- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Nanomedicine and Nanotechnology Journal
Formerly: The Open Nanomedicine Journal
(Discontinued)
ISSN: 2666-1500 ― Volume 6, 2020
Nanomedical Devices and Cancer Theranostics
Mohamed Moumaris1, 2, *, Jean-Michel Bretagne1, Nisen Abuaf2, 3
Abstract
The current therapies against cancer showed limited success. Nanotechnology is a promising strategy for cancer tracking, diagnosis, and therapy. The hybrid nanotechnology assembled several materials in a multimodal system to develop multifunctional approaches to cancer treatment. The quantum dot and polymer are some of these hybrid nanoparticle platforms. The quantum dot hybrid system possesses photonic and magnetic properties, allowing photothermal therapy and live multimodal imaging of cancer. These quantum dots were used to convey medicines to cancer cells. Hybrid polymer nanoparticles were utilized for the systemic delivery of small interfering RNA to malignant tumors and metastasis. They allowed non-invasive imaging to track in real-time the biodistribution of small interfering RNA in the whole body. They offer an opportunity to treat cancers by specifically silencing target genes. This review highlights the major nanotechnology approaches to effectively treat cancer and metastasis.
Article Information
Identifiers and Pagination:
Year: 2020Volume: 6
First Page: 1
Last Page: 11
Publisher Id: TONMJ-6-1
DOI: 10.2174/2666150002006010001
Article History:
Received Date: 30/10/2019Revision Received Date: 11/03/2020
Acceptance Date: 12/03/2020
Electronic publication date: 21/04/2020
Collection year: 2020
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Laboratoire d'Hématologie et d’Immunologie, Hôpital Rothschild, 5 Rue Santerre, 75012 Paris and Hôpital Tenon, 4 Rue de la Chine 75020 Paris, Groupe Hospitalier Universitaire Paris Est, AP-HP et Département d'Immunologie, Faculté de Médecine, Université Pierre et Marie Curie, Sorbonne Université, Paris and Hôtel-Dieu, Groupe Hospitalier Universitaire Paris Centre, Département des Investissements, AP-HP, 1 Place du Parvis Notre Dame, 75004 Paris, France; Tel: 0033762122825; E-mail: mohamed.moumaris@orange.fr
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 30-10-2019 |
Original Manuscript | Nanomedical Devices and Cancer Theranostics | |
1. INTRODUCTION
Cancer is one of the leading causes of death in the world. It is characterized by an uncontrolled cell proliferation within the body. The cells divide into infinity and lead to the formation of a primary tumor. Some cells of the primary tumor diffuse into the body and lead to the formation of secondary tumors called metastases. The cancer cell is characterized by its independence from the signals of cellular proliferation, to escape apoptosis, to proliferate to infinity, to become invasive and metastatic, and to induce angiogenesis (Fig. 1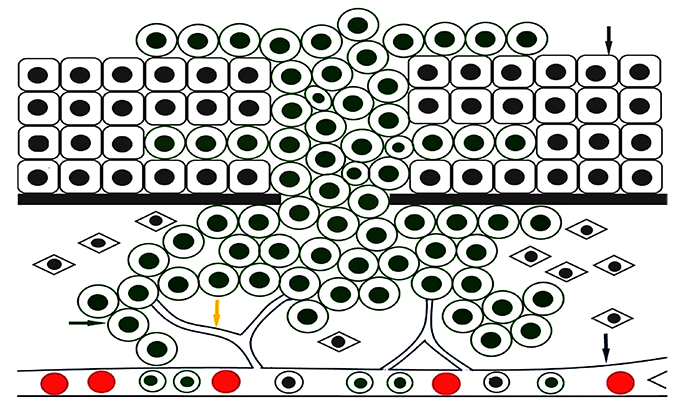 ). Cancers are linked to environmental and genetic factors. Three types of genes are responsible for cancerization, positive regulatory proto-oncogenes for normal cell proliferation, negative regulatory anti-oncogenes for cell proliferation and DNA repair genes [1Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100(1): 57-70.
). Cancers are linked to environmental and genetic factors. Three types of genes are responsible for cancerization, positive regulatory proto-oncogenes for normal cell proliferation, negative regulatory anti-oncogenes for cell proliferation and DNA repair genes [1Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100(1): 57-70.
[http://dx.doi.org/10.1016/S0092-8674(00)81683-9] [PMID: 10647931] -3Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J Control Release 2015; 200: 138-57.
[http://dx.doi.org/10.1016/j.jconrel.2014.12.030] [PMID: 25545217] ].
The diagnosis of cancer can be made by biopsy, biological analysis, endoscopy, and medical imaging modalities such as Pet, CT, and MRI [4Palsdottir T, Nordstrom T, Karlsson A, Grönberg H, Clements M, Eklund M. The impact of different prostate-specific antigen (PSA) testing intervals on Gleason score at diagnosis and the risk of experiencing false-positive biopsy recommendations: A population-based cohort study. BMJ Open 2019; 9(3): e027958.
[http://dx.doi.org/10.1136/bmjopen-2018-027958] [PMID: 30928965] -20Costa-Pinheiro P, Montezuma D, Henrique R, Jerónimo C. Diagnostic and prognostic epigenetic biomarkers in cancer. Epigenomics 2015; 7(6): 1003-15.
[http://dx.doi.org/10.2217/epi.15.56] [PMID: 26479312] ]. There are other options of therapies, as described in the references, such as immunotherapy and combination therapy [21Qureshi HA, Abouyared M, Barber B, Houlton JJ. Surgical options for locally advanced oropharyngeal cancer. Curr Treat Options Oncol 2019; 20(5): 36.
[http://dx.doi.org/10.1007/s11864-019-0621-x] [PMID: 30931485] -26Beardo P, Osman I, San José B, et al. Safety and outcomes of new generation hormone-therapy in elderly chemotherapy-naive metastatic castration-resistant prostate cancer patients in the real world. Arch Gerontol Geriatr 2019; 82: 179-85.
[http://dx.doi.org/10.1016/j.archger.2019.02.008] ]. Surgery and radiotherapy are curative treatments for localized tumors, they have undesirable effects. For metastatic tumors, chemotherapy is privileged, but lacks specificity and is associated with adverse side effects for patients. New targeted therapies or immunotherapies are more effective and less toxic, they are able to specifically recognize and treat cancer cells while preserving healthy cells and having fewer side effects [27Liu X, Liu S, Lyu H, Riker AI, Zhang Y, Liu B. Development of effective therapeutics targeting her3 for cancer treatment. Biol Proced Online 2019; 21: 5.
[http://dx.doi.org/10.1186/s12575-019-0093-1] [PMID: 30930695] -33Dong J, Li B, Lin D, Zhou Q, Huang D. Advances in targeted therapy and immunotherapy for non-small cell lung cancer based on accurate molecular typing. Front Pharmacol 2019; 10: 230.
[http://dx.doi.org/10.3389/fphar.2019.00230] [PMID: 30930778] ]. Nanomedicine is the set of processes for creating and manipulating devices at the nano-meter scale. These devices could perform cellular detections and repairs in the human body at the molecular level. Conventional imaging detects cancer at about a cm3 containing about a billion cancer cells, while nanotechnology devices have the ability to detect a single cancer cell. Through nanoparticles (NPs), nanotechnology is a promising strategy for the therapy and diagnosis of various diseases [34Xu Y, Ren H, Liu J, et al. A switchable NO-releasing nanomedicine for enhanced cancer therapy and inhibition of metastasis. Nanoscale 2019; 11(12): 5474-88.
[http://dx.doi.org/10.1039/C9NR00732F] [PMID: 30855625] -45Moumaris M, Abuaf N. Use of labeled dextran for in-vitro assessment of increased cell permeability, cell death and apoptosis. Brevet n°00/09235 2002; 2811682: A3].
2. NANOTECHNOLOGY APPROACHES
Nanomedical devices are designed to perform various biomedical applications in disease sites. They can be introduced into the body and guided to cancer sites to diagnose, administer drugs, and perform surgery on an anatomical, cellular and molecular scale. Biochips NPs can take single-cell captures and perform analytical cell separations [46Esfandyarpour R, DiDonato MJ, Yang Y, Durmus NG, Harris JS, Davis RW. Multifunctional, inexpensive, and reusable nanoparticle-printed biochip for cell manipulation and diagnosis. Proc Natl Acad Sci USA 2017; 114(8): E1306-15.
[http://dx.doi.org/10.1073/pnas.1621318114] [PMID: 28167769] ]. Nanotechnologies have theranostic applications, they have the ability to be used simultaneously for the diagnosis and targeted therapy of cancer [47Litwin MS, Tan HJ. The diagnosis and treatment of prostate Cancer: A Review. JAMA 2017; 317(24): 2532-42.
[http://dx.doi.org/10.1001/jama.2017.7248] [PMID: 28655021] , 48Lenaghan SC, Wang Y, Xi N, et al. Grand challenges in bioengineered nanorobotics for cancer therapy. IEEE Trans Biomed Eng 2013; 60(3): 667-73.
[http://dx.doi.org/10.1109/TBME.2013.2244599] [PMID: 23380844] ]. For the diagnostic function, nanotechnology has applications in non-invasive imaging, biocompatible NPs are used in tomography as imaging probes to measure and locate tumors [49Nahrendorf M, Keliher E, Marinelli B, et al. Hybrid PET-optical imaging using targeted probes. Proc Natl Acad Sci USA 2010; 107(17): 7910-5.
[http://dx.doi.org/10.1073/pnas.0915163107] [PMID: 20385821] ]. For the therapeutic function, nanotechnologies are applied in chemotherapy, small interfering RNA (siRNA) therapy, photodynamic therapy, and photothermal therapy. Nanoparticles were labeled with specific nucleic acid barcodes, which by their sequencing, allowed to measure the systemic and in vivo biodistribution of NPs siRNA delivered to tissues [50Dahlman JE, Kauffman KJ, Xing Y, et al. Barcoded nanoparticles for high throughput in vivo discovery of targeted therapeutics. Proc Natl Acad Sci USA 2017; 114(8): 2060-5.
