- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Ornithology Journal
(Discontinued)
ISSN: 1874-4532 ― Volume 13, 2020
Age-related Changes in the Response of Embryonic Motility to Acute Hypoxia During the Third Quarter of Chick Embryogenesis
Marina V. Nechaeva1, 2, *, Tatyana A. Alekseeva1
Abstract
Environmental factors may affect the growth, size, phenotype, behavior, and other characteristics of avian embryos at different developmental stages; however, the roles of individual embryonic physiological systems in these effects remain largely unclear. Embryonic motility is an important component of the prenatal development observed almost throughout embryogenesis and may be a precursor of post-hatching motor behavior. The influences of the environment on the development of motor behavior during embryogenesis (notably the embryonic motility affected by hypoxia) remain poorly studied. Consequently, using the chick embryo, we investigated the effect of acute hypoxia (10% or 5% О2 for 20 or 40 min) on embryonic cyclic motility at incubation days (D) 10, 12, 14, and 15 using in vivo video recording. Hypoxia inhibited motility; specifically, the average duration of activity and inactivity phases during hypoxic exposure were shortened and prolonged, respectively. Age-related changes in the responses to 10% and 5% O2 differed. The time course of the motility response to acute hypoxia varied during the D10-15 period and demonstrates that the embryo was capable of recovering motility under hypoxia. The recovery was likely enhanced with age due to maturation of regulatory capacity.
Article Information
Identifiers and Pagination:
Year: 2017Volume: 10
First Page: 10
Last Page: 22
Publisher Id: TOOENIJ-10-10
DOI: 10.2174/1874453201710010010
Article History:
Received Date: 29/09/2016Revision Received Date: 06/12/2016
Acceptance Date: 17/12/2016
Electronic publication date: 31/01/2017
Collection year: 2017
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution-Non-Commercial 4.0 International Public License (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/legalcode), which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Institute of Developmental Biology RAS, Vavilov Str. 26, Moscow 119334, Russia; Tel: 7(499) 135-63-27; Fax: 7(499) 135-80-12; E-mail: Mnechaeva2003@yahoo.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 29-09-2016 |
Original Manuscript | Age-related Changes in the Response of Embryonic Motility to Acute Hypoxia During the Third Quarter of Chick Embryogenesis | |
INTRODUCTION
Embryonic motility accompanies prenatal development of vertebrates, and it changes in a defined manner in the course of embryogenesis. Its nature and role in the normal development of the embryo are not entirely clear yet. It has been supposed that the embryonic motor activity is the precursor of motor behavior after birth or hatching [1Robinson SR. Behavioral embryology. In: Hopkins B, Ed. The cambridge encyclopedia of child development. Cambridge: Cambridge University Press 2005; pp. 469-73., 2Ryu YU, Bradley NS. Precocious locomotor behavior begins in the egg: development of leg muscle patterns for stepping in the chick. PLoS One 2009; 4(7): e6111.
[http://dx.doi.org/10.1371/journal.pone.0006111] [PMID: 19578536] ]. For instance, the repetitive leg movements in late stage chick embryos are locomotor-related and a fundamental link in the establishment of precocious locomotor skill [2Ryu YU, Bradley NS. Precocious locomotor behavior begins in the egg: development of leg muscle patterns for stepping in the chick. PLoS One 2009; 4(7): e6111.
[http://dx.doi.org/10.1371/journal.pone.0006111] [PMID: 19578536] ]. Further, embryonic motility is necessary for normal development of the musculoskeletal and nervous systems. There are many published studies on embryonic motility in chickens. They have shown, for example, that disturbances of the embryonic motor activity may result in developmental abnormalities and functional disorders, many of which persist in postnatal life [3Heywood JL, McEntee GM, Stickland NC. In ovo neuromuscular stimulation alters the skeletal muscle phenotype of the chick. J Muscle Res Cell Motil 2005; 26(1): 49-56.
[http://dx.doi.org/10.1007/s10974-005-9007-8] [PMID: 16088375] -6Pitsillides AA. Early effects of embryonic movement: a shot out of the dark. J Anat 2006; 208(4): 417-31.
[http://dx.doi.org/10.1111/j.1469-7580.2006.00556.x] [PMID: 16637868] ]. For example, the paralysis or hyperactivity of the chick embryo, provoked by the injection of some drugs, can affect joint, osteochondral, and muscle development and growth [6Pitsillides AA. Early effects of embryonic movement: a shot out of the dark. J Anat 2006; 208(4): 417-31.
[http://dx.doi.org/10.1111/j.1469-7580.2006.00556.x] [PMID: 16637868] ]. Moreover, via in ovo drug applications at precise developmental periods, it has been shown that normal patterns of spontaneous activity are required for correct motor axon guidance [7Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron 2004; 43(5): 687-701.
[http://dx.doi.org/10.1016/j.neuron.2004.08.018] [PMID: 15339650] ].
At the same time, little attention has been paid to the effects of external factors on chick embryonic motility. An important characteristic of this motility is its cyclic (or periodic) nature; i.e., it is organized in cycles consisting of active and inactive phases [8Hamburger V. Some aspects of the embryology of behavior. Q Rev Biol 1963; 38: 342-65.
[http://dx.doi.org/10.1086/403941] [PMID: 14111168] -11Oppenheim RW. The role of supraspinal input in embryonic motility: a re-examination in the chick. J Comp Neurol 1975; 160(1): 37-50.
[http://dx.doi.org/10.1002/cne.901600104] [PMID: 1112921] ]. The cyclic pattern of embryonic motility results from spontaneous periodic activity of the spinal neuronal network of the developing embryo [12Landmesser LT, ODonovan MJ. Activation patterns of embryonic chick hind limb muscles recorded in ovo and in an isolated spinal cord preparation. J Physiol 1984; 347: 189-204.
[http://dx.doi.org/10.1113/jphysiol.1984.sp015061] [PMID: 6707956] -14ODonovan MJ. Motor activity in the isolated spinal cord of the chick embryo: synaptic drive and firing pattern of single motoneurons. J Neurosci 1989; 9(3): 943-58.
[PMID: 2926486] ]. The ratio between the durations of the active and inactive phases varies during egg incubation, each stage of embryogenesis is being characterized by a specific value of this ratio. Therefore, this temporal parameter of embryonic motility is often used for quantitative estimation of the motility during embryogenesis as dependent on external factors [10Hamburger V, Balaban M, Oppenheim R, Wenger E. Periodic motility of normal and spinal chick embryos between 8 and 17 days of incubation. J Exp Zool 1965; 159(1): 1-13.
[http://dx.doi.org/10.1002/jez.1401590102] [PMID: 5215365] , 15Bradley NS. Transformations in embryonic motility in chick: kinematic correlates of type I and II motility at E9 and E12. J Neurophysiol 1999; 81(4): 1486-94.
[PMID: 10200185] -17Nechaeva M, Vladimirova I, Alexeeva T. Effect of acute hypoxia on the motor activity and heart rate of the 10-and 14-day chick embryo. Open Ornithol J 2010; 3: 127-33.
[http://dx.doi.org/10.2174/1874453201003010127] ].
Hypoxia is one of the most important factors affecting embryonic development. Oxygenation may vary considerably during the embryogenesis of birds. For example, a reduction in ambient oxygen (hypoxia) may occur naturally during embryogenesis of some bird species during periods of heavy rain. Additionally, many bird species are fossorial, laying their eggs at the end of long, narrow nests or within mounds, sites with poor air circulation and prone for development of hypoxic conditions. While nesting in a cavity can have advantages, such as minimizing exposure to predators, it may also pose difficulty with the fresh air supply leading to adverse effects on the survival of the incubating adults and their embryos [18Deeming DC. How does the bird-nest incubation unit work? Avian Biol Res 2016; 9: 103-13.
[http://dx.doi.org/10.3184/175815516X14567543242701] ]. The mechanisms of the effect of hypoxia on chick embryogenesis are being intensely studied; however, they remain unclear thus far. In addition, there are still limited data on how the chick embryonic motility changes under hypoxia [19Mortola JP, Louis AS, Simeonova M, Toro Velasquez PA. The motility of the chicken embryo: energetic cost and effects of hypoxia. Respir Physiol Neurobiol 2013; 188(2): 172-9.
[http://dx.doi.org/10.1016/j.resp.2013.05.030] [PMID: 23732509] , 20Nechaeva MV. Physiological responses to acute changes in temperature and oxygenation in bird and reptile embryos. Respir Physiol Neurobiol 2011; 178(1): 108-17.
[http://dx.doi.org/10.1016/j.resp.2011.04.003] [PMID: 21513821] ]. Earlier, we showed that acute hypoxia (10% O2 10 min) inhibited cyclic (or periodic) motility as estimated by its temporal parameters. This effect was found to depend on embryonic age: the motility inhibition was weak on day 10 of embryogenesis (D10), but it was substantial on D14 [17Nechaeva M, Vladimirova I, Alexeeva T. Effect of acute hypoxia on the motor activity and heart rate of the 10-and 14-day chick embryo. Open Ornithol J 2010; 3: 127-33.
