr Relat Cancer 2017; 24(4): 123-44.
[http://dx.doi.org/10.1530/ERC-16-0404] [PMID: 28193698] ].
Importantly, the TM is predominantly infiltrated with immunosuppressive factors that cripple T cell responses against the tumor. These factors are not present in normal tissues, but are components of tumor regulatory pathways in response to inflammatory or infectious etiologies [25Bussard KM, Mutkus L, Stumpf K, Gomez-Manzano C, Marini FC. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res 2016; 18(1): 84.
[http://dx.doi.org/10.1186/s13058-016-0740-2] [PMID: 27515302] ]. Thus, a balance between pro- and anti-malignancy factors in the microenvironment regulates the growth of the tumor [26Wu AA, Drake V, Huang HS, Chiu S, Zheng L. Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells. OncoImmunology 2015; 4(7): 1016700.
[http://dx.doi.org/10.1080/2162402X.2015.1016700] [PMID: 26140242] ].
2.3. TGF-β1 and Immune System
The immune system is a complex and well developed organized structure whose strict balance is required for a normal homeostasis along the human life. The immune system regulation requires a complex crosstalk between the innate and adaptive system by secretion of cytokines, growth factors and cell-cell interactions. Dysregulation of the immune system responses results in autoimmune diseases, inflammatory diseases and cancer [27Franks AL, Slansky JE. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res 2012; 32(4): 1119-36.
[PMID: 22493341] ].
In cancer, TGF-β1 is the most potent immune-suppressive cytokine; it can act on cancer cells, on “non-transformed cells” associated to tumor stroma and on distal cells in the host. TGF-β1 suppresses antitumor immune responses and creates an immune-tolerant microenvironment allowing cancer cells to escape from immune surveillance, which contributes to the tumor progression Fig. (2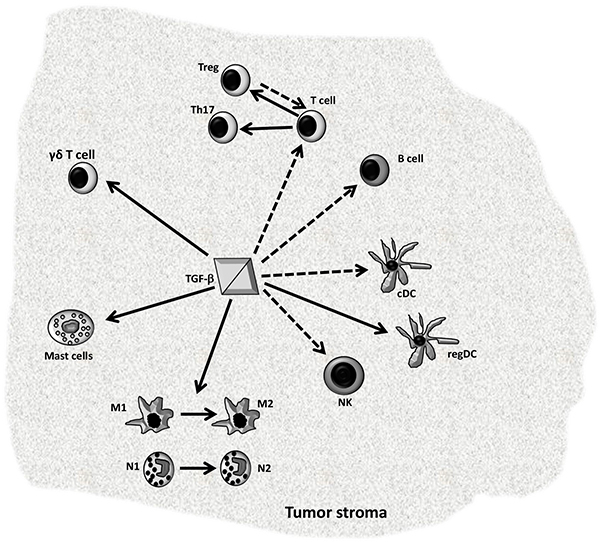 ) [28Worthington JJ, Fenton TM, Czajkowska BI, Klementowicz JE, Travis MA. Regulation of TGFβ in the immune system: An emerging role for integrins and dendritic cells. Immunobiology 2012; 217(12): 1259-65.
) [28Worthington JJ, Fenton TM, Czajkowska BI, Klementowicz JE, Travis MA. Regulation of TGFβ in the immune system: An emerging role for integrins and dendritic cells. Immunobiology 2012; 217(12): 1259-65.
[http://dx.doi.org/10.1016/j.imbio.2012.06.009] [PMID: 22902140] -31Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol 2010; 31(6): 220-7.
[http://dx.doi.org/10.1016/j.it.2010.04.002] [PMID: 20538542] ]. TGF-β1 importance as master regulator of mammalian immune system function and homeostasis was not highlighted until observing that tgfβ1-knockout mice exhibit a lethal multi-organ inflammation, primarily as consequence of deregulation in T cells responses [28Worthington JJ, Fenton TM, Czajkowska BI, Klementowicz JE, Travis MA. Regulation of TGFβ in the immune system: An emerging role for integrins and dendritic cells. Immunobiology 2012; 217(12): 1259-65.
[http://dx.doi.org/10.1016/j.imbio.2012.06.009] [PMID: 22902140] , 32Kulkarni AB, Huh CG, Becker D, et al. TGF-β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA 1993; 90: 770-4.
[http://dx.doi.org/10.1073/pnas.90.2.770] [PMID: 8421714] ]. This observation was further supported by Smad3-deficient mice, which exhibited multi-organ inflammatory injuries, as well as severe defects in the responsiveness and chemotaxis of neutrophils, T and B cells, and this primary defect in immune function results to be lethal [33Yang X, Letterio JJ, Lechleider RJ, et al. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-β. EMBO J 1999; 18(5): 1280-91.
[http://dx.doi.org/10.1093/emboj/18.5.1280] [PMID: 10064594] ]. In addition, the transgenic targeting of T cells with a truncated TβRII expression results in a severe autoimmune reaction characterized by multi-organ inflammation similar to that seen in TGF-β1 deficient mice, concomitantly to the autoantibody production [28Worthington JJ, Fenton TM, Czajkowska BI, Klementowicz JE, Travis MA. Regulation of TGFβ in the immune system: An emerging role for integrins and dendritic cells. Immunobiology 2012; 217(12): 1259-65.
[http://dx.doi.org/10.1016/j.imbio.2012.06.009] [PMID: 22902140] , 34Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 2000; 12(2): 171-81.
[http://dx.doi.org/10.1016/S1074-7613(00)80170-3] [PMID: 10714683] ].
Although cancer cells may express tumor specific antigens, potentially recognized by immune system, tumor immunotherapy is frequently unsuccessful as a result of cancer cells capacity to evade the immune surveillance by diverse strategies [29Park HY, Wakefield LM, Mamura M. Regulation of tumor immune surveillance and tumor immune subversion by tgf-β. Immune Netw 2009; 9(4): 122-6.
[http://dx.doi.org/10.4110/in.2009.9.4.122] [PMID: 20157598] ]. For instance, in malignant cells TGF-β1 downregulates the MHC Class I molecules making them invisible to the immune system. Moreover, TGF-β1 profoundly regulates the innate immune cells compartment. In tumors, TGF-β1 promotes both monocyte recruitment and macrophage differentiation [35Bierie B, Moses HL. Transforming growth factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth Factor Rev 2010; 21(1): 49-59.
[http://dx.doi.org/10.1016/j.cytogfr.2009.11.008] [PMID: 20018551] ]. Moreover, TGF-β1 suppresses mouse macrophage expression of TNF-α, MIP-1α, MIP-2 and it contributes to the resolution phase of inflammation [36McDonald PP, Fadok VA, Bratton D, Henson PM. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-beta in macrophages that have ingested apoptotic cells. J Immunol 1999; 163(11): 6164-72.
[PMID: 10570307] ]. Furthermore, TGF-β1 induces the polarization of TAMs M1 toward a protumorigenic M2 phenotype. Additionally, TAMs contribute to a tolerant tumor immunity microenvironment by producing TGF-β1 and supporting tumor growth [37Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002; 23(11): 549-55.
[http://dx.doi.org/10.1016/S1471-4906(02)02302-5] [PMID: 12401408] , 38Mantovani A, Locati M. Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization: lessons and open questions. Arterioscler Thromb Vasc Biol 2013; 33(7): 1478-83.
[http://dx.doi.org/10.1161/ATVBAHA.113.300168] [PMID: 23766387] ]. In neutrophils, TGF-β1 may inhibit the ability of these cells to eliminate cancer cells-expressing Fas-Ligand, and similarly to macrophages TGF-β1 promotes tumor-associated neutrophils switch from N1 to a protumorigenic N2 phenotype, thus further fomenting a permissive tumor microenvironment [39Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis 2012; 33(5): 949-55.
[http://dx.doi.org/10.1093/carcin/bgs123] [PMID: 22425643] ]. Besides, in tumor associated classical (c)DC, TGF-β1 represses the expression of MHC class II, CD40, CD80, and CD86, and TNF-α, IL-12, and CCL5/Rantes, and these DC become functionally defective because of their immature phenotype. Whereas, TGF-β is able to induce DC to adopt a tolerogenic phenotype, defined as regulatory (reg)DC [40Ghiringhelli F, Puig PE, Roux S, et al. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med 2005; 202(7): 919-29.
