- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Current Chemical Genomics and Translational Medicine
(Discontinued)
ISSN: 2213-9885 ― Volume 12, 2018
Development of a Cell-Based High-Throughput Assay to Screen for Inhibitors of Organic Anion Transporting Polypeptides 1B1 and 1B3
Chunshan Gui1, Amanda Obaidat1, Rathnam Chaguturu2, 3, Bruno Hagenbuch*, 1, 2
Abstract
The two organic anion transporting polypeptides (OATPs) 1B1 and 1B3 are expressed at the sinusoidal membrane of hepatocytes. They have a broad and overlapping substrate specificity and transport many endobiotics and drugs. Specific inhibitors are required to determine the contribution of each OATP to the hepatocellular uptake of common substrates. We have developed a cell-based high-throughput assay to screen chemical libraries in order to identify such inhibitors for OATP1B1 and OATP1B3. We have used OATP1B1- or OATP1B3-expressing Chinese Hamster Ovary cells on 96-well plates and determined uptake of fluorescein-methotrexate (FMTX). We validated the assay with known inhibitors and screened the well characterized Prestwick library of 1120 drugs. Along with several known OATP inhibitors including rifampicin, cyclosporine A and mifepristone we identified some new inhibitors. For inhibitors that seemed to be able to distinguish between OATP1B1- and OATP1B3-mediated FMTX uptake IC50 values were determined. Estropipate (estrone-3-sulfate stabilized with piperazine) was the most selective OATP1B1 inhibitor (IC50 = 0.06 μM vs. 19.3 μM for OATP1B3). Ursolic acid was the most selective OATP1B3 inhibitor (IC50 = 2.3 μM vs. 12.5 μM for OATP1B1). In conclusion, this cell-based assay should allow us to identify even more specific inhibitors by screening larger chemical libraries.
Article Information
Identifiers and Pagination:
Year: 2010Volume: 4
First Page: 1
Last Page: 8
Publisher Id: CCGTM-4-1
DOI: 10.2174/1875397301004010001
Article History:
Received Date: 24/9/2009Revision Received Date: 25/11/2009
Acceptance Date: 12/12/2009
Electronic publication date: 1/3/2010
Collection year: 2010
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Department of Pharmacology, Toxicology and Therapeutics, The University of Kansas Medical Center,3901 Rainbow Blvd, Kansas City, KS 66160, USA; Tel: +1-913-588-0028; Fax: +1-913-588-7501; E-mail: bhagenbuch@kumc.edu
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 24-9-2009 |
Original Manuscript | Development of a Cell-Based High-Throughput Assay to Screen for Inhibitors of Organic Anion Transporting Polypeptides 1B1 and 1B3 | |
INTRODUCTION
Organic anion transporting polypeptides (humans: OATPs; rodents: Oatps) form a large gene superfamily of transport proteins expressed in multiple organs [1Hagenbuch B, Gui C. Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family Xenobiotica 2008; 38: 778-801., 2Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties Pflugers Arch 2004; 447: 653-.]. Many of their members are multispecific transporters that mediate the uptake of numerous endo- and xeno-biotics. Human OATP1B1 and OATP1B3 are expressed in the sinusoidal membrane of hepatocytes and are thought to be liver-specific under normal physiological conditions. They are responsible for the uptake of numerous drugs, including statins, endothelin receptor antagonists, anticancer drugs like methotrexate and paclitaxel, as well as the antibiotic rifampicin [1Hagenbuch B, Gui C. Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family Xenobiotica 2008; 38: 778-801.]. Besides specific substrates like prostaglandin E2 for OATP1B1 and CCK-8 for OATP1B3 [3Kullak-Ublick GA, Ismair MG, Stieger B, et al. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver Gastroenterology 2001; 120: 525-33., 4Ismair MG, Stieger B, Cattori V, et al. Hepatic uptake of cholecystikinin octapeptide by organic anion-transporting polypeptides OATP4 and OATP8 of rat and human liver Gastroenterology 2001; 121: 1185-90.], OATP1B1 and OATP1B3 have a number of common drug substrates including bosentan [5Treiber A, Schneiter R, Hausler S, Stieger B. Bosentan is a substrate of human OATP1B1 and OATP1B3: inhibition of hepatic uptake as the common mechanism of its interactions with cyclosporin A, rifampicin, and sildenafil Drug Metab Dispos 2007; 35: 1400-7.], fluvastatin [6Kopplow K, Letschert K, Konig J, Walter B, Keppler D. Human hepatobiliary transport of organic anions analyzed by quadruple-transfected cells Mol Pharmacol 2005; 68: 1031-8.], methotrexate [7Abe T, Kakyo M, Tokui T, et al. Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1 J Biol Chem 1999; 274: 17159-63., 8Abe T, Unno M, Onogawa T, et al. LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers Gastroenterology 2001; 120: 1689-99.], olmesartan [9Nakagomi-Hagihara R, Nakai D, Kawai K, et al. OATP1B1, OATP1B3, and mrp2 are involved in hepatobiliary transport of olmesartan, a novel angiotensin II blocker Drug Metab Dispos 2006; 34: 862-9.], pitavastatin [10Hirano M, Maeda K, Shitara Y, Sugiyama Y. Contribution of OATP2 (OATP1B1) and OATP8 (OATP1B3) to the hepatic uptake of pitavastatin in humans J Pharmacol Exp Ther 2004; 311: 139-46.], rifampicin [11Vavricka SR, van Montfoort J, Ha HR, Meier PJ, Fattinger K. Interactions of rifamycin SV and rifampicin with organic anion uptake systems of human liver Hepatology 2002; 36: 164-72.], rosuvastatin [12Ho RH, Tirona RG, Leake BF, et al. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics Gastroenterology 2006; 130: 1793-806.], and valsartan [13Yamashiro W, Maeda K, Hirouchi M, Adachi Y, Hu Z, Sugiyama Y. Involvement of transporters in the hepatic uptake and biliary excretion of valsartan, a selective antagonist of the angiotensin II AT1-receptor, in humans Drug Metab Dispos 2006; 34: 1247-54.]. Given that both OATPs are expressed in human hepatocytes, it will be almost impossible without specific inhibitors to elucidate which of the two proteins is more important for the transport of a certain drug. Furthermore, these transporters can affect the bioavailability of drugs. In 1996 we demonstrated that the peptide-based thrombin inhibitor CRC 220 is a substrate of rat Oatp1a1 [14Eckhardt U, Horz JA, Petzinger E, et al. The peptide-based thrombin inhibitor CRC 220 is a new substrate of the basolateral rat-liver organic anion-transporting polypeptide Hepatology 1996; 24: 380-4.]. This explained the very high hepatic first pass elimination of CRC 220 which was partially responsible for its low bioavailability. Specific OATP1B1 or OATP1B3 inhibitors could be co-applied with a drug which is efficiently cleared by the liver and this could increase its bioavailability.