[http://dx.doi.org/10.2174/1874453201003010127] ]. At the same time, authors that estimated the overall motility of a chick embryo by measuring the pressure within the egg reported on variable and often insignificant effects of acute hypoxia (10% O2 30 min) on body motion in the period D10-18, except for D16, when a significant decrease in the motility was observed [19Mortola JP, Louis AS, Simeonova M, Toro Velasquez PA. The motility of the chicken embryo: energetic cost and effects of hypoxia. Respir Physiol Neurobiol 2013; 188(2): 172-9.
[http://dx.doi.org/10.1016/j.resp.2013.05.030] [PMID: 23732509] ]. In the same studies, a stronger hypoxia (5% O2) consistently reduced body motion on D18. In addition, in ovo estimation of the bioelectrical activity of the lumbosacral regions of the embryo spinal cord, which is related to the cyclic motor activity of the embryo, showed that acute hypoxia (10% O2) inhibited the burst activity on D14 and D19, but not on D16 [21Gonya-Magee T, Stokes BT. Acute modification of embryonic spinal cord activity induced by hypoxia. Dev Neurosci 1980; 3(1): 11-8.
[http://dx.doi.org/10.1159/000112372] [PMID: 7408706] ]. Thus, the existing data on the development of the cyclic motility response to acute hypoxia during incubation are fragmentary and ambiguous, which calls for additional research.
We previously found a characteristic time course of the motility response to hypoxia on D14, but it was much less pronounced on D10 [17Nechaeva M, Vladimirova I, Alexeeva T. Effect of acute hypoxia on the motor activity and heart rate of the 10-and 14-day chick embryo. Open Ornithol J 2010; 3: 127-33.
[http://dx.doi.org/10.2174/1874453201003010127] ]. The time course showed that during hypoxic exposure the embryonic motility initially stopped and then was partly restored while the hypoxic exposure continued. For detailed analysis of this phenomenon, it is necessary to determine at which stage of incubation this pattern appears, how it changes with embryonic age, whether it is expressed if the oxygen level is even lower, and how it is affected by the increase in the duration of the hypoxic exposure. In addition, the study of the time course of the hypoxic responses of embryonic motility could help in revealing the mechanisms underlying the hypoxia’s inhibitory effect on the motility and their changes with the embryonic age.
Therefore, the purpose of this study was, first, to describe the age-related changes in the response of the cyclic motility of the embryo to acute moderate and severe hypoxia (10% and 5%, respectively) with different durations (20 and 40 min) during the third quarter of chick embryogenesis and, second, to determine the time-course of the parameters of an embryo’s cyclic motility during the hypoxic response under these conditions in as much detail as possible.
MATERIALS AND METHODS
Fertile White Leghorn chicken eggs were obtained from a commercial supplier and incubated under normoxic conditions in a laboratory incubator at a temperature of 37.5 ± 0.5°C and a humidity of 60-70%. Eggs were taken from the incubator on D10, D12, D14, D15 or D16 and placed into a 300-ml temperature-controlled (37.5°C) Plexiglas experimental chamber with a continuous flow of atmospheric air (200 ml/min), warmed to 37.5°C and humidified by passing through a small container filled with hydro gel beads. Eggs were opened over the air-cell side by making a window approximately 2 - 2.5 cm in diameter in the egg shell above the developing embryo. A part of the inner shell membrane was removed, a small hole was cut in the chorioallantoic membrane and the amnion to reach the embryo, and the force transducer was attached to a limb of the embryo by means of a special grip (micro-serrefine); the signal from the transducer was recorded continuously and stored digitally on a computer [17Nechaeva M, Vladimirova I, Alexeeva T. Effect of acute hypoxia on the motor activity and heart rate of the 10-and 14-day chick embryo. Open Ornithol J 2010; 3: 127-33.
[http://dx.doi.org/10.2174/1874453201003010127] ]. The experimental setup is presented in Fig. (1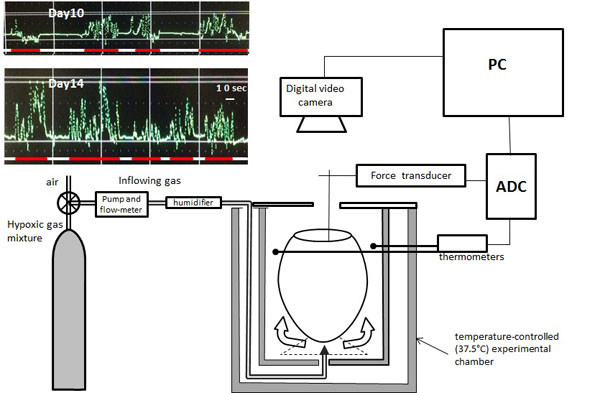 ).
).
A digital video camera eyepiece Scopetek DCM-800 (Shangrao TeleView Optical Instruments Co., Ltd, China) mounted on a dissecting microscope (OPTIKA SZM-2Led, Italy), was fixed at about 10 cm over the opened air cell of the egg and used to perform a continuous video recording with a capture rate of 30 fps using the software ScopePhoto3 to monitor the embryonic movements synchronously with the signal from the force transducer.
The videos were reviewed and calculated at a low speed by two reviewers using computer software (VLC media player with Motion-Detection) simultaneously compared with the corresponding mechanographic data. The durations of the activity phase (APh) and inactivity phase (IPh) were used to quantify the embryonic motility. As in previous studies [17Nechaeva M, Vladimirova I, Alexeeva T. Effect of acute hypoxia on the motor activity and heart rate of the 10-and 14-day chick embryo. Open Ornithol J 2010; 3: 127-33.
[http://dx.doi.org/10.2174/1874453201003010127] ], we defined an APh as any episode of embryonic movements (no shorter than 6 s) that was separated from other embryonic movements by at least 6 s of quiescence. When we studied the time-sequential changes in the APh and IPh, the durations of APh and IPh were represented as averages over every 5 min.
This method of the simultaneous video recording and mechanographical recording made it possible to accurately determine the time course of the cyclic embryo motility. For an additional control, at each studied age some embryos were run in normoxia for the whole duration of the test (120 min) and the results were analyzed for the control embryos the same way it was done for the experimental embryos. The results showed no reduction of cyclic motility during the 120 min period in normoxia (Fig. 2B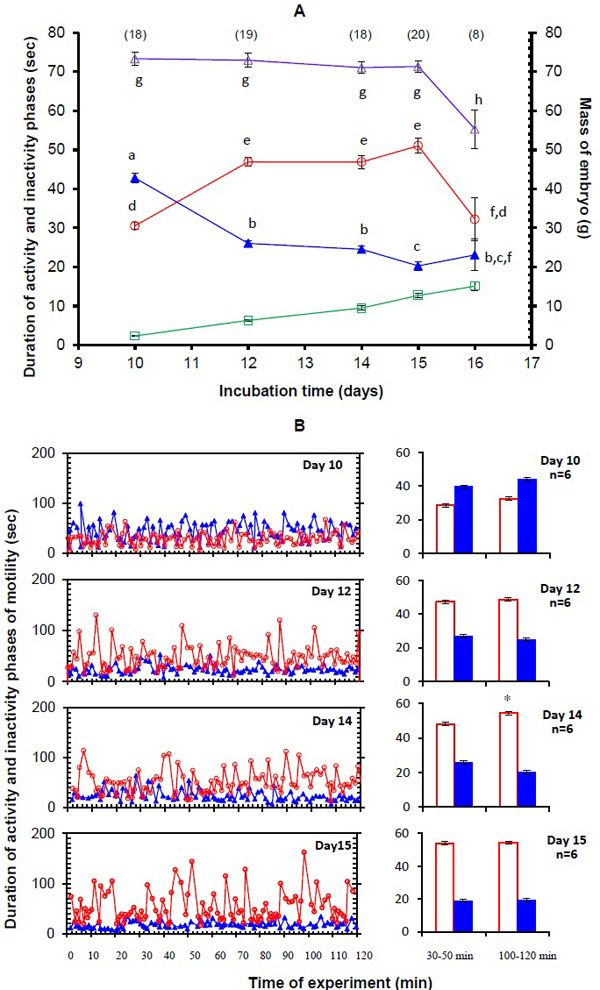 ).
).
During the experiment the egg was allowed to reach stable values of embryonic motility for 30 min postsurgery. Then the embryonic movements were sequentially recorded in normoxia (21% O2, control) for 20 min, then under hypoxia (in either a mixture of 10% O2 and 90% N2 or a mixture 5% O2 and 95% N2). In each case the gas mixture was supplied into the chamber at the same constant temperature 37.5°C, humidity and flow of 200 ml/min for 20 min or 40 min. Finally, the embryonic movements were recorded again under normoxic conditions for 30 min.
All experimental work was performed in accordance with the Guidelines for Humane Endpoints for Animals Used in Biomedical Research, Regulations for Laboratory Practice in Russian Federation, and under the supervision of the Ethics Committee for Animal Research of the Institute of Developmental Biology Russian Academy of Sciences. After the experiments, the embryos were staged according to the series for the chick embryo [22Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol 1951; 88(1): 49-92.
