- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Current Chemical Genomics and Translational Medicine
(Discontinued)
ISSN: 2213-9885 ― Volume 12, 2018
Comparison on Functional Assays for Gq-Coupled GPCRs by Measuring Inositol Monophospate-1 and Intracellular Calcium in 1536-Well Plate Format
Ke Liu#, Steve Titus#, Noel Southall, Pingjun Zhu, James Inglese, Christopher P Austin, Wei Zheng*
Abstract
Cell-based functional assays used for compound screening and lead optimization play an important role in drug discovery for G-protein coupled receptors (GPCRs). Cell-based assays can define the role of a compound as an agonist, antagonist or inverse agonist and can provide detailed information about the potency and efficacy of a compound. In addition, cell-based screens can be used to identify allosteric modulators that interact with sites other than the binding site of the endogenous ligand. Intracellular calcium assays which use a fluorescent calcium binding dye (such as Fluo-3, Fluo-4 or Fura-2) have been used in compound screening campaigns to measure the activity of Gq-coupled GPCRs. However, such screening methodologies require a special instrumentation to record the rapid change in intracellular free calcium concentration over time. The radioactive inositol 1,4,5- triphosphate (IP3) assay measures 3H-inositol incorporation and is another traditional assay for the assessment of Gq-coupled GPCR activity, but it is not suitable for screening of large size compound collections because it requires a cell wash step and generates radioactive waste. To avoid these limitations, we have optimized and miniaturized a TR-FRET based IP-One assay that measures inositol monophosphate in a 1536-well plate format. This assay is homogenous, non-radioactive and does not require a kinetic readout. It has been tested with the cell lines expressing M1 acetylcholine, FFAR1, vasopressin V1b, or Neuropeptide S receptors. The activities of antagonists determined in the IP-One assay correlated well with these measured in the intracellular calcium assay while the correlation of agonist activities might vary from cell line to cell line. This IP-One assay offers an alternative method for high throughput screening of Gq-coupled GPCRs without using costly kinetic plate readers.
Article Information
Identifiers and Pagination:
Year: 2008Volume: 1
First Page: 70
Last Page: 78
Publisher Id: CCGTM-1-70
DOI: 10.2174/1875397300801010070
Article History:
Received Date: 28/4/2008Revision Received Date: 17/5/2008
Acceptance Date: 21/5/2008
Electronic publication date: 11/7/2008
Collection year: 2008
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.5/), which permits unrestrictive use, distribution, and reproduction in any medium, provided the original work is properly cited.
* Address correspondence to this author at the NIH Chemical Genomics Center, National Human Genome Research Institute, National Institutes of Health, USA; E-mail: wzheng@mail.nih.gov# There authors contributed equally to this work.
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 28-4-2008 |
Original Manuscript | Comparison on Functional Assays for Gq-Coupled GPCRs by Measuring Inositol Monophospate-1 and Intracellular Calcium in 1536-Well Plate Format | |
INTRODUCTION
Guanine nucleotide triphosphate binding protein (G protein)-coupled receptors (GPCRs) are the largest and most important family of cell surface receptors for drug development. It is estimated that over 30% of all marketed small-molecule drugs target GPCRs [1Hopkins AL, Groom CR. The druggable genome Nat Rev Drug Discov 2002; 1(9): 727-30.]. When binding to appropriate ligands, GPCRs transduce these extracellular stimuli into intracellular second messengers through activation of one or several G proteins including the subtypes of Gs, Gi, and Gq. The Gs subtype activates adenylyl cyclase (AC), and thus increases the intracellular adenosine 3’,5’-cyclic monophosphate (cAMP), a secondary messenger that signals through the activation of protein kinase A (PKA). The Gi subtype inhibits AC and suppresses the signaling in the cAMP/PKA pathway. The Gq subtype activates phosholipase C (PLC), which increases the second messengers inositol 1,4,5-trisphosphate (IP3) and diacyl glycerol (DAG), resulting in an increase of intracellular free Ca2+ and activation of protein kinase C (PKC) [2Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors Nat Rev Mol Cell Biol 2002; 3(9): 639-50.].