[http://dx.doi.org/10.1073/pnas.1620874114] [PMID: 28167778] ] (Fig. 2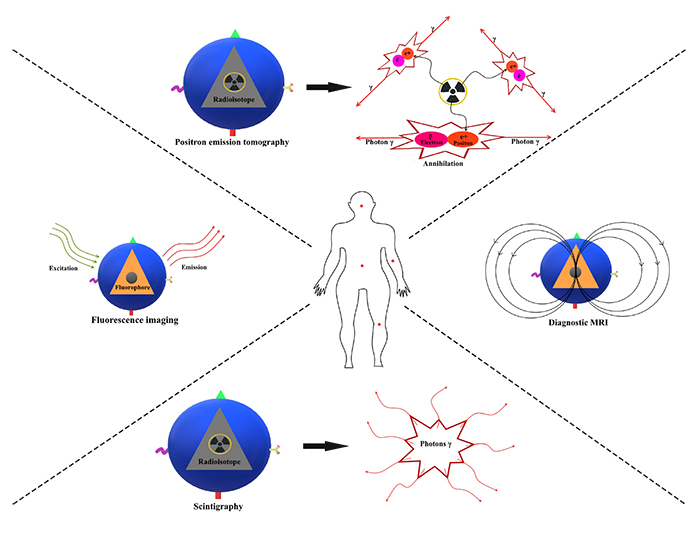 ).
).
Nanomedicine through NPs offers better treatments than conventional cancer therapies. NPs have the ability to encapsulate, protect, and release targeted drugs by having better pharmacokinetics, circulation half-life, and bioavailability. The characteristics of NPs, such as size, shape, and surface, offer better biological interactions and stability over large pH and temperature ranges [51Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm 2015; 93: 52-79.
[http://dx.doi.org/10.1016/j.ejpb.2015.03.018] [PMID: 25813885] ]. These platforms allow the selective distribution of drugs to tumor cells, reduce side effects, and avoid the body's defense mechanisms [52Xu X, Ho W, Zhang X, Bertrand N, Farokhzad O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol Med 2015; 21(4): 223-32.
[http://dx.doi.org/10.1016/j.molmed.2015.01.001] [PMID: 25656384] ]. The effectiveness of protein-based drugs is limited by the formation of antibodies, the combination of these drug proteins with NPs allows to increase their tolerance by the body and restore their antitumor activity [53Mazor R, King EM, Onda M, et al. Tolerogenic nanoparticles restore the antitumor activity of recombinant immunotoxins by mitigating immunogenicity. Proc Natl Acad Sci USA 2018; 115(4): E733-42.
[http://dx.doi.org/10.1073/pnas.1717063115] [PMID: 29311317] ].
The effectiveness of tumor administration of nanomedicines depends on biological barriers in the spleen, lymph nodes, and liver [54Tavares AJ, Poon W, Zhang YN, et al. Effect of removing Kupffer cells on nanoparticle tumor delivery. Proc Natl Acad Sci USA 2017; 114(51): E10871-80.
[http://dx.doi.org/10.1073/pnas.1713390114] [PMID: 29208719] ]. The NPs cross these natural barriers and selectively target biomarkers overexpressed by cancer cells such as ανβ3 integrin and folate receptors [55Han HD, Mangala LS, Lee JW, et al. Targeted gene silencing using RGD-labeled chitosan nanoparticles Clin Cancer Res 2010; 16: 3910-22.]. Physiological barriers may hinder the administration of nanomedicines. The NPs at 5 nm are eliminated by the kidneys. The gap between normal endothelial cells is 10 to 50 nm. In cancer sites, the gap between endothelial cells varies from 200 to 1200 nm. NPs greater than 200 nm accumulate in the liver, spleen and bone marrow, where they are taken up by the reticuloendothelial system (RES) and degraded by monocytes and macrophages. NPs at 100 nm leave the systemic circulation through the effect of permeability and increased retention (EPR) and accumulate in cancerous areas [56Stylianopoulos T, Wong C, Bawendi MG, Jain RK, Fukumura D. Multistage nanoparticles for improved delivery into tumor tissue. Methods Enzymol 2012; 508: 109-30.
[http://dx.doi.org/10.1016/B978-0-12-391860-4.00006-9] [PMID: 22449923] -58Ryu JH, Koo H, Sun IC, et al. Tumor-targeting multi-functional nanoparticles for theragnosis: new paradigm for cancer therapy. Adv Drug Deliv Rev 2012; 64(13): 1447-58.
[http://dx.doi.org/10.1016/j.addr.2012.06.012] [PMID: 22772034] ]. The NPs are able to distinguish target cancer cells based on their receptors profile allowing selective therapy with fewer side effects [59Curk T, Dobnikar J, Frenkel D. Optimal multivalent targeting of membranes with many distinct receptors. Proc Natl Acad Sci USA 2017; 114(28): 7210-5.
[http://dx.doi.org/10.1073/pnas.1704226114] [PMID: 28652338] ]. Nanomaterials such as graphene and nanoclays are able to interact with the cell membrane and activate protein kinase. These nanosilicates can provide information on cell signaling involved in tumor growth useful for the development of medical treatments [60Carrow JK, Cross LM, Reese RW, et al. Widespread changes in transcriptome profile of human mesenchymal stem cells induced by two-dimensional nanosilicates. Proc Natl Acad Sci USA 2018; 115(17): E3905-13.
[http://dx.doi.org/10.1073/pnas.1716164115] [PMID: 29643075] ].
 |
Fig. (1) Representation of cancer. The grey arrow indicates normal cells, the green arrow shows cancer cells, the yellow arrow shows angiogenesis, and the blue arrow indicates blood vessels. |
Nanomedical devices can be nanoliposomes, nanowires, carbon nanotubes, nanopores, gold nanoparticles (AuNPs), magnetic NPs, nanodiamonds, quantum dots, dendrimers, and nanosponges. There are NPs carrying drugs like liposome, mesoporous silica, polymer, and virus. There are NPs of photothermal therapy like gold NPs, and carbon tube. The devices of nanotechnology bring new hope in the targeted treatment of cancer (Table 1).
2.1. Liposomes
Liposomes are systems consisting of a lipid bilayer enveloping an aqueous central cavity. The cavity can carry hydrophilic drugs, whereas the envelope can carry hydrophobic drugs. Drug delivery into the cell cytoplasm is accomplished by the fusion of liposomes with cell membranes. The hydrophilic polar heads are directed outwards and the hydrophobic aliphatic tails are directed inwards. The size of the liposomes is of the order of 10 to 1000 nm. They are used to convey drugs and siRNAs to cancer cells because of their biodegradability. Liposomal doxorubicin (Doxil) is a chemotherapy drug used to treat various cancer. The fluorescent or radioactive labeling of liposomes is used in diagnostic techniques in medical imaging. Hybrid NPs in which liposomes are incorporated either within or at the surface have theranostic applications in cancer [61Gu Y, Li J, Li Y, et al. Nanomicelles loaded with doxorubicin and curcumin for alleviating multidrug resistance in lung cancer. Int J Nanomedicine 2016; 11: 5757-70.
[http://dx.doi.org/10.2147/IJN.S118568] [PMID: 27843316] ]. Liposomal NPs are used as an optical nanoprobe for in vivo chromogenic detection of H2O2 produced by cancer cells, and in photo-thermal therapy of target tumor sites [62Chen Q, Liang C, Sun X, et al. H2O2-responsive liposomal nanoprobe for photoacoustic inflammation imaging and tumor theranostics via in vivo chromogenic assay. Proc Natl Acad Sci USA 2017; 114(21): 5343-8.
[http://dx.doi.org/10.1073/pnas.1701976114] [PMID: 28484000] ]. The encapsulation of drugs by liposomes gives them steric stability, protects them against enzymes and the immune system and avoids their toxicity against the patient. To release the medications, the liposomes fuse with biological membranes and are endo-cytosed by cells. Molecules such as antibodies are added in liposomes systems to specifically recognize target cancer cells. The half-life of liposomes is short in biological media and their use requires frequent administration. They are chemically and physically unstable because they are sensitive to pH and temperature variations. They are opsonized and quickly eliminated by the reticuloendothelial system (RES). The modification of the surfaces of the liposomes with polyethylene glycol (PEG) chains reduces their immuno-genicity since the opsonins no longer cling to their surface, the organism does not recognize them as foreign and they are not destroyed by the RES [63Estanqueiro M, Amaral MH, Conceição J, Sousa Lobo JM. Nanotechnological carriers for cancer chemotherapy: The state of the art. Colloids Surf B Biointerfaces 2015; 126: 631-48.
[http://dx.doi.org/10.1016/j.colsurfb.2014.12.041] [PMID: 25591851] , 64Gharib A, Faezizadeh Z, Mesbah-Namin SA, Saravani R. Experimental treatment of breast cancer-bearing BALB/c mice by artemisinin and transferrin-loaded magnetic nanoliposomes. Pharmacogn Mag 2015; 11(Suppl. 1): S117-22.
[http://dx.doi.org/10.4103/0973-1296.157710] [PMID: 26109756] ].
2.2. Nanowires
Nanowires are wires of nanometric size, their diameter is of the order of 1 to 900 nanometers. They are made of plastic or metal conductive materials. The molecules of the nanowires are organic or inorganic. Nanowires applications are mainly in vitro and have many medical interests. They can be coated with antibodies that bind to target molecules for the diagnosis of cancer. Nanowires are highly more sensitive and specific than conventional biochemical tests and represent a powerful method of prognostic evaluation of cancer [65Lee MH, Lee DH, Jung SW, Lee KN, Park YS, Seong WK. Measurements of serum C-reactive protein levels in patients with gastric cancer and quantification using silicon nanowire arrays. Nanomedicine (Lond) 2010; 6(1): 78-83.
[http://dx.doi.org/10.1016/j.nano.2009.04.004] [PMID: 19446654] ].
2.3. Carbon Nanotubes
Carbon nanotubes (CNTs) are fibrous nanomaterials composed of layers of carbon atoms forming a tube. They have mechanical properties of rigidity, deformability, and lightness. Carbon nanotubes called “armchair” have a metallic character and they are conductors. Carbon nanotubes called “zig-zag” and carbon nanotubes called “chiral” are semiconductors, they have electrical conductivity between that of a conductor and an insulator. Owing to their physicochemical properties, they have many biomedical applications. They are used in drug delivery, chemotherapy, photodynamic therapy, and gene therapy. They have optical properties of luminescence with NIR absorbance, because of their fluorescent capacity they are employed in the detection of cancerous cells. They have properties of thermal conductivity; under laser irradiation, they produce heat allowing the thermotherapy of cancer cells [66Ji SR, Liu C, Zhang B, et al. Carbon nanotubes in cancer diagnosis and therapy. Biochim Biophys Acta 2010; 1806(1): 29-35.