In order to identify potentially specific inhibitors for OATP1B1 and OATP1B3, we decided to develop an assay for high-throughput screening. Fluorescent substrates are preferred for high-throughput screening because of the sensitivity and dynamic range that the fluorescence signal affords. So far only fluorescent bile acids [15Yamaguchi H, Okada M, Akitaya S, et al. Transport of fluorescent chenodeoxy-cholic acid via the human organic anion transporters OATP1B1 and OATP1B3 J Lipid Res 2006; 47: 1196-202.] and Fluo-3, a fluorescent calcium indicator [16Cui Y, Konig J, Keppler D. Vectorial transport by double-transfected cells expressing the human uptake transporter SLC21A8 and the apical export pump ABCC2 Mol Pharmacol 2001; 60: 934-43., 17Baldes C, Koenig P, Neumann D, Lenhof HP, Kohlbacher O, Lehr CM. Development of a fluorescence-based assay for screening of modulators of human Organic Anion Transporter 1B3 (OATP1B3) Eur J Pharm Biopharm 2006; 62: 39-43.] have been demonstrated to be substrates for OATP1B3 while fluorescein methotrexate (FMTX) has been reported as a substrate for OATP1B1 [18Hassan NJ, Pountney DJ, Ellis C, Mossakowska DE. BacMam recombinant baculovirus in transporter expression: a study of BCRP and OATP1B1 Protein Expr Purif 2006; 47: 591-8.]. However, methotrexate has been shown to be a substrate for both OATP1B1 and OATP1B3 [8Abe T, Unno M, Onogawa T, et al. LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers Gastroenterology 2001; 120: 1689-99.] and is commercially available as fluorescein methotrexate (FMTX) and Alexa Fluor® 488 methotrexate (AMTX). Preliminary data from our laboratory also demonstrated that the anticancer drug paclitaxel is a substrate for both OATPs. A fluorescent version of paclitaxel is also available commercially as Oregon Green® 488 Taxol (Flutax-2). Therefore, we compared these different fluorescent compounds along with fluorescein as substrates for OATP1B1 and OATP1B3. We further characterized FMTX transport by both OATPs and established an assay on 96-well plates for high-throughput screening. We used this assay to screen the Prestwick library for modulators (inhibitors or stimulators) and confirmed several hits by measuring IC50 or EC50 values for inhibition and stimulation of OATP1B1- and OATP1B3-mediated FMTX transport.
MATERIALS AND METHODS
Materials
Cell culture reagents, fluorescein-methotrexate (FMTX), Alexa Fluor® 488 methotrexate (AMTX), and Oregon Green® 488 Taxol (Flutax-2) were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum was from Hyclone (Logan, UT). The Prestwick library was obtained from the University of Kansas High Throughput Screening Laboratory. Individual chemicals for the confirmatory experiments were either from Prestwick Chemical or from Sigma and stock solutions were prepared in DMSO.
Cell Culture and Transport Assay on 24-well Plates
Chinese Hamster Ovary (CHO) cells stably expressing OATP1B1 and OATP1B3 were described previously [19Gui C, Miao Y, Thompson L, et al. Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3 Eur J Pharmacol 2008; 584: 57-65.] and were grown at 37 °C in a humidified 5% CO2 atmosphere in Dulbecco’s Modified Eagle Medium, containing 1 g/l D-glucose, 2 mM L-glutamine, 25 mM Hepes buffer and 110 mg/l sodium pyruvate, supplemented with 10% FBS (Hyclone, Logan, UT), 50 µg/ml L-proline, 100 U/ml penicillin and 100 µg/ml streptomycin, and 500 μg/ml G-418. Cells were passaged twice a week and used up to passage 55. Protein concentrations were very consistent, both on 24-well plates (0.150 ± 0.005 mg/well) as well as on 96-well plates (0.045 ± 0.002 mg/well). Transport assays on 24-well plates were performed as described [19Gui C, Miao Y, Thompson L, et al. Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3 Eur J Pharmacol 2008; 584: 57-65.] with the following modifications: cells were washed three times with 1 ml of pre-warmed (37 °C) uptake buffer (116.4 mM NaCl, 5.3 mM KCl, 1 mM NaH2PO4, 0.8 mM MgSO4, 5.5 mM D-glucose and 20 mM Hepes, pH adjusted to 7.4 with Trizma base) and uptake was started by adding 200 µl of uptake buffer containing the indicated concentrations of fluorescent compound. After the indicated time period at 37 °C, uptake was stopped by removing the uptake solution and washing the cells four times with 1 ml of ice-cold uptake buffer. The cell membranes were then disrupted with 300 µl of 1% Triton X-100 (dissolved in PBS) and fluorescence was measured in a Bio-Tek Synergy HT microplate reader (Winooski, VT) at an excitation wavelength of 485 nm and an emission wavelength of 528 nm.