[http://dx.doi.org/10.1002/jmor.1050880104] [PMID: 24539719] ] and weighed.
Statistical analysis was performed using Statistica (version 7.0, Statsoft). A U-Mann-Whitney comparison nonparametric test was conducted between days of incubation to determine changes in the mean durations of APh and IPh. The nonparametric Wilcoxon matched-pairs test was used to estimate the effect of hypoxia on the temporal parameters of embryonic motility and recovery in air. The differences were considered significant at P < 0.05. All data were expressed as the mean ± S.E.M.
RESULTS
Age-related Changes in Cyclic Motility During D10-16
Control measurements of cyclic motility in normoxia were made on a total of 83 chick embryos at five incubation time points: D10 (n = 18), D12 (n = 19), D14 (n = 18), D15 (n = 20), and D16 (n = 8). The control data were first examined for age-related motility changes, the control data having been obtained from the experiments with long embryo exposure to normoxia, and from the control values in normoxia that were obtained from the experiments in which embryos were exposed to hypoxia. The chick embryos reached stage 36 on D10 and stage 42 on D16. The mean embryo weight increased from 2.31 ± 0.15 g on D10 to 15.1 ± 1.26 g on D16 (Fig. 2A ).
).
In control (normoxia), the embryonic motility displayed а cyclic character and each cycle consisted of an APh which was separated by an IPh (Fig. 2B ). Long recordings (120 min) of embryonic motility in normoxia showed that the values of the average duration of APh and IPh at the beginning of exposure did not differ from their values at the end of exposure at all ages studied except Day 14 when the average duration of APh increased over the course of the recording (Fig. 2B
). Long recordings (120 min) of embryonic motility in normoxia showed that the values of the average duration of APh and IPh at the beginning of exposure did not differ from their values at the end of exposure at all ages studied except Day 14 when the average duration of APh increased over the course of the recording (Fig. 2B ).
).
The average APh duration significantly increased from D10 (30.5 ± 0.8 s; n = 18) to D12 (46.9 ± 1.2 s; n = 19) (P < 0.001) and remained constant between D12 to 15. During the next day, the APh duration significantly decreased (32.2 ± 5.4 s (n = 8), P = 0.008). The IPh duration significantly decreased in the period from D10 (42.8 ± 1.3 s; n = 18) to D12 (26.1 ± 1.0 s; n = 19) (P < 0.001), remained unchanged from D12 to D14 (n = 18; P = 0.15), then decreased until D15 (20.3 ± 1.1 s; n = 20) (P = 0.006), and remained constant until D16 (Fig. 2A ). The mean durations of APh and IPh on D10 and D14 were comparable with our earlier results for these embryonic ages [17Nechaeva M, Vladimirova I, Alexeeva T. Effect of acute hypoxia on the motor activity and heart rate of the 10-and 14-day chick embryo. Open Ornithol J 2010; 3: 127-33.
). The mean durations of APh and IPh on D10 and D14 were comparable with our earlier results for these embryonic ages [17Nechaeva M, Vladimirova I, Alexeeva T. Effect of acute hypoxia on the motor activity and heart rate of the 10-and 14-day chick embryo. Open Ornithol J 2010; 3: 127-33.
[http://dx.doi.org/10.2174/1874453201003010127] ]. The average duration of the motility cycle that consisted of the activity phase duration plus the duration of the subsequent inactivity phase remained unchanged from D10 to D15, and then was decreasing until D16 (Fig. 2A ).
).
Changes in the Parameters of Cyclic Motility During Acute Hypoxia
Effect of 20 min 10% or 5% O2 on the Cyclic Motility on D10 and 14
On D10, moderate hypoxia (20 min 10% O2) significantly increased average IPh duration during 20 min exposure, but average APh duration remained unchanged. In addition, insignificant changes in average APh and IPh durations were seen during 30 min recovery in air (Figs. 3A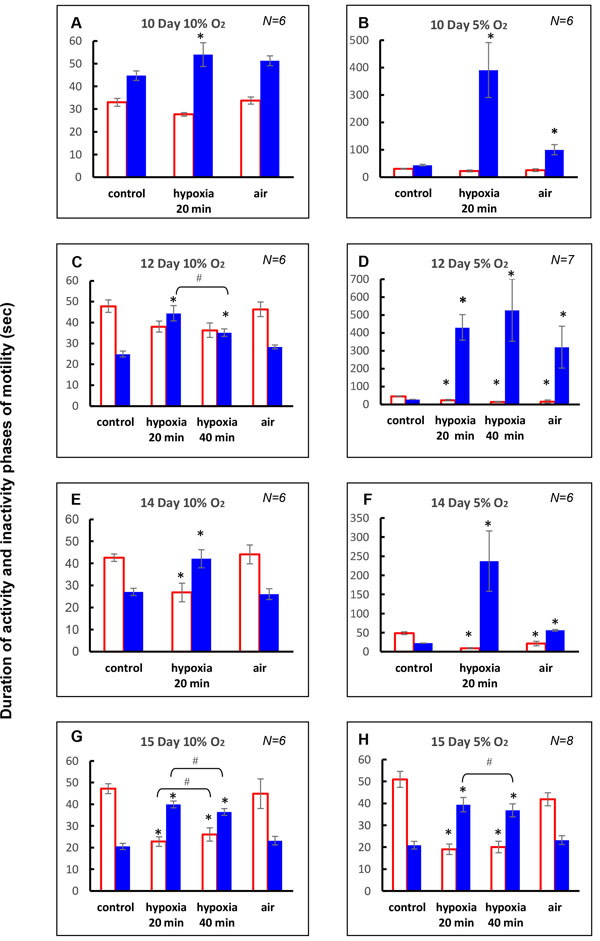 and 4A
and 4A ). The time course of the APh and IPh obtained from the detailed study of the response to hypoxia with the stepwise measurements at 5-min intervals showed that the APh duration did not change and IPh duration significantly exceeded the control value by the end of hypoxia (Fig. 4A
). The time course of the APh and IPh obtained from the detailed study of the response to hypoxia with the stepwise measurements at 5-min intervals showed that the APh duration did not change and IPh duration significantly exceeded the control value by the end of hypoxia (Fig. 4A ). During recovery in air, IPh duration during the first 5 min was longer than the control, but returned to the control during remaining period (Fig. 4A
). During recovery in air, IPh duration during the first 5 min was longer than the control, but returned to the control during remaining period (Fig. 4A ). The APh duration did not differ from the control level throughout the 30-min recovery period.
). The APh duration did not differ from the control level throughout the 30-min recovery period.
Under severe hypoxia (20 min 5% O2) on D10, the inhibitory effect on the cyclic motility was more pronounced. The average IPh duration during 20 min hypoxic exposure was increased considerably, but average APh duration did not differ from the control level (N = 6) (Figs. 3B and 4B
and 4B ). During 30 min recovery in air, average IPh duration was longer than the control, but average APh duration did not differ from the control level (Fig. 3B
). During 30 min recovery in air, average IPh duration was longer than the control, but average APh duration did not differ from the control level (Fig. 3B ). The time course of the motility response to hypoxia showed that embryonic movements stopped after about 3 min of the hypoxic exposure and were not restored until several minutes after the end of hypoxia (except for one embryo where single movements appeared by the end of the hypoxic exposure). The first embryonic movements appeared after 7-11 min of the recovery in air. After that, the motility was gradually restored, and the APh and IPh durations did not differ from the control values after 15 min of recovery (Fig. 4B
). The time course of the motility response to hypoxia showed that embryonic movements stopped after about 3 min of the hypoxic exposure and were not restored until several minutes after the end of hypoxia (except for one embryo where single movements appeared by the end of the hypoxic exposure). The first embryonic movements appeared after 7-11 min of the recovery in air. After that, the motility was gradually restored, and the APh and IPh durations did not differ from the control values after 15 min of recovery (Fig. 4B ).
).