[http://dx.doi.org/10.1084/jem.20050463] [PMID: 16186184] -42Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res 2007; 13(1): 5262-70.
[http://dx.doi.org/10.1158/1078-0432.CCR-07-1157] [PMID: 17875754] ]. Finally, TGF-β is a potent chemo attractant for mast cells, which depending of milieu can produce pro-tumorigenic or anti-tumorigenic factors [43Krstic J, Santibanez JF. Transforming growth factor-beta and matrix metalloproteinases: functional interactions in tumor stroma-infiltrating myeloid cells. Scientific World Journal 2014; 2014: 521754.
[http://dx.doi.org/10.1155/2014/521754] , 44Varricchi G, Galdiero MR, Loffredo S, et al. Are Mast Cells MASTers in Cancer? Front Immunol 2017; 8: 424.
[http://dx.doi.org/10.3389/fimmu.2017.00424] [PMID: 28446910] ].
Furthermore, TGF-β1 regulates also lymphoid compartment, this factor largely inhibits both T cells B cells responses, and hinders the effector cytokines production (including IL-2, IL-4 and IFN-γ); reduces NK cell proliferation and cytotoxicity; meanwhile, it induces the conversion of naive T-cells toward Tregs, and Th17 differentiation that increases the production and secretion of pro-inflammatory cytokine IL-17, which contributes to the immune tolerance as well as to tumor progression and metastasis [42Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res 2007; 13(1): 5262-70.
[http://dx.doi.org/10.1158/1078-0432.CCR-07-1157] [PMID: 17875754] , 45Marçais A, Viel S, Grau M, Henry T, Marvel J, Walzer T. Regulation of mouse NK cell development and function by cytokines. Front Immunol 2013; 4: 450.
[http://dx.doi.org/10.3389/fimmu.2013.00450] [PMID: 24376448] -48Fabre J, Giustiniani J, Garbar C, et al. Targeting the Tumor Microenvironment: The Protumor Effects of IL-17 Related to Cancer Type. Int J Mol Sci 2016; 17(9): 1433.
[http://dx.doi.org/10.3390/ijms17091433] [PMID: 27589729] ]. Furthermore, TGF-β1 contributes to the generation of γδ T cells, which are the major IL-17-producing cells in naïve animals, and tumor-infiltrating γδ T cells may promote tumorigenesis via IL-17 and PD-L1 upregulation [49Do JS, Fink PJ, Li L, et al. Cutting edge: Spontaneous development of IL-17-producing gamma delta T cells in the thymus occurs via a TGF-beta 1-dependent mechanism. J Immunol 2010; 184(4): 1675-9.
[http://dx.doi.org/10.4049/jimmunol.0903539] [PMID: 20061408] , 50Chitadze G, Oberg HH, Wesch D. Kabelitz D. The Ambiguous Role of γδ T Lymphocytes in Antitumor Immunity. Trends Immunol 2017; 38(9): 668-78.] Accordingly, TGF-β1 affects the initiation and stimulation of both primary and secondary immune responses and it also suppresses antitumor effectors cells [51Geiser AG, Letterio JJ, Kulkarni AB, Karlsson S, Roberts AB, Sporn MB. Transforming growth factor beta 1 (TGF-beta 1) controls expression of major histocompatibility genes in the postnatal mouse: aberrant histocompatibility antigen expression in the pathogenesis of the TGF-beta 1 null mouse phenotype. Proc Natl Acad Sci USA 1993; 90(21): 9944-8.
[http://dx.doi.org/10.1073/pnas.90.21.9944] [PMID: 8234339] , 52Kao JY, Gong Y, Chen CM, Zheng QD, Chen JJ. Tumor-derived TGF-β reduces the efficacy of dendritic cell/tumor fusion vaccine. J Immunol 2003; 170(7): 3806-11.
[http://dx.doi.org/10.4049/jimmunol.170.7.3806] [PMID: 12646647] ].
Therefore, active TGF-β1 produced by the tumor and local stroma cells contributes to the cancer progression and metastatic potential through autocrine and paracrine effects [53Tian M, Schiemann WP. The TGF-β paradox in human cancer: An update. Future Oncol 2009; 5(2): 259-71.
[http://dx.doi.org/10.2217/14796694.5.2.259] [PMID: 19284383] ]. Importantly, elevated TGF-β1 plasma levels have been associated with the advanced stage and poorer clinical outcome. For instance, in breast, prostate, pancreatic and renal cancer the increase in plasma TGF-β1 levels areassociated with the advanced stage of metastases [54Ivanović V, Todorović-Raković N, Demajo M, et al. Elevated plasma levels of transforming growth factor-beta 1 (TGF-beta 1) in patients with advanced breast cancer: Association with disease progression. Eur J Cancer 2003; 39(4): 454-61.
[http://dx.doi.org/10.1016/S0959-8049(02)00502-6] [PMID: 12751375] ]. Moreover, elevated serum levels of TGF-β1 have been observed in patients with myeloma, being both malignant cells and bone marrow stromal cells the source of TGF-β1. TGF-β1 levels also are elevated in non–Hodgkin’s lymphoma and are markedly elevated in high-grade lymphomas, cutaneous T cell lymphomas with a T-regulatory phenotype, and in splenic marginal zone lymphomas presented as myelofibrosis [30 and references therein].
3. MYELOID-DERIVED SUPPRESSORS CELLS IN CANCER AND TRANSFORMING GROWTH FACTOR-B1 INTERPLAY
3.1. Myeloid-Derived Suppressor Cells
It has been established that the cancer progression is commonly associated with increased number of immature myeloid cells, at various stages of differentiation, in spleen, peripheral blood and within TM. Currently these cells are recognized as MDSC, and they are a hallmark of cancer and a central mechanism of immune evasion [55Younos IH, Abe F, Talmadge JE. Myeloid-derived suppressor cells: Their role in the pathophysiology of hematologic malignancies and potential as therapeutic targets. Leuk Lymphoma 2015; 56(8): 2251-63.
[http://dx.doi.org/10.3109/10428194.2014.987141] [PMID: 25407654] , 56Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: Linking inflammation and cancer. J Immunol 2009; 182(8): 4499-506.
[http://dx.doi.org/10.4049/jimmunol.0802740] [PMID: 19342621] ]. MDSC were described first in mouse models bearing human tumor cells and later on they were described in the patients with head and neck squamous cancer [57Chen J, Ye Y, Liu P, et al. Suppression of T cells by myeloid-derived suppressor cells in cancer. Hum Immunol 2017; 78(2): 113-9.
[http://dx.doi.org/10.1016/j.humimm.2016.12.001] [PMID: 27939507] ].
MDSC primarily include immature myeloid cells (IMC), which in steady-state conditions, leave the bone marrow as myeloid precursor cells and migrate to peripheral tissues, such as the spleen, where they differentiate into mature myeloid cells in response to the specific tissue molecules. Whereas, under pathologic conditions associated with chronic inflammation IMC become to differentiate into functional immunosuppressive MDSC. Nevertheless, immunosuppressive MDSC do not expand under healthy conditions [58Gabrilovich DI. Myeloid-Derived Suppressor Cells. Cancer Immunol Res 2017; 5(1): 3-8.
[http://dx.doi.org/10.1158/2326-6066.CIR-16-0297] [PMID: 28052991] , 59Dai J, El Gazzar M, Li GY, Moorman JP, Yao ZQ. Myeloid-derived suppressor cells: Paradoxical roles in infection and immunity. J Innate Immun 2015; 7(2): 116-26.
[http://dx.doi.org/10.1159/000368233] [PMID: 25401944] ].
In general, MDSC include a small group of myeloid progenitors as well as immature mononuclear cells, that in humans can be identified as CD11b+,CD33+, CD15+ or CD66b+, CD14-for polymorphonuclear (PMN)-MSC, while monocytic(M)-MDSC are CD11b+ or CD33+,CD14+, HLA-DRlow which distinguish from HLA-DRhi monocytes. Moreover, the Lin− (including CD3, CD14, CD15, CD19, and CD56) and HLA-DR−CD33+ cells contain mixed groups of MDSC, which comprise more immature progenitors [60Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016; 7: 12150.