High-Throughput Uptake Experiments in 96-well Plates
For high-throughput uptake experiments, OATP-expressing CHO cells were plated at 12,000 cells per well on 96-well plates and 48 hours later medium was replaced with medium containing 5 mM Na-butyrate to induce nonspecific gene expression. Previous experiments with OATP expressing CHO cells have demonstrated that after treatment of CHO cells with Na-butyrate transporter function was increased due to an increase in the maximal transport rate [20Eckhardt U, Schroeder A, Stieger B, et al. Polyspecific substrate uptake by the hepatic organic anion transporter oatp1 in stably transfected CHO cells Am J Physiol 1999; 276: G1037-42.]. After another 24 hours in culture, the cells were used for uptake experiments. Cells were washed three times with 200 µl of room temperature uptake buffer (116.4 mM NaCl, 5.3 mM KCl, 1 mM NaH2PO4, 0.8 mM MgSO4, 5.5 mM D-glucose and 20 mM Hepes, pH adjusted to 7.4 with Trizma base) and uptake was started by adding 70 µl of uptake buffer containing 5 µM substrate in the presence or absence of inhibitors. After 30 min culture at room temperature, uptake was stopped by removing the uptake solution and washing the cells four times with 200 μl of room temperature uptake buffer. The cells were then solubilized with 100 µl of 1% Triton X-100 (dissolved in PBS). Fluorescence was measured in a Bio-Tek Synergy HT microplate reader (Winooski, VT) at an excitation wavelength of 485 nm and an emission wavelength of 528 nm.
Data Analysis
Z’ factor calculation and assay validation were done according to NIH assay guidance manual [21Company ELa, Center NCG. Assay Guidance Manual Version 5.0 http://www.ncgc.nih.gov/guidance/manual_toc.html 2008 [last accessed [11/18/2009]];]. The data were analyzed using Sigmaplot and Graphpad prism. Statistical significance was determined using the two-tailed unpaired Student’s t test.
RESULTS
Characterization of Fluorescent Substrate Transport by OATP1B1 and OATP1B3
In the first set of experiments, we compared the uptake of four new fluorescent compounds, fluorescein-methotrexate (FMTX), Alexa Fluor® 488 methotrexate (AMTX), Oregon Green® 488 Taxol (Flutax-2) and fluorescein to the uptake of the known OATP substrate Fluo-3. As can be seen in Fig. (1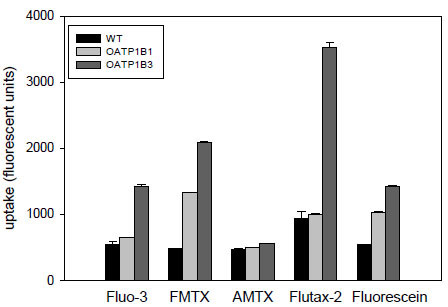 ), four of the five compounds namely Fluo-3, FMTX, Flutax-2 and fluorescein are substrates for OATP1B3, while AMTX was not transported. OATP1B1 on the other hand only transported FMTX and fluorescein. Given that radiolabeled methotrexate has previously been documented as a substrate for both OATP1B1 and OATP1B3 [8Abe T, Unno M, Onogawa T, et al. LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers Gastroenterology 2001; 120: 1689-99.] and that the uptake signal for both OATP1B3 (about 4.5 fold) and OATP1B1 (about 3 fold) was highest for FMTX, we decided to further characterize it. As the results in Fig. (2
), four of the five compounds namely Fluo-3, FMTX, Flutax-2 and fluorescein are substrates for OATP1B3, while AMTX was not transported. OATP1B1 on the other hand only transported FMTX and fluorescein. Given that radiolabeled methotrexate has previously been documented as a substrate for both OATP1B1 and OATP1B3 [8Abe T, Unno M, Onogawa T, et al. LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers Gastroenterology 2001; 120: 1689-99.] and that the uptake signal for both OATP1B3 (about 4.5 fold) and OATP1B1 (about 3 fold) was highest for FMTX, we decided to further characterize it. As the results in Fig. (2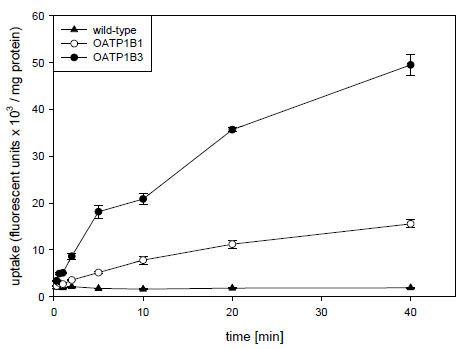 ) demonstrate, uptake of 10 μM FMTX was linear over a 5 min period. Thus, we used the 2 min time point to determine the kinetic parameters of OATP-mediated FMTX transport. Fig. (3
) demonstrate, uptake of 10 μM FMTX was linear over a 5 min period. Thus, we used the 2 min time point to determine the kinetic parameters of OATP-mediated FMTX transport. Fig. (3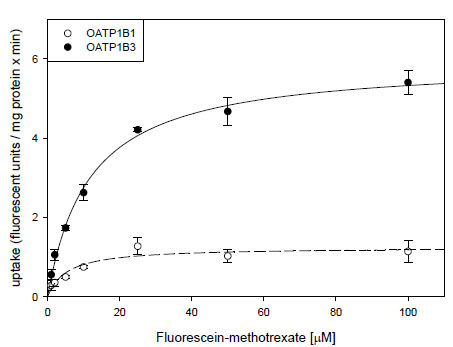 ) shows one of three independent experiments and demonstrates that OATP1B3 (closed circles) has a much higher capacity than OATP1B1 (open circles) to transport FMTX. Based on all three experiments we calculated for OATP1B1 an apparent Km value of 3.8 ± 0.7 μM and a Vmax value of 961 ± 198 fluorescent units/mg protein * min and for OATP1B3 an apparent Km value of 7.9 ± 2.0 μM and a Vmax value of 4772 ± 709 fluorescent units/mg protein * min.