[PMID: 20193746] ].
2.4. Nanopores
A nanopore is a hole of the order of 1 to 100 nm in a synthetic material such as graphene or biological material such as protein. Nanopore technology is used to characterize DNA molecules. The passage of individual nucleotides through the nanopore under tension triggers a signal which varies according to the bases of the DNA strand, making it possible to distinguish the four standard DNA bases and to read the genetic code. The sequencing of DNA by nanopores is less expensive and offers the possibility of detecting genetic mutations responsible for cancer. It has been utilized in patients with chronic lymphocytic leukemia and has been shown to be more sensitive than traditional sequencing methods. Nanopore-based DNA sequencing presents various advantages compared to traditional methods such as i) label-free ii) ultralong reads iii) high throughput reads iv) requires low material v) use unamplified genomic DNA [67Garaj S, Hubbard W, Reina A, Kong J, Branton D, Golovchenko JA. Graphene as a subnanometre trans-electrode membrane. Nature 2010; 467(7312): 190-3.
[http://dx.doi.org/10.1038/nature09379] [PMID: 20720538] -69Clarke J, Wu HC, Jayasinghe L, Patel A, Reid S, Bayley H. Continuous base identification for single-molecule nanopore DNA sequencing. Nat Nanotechnol 2009; 4(4): 265-70.
[http://dx.doi.org/10.1038/nnano.2009.12] [PMID: 19350039] ].
2.5. Gold Nanoparticles
AuNPs have optical properties of plasmons, they are contrast agents in biological imaging. In the treatment of cancer, they are used in imaging, photothermal therapy, photodynamic therapy and for targeted drug delivery. AuNPs are biocompatible and may have sites for attaching molecules to their shells that specifically recognize cancer cells. Nanoshells are AuNPs consisting of a dielectric core composed of silica and a metal shell made of gold (SiO2 core, Au shell). Nanorods are AuNPs of 1 to 100 nm containing semiconductor materials. Nanoshells and nanorods are used in photothermal therapy because of their NIR absorption. Under magnetic resonance guidance, the exposure to NIR light of tumors treated with AuNPs induces irreversible cancer cell lethality [70Sailor MJ, Park JH. Hybrid nanoparticles for detection and treatment of cancer. Adv Mater 2012; 24(28): 3779-802.
[http://dx.doi.org/10.1002/adma.201200653] [PMID: 22610698] -76Hirsch LR, Stafford RJ, Bankson JA, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci USA 2003; 100(23): 13549-54.
[http://dx.doi.org/10.1073/pnas.2232479100] [PMID: 14597719] ]. Multifunctional nanoshells have a greatly increased relaxivity of T1 enhancing MRI T1 properties used for quantitative monitoring in vivo therapeutics. These nano-particles of Au core-silica layer-Au shell contain internal gadolinium ions for T1 imaging contrast, encapsulated within the silica layer between an inner core and outer Au layer, in a multilayered geometry [77Marangoni VS, Neumann O, Henderson L, et al. Enhancing T1 magnetic resonance imaging contrast with internalized gadolinium(III) in a multilayer nanoparticle. Proc Natl Acad Sci USA 2017; 114(27): 6960-5.
[http://dx.doi.org/10.1073/pnas.1701944114] [PMID: 28630340] ]. The absorbing NIR is used to trigger the release, localized in space and time, of drugs conjugated to nanoshells in order to treat cancer cells while minimizing the toxicity of normal cells [78Goodman AM, Neumann O, Nørregaard K, et al. Near-infrared remotely triggered drug-release strategies for cancer treatment. Proc Natl Acad Sci USA 2017; 114(47): 12419-24.
[http://dx.doi.org/10.1073/pnas.1713137114] [PMID: 29109274] ]. Nanorods have been activated with NIR to generate photothermal therapy to treat metastases, they have the ability to inhibit the migration of cancer cells by targeting the cytoskeletons and integrins [79Ali MRK, Wu Y, Tang Y, et al. Targeting cancer cell integrins using gold nanorods in photothermal therapy inhibits migration through affecting cytoskeletal proteins. Proc Natl Acad Sci USA 2017; 114(28): E5655-63.
[http://dx.doi.org/10.1073/pnas.1703151114] [PMID: 28652358] ]. Gold nanocages are porous AuNPs of 10 to 150 nm, they are biocompatible and absorb in the NIR, they are contrast agents in medical imaging. Gold nanocages conjugated with antibodies are employed in cancer thermotherapy [80Chen J, Saeki F, Wiley BJ, et al. Gold nanocages: Bioconjugation and their potential use as optical imaging contrast agents. Nano Lett 2005; 5(3): 473-7.
[http://dx.doi.org/10.1021/nl047950t] [PMID: 15755097] , 81Au L, Zheng D, Zhou F, Li ZY, Li X, Xia Y. A quantitative study on the photothermal effect of immuno gold nanocages targeted to breast cancer cells. ACS Nano 2008; 2(8): 1645-52.
[http://dx.doi.org/10.1021/nn800370j] [PMID: 19206368] ].
2.6. Magnetic Nanoparticles
Magnetic NPs are nanomaterials of about 10 to 100 nm, they are composed of magnetic materials of iron, nickel or cobalt. The magnetic NPs most adapted for biomedical applications are nanomaterials composed of iron oxides, magnetite or maghemite. There are contrast agents in magnetic resonance imaging, using a magnetic field gradient, they can be directed to cancer sites. Magnetic nanoparticles are used in various applications for cancer therapy: i) hyperthermia-based therapy ii) selective photodynamic therapy of cancerous cells iii) targeting and extraction of cancer cells iv) targeted and controlled drug delivery to cancer cells [82Kralj S, Makovec D. Magnetic assembly of superparamagnetic iron oxide nanoparticle clusters into nanochains and nanobundles. ACS Nano 2015; 9(10): 9700-7.
[http://dx.doi.org/10.1021/acsnano.5b02328] [PMID: 26394039] -90Estelrich J, Escribano E, Queralt J, Busquets MA. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int J Mol Sci 2015; 16(4): 8070-101.
[http://dx.doi.org/10.3390/ijms16048070] [PMID: 25867479] ].
2.7. Nanodiamonds
Nanodiamonds are NPs of about 10 to 100 nm and can be produced by meteorite impacts. Due to their hardness and wear resistance, they are used in industrial and scientific applications. Due to their biocompatibility, photostability, electrostatic properties, and surface functionality, they are employed in biomedical applications to treat a wide range of diseases. These are platforms for localized drug delivery and monitoring of imaging-guided treatments. The nanodiamond technology system facilitates the targeted and sustainable delivery of drugs. The nanodiamond platform maximizes the effects of drugs by ensuring their prolonged administration. Nanodiamonds have a capacity in drugs sustained-release. The adsorption/desorption on nanodiamonds is the basic mechanism for targeted drug delivery. These are applications of cancer nanomedicine and cancer cell biomarkers [91Ho D. Nanodiamond-based chemotherapy and imaging. Cancer Treat Res 2015; 166: 85-102.
[http://dx.doi.org/10.1007/978-3-319-16555-4_4] [PMID: 25895865] -96Hong C, Song D, Lee DK, et al. Reducing posttreatment relapse in cleft lip palatal expansion using an injectable estrogen-nanodiamond hydrogel. Proc Natl Acad Sci USA 2017; 114(35): E7218-25.
[http://dx.doi.org/10.1073/pnas.1704027114] [PMID: 28808036] ].
2.8. Quantum Dot
The Quantum Dots (QDs) are fluorescent semiconductor nanocrystals of about 10 nm, composed of a core coated with an envelope. QD-containing NPs represent an approach in cancer medical imaging, cancer cell tracking, cancer photodynamic therapy, and cancer diagnosis. They are contrast agents and have tumor imaging properties. When injected into the body, they infiltrate cancerous sites and produce images of these sites. The toxicity of QDs limits their use for in vivo applications. Thus appropriately coated, QDs can be rendered non-toxic in biological media. Polymer-coated QDs have properties of biocompatibility, solubility, targeting cancerous cells and drug delivery. QDs based on a core with lead sulfide (PbS) and shell with cadmium sulfide (CdS) and covered with PEG, emitting at about 1600 nm allow 3D tumor fluorescence imaging in vivo [97Wang LW, Peng CW, Chen C, Li Y. Quantum dots-based tissue and in vivo imaging in breast cancer researches: current status and future perspectives. Breast Cancer Res Treat 2015; 151(1): 7-17.
[http://dx.doi.org/10.1007/s10549-015-3363-x] [PMID: 25833213] , 98Zhang M, Yue J, Cui R, et al. Bright quantum dots emitting at ∼1,600 nm in the NIR-IIb window for deep tissue fluorescence imaging. Proc Natl Acad Sci USA 2018; 115(26): 6590-5.
[http://dx.doi.org/10.1073/pnas.1806153115] [PMID: 29891702] ]. QDs can target specific receptors. Folate is vital for cell growth; the folate receptor is overexpressed in the cancer cell. QDs conjugated to folate are used to diagnose cancer. QDs have higher optical properties than organic dyes. They are photostable and possess quantum properties with excitation and emission of light in the NIR. The signal emitted is 100 times more intense than fluorescent dyes allowing deep tissue analysis [99Schroeder JE, Shweky I, Shmeeda H, Banin U, Gabizon A. Folate-mediated tumor cell uptake of quantum dots entrapped in lipid nanoparticles. J Control Release 2007; 124(1-2): 28-34.
[http://dx.doi.org/10.1016/j.jconrel.2007.08.028] [PMID: 17928088] , 100Smith AM, Dave S, Nie S, True L, Gao X. Multicolor quantum dots for molecular diagnostics of cancer. Expert Rev Mol Diagn 2006; 6(2): 231-44.