On D14, moderate hypoxia (20 min 10% О2) caused a more pronounced inhibitory effect on cyclic motility than on D10 (Figs. 3E , 4E
, 4E and 5A
and 5A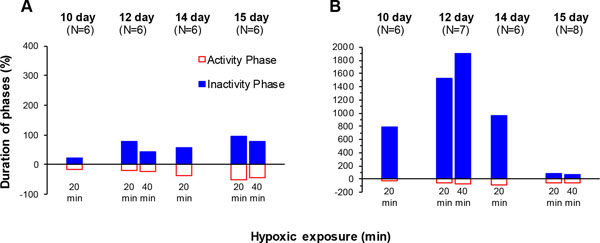 ). The average APh duration during 20 min exposure significantly decreased, and average IPh duration significantly increased (N = 6) (Fig. 3E
). The average APh duration during 20 min exposure significantly decreased, and average IPh duration significantly increased (N = 6) (Fig. 3E ). During recovery in air, cyclic motility recovered within 30 min and average APh and IPh durations reverted to the control values. The time course of the cyclic motility response was biphasic: embryonic motility stopped for 2-3 min and then was progressively restored under hypoxia. Specifically, for the first 5 min of hypoxic exposure, the IPh duration significantly increased and the APh duration significantly decreased (Fig. 4E
). During recovery in air, cyclic motility recovered within 30 min and average APh and IPh durations reverted to the control values. The time course of the cyclic motility response was biphasic: embryonic motility stopped for 2-3 min and then was progressively restored under hypoxia. Specifically, for the first 5 min of hypoxic exposure, the IPh duration significantly increased and the APh duration significantly decreased (Fig. 4E ). By the next 10 min, IPh returned to the control level with APh duration remaining below the control value. After that, the APh and IPh durations did not differ from the control values by the end of hypoxia (Fig. 4E
). By the next 10 min, IPh returned to the control level with APh duration remaining below the control value. After that, the APh and IPh durations did not differ from the control values by the end of hypoxia (Fig. 4E ). During recovery in air, IPh and APh durations did not differ from the control level, except for the 20th min when the APh duration was significantly higher than the pre-hypoxic value (Fig. 4E
). During recovery in air, IPh and APh durations did not differ from the control level, except for the 20th min when the APh duration was significantly higher than the pre-hypoxic value (Fig. 4E ).
).
Severe hypoxia (20 min 5% О2) on D14 caused a strong inhibitory effect on the cyclic motility (Figs. 3F , 4F
, 4F and 5B
and 5B ). Within 20 min of hypoxic exposure, the average APh duration significantly decreased and average IPh duration significantly increased (N=6) (Fig. 3F
). Within 20 min of hypoxic exposure, the average APh duration significantly decreased and average IPh duration significantly increased (N=6) (Fig. 3F ). During 30 min recovery in air, cyclic motility was not completely restored, and the average IPh and APh durations were different from the control values (Fig. 3F
). During 30 min recovery in air, cyclic motility was not completely restored, and the average IPh and APh durations were different from the control values (Fig. 3F ). The time course of the motility response to hypoxia showed that the embryonic movements initially disappeared for about 9 min, and the subsequent period of recovery was about 3 min. After that, the embryonic movements disappeared again until the end of exposure. The embryonic movements reappeared after 8-12 min of the recovery in air and then gradually increased, but they did not reach the pre-hypoxic level within 30 min in air. Specifically, after a peak on the 10th minute of hypoxia, the IPh duration significantly decreased by the 15th minute (P = 0.043), but did not reach the control value. After that, IPh duration significantly increased again by the 10th minute in air. The APh duration initially significantly decreased within 5 min of hypoxia and remained below the control value during hypoxia (Fig. 4F
). The time course of the motility response to hypoxia showed that the embryonic movements initially disappeared for about 9 min, and the subsequent period of recovery was about 3 min. After that, the embryonic movements disappeared again until the end of exposure. The embryonic movements reappeared after 8-12 min of the recovery in air and then gradually increased, but they did not reach the pre-hypoxic level within 30 min in air. Specifically, after a peak on the 10th minute of hypoxia, the IPh duration significantly decreased by the 15th minute (P = 0.043), but did not reach the control value. After that, IPh duration significantly increased again by the 10th minute in air. The APh duration initially significantly decreased within 5 min of hypoxia and remained below the control value during hypoxia (Fig. 4F ). During recovery in air, the IPh and APh durations gradually decreased and increased, respectively, but did not reach the control values within 30 min.
). During recovery in air, the IPh and APh durations gradually decreased and increased, respectively, but did not reach the control values within 30 min.
Effect of 40 min 10% or 5% O2 on the Cyclic Motility on D12 and 15
On D12, moderate hypoxia (40 min10% О2) significantly increased average IPh duration during 40 min exposure, but average APh duration remained unchanged (N = 6) (Figs. 3C , 4C
, 4C and 5A
and 5A ). During the first 20 min of hypoxia, the average IPh duration also increased, and average APh duration remained unchanged. Comparison of the first 20 min and the entire 40-min exposure showed that the respective average APh durations did not differ from each other (P = 0.22), while the average IPh duration was significantly higher for the first 20 min (P = 0.043). During 30 min of recovery in air, the average APh and IPh durations did not differ from the control values (Fig. 3C
). During the first 20 min of hypoxia, the average IPh duration also increased, and average APh duration remained unchanged. Comparison of the first 20 min and the entire 40-min exposure showed that the respective average APh durations did not differ from each other (P = 0.22), while the average IPh duration was significantly higher for the first 20 min (P = 0.043). During 30 min of recovery in air, the average APh and IPh durations did not differ from the control values (Fig. 3C ). The time course of the motility during hypoxia showed that the IPh duration significantly increased during the first 5 min of hypoxia, was restored to the pre-hypoxic value by the 15th minute of exposure, and then did not change until the end of exposure. The APh duration remained unchanged for 35 min of hypoxia and became significantly lower than the control level by the 40th minute (Fig. 4C
). The time course of the motility during hypoxia showed that the IPh duration significantly increased during the first 5 min of hypoxia, was restored to the pre-hypoxic value by the 15th minute of exposure, and then did not change until the end of exposure. The APh duration remained unchanged for 35 min of hypoxia and became significantly lower than the control level by the 40th minute (Fig. 4C ). During recovery in air, the cyclic motility was completely restored within 5 min, and IPh and APh durations did not differ from the control level throughout the 30-min recovery period (Fig. 4C
). During recovery in air, the cyclic motility was completely restored within 5 min, and IPh and APh durations did not differ from the control level throughout the 30-min recovery period (Fig. 4C ).
).
Severe hypoxia (40 min 5% O2) on D12 strongly inhibited the cyclic motility. The average IPh duration significantly increased, and the average APh duration significantly decreased (N=7) (Figs. 3D , 4D
, 4D and 5B
and 5B ). There were no significant differences of the average IPh and APh durations during 40 min of hypoxic exposure from the respective values during the first 20 min (P = 0.22 and P = 0.08, respectively). During 30 min recovery in air, the average IPh and APh durations were significantly different from the pre-hypoxic values (Fig. 3D
). There were no significant differences of the average IPh and APh durations during 40 min of hypoxic exposure from the respective values during the first 20 min (P = 0.22 and P = 0.08, respectively). During 30 min recovery in air, the average IPh and APh durations were significantly different from the pre-hypoxic values (Fig. 3D ). At this level of hypoxia, embryonic movements stopped after about 2 min of hypoxia and then were absent until the end of exposure. The first embryonic movements appeared after 10-20 min of recovery, and the IPh and APh durations did not reach the pre-hypoxic values within 30 min in air (Fig. 4D
). At this level of hypoxia, embryonic movements stopped after about 2 min of hypoxia and then were absent until the end of exposure. The first embryonic movements appeared after 10-20 min of recovery, and the IPh and APh durations did not reach the pre-hypoxic values within 30 min in air (Fig. 4D ).
).
On D15, moderate hypoxia (40 min 10% О2) significantly increased average IPh duration and significantly decreased average APh duration during 40 min exposure (N = 6) (Figs. 3G , 4G
, 4G and 5A
and 5A ). During the first 20 min of hypoxia, average IPh duration also significantly increased and average APh duration significantly decreased. The APh and IPh durations averaged over 40 min of hypoxic exposure were significantly higher and lower, respectively, than these values during the first 20 min (P = 0.043 and P = 0.038, respectively). During 30 min recovery in air, the average APh and IPh durations did not differ from the control values (Fig. 3G
). During the first 20 min of hypoxia, average IPh duration also significantly increased and average APh duration significantly decreased. The APh and IPh durations averaged over 40 min of hypoxic exposure were significantly higher and lower, respectively, than these values during the first 20 min (P = 0.043 and P = 0.038, respectively). During 30 min recovery in air, the average APh and IPh durations did not differ from the control values (Fig. 3G ). These results show that cyclic motility decreased starting from the first minutes of hypoxic exposure; however, in contrast to the hypoxic responses on D12 and D14, the embryo movements did not stop completely. Specifically, significant reduction of the APh duration and significant increase in the IPh duration were observed during the first 5 min of exposure. After that, APh duration gradually increased, reaching the control value by the 30th minute of hypoxia and remaining at the same level until the end of exposure. The IPh duration during the first 30 min of hypoxia remained longer than the control, but returned to the control during remaining hypoxic exposure (Fig. 4G
). These results show that cyclic motility decreased starting from the first minutes of hypoxic exposure; however, in contrast to the hypoxic responses on D12 and D14, the embryo movements did not stop completely. Specifically, significant reduction of the APh duration and significant increase in the IPh duration were observed during the first 5 min of exposure. After that, APh duration gradually increased, reaching the control value by the 30th minute of hypoxia and remaining at the same level until the end of exposure. The IPh duration during the first 30 min of hypoxia remained longer than the control, but returned to the control during remaining hypoxic exposure (Fig. 4G ). During recovery in air, APh and IPh durations did not differ from the control level throughout the 30-min recovery period (Fig. 4G
). During recovery in air, APh and IPh durations did not differ from the control level throughout the 30-min recovery period (Fig. 4G ).