[http://dx.doi.org/10.1038/ncomms12150] [PMID: 27381735] ]. Meanwhile, in mice MDSC are characterized by the expression of Gr-1 (that share common epitope to Ly6C and Ly6G) and CD11b. Two categories can be defined in mice, the PMN-MDSC as CD11b+Ly6G+Ly6Clo and M-MDSC as CD11b+Ly6G−Ly6Chi [1Trikha P, Carson WE 3rd. Signaling pathways involved in MDSC regulation. Biochim Biophys Acta 2014; 1846: 55-65., 60Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016; 7: 12150.
[http://dx.doi.org/10.1038/ncomms12150] [PMID: 27381735] ].
Currently, it is accepted that MDSC expansion and accumulation is regulated by two sequential set of factors: first the factors that are implicated in the expansion of MDSC such as GM-CSF, M-CSF and G-CSF, and also by factors produced by cancer cells and tumor stroma including TGF-β1; the second set of factors, such as IFN-γ, TNF-α, IL-4/13, IL-1β and COX2 significantly upregulates MDSC immunosuppressive functions [61Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest 2015; 125(9): 3356-64.
[http://dx.doi.org/10.1172/JCI80005] [PMID: 26168215] , 62Umansky V, Sevko A. Tumor microenvironment and myeloid-derived suppressor cells. Cancer Microenviron 2013; 6(2): 169-77.
[http://dx.doi.org/10.1007/s12307-012-0126-7] [PMID: 23242672] ].
Several studies reported the immunosuppressive effects of MDSC in HCC, melanoma, prostate cancer, bladder cancer, non–small cell lung cancer and head and neck squamous cell carcinoma, breast cancer, gastric cancer, colorectal cancer and others, which evidences their clinical significance [60Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016; 7: 12150.
[http://dx.doi.org/10.1038/ncomms12150] [PMID: 27381735] ]. The capacity of MDSC to support tumor growth and metastases can be defined according to the next functions: (i) protection of tumor cells from immune-surveillance; (ii) remodeling of the tumor microenvironment, (ii) participating in the formation of a pre-metastatic niche; and (iv) by their interaction with cancer cells facilitating the EMT [61Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest 2015; 125(9): 3356-64.
[http://dx.doi.org/10.1172/JCI80005] [PMID: 26168215] ].
3.2. Immunomodulatory Role of MDSC
The ability to suppress immune cells is one of the main characteristic of MDSC. Although MDSC are implicated in the suppression of different cells of the immune system such as NK and B cells the inhibition of T cells is the key for evaluation of MDSC function. Moreover, T-cell inhibition appears to be sufficient functional criteria for designation of cells as MDSC [58Gabrilovich DI. Myeloid-Derived Suppressor Cells. Cancer Immunol Res 2017; 5(1): 3-8.
[http://dx.doi.org/10.1158/2326-6066.CIR-16-0297] [PMID: 28052991] , 60Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016; 7: 12150.
[http://dx.doi.org/10.1038/ncomms12150] [PMID: 27381735] ].
MDSC express enzymes and metabolic by-products contributing to their immune-regulatory functions, such as arginase-1 (Arg-1) inducible nitric oxide synthase (iNOS)/ nitric oxide (NO), reactive nitrogen species (RNS), reactive oxygen species (ROS), Indoleamine 2, 3-dioxygenase (IDO) and programmed death-ligand 1 (PD-L1), among others. Furthermore, they produce high levels of anti-inflammatory cytokines, such as IL-10 and TGF-β1 [60Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016; 7: 12150.
[http://dx.doi.org/10.1038/ncomms12150] [PMID: 27381735] , 61Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest 2015; 125(9): 3356-64.
[http://dx.doi.org/10.1172/JCI80005] [PMID: 26168215] ]. Namely, T-cell expansion is highly susceptible and linked to the catabolism of L-arginine (Arg). In this sense, iNOS uses Arg as precursor for NO production; meanwhile Arg-1 catabolizes Arg to urea and ornithine. Therefore, the depletion of the T-cell essential nutrient Arg by elevated expression of iNOS and Arg-1 in tumor microenvironment inhibits T-cell proliferation. The main mechanisms of Arg depletion-induced T-cell inhibition are the downregulation of ζ-chains on T-cell receptors and the inhibition of cyclin D3 and cdk4 expression [26Wu AA, Drake V, Huang HS, Chiu S, Zheng L. Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells. OncoImmunology 2015; 4(7): 1016700.
[http://dx.doi.org/10.1080/2162402X.2015.1016700] [PMID: 26140242] , 63Pyzer AR, Cole L, Rosenblatt J, Avigan DE. Myeloid-derived suppressor cells as effectors of immune suppression in cancer. Int J Cancer 2016; 139(9): 1915-26.
[http://dx.doi.org/10.1002/ijc.30232] [PMID: 27299510] ]. In turn, the increase of iNOS expression in MDSC and therefore higher levels of NO contributes to T-cell proliferation arrest by blocking IL-2 production [64Blesson S, Thiery J, Gaudin C, et al. Analysis of the mechanisms of human cytotoxic T lymphocyte response inhibition by NO. Int Immunol 2002; 14(10): 1169-78.
[http://dx.doi.org/10.1093/intimm/dxf081] [PMID: 12356682] ]. In a similar way, IDO expression, which is a critical rate-limiting enzyme of tryptophan catabolism through the kynurenine pathway, produces tryptophan depletion and halts the effector T cell proliferation [65Munn DH, Mellor AL. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends Immunol 2016; 37(3): 193-207.
[http://dx.doi.org/10.1016/j.it.2016.01.002] [PMID: 26839260] ]
Furthermore, the production of RNS, such as perioxynitrites, drives T-cells apoptosis by nitrotyrosylating key proteins involved in the signaling of T-cell activation. For instance, the nitration of T-cell receptor (TCR) induces conformational changes in TCR-CD3 complex that diminishes the interaction between CD8 and TCR that results in the loss of antigen-specific stimulation [66Monu NR, Frey AB. Myeloid-derived suppressor cells and anti-tumor T cells: A complex relationship. Immunol Invest 2012; 41(6-7): 595-613.
[http://dx.doi.org/10.3109/08820139.2012.673191] [PMID: 23017137] ]. Whereas, the CD3 theta chain expression is reduced by MDSC through ROS (H2O2) dependant mechanisms [67Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res 2001; 61(12): 4756-60.
[PMID: 11406548] ]. Moreover, by cell-cell contact MDSC may suppress T-cell activation via induction of T-cell apoptosis through interaction of programmed death-1 (PD-1) molecules with its cognate ligands PD-L1 and PD-L2 [68Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 2014; 211(5): 781-90.
[http://dx.doi.org/10.1084/jem.20131916] [PMID: 24778419] ].
Additionally to direct T-cell inhibition, MDSC cells are able to induce and recruit Tregs by TGF-β1 and IL-10 expression [63Pyzer AR, Cole L, Rosenblatt J, Avigan DE. Myeloid-derived suppressor cells as effectors of immune suppression in cancer. Int J Cancer 2016; 139(9): 1915-26.
[http://dx.doi.org/10.1002/ijc.30232] [PMID: 27299510] ]. Moreover, M-MDSC-derived TGF-β1 and retinoic acid participates in the transdifferentiation of TH17 cell towards Tregs [69Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood 2011; 117(24): 6532-41.
[http://dx.doi.org/10.1182/blood-2010-11-317321] [PMID: 21493801] ]. Furthermore, MDSC inhibit cytotoxicity of NK Cells towards autologous activated T cells in an in vitro model [70Hoechst B, Voigtlaender T, Ormandy L, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009; 50(3): 799-807.
[http://dx.doi.org/10.1002/hep.23054] [PMID: 19551844] ]. Finally, MDSC inhibit DC function via IL-10 by reducing the DC-mediated T-cells activation [71Hu C-E, Gan J, Zhang R-D, Cheng YR, Huang GJ. Up-regulated myeloid-derived suppressor cell contributes to hepatocellular carcinoma development by impairing dendritic cell function. Scand J Gastroenterol 2011; 46(2): 156-64.
[http://dx.doi.org/10.3109/00365521.2010.516450] [PMID: 20822377] ].
Thus, MDSC possesses several mechanisms to counteract T-cell activation and response that contributes to produce a supportive TM for cancer cell development.