) shows one of three independent experiments and demonstrates that OATP1B3 (closed circles) has a much higher capacity than OATP1B1 (open circles) to transport FMTX. Based on all three experiments we calculated for OATP1B1 an apparent Km value of 3.8 ± 0.7 μM and a Vmax value of 961 ± 198 fluorescent units/mg protein * min and for OATP1B3 an apparent Km value of 7.9 ± 2.0 μM and a Vmax value of 4772 ± 709 fluorescent units/mg protein * min.
Establishing a High-Throughput Assay on 96-well Plates
In order to test whether this cell-based assay could be run on 96-well plates instead of on 24-well plates, we determined uptake of 5 μM FMTX in the absence or presence of known OATP substrates or inhibitors for 30 min at room temperature on 96-well plates. We could detect FMTX uptake mediated by both OATPs. A typical uptake resulted in 4,400 ± 80 fluorescent units for wild-type CHO cells, 15,060 ± 1,540 fluorescent units for OATP1B1-expressing cells and 33,880 ± 3,645 fluorescent units for OATP1B3-expressing cells. Thus, signal-to-basal ratios of 3.4 for OATP1B1 and of 7.7 for OATP1B3 were obtained. As can be seen from Fig. (4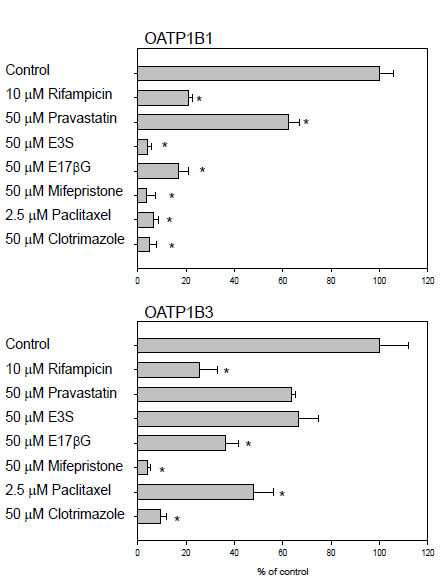 ), uptake by OATP1B1 and OATP1B3 was inhibited by all tested compounds. In additional experiments (data not shown), we determined that uptake at room temperature was linear over at least 30 min. Furthermore, we calculated the Z’ factors for the assays (0.26 for OATP1B1 and 0.46 for OATP1B3) in the absence (negative control) and presence (positive control) of 10 μM rifampicin with three independent 96-well plates. Validation was performed following the recommendations of the NIH assay guidance manual [21Company ELa, Center NCG. Assay Guidance Manual Version 5.0 http://www.ncgc.nih.gov/guidance/manual_toc.html 2008 [last accessed [11/18/2009]];]. The coefficient of variance for all maximal signals, unnormalized middle signals and minimal signals was less than 20%. Furthermore, all minimal signal standard deviations were smaller than both, the standard deviations of the middle and the maximal signals.
), uptake by OATP1B1 and OATP1B3 was inhibited by all tested compounds. In additional experiments (data not shown), we determined that uptake at room temperature was linear over at least 30 min. Furthermore, we calculated the Z’ factors for the assays (0.26 for OATP1B1 and 0.46 for OATP1B3) in the absence (negative control) and presence (positive control) of 10 μM rifampicin with three independent 96-well plates. Validation was performed following the recommendations of the NIH assay guidance manual [21Company ELa, Center NCG. Assay Guidance Manual Version 5.0 http://www.ncgc.nih.gov/guidance/manual_toc.html 2008 [last accessed [11/18/2009]];]. The coefficient of variance for all maximal signals, unnormalized middle signals and minimal signals was less than 20%. Furthermore, all minimal signal standard deviations were smaller than both, the standard deviations of the middle and the maximal signals.
Screening of Prestwick Library
To screen the 1120 compounds in the Prestwick library, we included 8 wells of negative (DMSO controls) and 8 wells of positive (10 μM rifampicin in DMSO) controls in each plate. As can be seen from the screening statistics shown in Table 1, we have developed a useful assay to screen for OATP1B3 inhibitors but the assay needs to be improved to be used with OATP1B1. Z’ values for the OATP1B1 part were close to zero or even negative, while the values for the OATP1B3 part were between 0.41 and 0.79. Nevertheless, we identify inhibitors for both OATP1B1 and OATP1B3 mediated uptake and a stimulator for OATP1B3. Among the strongest inhibitors found were known OATP substrates and/or inhibitors like cyclosporine A, rifampicin, saquinavir and several statins. In addition to these compounds, several new substances were found to inhibit FMTX transport mediated by either OATP1B1 or OATP1B3 or by both.