).
Severe hypoxia (40 min 5% О2) on D15 strongly inhibited the cyclic motility. During the first 20 min of hypoxic exposure, the average APh duration significantly decreased and the average IPh duration significantly increased (N = 8) (Figs. 3H , 4H
, 4H and 5B
and 5B ). As the hypoxic exposure was prolonged to 40 min, the average IPh duration significantly decreased (P = 0.043), and the average APh duration did not change (P = 0.08) (Fig. 3H
). As the hypoxic exposure was prolonged to 40 min, the average IPh duration significantly decreased (P = 0.043), and the average APh duration did not change (P = 0.08) (Fig. 3H ). During 30 min recovery in air, the average IPh and APh durations did not differ from the control values. The time course of the cyclic motility response to hypoxia showed that the IPh duration significantly increased during the first 5 min of hypoxia, was restored to the control value by the 25th minute of exposure and then significantly exceeded the control value until the end of exposure (Fig. 4H
). During 30 min recovery in air, the average IPh and APh durations did not differ from the control values. The time course of the cyclic motility response to hypoxia showed that the IPh duration significantly increased during the first 5 min of hypoxia, was restored to the control value by the 25th minute of exposure and then significantly exceeded the control value until the end of exposure (Fig. 4H ). The APh duration significantly decreased during the first 5 min of hypoxia and remained at about the same level until the end of exposure (Fig. 4H
). The APh duration significantly decreased during the first 5 min of hypoxia and remained at about the same level until the end of exposure (Fig. 4H ). During recovery in air, the IPh duration during the first 5 min was longer than the control, but returned to the control during remaining period. The APh duration did not differ from the control level throughout the 30-min recovery period (Fig. 4H
). During recovery in air, the IPh duration during the first 5 min was longer than the control, but returned to the control during remaining period. The APh duration did not differ from the control level throughout the 30-min recovery period (Fig. 4H ).
).
DISCUSSION
Age-related Changes in Cyclic Motility Under Normoxic Conditions During the D10-16 Period of Incubation
Changes in the embryo weight during development shown in Fig. (2A ) agree with the results of many previous studies, which confirm that the embryos grew at normal rates.
) agree with the results of many previous studies, which confirm that the embryos grew at normal rates.
Our study of the changes in cyclic motility during the D10-16 period of incubation showed that the APh lengthened and the IPh shortened in the period from D10 to 12; then, neither APh nor IPh durations changed between D12 and 14; from D14 to D15, IPh duration decreased and APh duration remained unchanged (Fig. 2A ). At the same time, the average duration of the activity - inactivity cycle which is defined as the sum of the durations of two consecutive phases (APh and IPh), remained constant from D10 to D15 (Fig. 2A
). At the same time, the average duration of the activity - inactivity cycle which is defined as the sum of the durations of two consecutive phases (APh and IPh), remained constant from D10 to D15 (Fig. 2A ). On D16, the average cycle duration was substantially decreased because the APh duration was considerably shorter than on D15 (P = 0.008), and the IPh duration remained unchanged. We studied the hypoxic effect on cyclic motility until D15 inclusive. Then the motility character was beginning to gradually change mainly due to the appearance of repetitive limb movements that have been described by Bradley et al. [23Bradley NS, Ryu YU, Lin J. Fast locomotor burst generation in late stage embryonic motility. J Neurophysiol 2008; 99(4): 1733-42.
). On D16, the average cycle duration was substantially decreased because the APh duration was considerably shorter than on D15 (P = 0.008), and the IPh duration remained unchanged. We studied the hypoxic effect on cyclic motility until D15 inclusive. Then the motility character was beginning to gradually change mainly due to the appearance of repetitive limb movements that have been described by Bradley et al. [23Bradley NS, Ryu YU, Lin J. Fast locomotor burst generation in late stage embryonic motility. J Neurophysiol 2008; 99(4): 1733-42.
[http://dx.doi.org/10.1152/jn.01393.2007] [PMID: 18272869] ], and the cyclic character of the motility became less pronounced. Other authors have also reported an increased variation of the parameters of cyclic motility (APh and IPh) with embryonic age [16Bradley NS. Age-related changes and condition-dependent modifications in distribution of limb movements during embryonic motility. J Neurophysiol 2001; 86(4): 1511-22.
[PMID: 11600617] , 24Ganley KJ, Bradley NS. Transformations in embryonic motility in chick: E9 to E18. Soc Neurosci Abstr 1999; 25: 2177., 25Rose D, Ganley K, Bradley N. Transformations in embryonic motility in chick: E9 to E15. Soc Neurosci Abstr 1998; 24: 1153.]. This prevents the use of these parameters as quantitative estimates of the motility after D15, especially in studying the effects of exogenous factors.
Age-related changes in the parameters of cyclic motility during the period studied (Fig. 2A ) generally agree with the results of earlier studies [7Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron 2004; 43(5): 687-701.
) generally agree with the results of earlier studies [7Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron 2004; 43(5): 687-701.
[http://dx.doi.org/10.1016/j.neuron.2004.08.018] [PMID: 15339650] , 16Bradley NS. Age-related changes and condition-dependent modifications in distribution of limb movements during embryonic motility. J Neurophysiol 2001; 86(4): 1511-22.
[PMID: 11600617] , 17Nechaeva M, Vladimirova I, Alexeeva T. Effect of acute hypoxia on the motor activity and heart rate of the 10-and 14-day chick embryo. Open Ornithol J 2010; 3: 127-33.
[http://dx.doi.org/10.2174/1874453201003010127] , 26Wu KC, Streicher J, Lee ML, Hall BK, Müller GB. Role of motility in embryonic development I: Embryo movements and amnion contractions in the chick and the influence of illumination. J Exp Zool 2001; 291(2): 186-94.
[http://dx.doi.org/10.1002/jez.1068] [PMID: 11479917] ], where these parameters were also used for qualitative and quantitative estimation of the motility. However, note that Bradley [16Bradley NS. Age-related changes and condition-dependent modifications in distribution of limb movements during embryonic motility. J Neurophysiol 2001; 86(4): 1511-22.
[PMID: 11600617] ] and Wu et al. [26Wu KC, Streicher J, Lee ML, Hall BK, Müller GB. Role of motility in embryonic development I: Embryo movements and amnion contractions in the chick and the influence of illumination. J Exp Zool 2001; 291(2): 186-94.
[http://dx.doi.org/10.1002/jez.1068] [PMID: 11479917] ] found that the APh and IPh durations were close to each other on D10.
Effects of Acute Hypoxia on the Cyclic Motility of D10-15 Embryos
Our results have demonstrated a distinct dependence of the cyclic motility response to 20-min hypoxia during the D10-15 period of incubation on the embryonic age, the dependence being observed at both degrees of hypoxia studied (10 or 5% O2) (Figs. 3 and 5
and 5 ). In all cases, the cyclic motility was decreased compared to normoxia (control). The changes in the average durations of APh and IPh during hypoxic exposure reflected the strength of the inhibitory effect on cyclic motility but did not allow detailed analysis of the events within hypoxic exposure. Therefore, it was necessary to trace the time course of these parameters. Stepwise measurements of the APh and IPh at 5-min intervals during hypoxic exposure and the subsequent recovery in air have revealed the details of cyclic motility changes during the hypoxia (Fig. 4
). In all cases, the cyclic motility was decreased compared to normoxia (control). The changes in the average durations of APh and IPh during hypoxic exposure reflected the strength of the inhibitory effect on cyclic motility but did not allow detailed analysis of the events within hypoxic exposure. Therefore, it was necessary to trace the time course of these parameters. Stepwise measurements of the APh and IPh at 5-min intervals during hypoxic exposure and the subsequent recovery in air have revealed the details of cyclic motility changes during the hypoxia (Fig. 4 ). We have found that the time course of the cyclic motility response to hypoxia depends not only on the embryonic age (D10-15), but also the degree (10 or 5% O2) and duration (20 or 40 min) of hypoxia.
). We have found that the time course of the cyclic motility response to hypoxia depends not only on the embryonic age (D10-15), but also the degree (10 or 5% O2) and duration (20 or 40 min) of hypoxia.
The most important result of our research was the discovery of a new phenomenon, namely, the ability of a chick embryo to maintain motility in hypoxia, not only on D14 as we first discovered [17Nechaeva M, Vladimirova I, Alexeeva T. Effect of acute hypoxia on the motor activity and heart rate of the 10-and 14-day chick embryo. Open Ornithol J 2010; 3: 127-33.
[http://dx.doi.org/10.2174/1874453201003010127] ], but also at other developmental stages. In addition we succeeded in quantitatively measuring motility during hypoxia, studying its change with growth, and analyzing its dependence on the hypoxia level. The capacity of the embryo to maintain the cyclic motility under acute hypoxia was manifested, first, in the experiments under prolonged hypoxia, that is the experiments where the hypoxia duration increased twofold; and, second, the experiments in which the time course of the cyclic motility response to hypoxia was measured at 5-min intervals.