3.3. MDSC Contribution to Cancer Progression
One of the main aspects, beyond immunomodulatory functions, that support tumor growth is the MDSC contribution to the remodeling of tumor microenvironment by producing VEGF, bFGF and Matrix metalloproteinases (MMPs), which contribute to tumor neoangiogenesis and to the increase of cancer cell motility and invasion [58Gabrilovich DI. Myeloid-Derived Suppressor Cells. Cancer Immunol Res 2017; 5(1): 3-8.
[http://dx.doi.org/10.1158/2326-6066.CIR-16-0297] [PMID: 28052991] , 61Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest 2015; 125(9): 3356-64.
[http://dx.doi.org/10.1172/JCI80005] [PMID: 26168215] ]. Intriguingly, MDSC can also contribute to the tumor endothelium by transdifferentiation toward endothelial cells as is demonstrated by VEGFR2 expression [72Yang L, DeBusk LM, Fukuda K, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 2004; 6(4): 409-21.
[http://dx.doi.org/10.1016/j.ccr.2004.08.031] [PMID: 15488763] ]. Moreover, MDSC may contribute to the cancer-associated EMT by the expression of TGF-β1, HGF, EGF, and IL-6. In in vitro studies it has been demonstrated that co-culture of cancer cells with MDSC produces a stem-like phenotype of cancer cells, whereas in vivo depletion of PMN-MDSC decreases the frequency of cancer cell displaying EMT phenotype in the primary tumor [73Toh B, Wang X, Keeble J, et al. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol 2011; 9(9): 1001162.
[http://dx.doi.org/10.1371/journal.pbio.1001162] [PMID: 21980263] ].
Due to the increasing evidences that support the relation between MDSC frequency and clinical outcome in cancer, these cells have been postulated as cellular biomarkers for monitoring tumor progression and cancer patients response to chemotherapy [74Umansky V, Blattner C, Gebhardt C, Utikal J. The Role of Myeloid-Derived Suppressor Cells (MDSC) in Cancer Progression. Vaccines (Basel) 2016; 4(4): 36.
[http://dx.doi.org/10.3390/vaccines4040036] [PMID: 27827871] ]. Circulating MDSC number is elevated in cancer patients and can be inversely correlated with clinical response. For instance, in renal cell carcinoma the resistance to the TK inhibitor sunitinib is associated with elevated peripheral blood MDSC levels [75Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol 2011; 11(7): 856-61.
[http://dx.doi.org/10.1016/j.intimp.2011.01.030] [PMID: 21315783] ], while in small cell lung cancer they predict a poorer response to cisplatin chemotherapy, the level of M-MDSC is associated with poor response to bevacizumab and disease progression [76Feng PH, Lee KY, Chang YL, et al. CD14(+)S100A9(+) monocytic myeloid-derived suppressor cells and their clinical relevance in non-small cell lung cancer. Am J Respir Crit Care Med 2012; 186(10): 1025-36.
[http://dx.doi.org/10.1164/rccm.201204-0636OC] [PMID: 22955317] , 77Koinis F, Vetsika EK, Aggouraki D, et al. Effect of First-Line Treatment on Myeloid-Derived Suppressor Cells’ Subpopulations in the Peripheral Blood of Patients with Non-Small Cell Lung Cancer. J Thorac Oncol 2016; 11(8): 1263-72.
[http://dx.doi.org/10.1016/j.jtho.2016.04.026] [PMID: 27178984] ]. Similarly, in breast cancer patients M-MDSC levels may represent biomarker for monitoring disease progression [78Bergenfelz C, Larsson AM, von Stedingk K, et al. Systemic Monocytic-MDSCs Are Generated from Monocytes and Correlate with Disease Progression in Breast Cancer Patients. PLoS One 2015; 10(5): 0127028.
[http://dx.doi.org/10.1371/journal.pone.0127028] [PMID: 25992611] ]. Meanwhile, in B cell acute lymphoblastic leukemia (B-ALL), PMN-MDSC levels correlated positively with therapeutic responses and B-ALL prognostic markers, including minimal residual disease, and the frequencies of CD20+ and blast cells [79Liu YF, Chen YY, He YY, et al. Expansion and activation of granulocytic, myeloid-derived suppressor cells in childhood precursor B cell acute lymphoblastic leukemia. J Leukoc Biol 2017; 102(2): 449-58.
[http://dx.doi.org/10.1189/jlb.5MA1116-453RR] [PMID: 28619949] ]. Furthermore, MDSC also seems to impact the clinical course and prognosis of adult acute myeloid leukemia [80Sun H, Li Y, Zhang ZF, et al. Increase in myeloid-derived suppressor cells (MDSCs) associated with minimal residual disease (MRD) detection in adult acute myeloid leukemia. Int J Hematol 2015; 102(5): 579-86.
[http://dx.doi.org/10.1007/s12185-015-1865-2] [PMID: 26358057] ].
3.4. TGF-β1 and MDSC
Clinical data demonstrated that TM–associated TGF-β1 levels correlate with poor prognosis in cancer patients. Beyond cancer cell production of TGF-β1, several stromal cells are also able to increase the level of TGF-β1 the tumor, which include cancer associated fibroblast , mesenchymal stromal cells, and also the innate immune system cooperates to the augment of TGF-β1 tumor levels [31Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol 2010; 31(6): 220-7.
[http://dx.doi.org/10.1016/j.it.2010.04.002] [PMID: 20538542] , 81Costanza B, Umelo IA, Bellier J, Castronovo V, Turtoi A. Stromal Modulators of TGF-β in Cancer. J Clin Med 2017; 6(1): 7.
[http://dx.doi.org/10.3390/jcm6010007] [PMID: 28067804] ].
Tumor production of TGF-β1 can contribute to the increment of MDSC frequency and function Fig. (3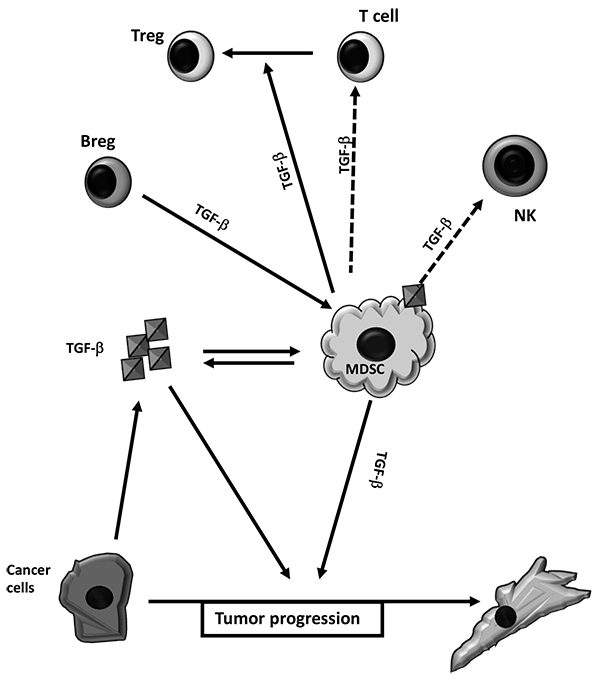 ). In vitro TGF-β1 is able to induce functional M-MDSC from purified human monocytes [82Casacuberta-Serra S, Parés M, Golbano A, Coves E, Espejo C, Barquinero J. Myeloid-derived suppressor cells can be efficiently generated from human hematopoietic progenitors and peripheral blood monocytes. Immunol Cell Biol 2017; 95(6): 538-48.
). In vitro TGF-β1 is able to induce functional M-MDSC from purified human monocytes [82Casacuberta-Serra S, Parés M, Golbano A, Coves E, Espejo C, Barquinero J. Myeloid-derived suppressor cells can be efficiently generated from human hematopoietic progenitors and peripheral blood monocytes. Immunol Cell Biol 2017; 95(6): 538-48.