[http://dx.doi.org/10.1586/14737159.6.2.231] [PMID: 16512782] ]. When placed in the body and illuminated by light, QDs heat the surrounding tissue allowing hyperthermic cancer therapy. Considering that hyperthermia between 42°C and 45°C induces cell apoptosis while a temperature above 45°C causes cell necrosis. In photodynamic cancer therapy, QDs react with molecular oxygen producing peroxides and hydroxyl radicals, which lead to the death of cancer cells [101Allison R, Moghissi K, Downie G, Dixon K. Photodynamic therapy (PDT) for lung cancer Photodiagnosis Photodyn Ther 2011; 8: 231-9., 102Young JK, Figueroa ER, Drezek RA. Tunable nanostructures as photothermal theranostic agents. Ann Biomed Eng 2012; 40(2): 438-59.
[http://dx.doi.org/10.1007/s10439-011-0472-5] [PMID: 22134466] ].
2.9. Polymer-based Nanoparticles
Dendrimers and nanosponges are polymers of size from about 10 nm to 1000 nm. They have better solubility, stability, absorption, and in vivo bioavailability. They are the most promising drug carriers in targeted cancer therapy [103Kim HS, Han HD, Armaiz-Pena GN, et al. Functional roles of Src and Fgr in ovarian carcinoma. Clin Cancer Res 2011; 17(7): 1713-21.
[http://dx.doi.org/10.1158/1078-0432.CCR-10-2081] [PMID: 21300758] ]. Dendrimers are polymers of macromolecules, their size and shape are variable. Dendrimers are tree branch molecules composed of a nucleus of branching units and functional end groups. Drugs can be incorporated into the central nucleus or conjugated to the functional extremity, to be transported to the cancer cells. These are contrast agents used in MRI for the diagnosis of cancer [104Astruc D, Boisselier E, Ornelas C. Dendrimers designed for functions: from physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem Rev 2010; 110(4): 1857-959.
[http://dx.doi.org/10.1021/cr900327d] [PMID: 20356105] , 105Yao H, Veine DM, Fay KS, Staszewski ED, Zeng ZZ, Livant DL. The PHSCN dendrimer as a more potent inhibitor of human breast cancer cell invasion, extravasation, and lung colony formation. Breast Cancer Res Treat 2011; 125(2): 363-75.
[http://dx.doi.org/10.1007/s10549-010-0826-y] [PMID: 20300829] ]. Nanosponges are a scaffold of polymers filled with nanocavities in which molecules can be stored. These are NPs that take the form of red blood cells and move throughout the living body. They can trap bacteria and toxins in their scaffolds and filter them into the liver. Nanosponges injected into the living body are used to deliver drugs to cancer cells [106S S S A, Krishnamoorthy K, Rajappan M. Nanosponges: A novel class of drug delivery system--review. J Pharm Pharm Sci 2012; 15: 103-11.
[http://dx.doi.org/10.18433/J3K308] -108Osmani RA, Hani U, Bhosale RR, Kulkarni PK, Shanmuganathan S. Nanosponge carriers- an archetype swing in cancer therapy: A comprehensive review. Curr Drug Targets 2017; 18(1): 108-18.
[http://dx.doi.org/10.2174/1389450116666151001105449] [PMID: 26424399] ]. Human ferritin heavy-chain nanocages coupled to polyethylene glycol constitute a drug delivery system able to overcome multiple biological barriers, to penetrate preferentially into tumor tissues, to distribute selectively and dependently on transferrin receptor in cancer cells [109Huang X, Chisholm J, Zhuang J, et al. Protein nanocages that penetrate airway mucus and tumor tissue. Proc Natl Acad Sci USA 2017; 114(32): E6595-602.
[http://dx.doi.org/10.1073/pnas.1705407114] [PMID: 28739953] ]. Polymer-based NPs can be wrapped in macrophage-derived cell membranes which neutralize endotoxins, sequester pro-inflammatory cytokines, and inhibit the onset of immune activation against these NPs [110Thamphiwatana S, Angsantikul P, Escajadillo T, et al. Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc Natl Acad Sci USA 2017; 114(43): 11488-93.
[http://dx.doi.org/10.1073/pnas.1714267114] [PMID: 29073076] ]. To develop interfering RNA (RNAi) - based cancer treatments, albumin is conjugated to siRNA to increase their half-life in the circulation, their bioavailability, their accumulation in tumors and their uptake by tumor cells [111Sarett SM, Werfel TA, Lee L, et al. Lipophilic siRNA targets albumin in situ and promotes bioavailability, tumor penetration, and carrier-free gene silencing. Proc Natl Acad Sci USA 2017; 114(32): E6490-7.
[http://dx.doi.org/10.1073/pnas.1621240114] [PMID: 28739942] ]. Nano-encapsulation of multiplexed RNAi in lipopolymeric NPs is a therapeutic targeting strategy to meet the challenges of therapeutic resistance and tumor heterogeneity [112Yu D, Khan OF, Suvà ML, et al. Multiplexed RNAi therapy against brain tumor-initiating cells via lipopolymeric nanoparticle infusion delays glioblastoma progression. Proc Natl Acad Sci USA 2017; 114(30): E6147-56.
[http://dx.doi.org/10.1073/pnas.1701911114] [PMID: 28696296] ]. Spherical nucleic acids (SNAs) nanoconjugate-based RNAi constitute an in vivo nanotherapeutic strategy, they are capable of performing non-invasive imaging and inactivating in vivo intratumoral proteins [113Sita TL, Kouri FM, Hurley LA, et al. Dual bioluminescence and near-infrared fluorescence monitoring to evaluate spherical nucleic acid nanoconjugate activity in vivo. Proc Natl Acad Sci USA 2017; 114(16): 4129-34.
[http://dx.doi.org/10.1073/pnas.1702736114] [PMID: 28373576] ].
2.10. Hybrid Nanoparticles
Hybrid NPs are multifunctional systems in which nanostructures such as liposomes, polymers, noble metals, and nanotubes are incorporated inside or on the surface of a nano-assembly. They combine both diagnostic and therapeutic functions. These nanodevices are able to perform multiple tasks, they are utilized in drug therapy and medical imaging for in vivo targeting of cancer sites (Figs. 3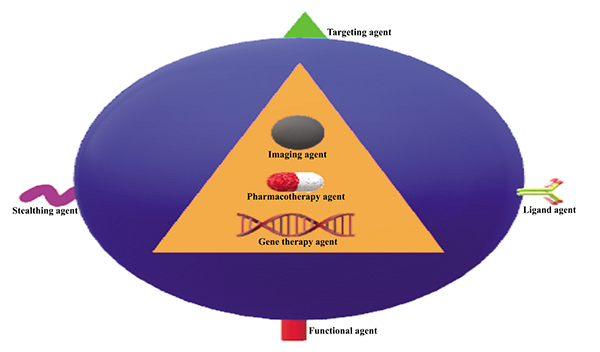 and 4
and 4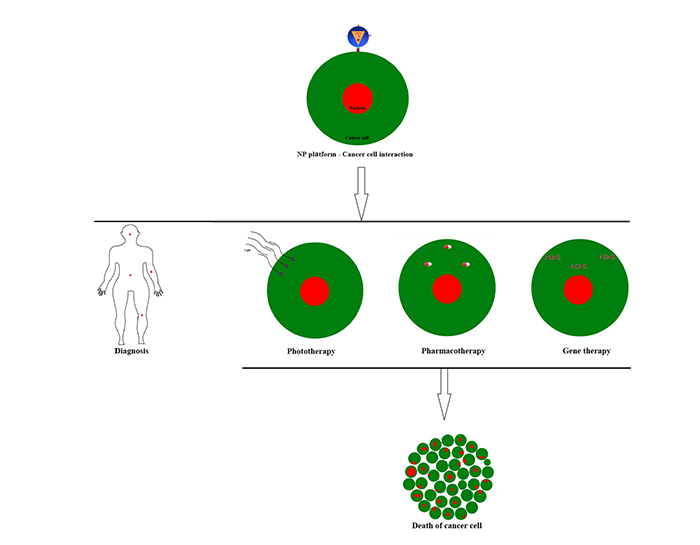 ). Polymer hybrid NPs aim to offer biocompatible and biodegradable materials suitable for therapeutics and diagnosis applications. They can incorporate QD and magnetic NPs. They combine medical imaging and drug delivery in cancer therapy. Conjugated with folate, they selectively target cancer sites.
). Polymer hybrid NPs aim to offer biocompatible and biodegradable materials suitable for therapeutics and diagnosis applications. They can incorporate QD and magnetic NPs. They combine medical imaging and drug delivery in cancer therapy. Conjugated with folate, they selectively target cancer sites.
Mesoporous silica-based hybrid NPs are the best drug carriers because of their large surface area, size, porosity, chemical stability, and biocompatibility. They can incorporate theranostic molecules and magnetic nanocrystals [70Sailor MJ, Park JH. Hybrid nanoparticles for detection and treatment of cancer. Adv Mater 2012; 24(28): 3779-802.
[http://dx.doi.org/10.1002/adma.201200653] [PMID: 22610698] ] (Table 2). AuNPs have photonic and magnetic properties, but their toxicity to cells and their lack of solvent stability limit their use. The creation of mesoporous silica-based hybrid systems stabilizes AuNPs by allowing them to retain their properties in biological media while being biocompatible. In these hybrid systems, the AuNPs absorb light in the NIR, convert it into photons through the tissues and deliver treatment. QDs are promising in tumor multimodal imaging, hyperthermic therapy, and photodynamic therapy. Mesoporous silica is employed in these systems to host the drug load and control its release [114Hembury M, Chiappini C, Bertazzo S, et al. Gold-silica quantum rattles for multimodal imaging and therapy. Proc Natl Acad Sci USA 2015; 112(7): 1959-64.
[http://dx.doi.org/10.1073/pnas.1419622112] [PMID: 25653336] ].