Thus, it was found that when the exposure to hypoxia was prolonged from 20 min to 40 min, on D12, the inhibitory effect on cyclic motility decreased in 10% hypoxia (average IPh decreased) and increased in 5% hypoxia (average IPh was increased and APh decreased) as compared to what was observed during the first 20 min of exposure (Figs. 3 and 5
and 5 ). At an older embryonic age (D15), the capacity of the embryo to maintain the cyclic motility under acute hypoxia was expressed to a larger extent and the inhibitory effect observed during the prolonged hypoxia (40 min) was weaker than that observed after the first 20 min at both levels of hypoxia, the weakening being more pronounced in 10% hypoxia (Fig. 5
). At an older embryonic age (D15), the capacity of the embryo to maintain the cyclic motility under acute hypoxia was expressed to a larger extent and the inhibitory effect observed during the prolonged hypoxia (40 min) was weaker than that observed after the first 20 min at both levels of hypoxia, the weakening being more pronounced in 10% hypoxia (Fig. 5 ). While the mechanisms that maintain and even enhance embryonic motility are unknown, they are already effective on D12 in 10% hypoxia and on D15 at both levels of hypoxia studied.
). While the mechanisms that maintain and even enhance embryonic motility are unknown, they are already effective on D12 in 10% hypoxia and on D15 at both levels of hypoxia studied.
The analysis of embryonic cyclic motility (duration of APh and IPh) during hypoxia also showed the embryo’s capacity to maintain the cyclic motility under 20 min 10% hypoxia. Embryonic movements first stop completely (thus IPh continues without any APh duration) and then begin to recover before the end of hypoxic exposure (APh and IPh occur alternately). We first observed this characteristic biphasic motility response to hypoxia (10 min 10% O2) on D14 [17Nechaeva M, Vladimirova I, Alexeeva T. Effect of acute hypoxia on the motor activity and heart rate of the 10-and 14-day chick embryo. Open Ornithol J 2010; 3: 127-33.
[http://dx.doi.org/10.2174/1874453201003010127] ]; however, the duration of the exposure was insufficient for embryonic motility to be completely restored under hypoxia. The present study has shown that prolongation of the hypoxic exposure to 20 min at the same embryonic age (D14) leads to a complete restoration of cyclic motility during hypoxia (Fig. 4E ).
).
A question arises as to the mechanisms underlying the recovery or maintenance of motility under hypoxia and the causes of its changes with embryonic age. Two main factors may determine it at each embryonic age: first, the degree of morphological and functional development of the embryo's motor system and the systems involved in oxygen supply to embryonic tissues; second, the maturation level of physiological regulatory mechanisms.
The involvement of these factors and their correlation can be traced in the incubation process. On D10, the biphasic motility response to 10% O2 was not pronounced and the hypoxic effect on cyclic motility developed gradually (Fig. 4A ). We assume that this response was slow because the system of oxygen supply to the embryo was not yet developed enough. This resulted from a small gas-exchange area on the egg surface on D10, when the chorioallantoic membrane (CAM) had not covered the entire egg [27Romanoff AL. The Avian Embryo: Structural and Functional Development. New York: Macmillan 1960.], as well as low blood flow rate [28van Golde J, Mulder T, v Straaten H, Blanco CE. The chorioallantoic artery blood flow of the chick embryo from stage 34 to 43. Pediatr Res 1996; 40(6): 867-71.
). We assume that this response was slow because the system of oxygen supply to the embryo was not yet developed enough. This resulted from a small gas-exchange area on the egg surface on D10, when the chorioallantoic membrane (CAM) had not covered the entire egg [27Romanoff AL. The Avian Embryo: Structural and Functional Development. New York: Macmillan 1960.], as well as low blood flow rate [28van Golde J, Mulder T, v Straaten H, Blanco CE. The chorioallantoic artery blood flow of the chick embryo from stage 34 to 43. Pediatr Res 1996; 40(6): 867-71.
[http://dx.doi.org/10.1203/00006450-199612000-00016] [PMID: 8947964] ] and blood oxygen transport capacity [29Tazawa H, Andrewartha SJ, Burggren WW. Development of hematological respiratory variables in late chicken embryos: the relative importance of incubation time and embryo mass. Comp Biochem Physiol A Mol Integr Physiol 2011; 159(3): 225-33.
[http://dx.doi.org/10.1016/j.cbpa.2011.02.024] [PMID: 21377532] ] at this embryonic age. The considerable inertia of the hypoxic response on D10 was also expressed in the slow restoration of embryonic motility after the hypoxic exposure: the IPh duration continued increasing for 5 min after the hypoxic gas mixture had been replaced with air (Fig. 4A ).
).
At later stages (on D12 and D14), hypoxia (10% O2) caused the distinct biphasic response of cyclic motility. This was more pronounced on D12 than on D14, while the initial temporary cessation of embryonic motility under hypoxia was longer on D12 than on D14 (Fig. 4C, E ). The initial arrest of embryonic motility under hypoxia was apparently caused by a rapid decrease in O2 supply to the spinal neurons responsible for the rhythmic embryonic motility. This resulted from (i) an increase of gas exchange efficiency due to the growth of the CAM that had surrounded the entire egg contents by D11-12 [30Freeman BM, Vince MA. Development of the avian embryo: a behavioral and physiological study. London: Chapman and Hall 1974.
). The initial arrest of embryonic motility under hypoxia was apparently caused by a rapid decrease in O2 supply to the spinal neurons responsible for the rhythmic embryonic motility. This resulted from (i) an increase of gas exchange efficiency due to the growth of the CAM that had surrounded the entire egg contents by D11-12 [30Freeman BM, Vince MA. Development of the avian embryo: a behavioral and physiological study. London: Chapman and Hall 1974.
[http://dx.doi.org/10.1007/978-94-009-5710-7] , 31Gabrielli MG, Accili D. The chick chorioallantoic membrane: a model of molecular, structural, and functional adaptation to trans epithelial ion transport and barrier function during embryonic development. J Biomed Biotechnol 2010; 2010: 940741.] and development of the chorioallantoic vascular network; (ii) considerable acceleration of blood circulation [28van Golde J, Mulder T, v Straaten H, Blanco CE. The chorioallantoic artery blood flow of the chick embryo from stage 34 to 43. Pediatr Res 1996; 40(6): 867-71.
[http://dx.doi.org/10.1203/00006450-199612000-00016] [PMID: 8947964] ]; and (iii) an increase in the blood oxygen transport capacity [29Tazawa H, Andrewartha SJ, Burggren WW. Development of hematological respiratory variables in late chicken embryos: the relative importance of incubation time and embryo mass. Comp Biochem Physiol A Mol Integr Physiol 2011; 159(3): 225-33.
[http://dx.doi.org/10.1016/j.cbpa.2011.02.024] [PMID: 21377532] ]. The recovery of cyclic motility to the control level by the 20th minute of hypoxia may be accounted for by activation of regulatory mechanisms that were already sufficiently mature on D12-14. These may be, e.g., heart rate restoration against the background of hypoxia [17Nechaeva M, Vladimirova I, Alexeeva T. Effect of acute hypoxia on the motor activity and heart rate of the 10-and 14-day chick embryo. Open Ornithol J 2010; 3: 127-33.
[http://dx.doi.org/10.2174/1874453201003010127] ], changes in hematological respiratory variables [32Andrewartha SJ, Tazawa H, Burggren WW. Acute regulation of hematocrit and acid-base balance in chicken embryos in response to severe intrinsic hypercapnic hypoxia. Respir Physiol Neurobiol 2014; 195: 1-10.
[http://dx.doi.org/10.1016/j.resp.2014.01.019] [PMID: 24509299] , 33Tazawa H, Andrewartha SJ, Burggren WW. Acute regulation of hematocrit and blood acid-base balance during severe hypoxic challenges in late chicken embryos (Gallus gallus). Respir Physiol Neurobiol 2012; 184(1): 86-96.
[http://dx.doi.org/10.1016/j.resp.2012.08.002] [PMID: 22902513] ], and redistribution of blood flow towards the most important organs (including brain) in response to hypoxia, which is known to become more pronounced with embryonic age [34Mulder AL, van Golde JC, Prinzen FW, Blanco CE. Cardiac output distribution in response to hypoxia in the chick embryo in the second half of the incubation time. J Physiol 1998; 508(Pt 1): 281-7.
[http://dx.doi.org/10.1111/j.1469-7793.1998.281br.x] [PMID: 9490852] ]. These regulatory mechanisms may contribute to the restoration of motility under hypoxia. The mechanisms are more developed on D14 than on D12; therefore, the restoration of embryonic motility begins earlier, and the initial cessation of motility under hypoxia on D14 is shorter than on D12. If the hypoxia on D12 was prolonged from 20 to 40 min, the restored motility decreased again during the second half of the exposure (Fig. 4C ). Apparently, the maturation level of the regulatory mechanisms underlying this recovery was not sufficient for maintaining the motility for a long time.
). Apparently, the maturation level of the regulatory mechanisms underlying this recovery was not sufficient for maintaining the motility for a long time.