[http://dx.doi.org/10.1038/icb.2017.4] [PMID: 28108746] ]. One interesting mechanism implicated tumor exosomes (TEXs) in the induction and accumulation of MDSC. Generally, exosomes are described as 30 to 100 nm size vesicles originated from the endosome organelles carrying different genes, lipids, proteins, and microRNAs (miRS). Many cells, including tumor cells, have the capacity to release exosomes. Recently, increased evidence has suggested that TEXs might act as a vehicle for transmitting signals for suppression thus having negative effects on antitumor immune responses [83Chen W, Jiang J, Xia W, Huang J. Tumor-Related Exosomes Contribute to Tumor-Promoting Microenvironment: An Immunological Perspective. J Immunol Res 2017; 2017: 1073947.]. Furthermore, TEXs-associated TGF-β1 also contributes to MDSC expansion. Namely, in breast cancer mouse model, TEXs-associated TGF-β1, in addition to PGE2, contributes to in vivo MDSC induction and tumor growth [84Xiang X, Poliakov A, Liu C, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer 2009; 124(11): 2621-33.
[http://dx.doi.org/10.1002/ijc.24249] [PMID: 19235923] ].
One of the mechanisms involved in TGF-β-induced MDSC is its capacity to regulate miRs expression. MiRs are noncoding single-stranded RNAs with an average of 22 nucleotides long, which post-transcriptionally regulate gene expression trough its capacity to interfere RNAs by binding either to the 3′UTR or 5′UTR or the coding sequence of mRNAs [85Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116(2): 281-97.
[http://dx.doi.org/10.1016/S0092-8674(04)00045-5] [PMID: 14744438] ]. Specifically, TGF-β1 induces both M-MDSC and PMN-MDSC from mouse bone marrow mononuclear cells by up-regulating the expression of miR-21 and miR-155 [86Li L, Zhang J, Diao W, et al. MicroRNA-155 and MicroRNA-21 promote the expansion of functional myeloid-derived suppressor cells. J Immunol 2014; 192(3): 1034-43.
[http://dx.doi.org/10.4049/jimmunol.1301309] [PMID: 24391219] ]. In addition, TGF-β1 is able to regulate mouse MDSC proliferation by induction of miR-494 in a Smad3 dependent way [87Liu Y, Lai L, Chen Q, et al. MicroRNA-494 is required for the accumulation and functions of tumor-expanded myeloid-derived suppressor cells via targeting of PTEN. J Immunol 2012; 188(11): 5500-10.
[http://dx.doi.org/10.4049/jimmunol.1103505] [PMID: 22544933] ].
The capacity of TGF-β to contribute to MDSC immunosuppressive properties is also pictured by the capacity of B regulatory cells (Breg) to educate the MDSC. Cancer associated Breg can educate both M- MDSC and PMN-MDSC subpopulations to suppress T cell proliferation. This relays in part to the presence and activation of TGF-β1 receptors in MDSC, since the use of TβRI inhibitor or mice with TβRII deficient myeloid cells reduces the capacity of Bregs to fully activate the regulatory capacity of MDSC concomitantly to the inhibition of metastasis [88Bodogai M, Moritoh K, Lee-Chang C, et al. Immunosuppressive and Prometastatic Functions of Myeloid-Derived Suppressive Cells Rely upon Education from Tumor-Associated B Cells. Cancer Res 2015; 75(17): 3456-65.
[http://dx.doi.org/10.1158/0008-5472.CAN-14-3077] [PMID: 26183924] ].
TGF-β1, beyond its capacity to induce MDSC, also contributes to the immune suppressive capacity of MDSC, as well as to their contribution to tumor malignancy. For instance, mouse mammary carcinomas with Tgfbr2 deletion provoke increased infiltration of Gr-1+CD11b+ MDSC to the invasive front, moreover these MDSC were the main source of TGF-β1 within the tumors. The increment in TGF-β1 levels within the tumor contribute to the enhancement of invasive capacities of mammary cancer cells [89Yang L, Huang J, Ren X, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 2008; 13(1): 23-35.
[http://dx.doi.org/10.1016/j.ccr.2007.12.004] [PMID: 18167337] ]. Human peripheral blood CD14+HLA-DR− MDSC subset in squamous cell carcinoma of the head and neck are high producers of TGF-β1 and by blocking this factor with anti-TGF-β1 monoclonal antibody the T-cell immunosuppression is reduced [90Chikamatsu K, Sakakura K, Toyoda M, Takahashi K, Yamamoto T, Masuyama K. Immunosuppressive activity of CD14+ HLA-DR- cells in squamous cell carcinoma of the head and neck. Cancer Sci 2012; 103(6): 976-83.
[http://dx.doi.org/10.1111/j.1349-7006.2012.02248.x] [PMID: 22360618] ]. Also, IL-13 activation of CD11b+Gr-1int MDSC induces TGF-β1 production, and the inhibition of IL-13 receptor alpha restores in vivo tumor immunosurveillance in a murine syngeneic model of colon carcinoma [91Fichtner-Feigl S, Terabe M, Kitani A, et al. Restoration of tumor immunosurveillance via targeting of interleukin-13 receptor-alpha 2. Cancer Res 2008; 68(9): 3467-75.
[http://dx.doi.org/10.1158/0008-5472.CAN-07-5301] [PMID: 18451175] ]. Interestingly, the exposition of C57BL6/J mice to an acute dose of single-walled carbon nanotubes (CNT) provokes the recruitment and accumulation of MDSC into the lung. These CNT-induced MDSC produce TGF-β1 resulting in an immunosuppressive microenvironment and increased lung tumor burden. However, in TGF-β1-deficient mice the CNT do not enhance the tumor growth. In this model, TGF-β1 was not involved in the initial recruitment of MDSC to exposed lungs to CNT, while it was critical to the MDSC-dependant stimulation of tumor growth [92Shvedova AA, Kisin ER, Yanamala N, et al. MDSC and TGFβ Are Required for Facilitation of Tumor Growth in the Lungs of Mice Exposed to Carbon Nanotubes. Cancer Res 2015; 75(8): 1615-23.
[http://dx.doi.org/10.1158/0008-5472.CAN-14-2376] [PMID: 25744719] ].
Although the immunosuppressive capacity of the MDSC is in part due to its capacity to produce TGF-β1, they can also use TGF-β1 to regulate other immunoregulatory cells. As aforementioned, TGF-β1 induces the potent immunosuppressive cells Treg cells [93Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 2003; 198(12): 1875-86.
[http://dx.doi.org/10.1084/jem.20030152] [PMID: 14676299] ]. In this aspect, mouse MDSC, in an IL-10 and TGF-β1 dependant manner, induce Tregs cells in tumor-bearing host model, which contributes to the downregulation of T-cell mediate immunity [94Huang B, Pan PY, Li Q, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 2006; 66(2): 1123-31.
[http://dx.doi.org/10.1158/0008-5472.CAN-05-1299] [PMID: 16424049] ]. Conversely, PMN-MDSC obtained from tumor bearing mice have been shown to inhibit the in vitro capacity of TGF-β1 to induce Tregs cells in ROS/IDO-dependant pathways. These data point out that PMN-MDSC plays fundamental roles in the generation of Tregs cells during tumorigenesis [95Centuori SM, Trad M, LaCasse CJ, et al. Myeloid-derived suppressor cells from tumor-bearing mice impair TGF-β-induced differentiation of CD4+CD25+FoxP3+ Tregs from CD4+CD25-FoxP3- T cells. J Leukoc Biol 2012; 92(5): 987-97.
[http://dx.doi.org/10.1189/jlb.0911465] [PMID: 22891289] ]. In addition, MDSC are able to regulate NK cells activity in mouse model of liver cancer. Furthermore, MDSC by membrane bounded TGF-β1 impair NK function in orthotropic liver cancer-bearing mice, therefore MDSC induce an immune-tolerant tumor microenvironment by also inhibiting NK cytotoxic activity [96Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol 2009; 182(1): 240-9.
[http://dx.doi.org/10.4049/jimmunol.182.1.240] [PMID: 19109155] , 97Zhang H, Li Z, Wang L, et al. Critical Role of Myeloid-Derived Suppressor Cells in Tumor-Induced Liver Immune Suppression through Inhibition of NKT Cell Function. Front Immunol 2017; 8: 129.
[http://dx.doi.org/10.3389/fimmu.2017.00129] [PMID: 28243237] ].