Drug delivery is focused on lipid NPs which have pharmacokinetic, stability, biodistribution and toxicity limitations. Polymeric NPs are drug delivery systems that can escape endosomal activities and offer greater anticancer efficiency [115Haabeth OAW, Blake TR, McKinlay CJ, Waymouth RM, Wender PA, Levy R. mRNA vaccination with charge-altering releasable transporters elicits human T cell responses and cures established tumors in mice Proc Natl Acad Sci 2018; 115: E9153-61.]. NPs composed of a combination of mixed lipids are vectors for delivering oligonucleotides across the cell membrane. The mechanism for releasing oligonucleotides is endocytotic. These various lipid block hybrid NPs have a high efficiency of transfection of cells with the ribonucleic acid messenger (mRNA), allowing protein expression. These lipid hybrid NPs have minimal toxicity and promising medical strategies [116Liu Y, Gunda V, Zhu X, et al. Theranostic near-infrared fluorescent nanoplatform for imaging and systemic siRNA delivery to metastatic anaplastic thyroid cancer. Proc Natl Acad Sci USA 2016; 113(28): 7750-5.
[http://dx.doi.org/10.1073/pnas.1605841113] [PMID: 27342857] , 117McKinlay CJ, Benner NL, Haabeth OA, Waymouth RM, Wender PA. Enhanced mRNA delivery into lymphocytes enabled by lipid-varied libraries of charge-altering releasable transporters. Proc Natl Acad Sci USA 2018; 115(26): E5859-66.
[http://dx.doi.org/10.1073/pnas.1805358115] [PMID: 29891683] ]. Mechanisms that involve tumor growth and metastasis depend on the genetic mutations of oncogenes. RNAi represents a promising strategy for the treatment of human diseases, including cancer. Hybrid polymer NPs used for in vivo and systemic delivery of siRNA to tumors specifically deactivate the expression of mutated oncogenes. These siRNA-conjugated polymeric NPs have no toxicity risk to patients and are photostable. They provide theranostic follow-up by non-invasive imaging of tumors ; also long-term circulation, prolonged-release, and significant accumulation of drugs in tumors. They are capable of efficient gene silencing and have negligible side effects [118Zhu X, Xu Y, Solis LM, et al. Long-circulating siRNA nanoparticles for validating Prohibitin1-targeted non-small cell lung cancer treatment. Proc Natl Acad Sci USA 2015; 112(25): 7779-84.
[http://dx.doi.org/10.1073/pnas.1505629112] [PMID: 26056316] ].
Fluidic microchips were used to probe the translocation of hybrid polymeric NPs through endothelial cells and in vivo imaging monitoring of their transvascular permeability [119Kim Y, Lobatto ME, Kawahara T, et al. Probing nanoparticle translocation across the permeable endothelium in experimental atherosclerosis. Proc Natl Acad Sci USA 2014; 111(3): 1078-83.
[http://dx.doi.org/10.1073/pnas.1322725111] [PMID: 24395808] ]. The hybrid anti-cancer systems integrate Yttrium 90 (90Y) beta-emitting radionuclides inside of nanocrystalline matrix coated with NIR light upconversion polymer and coupled to recombinant proteins composed of two modules: i) the DARP in module targeting cells overexpressing the HER2 receptor oncomarker ii) the Exotoxin A cytotoxic module inhibiting the synthesis of HER2-positive cell proteins. These nanocomple-xes have diagnostic functions and selective drug therapy of cancer cells [120Guryev EL, Volodina NO, Shilyagina NY, et al. Radioactive (90Y) upconversion nanoparticles conjugated with recombinant targeted toxin for synergistic nanotheranostics of cancer. Proc Natl Acad Sci USA 2018; 115(39): 9690-5.
[http://dx.doi.org/10.1073/pnas.1809258115] [PMID: 30194234] ].
CONCLUSION
The tools of nanotechnology are promising for the treatment of a wide range of diseases. Several nanotechnology platforms have been developed and several clinical trials have demonstrated their anticancer benefits, thereby liposomes are widely used. Dendrimers, cantilevers, and CNTs are emerging nano-applications in cancer medicine. Drug resistance results from the accumulation of lactic acid in poorly vascularized cancer sites, the NPs make it possible to overcome these problems of administration of the drugs to the cancerous tissues. NPs are characterized by their modular size, their high surface-volume ratio, their loadable surface with biological substances of interest, and their stability in wide ranges of temperature and pH. Nevertheless, nanoparticles have many challenges to overcome, notably their accumulation in the liver and the spleen, as well as the different barriers to cross to reach the cancer cells. Antisense oligonucleotides or RNAi offer an opportunity to treat cancers by specifically disabling the expression of target genes that lead to tumor growth. Nevertheless, this approach confronts problems of side effects related to their pairing with non-target mRNA, the induction of the innate immune response, their short half-life and their destruction by serum nucleases. Hybrid NP polymers effectively protect siRNA and allow NIR imaging to track in real-time the systemic and in vivo biodistribution of siRNA to tumors. Polymeric hybrid NPs show photostability and significant systemic circulation time. Nanotechnology presents new ways to diagnose, treat and follows patients. The characterization and lack of biodegradation of NPs raise concerns about the safety of their clinical use. The toxic effects of NPs are not sufficiently known. Regulations hinder the widespread application of NPs in cancer medicine because of potential health risks for patients. Successful clinical trials and compliance with quality guidelines would address the challenges facing the NPs on the marketplace.
LIST OF ABBREVIATIONS
| AuNPs | = Gold Nanoparticles |
| BRAF | = B-Raf proto-oncogene |
| CCD | = Charge Coupled Device |
| CdS | = Cadmium Sulfide |
| CNTs | = Carbon Nanotubes |
| CT | = Computerized Tomography |
| DARPin | = Designed Ankyrin Repeat Proteins |
| DNA | = Deoxyribonucleic acid |
| ERBB3 | = Receptor tyrosine-protein kinase erbB-3 |
| EPR | = Effect of Permeability and increased Retention |
| HER2 receptor | = Human Epidermal Growth Factor Receptor-2 |
| MRI | = Magnetic Resonance Imaging |
| NIR | = Near-infrared Region |
| NP | = Nanoparticle |
| (NMR) | = Nuclear Magnetic Resonance |
| PbS | = Lead Sulfide |
| PEG | = Polyethylene Glycol |
| Pet | = Positron Emission Tomography |
| pH | = Potentiel Hydrogen |
| QD | = Quantum Dot |
| RES | = reticuloendothelial System |
| RES | = Reticuloendothelial System |
| RNAi | = Interfering RNA |
| mRNA | = Ribonucleic Acid Messenger |
| siRNA | = Small Interfering RNA |
| SNAs | = Spherical Nucleic Acids |
| T1 | = spin-lattice Relaxation Time in Contrast |
| Theranostics | = Diagnosis and Therapy Coupled in the Same System |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The author acknowledges Norri Zahra and Regragui Moumaris. The author thanks Marie-Hélène Maës, Comtesse Marie-Françoise d'Andigné, Anne de Maisonneuve and Benoît de Maisonneuve, Monique Abuaf, Lucienne Masse AP-HP and Association Recherche et Développement Biomédical.
REFERENCES
| [1] | Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100(1): 57-70. [http://dx.doi.org/10.1016/S0092-8674(00)81683-9] [PMID: 10647931] |
| [2] | Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011; 144(5): 646-74. [http://dx.doi.org/10.1016/j.cell.2011.02.013] [PMID: 21376230] |
| [3] | Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J Control Release 2015; 200: 138-57. [http://dx.doi.org/10.1016/j.jconrel.2014.12.030] [PMID: 25545217] |
| [4] | Palsdottir T, Nordstrom T, Karlsson A, Grönberg H, Clements M, Eklund M. The impact of different prostate-specific antigen (PSA) testing intervals on Gleason score at diagnosis and the risk of experiencing false-positive biopsy recommendations: A population-based cohort study. BMJ Open 2019; 9(3): e027958. [http://dx.doi.org/10.1136/bmjopen-2018-027958] [PMID: 30928965] |
| [5] | Sheng M, Xie X, Wang J, Gu W. A Pathway-Based Strategy to Identify Biomarkers for Lung Cancer Diagnosis and Prognosis. Evol Bioinform Online 2019; 15: 1176934319838494. [http://dx.doi.org/10.1177/1176934319838494] [PMID: 30923439] |
| [6] | Suzuki T, Kitagawa Y, Nankinzan R, Yamaguchi T. Early gastric cancer diagnostic ability of ultrathin endoscope loaded with laser light source. World J Gastroenterol 2019; 25(11): 1378-86. [http://dx.doi.org/10.3748/wjg.v25.i11.1378] [PMID: 30918430] |
| [7] | García-Figueiras R, Baleato-González S, Padhani AR, et al. How clinical imaging can assess cancer biology. Insights Imaging 2019; 10(1): 28. [http://dx.doi.org/10.1186/s13244-019-0703-0] [PMID: 30830470] |