A variety of methods have been used to screen for compounds that modulate GPCR signaling. These include measuring direct ligand-receptor binding, level of second messengers, receptor internalization, or expression of reporter genes [3Eglen RM, Bosse R, Reisine T. Emerging concepts of guanine nucleotide-binding protein-coupled receptor (GPCR) function and implications for high throughput screening Assay Drug Dev Technol 2007; 5(3): 425-51.,4Inglese J, Johnson RL, Simeonov A, et al. High-throughput screening assays for the identification of chemical probes Nat Chem Biol 2007; 3(8): 466-79.]. Ligand binding assays are used to determine the binding affinity of compounds to a particular GPCR, either directly (saturation binding) or indirectly (displacement binding). However, this type of assay can not differentiate the function of compounds and is limited by the availability of labeled ligand. Moreover, compounds that bind to a different site on the receptor may not affect the binding of labeled ligand in this type of assay. Reporter gene assays couple GPCR signaling transduction pathway to the expression level of an exogenous enzyme such as luciferase or beta-lactamase. This type of assay is usually very sensitive to stimulation by an agonist and can be used as a functional assay for compound screening. However, the false positive rate in reporter gene assays is often much higher than other types of assays, which is resulted from the compound’s actions on other proteins in the signaling pathway as well as on the receptor protein’s transcription and synthesis. Second messenger assays are usually cell-based and can be used to screen for agonists, antagonists and inverse agonists. Because second messengers are closer to the initial GPCR signaling events, second messenger assays directly reflect the effect of a compound and thus are more trustworthy than the reporter gene assay. Several types of cAMP assays are currently available for screening of compound libraries against the Gs/Gi-coupled receptors [3Eglen RM, Bosse R, Reisine T. Emerging concepts of guanine nucleotide-binding protein-coupled receptor (GPCR) function and implications for high throughput screening Assay Drug Dev Technol 2007; 5(3): 425-51.,4Inglese J, Johnson RL, Simeonov A, et al. High-throughput screening assays for the identification of chemical probes Nat Chem Biol 2007; 3(8): 466-79.], including fluorescence polarization (FP) [5Prystay L, Gagne A, Kasila P, Yeh LA, Banks P. Homogeneous cell-based fluorescence polarization assay for the direct detection of cAMP J Biomol Screen 2001; 6(2): 75-82.] , time-resolved fluorescence resonance energy transfer (TR-FRET) such as HTRF (homogeneous time resolved fluorescence) [6Gabriel D, Vernier M, Pfeifer MJ, Dasen B, Tenaillon L, Bouhelal R. High throughput screening technologies for direct cyclic AMP measurement Assay Drug Dev Technol 2003; 1(2): 291-303.] and Lance, AlphaScreen [7Eglen RM, Reisine T, Roby P, Rouleau N, Bosse RI, Bielefeld M. The Use of AlphaScreen Technology in HTS: Current Status Curr Chem Genomics 2008; 1: 2-10.], enzyme fragmentation complementation (EFC) 8 and cyclic nucleotide gated ion channel (CNG)-coupled [9Reinscheid RK, Kim J, Zeng J, Civelli O. High-throughput real-time monitoring of Gs-coupled receptor activation in intact cells using cyclic nucleotide-gated channels Eur J Pharmacol 2003; 478(1): 27-34.] assays.
The radioactive IP3 assay has been used to measure the function of Gq coupled GPCRs for many years since the discovery of IP3 as a second messenger [10Berridge MJ. Inositol trisphosphate and calcium signalling Nature 1993; 361(6410): 315-25.], but the screening throughput of an IP3 assay, based on 3H-inositol incorporation and ion exchange chromatography, is very small and is not suitable for HTS. Despite the recent improvement of the IP3 assay throughput with the scintillation proximity assay (SPA) technology, it still requires a cell wash step and is not practical for compound screening with large compound libraries [11Brandish PE, Hill LA, Zheng W, Scolnick EM. Scintillation proximity assay of inositol phosphates in cell extracts: high-throughput measurement of G-protein-coupled receptor activation Anal Biochem 2003; 313(2): 311-8.,12Chambers C, Smith F, Williams C, et al. Measuring intracellular calcium fluxes in high throughput mode Comb Chem High Throughput Screen 2003; 6(4): 355-62.]. Alternatively, the function of Gq-coupled GPCRs can be determined by the measurement of intracellular calcium concentration [12Chambers C, Smith F, Williams C, et al. Measuring intracellular calcium fluxes in high throughput mode Comb Chem High Throughput Screen 2003; 6(4): 355-62.,13Zhang Y, Kowal D, Kramer A, Dunlop J. Evaluation of FLIPR Calcium 3 Assay Kit--a new no-wash fluorescence calcium indicator reagent J Biomol Screen 2003; 8(5): 571-7.]. In the last 10 years, the intracellular calcium assays, based on fluorescence dyes including Fluo-3, Fluo-4 or Fura-2 that become fluorescent upon their binding to free Ca2+, have been developed and applied for compound screening; along with the available instruments including Fluorescent-Imaging Plate Reader (FLIPR) and Functional Drug Screening System (FDSS). But use of this assay format has been limited by the need for these special and costly florescence kinetic plate readers. Recently, an IP-One assay which utilizes the HTRF detection format has emerged as an alternative method for the functional measurement of Gq-coupled GPCRs [14Trinquet E, Fink M, Bazin H, et al. D-myo-inositol 1-phosphate as a surrogate of D-myo-inositol 1,4,5-tris phosphate to monitor G protein-coupled receptor activation Anal Biochem 2006; 358(1): 126-35.]. This assay applies a specific antibody to quantify the amount of inositol-1-phosphate (IP1) accumulation in cells and it is sensitive since HTRF is used as the detection method. We report here the miniaturization and optimization of this IP-One assay with four GPCRs as well as a comparison of antagonist screens against M1 muscarinic acetylcholine receptor expressed in Chinese hamster ovary (CHO) cells using both the IP-One assay and the intracellular calcium assay.
MATERIALS AND METHODS
Materials
All cell culture reagents were obtained from Invitrogen (Carlsbad, CA). Carbachol, pirenzepine, atropine and probenecid were purchased from Sigma Aldrich (St. Louis, MO). The 1536-well tissue culture-treated, clear-bottom black plates and solid white plates were purchased from Kalypsys (San Diego, CA). The IP-One HTRF assay kit was obtained from Cisbio (Bedford, MA). The Fluo-4 containing no-wash PBX calcium assay kit was purchased from BD Biosciences (Rockville, MD). A CHO cell line expressing the murine M1 muscarinic acetylcholine receptor (CHO-M1) was obtained from American Type Culture Collection (ATCC, Manassas, VA)
Compound Library
A compound library containing 1208 compounds (Library of Pharmacologically Active Compounds, LOPAC) was purchased from Sigma-Aldrich and dissolved in DMSO to a concentration of 10 mM. All compounds in the LOPAC Library were serially diluted in DMSO to fifteen concentrations in 384-well plates at a ratio of the square root of 5 (1:2.236) as described previously [15Inglese J, Auld DS, Jadhav A, et al. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries Proc Natl Acad Sci U S A 2006; 103(31): 11473-8.]. Four sets of the interplate dilution plates were subsequently reformatted into one set of 1536-well plates at 7 μl/well with concentrations ranging from 0.29 μM to 10 mM as the compound source plates.