As development continued (D15), however, there was no distinct cessation of embryo movements at the beginning of hypoxic exposure, and the motility decreased due to a considerable prolongation of IPh and shortening of APh, which continued to occur alternately (Fig. 4G ). It may be assumed that the regulatory mechanisms by this age were already mature enough to maintain the cyclic motility under hypoxia; hence, they were triggered more quickly and cyclic motility did not cease at the beginning of hypoxia. On the other hand, the APh and IPh were restored to the pre-hypoxic values only by the 35th minute of hypoxia, in contrast to D12 and D14, when the motility rate reached the control level by the 20th minute of hypoxia. Apparently, some additional factors hindered the rapid restoration of motility. For example, it was previously shown that the structure of the APh changes with age and it is determined by the activity not only of spinal neurons, but also the brain, muscles, and proprioceptors [10Hamburger V, Balaban M, Oppenheim R, Wenger E. Periodic motility of normal and spinal chick embryos between 8 and 17 days of incubation. J Exp Zool 1965; 159(1): 1-13.
). It may be assumed that the regulatory mechanisms by this age were already mature enough to maintain the cyclic motility under hypoxia; hence, they were triggered more quickly and cyclic motility did not cease at the beginning of hypoxia. On the other hand, the APh and IPh were restored to the pre-hypoxic values only by the 35th minute of hypoxia, in contrast to D12 and D14, when the motility rate reached the control level by the 20th minute of hypoxia. Apparently, some additional factors hindered the rapid restoration of motility. For example, it was previously shown that the structure of the APh changes with age and it is determined by the activity not only of spinal neurons, but also the brain, muscles, and proprioceptors [10Hamburger V, Balaban M, Oppenheim R, Wenger E. Periodic motility of normal and spinal chick embryos between 8 and 17 days of incubation. J Exp Zool 1965; 159(1): 1-13.
[http://dx.doi.org/10.1002/jez.1401590102] [PMID: 5215365] , 16Bradley NS. Age-related changes and condition-dependent modifications in distribution of limb movements during embryonic motility. J Neurophysiol 2001; 86(4): 1511-22.
[PMID: 11600617] ]. The considerably greater decrease in the APh duration during hypoxia on D15 compared to earlier ages was probably accounted for by switching off of these additional components, which might have been restored under hypoxia more slowly than the activity of spinal motor networks.
The results of experiments with 5% hypoxia also confirm a high maturation level of the regulatory mechanisms on D15. In contrast to earlier embryonic ages, when such hypoxia rapidly led to an almost complete immobility of the embryo, the same exposure on D15 did not cause cessation of embryonic motility; moreover, there was a tendency towards motility restoration during the exposure, although its rate did not reach the control level.
The time period we indentified for the gradual development of the embryonic capacity to maintain motility under acute hypoxia is similar to the time course of development of some other embryonic capacities, including embryonic resilience to chronic hypoxia [35Dzialowski EM, von Plettenberg D, Elmonoufy NA, Burggren WW. Chronic hypoxia alters the physiological and morphological trajectories of developing chicken embryos. Comp Biochem Physiol A Mol Integr Physiol 2002; 131(4): 713-24.
[http://dx.doi.org/10.1016/S1095-6433(02)00009-0] [PMID: 11897182] ], and the period for changing the damaging hypoxia influence on the chick embryo brain [36Rodricks CL, Gibbs ME, Castillo-Melendez M, Miller SL. The effect of hypoxia on the functional and structural development of the chick brain. Int J Dev Neurosci 2010; 28(4): 343-50.
[http://dx.doi.org/10.1016/j.ijdevneu.2010.02.004] [PMID: 20171268] ]. Our results supplement the available data on the existence of the so called window in development when chick embryos are capable of compensating for the hypoxia effect [35Dzialowski EM, von Plettenberg D, Elmonoufy NA, Burggren WW. Chronic hypoxia alters the physiological and morphological trajectories of developing chicken embryos. Comp Biochem Physiol A Mol Integr Physiol 2002; 131(4): 713-24.
[http://dx.doi.org/10.1016/S1095-6433(02)00009-0] [PMID: 11897182] ]. Additionally, data concerning the development of the stress response during this same period of chick embryogenesis [37Epple A, Gower B, Ten Busch M, Gill T, Milakofsky L, Piechotta R, et al. Stress responses in avian embryos. Am Zool 1997; 37: 536-45.
[http://dx.doi.org/10.1093/icb/37.6.536] -39Tona K, Onagbesan O, Bruggeman V, Mertens K, Decuypere E. Effects of turning duration during incubation on embryo growth, utilization of albumen, and stress regulation. Poult Sci 2005; 84(2): 315-20.
[http://dx.doi.org/10.1093/ps/84.2.315] [PMID: 15742969] ], suggests that the gradual development of the embryo’s capacity to maintain motility under acute hypoxia parallels the development of the stress response. Consequently, the physiological compensatory response to acute hypoxia could develop during the period studied.
In summary, the results of the study indicate that the response of cyclic motility to acute hypoxia varies during D10-15 of incubation. The age-related changes in the responses to moderate (10%) and severe (5%) hypoxia differ from each other. In 10% hypoxia, the strongest inhibitory effect on the cyclic motility was observed on D15; in 5% hypoxia, the strongest effect was on D12, with severe hypoxia inhibiting cyclic motility more than moderate hypoxia at all embryonic ages except D15 when the effect of both levels of hypoxia was about the same (Figs. 3 and 5
and 5 ). We believe that the cyclic motility response to acute hypoxia at different embryonic ages is mainly determined by the strength of the inhibitory influence of hypoxia, on the one hand, and, by the capacity of the embryo to maintain cyclic motility upon a decrease in the О2 concentration, on the other. The interaction between these two factors determines the degree of reduction of embryonic motility during hypoxia relative to the normal level characteristic of the same embryonic age. It is still unclear what regulatory mechanisms are involved in the hypoxic response. However, analysis of the changes in the parameters of cyclic motility under hypoxia at different developmental ages revealed that the embryo became capable of maintaining motility under hypoxia during D10-15. This capacity was increased with embryonic age, which we relate to the maturation of the regulatory mechanisms. For example, on D12, cyclic motility was partly restored during 10% hypoxia, but the regulatory mechanisms were not mature enough to maintain the motility under more severe hypoxia (5% О2), where the motility stopped completely. By D15, the maturation of the regulatory capacity reached the level at which embryonic motility was maintained under both 10% and 5% hypoxia. Further studies are necessary in order to better understand the role of the regulatory mechanisms involved in the cyclic motor response to hypoxia at different stages of incubation.
). We believe that the cyclic motility response to acute hypoxia at different embryonic ages is mainly determined by the strength of the inhibitory influence of hypoxia, on the one hand, and, by the capacity of the embryo to maintain cyclic motility upon a decrease in the О2 concentration, on the other. The interaction between these two factors determines the degree of reduction of embryonic motility during hypoxia relative to the normal level characteristic of the same embryonic age. It is still unclear what regulatory mechanisms are involved in the hypoxic response. However, analysis of the changes in the parameters of cyclic motility under hypoxia at different developmental ages revealed that the embryo became capable of maintaining motility under hypoxia during D10-15. This capacity was increased with embryonic age, which we relate to the maturation of the regulatory mechanisms. For example, on D12, cyclic motility was partly restored during 10% hypoxia, but the regulatory mechanisms were not mature enough to maintain the motility under more severe hypoxia (5% О2), where the motility stopped completely. By D15, the maturation of the regulatory capacity reached the level at which embryonic motility was maintained under both 10% and 5% hypoxia. Further studies are necessary in order to better understand the role of the regulatory mechanisms involved in the cyclic motor response to hypoxia at different stages of incubation.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
This research was supported by the Grant RFBR No: 11-04-01362a and RSF Grant No: 15-15-20008.