| [8] | Ahdi Rezaeieh S, Zamani A, Bialkowski KS, Macdonald GA, Abbosh AM. Three-dimensional electromagnetic torso scanner. Sensors (Basel) 2019; 19(5): E1015. [http://dx.doi.org/10.3390/s19051015] [PMID: 30818868] |
| [9] | Badawi RD, Shi H, Hu P, et al. First human imaging studies with the explorer total-body pet scanner. J Nucl Med 2019; 60(3): 299-303. [http://dx.doi.org/10.2967/jnumed.119.226498] [PMID: 30733314] |
| [10] | Avram AM, Dewaraja YK. Thyroid cancer radiotheragnostics: the case for activity adjusted 131I therapy. Clin Transl Imaging 2018; 6(5): 335-46. [http://dx.doi.org/10.1007/s40336-018-0291-x] [PMID: 30911535] |
| [11] | Li C, Liang H, Zhong N, He J, Liang W. Optimal starting age for lung cancer screening with low-dose computed tomography: A Population Level Analysis. J Thorac Oncol 2019; 14(4): 82-4. |
| [12] | Verschueren J, Huyghe I, Van den wt T. Ovarian cancer unmasked by technetium-99m bone scintigraphy and single-photon emission computed tomography-computed tomography. World J Nucl Med 2019; 18(1): 58-60. [http://dx.doi.org/10.4103/wjnm.WJNM_66_17] [PMID: 30774548] |
| [13] | Renard-Penna R, Gauthé M. The future of molecular and functional imaging in prostate cancer. Arch Esp Urol 2019; 72(2): 150-6. [PMID: 30855016] |
| [14] | Simon K, Dodelzon K, Drotman M, et al. Accuracy of synthetic 2d mammography compared with conventional 2d digital mammography obtained with 3d tomosynthesis. AJR Am J Roentgenol 2019; 1-6. [http://dx.doi.org/10.2214/AJR.18.20520] [PMID: 30917028] |
| [15] | Mao MH, Wang S, Feng ZE, et al. Accuracy of magnetic resonance imaging in evaluating the depth of invasion of tongue cancer. A prospective cohort study. Oral Oncol 2019; 91: 79-84. [http://dx.doi.org/10.1016/j.oraloncology.2019.01.021] [PMID: 30926067] |
| [16] | Rehman O, Zhuang H, Muhamed Ali A, Ibrahim A, Li Z. Validation of mirnas as breast cancer biomarkers with a machine learning approach. Cancers (Basel) 2019; 11(3): E431. [http://dx.doi.org/10.3390/cancers11030431] [PMID: 30917548] |
| [17] | Filipów S, Łaczmański Ł. Blood circulating mirnas as cancer biomarkers for diagnosis and surgical treatment response. Front Genet 2019; 10: 169. [http://dx.doi.org/10.3389/fgene.2019.00169] [PMID: 30915102] |
| [18] | Sharma B, Kanwar SS. Phosphatidylserine: A cancer cell targeting biomarker. Semin Cancer Biol 2018; 52(Pt 1): 17-25. [http://dx.doi.org/10.1016/j.semcancer.2017.08.012] [PMID: 28870843] |
| [19] | i H, Cho JY. I H. Lung cancer biomarkers. Adv Clin Chem 2015; 72: 107-70. [http://dx.doi.org/10.1016/bs.acc.2015.07.003] [PMID: 26471082] |
| [20] | Costa-Pinheiro P, Montezuma D, Henrique R, Jerónimo C. Diagnostic and prognostic epigenetic biomarkers in cancer. Epigenomics 2015; 7(6): 1003-15. [http://dx.doi.org/10.2217/epi.15.56] [PMID: 26479312] |
| [21] | Qureshi HA, Abouyared M, Barber B, Houlton JJ. Surgical options for locally advanced oropharyngeal cancer. Curr Treat Options Oncol 2019; 20(5): 36. [http://dx.doi.org/10.1007/s11864-019-0621-x] [PMID: 30931485] |
| [22] | Koppiker CB, Noor AU, Dixit S, et al. Extreme oncoplastic surgery for multifocal/multicentric and locally advanced breast cancer. Int J Breast Cancer 2019; 2019: 4262589. [http://dx.doi.org/10.1155/2019/4262589] [PMID: 30915240] |
| [23] | Solanki AA, Bossi A, Efstathiou JA, et al. Combining immunotherapy with radiotherapy for the treatment of genitourinary malignancies. Eur Urol Oncol 2019; 2(1): 79-87. [http://dx.doi.org/10.1016/j.euo.2018.09.013] [PMID: 30929848] |
| [24] | Jha AK, Neupane P, Pradhan M, et al. Ewing sarcoma in Nepal treated with combined chemotherapy and definitive radiotherapy. J Glob Oncol 2019; 5: 1-10. [http://dx.doi.org/10.1200/JGO.19.00015] [PMID: 30917070] |
| [25] | Harlid S, Xu Z, Kirk E, Wilson LE, Troester MA, Taylor JA. Hormone therapy use and breast tissue DNA methylation: Analysis of epigenome wide data from the normal breast study. Epigenetics 2019; 14(2): 146-57. [http://dx.doi.org/10.1080/15592294.2019.1580111] [PMID: 30821641] |
| [26] | Beardo P, Osman I, San José B, et al. Safety and outcomes of new generation hormone-therapy in elderly chemotherapy-naive metastatic castration-resistant prostate cancer patients in the real world. Arch Gerontol Geriatr 2019; 82: 179-85. [http://dx.doi.org/10.1016/j.archger.2019.02.008] |
| [27] | Liu X, Liu S, Lyu H, Riker AI, Zhang Y, Liu B. Development of effective therapeutics targeting her3 for cancer treatment. Biol Proced Online 2019; 21: 5. [http://dx.doi.org/10.1186/s12575-019-0093-1] [PMID: 30930695] |
| [28] | Griguolo G, Pascual T, Dieci MV, Guarneri V, Prat A. Interaction of host immunity with HER2-targeted treatment and tumor heterogeneity in HER2-positive breast cancer. J Immunother Cancer 2019; 7(1): 90. [http://dx.doi.org/10.1186/s40425-019-0548-6] [PMID: 30922362] |
| [29] | Bao J, Qiao L. New developments on targeted cancer therapy: Multi-faceted issues in targeted cancer therapy. Cancer Lett 2017; 387: 1-2. [http://dx.doi.org/10.1016/j.canlet.2016.12.016] [PMID: 28038727] |
| [30] | Gotwals P, Cameron S, Cipolletta D, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer 2017; 17(5): 286-301. [http://dx.doi.org/10.1038/nrc.2017.17] [PMID: 28338065] |
| [31] | Peranzoni E, Donnadieu E. Improving efficacy of cancer immunotherapy through targeting of macrophages. Med Sci (Paris) 2019; 35(3): 207-9. [http://dx.doi.org/10.1051/medsci/2019043] [PMID: 30931902] |
| [32] | Aroldi F, Zaniboni A. Immunotherapy for pancreatic cancer: present and future. Immunotherapy 2017; 9(7): 607-16. [http://dx.doi.org/10.2217/imt-2016-0142] [PMID: 28595517] |
| [33] | Dong J, Li B, Lin D, Zhou Q, Huang D. Advances in targeted therapy and immunotherapy for non-small cell lung cancer based on accurate molecular typing. Front Pharmacol 2019; 10: 230. [http://dx.doi.org/10.3389/fphar.2019.00230] [PMID: 30930778] |
| [34] | Xu Y, Ren H, Liu J, et al. A switchable NO-releasing nanomedicine for enhanced cancer therapy and inhibition of metastasis. Nanoscale 2019; 11(12): 5474-88. [http://dx.doi.org/10.1039/C9NR00732F] [PMID: 30855625] |
| [35] | Wen R, Umeano AC, Kou Y, Xu J, Farooqi AA. Nanoparticle systems for cancer vaccine. Nanomedicine (Lond) 2019; 14(5): 627-48. [http://dx.doi.org/10.2217/nnm-2018-0147] [PMID: 30806568] |
| [36] | Dhandapani R, Sethuraman S, Subramanian A. Nanohybrids - cancer theranostics for tiny tumor clusters. J Control Release 2019; 299: 21-30. [http://dx.doi.org/10.1016/j.jconrel.2019.02.027] [PMID: 30797000] |
| [37] | Navyatha B, Nara S. Gold nanostructures as cancer theranostic probe: promises and hurdles. Nanomedicine (Lond) 2019; 14(6): 766-96. [http://dx.doi.org/10.2217/nnm-2018-0170] [PMID: 30794100] |
| [38] | Bor G, Mat Azmi ID, Yaghmur A. Nanomedicines for cancer therapy: Current status, challenges and future prospects. Ther Deliv 2019; 10(2): 113-32. [http://dx.doi.org/10.4155/tde-2018-0062] [PMID: 30678550] |
| [39] | Li R, Liu B, Gao J. The application of nanoparticles in diagnosis and theranostics of gastric cancer. Cancer Lett 2017; 386: 123-30. [http://dx.doi.org/10.1016/j.canlet.2016.10.032] [PMID: 27845158] |
| [40] | Wu A. Cancer imaging, therapy and theranostics based on different types of functional materials. Curr Med Chem 2018; 25(25): 2874-5. [http://dx.doi.org/10.2174/092986732525180719103001] [PMID: 30191774] |
| [41] | Badu-Peprah A, Adu-Sarkodie Y. Accuracy of clinical diagnosis, mammography and ultrasonography in preoperative assessment of breast cancer. Ghana Med J 2018; 52: 133-9. [http://dx.doi.org/10.4314/gmj.v52i4.11] |
| [42] | Cochran JM, Busch DR, Lin L, et al. Hybrid time-domain and continuous-wave diffuse optical tomography instrument with concurrent, clinical magnetic resonance imaging for breast cancer imaging. J Biomed Opt 2019; 24(5): 1-11. [http://dx.doi.org/10.1117/1.JBO.24.5.051409] [PMID: 30680976] |
| [43] | Moumaris M, Bretagne JM, Abuaf N. Hospital engineering of medical devices in France. Open Med Devices J 2018; 06: 10-20. [http://dx.doi.org/10.2174/1875181401806010010] |
| [44] | Moumaris M, Rajoely B, Abuaf N. Fluorescein isothiocyanate-dextran can track apoptosis and necrosis induced by heat shock of peripheral blood mononuclear cells and hela cells. Open Biol Sci J 2015; 1: 7-15. [http://dx.doi.org/10.2174/2352633501501010007] |
| [45] | Moumaris M, Abuaf N. Use of labeled dextran for in-vitro assessment of increased cell permeability, cell death and apoptosis. Brevet n°00/09235 2002; 2811682: A3 |