To confirm these hits of the screening results, we determined IC50 values for several compounds that preferentially inhibited either OATP1B1 or OATP1B3 or seemed to stimulate one or the other OATP. Among the tested compounds we identified five compounds that inhibited OATP1B1 more potently than OATP1B3 and three that preferentially inhibited OATP1B3 mediated FMTX transport (Table 2, Fig. 5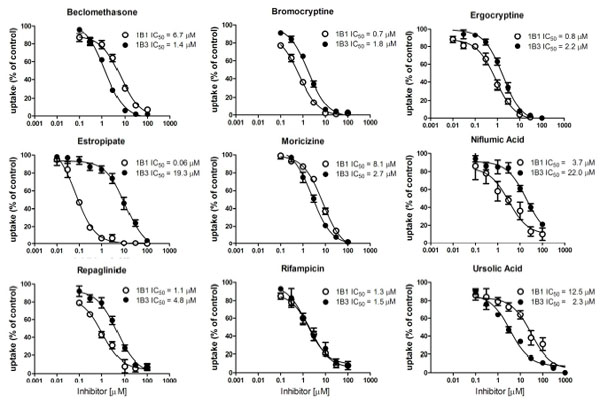 ). Estropipate, which is estrone-sulfate stabilized with piperazine, was the strongest OATP1B1 inhibitor with an IC50 of 0.06 ± 0.01 μM, while it hardly inhibited OATP1B3 at concentrations up to 1 μM (IC50 of 19.3 ± 6.3 μM) (Table 2). In addition to estropipate, we found that bromocryptine, ergocryptine, fipexide, niflumic acid and repaglinide preferentially inhibited OATP1B1 (Table 2, Fig. 5
). Estropipate, which is estrone-sulfate stabilized with piperazine, was the strongest OATP1B1 inhibitor with an IC50 of 0.06 ± 0.01 μM, while it hardly inhibited OATP1B3 at concentrations up to 1 μM (IC50 of 19.3 ± 6.3 μM) (Table 2). In addition to estropipate, we found that bromocryptine, ergocryptine, fipexide, niflumic acid and repaglinide preferentially inhibited OATP1B1 (Table 2, Fig. 5 ), while beclamethasone, moricizine and ursolic acid preferentially inhibited OATP1B3 (Table 2, Fig. 5
), while beclamethasone, moricizine and ursolic acid preferentially inhibited OATP1B3 (Table 2, Fig. 5 ). Among the potential stimulators we could confirm that progesterone stimulated OATP1B3-mediated FMTX uptake with an EC50 of 0.2 μM, while it inhibited OATP1B1-mediated FMTX uptake with an IC50 of 5.4 ± 1.9 μM (Fig. 6
). Among the potential stimulators we could confirm that progesterone stimulated OATP1B3-mediated FMTX uptake with an EC50 of 0.2 μM, while it inhibited OATP1B1-mediated FMTX uptake with an IC50 of 5.4 ± 1.9 μM (Fig. 6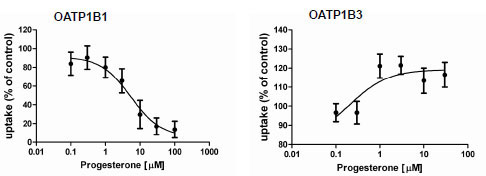 ).
).
DISCUSSION
In this study, we established a good high-throughput assay for OATP1B3 (Z’ factor > 0.4) that allows screening for inhibitors and stimulators of OATP1B3. The assay established for OATP1B1 needs more optimization perhaps using a different substrate to be as valuable as the one for OATP1B3. Initially we tested several fluorescent compounds as potential substrates for OATP1B1 and OATP1B3 and compared them to the known OATP1B1 and OATP1B3 substrate Fluo-3 [16Cui Y, Konig J, Keppler D. Vectorial transport by double-transfected cells expressing the human uptake transporter SLC21A8 and the apical export pump ABCC2 Mol Pharmacol 2001; 60: 934-43., 17Baldes C, Koenig P, Neumann D, Lenhof HP, Kohlbacher O, Lehr CM. Development of a fluorescence-based assay for screening of modulators of human Organic Anion Transporter 1B3 (OATP1B3) Eur J Pharm Biopharm 2006; 62: 39-43., 19Gui C, Miao Y, Thompson L, et al. Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3 Eur J Pharmacol 2008; 584: 57-65.]. Because fluorescein-methotrexate (FMTX) resulted in the highest signal for both OATP1B1 and OATP1B3 and because radiolabeled methotrexate has previously been identified as a substrate for both OATP1B1 and OATP1B3 [8Abe T, Unno M, Onogawa T, et al. LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers Gastroenterology 2001; 120: 1689-99.], we decided to continue with this substrate and further characterize its transport. Our results show that FMTX is transported by both OATPs (Figs. 1 , 2
, 2 and 3
and 3 ) and the kinetic parameters suggest that OATP1B3 has a 2–3 fold higher efficiency (Vmax/Km = 604) than OATP1B1 (Vmax/Km = 252) to transport FMTX. This is similar to the transport of Fluo-3 which is also transported much better by OATP1B3 than by OATP1B1 [19Gui C, Miao Y, Thompson L, et al. Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3 Eur J Pharmacol 2008; 584: 57-65.], suggesting that the fluorescent moiety might be more important for recognition of FMTX by these two OATPs than the methotrexate moiety. This hypothesis is further supported by the finding that inhibition of OATP1B1-mediated pitavastatin uptake by methotrexate was very weak (Ki > 300 μM) [22Hirano M, Maeda K, Shitara Y, Sugiyama Y. Drug-drug interaction between pitavastatin and various drugs via OATP1B1 Drug Metab Dispos 2006; 34: 1229-36.] and by our own observations that methotrexate at 100 μM only inhibited OATP-mediated FMTX uptake by 30% (data not shown). Therefore, it is likely that additional fluorescein-conjugates are transported by OATP1B1 and OATP1B3. Along the same lines, we also tested fluorescein itself and found that it is indeed a substrate for both transporters. However, the apparent affinity constants were about 10 fold higher for fluorescein (Km OATP1B1 38 ± 5 μM; Km OATP1B3 82 ± 10 μM) than for FMTX (Km OATP1B1 3.8 ± 0.7 μM; Km OATP1B3 7.9 ± 2.0 μM), suggesting that FMTX is the better substrate and that more than just the fluorescein moiety of the FMTX molecule is recognized by the transporters.