CONCLUSION
In tumor microenvironment TGF−β1 plays an important role by contributing to the reduction of immunosurveillance, either by direct induction of MDSC, or by contributing to MDSC regulation of T-cell mediate immune-responses as well as indirectly by mediating the capacity of MDSC to modulate Tregs and cytotoxic NK cells. However, the capacity of MDSC to produce TGF-β1 suggests a positive feedback that amplifies the role of MDSC in establishing an immune-tolerant microenvironment to promote tumor progression.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are grateful to Ms. Marija Bozic for her excellent and valuable editorial assistance. We apologize to those colleagues whose work, although relevant to the issues dealt within this review, has not been included due to space limitations. This work was supported by Ministry of education, Science and Technological Development of the Republic of Serbia (grants 175053 and 175024). We also thank to the support of visiting professor program of UBO to J.F.S.
This article is based upon work from the COST Action BM1404 Mye-EUNITER (www.mye-euniter.eu), supported by COST (European Cooperation in Science and Technology) to JFS.
REFERENCES
| [1] | Trikha P, Carson WE 3rd. Signaling pathways involved in MDSC regulation. Biochim Biophys Acta 2014; 1846: 55-65. |
| [2] | Santibanez JF, Krstic J, Quintanilla M, Bernabeu C. TGF–β Signalling and Its Role in Cancer Progression and Metastasis. eLS John Wiley and Sons Ltd 2016; 1-9. [http://dx.doi.org/10.1002/9780470015902.a0025045] |
| [3] | Colak S, Ten Dijke P. Targeting TGF-β Signaling in Cancer. Trends Cancer 2017; 3(1): 56-71. [http://dx.doi.org/10.1016/j.trecan.2016.11.008] [PMID: 28718426] |
| [4] | Morikawa M, Derynck R, Miyazono K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb Perspect Biol 2016; 8(5): 27141051. |
| [5] | Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011; 20(5): 576-90. [http://dx.doi.org/10.1016/j.ccr.2011.09.009] [PMID: 22094253] |
| [6] | Yang L, Pang Y, Moses HL. TGF-β and immune cells: An important regulatory axis in the tumor microenvironment and progression. Trends Immunol 2010; 31(6): 220-7. [http://dx.doi.org/10.1016/j.it.2010.04.002] [PMID: 20538542] |
| [7] | Sica A, Porta C, Amadori A, Pastò A. Tumor-associated myeloid cells as guiding forces of cancer cell stemness. Cancer Immunol Immunother 2017; 66(8): 1025-36. [http://dx.doi.org/10.1007/s00262-017-1997-8] [PMID: 28401258] |
| [8] | Barnie PA, Zhang P, Lv H, et al. Myeloid-derived suppressor cells and myeloid regulatory cells in cancer and autoimmune disorders. Exp Ther Med 2017; 13(2): 378-88. [http://dx.doi.org/10.3892/etm.2016.4018] [PMID: 28352304] |
| [9] | Anzano MA, Roberts AB, Smith JM, Sporn MB, De Larco JE. Sarcoma growth factor from conditioned medium of virally transformed cells is composed of both type alpha and type beta transforming growth factors. Proc Natl Acad Sci USA 1983; 80(20): 6264-8. [http://dx.doi.org/10.1073/pnas.80.20.6264] [PMID: 6604914] |
| [10] | Govinden R, Bhoola KD. Genealogy, expression, and cellular function of transforming growth factor-beta. Pharmacol Ther 2003; 98(2): 257-65. [http://dx.doi.org/10.1016/S0163-7258(03)00035-4] [PMID: 12725873] |
| [11] | Santibañez JF, Quintanilla M, Bernabeu C. TGF-β/TGF-β receptor system and its role in physiological and pathological conditions. Clin Sci 2011; 121(6): 233-51. [http://dx.doi.org/10.1042/CS20110086] [PMID: 21615335] |
| [12] | Gordon KJ, Blobe GC. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta 2008; 197-228. |
| [13] | Sun N, Taguchi A, Hanash S. Switching Roles of TGF-β in Cancer Development: Implications for Therapeutic Target and Biomarker Studies. J Clin Med 2016; 5(12): 109. [http://dx.doi.org/10.3390/jcm5120109] [PMID: 27916872] |
| [14] | Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci 2003; 116(2): 217-24. [http://dx.doi.org/10.1242/jcs.00229] [PMID: 12482908] |
| [15] | Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 2000; 14(2): 163-76. [PMID: 10652271] |
| [16] | Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003; 113(6): 685-700. [http://dx.doi.org/10.1016/S0092-8674(03)00432-X] [PMID: 12809600] |
| [17] | Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol 2005; 21: 659-93. [http://dx.doi.org/10.1146/annurev.cellbio.21.022404.142018] [PMID: 16212511] |
| [18] | Itoh S, ten Dijke P. Negative regulation of TGF-beta receptor/Smad signal transduction. Curr Opin Cell Biol 2007; 19(2): 176-84. [http://dx.doi.org/10.1016/j.ceb.2007.02.015] [PMID: 17317136] |
| [19] | Mu Y, Gudey SK, Landström M. Non-Smad signaling pathways. Cell Tissue Res 2012; 347(1): 11-20. [http://dx.doi.org/10.1007/s00441-011-1201-y] [PMID: 21701805] |
| [20] | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144(5): 646-74. [http://dx.doi.org/10.1016/j.cell.2011.02.013] [PMID: 21376230] |
| [21] | Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986; 315(26): 1650-9. [http://dx.doi.org/10.1056/NEJM198612253152606] [PMID: 3537791] |
| [22] | Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21(3): 309-22. [http://dx.doi.org/10.1016/j.ccr.2012.02.022] [PMID: 22439926] |
| [23] | Pandya PH, Murray ME, Pollok KE, Renbarger JL. The Immune System in Cancer Pathogenesis: Potential Therapeutic Approaches. J Immunol Res 2016; 2016: 4273943. |
| [24] | Law AM, Lim E, Ormandy CJ, Gallego-Ortega D. The innate and adaptive infiltrating immune systems as targets for breast cancer immunotherapy. Endocr Relat Cancer 2017; 24(4): 123-44. [http://dx.doi.org/10.1530/ERC-16-0404] [PMID: 28193698] |
| [25] | Bussard KM, Mutkus L, Stumpf K, Gomez-Manzano C, Marini FC. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res 2016; 18(1): 84. [http://dx.doi.org/10.1186/s13058-016-0740-2] [PMID: 27515302] |
| [26] | Wu AA, Drake V, Huang HS, Chiu S, Zheng L. Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells. OncoImmunology 2015; 4(7): 1016700. [http://dx.doi.org/10.1080/2162402X.2015.1016700] [PMID: 26140242] |
| [27] | Franks AL, Slansky JE. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res 2012; 32(4): 1119-36. [PMID: 22493341] |
| [28] | Worthington JJ, Fenton TM, Czajkowska BI, Klementowicz JE, Travis MA. Regulation of TGFβ in the immune system: An emerging role for integrins and dendritic cells. Immunobiology 2012; 217(12): 1259-65. [http://dx.doi.org/10.1016/j.imbio.2012.06.009] [PMID: 22902140] |
| [29] | Park HY, Wakefield LM, Mamura M. Regulation of tumor immune surveillance and tumor immune subversion by tgf-β. Immune Netw 2009; 9(4): 122-6. [http://dx.doi.org/10.4110/in.2009.9.4.122] [PMID: 20157598] |
| [30] | Teicher BA. Transforming growth factor-beta and the immune response to malignant disease. Clin Cancer Res 2007; 13(21): 6247-51. [http://dx.doi.org/10.1158/1078-0432.CCR-07-1654] [PMID: 17975134] |
| [31] | Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol 2010; 31(6): 220-7. [http://dx.doi.org/10.1016/j.it.2010.04.002] [PMID: 20538542] |
| [32] | Kulkarni AB, Huh CG, Becker D, et al. TGF-β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA 1993; 90: 770-4. [http://dx.doi.org/10.1073/pnas.90.2.770] [PMID: 8421714] |