| [46] | Esfandyarpour R, DiDonato MJ, Yang Y, Durmus NG, Harris JS, Davis RW. Multifunctional, inexpensive, and reusable nanoparticle-printed biochip for cell manipulation and diagnosis. Proc Natl Acad Sci USA 2017; 114(8): E1306-15. [http://dx.doi.org/10.1073/pnas.1621318114] [PMID: 28167769] |
| [47] | Litwin MS, Tan HJ. The diagnosis and treatment of prostate Cancer: A Review. JAMA 2017; 317(24): 2532-42. [http://dx.doi.org/10.1001/jama.2017.7248] [PMID: 28655021] |
| [48] | Lenaghan SC, Wang Y, Xi N, et al. Grand challenges in bioengineered nanorobotics for cancer therapy. IEEE Trans Biomed Eng 2013; 60(3): 667-73. [http://dx.doi.org/10.1109/TBME.2013.2244599] [PMID: 23380844] |
| [49] | Nahrendorf M, Keliher E, Marinelli B, et al. Hybrid PET-optical imaging using targeted probes. Proc Natl Acad Sci USA 2010; 107(17): 7910-5. [http://dx.doi.org/10.1073/pnas.0915163107] [PMID: 20385821] |
| [50] | Dahlman JE, Kauffman KJ, Xing Y, et al. Barcoded nanoparticles for high throughput in vivo discovery of targeted therapeutics. Proc Natl Acad Sci USA 2017; 114(8): 2060-5. [http://dx.doi.org/10.1073/pnas.1620874114] [PMID: 28167778] |
| [51] | Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm 2015; 93: 52-79. [http://dx.doi.org/10.1016/j.ejpb.2015.03.018] [PMID: 25813885] |
| [52] | Xu X, Ho W, Zhang X, Bertrand N, Farokhzad O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol Med 2015; 21(4): 223-32. [http://dx.doi.org/10.1016/j.molmed.2015.01.001] [PMID: 25656384] |
| [53] | Mazor R, King EM, Onda M, et al. Tolerogenic nanoparticles restore the antitumor activity of recombinant immunotoxins by mitigating immunogenicity. Proc Natl Acad Sci USA 2018; 115(4): E733-42. [http://dx.doi.org/10.1073/pnas.1717063115] [PMID: 29311317] |
| [54] | Tavares AJ, Poon W, Zhang YN, et al. Effect of removing Kupffer cells on nanoparticle tumor delivery. Proc Natl Acad Sci USA 2017; 114(51): E10871-80. [http://dx.doi.org/10.1073/pnas.1713390114] [PMID: 29208719] |
| [55] | Han HD, Mangala LS, Lee JW, et al. Targeted gene silencing using RGD-labeled chitosan nanoparticles Clin Cancer Res 2010; 16: 3910-22. |
| [56] | Stylianopoulos T, Wong C, Bawendi MG, Jain RK, Fukumura D. Multistage nanoparticles for improved delivery into tumor tissue. Methods Enzymol 2012; 508: 109-30. [http://dx.doi.org/10.1016/B978-0-12-391860-4.00006-9] [PMID: 22449923] |
| [57] | Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov 2010; 9(8): 615-27. [http://dx.doi.org/10.1038/nrd2591] [PMID: 20616808] |
| [58] | Ryu JH, Koo H, Sun IC, et al. Tumor-targeting multi-functional nanoparticles for theragnosis: new paradigm for cancer therapy. Adv Drug Deliv Rev 2012; 64(13): 1447-58. [http://dx.doi.org/10.1016/j.addr.2012.06.012] [PMID: 22772034] |
| [59] | Curk T, Dobnikar J, Frenkel D. Optimal multivalent targeting of membranes with many distinct receptors. Proc Natl Acad Sci USA 2017; 114(28): 7210-5. [http://dx.doi.org/10.1073/pnas.1704226114] [PMID: 28652338] |
| [60] | Carrow JK, Cross LM, Reese RW, et al. Widespread changes in transcriptome profile of human mesenchymal stem cells induced by two-dimensional nanosilicates. Proc Natl Acad Sci USA 2018; 115(17): E3905-13. [http://dx.doi.org/10.1073/pnas.1716164115] [PMID: 29643075] |
| [61] | Gu Y, Li J, Li Y, et al. Nanomicelles loaded with doxorubicin and curcumin for alleviating multidrug resistance in lung cancer. Int J Nanomedicine 2016; 11: 5757-70. [http://dx.doi.org/10.2147/IJN.S118568] [PMID: 27843316] |
| [62] | Chen Q, Liang C, Sun X, et al. H2O2-responsive liposomal nanoprobe for photoacoustic inflammation imaging and tumor theranostics via in vivo chromogenic assay. Proc Natl Acad Sci USA 2017; 114(21): 5343-8. [http://dx.doi.org/10.1073/pnas.1701976114] [PMID: 28484000] |
| [63] | Estanqueiro M, Amaral MH, Conceição J, Sousa Lobo JM. Nanotechnological carriers for cancer chemotherapy: The state of the art. Colloids Surf B Biointerfaces 2015; 126: 631-48. [http://dx.doi.org/10.1016/j.colsurfb.2014.12.041] [PMID: 25591851] |
| [64] | Gharib A, Faezizadeh Z, Mesbah-Namin SA, Saravani R. Experimental treatment of breast cancer-bearing BALB/c mice by artemisinin and transferrin-loaded magnetic nanoliposomes. Pharmacogn Mag 2015; 11(Suppl. 1): S117-22. [http://dx.doi.org/10.4103/0973-1296.157710] [PMID: 26109756] |
| [65] | Lee MH, Lee DH, Jung SW, Lee KN, Park YS, Seong WK. Measurements of serum C-reactive protein levels in patients with gastric cancer and quantification using silicon nanowire arrays. Nanomedicine (Lond) 2010; 6(1): 78-83. [http://dx.doi.org/10.1016/j.nano.2009.04.004] [PMID: 19446654] |
| [66] | Ji SR, Liu C, Zhang B, et al. Carbon nanotubes in cancer diagnosis and therapy. Biochim Biophys Acta 2010; 1806(1): 29-35. [PMID: 20193746] |
| [67] | Garaj S, Hubbard W, Reina A, Kong J, Branton D, Golovchenko JA. Graphene as a subnanometre trans-electrode membrane. Nature 2010; 467(7312): 190-3. [http://dx.doi.org/10.1038/nature09379] [PMID: 20720538] |
| [68] | Minervini CF, Cumbo C, Orsini P, et al. TP53 gene mutation analysis in chronic lymphocytic leukemia by nanopore MinION sequencing. Diagn Pathol 2016; 11(1): 96. [http://dx.doi.org/10.1186/s13000-016-0550-y] [PMID: 27724982] |
| [69] | Clarke J, Wu HC, Jayasinghe L, Patel A, Reid S, Bayley H. Continuous base identification for single-molecule nanopore DNA sequencing. Nat Nanotechnol 2009; 4(4): 265-70. [http://dx.doi.org/10.1038/nnano.2009.12] [PMID: 19350039] |
| [70] | Sailor MJ, Park JH. Hybrid nanoparticles for detection and treatment of cancer. Adv Mater 2012; 24(28): 3779-802. [http://dx.doi.org/10.1002/adma.201200653] [PMID: 22610698] |
| [71] | Dykman L, Khlebtsov N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem Soc Rev 2012; 41: 2256-82. [http://dx.doi.org/10.1039/C1CS15166E] |
| [72] | Loo C, Lin A, Hirsch L, et al. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol Cancer Res Treat 2004; 3(1): 33-40. [http://dx.doi.org/10.1177/153303460400300104] [PMID: 14750891] |
| [73] | Abbasi A, Park K, Bose A, Bothun GD. Near-infrared responsive gold-layersome nanoshells. Langmuir 2017; 33(21): 5321-7. [http://dx.doi.org/10.1021/acs.langmuir.7b01273] [PMID: 28486807] |
| [74] | Bardhan R, Grady NK, Halas NJ. Nanoscale control of near-infrared fluorescence enhancement using Au nanoshells. Small 2008; 4(10): 1716-22. [http://dx.doi.org/10.1002/smll.200800405] [PMID: 18819167] |
| [75] | Choi MR, Stanton-Maxey KJ, Stanley JK, et al. A cellular Trojan Horse for delivery of therapeutic nanoparticles into tumors. Nano Lett 2007; 7(12): 3759-65. [http://dx.doi.org/10.1021/nl072209h] [PMID: 17979310] |
| [76] | Hirsch LR, Stafford RJ, Bankson JA, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci USA 2003; 100(23): 13549-54. [http://dx.doi.org/10.1073/pnas.2232479100] [PMID: 14597719] |
| [77] | Marangoni VS, Neumann O, Henderson L, et al. Enhancing T1 magnetic resonance imaging contrast with internalized gadolinium(III) in a multilayer nanoparticle. Proc Natl Acad Sci USA 2017; 114(27): 6960-5. [http://dx.doi.org/10.1073/pnas.1701944114] [PMID: 28630340] |
| [78] | Goodman AM, Neumann O, Nørregaard K, et al. Near-infrared remotely triggered drug-release strategies for cancer treatment. Proc Natl Acad Sci USA 2017; 114(47): 12419-24. [http://dx.doi.org/10.1073/pnas.1713137114] [PMID: 29109274] |
| [79] | Ali MRK, Wu Y, Tang Y, et al. Targeting cancer cell integrins using gold nanorods in photothermal therapy inhibits migration through affecting cytoskeletal proteins. Proc Natl Acad Sci USA 2017; 114(28): E5655-63. [http://dx.doi.org/10.1073/pnas.1703151114] [PMID: 28652358] |
| [80] | Chen J, Saeki F, Wiley BJ, et al. Gold nanocages: Bioconjugation and their potential use as optical imaging contrast agents. Nano Lett 2005; 5(3): 473-7. [http://dx.doi.org/10.1021/nl047950t] [PMID: 15755097] |
| [81] | Au L, Zheng D, Zhou F, Li ZY, Li X, Xia Y. A quantitative study on the photothermal effect of immuno gold nanocages targeted to breast cancer cells. ACS Nano 2008; 2(8): 1645-52. [http://dx.doi.org/10.1021/nn800370j] [PMID: 19206368] |
| [82] | Kralj S, Makovec D. Magnetic assembly of superparamagnetic iron oxide nanoparticle clusters into nanochains and nanobundles. ACS Nano 2015; 9(10): 9700-7. [http://dx.doi.org/10.1021/acsnano.5b02328] [PMID: 26394039] |
| [83] | Ungureanu BS, Teodorescu CM, Săftoiu A. Magnetic nanoparticles for hepatocellular carcinoma diagnosis and therapy. J Gastrointestin Liver Dis 2016; 25(3): 375-83. [PMID: 27689203] |
| [84] | Rabias I, Tsitrouli D, Karakosta E, et al. Rapid magnetic heating treatment by highly charged maghemite nanoparticles on Wistar rats exocranial glioma tumors at microliter volume. Biomicrofluidics 2010; 4(2)024111 [http://dx.doi.org/10.1063/1.3449089] [PMID: 20697578] |