) and the kinetic parameters suggest that OATP1B3 has a 2–3 fold higher efficiency (Vmax/Km = 604) than OATP1B1 (Vmax/Km = 252) to transport FMTX. This is similar to the transport of Fluo-3 which is also transported much better by OATP1B3 than by OATP1B1 [19Gui C, Miao Y, Thompson L, et al. Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3 Eur J Pharmacol 2008; 584: 57-65.], suggesting that the fluorescent moiety might be more important for recognition of FMTX by these two OATPs than the methotrexate moiety. This hypothesis is further supported by the finding that inhibition of OATP1B1-mediated pitavastatin uptake by methotrexate was very weak (Ki > 300 μM) [22Hirano M, Maeda K, Shitara Y, Sugiyama Y. Drug-drug interaction between pitavastatin and various drugs via OATP1B1 Drug Metab Dispos 2006; 34: 1229-36.] and by our own observations that methotrexate at 100 μM only inhibited OATP-mediated FMTX uptake by 30% (data not shown). Therefore, it is likely that additional fluorescein-conjugates are transported by OATP1B1 and OATP1B3. Along the same lines, we also tested fluorescein itself and found that it is indeed a substrate for both transporters. However, the apparent affinity constants were about 10 fold higher for fluorescein (Km OATP1B1 38 ± 5 μM; Km OATP1B3 82 ± 10 μM) than for FMTX (Km OATP1B1 3.8 ± 0.7 μM; Km OATP1B3 7.9 ± 2.0 μM), suggesting that FMTX is the better substrate and that more than just the fluorescein moiety of the FMTX molecule is recognized by the transporters.
After having established that FMTX is a substrate for both OATP1B1 and OATP1B3, we tested the transport assay on 96-well plates at room temperature which would allow us to perform high-throughput screening. There was clear uptake signal for OATP1B1 and OATP1B3 (3.5 fold for OATP1B1 and 7.7 fold for OATP1B3) which could be inhibited by several common OATP substrates (Fig. 4 ).
).
Although the Z’ factors were not very high (0.26 for OATP1B1 and 0.46 for OATP1B3), we screened the Prestwick library and observed several hits for both OATPs. The screening statistics (Table 1) confirmed that the assay is very useful with respect to OATP1B3 but needs improvement for OATP1B1. We are currently testing additional fluorescent compounds as substrates, in particular for OATP1B1, in order to increase the signal and establish a reliable assay also for OATP1B1. In the follow-up experiments, we determined IC50 values for several inhibitors identified by the primary screening for the inhibition of 5 μM FMTX transported by either OATP. Some of the compounds that seemed to be stimulators during the primary screening could not be confirmed as stimulators, but inhibited slightly at higher concentrations and are listed with IC50 values larger than 100 μM (2-aminobenxenesulfonamide, aconitine, etomidate, metronidazole and trifluridine). The only stimulator that could be confirmed was progesterone which stimulated OATP1B3-mediated transport but inhibited OATP1B1-mediated transport. Progesterone has previously been shown to also stimulate OATP2B1- mediated estrone-3-sulfate uptake [23Grube M, Kock K, Karner S, et al. Modification of OATP2B1-mediated transport by steroid hormones Mol Pharmacol 2006; 70: 1735-41.].