Cell Culture and Frozen Cell Preparation
CHO-M1 cells were maintained in F12 Kaighn’s media supplemented with 10 % FBS, 100 units/ml penicillin, 100 μg/ml streptomycin and 250 μg/ml geneticin at 37 ºC, 5 % CO2 in a humidified atmosphere. For the frozen cell preparation, 5 × 106 cells in 60 ml of media were seeded in each Nunclon triple layer flask (Nalge Nunc International, Rochester, NY) and were cultured for 3 to 4 days to reach 90-95 % confluence. The cells were then detached by incubation with 15 ml 0.12 % trypsin at 37 °C for 3 minutes and centrifuged at 1000 RPM to remove the trypsin solution. The resulting cell pellet was resuspended in the cell freezing media containing 10 % DMSO (Invitrogen) at a density of 4 × 107 cells/ml. One triple layer flask (500 cm2) yielded approximately 4 to 5 × 107 cells. Aliquots of cells with 0.5 – 1 ml per vial were put in a Cryo 1°C freezing container (Nalgene Nunc, Rochster, NY) and slowly frozen down overnight in a -80°C freezer at 1°C/min. The frozen cells were then transferred to liquid nitrogen for storage for up to two years.
Instruments for Liquid Handling and Plate Detection
Cells and other reagents were dispensed into 1536-well plates by either a flying reagent dispenser (FRD) (Aurora Discovery, San Diego, CA) or a Multidrop Combi dispenser (Thermo Fisher Scientific Inc., Waltham, MA). The control compounds were serially diluted in DMSO in 384-well plates manually and then reformatted into 1536-well plates at 7 μl/well using Cybi-well dispensing station (Cybio, Inc., Woburn, MA). A pintool station (Kalypsys, San Diego, CA) was used to transfer 23 nl of compound in DMSO solution to the 1536-well assay plate in which the final DMSO concentration was under 0.5%. A CCD-based imaging plate reader, ViewLux (PerkinElmer, Boston, MA), was used for the detection of IP-One assay plates in the TR-FRET mode with the excitation of 320 nm and the dual emissions of 620 and 665 nm. A kinetic fluorescence plate reader, Functional Drug Screening System (FDSS) 7000 (Hamamatsu Corp., Hamamatsu City, Japan), was used to measure changes in the intracellular free calcium.
HTRF IP-One Assay
Frozen CHO-M1 Cells were resuspended in the culture media and seeded at 4 μl/well (2000 cells/well) in white, solid bottom, tissue culture-treated 1536-well plates. The cells were cultured at 37 ºC, 5 % CO2 overnight to allow recovery from the frozen state. Serial dilutions of compounds were then added at 23 nl/well on the second day and incubated for 10 min. The agonist carbachol was prepared in the stimulation buffer (10mM Hepes, 1mM CaCl2, 0.5 mM MgCl2, 4.2 mM KCl, 146 mM NaCl, 5.5 mM glucose 250mM LiCl 50mM pH7.4) and was added to the assay plate at 1 μl/well. After 30 min incubation with carbachol at 37 ºC with 5 % CO2, all wells in the assay plate were dispensed with 1 μl/well of d2-conjugated IP1 (d2-IP1) and 1 μl/well of Eu+ cryptate-conjugated anti-IP1 antibody (Eu+-Ab), both from the HTRF IP-One kit and prepared in the cell lysis buffer supplied in the kit. After 10 min incubation at room temperature, the assay plates were measured in the TR-FRET detection mode with a ViewLux plate reader. The results were calculated as a ratio of the acceptor fluorescence intensity divided by the donor fluorescence intensity [16Harbert C, Marshall J, Soh S, Steger K. Development of a HTRF® Kinase Assay for Determination of Syk Activity Curr Chem Genomics 2008; 1: 20-6.].
Intracellular Calcium Assay
The resuspended frozen cells were plated at 3 μl/well with 2000 cells in black, tissue culture treated, clear bottom 1536-well plates. After overnight incubation at 37 ºC, 5% CO2, 2.5 μl of the calcium dye (1x calcium indicator in HBSS with 2.5 mM Probenecid as per manufacturer’s instructions) was dispensed to all wells and plates were incubated at 37 ºC, 5 % CO2 for 1 hour followed by 10 min incubation with 23 nl compound in DMSO solution. The assay plates were then placed in the kinetic fluorescence plate reader (FDSS-7000) for a kinetic measurement of changes in intracellular free calcium. The basal fluorescence signal was recorded 6 times at 1 Hz followed by an addition of 1 μl of Carbachol and 4-minute continuously recording at 1 Hz.
Data Analysis
The primary screening data was first analyzed using software developed internally in NIH Chemical Genomics Center for the curve fitting and curve classification that is available for open access (www.ncgc.nih.gov) [15Inglese J, Auld DS, Jadhav A, et al. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries Proc Natl Acad Sci U S A 2006; 103(31): 11473-8.]. The results from experiments with control compounds were analyzed with Prism® software (GraphPad, San Diego, CA) for the curve fitting and EC50/IC50 calculation.