| [85] | Kumar CS, Mohammad F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv Drug Deliv Rev 2011; 63: 789-808. [http://dx.doi.org/10.1016/j.addr.2011.03.008] |
| [86] | Scarberry KE, Dickerson EB, McDonald JF, Zhang ZJ. Magnetic nanoparticle-peptide conjugates for in vitro and in vivo targeting and extraction of cancer cells. J Am Chem Soc 2008; 130(31): 10258-62. [http://dx.doi.org/10.1021/ja801969b] [PMID: 18611005] |
| [87] | Colombo M, Carregal-Romero S, Casula MF, et al. Biological applications of magnetic nanoparticles. Chem Soc Rev 2012; 41(11): 4306-34. [http://dx.doi.org/10.1039/c2cs15337h] [PMID: 22481569] |
| [88] | Javidi M, Heydari M, Attar MM, et al. Cylindrical agar gel with fluid flow subjected to an alternating magnetic field during hyperthermia. Int J Hyperthermia 2015; 31(1): 33-9. [http://dx.doi.org/10.3109/02656736.2014.988661] [PMID: 25523967] |
| [89] | Javidi M, Heydari M, Karimi A, Haghpanahi M, Navidbakhsh M, Razmkon A. Evaluation of the effects of injection velocity and different gel concentrations on nanoparticles in hyperthermia therapy. J Biomed Phys Eng 2014; 4(4): 151-62. [PMID: 25599061] |
| [90] | Estelrich J, Escribano E, Queralt J, Busquets MA. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int J Mol Sci 2015; 16(4): 8070-101. [http://dx.doi.org/10.3390/ijms16048070] [PMID: 25867479] |
| [91] | Ho D. Nanodiamond-based chemotherapy and imaging. Cancer Treat Res 2015; 166: 85-102. [http://dx.doi.org/10.1007/978-3-319-16555-4_4] [PMID: 25895865] |
| [92] | Gupta C, Prakash D, Gupta S. Cancer treatment with nano-diamonds. Front Biosci (Schol Ed) 2017; 9: 62-70. [http://dx.doi.org/10.2741/s473] [PMID: 27814575] |
| [93] | Mochalin VN, Pentecost A, Li XM, et al. Adsorption of drugs on nanodiamond: Toward development of a drug delivery platform. Mol Pharm 2013; 10(10): 3728-35. [http://dx.doi.org/10.1021/mp400213z] [PMID: 23941665] |
| [94] | Vaijayanthimala V, Chang HC. Functionalized fluorescent nanodiamonds for biomedical applications. Nanomedicine (Lond) 2009; 4(1): 47-55. [http://dx.doi.org/10.2217/17435889.4.1.47] [PMID: 19093895] |
| [95] | Lee DK, Kee T, Liang Z, et al. Clinical validation of a nanodiamond-embedded thermoplastic biomaterial. Proc Natl Acad Sci USA 2017; 114(45): E9445-54. [http://dx.doi.org/10.1073/pnas.1711924114] [PMID: 29078364] |
| [96] | Hong C, Song D, Lee DK, et al. Reducing posttreatment relapse in cleft lip palatal expansion using an injectable estrogen-nanodiamond hydrogel. Proc Natl Acad Sci USA 2017; 114(35): E7218-25. [http://dx.doi.org/10.1073/pnas.1704027114] [PMID: 28808036] |
| [97] | Wang LW, Peng CW, Chen C, Li Y. Quantum dots-based tissue and in vivo imaging in breast cancer researches: current status and future perspectives. Breast Cancer Res Treat 2015; 151(1): 7-17. [http://dx.doi.org/10.1007/s10549-015-3363-x] [PMID: 25833213] |
| [98] | Zhang M, Yue J, Cui R, et al. Bright quantum dots emitting at ∼1,600 nm in the NIR-IIb window for deep tissue fluorescence imaging. Proc Natl Acad Sci USA 2018; 115(26): 6590-5. [http://dx.doi.org/10.1073/pnas.1806153115] [PMID: 29891702] |
| [99] | Schroeder JE, Shweky I, Shmeeda H, Banin U, Gabizon A. Folate-mediated tumor cell uptake of quantum dots entrapped in lipid nanoparticles. J Control Release 2007; 124(1-2): 28-34. [http://dx.doi.org/10.1016/j.jconrel.2007.08.028] [PMID: 17928088] |
| [100] | Smith AM, Dave S, Nie S, True L, Gao X. Multicolor quantum dots for molecular diagnostics of cancer. Expert Rev Mol Diagn 2006; 6(2): 231-44. [http://dx.doi.org/10.1586/14737159.6.2.231] [PMID: 16512782] |
| [101] | Allison R, Moghissi K, Downie G, Dixon K. Photodynamic therapy (PDT) for lung cancer Photodiagnosis Photodyn Ther 2011; 8: 231-9. |
| [102] | Young JK, Figueroa ER, Drezek RA. Tunable nanostructures as photothermal theranostic agents. Ann Biomed Eng 2012; 40(2): 438-59. [http://dx.doi.org/10.1007/s10439-011-0472-5] [PMID: 22134466] |
| [103] | Kim HS, Han HD, Armaiz-Pena GN, et al. Functional roles of Src and Fgr in ovarian carcinoma. Clin Cancer Res 2011; 17(7): 1713-21. [http://dx.doi.org/10.1158/1078-0432.CCR-10-2081] [PMID: 21300758] |
| [104] | Astruc D, Boisselier E, Ornelas C. Dendrimers designed for functions: from physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem Rev 2010; 110(4): 1857-959. [http://dx.doi.org/10.1021/cr900327d] [PMID: 20356105] |
| [105] | Yao H, Veine DM, Fay KS, Staszewski ED, Zeng ZZ, Livant DL. The PHSCN dendrimer as a more potent inhibitor of human breast cancer cell invasion, extravasation, and lung colony formation. Breast Cancer Res Treat 2011; 125(2): 363-75. [http://dx.doi.org/10.1007/s10549-010-0826-y] [PMID: 20300829] |
| [106] | S S S A, Krishnamoorthy K, Rajappan M. Nanosponges: A novel class of drug delivery system--review. J Pharm Pharm Sci 2012; 15: 103-11. [http://dx.doi.org/10.18433/J3K308] |
| [107] | Rigo C, Ferroni L, Tocco I, et al. Active silver nanoparticles for wound healing. Int J Mol Sci 2013; 14(3): 4817-40. [http://dx.doi.org/10.3390/ijms14034817] [PMID: 23455461] |
| [108] | Osmani RA, Hani U, Bhosale RR, Kulkarni PK, Shanmuganathan S. Nanosponge carriers- an archetype swing in cancer therapy: A comprehensive review. Curr Drug Targets 2017; 18(1): 108-18. [http://dx.doi.org/10.2174/1389450116666151001105449] [PMID: 26424399] |
| [109] | Huang X, Chisholm J, Zhuang J, et al. Protein nanocages that penetrate airway mucus and tumor tissue. Proc Natl Acad Sci USA 2017; 114(32): E6595-602. [http://dx.doi.org/10.1073/pnas.1705407114] [PMID: 28739953] |
| [110] | Thamphiwatana S, Angsantikul P, Escajadillo T, et al. Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc Natl Acad Sci USA 2017; 114(43): 11488-93. [http://dx.doi.org/10.1073/pnas.1714267114] [PMID: 29073076] |
| [111] | Sarett SM, Werfel TA, Lee L, et al. Lipophilic siRNA targets albumin in situ and promotes bioavailability, tumor penetration, and carrier-free gene silencing. Proc Natl Acad Sci USA 2017; 114(32): E6490-7. [http://dx.doi.org/10.1073/pnas.1621240114] [PMID: 28739942] |
| [112] | Yu D, Khan OF, Suvà ML, et al. Multiplexed RNAi therapy against brain tumor-initiating cells via lipopolymeric nanoparticle infusion delays glioblastoma progression. Proc Natl Acad Sci USA 2017; 114(30): E6147-56. [http://dx.doi.org/10.1073/pnas.1701911114] [PMID: 28696296] |
| [113] | Sita TL, Kouri FM, Hurley LA, et al. Dual bioluminescence and near-infrared fluorescence monitoring to evaluate spherical nucleic acid nanoconjugate activity in vivo. Proc Natl Acad Sci USA 2017; 114(16): 4129-34. [http://dx.doi.org/10.1073/pnas.1702736114] [PMID: 28373576] |
| [114] | Hembury M, Chiappini C, Bertazzo S, et al. Gold-silica quantum rattles for multimodal imaging and therapy. Proc Natl Acad Sci USA 2015; 112(7): 1959-64. [http://dx.doi.org/10.1073/pnas.1419622112] [PMID: 25653336] |
| [115] | Haabeth OAW, Blake TR, McKinlay CJ, Waymouth RM, Wender PA, Levy R. mRNA vaccination with charge-altering releasable transporters elicits human T cell responses and cures established tumors in mice Proc Natl Acad Sci 2018; 115: E9153-61. |
| [116] | Liu Y, Gunda V, Zhu X, et al. Theranostic near-infrared fluorescent nanoplatform for imaging and systemic siRNA delivery to metastatic anaplastic thyroid cancer. Proc Natl Acad Sci USA 2016; 113(28): 7750-5. [http://dx.doi.org/10.1073/pnas.1605841113] [PMID: 27342857] |
| [117] | McKinlay CJ, Benner NL, Haabeth OA, Waymouth RM, Wender PA. Enhanced mRNA delivery into lymphocytes enabled by lipid-varied libraries of charge-altering releasable transporters. Proc Natl Acad Sci USA 2018; 115(26): E5859-66. [http://dx.doi.org/10.1073/pnas.1805358115] [PMID: 29891683] |
| [118] | Zhu X, Xu Y, Solis LM, et al. Long-circulating siRNA nanoparticles for validating Prohibitin1-targeted non-small cell lung cancer treatment. Proc Natl Acad Sci USA 2015; 112(25): 7779-84. [http://dx.doi.org/10.1073/pnas.1505629112] [PMID: 26056316] |
| [119] | Kim Y, Lobatto ME, Kawahara T, et al. Probing nanoparticle translocation across the permeable endothelium in experimental atherosclerosis. Proc Natl Acad Sci USA 2014; 111(3): 1078-83. [http://dx.doi.org/10.1073/pnas.1322725111] [PMID: 24395808] |
| [120] | Guryev EL, Volodina NO, Shilyagina NY, et al. Radioactive (90Y) upconversion nanoparticles conjugated with recombinant targeted toxin for synergistic nanotheranostics of cancer. Proc Natl Acad Sci USA 2018; 115(39): 9690-5. [http://dx.doi.org/10.1073/pnas.1809258115] [PMID: 30194234] |