Rifampicin which inhibited FMTX uptake of both OATPs with an IC50 of 1.3 to 1.5 µM (Table 2) has previously been shown to be an inhibitor and substrate for both OATPs [11Vavricka SR, van Montfoort J, Ha HR, Meier PJ, Fattinger K. Interactions of rifamycin SV and rifampicin with organic anion uptake systems of human liver Hepatology 2002; 36: 164-72.] and has since been used to characterize OATP mediated transport by many groups. However, inhibition by rifampicin is dependent on the transported substrate. Fexofenadine has been inhibited by rifampicin with Ki values of 0.5 µM for OATP1B1 and of 1.5 µM for OATP1B3 [24Matsushima S, Maeda K, Ishiguro N, Igarashi T, Sugiyama Y. Investigation of the inhibitory effects of various drugs on the hepatic uptake of fexofenadine in humans Drug Metab Dispos 2008; 36: 663-9.] while BSP was inhibited with Ki values of 17 µM for OATP1B1 and 5 µM for OATP1B3 [11Vavricka SR, van Montfoort J, Ha HR, Meier PJ, Fattinger K. Interactions of rifamycin SV and rifampicin with organic anion uptake systems of human liver Hepatology 2002; 36: 164-72.]. Treiber et al. [5Treiber A, Schneiter R, Hausler S, Stieger B. Bosentan is a substrate of human OATP1B1 and OATP1B3: inhibition of hepatic uptake as the common mechanism of its interactions with cyclosporin A, rifampicin, and sildenafil Drug Metab Dispos 2007; 35: 1400-7.] reported inhibition of bosentan transport with IC50 values of 3.2 µM for OATP1B1 and 1.6 µM for OATP1B3 while uptake of the bosentan metabolite Ro 48-5033 was inhibited with IC50 values of about 50 µM for OATP1B1 and 0.8 µM for OATP1B3. These data clearly demonstrate substrate dependent inhibition patterns for OATP1B1 and OATP1B3-mediated transport. Previously, IC50 values between 0.9 and 1.5 µM for inhibition of OATP1B1 and 2.6 µM for OATP1B3 mediated estradiol-17β-glucuronide have been reported [19Gui C, Miao Y, Thompson L, et al. Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3 Eur J Pharmacol 2008; 584: 57-65., 25Tirona RG, Leake BF, Wolkoff AW Kim RB. Human organic anion transporting polypeptide-C (SLC21A6) is a major determinant of rifampin-mediated pregnane X receptor activation J Pharmacol Exp Ther 2003; 304: 223-8.]. These values are in good agreement with the values obtained in this study with FMTX and could indicate that estradiol-17β-glucuronide and FMTX are handled by the transporters in a very similar way while bosentan and its metabolite are probably transported via a different substrate binding site or translocation pathway given that the inhibition affinities are reversed. Based on the same assumptions it can be concluded that BSP and FMTX are probably handled in a very similar way given that IC50 values of repaglinide inhibition for OATP1B1 and OATP1B3 obtained in this study are very similar to the ones obtained with BSP as a substrate [26Bachmakov I, Glaeser H, Fromm MF, Konig J. Interaction of oral antidiabetic drugs with hepatic uptake transporters: focus on organic anion transporting polypeptides and organic cation transporter 1 Diabetes 2008; 57: 1463-9.]. Thus, the results of this study confirm that it is important to keep in mind that multispecific transporters like OATP1B1 and OATP1B3 seem to have multiple overlapping substrate binding/transport sites that are also affected by inhibitors in a substrate dependent way.
Estropipate (estrone-sulfate stabilized with piperazine) was the only compound among the real inhibitors that can be considered selective for OATP1B1-mediated FMTX transport. It inhibited OATP1B1 with an IC50 value of 0.06 μM, while the IC50 value for OATP1B3-mediated FMTX transport was approximately 320-fold higher (Table 2). Besides indocyanine green which has been shown to be a specific inhibitor for OATP1B1-mediated estradiol-17β-glucuronide transport while not affecting OATP1B3-mediated estradiol-17β-glucuronide transport at concentrations up to 10 μM [27Cui Y, König J, Leier I, Buchholz U, Keppler D. Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6 J Biol Chem 2001; 276: 9626-30.], estropipate is as far as we know the only other selective inhibitor that inhibits OATP1B1 with hardly any effect on OATP1B3 at concentrations up to 1 μM at which concentration it completely inhibits OATP1B1 (Fig. 5 ). The most selective OATP1B3 inhibitors we identified were ursolic acid and beclomethasone that were about 5-fold more selective for OATP1B3 than for OATP1B1, but they were not as selective as the two OATP1B3 selective inhibitors T-3095 and T-3157 [28Nakakariya M, Shimada T, Irokawa M, Maeda T, Tamai I. Identification and species similarity of OATP transporters responsible for hepatic uptake of beta-lactam antibiotics Drug Metab Pharmacokinet 2008; 23: 347-55.].
). The most selective OATP1B3 inhibitors we identified were ursolic acid and beclomethasone that were about 5-fold more selective for OATP1B3 than for OATP1B1, but they were not as selective as the two OATP1B3 selective inhibitors T-3095 and T-3157 [28Nakakariya M, Shimada T, Irokawa M, Maeda T, Tamai I. Identification and species similarity of OATP transporters responsible for hepatic uptake of beta-lactam antibiotics Drug Metab Pharmacokinet 2008; 23: 347-55.].
In conclusion, we have established that FMTX is a substrate for OATP1B1 and OATP1B3 and that it can be used for high-throughput screening with OATP1B3. Using this assay we have identified estropipate as a selective inhibitor for OATP1B1-mediated FMTX transport, and ursolic acid and beclomethasone as preferential inhibitors for OATP1B3-mediated FMTX transport. To identify additional more specific OATP1B1 and OATP1B3 inhibitors, we are currently improving the assay especially for OATP1B1 and then plan to extend the screening to larger libraries that are available at the High Throughput Laboratory of the University of Kansas.
ACKNOWLEDGEMENTS
This work was supported by National Institute of Health grants RR021940, GM077336 and T32-ES07079. The University of Kansas high throughput screening laboratory is a KU Cancer Center Shared Resource, and is funded in part by NIH/NCRR COBRE grant P20 RR015563.