RESULTS AND DISCUSSION
Agonist binding to a Gq-coupled GPCR results in activation of PLC, which hydrolyzes phosphatidylinositol bisphosphate (PIP2) to form two second messengers, IP3 and DAG (Fig. 1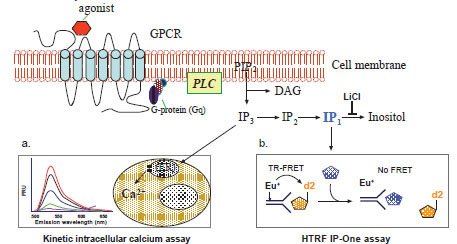 ). While DAG activates protein kinase C (PKC), IP3 activates the IP3 receptor on the endoplasmic reticulum (ER) that results in an efflux of Ca2+ from ER to cytoplasm and elevation of intracellular free Ca2+. These events transfer the extracellular signal to biological responses in cells [10Berridge MJ. Inositol trisphosphate and calcium signalling Nature 1993; 361(6410): 315-25.]. The IP3 is very rapidly hydrolyzed to IP2, then to IP1 and finally to inositol by a series of enzymes; meanwhile Ca2+ is quickly pumped back into ER by a calcium pump on the ER membrane. Both processes effectively switch off the IP3 and Ca2+ signaling. The time frames for intracellular free Ca2+ and IP3 measurements are usually within a few seconds to a few minutes [3Eglen RM, Bosse R, Reisine T. Emerging concepts of guanine nucleotide-binding protein-coupled receptor (GPCR) function and implications for high throughput screening Assay Drug Dev Technol 2007; 5(3): 425-51.,17Sullivan E, Tucker EM, Dale IL. Measurement of [Ca2+] using the Fluorometric Imaging Plate Reader (FLIPR) Methods Mol Biol 1999; 114: 125-33.]. For compound screens, the changes in intracellular free Ca2+ concentration can be measured by fluorescence calcium dyes with a fluorescence kinetic plate reader (Fig. 1a
). While DAG activates protein kinase C (PKC), IP3 activates the IP3 receptor on the endoplasmic reticulum (ER) that results in an efflux of Ca2+ from ER to cytoplasm and elevation of intracellular free Ca2+. These events transfer the extracellular signal to biological responses in cells [10Berridge MJ. Inositol trisphosphate and calcium signalling Nature 1993; 361(6410): 315-25.]. The IP3 is very rapidly hydrolyzed to IP2, then to IP1 and finally to inositol by a series of enzymes; meanwhile Ca2+ is quickly pumped back into ER by a calcium pump on the ER membrane. Both processes effectively switch off the IP3 and Ca2+ signaling. The time frames for intracellular free Ca2+ and IP3 measurements are usually within a few seconds to a few minutes [3Eglen RM, Bosse R, Reisine T. Emerging concepts of guanine nucleotide-binding protein-coupled receptor (GPCR) function and implications for high throughput screening Assay Drug Dev Technol 2007; 5(3): 425-51.,17Sullivan E, Tucker EM, Dale IL. Measurement of [Ca2+] using the Fluorometric Imaging Plate Reader (FLIPR) Methods Mol Biol 1999; 114: 125-33.]. For compound screens, the changes in intracellular free Ca2+ concentration can be measured by fluorescence calcium dyes with a fluorescence kinetic plate reader (Fig. 1a ). As an alternative method for assessing the function of Gq-coupled GPCRs, IP3 or total IPs including IP1, IP2 and IP3 are usually measured in the presence of lithium, an inhibitor of inositol monophosphatase (Fig. 1b
). As an alternative method for assessing the function of Gq-coupled GPCRs, IP3 or total IPs including IP1, IP2 and IP3 are usually measured in the presence of lithium, an inhibitor of inositol monophosphatase (Fig. 1b ). In this newly available HTRF based IP-One assay, an IP1 specific antibody is used to detect the labeled IP1 tracer. When the IP1 level in the cell lysate elevates that is proportional to an increase in IP3, the TR-FRET between the labeled antibody and IP1 tracer is disrupted (Fig. 1b
). In this newly available HTRF based IP-One assay, an IP1 specific antibody is used to detect the labeled IP1 tracer. When the IP1 level in the cell lysate elevates that is proportional to an increase in IP3, the TR-FRET between the labeled antibody and IP1 tracer is disrupted (Fig. 1b ).
).
 |
Fig. (1) Schematic representation of the principles for the intracellular calcium assay and the IP-One assay. |
IP-One Assay in the 1536-Well Plate Format
The original IP-One assay was developed in 96/384-well plate formats and requires a medium change for adherent cells or use of freshly detached suspension cells [14Trinquet E, Fink M, Bazin H, et al. D-myo-inositol 1-phosphate as a surrogate of D-myo-inositol 1,4,5-tris phosphate to monitor G protein-coupled receptor activation Anal Biochem 2006; 358(1): 126-35.,18Cassutt KJ, Orsini MJ, Abousleiman M, Colone D, Tang W. Identifying nonselective hits from a homogeneous calcium assay screen J Biomol Screen 2007; 12(2): 285-7.]. The additional cell wash step is obviously not suitable for HTS especially in 1536-well plate format. The automated robotic operation in HTS requires continuous supply of live cells over many hours. Adherent cells cultured overnight in assay plates are usually the convenient source of live cells for this purpose. Alternatively, for screenings using the compound pre-dispensed assay plates, suspension cells are needed in which the cells can be washed in batch before dispensing into the assay plates. Thus, we had miniaturized the IP-One assay in both adherent and suspension cell modes in 1536-well plates.