| [33] | Yang X, Letterio JJ, Lechleider RJ, et al. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-β. EMBO J 1999; 18(5): 1280-91. [http://dx.doi.org/10.1093/emboj/18.5.1280] [PMID: 10064594] |
| [34] | Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 2000; 12(2): 171-81. [http://dx.doi.org/10.1016/S1074-7613(00)80170-3] [PMID: 10714683] |
| [35] | Bierie B, Moses HL. Transforming growth factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth Factor Rev 2010; 21(1): 49-59. [http://dx.doi.org/10.1016/j.cytogfr.2009.11.008] [PMID: 20018551] |
| [36] | McDonald PP, Fadok VA, Bratton D, Henson PM. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-beta in macrophages that have ingested apoptotic cells. J Immunol 1999; 163(11): 6164-72. [PMID: 10570307] |
| [37] | Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002; 23(11): 549-55. [http://dx.doi.org/10.1016/S1471-4906(02)02302-5] [PMID: 12401408] |
| [38] | Mantovani A, Locati M. Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization: lessons and open questions. Arterioscler Thromb Vasc Biol 2013; 33(7): 1478-83. [http://dx.doi.org/10.1161/ATVBAHA.113.300168] [PMID: 23766387] |
| [39] | Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis 2012; 33(5): 949-55. [http://dx.doi.org/10.1093/carcin/bgs123] [PMID: 22425643] |
| [40] | Ghiringhelli F, Puig PE, Roux S, et al. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med 2005; 202(7): 919-29. [http://dx.doi.org/10.1084/jem.20050463] [PMID: 16186184] |
| [41] | Tesone AJ, Svoronos N, Allegrezza MJ, Conejo-Garcia JR. Pathological mobilization and activities of dendritic cells in tumor-bearing hosts: challenges and opportunities for immunotherapy of cancer. Front Immunol 2013; 4: 435. [http://dx.doi.org/10.3389/fimmu.2013.00435] [PMID: 24339824] |
| [42] | Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res 2007; 13(1): 5262-70. [http://dx.doi.org/10.1158/1078-0432.CCR-07-1157] [PMID: 17875754] |
| [43] | Krstic J, Santibanez JF. Transforming growth factor-beta and matrix metalloproteinases: functional interactions in tumor stroma-infiltrating myeloid cells. Scientific World Journal 2014; 2014: 521754. [http://dx.doi.org/10.1155/2014/521754] |
| [44] | Varricchi G, Galdiero MR, Loffredo S, et al. Are Mast Cells MASTers in Cancer? Front Immunol 2017; 8: 424. [http://dx.doi.org/10.3389/fimmu.2017.00424] [PMID: 28446910] |
| [45] | Marçais A, Viel S, Grau M, Henry T, Marvel J, Walzer T. Regulation of mouse NK cell development and function by cytokines. Front Immunol 2013; 4: 450. [http://dx.doi.org/10.3389/fimmu.2013.00450] [PMID: 24376448] |
| [46] | Nam JS, Terabe M, Kang MJ, et al. TGF-β subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res 2008; 68: 3915-23. [http://dx.doi.org/10.1158/0008-5472.CAN-08-0206] [PMID: 18483277] |
| [47] | Yoshimura A, Wakabayashi Y, Mori T. Cellular and molecular basis for the regulation of inflammation by TGF-beta. J Biochem 2010; 147(6): 781-92. [http://dx.doi.org/10.1093/jb/mvq043] [PMID: 20410014] |
| [48] | Fabre J, Giustiniani J, Garbar C, et al. Targeting the Tumor Microenvironment: The Protumor Effects of IL-17 Related to Cancer Type. Int J Mol Sci 2016; 17(9): 1433. [http://dx.doi.org/10.3390/ijms17091433] [PMID: 27589729] |
| [49] | Do JS, Fink PJ, Li L, et al. Cutting edge: Spontaneous development of IL-17-producing gamma delta T cells in the thymus occurs via a TGF-beta 1-dependent mechanism. J Immunol 2010; 184(4): 1675-9. [http://dx.doi.org/10.4049/jimmunol.0903539] [PMID: 20061408] |
| [50] | Chitadze G, Oberg HH, Wesch D. Kabelitz D. The Ambiguous Role of γδ T Lymphocytes in Antitumor Immunity. Trends Immunol 2017; 38(9): 668-78. |
| [51] | Geiser AG, Letterio JJ, Kulkarni AB, Karlsson S, Roberts AB, Sporn MB. Transforming growth factor beta 1 (TGF-beta 1) controls expression of major histocompatibility genes in the postnatal mouse: aberrant histocompatibility antigen expression in the pathogenesis of the TGF-beta 1 null mouse phenotype. Proc Natl Acad Sci USA 1993; 90(21): 9944-8. [http://dx.doi.org/10.1073/pnas.90.21.9944] [PMID: 8234339] |
| [52] | Kao JY, Gong Y, Chen CM, Zheng QD, Chen JJ. Tumor-derived TGF-β reduces the efficacy of dendritic cell/tumor fusion vaccine. J Immunol 2003; 170(7): 3806-11. [http://dx.doi.org/10.4049/jimmunol.170.7.3806] [PMID: 12646647] |
| [53] | Tian M, Schiemann WP. The TGF-β paradox in human cancer: An update. Future Oncol 2009; 5(2): 259-71. [http://dx.doi.org/10.2217/14796694.5.2.259] [PMID: 19284383] |
| [54] | Ivanović V, Todorović-Raković N, Demajo M, et al. Elevated plasma levels of transforming growth factor-beta 1 (TGF-beta 1) in patients with advanced breast cancer: Association with disease progression. Eur J Cancer 2003; 39(4): 454-61. [http://dx.doi.org/10.1016/S0959-8049(02)00502-6] [PMID: 12751375] |
| [55] | Younos IH, Abe F, Talmadge JE. Myeloid-derived suppressor cells: Their role in the pathophysiology of hematologic malignancies and potential as therapeutic targets. Leuk Lymphoma 2015; 56(8): 2251-63. [http://dx.doi.org/10.3109/10428194.2014.987141] [PMID: 25407654] |
| [56] | Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: Linking inflammation and cancer. J Immunol 2009; 182(8): 4499-506. [http://dx.doi.org/10.4049/jimmunol.0802740] [PMID: 19342621] |
| [57] | Chen J, Ye Y, Liu P, et al. Suppression of T cells by myeloid-derived suppressor cells in cancer. Hum Immunol 2017; 78(2): 113-9. [http://dx.doi.org/10.1016/j.humimm.2016.12.001] [PMID: 27939507] |
| [58] | Gabrilovich DI. Myeloid-Derived Suppressor Cells. Cancer Immunol Res 2017; 5(1): 3-8. [http://dx.doi.org/10.1158/2326-6066.CIR-16-0297] [PMID: 28052991] |
| [59] | Dai J, El Gazzar M, Li GY, Moorman JP, Yao ZQ. Myeloid-derived suppressor cells: Paradoxical roles in infection and immunity. J Innate Immun 2015; 7(2): 116-26. [http://dx.doi.org/10.1159/000368233] [PMID: 25401944] |
| [60] | Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016; 7: 12150. [http://dx.doi.org/10.1038/ncomms12150] [PMID: 27381735] |
| [61] | Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest 2015; 125(9): 3356-64. [http://dx.doi.org/10.1172/JCI80005] [PMID: 26168215] |
| [62] | Umansky V, Sevko A. Tumor microenvironment and myeloid-derived suppressor cells. Cancer Microenviron 2013; 6(2): 169-77. [http://dx.doi.org/10.1007/s12307-012-0126-7] [PMID: 23242672] |
| [63] | Pyzer AR, Cole L, Rosenblatt J, Avigan DE. Myeloid-derived suppressor cells as effectors of immune suppression in cancer. Int J Cancer 2016; 139(9): 1915-26. [http://dx.doi.org/10.1002/ijc.30232] [PMID: 27299510] |
| [64] | Blesson S, Thiery J, Gaudin C, et al. Analysis of the mechanisms of human cytotoxic T lymphocyte response inhibition by NO. Int Immunol 2002; 14(10): 1169-78. [http://dx.doi.org/10.1093/intimm/dxf081] [PMID: 12356682] |
| [65] | Munn DH, Mellor AL. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends Immunol 2016; 37(3): 193-207. [http://dx.doi.org/10.1016/j.it.2016.01.002] [PMID: 26839260] |