REFERENCES
| [1] | Hagenbuch B, Gui C. Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family Xenobiotica 2008; 38: 778-801. |
| [2] | Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties Pflugers Arch 2004; 447: 653-. |
| [3] | Kullak-Ublick GA, Ismair MG, Stieger B, et al. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver Gastroenterology 2001; 120: 525-33. |
| [4] | Ismair MG, Stieger B, Cattori V, et al. Hepatic uptake of cholecystikinin octapeptide by organic anion-transporting polypeptides OATP4 and OATP8 of rat and human liver Gastroenterology 2001; 121: 1185-90. |
| [5] | Treiber A, Schneiter R, Hausler S, Stieger B. Bosentan is a substrate of human OATP1B1 and OATP1B3: inhibition of hepatic uptake as the common mechanism of its interactions with cyclosporin A, rifampicin, and sildenafil Drug Metab Dispos 2007; 35: 1400-7. |
| [6] | Kopplow K, Letschert K, Konig J, Walter B, Keppler D. Human hepatobiliary transport of organic anions analyzed by quadruple-transfected cells Mol Pharmacol 2005; 68: 1031-8. |
| [7] | Abe T, Kakyo M, Tokui T, et al. Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1 J Biol Chem 1999; 274: 17159-63. |
| [8] | Abe T, Unno M, Onogawa T, et al. LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers Gastroenterology 2001; 120: 1689-99. |
| [9] | Nakagomi-Hagihara R, Nakai D, Kawai K, et al. OATP1B1, OATP1B3, and mrp2 are involved in hepatobiliary transport of olmesartan, a novel angiotensin II blocker Drug Metab Dispos 2006; 34: 862-9. |
| [10] | Hirano M, Maeda K, Shitara Y, Sugiyama Y. Contribution of OATP2 (OATP1B1) and OATP8 (OATP1B3) to the hepatic uptake of pitavastatin in humans J Pharmacol Exp Ther 2004; 311: 139-46. |
| [11] | Vavricka SR, van Montfoort J, Ha HR, Meier PJ, Fattinger K. Interactions of rifamycin SV and rifampicin with organic anion uptake systems of human liver Hepatology 2002; 36: 164-72. |
| [12] | Ho RH, Tirona RG, Leake BF, et al. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics Gastroenterology 2006; 130: 1793-806. |
| [13] | Yamashiro W, Maeda K, Hirouchi M, Adachi Y, Hu Z, Sugiyama Y. Involvement of transporters in the hepatic uptake and biliary excretion of valsartan, a selective antagonist of the angiotensin II AT1-receptor, in humans Drug Metab Dispos 2006; 34: 1247-54. |
| [14] | Eckhardt U, Horz JA, Petzinger E, et al. The peptide-based thrombin inhibitor CRC 220 is a new substrate of the basolateral rat-liver organic anion-transporting polypeptide Hepatology 1996; 24: 380-4. |
| [15] | Yamaguchi H, Okada M, Akitaya S, et al. Transport of fluorescent chenodeoxy-cholic acid via the human organic anion transporters OATP1B1 and OATP1B3 J Lipid Res 2006; 47: 1196-202. |
| [16] | Cui Y, Konig J, Keppler D. Vectorial transport by double-transfected cells expressing the human uptake transporter SLC21A8 and the apical export pump ABCC2 Mol Pharmacol 2001; 60: 934-43. |
| [17] | Baldes C, Koenig P, Neumann D, Lenhof HP, Kohlbacher O, Lehr CM. Development of a fluorescence-based assay for screening of modulators of human Organic Anion Transporter 1B3 (OATP1B3) Eur J Pharm Biopharm 2006; 62: 39-43. |
| [18] | Hassan NJ, Pountney DJ, Ellis C, Mossakowska DE. BacMam recombinant baculovirus in transporter expression: a study of BCRP and OATP1B1 Protein Expr Purif 2006; 47: 591-8. |
| [19] | Gui C, Miao Y, Thompson L, et al. Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3 Eur J Pharmacol 2008; 584: 57-65. |
| [20] | Eckhardt U, Schroeder A, Stieger B, et al. Polyspecific substrate uptake by the hepatic organic anion transporter oatp1 in stably transfected CHO cells Am J Physiol 1999; 276: G1037-42. |
| [21] | Company ELa, Center NCG. Assay Guidance Manual Version 5.0 http://www.ncgc.nih.gov/guidance/manual_toc.html 2008 [last accessed [11/18/2009]]; |
| [22] | Hirano M, Maeda K, Shitara Y, Sugiyama Y. Drug-drug interaction between pitavastatin and various drugs via OATP1B1 Drug Metab Dispos 2006; 34: 1229-36. |
| [23] | Grube M, Kock K, Karner S, et al. Modification of OATP2B1-mediated transport by steroid hormones Mol Pharmacol 2006; 70: 1735-41. |
| [24] | Matsushima S, Maeda K, Ishiguro N, Igarashi T, Sugiyama Y. Investigation of the inhibitory effects of various drugs on the hepatic uptake of fexofenadine in humans Drug Metab Dispos 2008; 36: 663-9. |
| [25] | Tirona RG, Leake BF, Wolkoff AW Kim RB. Human organic anion transporting polypeptide-C (SLC21A6) is a major determinant of rifampin-mediated pregnane X receptor activation J Pharmacol Exp Ther 2003; 304: 223-8. |
| [26] | Bachmakov I, Glaeser H, Fromm MF, Konig J. Interaction of oral antidiabetic drugs with hepatic uptake transporters: focus on organic anion transporting polypeptides and organic cation transporter 1 Diabetes 2008; 57: 1463-9. |
| [27] | Cui Y, König J, Leier I, Buchholz U, Keppler D. Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6 J Biol Chem 2001; 276: 9626-30. |
| [28] | Nakakariya M, Shimada T, Irokawa M, Maeda T, Tamai I. Identification and species similarity of OATP transporters responsible for hepatic uptake of beta-lactam antibiotics Drug Metab Pharmacokinet 2008; 23: 347-55. |