Plate Wash vs no Plate Wash
Medium removing or cell wash was suggested previously for the IP-One assay in order to reduce to potential interference from serum and phosphates present in the cell culture medium, reducing the liquid volume in wells, and maintaining the final lithium concentration [14Trinquet E, Fink M, Bazin H, et al. D-myo-inositol 1-phosphate as a surrogate of D-myo-inositol 1,4,5-tris phosphate to monitor G protein-coupled receptor activation Anal Biochem 2006; 358(1): 126-35.,18Cassutt KJ, Orsini MJ, Abousleiman M, Colone D, Tang W. Identifying nonselective hits from a homogeneous calcium assay screen J Biomol Screen 2007; 12(2): 285-7.]. We performed an assay with a cell wash step (cell culture medium was changed to buffer) to compare with that without a cell wash using adherent cells cultured overnight in 1536-well plates. LiCl, an inositol monophosphatase inhibitor, was added at a final concentration of 50 mM to accumulate inositol phosphates including IP1. We found that eliminating cell wash step did not reduce the fluorescence intensities of either donor or acceptor fluorophores or change the acceptor/donor fluorescence ratio (Fig. 2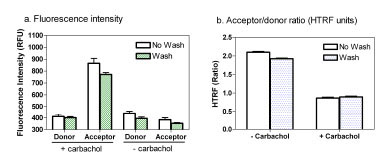 ). In fact, the signal-to-basal (S/B) ratio was slightly higher in the assay without a cell wash compared with that with a cell wash. This indicates that the cell wash step in 1536-well plates with small assay volume is not necessary for this IP-One assay. The IP-One assay in homogenous assay format is more suitable for HTS. The small amount (4 μl/well) of cell culture media in 1536-well plates might reduce the negative effect of phosphate containing medium on the IP-One assay.
). In fact, the signal-to-basal (S/B) ratio was slightly higher in the assay without a cell wash compared with that with a cell wash. This indicates that the cell wash step in 1536-well plates with small assay volume is not necessary for this IP-One assay. The IP-One assay in homogenous assay format is more suitable for HTS. The small amount (4 μl/well) of cell culture media in 1536-well plates might reduce the negative effect of phosphate containing medium on the IP-One assay.
Adherent Cells vs. Suspension Cells
In the adherent cell mode of the IP-One assay, resuspended frozen cells were used for cell plating in 1536-well assay plates instead of freshly cultured cells because frozen cells were readily available and convenient for HTS [19Kunapuli P, Zheng W, Weber M, et al. Application of division arrest technology to cell-based HTS: comparison with frozen and fresh cells Assay Drug Dev Technol 2005; 3(1): 17-26.]. We found that the S/B ratios in the adherent cell mode with 1000, 2000, 3000, 4000 cells/well were 1.84, 2.40, 3.01 and 3.39 fold, respectively. The EC50 values of Carbachol remained the same in these cell densities (Fig. 3a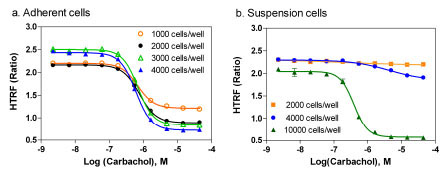 ). In the suspension cell mode of IP-One assay, freshly detached cultured cells were resuspended in a phosphates-free buffer included in the IP-One assay kit. The IP-One assay was then performed immediately after the cells were dispensed to 1536-well assay plates. We found that 2000 and 4000 cells/well in the suspension cell mode did not produce the same levels of signal as these in the adherent cell mode (Fig. 3b
). In the suspension cell mode of IP-One assay, freshly detached cultured cells were resuspended in a phosphates-free buffer included in the IP-One assay kit. The IP-One assay was then performed immediately after the cells were dispensed to 1536-well assay plates. We found that 2000 and 4000 cells/well in the suspension cell mode did not produce the same levels of signal as these in the adherent cell mode (Fig. 3b ). A 3.56 fold of S/B ratio was obtained in the suspension assay mode only after the cell density increased to 10,000 cells/well as it was reported previously in 384-well plates [14Trinquet E, Fink M, Bazin H, et al. D-myo-inositol 1-phosphate as a surrogate of D-myo-inositol 1,4,5-tris phosphate to monitor G protein-coupled receptor activation Anal Biochem 2006; 358(1): 126-35.,18Cassutt KJ, Orsini MJ, Abousleiman M, Colone D, Tang W. Identifying nonselective hits from a homogeneous calcium assay screen J Biomol Screen 2007; 12(2): 285-7.]. The results indicate that a higher cell density in the suspension cell mode is needed and the adherent cell mode requires much less cells. Frozen cells were tested in the suspension cell mode and they did not produce enough signals, while use of cell culture medium with 10% FBS produced similar signal as the phosphate-free buffer in the suspension cell mode (data not shown). Two reasons are likely to account for the difference of cell numbers in these two assay modes. First, cell numbers increase or even double after overnight culture in the adherent cell mode. Second, the cell health condition in assay plates can also affect the assay signal. The process for preparing freshly suspended cells involves cell detachment, centrifugation, resuspension and plating that can cause the stress and/or injury to cells. Thus, more cell numbers are needed to produce the same amount of IP1 in the suspension cell mode because the freshly plated cells are not fully recovered from the stress/injury, whereas the cells in the adherent cell mode are recovered after overnight cell culture and have better responses in the experiment. We selected 2000 cells/well for the compound screen in the adherent cell mode for the rest of study considering the cells reached 80 to 90 % confluence after overnight incubation. A density of 8000 to 10,000 cells/well in 1536-well plates is recommended for the IP-One assay in the suspension cell mode.