REFERENCES
| [1] | Robinson SR. Behavioral embryology. In: Hopkins B, Ed. The cambridge encyclopedia of child development. Cambridge: Cambridge University Press 2005; pp. 469-73. |
| [2] | Ryu YU, Bradley NS. Precocious locomotor behavior begins in the egg: development of leg muscle patterns for stepping in the chick. PLoS One 2009; 4(7): e6111. [http://dx.doi.org/10.1371/journal.pone.0006111] [PMID: 19578536] |
| [3] | Heywood JL, McEntee GM, Stickland NC. In ovo neuromuscular stimulation alters the skeletal muscle phenotype of the chick. J Muscle Res Cell Motil 2005; 26(1): 49-56. [http://dx.doi.org/10.1007/s10974-005-9007-8] [PMID: 16088375] |
| [4] | Hogg DA, Hosseini A. The effects of paralysis on skeletal development in the chick embryo. Comp Biochem Physiol Comp Physiol 1992; 103(1): 25-8. [http://dx.doi.org/10.1016/0300-9629(92)90237-K] [PMID: 1356698] |
| [5] | Oppenheim RW, Prevette D, Houenou LJ, et al. Neuromuscular development in the avian paralytic mutant crooked neck dwarf (cn/cn): further evidence for the role of neuromuscular activity in motoneuron survival. J Comp Neurol 1997; 381(3): 353-72. [http://dx.doi.org/10.1002/(SICI)1096-9861(19970512)381:3<353::AID-CNE7>3.0.CO;2-1] [PMID: 9133573] |
| [6] | Pitsillides AA. Early effects of embryonic movement: a shot out of the dark. J Anat 2006; 208(4): 417-31. [http://dx.doi.org/10.1111/j.1469-7580.2006.00556.x] [PMID: 16637868] |
| [7] | Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron 2004; 43(5): 687-701. [http://dx.doi.org/10.1016/j.neuron.2004.08.018] [PMID: 15339650] |
| [8] | Hamburger V. Some aspects of the embryology of behavior. Q Rev Biol 1963; 38: 342-65. [http://dx.doi.org/10.1086/403941] [PMID: 14111168] |
| [9] | Hamburger V, Balaban M. Observations and experiments on spontaneous rhythmical behavior in the chick embryo. Dev Biol 1963; 6: 533-45. [http://dx.doi.org/10.1016/0012-1606(63)90140-4] [PMID: 13952299] |
| [10] | Hamburger V, Balaban M, Oppenheim R, Wenger E. Periodic motility of normal and spinal chick embryos between 8 and 17 days of incubation. J Exp Zool 1965; 159(1): 1-13. [http://dx.doi.org/10.1002/jez.1401590102] [PMID: 5215365] |
| [11] | Oppenheim RW. The role of supraspinal input in embryonic motility: a re-examination in the chick. J Comp Neurol 1975; 160(1): 37-50. [http://dx.doi.org/10.1002/cne.901600104] [PMID: 1112921] |
| [12] | Landmesser LT, ODonovan MJ. Activation patterns of embryonic chick hind limb muscles recorded in ovo and in an isolated spinal cord preparation. J Physiol 1984; 347: 189-204. [http://dx.doi.org/10.1113/jphysiol.1984.sp015061] [PMID: 6707956] |
| [13] | ODonovan MJ, Landmesser LT. The development of hind limb motor activity studied in an isolated preparation of the chick spinal cord. J Neurosci 1987; 7: 3256-64. [PMID: 3668626] |
| [14] | ODonovan MJ. Motor activity in the isolated spinal cord of the chick embryo: synaptic drive and firing pattern of single motoneurons. J Neurosci 1989; 9(3): 943-58. [PMID: 2926486] |
| [15] | Bradley NS. Transformations in embryonic motility in chick: kinematic correlates of type I and II motility at E9 and E12. J Neurophysiol 1999; 81(4): 1486-94. [PMID: 10200185] |
| [16] | Bradley NS. Age-related changes and condition-dependent modifications in distribution of limb movements during embryonic motility. J Neurophysiol 2001; 86(4): 1511-22. [PMID: 11600617] |
| [17] | Nechaeva M, Vladimirova I, Alexeeva T. Effect of acute hypoxia on the motor activity and heart rate of the 10-and 14-day chick embryo. Open Ornithol J 2010; 3: 127-33. [http://dx.doi.org/10.2174/1874453201003010127] |
| [18] | Deeming DC. How does the bird-nest incubation unit work? Avian Biol Res 2016; 9: 103-13. [http://dx.doi.org/10.3184/175815516X14567543242701] |
| [19] | Mortola JP, Louis AS, Simeonova M, Toro Velasquez PA. The motility of the chicken embryo: energetic cost and effects of hypoxia. Respir Physiol Neurobiol 2013; 188(2): 172-9. [http://dx.doi.org/10.1016/j.resp.2013.05.030] [PMID: 23732509] |
| [20] | Nechaeva MV. Physiological responses to acute changes in temperature and oxygenation in bird and reptile embryos. Respir Physiol Neurobiol 2011; 178(1): 108-17. [http://dx.doi.org/10.1016/j.resp.2011.04.003] [PMID: 21513821] |
| [21] | Gonya-Magee T, Stokes BT. Acute modification of embryonic spinal cord activity induced by hypoxia. Dev Neurosci 1980; 3(1): 11-8. [http://dx.doi.org/10.1159/000112372] [PMID: 7408706] |
| [22] | Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol 1951; 88(1): 49-92. [http://dx.doi.org/10.1002/jmor.1050880104] [PMID: 24539719] |
| [23] | Bradley NS, Ryu YU, Lin J. Fast locomotor burst generation in late stage embryonic motility. J Neurophysiol 2008; 99(4): 1733-42. [http://dx.doi.org/10.1152/jn.01393.2007] [PMID: 18272869] |
| [24] | Ganley KJ, Bradley NS. Transformations in embryonic motility in chick: E9 to E18. Soc Neurosci Abstr 1999; 25: 2177. |
| [25] | Rose D, Ganley K, Bradley N. Transformations in embryonic motility in chick: E9 to E15. Soc Neurosci Abstr 1998; 24: 1153. |
| [26] | Wu KC, Streicher J, Lee ML, Hall BK, Müller GB. Role of motility in embryonic development I: Embryo movements and amnion contractions in the chick and the influence of illumination. J Exp Zool 2001; 291(2): 186-94. [http://dx.doi.org/10.1002/jez.1068] [PMID: 11479917] |
| [27] | Romanoff AL. The Avian Embryo: Structural and Functional Development. New York: Macmillan 1960. |
| [28] | van Golde J, Mulder T, v Straaten H, Blanco CE. The chorioallantoic artery blood flow of the chick embryo from stage 34 to 43. Pediatr Res 1996; 40(6): 867-71. [http://dx.doi.org/10.1203/00006450-199612000-00016] [PMID: 8947964] |
| [29] | Tazawa H, Andrewartha SJ, Burggren WW. Development of hematological respiratory variables in late chicken embryos: the relative importance of incubation time and embryo mass. Comp Biochem Physiol A Mol Integr Physiol 2011; 159(3): 225-33. [http://dx.doi.org/10.1016/j.cbpa.2011.02.024] [PMID: 21377532] |
| [30] | Freeman BM, Vince MA. Development of the avian embryo: a behavioral and physiological study. London: Chapman and Hall 1974. [http://dx.doi.org/10.1007/978-94-009-5710-7] |
| [31] | Gabrielli MG, Accili D. The chick chorioallantoic membrane: a model of molecular, structural, and functional adaptation to trans epithelial ion transport and barrier function during embryonic development. J Biomed Biotechnol 2010; 2010: 940741. |
| [32] | Andrewartha SJ, Tazawa H, Burggren WW. Acute regulation of hematocrit and acid-base balance in chicken embryos in response to severe intrinsic hypercapnic hypoxia. Respir Physiol Neurobiol 2014; 195: 1-10. [http://dx.doi.org/10.1016/j.resp.2014.01.019] [PMID: 24509299] |
| [33] | Tazawa H, Andrewartha SJ, Burggren WW. Acute regulation of hematocrit and blood acid-base balance during severe hypoxic challenges in late chicken embryos (Gallus gallus). Respir Physiol Neurobiol 2012; 184(1): 86-96. [http://dx.doi.org/10.1016/j.resp.2012.08.002] [PMID: 22902513] |
| [34] | Mulder AL, van Golde JC, Prinzen FW, Blanco CE. Cardiac output distribution in response to hypoxia in the chick embryo in the second half of the incubation time. J Physiol 1998; 508(Pt 1): 281-7. [http://dx.doi.org/10.1111/j.1469-7793.1998.281br.x] [PMID: 9490852] |
| [35] | Dzialowski EM, von Plettenberg D, Elmonoufy NA, Burggren WW. Chronic hypoxia alters the physiological and morphological trajectories of developing chicken embryos. Comp Biochem Physiol A Mol Integr Physiol 2002; 131(4): 713-24. [http://dx.doi.org/10.1016/S1095-6433(02)00009-0] [PMID: 11897182] |
| [36] | Rodricks CL, Gibbs ME, Castillo-Melendez M, Miller SL. The effect of hypoxia on the functional and structural development of the chick brain. Int J Dev Neurosci 2010; 28(4): 343-50. [http://dx.doi.org/10.1016/j.ijdevneu.2010.02.004] [PMID: 20171268] |
| [37] | Epple A, Gower B, Ten Busch M, Gill T, Milakofsky L, Piechotta R, et al. Stress responses in avian embryos. Am Zool 1997; 37: 536-45. [http://dx.doi.org/10.1093/icb/37.6.536] |
| [38] | Tazawa H, Nakagawa S. Response of egg temperature, heart rate and blood pressure in the chick embryo to hypothermal stress. J Comp Physiol B 1985; 155: 195-200. [http://dx.doi.org/10.1007/BF00685213] |
| [39] | Tona K, Onagbesan O, Bruggeman V, Mertens K, Decuypere E. Effects of turning duration during incubation on embryo growth, utilization of albumen, and stress regulation. Poult Sci 2005; 84(2): 315-20. [http://dx.doi.org/10.1093/ps/84.2.315] [PMID: 15742969] |