| [66] | Monu NR, Frey AB. Myeloid-derived suppressor cells and anti-tumor T cells: A complex relationship. Immunol Invest 2012; 41(6-7): 595-613. [http://dx.doi.org/10.3109/08820139.2012.673191] [PMID: 23017137] |
| [67] | Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res 2001; 61(12): 4756-60. [PMID: 11406548] |
| [68] | Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 2014; 211(5): 781-90. [http://dx.doi.org/10.1084/jem.20131916] [PMID: 24778419] |
| [69] | Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood 2011; 117(24): 6532-41. [http://dx.doi.org/10.1182/blood-2010-11-317321] [PMID: 21493801] |
| [70] | Hoechst B, Voigtlaender T, Ormandy L, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009; 50(3): 799-807. [http://dx.doi.org/10.1002/hep.23054] [PMID: 19551844] |
| [71] | Hu C-E, Gan J, Zhang R-D, Cheng YR, Huang GJ. Up-regulated myeloid-derived suppressor cell contributes to hepatocellular carcinoma development by impairing dendritic cell function. Scand J Gastroenterol 2011; 46(2): 156-64. [http://dx.doi.org/10.3109/00365521.2010.516450] [PMID: 20822377] |
| [72] | Yang L, DeBusk LM, Fukuda K, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 2004; 6(4): 409-21. [http://dx.doi.org/10.1016/j.ccr.2004.08.031] [PMID: 15488763] |
| [73] | Toh B, Wang X, Keeble J, et al. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol 2011; 9(9): 1001162. [http://dx.doi.org/10.1371/journal.pbio.1001162] [PMID: 21980263] |
| [74] | Umansky V, Blattner C, Gebhardt C, Utikal J. The Role of Myeloid-Derived Suppressor Cells (MDSC) in Cancer Progression. Vaccines (Basel) 2016; 4(4): 36. [http://dx.doi.org/10.3390/vaccines4040036] [PMID: 27827871] |
| [75] | Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol 2011; 11(7): 856-61. [http://dx.doi.org/10.1016/j.intimp.2011.01.030] [PMID: 21315783] |
| [76] | Feng PH, Lee KY, Chang YL, et al. CD14(+)S100A9(+) monocytic myeloid-derived suppressor cells and their clinical relevance in non-small cell lung cancer. Am J Respir Crit Care Med 2012; 186(10): 1025-36. [http://dx.doi.org/10.1164/rccm.201204-0636OC] [PMID: 22955317] |
| [77] | Koinis F, Vetsika EK, Aggouraki D, et al. Effect of First-Line Treatment on Myeloid-Derived Suppressor Cells’ Subpopulations in the Peripheral Blood of Patients with Non-Small Cell Lung Cancer. J Thorac Oncol 2016; 11(8): 1263-72. [http://dx.doi.org/10.1016/j.jtho.2016.04.026] [PMID: 27178984] |
| [78] | Bergenfelz C, Larsson AM, von Stedingk K, et al. Systemic Monocytic-MDSCs Are Generated from Monocytes and Correlate with Disease Progression in Breast Cancer Patients. PLoS One 2015; 10(5): 0127028. [http://dx.doi.org/10.1371/journal.pone.0127028] [PMID: 25992611] |
| [79] | Liu YF, Chen YY, He YY, et al. Expansion and activation of granulocytic, myeloid-derived suppressor cells in childhood precursor B cell acute lymphoblastic leukemia. J Leukoc Biol 2017; 102(2): 449-58. [http://dx.doi.org/10.1189/jlb.5MA1116-453RR] [PMID: 28619949] |
| [80] | Sun H, Li Y, Zhang ZF, et al. Increase in myeloid-derived suppressor cells (MDSCs) associated with minimal residual disease (MRD) detection in adult acute myeloid leukemia. Int J Hematol 2015; 102(5): 579-86. [http://dx.doi.org/10.1007/s12185-015-1865-2] [PMID: 26358057] |
| [81] | Costanza B, Umelo IA, Bellier J, Castronovo V, Turtoi A. Stromal Modulators of TGF-β in Cancer. J Clin Med 2017; 6(1): 7. [http://dx.doi.org/10.3390/jcm6010007] [PMID: 28067804] |
| [82] | Casacuberta-Serra S, Parés M, Golbano A, Coves E, Espejo C, Barquinero J. Myeloid-derived suppressor cells can be efficiently generated from human hematopoietic progenitors and peripheral blood monocytes. Immunol Cell Biol 2017; 95(6): 538-48. [http://dx.doi.org/10.1038/icb.2017.4] [PMID: 28108746] |
| [83] | Chen W, Jiang J, Xia W, Huang J. Tumor-Related Exosomes Contribute to Tumor-Promoting Microenvironment: An Immunological Perspective. J Immunol Res 2017; 2017: 1073947. |
| [84] | Xiang X, Poliakov A, Liu C, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer 2009; 124(11): 2621-33. [http://dx.doi.org/10.1002/ijc.24249] [PMID: 19235923] |
| [85] | Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116(2): 281-97. [http://dx.doi.org/10.1016/S0092-8674(04)00045-5] [PMID: 14744438] |
| [86] | Li L, Zhang J, Diao W, et al. MicroRNA-155 and MicroRNA-21 promote the expansion of functional myeloid-derived suppressor cells. J Immunol 2014; 192(3): 1034-43. [http://dx.doi.org/10.4049/jimmunol.1301309] [PMID: 24391219] |
| [87] | Liu Y, Lai L, Chen Q, et al. MicroRNA-494 is required for the accumulation and functions of tumor-expanded myeloid-derived suppressor cells via targeting of PTEN. J Immunol 2012; 188(11): 5500-10. [http://dx.doi.org/10.4049/jimmunol.1103505] [PMID: 22544933] |
| [88] | Bodogai M, Moritoh K, Lee-Chang C, et al. Immunosuppressive and Prometastatic Functions of Myeloid-Derived Suppressive Cells Rely upon Education from Tumor-Associated B Cells. Cancer Res 2015; 75(17): 3456-65. [http://dx.doi.org/10.1158/0008-5472.CAN-14-3077] [PMID: 26183924] |
| [89] | Yang L, Huang J, Ren X, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 2008; 13(1): 23-35. [http://dx.doi.org/10.1016/j.ccr.2007.12.004] [PMID: 18167337] |
| [90] | Chikamatsu K, Sakakura K, Toyoda M, Takahashi K, Yamamoto T, Masuyama K. Immunosuppressive activity of CD14+ HLA-DR- cells in squamous cell carcinoma of the head and neck. Cancer Sci 2012; 103(6): 976-83. [http://dx.doi.org/10.1111/j.1349-7006.2012.02248.x] [PMID: 22360618] |
| [91] | Fichtner-Feigl S, Terabe M, Kitani A, et al. Restoration of tumor immunosurveillance via targeting of interleukin-13 receptor-alpha 2. Cancer Res 2008; 68(9): 3467-75. [http://dx.doi.org/10.1158/0008-5472.CAN-07-5301] [PMID: 18451175] |
| [92] | Shvedova AA, Kisin ER, Yanamala N, et al. MDSC and TGFβ Are Required for Facilitation of Tumor Growth in the Lungs of Mice Exposed to Carbon Nanotubes. Cancer Res 2015; 75(8): 1615-23. [http://dx.doi.org/10.1158/0008-5472.CAN-14-2376] [PMID: 25744719] |
| [93] | Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 2003; 198(12): 1875-86. [http://dx.doi.org/10.1084/jem.20030152] [PMID: 14676299] |
| [94] | Huang B, Pan PY, Li Q, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 2006; 66(2): 1123-31. [http://dx.doi.org/10.1158/0008-5472.CAN-05-1299] [PMID: 16424049] |
| [95] | Centuori SM, Trad M, LaCasse CJ, et al. Myeloid-derived suppressor cells from tumor-bearing mice impair TGF-β-induced differentiation of CD4+CD25+FoxP3+ Tregs from CD4+CD25-FoxP3- T cells. J Leukoc Biol 2012; 92(5): 987-97. [http://dx.doi.org/10.1189/jlb.0911465] [PMID: 22891289] |
| [96] | Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol 2009; 182(1): 240-9. [http://dx.doi.org/10.4049/jimmunol.182.1.240] [PMID: 19109155] |
| [97] | Zhang H, Li Z, Wang L, et al. Critical Role of Myeloid-Derived Suppressor Cells in Tumor-Induced Liver Immune Suppression through Inhibition of NKT Cell Function. Front Immunol 2017; 8: 129. [http://dx.doi.org/10.3389/fimmu.2017.00129] [PMID: 28243237] |