). A 3.56 fold of S/B ratio was obtained in the suspension assay mode only after the cell density increased to 10,000 cells/well as it was reported previously in 384-well plates [14Trinquet E, Fink M, Bazin H, et al. D-myo-inositol 1-phosphate as a surrogate of D-myo-inositol 1,4,5-tris phosphate to monitor G protein-coupled receptor activation Anal Biochem 2006; 358(1): 126-35.,18Cassutt KJ, Orsini MJ, Abousleiman M, Colone D, Tang W. Identifying nonselective hits from a homogeneous calcium assay screen J Biomol Screen 2007; 12(2): 285-7.]. The results indicate that a higher cell density in the suspension cell mode is needed and the adherent cell mode requires much less cells. Frozen cells were tested in the suspension cell mode and they did not produce enough signals, while use of cell culture medium with 10% FBS produced similar signal as the phosphate-free buffer in the suspension cell mode (data not shown). Two reasons are likely to account for the difference of cell numbers in these two assay modes. First, cell numbers increase or even double after overnight culture in the adherent cell mode. Second, the cell health condition in assay plates can also affect the assay signal. The process for preparing freshly suspended cells involves cell detachment, centrifugation, resuspension and plating that can cause the stress and/or injury to cells. Thus, more cell numbers are needed to produce the same amount of IP1 in the suspension cell mode because the freshly plated cells are not fully recovered from the stress/injury, whereas the cells in the adherent cell mode are recovered after overnight cell culture and have better responses in the experiment. We selected 2000 cells/well for the compound screen in the adherent cell mode for the rest of study considering the cells reached 80 to 90 % confluence after overnight incubation. A density of 8000 to 10,000 cells/well in 1536-well plates is recommended for the IP-One assay in the suspension cell mode.
The results also indicated that the EC50 values of carbachol obtained from both adherent and suspension cells were similar (Fig. 3 ). This result was consistent with previous reports that the responses of frozen cells after overnight culture were comparable to the freshly detached cells in several other assays [19Kunapuli P, Zheng W, Weber M, et al. Application of division arrest technology to cell-based HTS: comparison with frozen and fresh cells Assay Drug Dev Technol 2005; 3(1): 17-26.,20Ding M, Stjernborg L, Albertson N. Application of cryopreserved cells to HERG screening using a non-radioactive Rb+ efflux assay Assay Drug Dev Technol 2006; 4(1): 83-.].
). This result was consistent with previous reports that the responses of frozen cells after overnight culture were comparable to the freshly detached cells in several other assays [19Kunapuli P, Zheng W, Weber M, et al. Application of division arrest technology to cell-based HTS: comparison with frozen and fresh cells Assay Drug Dev Technol 2005; 3(1): 17-26.,20Ding M, Stjernborg L, Albertson N. Application of cryopreserved cells to HERG screening using a non-radioactive Rb+ efflux assay Assay Drug Dev Technol 2006; 4(1): 83-.].
The effect of FBS concentration on the assay performance was also tested in the M1-CHO cells. We found that although the EC50 value of Carbachol was not changed in the 1% FBS medium compared to that in the 10 % FBS medium (Fig. 4a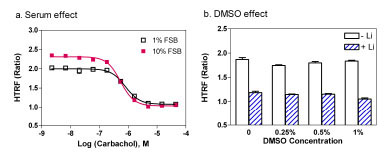 ), the S/B ratio decreased from 2.21 fold in 10 % FBS to 1.87 fold in 1 % FBS medium. This can be explained by the decreased amount of IP1 produced in 1% FBS cultured cells compared with the 10% FBS cultured cells. With a consideration of better S/B ratio for the antagonist screening, 10 % FBS medium was selected for this IP-One assay. We also found that the DMSO concentration was tolerated up to 1 % in this IP-One assay (Fig. 4b
), the S/B ratio decreased from 2.21 fold in 10 % FBS to 1.87 fold in 1 % FBS medium. This can be explained by the decreased amount of IP1 produced in 1% FBS cultured cells compared with the 10% FBS cultured cells. With a consideration of better S/B ratio for the antagonist screening, 10 % FBS medium was selected for this IP-One assay. We also found that the DMSO concentration was tolerated up to 1 % in this IP-One assay (Fig. 4b ). If the use of 10% FBS concentration is a concern, either 1% FBS medium or the suspension cell mode with a higher cell density can be used.
). If the use of 10% FBS concentration is a concern, either 1% FBS medium or the suspension cell mode with a higher cell density can be used.
 |
Fig. (4) Effects of serum and DMSO on the performance of the IP-One assay. (a) Effect of FBS concentration on the assay performance. (b) Effect of DMSO concentration on the assay performance. |
Comparison of Agonist Responses Between the IP-One and Intracellular Calcium Assays
Carbachol concentration responses in the same M1-CHO cells were determined in both the IP-One assay and intracellular calcium assay with a fluorescent calcium dye using a fluorescence kinetic plate reader. We found that EC50 value of carbachol was 535 nM determined in the IP-One assay (Fig. 5a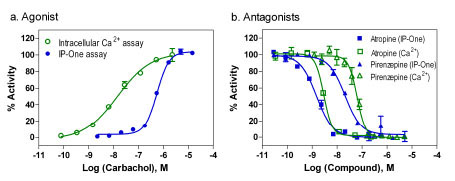 ), similar to that reported previously obtained in the radioactive IP1 assay with 3H-inositol incorporation [11Brandish PE, Hill LA, Zheng W, Scolnick EM. Scintillation proximity assay of inositol phosphates in cell extracts: high-throughput measurement of G-protein-coupled receptor activation Anal Biochem 2003; 313(2): 311-8.,14Trinquet E, Fink M, Bazin H, et al. D-myo-inositol 1-phosphate as a surrogate of D-myo-inositol 1,4,5-tris phosphate to monitor G protein-coupled receptor activation Anal Biochem 2006; 358(1): 126-35.]. The EC50 value of carbachol determined from the intracellular calcium assay in the fluorescence kinetic measurement was 15.1 nM that is similar to that of 23 nM reported elsewhere (www.invitrogen.com/) but was 35 fold more potent than that determined from the IP-One assay (Fig. 5a
), similar to that reported previously obtained in the radioactive IP1 assay with 3H-inositol incorporation [11Brandish PE, Hill LA, Zheng W, Scolnick EM. Scintillation proximity assay of inositol phosphates in cell extracts: high-throughput measurement of G-protein-coupled receptor activation Anal Biochem 2003; 313(2): 311-8.,14Trinquet E, Fink M, Bazin H, et al. D-myo-inositol 1-phosphate as a surrogate of D-myo-inositol 1,4,5-tris phosphate to monitor G protein-coupled receptor activation Anal Biochem 2006; 358(1): 126-35.]. The EC50 value of carbachol determined from the intracellular calcium assay in the fluorescence kinetic measurement was 15.1 nM that is similar to that of 23 nM reported elsewhere (www.invitrogen.com/) but was 35 fold more potent than that determined from the IP-One assay (Fig. 5a ). This discrepancy of carbachol potency can be explained by the receptor reserve (spare receptor) theory. The carbachol dissociation constant for the receptor binding was reported previously to be 1-3 μM [21Buck MA, Fraser CM. Muscarinic acetylcholine receptor subtypes which selectively couple to phospholipase C: pharmacological and biochemical properties Biochem Biophys Res Commun 1990; 173(2): 666-72.,22Huang XP, Nagy PI, Williams FE, Peseckis SM, Messer WS Jr. Roles of threonine 192 and asparagine 382 in agonist and antagonist interactions with M1 muscarinic receptors Br J Pharmacol 1999; 126(3): 735-45.] while its EC50 for the intracellular calcium assay was 15.1 to 23 nM. This suggested that only a small portion of receptors in this M1-CHO cell line need to be activated to stimulate a full calcium response. This discrepancy of the carbachol potency might be partly due to the different agonist incubation time in two assays. The agonist response in the intracellular calcium assay was measured at 1-2 minutes as it reaches its peak whereas the agonist incubation time in the IP-One assay was 30 minutes in order to reach its peak response. In addition, other unknown factors related to a specific receptor may be responsible for this discrepancy.
). This discrepancy of carbachol potency can be explained by the receptor reserve (spare receptor) theory. The carbachol dissociation constant for the receptor binding was reported previously to be 1-3 μM [21Buck MA, Fraser CM. Muscarinic acetylcholine receptor subtypes which selectively couple to phospholipase C: pharmacological and biochemical properties Biochem Biophys Res Commun 1990; 173(2): 666-72.,22Huang XP, Nagy PI, Williams FE, Peseckis SM, Messer WS Jr. Roles of threonine 192 and asparagine 382 in agonist and antagonist interactions with M1 muscarinic receptors Br J Pharmacol 1999; 126(3): 735-45.] while its EC50 for the intracellular calcium assay was 15.1 to 23 nM. This suggested that only a small portion of receptors in this M1-CHO cell line need to be activated to stimulate a full calcium response. This discrepancy of the carbachol potency might be partly due to the different agonist incubation time in two assays. The agonist response in the intracellular calcium assay was measured at 1-2 minutes as it reaches its peak whereas the agonist incubation time in the IP-One assay was 30 minutes in order to reach its peak response. In addition, other unknown factors related to a specific receptor may be responsible for this discrepancy.
The agonist responses of three other Gq-coupled GPCR cell lines including the free fatty acid receptor 1 (FFAR1), the vasopressin V1b receptor and the neuropeptide S (NPS) receptor were also determined in both IP-One and intracellular calcium assays. We found that the EC50 values of agonists determined in the intracellular calcium assay for FFAR1 receptor and NPS receptor were similar to these determined from IP-One assay (Table 1). The EC50 values of NPS in the intracellular calcium assay and IP-One assay were 0.22 and 0.41 nM, respectively. The Kd value of NPS receptor binding was 0.33 nM reported previously [23Xu YL, Reinscheid RK, Huitron-Resendiz S, et al. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects Neuron 2004; 43(4): 487-97.], correlating well with the activities determined from both functional assays. However, the agonist activity to V1b receptor was 34.4 times more potent than that obtained in the IP-One assay (Table 1). The extent of left shift of the agonist responses in the intracellular calcium assay with the V1b receptors was similar to that observed in the M1-CHO cell line.
Comparison of Antagonist Responses Between the IP-One and Intracellular Calcium Assays
Two muscarinic acetylcholine receptor antagonists, atropine and pirenzepine were tested in both assays to compare their activities. Atropine is a nonselective muscarinic acetylcholine receptor antagonist and pirenzepine is a M1 selective antagonist. In the experiments with antagonist screening, an EC80 amount of carbachol (agonist) was added to stimulate the M1 receptor responses. Because of the difference of agonist potency in these two assays, 0.1 and 5 μM Carbachol were used in the intracellular calcium assay and IP-One assay, respectively. Under these conditions, the S/B ratios were similar in both the IP-One assay and intracellular calcium assay for measurement of antagonist activities. The IC50 value of atropine was 2.93 nM determined from the intracellular calcium assay which is similar to 1.82 nM obtained from the IP-One assay (Fig. 5b ). For pirenzepine, the IC50 values were 63.3 and 20.8 nM, respectively in intracellular calcium assay and IP-One assay (Fig. 5b
). For pirenzepine, the IC50 values were 63.3 and 20.8 nM, respectively in intracellular calcium assay and IP-One assay (Fig. 5b ). This result indicates that the potencies of antagonists are similar and comparable in both assays using EC80 amount of agonist.
). This result indicates that the potencies of antagonists are similar and comparable in both assays using EC80 amount of agonist.
LOPAC Library Screenings in the IP-One and Intracellular Calcium Assays
A DMSO plate was first tested to evaluate both assays in the antagonist screening mode with the EC80 concentration of carbachol. The IP-One assay in 1536-well plates was performed in a homogenous assay format (Table 2). The S/B ratio was 1.86 fold and Z’ factor was 0.78 (Fig. 6a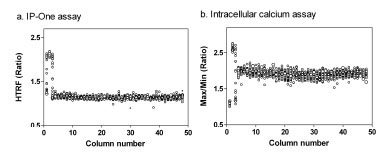 ), indicating a robust screening assay. The no-wash calcium dye was used in the intracellular calcium assay with a newly available kinetic plate reader specifically designed for 1536-well plate assays (Table 3). The S/B ratio and Z’ determined from the intracellular calcium assay were 1.80 fold and 0.64, respectively (Fig. 6b
), indicating a robust screening assay. The no-wash calcium dye was used in the intracellular calcium assay with a newly available kinetic plate reader specifically designed for 1536-well plate assays (Table 3). The S/B ratio and Z’ determined from the intracellular calcium assay were 1.80 fold and 0.64, respectively (Fig. 6b ). Although the S/B ratios in both assays were smaller than these in the agonist assay mode, the Z’ factors were above 0.5 indicating that both assay were robust for compound screening.
). Although the S/B ratios in both assays were smaller than these in the agonist assay mode, the Z’ factors were above 0.5 indicating that both assay were robust for compound screening.
The results of both the IP-One assay and intracellular calcium assays are calculated ratiometrically which compensates for the relatively small S/B ratios for the assay performance. Both IP-One and intracellular calcium assays described here do not require cell wash nor medium aspiration and are thus suitable for high throughput screening. While the intracellular calcium assay requires a costly kinetic fluorescence plate reader, the IP-One assay can be carried out using a standard fluorescence plate reader that is obviously a convenient and cost-effective alternative for compound screening with Gq-coupled GPCRs.
We then screened the LOPAC library in a quantitative high throughput screening (qHTS) format with both the IP-One and the intracellular calcium assays. Since all compounds were titrated in 15-concentrations, the active compounds were evaluated by their potencies and the curve classes as it was discussed in detail previously [15Inglese J, Auld DS, Jadhav A, et al. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries Proc Natl Acad Sci U S A 2006; 103(31): 11473-8.]. The results of cholinergic antagonists were particularly examined in details and their potencies in two assays are compared in Table 4.
Correlation of Antagonist Activities Between IP-One and Intracellular Calcium Assays
The LOPAC collection contains 30 known cholinergic antagonists including M1 specific, other subtype specific and nonselective antagonists. The IC50 values of these 30 known antagonists determined from the IP-One assay correlated with these measured from the intracellular calcium assay (Fig. 7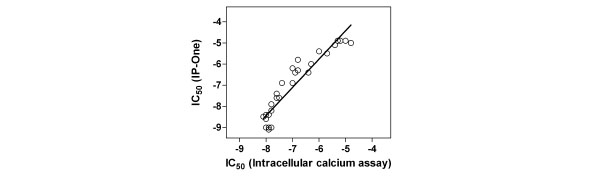 ). Together with the results of atropine and pirenzepine, it further demonstrated that the antagonist activities determined in this IP-One assay were similar to those obtained from the intracellular calcium assay in the kinetic assay mode.
). Together with the results of atropine and pirenzepine, it further demonstrated that the antagonist activities determined in this IP-One assay were similar to those obtained from the intracellular calcium assay in the kinetic assay mode.
Taken together, the intracellular calcium assay measures the real-time kinetic changes of free Ca2+ in live cells. It is a reliable assay method for screening compounds acting on Gq-coupled GPCRs; however, this method requires a special kinetic plate reader with the liquid handling capability for the addition of agonist to all the wells in an assay plate simultaneously. The tips on the multiple channel pipette head must be washed extensively before being used for the next assay plate. This tip washing step significantly limits the screening throughput and assay plate density in the previous version of instruments including FDSS-6000 and FLIPR Tetra. We have applied a newly designed FDSS-7000 plate reader which has one 1536-well pipetting head and three wash stations with sonicators. It can effectively and quickly wash tips for reuse and make the intracellular calcium assays robust in a 1536-well plate format; although the instrument is still costly.
Alternatively, the IP-One assay can be carried out with a standard plate reader without the requirement of a kinetic measurement. The HTRF assay format of the IP-One assay also reduces the well-to-well variation because the result is calculated in a ratiometric manner which can minimize the variations caused by cell number and dispensing errors among wells. The IP-One assay is also a homogenous assay format and has been miniaturized to 1536-well plates. The EC50 values of the agonists determined in the IP-One assay were similar to these reported in the radioactive IP assay. Thus, it is a useful alternative method for compound screening with Gq-protein coupled GPCRs. Compared with the intracellular calcium assay, the EC50 values of agonists in the IP-One assay could be less potent but the antagonist potencies are similar in both assays.
In summary, we have optimized and miniaturized an IP-One assay for the M1R, V1bR, FFAR1, and NPSR cell lines. The potencies of agonists determined from this IP-One assay are similar to these obtained from the traditional radioactive IP3 assay. The EC50 values of agonists determined from some GPCRs (FFAR1 and NPSR) were similar in both intracellular calcium and IP-One assays, while these determined from others (M1R and V1bR) were less potent in the IP-One assay, probably due to the receptor reserve. In comparison with the intracellular calcium assay using the kinetic detection mode, the potencies of antagonist responses are similar in both assays. The IP-One assay is homogenous and robust without requirement of a costly kinetic plate reader. These results indicate that the TR-FRET based IP-One assay is an alternative method to screen large sizes of compound collections for the Gq-coupled GPCRs.
ACKNOWLEDGEMENTS
This research was supported by the Molecular Libraries Initiative of the NIH Roadmap for Medical Research and the Intramural Research Programs of the National Human Genome Research Institute. The authors thank Dr. Marvin Gershengorn for providing with the FFAR1 cell line, Dr. Roger Bosse for the V1b cell line and Dr. Markus Heilig for the NPS cell line. We thank Paul Shinn and Adam Yasgar for assistance with compound management.